Abstract
The goal in this study was to determine the impact of colorectal cancer and cumulative chemotherapeutic dose on sensory function to gain mechanistic insight to the subtypes of primary afferent fibers damaged by chemotherapy. Patients with colorectal cancer underwent quantitative sensory testing (QST) before and then prior to each cycle of oxaliplatin. These data were compared to that from age- and sex-matched healthy volunteers. The patients showed significant subclinical deficits in sensory function prior to any therapy compared to healthy volunteers. Sensory deficits became more pronounced in patients with chemotherapy. Sensory deficits were most pronounced for modalities mediated by large Aβ myelinated fibers and unmyelinated C fibers whereas those modalities of sensation conveyed by thinly myelinated Aδ fibers appeared showed less sensitivity to chemotherapy. Patients with baseline sensory deficits went on to develop more symptom complaints during chemotherapy than those who had no baseline deficit. Patients who were re-tested 6 to 12 months following chemotherapy showed the most numbness and pain as well as the most pronounced sensory deficits. The pattern of effects on sensory function has clear mechanistic implications for the fibers types that are vulnerable to the toxicity of chemotherapy.
Keywords: neuropathy, colorectal cancer, oxaliplatin, peripheral nerve fibers
INTRODUCTION
Neuropathy induced by chemotherapy can seriously impede successful treatment for many cancers as it often leads to reduction or cessation of frontline treatment; and can drastically impact patients’ quality of life both during and following therapy (1). The mechanism for chemotherapy induced peripheral neuropathy (CIPN) is poorly understood. Primary afferent neurons appear to be the most vulnerable as most commonly sensory symptoms alone start in the tips of the toes and fingers and then advance over time proximally in a “stocking-glove” distribution (2-5). More specifically, pain is typically reported in the tips of the toes and fingers; numbness and tingling, but not necessarily pain is present in the soles of the feet and palms; while hairy skin is typically outside the areas of patient complaint (3'6).Yet specific data concerning the vulnerability of primary afferent fiber subtypes to toxic insult by cancer and its treatment are not known. Here, quantitative sensory tests (QST) were conducted in patients before and after various cumulative doses of oxaliplatin to fill this gap in knowledge. QST is a psychophysical method used to study human somatic sensory physiology including pain perception (7). Small-fiber sensory function is assessed by measuring the threshold to detect warmth, hot and cold pain; thinly myelinated fibers are assessed by measurement of threshold to detect skin cooling and sharpness; and large-fiber sensory function is measured by detection thresholds for cutaneous mechanical stimuli (8). The pattern of effects of chemotherapy on sensory function has clear mechanistic implications for the fibers types that are vulnerable to the toxicity of chemotherapy.
METHODS
Patients
Patients starting initial chemotherapy with oxaliplatin for stage II, III or IV colorectal cancer at MD Anderson were recruited for the study that was approved by the Institutional Review Board. Seventy eight patients were enrolled and gave informed consent. Patients with any history of neuropathy or other factors known to contribute to neuropathy including diabetes mellitus, history of alcohol intake greater than 100gm per week, vitamin deficiency, nerve compression or any CNS metastasis were excluded. The typical chemotherapy protocol consisted of oxaliplatin administered at a dose of 85 mg/m2 on two week cycles. Patients underwent QST prior to each treatment with oxaliplatin. In addition, 47 age-, and sex-matched, volunteers were recruited to provide comparative data.
QST
QST data was collected with the patients comfortably seated in a quiet, dedicated psychophysics laboratory in the daytime hours with the subjects not on analgesics that might interfere with the tasks. Three sites were tested in each subject, the tip of the index finger, the thenar eminence, and the volar surface of the forearm to encompass the areas of skin that are typically affected in chronic CIPN patients (2-5). QST were conducted by two clinical data coordinators with many years’ experience and with previously verified excellent inter-rater reliability (2-5). The specific tests that were performed included the following and were performed in the order described.
Basal skin temperature was measured using an infrared thermistor positioned against the skin at each site.
The Slotted Peg Board Test was used to evaluate sensorimotor function (9). Participants filled a 5X5 slotted pegboard with spindles in non-random fashion by one row or column at a time with the dominant hand and then with the non-dominant hand (10). The time for each participant to complete the task was recorded with a five minute (300s) cutoff.
Bumps detection was used to assess low threshold mechanosensation (11'12). Participants used their index finger to probe a smooth plate that was divided into nine blocks, with each block marked by five colored circles. Over one of the circles in each block a bump of varying height (500 μm in diameter, 2.5 to 22.5 μm tall) was concealed such that it was not visible to the patient (3 plates total in the set). The threshold was defined as the lowest height bump correctly detected with the next two higher bumps also correctly detected.
Touch detection threshold was determined using von Frey monofilaments (Semmes-Weinstein) in an up/down manner as previously described (2). The filaments were applied for 1 second at each testing site starting with a force of 0.5 g and the patients unable to see the stimulus application. If a participant did not feel a given filament, the next higher force filament was applied. If a participant felt a stimulus the next lower force filament was applied. Threshold was defined as the first filament force detected by the participant three times.
Sharpness detection threshold was determined using blunted 30-gauge needles with force determined by weights graded from 8 to 128g (10'13). Weighted needles were applied in order from lightest to heaviest at each site for 1 second, and participants were asked to report each stimulus as touch, pressure, sharp, or pain. The lowest force at which the report of “sharp” or “painful” was given determined the end point for each trial. The final threshold was the mean of three trials separated by 30-90 seconds. The starting weight was modified between trials based to manage errors in anticipation.
Thermal detection threshold was determined using a 3.6 x 3.6cm Peltier probe set at a baseline temperature of 32°C (2). The probe temperature was ramped upward at a rate of 0.30°C/s for detection of warmth and heat pain thresholds, whereas cool detection and cold pain threshold was determined using a decreasing ramp of 0.50°C/s. Participants were not given any cue to the onset of a given trial, nor whether the probe would heat or cool. Participants were instructed to indicate when they could first detect a change in temperature and then when the temperature became painful at which point the probe was immediately returned to the baseline temperature. The final threshold was the average of three heating and cooling trials separated by 30-90 seconds.
Descriptors of symptoms were assessed using questionnaires and a standardized body map presented to the participants at each meeting (2). The participants marked areas where they felt pain with a red pen and areas where they felt tingling or numbness with a green pen. Participants also selected descriptors for their symptoms from a standardized list (2) that was previously validated (14).
Data Analysis
Analysis of the data was based on total cumulative oxaliplatin dose that patients received prior to each test. In this manner, patient data was stratified into baseline (cumulative dose 0), low (115.7 to 345.1 mg), medium (347.1-737.8 mg), and high dose (739.5-2328.2 mg) categories established by empirical analysis. Patients only contributed one set of data per dose category with that included at the highest dose if sampled more than once within a given category. Finally, patients were also tested at approximately 6 months after chemotherapy. The non-parametric Kruskal-Wallis test was applied to all data. Patient data was compared to that from the health volunteers only for the baseline time point. The patient data collected at the time points during and following chemotherapy were compared to the patient baseline dataset. Significance was defined as any P value < 0.05.
RESULTS
Patient demographics and clinical data are shown in Table 1. The breakdown by treatment category resulted in 51 patients in the baseline group because some had not agreed to take part in the study before starting chemotherapy. Sixty-two (62) patients were included in the low dose category; 54 in the medium dose category; and 49 in the high cumulative dose category. Finally, 27 patients underwent QST at a post-chemotherapy follow-up examination at a mean of 165 (±12) days after the last chemotherapy. The numbers vary due to missed visits and/or loss of subjects to the study over time.
Table I Demographic and Clinical Characteristics
| Patients (N=78) | Volunteers (N=47) | |
|---|---|---|
| Age (mean ± SD) | 55.7 ± 1.45 years | 53.87±2.02 years |
| Male (%) | 48 (62) | 29 (62) |
| Female (%) | 30 (39) | 18 (38) |
| White (%) | 47 (60) | 30 (64) |
| Black (%) | 14 (18) | 13 (28) |
| Hispanic or Latino (%) | 12 (15) | 4 (8) |
| Asian (%) | 4 (5) | 0 (0) |
| Unknown (%) | 1 ( 1) | 0 (0) |
| Smoke > 10 pack years (%) | 19 (24) | 0 (0) |
| Married | 54 (70) | 35 (74) |
| TNM Stage† | ||
| I | 0 (0) | NA |
| II | 11(14) | NA |
| III or IV | 67 (86) | NA |
| Mean Cycles (mean ± SD) | 6.7 ± 3.18 | NA |
American Joint Committee on Cancer Staging Manual [7th edition] criteria
Pegboard Test
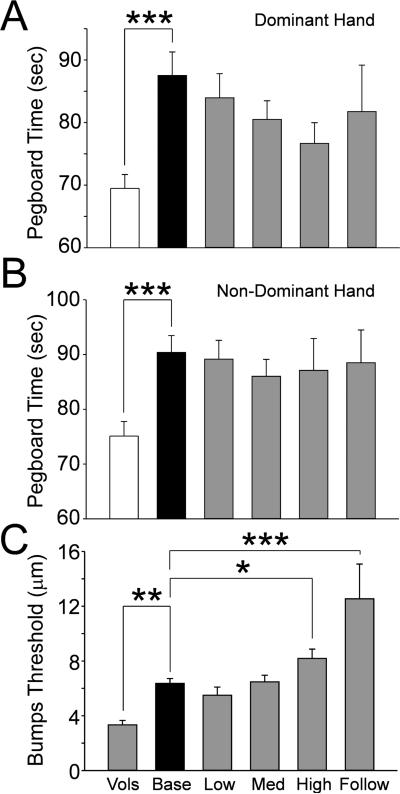
Patients at baseline took significantly more time to complete the pegboard test with the dominant hand than volunteers did (Fig.1A, p<0.001). The same was observed for the non-dominant hand (Fig.1B, p<0.001). Subsequent pegboard tests collected during chemotherapy did not show any differences from the patient baseline value. Indeed, there was a trend in both hands for a slight decline in pegboard time, most likely reflecting a training effect (none of the volunteers were allowed training prior to their data collection).
Figure 1.
The bar graphs show the mean and standard error for the slotted peg board times in the dominant (A) and non-dominant hand (B) and the Bumps detection threshold (C) for the healthy volunteers (Vols, open bars) and the patients at the pretreatment baseline (Base, black bars) and at each cumulative dose treatment category (gray bars, “Low” is up to 370.6 mg; medium (Med) is up to 795.6 mg; “High” is up to 2328.2 mg and “Follow” is 6 months after tretament). The horizontal lines indicate the comparions made and * = p<0.05, ** = p<0.01, *** = p<0.00.1.
Bumps Detection
The Bumps test is an assessment of large diameter Aβ fiber mechanoreceptor function best correlated to transduction by Meissner's corpuscles (12'15). The patients showed a significantly elevated Bumps threshold at baseline compared to healthy volunteers (Fig1C, p<0.01). There was a clear trend for the impairment seen at the patient baseline QST to worsen during therapy, with this difference becoming statistically significant from the patient baseline in the high dose chemotherapy group (Fig 1C, p<0.05). The mean bump detection score in patients who had undergone high cumulative doses of oxaliplatin was 2.1 times that of volunteers (6.33±0.68 in patients versus 3.32±0.32 in healthy volunteers; p< 0.05; Fig. 2A). However, patients in the follow-up group displayed the most significant impairment in bump detection compared with all other groups (p< 0.01 to < 0.0001) consistent with the coasting phenomenon often attributed to platin-based chemotherapeutics.
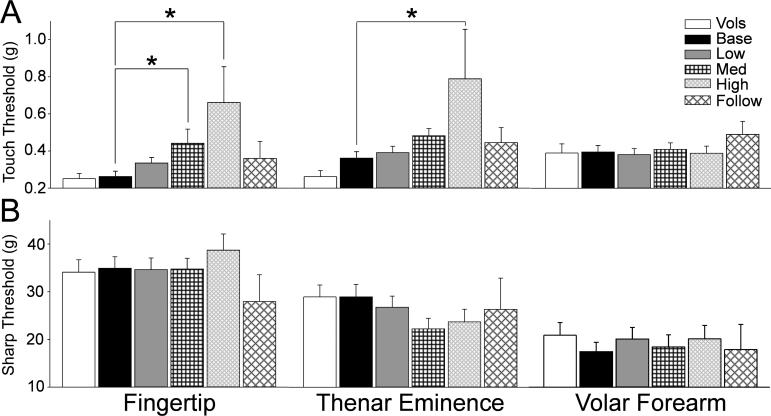
Figure 2.
The bar graphs show the mean and standard error for the VonFrey touch detection threshold (A) and the sharpness dection threhsold (B) for the healthy volunteers (open bars) and the patients at the pretreatment baseline (black bars) and at each cumulative dose treatment category (“Low” is up to 370.6 mg, gray bars; medium (Med) is up to 795.6 mg, black cross hatch; “High” is up to 2328.2 mg, gray diagonal narrow cross hatch; and “Follow” is 6 months after tretament, black diagonal wide cross hatch). The horizontal lines indicate the comparions made and * = p<0.05.
Touch Detection
The detection of touch using VonFrey filaments engages large diameter Aβ slowly-adapting Merkel complex mechanoreceptors (16'17). There was no difference between touch detection threshold between healthy volunteers and patients prior to therapy. Touch threshold did show increasing deficit with dose during chemotherapy that became statistically significant in the fingertips at middle and high chemotherapy doses (Fig. 2A, p<0.05). Similarly, touch threshold in the thenar eminence gradually increased with chemotherapy dose and was statistically significant between the patient baseline for those patients receiving high dose chemotherapy (Fig.2A, p<0.05). Interestingly, these deficits resolved at the 6 month follow-up test. Touch threshold in hairy skin showed no change at any time point.
Sharpness Detection
Sharpness detection threshold is an assessment of thinly myelinated Aδ fibers. There were no differences observed in sharpness detection between healthy volunteers and patients prior to chemotherapy (Fig.2B). There was also no change shown in sharpness detection in patients at the various cumulative doses of chemotherapy compared to the patient baseline.
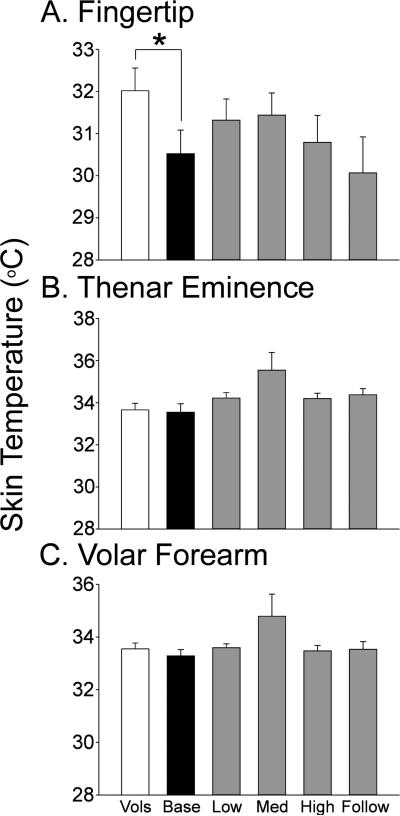
Basal Skin Temperature
The patient group showed significantly cooler skin temperature in the fingertips prior to chemotherapy than found in the healthy volunteer group (Fig.3). Interestingly, skin temperature returned toward that of healthy volunteers in the treatment groups, but this change was not significant compared to the patient baseline group.
Figure 3.
The bar graphs show the mean and standard error for skin temperature in the index finger (A) the thenar eminence (B) and the volar forearm (C) for the healthy volunteers (Vols, open bars) and the patients at the pretreatment baseline (Base, black bars) and at each cumulative dose treatment category (gray bars, “Low” is up to 370.6 mg; medium (Med) is up to 795.6 mg; “High” is up to 2328.2 mg and “Follow” is 6 months after tretament). The horizontal lines indicate the comparions made and * = p<0.05.
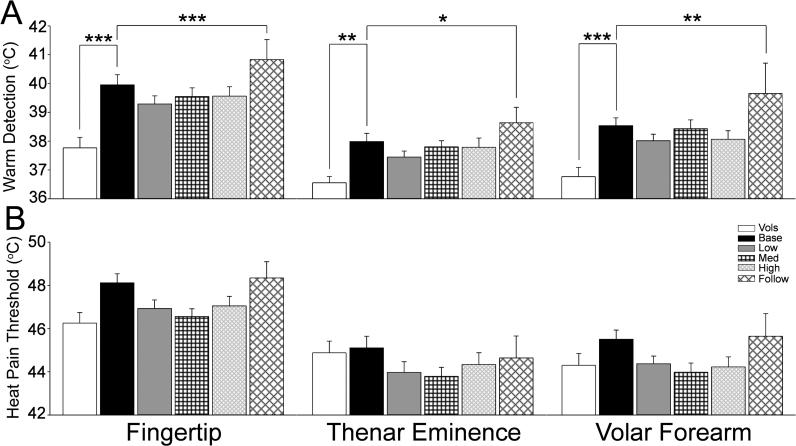
Temperature Detection
The detection of warming is dependent on inputs from subgroups of unmyelinated C fibers given the relatively slow heat ramps that were used in this study. The patient baseline warm detection threshold was significantly elevated from that of healthy volunteers at all three test sites (Fig.4A, p<0.01 to 0.001). Warm detection in the treatment groups showed no change from the patient baseline. However, warm detection threshold was significantly increased from the patient baseline in the 6 month follow-up group (Fig.4A, p< 0.05 to 0.001). Heat pain threshold also mediated by unmyelinated C fibers tended to be higher in the patient group at baseline compared to that of the healthy volunteers, but this difference did not achieve statistical significance. No change in heat pain threshold from the patient baseline was observed in any of the treatment groups or at 6 months follow-up (Fig.4B).
Figure 4.
The bar graphs show the mean and standard error for warm detection threshold (A) and heat pain threhsold (B) for the healthy volunteers (open bars) and the patients at the pretreatment baseline (black bars) and at each cumulative dose treatment category (“Low” is up to 370.6 mg, gray bars; medium (Med) is up to 795.6 mg, black cross hatch; “High” is up to 2328.2 mg, gray diagonal narrow cross hatch; and “Follow” is 6 months after tretament, black diagonal wide cross hatch). The horizontal lines indicate the comparions made and * = p<0.05; ** = p<0.01; *** = p<0.001.
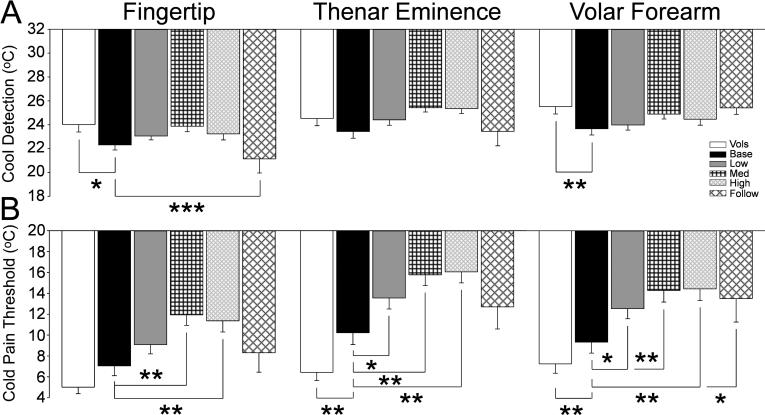
Cool and cold pain detection is mediated by activity in thinly myelinated Aδ and unmyelinated C fibers, respectively. The patients had a significantly lower threshold to detect skin cooling at the baseline measure prior to chemotherapy than the healthy volunteers in both the fingertips and the hairy skin of the volar forearm (Fig.5A, p<0.05 to 0.01). This threshold showed a further deficit at 6 months follow-up compared to the patient baseline. The patient baseline cold pain threshold was significantly elevated compared to the healthy volunteers in both the thenar eminence and volar forearm (Fig. 5B, p<0.01). Chemotherapy treatment increased this deficit such that the moderate and high doses resulted in pain at significantly warmer temperatures than at the patient baseline (Fig.5B, p<0.05 to 0.01). This deficit showed some resolution at 6months follow-up back toward the original patient baseline, though cold pain in the volar forearm remained significantly different.
Figure 5.
The bar graphs show the mean and standard error for cool detection threshold (A) and cold pain threhsold (B) for the healthy volunteers (open bars) and the patients at the pretreatment baseline (black bars) and at each cumulative dose treatment category (“Low” is up to 370.6 mg, gray bars; medium (Med) is up to 795.6 mg, black cross hatch; “High” is up to 2328.2 mg, gray diagonal narrow cross hatch; and “Follow” is 6 months after tretament, black diagonal wide cross hatch). The horizontal lines indicate the comparions made and * = p<0.05; ** = p<0.01; *** = p<0.001.
Neuropathy Score and Symptom Complaints
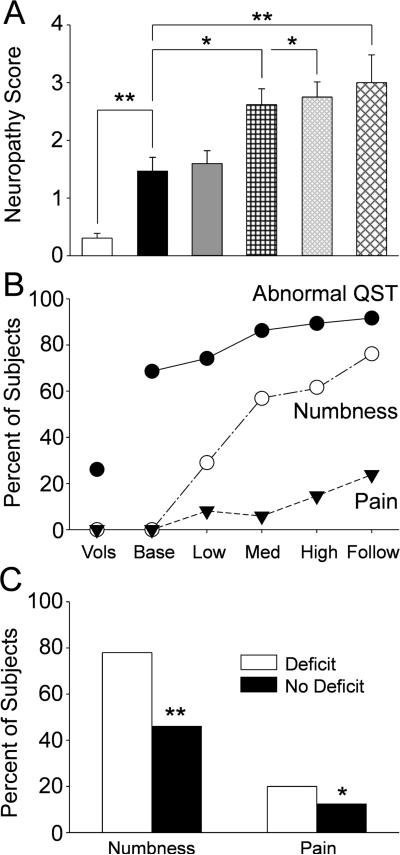
Figure 6A shows the overall neuropathy scores for the healthy volunteers and for each of the patient groups tabulated by determining the number of measures in the QST battery for each subject that were 2 standard deviations or more outside the healthy volunteer mean values. The mean neuropathy score for the healthy volunteers was predictably very low, and as detailed in the previous sections the value for the patients at baseline was significantly higher (Fig.6A, p<0.01). The mean neuropathy scores showed significant further increases from the patient baseline value with chemotherapy dose and had a peak at the 6 month follow-up (Fig.6A, p<0.05 to 0.01). Figure 6B shows that the percentage of subjects in each group that had QST measures that were 2 standard deviations or more outside the healthy volunteer mean values (Filled circles). Approximately 25% of the healthy volunteers had at least one out-of-range measure compared to roughly three quarters of the patients (p < 0.01). Notably, the QST deficits in the patients at baseline were sub-clinical as none reported any numbness or pain at the baseline measure (Fig.6B, open circles and filled triangles). The percentage of patients with abnormal QST measures showed a continuous increase across the treatment groups with the final peak at the 6 month follow-up (Fig6B, filled circles). The decay in sensory function was paralleled by increasing reports of numbness and pain in the patient groups such that by 6 months follow-up roughly three quarters of the patients reported numbness and roughly one fifth reported pain. Finally, Figure 6C shows the rates of symptom complaint that developed during chemotherapy or present at follow-up based on whether the patients had a baseline QST deficit. The frequency of both numbness and pain was significantly increased in the patients who presented with subclinical neuropathy versus those who did not.
Figure 6.
The bar graphs in A show the mean and standard error for the neuropathy score (total number of QST measures 2 standard deviations or more from the healthy volunteer mean) for the for the healthy volunteers (“Vols” open bars) and the patients at the pretreatment baseline (“Base”, black bars) and at each cumulative dose treatment category (“Low” is up to 370.6 mg, gray bars; medium (Med) is up to 795.6 mg, black cross hatch; “High” is up to 2328.2 mg, gray diagonal narrow cross hatch; and “Follow” is 6 months after tretament, black diagonal wide cross hatch). The horizontal lines indicate the statistical comparions made. The scatter and line plot in B shows the percentage of healthy volunteers and patients at each treatment category (abbreviations as above) that had abnormal QST measures (out of range measures as defined above, black circles) or that reported numbness (open circles) or pain (filled triangles). Finally, the bar graphs in C shows the percentage of patients that reported numbness or pain at the end of treatment categorized on whether they had any QST deficit (open bars) or not (black bars) at the pretretament baseline assessment. * = p<0.05; ** = p<0.01; *** = p<0.001.
DISCUSSION
This is the first study to employ repeated QST in the study of the development of CIPN bringing a highly sensitive method to detect sensory impairments to this field (8). A key finding from this approach was the detection of pre-existing subclinical sensory deficits in a large cohort of colorectal cancer patients prior to treatment that appears to be disease driven and that when present appears to increase the risk for the later development of clinical CIPN. This observation therefore provides a generalization of a correlation between apparent subclinical pretreatment neuropathy and risk for CIPN as previously suggested in multiple myeloma patients (10'11'15). A caveat however, is that QST was not performed on the feet for convenience of the patients, yet CIPN often first presents in the feet. Hence, this study may present an overly conservative survey of QST deficits, particularly those that remained subclinical, that occurred in this patient cohort. Is should be noted however that all of our subjects who became symptomatic complained of symptoms in the hands as well as the feet.
Perhaps the most important findings of this study are the mechanistic implications for impact of chemotherapy on specific groups of primary afferent fibers. Touch detection using the Bumps and von Frey assays are transduced by large diameter myelinated axons that terminate in or near Meissner's corpuscles (15) and Merkel disk complexes (16-18), respectively. The slotted peg board task, although also dependent to a degree on cutaneous mechanoreceptors, is more dependent on sensorimotor coordination involving neural inputs from muscle and joint mechanoreceptors that engage spino-cerebellar and cortical cognitive processing. The pegboard test was significantly worse for patients at baseline compared to healthy controls, but then did not show any decay from that level and even a trend toward improvement. On the other hand, patient mechanoreceptor function tested using the Bumps test not only showed a difference at baseline from healthy volunteers, but showed significant further deterioration with increasing chemotherapy doses and evidence of coasting following the termination of chemotherapy. Mechanoreceptor function assessed using von Frey monofilaments showed much of the same results as in the Bumps test. The difference between the peg board to the Bumps and VonFrey tests could be explained by assuming the patients learned to cognitively overcome mechanoreceptor deficits in the former whereas the lack of learning cues in the latter tests prevented this compensation. Alternatively, this paradox in results could also indicate that the myelinated fibers innervating the different tissues involved in these tasks show differential toxic deficit to oxaliplatin.
The Aδ fiber-dependent tasks seem to provide clear psychophysical evidence in support of a differential susceptibility of primary afferent fibers to toxic insult by chemotherapy. Whereas the percept of sharpness/sharp pain evoked by the weighted needles showed little change at baseline or with chemotherapy, the detection of skin cooling showed a clear impairment. Similarly, various groups of C fibers are recruited in the detection of warm, heat and cold pain yet the patients showed preserved heat pain in the context of altered warmth and cold pain detection. Thus, for each group of fibers psychophysical evidence indicates that the mechanism of toxicity engaged by chemotherapeutics differentially impacts function in different subtypes of primary afferent fibers resulting in the clinical phenotype that is observed.
One possible explanation that fits this criterion well is the recent demonstration of an interaction of the chemotherapeutic paclitaxel with toll-like receptor 4 (TLR4) (19). Not all, but only subsets of small (C-fiber) and medium sized (Aδ fiber) DRG neurons express TLR4 and following chemotherapy treatment and show signs of an activated innate immune response including an increase in the expression of pro-inflammatory cytokines such as MCP-1 (20). The effects of MCP-1 and other cytokines on peripheral nerves could account for the clinical presentation of CIPN and the known risk and protective factors. Schwann cells express cytokine receptors that when activated lead to dedifferentiation and down regulation of myelin synthesis (21-24). This would consequently have pronounced functional impact on Aβ fibers that require extensive myelination, but less so on C-fibers, thus generating a clinical picture like that observed in the patient studies as described here. Finally, this mechanism would explain the observed effects of anti-cytokine treatments, such as minocycline, in preventing CIPN (25'26) and suggests a clear short-term target for clinical evaluation in preventing a major complication of cancer treatment.
Precis.
Results suggest that pre-treatment test may predict complications to chemotherapy in colorectal cancers.
Acknowledgments
This work was supported by National Institutes of Health grant NS046606 and National Cancer Institute grant CA124787. The authors wish to acknowledge Tina Peters and Tony Perez for QST data collection.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest. Dr. William Kennedy's son holds a financial and business interest in Neuro Devices, Inc., the company involved in this study in producing the Bumps device. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest polices.
REFERENCES
- 1.Giglio P, Gilbert MR. Neurologic complications of cancer and its treatment. Curr Oncol Rep. 2010;12:50–9. doi: 10.1007/s11912-009-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyette-Davis JA, Cata JP, Zhang H, Driver LC, Wendelschafer-Crabb G, Kennedy WR, et al. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain. 2011;12:1017–24. doi: 10.1016/j.jpain.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyette-Davis JA, Cata JP, Driver LC, Novy DM, Bruel BM, Mooring DL, et al. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother Pharmacol. 2013;71:619–26. doi: 10.1007/s00280-012-2047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder S, Beckmann K, Franconi G, Meyer-Hamme G, Friedemann T, Greten HJ, et al. Can medical herbs stimulate regeneration or neuroprotection and treat neuropathic pain in chemotherapy-induced peripheral neuropathy? Evid Based Complement Alternat Med. 2013;2013:423713. doi: 10.1155/2013/423713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder A, Stengel M, Maag R, Wasner G, Schoch R, Moosig F, et al. Pain in oxaliplatin-induced neuropathy--sensitisation in the peripheral and central nociceptive system. Eur J Cancer. 2007;43:2658–63. doi: 10.1016/j.ejca.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng H-R. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–42. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25:641–7. doi: 10.1097/AJP.0b013e3181a68c7e. [DOI] [PubMed] [Google Scholar]
- 8.Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154:1807–19. doi: 10.1016/j.pain.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 9.Ruff RM, Parker SB. Gender and age-specific changes in motor speed and eye-hand coordination in adults: normative values for finger tapping and grooved pegboard tests. Percept Motor Skills. 1993;76:1219–30. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 10.Vichaya EG, Wang XS, Boyette-Davis JA, He Z, Thomas SK, Shah N, et al. Baseline subclinical sensory deficits are associated with the development of pain and numbness in mutiple myeloma patients being treated with chemotherapy. Cancer Chemother Pharmacol. 2013;71:1531–40. doi: 10.1007/s00280-013-2152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyette-Davis JA, Eng C, Wang XS, Cleeland CS, Wendelschafer-Crabb G, Kennedy WR, et al. Subclinical peripheral neuropathy is a common finding in colo-rectal cancer patients prior to chemotherapy. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-0205. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy WR, Selim MM, Brink TS, Hodges JS, Wendelschafer-Crabb G, Foster SX, et al. A new device to quantify tactile sensation in neuropathy. Neurology. 2011;76:1642–9. doi: 10.1212/WNL.0b013e318219fadd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84:141–9. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- 14.Gracely RH, McGrath P, Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: Manipulation of affect by Diazepam. Pain. 1978;5:19–29. doi: 10.1016/0304-3959(78)90021-0. [DOI] [PubMed] [Google Scholar]
- 15.Kosturakis AK, He Z, Li Y, Boyette-Davis JA, Shah N, Thomas SK, et al. Subclinical peripheral neuropathy in multiple myeloma patients prior to chemotherapy is correlated with decreased fingertip innervation density. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.54.5418. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014 doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haeberle H, Lumpkin EA. Merkel Cells in Somatosensation. Chemosens Percept. 2008;1:110–8. doi: 10.1007/s12078-008-9012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, et al. Merkel cells are essential for light-touch responses. Science. 2009;324:1580–2. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling in primary sensory neurons and spinal astrocytes contribute to paclitaxel-induced peripheral neuropathy. J Pain. 2014 doi: 10.1016/j.jpain.2014.04.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Boyette-Davis JA, Kosturakis AK, Li Y, Yoon SY, Walters ET, et al. Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. J Pain. 2012 doi: 10.1016/j.jpain.2013.03.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benn T, Halfpenny C, Scolding N. Glial cells as targets for cytotoxic immune mediators. GLIA. 2001;36:200–11. doi: 10.1002/glia.1109. [DOI] [PubMed] [Google Scholar]
- 22.Lisak RP, Bealmear B, Benjamins J, Skoff A. Inflammatory cytokines inhibit upregulation of glycolipid expression by Schwann cells in vitro. Neurology. 1998;51:1661–5. doi: 10.1212/wnl.51.6.1661. [DOI] [PubMed] [Google Scholar]
- 23.Conti G, De Pol A, Scarpini E, Vaccina F, De Riz M, Baron P, et al. Interleukin-1 beta and interferon-gamma induce proliferation and apoptosis in cultured Schwann cells. J Neuroimmunol. 2002;124:29–35. doi: 10.1016/s0165-5728(02)00003-6. [DOI] [PubMed] [Google Scholar]
- 24.Shubayev VI, Myers RR. Endoneurial remodeling by TNFa- and TNFa-releasing proteases. A spatial and temporal co-localization study in painful neuropathy. J Peripher Nerv Syst. 2002;7:28–36. doi: 10.1046/j.1529-8027.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- 25.Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–13. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyette-Davis J, Dougherty PM. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Exp Neurol. 2011;229:353–7. doi: 10.1016/j.expneurol.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]