Abstract
Purpose
This phase I/II study sought to determine the safety and maximum-tolerated dose (MTD) of a novel schedule of belinostat, a histone deacetylase inhibitor administered prior to and in combination with cisplatin (P), doxorubicin (A) and cyclophosphamide (C) in thymic epithelial tumors (TET). Anti-tumor activity, pharmacokinetics, and biomarkers of response were also assessed.
Patients and methods
Patients with advanced, unresectable TET received increasing doses of belinostat as a continuous intravenous infusion over 48-hours with chemotherapy in 3-week cycles. In phase II, belinostat at the MTD was used.
Results
26 patients were enrolled (thymoma: 12; thymic carcinoma: 14). Dose-limiting toxicities at 2000 mg/m2 belinostat were grade 3 nausea and diarrhea and grade 4 neutropenia and thrombocytopenia, respectively, in two patients. 24 patients were treated at the MTD of 1000 mg/m2 with chemotherapy (P 50 mg/m2 on day 2; A 25 mg/m2 on days 2, 3; C 500 mg/m2 on day 3). Objective response rates in thymoma and thymic carcinoma were 64% [95% confidence interval: 30.8%–89.1%] and 21% (4.7%–50.8%) respectively. Modulation of pharmacodynamic markers of HDAC-inhibition and declines in regulatory T cell (Tregs) and exhausted CD8+ T cell populations were observed. Decline in Tregs was associated with response (p=0.0041) and progression-free survival (p=0.021). Declines in TIM-3+ CD8+T cells were larger in responders than non-responders (p=0.049).
Conclusion
This study identified the MTD of belinostat in combination with PAC and indicates that the combination is active and feasible in TETs. Immunomodulatory effects on regulatory T cells and TIM3+ CD8+ T cells warrant further study.
Keywords: thymic epithelial tumors, thymoma, thymic carcinoma, histone deacetylase inhibitor
Background
Thymic epithelial tumors (TETs) are rare malignancies with an incidence of 0.13 per 100,000 person-years in the United States.1 Depending on morphology and atypia of the neoplastic epithelial cells and their relative proportion compared with lymphocytes, the World Health Organization (WHO) classification groups TETs into a continuum of tumors with increasing degrees of aggressiveness: types A, AB, B1, B2, B3 and C, the latter also referred to as thymic carcinoma.2 The WHO classification and the Masaoka staging system,3 which takes into account the integrity of the thymic capsule, degree of local tumor invasion and presence of distant metastases are useful predictors of the biologic behavior and prognosis of TETs. Type A thymoma has an excellent overall survival rate of more than 95% at 10 years, whereas the 5-year survival for types B2, B3, and thymic carcinoma are 75%, 70%, and 48%, respectively.4 Thymic carcinomas account for less than 1% of TETs and display a more aggressive phenotype with higher likelihood of distant metastases compared with thymomas.5
Chemotherapy is used in patients with unresectable or recurrent disease. Several regimens have been used, but a combination of cisplatin, doxorubicin and cyclophosphamide (PAC) is one of the most commonly employed regimens.5,6 In a phase II trial of 30 patients among whom only one patient had thymic carcinoma, this combination resulted in an objective response rate (ORR) of 50%.7 The median duration of response and overall survival were 12 months and 38 months respectively. Largely due to the rarity of the disease, there are no randomized control trials comparing chemotherapies.5
Acetylation and deacetylation of histones, the protein component of the nucleosome core particle, plays an important role in the regulation of gene expression. The inhibition of histone deacetylase (HDAC) enzymes by HDAC inhibitors (HDACi) shifts the dynamic equilibrium between the deacetylation activity of HDACs, which has been associated with gene silencing, and the acetylation activity of histone acetyltransferases (HATs), which has been associated with chromatin decondensation and gene expression. In particular, HDACi have been reported to induce the expression of genes suppressing the malignant phenotype and have demonstrated promising anticancer activity against a variety of hematological malignancies. HDACi also mediate anti-cancer effects by inhibiting deacetylation of a number of non-histone proteins.8,9
Belinostat, N-hydroxy-3-(phenylsulphamoylphenyl) acrylamide, is an HDACi of the hydroxymate class which inhibits both HDAC class I and II enzymes in nanomolar concentrations in vitro.10 In a phase II trial of patients with TETs who had failed prior platinum-containing chemotherapy, we showed that belinostat has modest antitumor activity with minimal toxicity.11 Of 41 patients, two with thymoma achieved a partial response and 25 had stable disease, but no responses were seen in patients with thymic carcinoma. Median times to progression and overall survival were 5.8 and 19.1 months, respectively in the overall population.
Belinostat has been shown to potentiate the antitumor activity of platinum-containing agents in vitro.12 Potentiation of chemotherapy by HDAC inhibition is likely secondary to altered gene expression patterns allowing increased chemotherapy-sensitivity.13 Chromatin decondensation could also facilitate increased access to DNA-damaging agents and target drug-tolerant subpopulations which evolve via an altered chromatin state in response to stressful exposures such as chemotherapy.14
Here, we report results of a phase I/II study of belinostat in combination with PAC in the first- line treatment of advanced or recurrent TETs. We also evaluated the pharmacokinetics of belinostat when administered with chemotherapy and pharmacodynamic effects in peripheral blood.
Materials and Methods
Patients
Eligible patients were those more than 18 years of age with histologically-confirmed advanced (Masaoka stage III or IV) TETs who had not received prior systemic therapy for advanced disease with Eastern Cooperative Oncology Group (ECOG) performance status score 0 or 1, life expectancy more than 3 months, and adequate organ function. Patients at increased cardiac risk were excluded, including those with unstable angina, myocardial infarction within the previous 12 months, baseline prolongation of QT/QTc interval as demonstrated by repeated QTc interval > 500 ms and long QT syndrome. Other exclusion criteria included: resectable disease, untreated brain metastases, radiotherapy or chemotherapy within 3 weeks before study drug administration, or positive serology for human immunodeficiency virus.
The study was approved by the National Cancer Institute Institutional Review Board. The study was overseen by a Safety Monitoring Committee. All patients provided written informed consent to participate in the study before undergoing any study-related procedures. This trial was registered with ClinicalTrials.gov, number NCT01100944.
Study design
Patients received 250 mg/m2 or 500 mg/m2 of belinostat via four consecutive 12-hour continuous intravenous infusions (CIVI) for 48 hrs starting on day 1. At dose levels 1 and 2, patients received the same doses of doxorubicin (25 mg/m2 on days 2 and 3, IV push over 3–5 min), cisplatin (50 mg/m2 over 60 min IV on day 2 after doxorubicin), and cyclophosphamide (500 mg/m2 over 60 min IV on day 3 after doxorubicin). Combination of chemotherapy and belinostat was repeated every 21 days for a total of 6 cycles unless there was evidence of disease progression or intolerance of the study treatment.
The study was divided into 2 phases, a dose escalation phase (phase I) and an expansion phase (phase II). The phase I portion of the trial consisted of a stepwise increase in belinostat and chemotherapy using a standard phase I design with 4 planned dose levels to determine the safety and tolerability of this combination. A conventional 3 + 3 dose-escalation design was used with up to 3 additional patients added if one patient exhibited a dose-limiting toxicity (DLT). Dose escalation was halted if at least 2 out of a maximum of 6 patients within a cohort exhibited a DLT. Intra-patient dose escalations were not allowed. Table S1 contains the dose escalation schema. Criteria for defining DLT and maximum-tolerated dose (MTD) are in Supplementary Methods.
During the phase II, additional patients were enrolled to evaluate antitumor activity, safety, pharmacokinetics and pharmacodynamics. In this phase, patients were treated at the MTD. Treatment was discontinued in the event of progressive disease (PD) or unacceptable toxicity.
Patients with non-progressive disease after combination therapy could receive maintenance belinostat every 4 weeks until disease progression or unacceptable toxic effects developed. If however, disease was felt to be resectable, they were offered multimodality therapy including surgical resection and radiation. Patients with resectable disease did not receive maintetance belinostat.
Safety evaluations
Safety assessments included monitoring for treatment-related adverse events, clinical laboratory tests, vital signs, physical examinations, and 12-lead electrocardiograms (ECG). Adverse events were evaluated in accordance with National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.0, and were followed to a satisfactory resolution or a clinically stable endpoint.
Pharmacokinetic evaluations
During cycle 1, plasma samples were drawn pre-treatment, 0.5, 1, and 2 hours post-start of belinostat infusion on day 1; immediately prior to, immediately following, and 1-hr post-cisplatin on day 2; immediately prior to, immediately following, and 1- hour post-cyclophosphamide on day 3, as well as immediately following end of 48-hour belinostat infusion, and 5, 15, 30 minutes, 1, 2, 4, 6, 8, 12, and 24 hours after completion of belinostat infusion. This allowed for steady-state pharmacokinetics and the elimination of belinostat following the end of the 48-hour CIVI. Details of pharmacokinetic analyses are in Supplementary Methods.
Pharmacodynamic evaluations
Whole blood samples were collected in cell preparation (CPT) tubes with sodium citrate (BD Biosciences, San Jose, CA) on days 1–3 of cycle 1 and prior to treatment on day 1 of cycle 2. Peripheral blood mononuclear cells (PBMC) were obtained by centrifugation and viably frozen until analysis. All analyses were performed by multiparametric flow cytometry (MACSQuant, Miltenyi Biotec, Bergisch Gladbach, DE) and flow cytometric data were analyzed using FlowJo (Tree Star Inc., Ashland, OR) software.
PBMCs were analyzed for global protein acetylation, tubulin acetylation,11 and γ-H2AX expression.15 Regulatory T cells (CD4+CD25hiFoxp3+) and CD8+ T cells were evaluated for an immunosuppressive/exhausted phenotype (CTLA-4, T-cell immunoglobulin- and mucin-domain-containing molecule 3, TIM-3; programmed death-1, PD-1, p21). Details of pharmacodynamic analyses are in Supplementary Methods.
Efficacy evaluations
Baseline tumor assessments were conducted by computed tomography (CT) scan within 4 weeks of starting study treatment, and efficacy was evaluated by repeat imaging after every 6 weeks during both combination and maintenance treatments. Tumor assessments were conducted using Response Evaluation Criteria in Solid Tumors version 1.1.16 Confirmatory scans were obtained 6 weeks following initial documentation of an objective response.
Statistical considerations in design and analysis
The primary objective of phase I was to determine the MTD of belinostat when combined with PAC. The phase I dose escalation portion of the trial used a standard 3 + 3 design to determine MTD over 4 dose levels of belinostat with varying doses of cytotoxic chemotherapy.
The primary objective of phase II was to determine if this drug combination was associated with ORRs exceeding those historically seen with PAC in metastatic or recurrent thymomas.7 The phase II used a Simon two-stage optimal design in order to rule out an unacceptably low 50% ORR [partial response (PR)+ complete response (CR)], in favor of a modestly high ORR of 75%. With an alpha=0.10 and beta=0.10, this portion of the study was to initially enroll 12 evaluable patients with thymoma and if 7 or more of the first 12 had a response, then accrual would continue until a total of 28 patients were enrolled in the phase II portion. Patients who were treated at the MTD during phase I were to be included among the initial 12 patients constituting the first stage. If there were 7 to 17 responses in 28 patients, this would be an uninterestingly low ORR, while if there were 18 or more responses, it would be sufficiently interesting to warrant further study in later trials. Under the null hypothesis (50% ORR), the probability of early termination was 61%.
Patients with thymic carcinoma, which is less common than thymoma, were to be treated using the identified phase II doses, but they were not to be included in the two-stage design. Patients with thymic carcinoma were to have a separate ORR determined and reported with 95% confidence interval (CI).
The safety analysis included all patients who received at least one dose of belinostat. Descriptive statistics were used to summarize the pharmacokinetic and pharmacodynamic data, and safety. The expected accrual rate was up to 2 patients per month and it was anticipated that the trial would be completed in 2–3 years. Additional details of statistical analyses are in Supplementary Methods.
Results
Patient disposition and baseline characteristics
Between April 2010 and June 2013, 26 patients were enrolled in the study. Baseline demographic characteristics are summarized in Table 1. Eight patients were treated in the dose escalation phase of the protocol (phase I) which explored two dose levels (3 patients at dose level 1, 2 patients at dose level 2 and the last 3 patients at dose level 1) and 18 patients were treated on phase II. In total 24 patients were treated at the recommended phase II dose (MTD).
Table 1.
Demographic and baseline patient characteristics (N=26)
| Age, y | |
| Median (range) | 53 (23–76) |
|
| |
| Sex, n (%) | |
| Women | 13 (50) |
| Men | 13 (50) |
|
| |
| Race, n (%) | |
| White | 19 (73) |
| Black | 4 (15) |
| Asian | 2 (8) |
| Hispanic | 1 (4) |
|
| |
| ECOGa performance status | |
| 0 | 18 (69) |
| 1 | 8 (31) |
|
| |
| Tumor type, n (%) | |
| Thymoma | 12 (46) |
| B1 | 2 (8) |
| B2 | 7 (27) |
| B3 | 3 (11) |
| Thymic carcinoma | 14 (54) |
|
| |
| Stage at enrolment | |
| IVA | 12 (46) |
| IVB | 14 (54) |
|
| |
| Prior Surgery^ | |
| Radical | 11 (42) |
| Debulking | 2 (8) |
|
| |
| Prior Radiation | 7 (27) |
|
| |
| Prior neoadjuvant or adjuvant chemotherapy | 4 (16) |
Eastern Cooperative Oncology Group
11 patients had radical surgery including 2 who underwent debulking subsequently
All 26 patients received at least one cycle of treatment. The median number of combination treatment cycles administered was 6 (range: 1–6); 20 (77%) patients received the planned 6 cycles. After 5 cycles, treatment was discontinued in both patients who were treated at dose level 2 due to adverse events. Adverse events precluded treatment beyond 4 cycles in two more patients. One patient discontinued treatment after one cycle due to treatment delay secondary to viral meningoencephalitis. One patient had progressive disease after 2 cycles.
Of 20 patients who completed all 6 cycles of combination treatment and were eligible, only 4 (20%) proceeded to receive maintenance belinostat. As of October 15, 2013, maintenance belinostat was discontinued in two patients due to PD after 1 and 5 cycles, respectively, whereas two other patients remain on treatment and have received 1 and 3 cycles of belinostat.
After enrolling 26 patients including the first 12 patients with thymoma to complete the first-stage of the two-stage design, the study was closed due to slower than projected accrual.
Efficacy
Table 2 shows responses by histological cohort. Twenty-five of 26 (96%) patients were evaluable for response. One patient with thymoma, who was treated at dose level 1, was hospitalized for viral meningoencephalitis during the first cycle and was not evaluable for response.
Table 2.
Efficacy
| Histology | Thymoma | Thymic carcinoma | Total |
|---|---|---|---|
| Number of evaluable patients | 11 | 14 | 25 |
| Complete response, n (%) | 1 (9) | 0 | 1 (4) |
| Partial Response, n (%) | 6 (55) | 3 (21) | 9 (36) |
| Stable Disease, n (%) | 4 (36) | 10 (72) | 14 (56) |
| Progressive Disease, n (%) | 0 | 1 (7) | 1 (4) |
| Objective Response Rate, % (95% confidence interval) | 64 (30.8–89.1) | 21 (4.7–50.8) | 40 (21.1 –61.3) |
| Disease control rate, % (95% confidence interval) | 100 (71.5–100.0) | 93 (66.1–99.8) | 96 (79.7 –99.9) |
| Median progression-free survival, months | Not reached | 7.2 | 9.0 |
| Median overall survival, months | Not reached | 21.4 | 28.5 |
Both patients who were treated at dose level 2, one each with thymoma and thymic carcinoma, had PRs. Among the 23 evaluable patients who were treated at dose level 1, one patient (4%) had a CR, 7 (30%) had PRs, 14 (61%) had stable disease (SD) and one patient (4%) had PD.
In the overall population of 25 evaluable patients, one patient (4%) had a CR, 9 (36%) had PRs, 14 (56%) had stable disease (SD) and one (4%) patient had PD. The ORR in the overall population was 40% [95% CI: 21.1%–61.3%) and disease control rate (DCR: SD+PR+CR) 96% (95% CI: 79.7%–99.9%). The median duration of response (DOR) was 7.4 months (95% CI: 5.2 months to undefined upper limit). After a median potential follow up of 20.5 months, the median OS was 28.5 months and median PFS 9 months.
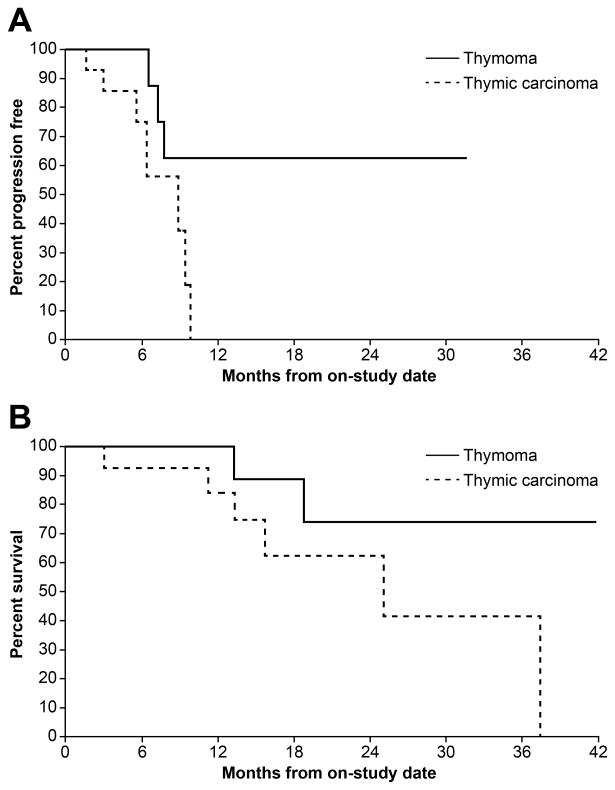
Among 11 evaluable patients with thymoma which included 10 who were treated at the MTD, one patient (9%) had a confirmed and durable CR: a 61-year old woman with B2 thymoma who had undergone surgical resection 3 years prior with recurrent disease involving bilateral lung parenchyma achieved a CR after 4 cycles of treatment which lasted for 21 months. There were 6 PRs (55%) and 4 SD (36%). The ORR for thymoma was 64% (7/11; 95% CI: 30.8%–89.1%) and the DCR 100% (95% CI: 71.5%–100.0%). The median DOR, PFS and OS for thymoma were not reached (Figure 1 A, B).
Figure 1.
Kaplan-Meier plot showing progression-free survival (A) and overall survival (B) of patients with thymoma and thymic carcinoma
Among 14 patients with thymic carcinoma which included 11 who were treated at the MTD, there were no CRs. Three of 14 patients (21%) had PRs and 10 of 14 (71%) patients had SD. The ORR for thymic carcinoma was 21% (3/14; 95% CI: 4.7%–50.8%) and DCR 93% (13/14; 95% CI, 66.1%–99.8%). The median DOR for thymic carcinoma was 7.4 months (95% CI: 4.2 to 8.5 months). The median PFS and OS were 7.2 months and 21.4 months respectively (Figure 1 A, B). The 6-month probability of PFS was 75%.
Additional Treatments
Patients with non-progressive disease after at least 4 cycles of combination chemotherapy were evaluated for multimodality therapy including surgical resection of residual tumor and radiation. Of the 23 patients who were assessed, 12 (52%) were deemed unresectable due to the following reasons: presence of extra-thoracic disease (n=6), co-morbidities (n=2), myocardial and intra-cardiac tumor involvement (n=1), extensive mediastinal involvement (n=1), poor cardiac function (n=1) and PD (n=1). Eleven (48%) patients underwent surgery: 1 patient was found to be inoperable intra-operatively due to extensive epicardial tumor involvement; 1 patient had extensive fibrotic changes which were non-malignant on intra-operative biopsy; 5 patients had complete resection of all visible tumors with negative margins (R0); 4 patients had complete resection of all visible tumors, but with positive margins (R1). In total, 9 of 23 (39%) patients underwent R0 or R1 resection including 6 patients with thymic carcinoma and 3 patients with thymoma. Eight of these patients received post-operative radiation therapy. Three patients had disease-free intervals of 6 weeks, 4 months and 18 months after surgery. Six of 23 (26%) patients have no evidence of disease at the time of reporting with follow-up intervals of 4, 6, 10, 15, 20, and 23 months from surgery. Table S2 shows the disease and treatment characteristics and outcomes of patients who underwent multimodality therapy.
Safety
All patients who received at least one cycle of treatment (n=26) were assessed for toxicity. All treatment-related grade 3/4 adverse events for each dose level and for the overall trial population are listed in Table 3. DLTs were encountered at dose level 2: grade 3 nausea and diarrhea and grade 4 neutropenia and thrombocytopenia, respectively, in two patients. Four patients discontinued treatment due to adverse events: two patients, both of whom were treated at dose level 2, after cycle 5 due to grade 4 thrombocytopenia and grade 4 neutropenia; one patient who had previously received cisplatin post-operatively, after 4 cycles due to grade 3 hearing loss; one patient after cycle 4 due to grade 3 decrease in left ventricular ejection fraction (LVEF) from 60% at baseline to 30% prior to cycle 4. The latter, a 76-year old lady with hypertension, had already required chemotherapy dose-reduction of 25% for febrile neutropenia and pulmonary embolism after cycle 1.
Table 3.
Treatment-related grade 3 and 4 adverse events (highest grade per event per patient)
| Adverse event | Dose level 1 (n=24) | Dose level 2 (n=2) | All dose levels (n= 26) |
|---|---|---|---|
| Grade 3/4 | Grade 3/4 | Grade 3/4 (%) | |
| Hematologic | |||
| Lymphocyte count decreased | 24 | 2 | 26 (100) |
| White blood cell decreased | 22 | 2 | 24 (92) |
| Neutrophil count decreased | 19 | 2 | 21 (81) |
| Platelet count decreased | 10 | 2 | 12 (46) |
| Anemia | 7 | 2 | 9 (35) |
| Hepatic and Renal | |||
| Hypophosphatemia | 4 | 2 | 6(23) |
| Aspartate aminotransferase increased | 4 | 2 | 6 (23) |
| Alanine aminotransferase increased | 3 | 1 | 4 (15) |
| Hypokalemia | 3 | 1 | 4(15) |
| Hypermagnesemia | 3 | 3 (12) | |
| Hypoalbuminemia | 1 | 1 | 2(8) |
| Hypocalcemia | 1 | 1 | 2(8) |
| Hypomagnesemia | 1 | 1(4) | |
| Hyponatremia | 1 | 1(4) | |
| Cardiovascular | |||
| Thromboembolic event | 3 | 3(12) | |
| Atrial fibrillation | 1 | 1 (4) | |
| Ejection fraction decreased | 1 | 1(4) | |
| Electrocardiogram QT corrected interval prolonged | 1 | 1(4) | |
| Others | |||
| Nausea | 1 | 2 | 3(12) |
| Febrile neutropenia | 3 | 3(12) | |
| Diarrhea | 1 | 1 | 2 (8) |
| Lung infection | 2 | 2(8) | |
| Enterocolitis infectious | 1 | 1 | 2(8) |
| Fatigue | 2 | 2(8) | |
| Hearing impaired | 1 | 1(4) | |
| Hypoxia | 1 | 1(4) | |
| Non-cardiac chest pain | 1 | 1(4) | |
| Pain in extremity | 1 | 1(4) | |
| Syncope | 1 | 1(4) | |
| Tumor lysis syndrome | 1 | 1(4) | |
| Limb edema | 1 | 1 (4) | |
| Urinary tract infection | 1 | 1(4) | |
| Vomiting | 1 | 1(4) | |
Nine (35%) patients required dose reductions of PAC due to toxicities, which included febrile neutropenia (n=3), thrombocytopenia (n=2), renal insufficiency (n=1), pulmonary embolism (n=1), diarrhea (n=1), and nausea (n=1). Six (23%) patients required dose reductions of belinostat for QTc prolongation (n=5) and bradycardia (n=1). One patient had persistent QTc prolongation despite two dose reductions of belinostat of 25% each during cycles 3 and 4. Hence further administration of belinostat was discontinued. QTc prolongation was observed in 9 (35%) patients and included one grade 3 event (>500 milliseconds): two of these patients had electrolyte abnormalities and dehydration, which could have contributed to QTc prolongation. Adriamycin was discontinued in two patients after three cycles since they had received cumulative doses of 450 mg/m2.
Pharmacokinetic evaluations
Figure S1 depicts the mean plasma concentration vs. time curve at the 250 mg/m2 belinostat dose. During the 48-hour infusion, the plasma concentration of belinostat remained around 1 μM.
Table S4 shows belinostat pharmacokinetic data following a 48-hr CIVI on cycle 1. The mean half-life of belinostat (n=25) was 0.47 hours (range 0.2–0.91 hr; 40% inter-patient variability). The mean clearance of belinostat was 133 L/hr (range 41–790 L/hr; 106% inter-patient variability). After excluding a patient with normal renal and liver function tests who had a clearance of 790 L/hr, a statistical outlier, the mean clearance was 106 L/hr (range 41–201 L/hr; 36% inter-patient variability). The only patient with high serum creatinine (1.43 mg/dL, normal range 0.5–1.16 mg/dL), indicating mildly impaired kidney function, had a normal belinostat total systemic clearance of 106 L/hr.
With only two dose levels, and only one patient with adequate sampling at dose level 2, conclusions could not be drawn regarding dose proportionality or linearity of pharmacokinetic parameters. There were no statistically significant differences in CMAX, AUCINF, clearance, and half-life between different genders and races.
Pharmacodynamic evaluations
Histone acetylation
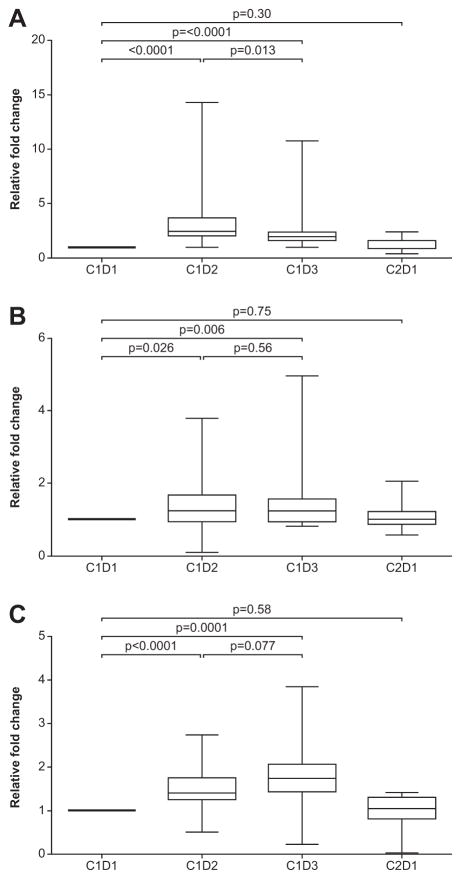
Figure 2 A, B and C show relative changes in total protein hyperacetylation of CD3+ T cells, and CD14+ monocytes and tubulin-specific hyperacetylation of total PBMCs with treatment. Total protein hyperacetylation of CD3+ cells increased significantly after belinostat (p< 0.0001). Increases ranged between 1.4 fold and 14.3 fold (median 2.5 fold) and were observed in 21 of 23 (91%) patients. Although total protein hyperacetylation of CD14+ cells was higher than CD3+ cells at baseline, the magnitude of response to treatment was low. Total protein hyperacetylation of CD14+ cells increased only marginally after belinostat (p= 0.026). PBMC acetylated tubulin increased significantly after belinostat (p<0.0001) and following belinostat plus chemotherapy (p=0.0001). Changes in hyperacetylation with treatment were not associated with response or survival.
Figure 2.
Relative changes (mean and standard deviation) in total protein hyperacetylation of CD3+ T cells (A), and CD14+ monocytes (B) and tubulin-specific hyperacetylation of PBMCs (C) with treatment.
p21 expression in CD14+ cells increased after belinostat alone (p=0.019) and following belinostat plus chemotherapy (p<0.0001). Although these changes were not associated with responses, higher p21 expression in CD14+ cells post-belinostat monotherapy, was associated with a trend towards improved OS (p=0.074) and PFS (p=0.0058) (results not shown).
T cell subsets and expression of immune markers
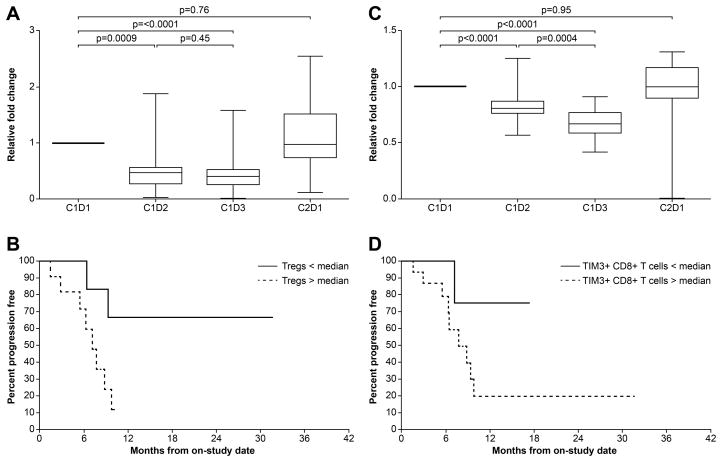
Relative changes in the number of Tregs with treatment are shown in Figure 3A. Tregs decreased significantly following belinostat alone (p=0.0009) and following belinostat plus chemotherapy (p <0.0001). Declines in Tregs with treatment were significantly larger in patients who responded to treatment compared with those who did not (p=0.0041). Furthermore, patients with greater decline in Tregs post-combination therapy had improved PFS compared with those who had lesser decreases (median progression-free survival not reached vs. 7.3 months; p=0.021) (Figure 3B). There were no significant differences in Treg numbers between thymoma and thymic carcinoma. Although the total Treg numbers declined with treatment, the fraction of CTLA4-expressing Tregs increased significantly after belinostat alone (p=0.0078) and after belinostat plus chemotherapy (p<0.0001) (Figure S2A). Patients with higher pre-treatment CTLA4-expressing Tregs had inferior OS compared with those who had lower CTLA4-expressing Tregs (median OS 18.8 months vs. 37.5 months; p=0.03) (Figure S2B).
Figure 3.
Relative changes (mean and standard deviation) in the number of Tregs (A) and TIM3-expressing CD8+T cells (C) with treatment. Progression-free survival of patients with post-treatment Treg (B) and TIM3+ CD8+T cell (D) numbers above and below the median.
Relative changes in TIM3-expressing CD8+T cells, a phenotype of exhausted CD8+ T cells, with treatment are shown in Figure 3C. Compared with baseline, TIM3+ CD8+T cells decreased significantly following belinostat alone (p < 0.0001) and following belinostat with chemotherapy (p <0.0001). Declines in TIM3+ CD8+T cells with treatment were larger in patients who responded to treatment compared with those who did not (p=0.049). Patients with greater decline in post-combination therapy TIM3+ CD8+T cells had non-statistically significant improvements in PFS compared with those who had lesser decreases (median progression-free survival not reached vs. 7.8 months; p=0.081) (Figure 3D). PD-1+ CD8+ T cells decreased significantly following belinostat alone compared with baseline (p=0.01) (results not shown). No statistically significant associations were noted between PD-1+ CD8+ T cell changes and response or survival.
DNA damage
Figure S3A and B show relative changes in γ-H2AX expression on CD3+ and CD14+ cells before and after treatment. γ-H2AX expression on CD3+ cells increased after belinostat alone (p=0.033) and following belinostat plus chemotherapy (p=0.01). γ-H2AX expression on CD14+ cells did not increase significantly after belinostat alone (p=0.41), but was increased after belinostat plus chemotherapy (p=0.012). γ-H2AX expression was not associated with responses or survival. Figure S3C shows representative γ-H2AX foci formation in PBMCs by microscopy.
Discussion
This is the first clinical trial to evaluate combination of chemotherapy with an epigenetic modifier in TETs. In this phase I/II study, we evaluated the combination of a novel schedule of belinostat administered as a CIVI prior to and in combination with PAC in 3-week cycles in patients with advanced TETs. We established belinostat 1000 mg/m2 over 48 hours administered in combination with PAC as the MTD. The dose-limiting toxicities of this regimen were consistent with known side effects of PAC and belinostat and included nausea, diarrhea, neutropenia, and thrombocytopenia.
Responses to monotherapy with HDACi have been observed in advanced hematologic malignancies including T-cell lymphoma, Hodgkin lymphoma, and myeloid malignancies. Solid tumors, in general have proven refractory to monotherapy with HDACi. Available preclinical and clinical data suggest that most HDACi are optimally used in combination with chemotherapies,12,17 targeted therapies,18 radiation,19 or other epigenetic modifiers,20 rather than as single agents.
Unlike previous studies which used a 30 min IV infusion (days 1–5 on a 21-day cycle), we administered belinostat over 48 hrs.21–24 The rationale for CIVI was derived from in vitro studies with belinostat and other HDACi which showed markedly increased gene expression and activity with continuous exposure of 24 or 48 hours. We hypothesized that given the short half-life of belinostat, the daily 30 minute infusions would lead to significant reversal of effects following drug clearance and that a 48-hr CIVI would allow prolonged exposure of belinostat and increased pharmacological effects. Elimination half-life of belinostat in this study was comparable to its administration over 30 minutes (0.52– 0.54 hours),21 but was shorter than when administered IV with paclitaxel and carboplatin (1.7 hours),22 or orally alone (1.9 hours).23 No drug-drug interactions were predicted with belinostat and the co-administered drugs as none of them predominantly utilizes the UGT1A family, the primary pathway for metabolism of belinostat.
Premature closure of the trial precluded definitive conclusions on the efficacy of the combination, but the ORR of 64% and 21% respectively for thymoma and thymic carcinoma, is in agreement with historic controls treated with anthracycline- and platinum-containing chemotherapy.7,26–29
This study describes one of the largest series of patients with TET who underwent multimodality treatment including neoadjuvant chemotherapy, surgery and post-operative radiotherapy. All patients in this study had surgically unresectable disease at enrolment and 42% had recurred after previous radical surgery. Patients with non-progressive disease after belinostat plus chemotherapy were evaluated for multimodality therapy including surgery and post-operative radiation. The resectability rate (R0 or R1) of 39% was lower than published reports.29 This reflects the substantial proportion of our patients who had disseminated disease and prior radical surgeries. Resection was complete with negative margins in 56% of patients who underwent surgery. Although complete surgical resection is the mainstay of treatment of TETs and is the most important predictor of long-term survival,30 debulking is also reported to provide palliation and prolong survival for selected patients incapable of being rendered completely disease-free.31 Considering that all our patients had surgically unresectable disease at enrolment including many who had disseminated disease and prior radical surgeries, the disease-free survival rate of 26% with multimodality therapy is noteworthy and has not been reported previously. Nevertheless, the benefit, if any, of combining belinostat with chemotherapy in improving resectability is unclear.
Although dose reductions were required in 35% of patients, a median of 6 cycles was administered in patients treated at the MTD. Adverse events were consistent with known toxicities of PAC and belinostat. Dose and schedule of administration-dependent ECG changes including mild to moderate QTc prolongation are known class effects of HDACi.10 Although the frequency of QTc interval prolongation seen in this study is comparable to our previous study of single-agent belinostat in TETs,11 it occurred more frequently than in previous studies of belinostat.32 Concurrent risk factors for QTc prolongation in our patient population included drugs associated with QT prolongation (e.g. ondansetron), chemotherapy and anorexia-related electrolyte changes, prior exposure to anthracyclines (in the adjuvant setting) and radiation. Furthermore, high rates of subclinical cardiac involvement of TETs can by itself predispose to increased cardiac toxicity.33 Close cardiac monitoring is needed when HDACi are administered intravenously in combination with chemotherapy and patient selection is crucial to minimize concurrent risk factors.
Histone acetylation is the basic epigenetic mechanism whereby HDACi regulate growth arrest, apoptosis and differentiation. A marked and significant increase in total protein hyperacetylation in CD3+ and CD14+ cells and tubulin-specific hyperacetylation in PBMCs was observed after belinostat and after belinostat plus chemotherapy. Since HDACi induce hyperacetylation of many histone and non-histone proteins, we used a multiparameter flow cytometric assay that detects total protein acetylation rather than just histone acetylation. Increase in tubulin-specific hyperacetylation is indicative of the pan-HDACi effect of belinostat, which is similar to other hydroxamate HDACi such as vorinostat and panobinostat. Although histone acetylation was seen in peripheral blood cells, this did not correlate with clinical response.
Immunosuppressive cells such as Tregs play a major role in maintaining an immunosuppressive milieu in cancer. A number of chemotherapeutic agents including cyclophosphamide have been described to selectively suppress inhibitory cell subsets including Tregs.34 Class I/II HDACi have generally been reported to enhance Treg number and function.35 However, we found a marked and significant decrease in Tregs after belinostat monotherapy. This discrepancy between our observations and previous reports is possibly due to dose-dependent effects of pan-HDACi on Tregs. In vitro data suggests that pan HDACi may target class I HDACs at low doses and impair Treg function whereas at higher doses, they may target class II HDAC and show a dominant Treg promoting effect.36
We found that patients with larger declines in Tregs with treatment were more likely to respond to treatment and had longer PFS. Although previous studies have shown that thymomas have low numbers of Tregs, which are functionally defective,37 the predictive role of Tregs in TETs has not been described previously. Significant decreases after belinostat monotherapy were also observed in TIM-3+ and PD-1+ T cells, which exhibit a severe exhausted phenotype as defined by failure to proliferate and produce IL-2, TNF-alpha, and IFN-gamma.38 Blockade of both TIM-3 and PD-1 pathways is reported to be more effective in controlling tumor growth than targeting either pathway alone.39 Furthermore, declines in TIM-3+ CD8+ T cells with treatment were significantly larger in patients who responded to treatment. Taken together, a beneficial impact of belinostat on Treg and exhausted CD8+ T cells may play an important role in the prognosis of TET. The data presented here suggest that TETs, may be amenable to immunomodulatory strategies, including therapeutic effects on Tregs and effector T cells, and that clinical efficacy may be enhanced by a combinatorial immunomodulatory strategy.
The cyclin-dependent kinase inhibitor, p21 promotes cell cycle arrest in response to many stimuli and several studies have shown that HDACi strongly activate the expression of p21.40 The prognostic role of p21 expression in radically-resected thymoma has been described by Mineo et al who found an association between low tumoral expression of p21 and reduced disease-free survival.41 Although our data indicate a possible predictive role of p21 expression, given the small sample sizes, the significance of this finding will have to be further evaluated in larger samples. Detection and visualization of γ-H2AX, the phosphorylated form of the H2AX protein, provides a useful assessment of DNA damage.42 γ-H2AX expression increased in both CD3+ and CD14+ cells as expected following concurrent chemotherapy and belinostat.
This study was closed before the primary objective of phase II i.e. to improve ORR in thymoma compared to historical controls, was met. Nevertheless, among the patients who enrolled, the ORR was comparable to those of historic controls. In the absence of a randomized comparison, the questions as to whether the observed responses can be entirely explained by chemotherapy alone will remain. In conclusion, this phase I/II trial identified the MTD of belinostat in combination with PAC and indicates that the combination is active without additive toxicities. In one of the largest series of patients with TETs, this study demonstrates the effectiveness of multimodality therapy in rendering select patients disease-free and suggests the possible predictive role of T cell populations such as Tregs and TIM3+ CD8+ T cells in TETs.
Supplementary Material
Translational Relevance.
Belinostat is a histone deacetylase (HDAC) inhibitor which inhibits both HDAC class I and II enzymes in nanomolar concentrations in vitro. Belinostat has known anti-tumor activity in previously treated patients with thymoma with minimal toxicity. In pre-clinical studies, belinostat potentiated anti-tumor activity of platinum-containing chemotherapy. In this phase I/II trial, belinostat was administered as a continuous intravenous infusion prior to and in combination with cisplatin, doxorubicin and cyclophosphamide (PAC) in patients with advanced or recurrent thymic epithelial tumors (TET). This trial identified the maximum-tolerated dose of belinostat in combination with PAC and demonstrate that the combination is active and feasible in TETs. Modulation of pharmacodynamic markers of HDAC-inhibition and declines in regulatory T cell (Tregs) and exhausted CD8+ T cell populations were observed with belinostat. Declines in Tregs and TIM-3+ CD8+T cells were associated with responses. The immunomodulatory effects of this novel schedule of administration of belinostat needs further evaluation.
Acknowledgments
Financial Support: This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
References
- 1.Engels EA, Pfeiffer RM. Malignant thymoma in the United States: Demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105:546–551. doi: 10.1002/ijc.11099. [DOI] [PubMed] [Google Scholar]
- 2.Suster S, Moran CA. Histologic classification of thymoma: the World Health Organization and beyond. Hematol Oncol Clin North Am. 2008;22:381–392. doi: 10.1016/j.hoc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up-study of thymomas with Special Reference to their clinical stages. Cancer. 1981;48:2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Marx A, Chen WH, Yong J, Puppe B, Stroebel P, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors - A clinicopathologic study of 200 thymoma cases from China. Cancer. 2002;95:420–429. doi: 10.1002/cncr.10665. [DOI] [PubMed] [Google Scholar]
- 5.Kelly RJ, Petrini I, Rajan A, Wang Y, Giaccone G. Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol. 2011;29:4820–4827. doi: 10.1200/JCO.2011.36.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The National Comprehensive Cancer Network Guidelines. [Accessed November 24, 2013];Thymomas and thymic carcinomas. 2013 doi: 10.6004/jnccn.2013.0072. http://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf. [DOI] [PubMed]
- 7.Loehrer PJ, Sr, Kim K, Aisner SC, Livingston R, Einhorn LH, Johnson D, et al. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group. J Clin Oncol. 1994;12:1164–1168. doi: 10.1200/JCO.1994.12.6.1164. [DOI] [PubMed] [Google Scholar]
- 8.Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- 9.Marks PA, Rifkind RA, Richon VM, Breslow R. Inhibitors of histone deacetylase are potentially effective anticancer agents. Clin Cancer Res. 2001;7:759–760. [PubMed] [Google Scholar]
- 10.Molife LR, de Bono JS. Belinostat: clinical applications in solid tumors and lymphoma. Expert Opin Investig Drugs. 2011;20:1723–1732. doi: 10.1517/13543784.2011.629604. [DOI] [PubMed] [Google Scholar]
- 11.Giaccone G, Rajan A, Berman A, Kelly RJ, Szabo E, Lopez-Chavez A, et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J Clin Oncol. 2011;29:2052–2059. doi: 10.1200/JCO.2010.32.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63:7291–7300. [PubMed] [Google Scholar]
- 13.Kurz EU, Wilson SE, Leader KB, Sampey BP, Allan WP, Yalowich JC, et al. The histone deacetylase inhibitor sodium butyrate induces DNA topoisomerase II alpha expression and confers hypersensitivity to etoposide in human leukemic cell lines. Mol Cancer Ther. 2001;1:121–131. [PubMed] [Google Scholar]
- 14.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redon CE, Nakamura AJ, Gouliaeva K, Rahman A, Blakely WF, Bonner WM. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PLoS One. 2010;5:e15544. doi: 10.1371/journal.pone.0015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Luchenko VL, Salcido CD, Zhang Y, Agama K, Komlodi-Pasztor E, Murphy RF, et al. Schedule-dependent synergy of histone deacetylase inhibitors with DNA damaging agents in small cell lung cancer. Cell Cycle. 2011;10:3119–3128. doi: 10.4161/cc.10.18.17190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa T, Takeuchi S, Yamada T, Ebi H, Sano T, Nanjo S, et al. EGFR-TKI resistance due to BIM polymorphism can be circumvented in combination with HDAC inhibition. Cancer Res. 2013;73:2428–2434. doi: 10.1158/0008-5472.CAN-12-3479. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Jung M, Dritschilo A. Enhancement of radiation sensitivity of human squamous carcinoma cells by histone deacetylase inhibitors. Radiat Res. 2004;161:667–674. doi: 10.1667/rr3192. [DOI] [PubMed] [Google Scholar]
- 20.Chai G, Li L, Zhou W, Wu L, Zhao Y, Wang D, et al. HDAC inhibitors act with 5-aza-2′-deoxycytidine to inhibit cell proliferation by suppressing removal of incorporated abases in lung cancer cells. PLoS One. 2008;3:e2445. doi: 10.1371/journal.pone.0002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steele NL, Plumb JA, Vidal L, Tjørnelund J, Knoblauch P, Rasmussen A, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14:804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 22.Lassen U, Molife LR, Sorensen M, Engelholm SA, Vidal L, Sinha R, et al. A phase I study of the safety and pharmacokinetics of the histone deacetylase inhibitor belinostat administered in combination with carboplatin and/or paclitaxel in patients with solid tumours. Br J Cancer. 2010;103:12–17. doi: 10.1038/sj.bjc.6605726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steele NL, Plumb JA, Vidal L, Tjørnelund J, Knoblauch P, Buhl-Jensen P, et al. Pharmacokinetic and pharmacodynamic properties of an oral formulation of the histone deacetylase inhibitor Belinostat (PXD101) Cancer Chemother Pharmacol. 2011;67:1273–1279. doi: 10.1007/s00280-010-1419-5. [DOI] [PubMed] [Google Scholar]
- 24.Yeo W, Chung HC, Chan SL, Wang LZ, Lim R, Picus J, et al. Epigenetic therapy using belinostat for patients with unresectable hepatocellular carcinoma: a multicenter phase I/II study with biomarker and pharmacokinetic analysis of tumors from patients in the Mayo Phase II Consortium and the Cancer Therapeutics Research Group. J Clin Oncol. 2012;30:3361–3367. doi: 10.1200/JCO.2011.41.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoi K, Matsuguma H, Nakahara R, Kondo T, Kamiyama Y, Mori K, et al. Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone. J Thorac Oncol. 2007;2:73–78. doi: 10.1097/JTO.0b013e31802bafc8. [DOI] [PubMed] [Google Scholar]
- 27.Fornasiero A, Daniele O, Ghiotto C, Sartori F, Rea F, Piazza M, et al. Chemotherapy of invasive thymoma. J Clin Oncol. 1990;8:1419–1423. doi: 10.1200/JCO.1990.8.8.1419. [DOI] [PubMed] [Google Scholar]
- 28.Kawashima Y, Inoue A, Sugawara S, et al. Phase II study of amrubicin and carboplatin for invasive thymoma and thymic carcinoma: NJLCG0803. Proc Am Soc Clin Oncol. 2013;31(suppl):abstr 7530. [Google Scholar]
- 29.Kim ES, Putnam JB, Komaki R, Walsh GL, Ro JY, Shin HJ, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer. 2004;44:369–79. doi: 10.1016/j.lungcan.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg. 2003;76:1859–64. doi: 10.1016/s0003-4975(03)01017-8. [DOI] [PubMed] [Google Scholar]
- 31.Lin CS, Kuo KT, Hsu WH, Huang BS, Wu YC, Hsu HS, et al. Management of locally advanced unresectable thymic epithelial tumors. J Chin Med Assoc. 2004;67:172–178. [PubMed] [Google Scholar]
- 32.Cashen A, Juckett M, Jumonville A, Litzow M, Flynn PJ, Eckardt J, et al. Phase II study of the histone deacetylase inhibitor belinostat (PXD101) for the treatment of myelodysplastic syndrome (MDS) Ann Hematol. 2012;91:33–38. doi: 10.1007/s00277-011-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas A, Shanbhag S, Haglund K, Berman A, Jakopovic M, Szabo E, et al. Characterization and management of cardiac involvement of thymic epithelial tumors. J Thorac Oncol. 2013;8:246–249. doi: 10.1097/JTO.0b013e31827bd931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brode S, Cooke A. Immune-potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol. 2008;28:109–26. doi: 10.1615/critrevimmunol.v28.i2.20. [DOI] [PubMed] [Google Scholar]
- 35.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 36.Shen L, Pili R. Class I histone deacetylase inhibition is a novel mechanism to target regulatory T cells in immunotherapy. Oncoimmunology. 2012;1:948–950. doi: 10.4161/onci.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strobel P, Rosenwald A, Beyersdorf N, Kerkau T, Elert O, Murumägi A, et al. Selective loss of regulatory T cells in thymomas. Ann Neurol. 2004;56:901–904.38. doi: 10.1002/ana.20340. [DOI] [PubMed] [Google Scholar]
- 38.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gartel AL. The conflicting roles of the cdk inhibitor p21(CIP1/WAF1) in apoptosis. Leuk Res. 2005;29:1237–1238. doi: 10.1016/j.leukres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Mineo TC, Ambrogi V, Mineo D, Baldi A. Long-term disease-free survival of patients with radically resected thymomas: relevance of cell-cycle protein expression. Cancer. 2005;104:2063–2071. doi: 10.1002/cncr.21433. [DOI] [PubMed] [Google Scholar]
- 42.Kuo LJ, Yang LX. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In Vivo. 2008;22:305–309. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.