Abstract
Specific mutations were created in the cytoplasmic domain of the gp41 transmembrane protein of simian immunodeficiency virus strain 239 (SIV239). The resultant strains included a mutant in which Env residue 767 was changed to a stop codon, a double mutant in which positions 738 and 739 were changed to stop codons, another mutant in which a prominent endocytosis motif was changed from YRPV to GRPV by the substitution of tyrosine 721, and a final combination mutant bearing Q738stop, Q739stop, and Y721G mutations. The effects of these mutations on cell surface expression, on Env incorporation into virions, and on viral infectivity were examined. The molar ratio of Gag to gp120 of 54:1 that we report here for SIV239 virions agrees very well with the ratio of 60:1 reported previously by Chertova et al. (E. Chertova, J. W. Bess, Jr., B. J. Crise, R. C. Sowder II, T. M. Schaden, J. M. Hilburn, J. A. Hoxie, R. E. Benveniste, J. D. Lifson, L. E. Henderson, and L. O. Arthur, J. Virol. 76:5315-5325, 2002), although they were determined by very different methodologies. Assuming 1,200 to 2,500 Gag molecules per virion, this corresponds to 7 to 16 Env trimers per SIV239 virion particle. Although all of the mutations increased Env levels in virions, E767stop had the most dramatic effect, increasing the Env content per virion 25- to 50-fold. Increased levels of Env content in virions correlated strictly with higher levels of Env expression on the cell surface. The increased Env content with the E767stop mutation also correlated with an increased infectivity, but the degree of change was not proportional: the 25- to 50-fold increase in Env content only increased infectivity 2- to 3-fold. All of the mutants replicated efficiently in the CEMx174 and Rh221-89 cell lines. Although some of these findings have been reported previously, our findings show that the effects of the cytoplasmic domain of gp41 on the Env content in virions can be dramatic, that the Env content in virions correlates strictly with the levels of cell surface expression, and that the Env content in virions can determine infectivity; furthermore, our results define a particular change with the most dramatic effects.
Lentiviruses have transmembrane glycoproteins (TMs) with unusually long cytoplasmic domains compared to those of other retroviruses (12). The unusual length of lentiviral TM cytoplasmic domains (usually 150 amino acids or longer) suggests that these sequences may have evolved functions that are specific for lentiviruses. What these functions may be is not completely understood. In simian immunodeficiency virus (SIV), the cytoplasmic domain of the TM gp41 is not absolutely required for replication. In vitro passaging of SIVmac in certain CD4+ human cell lines and human peripheral blood mononuclear cells has been shown to select for variants with truncated cytoplasmic tails (29). Passaging of these truncated variants in monkey peripheral blood mononuclear cell cultures or their replication in rhesus monkeys leads to reversion to the full-length sequence (29, 33). Consistent with the requirement of a full-length cytoplasmic tail for optimal replication in rhesus cells, Shacklett et al. reported attenuated replication in rhesus macaques for viruses with truncated intracytoplasmic tails (51). Early studies showed that the replication of human immunodeficiency virus type 1 (HIV-1) is less tolerant to truncation of the gp41 cytoplasmic domain (13, 18). However, some cytoplasmic domain truncations in HIV-1 are compatible with replication (44, 56). Truncation of the cytoplasmic domain of SIV TMs can increase the incorporation of the envelope protein into virions (35, 59), the fusogenicity of the virions (40, 47, 52, 59), and viral infectivity (35, 59).
Several cellular proteins have been found to interact with the gp41 cytoplasmic domain of SIV and HIV-1. These include the clathrin-associated adapter complexes AP-1 and AP-2 (3), calmodulin (53), p115-RhoGEF (57), α-catenin (28), the prenylated Rab acceptor (14), and Tip47 (4). These cellular proteins are all known to influence the trafficking of proteins to and from the plasma membrane. An interaction between the cytoplasmic domain of gp41 and the viral matrix protein (p17) also appears to modulate envelope glycoprotein incorporation into virions (15, 16, 34, 55).
The envelope proteins of both SIV and HIV-1 are efficiently endocytosed in a clathrin-dependent manner. The cytoplasmic domains of SIV and HIV-1 TMs contain multiple endocytosis signals to mediate clathrin-dependent endocytosis. In HIV-1, endocytosis is mediated at least in part by a YXXφ motif (X, any amino acid; φ, an amino acid with a bulky hydrophobic side chain) located in the membrane-proximal region of the TM (Y712 in HIV-1IIIB) (48). This signal can bind clathrin adapters and target proteins for endocytosis. Another potential endocytosis signal at residues 760 to 766 that interacts with clathrin adapters has been identified, although it appears to be less important for internalization (5, 41). Tyrosine 721 in SIVmac239 corresponds to Y712 in HIV-1, and it too appears to function as an endocytosis signal by interaction with components of the clathrin adapter complex (3, 30, 49). Mutation of this tyrosine residue in SIV reduces endocytosis rates in the context of a tail-truncated TM (49). However, other signals downstream of Y721 in SIV Env appear to be important, since the effects of Y721 on endocytosis are marginal in the context of a full-length cytoplasmic domain (6).
Despite intensive investigation of the envelope proteins of HIV-1 and SIV, there have been surprisingly few attempts to estimate the numbers of envelope molecules per virion. Early studies using electron microscopy suggested the incorporation of 72 spikes on immature, budding HIV-1 virions (19, 20). Smaller numbers on mature virions were suggested to result from shedding of the envelope surface protein SU (gp120) from virions. Again, using electron microscopy, Layne et al. (32) observed fewer “knobs” on mature HIV-1 virions and estimated their number to be 10 per mature virion. Using a biochemical analysis of purified SIV239, Chertova et al. (10) found a ratio of Gag to Env molecules of 60 to 1. Assuming 1,200 to 2,500 Gag molecules per virion (10, 32, 43, 54) and three envelope molecules per spike (7, 8), this corresponds to 7 to 14 trimer spikes per SIV239 virion. Chertova et al. (10) also noticed an increased SU content in an SIV with a truncated TM.
In this report, we describe the effects of a number of different mutations within the cytoplasmic domain of SIV239 on levels of envelope protein expression on the cell surface, on envelope protein incorporation into virions, and on viral infectivity.
MATERIALS AND METHODS
Site-specific mutagenesis and subcloning.
The ClaI-NdeI fragment of the proviral SIVmac239 DNA, containing 1,578 nucleotides of env coding sequence (proviral nucleotides 8072 to 9751 in the numbering of Regier and Desrosiers [46]), was subcloned into pSP72 (Promega, Madison, Wis.). Mutations in env were created by site-directed mutagenesis using a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). The following mutagenic primers were used: for Y721G, 5′-GCTAAGTTAAGGCAGGGGGGAAGGCCAGTGTTCTCTTCC-3′ (nucleotides 8746 to 8784) and 5′-GGAAGAGAACACTGGCCTTCCCCCCTGCCTTAACTTAGC-3′ (nucleotides 8784 to 8746); for Q738stop and Q739stop, 5′-CCCACCCTCTTATTTCTGATAGACCCATATCCAACAGG-3′ (nucleotides 8784 to 8821) and 5′-CCTGTTGGATATGGGTCTATCAGAAATAAGAGGGTGGG-3′ (nucleotides 8821 to 8784); and for E767stop, 5′-CCTGGCCTTGGCAGATATAATATATTCATTTCCTGATCCGCC-3′ (nucleotides 8884 to 8926) and 5′-GGCGGATCAGGAAATGAATATATTATATCTGCCAAGGCCAGG-3′ (nucleotides 8926 to 8884). The primers were purchased from Sigma-Genosys Biotechnologies, Inc. (The Woodlands, Tex.). For cell surface expression, all mutant env genes were inserted into pSIVΔgpV (36). pSIVΔgpV contains a full-length SIVmac239 genome from which a substantial portion of the gag-pol region has been deleted (nucleotide positions 1770 to 5441 [46]). This construct expresses the envelope glycoprotein of SIV239 under the control of the viral long terminal repeat (LTR) as well as the viral regulatory proteins Vpx, Vpr, Tat, and Rev. For infectivity assays using T-Rex CD4 cells (45), the E767stop env gene was inserted into SIVΔnefEGFP. SIVΔnefEGFP is an engineered recombinant derivative of SIVmac239 that expresses enhanced green fluorescent protein (2).
DNA sequencing.
Cloned fragments containing mutated envelope genes were sequenced with an ABI377 automated DNA sequencer by using a dye terminator cycle sequencing kit as specified by the manufacturer (Perkin-Elmer Inc., Foster City, Calif.).
Virus stocks and cell culture.
For the generation of viral stocks, 3 μg of the 5′ and 3 μg of the 3′ clones of SIVmac239 were digested with SphI. Each 3′ clone was ligated with the 5′ clone p239SpSp5′ by using T4 DNA ligase. The ligated DNA was used to transfect 293T cells by the calcium phosphate method (Promega). 293T, COS-1, CEMx174, and Rh221-89 cells were maintained as previously described (38, 39). For virus stocks, 293T and COS cells were transfected as described above. The culture medium was changed on day 2 posttransfection, and supernatants were harvested on day 3. The virus was quantified by determining the concentration of p27 capsid in the supernatant by an antigen capture assay (Coulter Corp., Hialeah, Fla.). T-Rex CD4 cells were maintained in Dulbecco's modified Eagle medium-10 supplemented with 200 μg of zeocin (Invitrogen, Carlsbad, Calif.)/ml, 5 μg of blasticidin (Invitrogen)/ml, and 0.5 mg of G418 (GIBCO, Grand Island, N.Y.)/ml to maintain the ccr5, Tet repressor, and CD4 genes, respectively. Both the ccr5 and CD4 genes were of human origin. Variable levels of CCR5 expression were induced by the addition of 0.1 or 1 ng of deoxycycline (Sigma, St. Louis, Mo.)/ml to the culture medium. CD4 and CCR5 expression levels were determined by flow cytometry analysis of cells immunostained with a phycoerythrin-conjugated CCR5-specific antibody (Pharmingen).
Viral growth curves.
For growth curves of the viruses in CEMx174 and Rh221-89 cells, 2 × 106 cells were infected with virus stocks containing 10 ng of p27 from each virus. At 1 day postinfection, the cells were pelleted and resuspended in 10 ml of virus-free RPMI 1640-10 or 20% fetal calf serum and 20% interleukin-2. Five milliliters of the supernatant was replaced with fresh medium every 3 to 4 days. The cell-free supernatant was harvested on the indicated days, and the amount of p27 antigen was determined with a commercial antigen capture kit (Coulter Co.).
Infectivity assay.
Viral infectivity was measured with LTR-SEAP-CEMx174 indicator cells (37). A 96-well plate was set up, with each row containing two uninfected wells and two sets of five twofold dilutions of virus. To these wells, 3 × 104 LTR-SEAP-CEMx174 cells were added, and the plate was transferred to a humidified CO2 incubator at 37°C. After 3 days, the secreted alkaline phosphatase (SEAP) activity was measured with a Phosphalight kit (Applied-Biosystems, Foster City, Calif.).
Viral pellets.
Virus-containing supernatants were first clarified by two consecutive centrifugation steps for 10 min at 2,600 × g. The virus was then pelleted by centrifugation for 2 h at a high speed (16,000 × g) in a refrigerated microcentrifuge. The viral pellet was washed by resuspension in 1 ml of phosphate-buffered saline (PBS) and pelleted again by centrifugation at a high speed. After this second ultracentrifugation, the viral pellets were resuspended in 50 μl of PBS and the amount of p27 was quantified by antigen capture as described above.
Western blotting.
Identical quantities of p27 were mixed with Laemmli buffer (31) and boiled for 4 min. The samples were then electrophoresed through an 8 to 16% polyacrylamide-sodium dodecyl sulfate (SDS) gradient gel. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). The membranes were blocked with 5% skim milk in PBS-0.05% Tween 20 for 1 h and then incubated with antibodies recognizing the gp120 (3.11H) (11) and gp41 subunits (KK41) (26) as well as p27 (2F12) (21). Horseradish peroxidase-conjugated anti-rhesus immunoglobulin G was used to detect antibody 3.11H and horseradish peroxidase-conjugated anti-mouse immunoglobulin G was used to detect monoclonal antibodies 2F12 and KK41. The rhesus monoclonal antibody 3.11H was a gift of J. E. Robinson (Tulane University Medical School). The KK41 and 2F12 murine monoclonal antibodies were obtained through the NIH AIDS Research and Reference Reagent Program. A recombinant Gag p27 SIV251-glutathione S-transferase (GST) fusion protein (Immunodiagnostics, Woburn, Mass.) and SIVmac239 gp130 (NIH AIDS Research and Reference Reagent Program) were used to prepare standard curves. For the molecular weight of gp120, only the protein component was considered (minus the glycans). The purity of Gag p27 SIV251-GST was >98% and the purity of SIVmac239 gp130 was approximately 55%. The purity was verified by SDS-polyacrylamide gel electrophoresis and silver staining (Bio-Rad, Hercules, Calif.) and the concentration was verified by the Bradford dye-binding procedure (Bio-Rad). The membranes were treated with a chemiluminescent substrate (Pierce, Rockford, Ill.). The bands were visualized and analyzed with a Fuji phosphorimager.
Flow cytometry.
Env expression on the surfaces of transfected cells was monitored by flow cytometry. pSIVΔgpV constructions were used to transfect 293T cells as described above. The cells were harvested at day 3 posttransfection and washed twice with PBS-2% fetal calf serum. The cells (5 × 105) were incubated with the 1.9C antibody followed by a phycoerythrin-conjugated anti-human immunoglobulin G and were fixed in 2% formaldehyde-PBS. CD4 and CCR5 expression levels in T-Rex/CCR5 and T-Rex/CCR5 CD4 cells were determined by flow cytometry analysis of cells that were immunostained with a fluorescein isothiocyanate-conjugated anti-human CD4 antibody (Pharmingen) and a Cy-chrome-conjugated anti-human CCR5 antibody (Pharmingen) and fixed with 2% formaldehyde-PBS. The green fluorescent protein (GFP) expression of infected T-Rex/CCR5 and T-Rex/CCR5 CD4 cells was monitored by flow cytometry. At day 2 postinfection, the cells were harvested, washed, and fixed as described above.
RESULTS
Selection of mutations.
To investigate the effects of different sequence changes within the cytoplasmic domain of TM, we used site-specific mutagenesis to introduce a variety of mutations into SIV239. SIV239 was selected as the parent strain since it replicates well in primary rhesus monkey cells, in the rhesus monkey 221 cell line, in some human cell lines, and in rhesus monkeys, in which it consistently causes a progressive AIDS-like illness (1, 24, 27). We changed the glutamine codons at Env residues 738 and 739 to stop codons, which resulted in the truncation of 141 amino acids from the C-terminal tail of gp41 (TM) (Fig. 1). These mutations did not affect the second exons of tat and rev, which are located just downstream. These particular mutations were selected because truncation at these residues has been noted previously in SIV passaged in human cells (9, 22, 29) and because others have studied the effects of truncation at this location (6, 25, 40, 59). In a separate construction, we introduced a stop codon at residue 767, resulting in the truncation of 119 residues from the C-terminal tail (Fig. 1). This mutation did alter the second exon of rev by changing an AGA Arg codon to an AUA Ile codon and the second exon of tat by changing a UAG stop codon to a Tyr codon, adding six amino acids at the end of Tat (YNIPIS). This mutation was noted previously in an SIV strain recovered from the lung compartment of an infected rhesus monkey (38), but otherwise it has not been studied. We also introduced a Y721G mutation into the wild-type (WT) SIV239 background and into the Q738stop/Q739stop background (Fig. 1). Y721 is part of a YXXφ sequence that has previously been shown to function as an endocytosis signal (17, 30, 49).
FIG. 1.
Schematic representation of mutations in the gp41 cytoplasmic domain of SIV239 relative to the locations of putative endocytosis signals. The overlapping reading frames for tat, rev, and nef are also indicated.
Effects of gp41 cytoplasmic tail mutations on Env incorporation into virions.
We originally set out to make a variety of measurements in CD4+ cells permissively infected with the five forms of SIV shown in Fig. 1. However, cytolytic effects caused by the permissive replication produced considerable cell debris and caused difficulty with the reliability of the measurements. We therefore used parallel transfections of cloned DNA in transient assays to assess the effects of the mutations on the parameters of interest. The cloned DNA used in these transfection assays produces a virus that is fully replication competent (see below), and none of the mutations affected the quantity of virions produced. To assess Env incorporation into virions, we pelleted the virus from the supernatant of transfected cells. The amounts of p27 Gag antigen in pelleted virions were assessed by an antigen capture assay, and normalized amounts of p27-containing virions were analyzed by Western blotting for SU (gp120) and TM content (Fig. 2 and 3). Strongly reactive monoclonal antibodies to the ectodomain of TM (KK41) and to the V3 loop of gp120 (3.11H) were used for detection. No incorporation of uncleaved gp160 was detected in virions by probing the membrane with the antibody that was reactive to the ectodomain of TM (KK41). All four of the mutated forms of TM displayed elevated levels of Env incorporation into virions after the transfection of 293T cells (Fig. 2 and 3). The largest effect by far was observed with the truncation at Env residue 767, which resulted in 25- to 50-fold increases in the amount of Env in virions. In general, the levels of gp120 in virions paralleled those of TM. However, the increases in gp120 content for the E767stop and Y721G/Q738stop/Q739stop mutants lagged a bit behind the gp41 increases for these viruses. It is possible that the shedding of gp120 could have contributed to the magnitude of the effect on gp120 versus that on TM for these two strains; however, the magnitude of the difference between the gp120 increase and the TM increase (26-fold versus 47-fold for the E767stop mutant and 8-fold versus 20-fold for the Y721G/Q738stop/Q739stop mutant) was not large, and it is difficult to be certain if shedding did indeed contribute to the slight differential. The magnitude of the effect was similar in replicate experiments. When transfected COS-1 cells were used for an analysis of SIV239 and the E767stop mutant, a similar increase in Env content in the virions was observed (data not shown).
FIG. 2.
Effect of different mutations in Env on incorporation into virions. Viruses were produced by transfection into 293T cells, and virions were pelleted from the clarified supernatants. gp120 (A), gp41 (B), and p27 (C) were detected by Western blotting using 3.11H, KK41, and 2F12 monoclonal antibodies.
FIG. 3.
Relative Env incorporation into virions. The ratios of gp120 to p27 (A) and of gp41 to p27 (B) were calculated by using phosphorimaging analysis. Data for the mutants are presented relative to the ratios found for SIV239 virions.
Estimation of the number of spikes per virion.
To determine the ratio of Gag to Env proteins in virion samples, we employed quantitative Western blotting analysis. Standard curves were generated by using recombinant SIV p27 and SIV gp120, taking into account the concentration and purity of the proteins. Pelleted viruses and the proteins for standard curves were resolved in an 8 to 16% polyacrylamide-SDS gradient gel and probed for p27 and gp120 with the monoclonal antibodies 2F12 and 3.11H (Fig. 4). Using these standard curves, we were able to determine the amounts of p27 and gp120 proteins (in nanograms) in each viral sample. The amount of p27 protein was estimated by using a standard curve instead of the antigen capture assay because we have observed an underestimation of the amount of p27 in viral lysate compared to that of purified protein when using the antigen capture assay. This underestimation is likely due to incomplete virion lysis or release of Gag proteins under the conditions of the antigen capture assay. By taking into consideration the molecular weights of these proteins, we were able to estimate the molar ratio of p27 Gag to gp120 Env. According to this immunoblot analysis, we estimated a molar ratio of Gag to gp120 of 54:1 for SIV239 virions. Similar results were obtained in independent experiments. If an estimate of 1,200 to 2,500 Gag molecules per viral particle is used (32, 43, 54), this Gag-to-gp120 ratio corresponds to an average of 22 to 47 SU molecules per virion for SIV239. By considering the organization of Env molecules in virions into trimers (7, 8), we thus estimate that there are 7 to 16 spikes per SIV239 virion. For the mutant with the highest spike density (E767stop), values of 200 to 417 spikes per virion were calculated (Table 1).
FIG. 4.
Estimation of Gag/Env molar ratio. Quantification of the relative amounts of p27 and gp120 in the pelleted SIV239 virus was achieved by using standard curves. Antibodies 3.11H and 2F12 were used for the Western blot analysis.
TABLE 1.
Estimated numbers of spikes per virion for SIV mutantsa
| Virus | No. of spikes per virion (range) |
|---|---|
| SIV239 | 7-16 |
| Y721G | 25-51 |
| Q738stop, Q739stop | 87-181 |
| E767stop | 200-417 |
| Y721G, Q738stop, Q739stop | 60-124 |
The numbers of spikes per virion were estimated based upon our quantitation of p27 Gag-to-gp120 Env molar ratios. p27 Gag-to-gp120 Env ratios were estimated by using standard curves generated with purified recombinant GST-p27 and gp120 proteins. Pelleted virions and standards were resolved in an 8 to 16% polyacrylamide-SDS gel and probed with antibodies recognizing p27 and gp120 (Fig. 4). The numbers of spikes per virion were calculated by using the p27-to-gp120 molar ratios estimated from the Western blot analysis, a value of 1,200 to 2,500 Gag molecules per virion (10, 32, 43, 54), and three gp120 molecules per spike (7, 8).
Effects of gp41 cytoplasmic tail mutations on Env expression on the cell surface.
For Env cell surface expression analysis, 293T cells were transiently transfected with Env expression constructs (see Materials and Methods). Envelope glycoprotein expression on the surfaces of transfected cells was quantitated by fluorescence-activated cell sorting analysis using a monoclonal antibody recognizing SIV gp120 (Fig. 5). Mean channel fluorescence intensities (MFIs) and percentages of positive cells for 293T cells transfected with each of the cytoplasmic domain mutants are shown in Fig. 6. All of the truncating mutations within the TM cytoplasmic domain (E767stop, Q738stop/Q739stop, and Y721G/Q738stop/Q739stop) markedly increased the levels of surface envelope glycoproteins compared with those in SIVmac239. A smaller but reproducible increase was also observed for cells transfected with the YXXφ endocytosis motif mutant (Y721G).
FIG. 5.
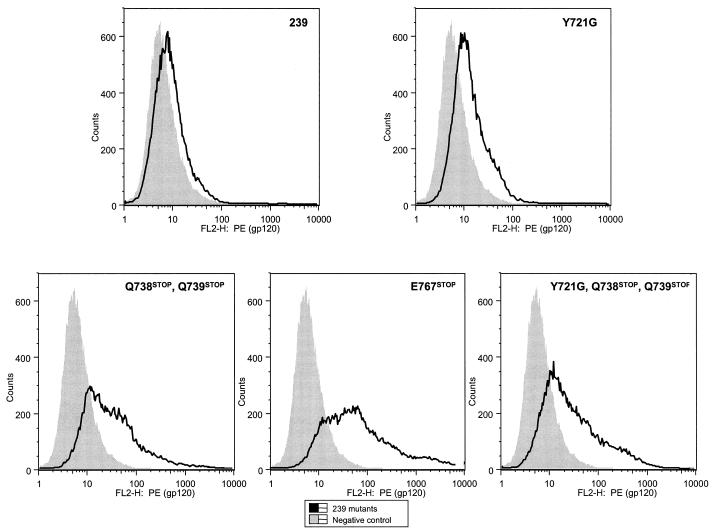
Analysis of cell surface expression by flow cytometry. 293T cells were transfected with Env expression cassettes in vector SIVΔgpΔNEFGFP. Envelope expression on the surface was analyzed with the 1.9C monoclonal antibody.
FIG. 6.
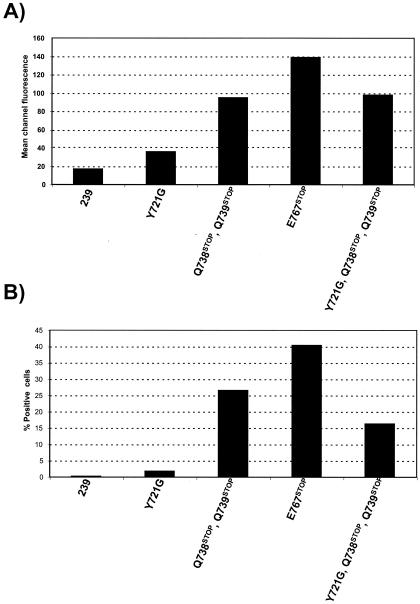
Surface expression of Env in 293T cells transfected with the Env expression vector pSIVΔgpV. (A) Mean fluorescence per channel. (B) Percentages of positive cells.
Infectivity.
LTR-SEAP-CEMx174 cells were used to quantitate the infectivities of the viruses under conditions that approximated a single cycle of infection. LTR-SEAP-CEMx174 cells were infected with SIV239 and each of the viruses bearing mutations in the cytoplasmic domain of the TM. LTR-SEAP-CEMx174 cells secrete engineered, placental SEAP into the medium in response to infection by SIV (37). The amount of SEAP secreted correlates directly with the amount of infecting virus and can be sensitively and rapidly measured by a chemiluminescence assay. The results of a representative experiment are shown in Fig. 7, and the infectivities per nanogram of p27 are also shown. The only mutant with a believable increase in infectivity was the E767stop mutant, with an increase in SEAP activity of about twofold. The infectivity assay for the E767stop mutant was assessed independently on two additional occasions with separate stocks; the increases in infectivity observed were 3.8- and 2-fold. Slight differences in SEAP activity were observed for the Q738stop/Q739stop and Y721G/Q738stop/Q739stop mutants, but it is uncertain whether they were significant.
FIG. 7.
Effect of different mutations on viral infectivity. Virus stocks were obtained from the transfection of 293T cells. Stocks were normalized to the amount of p27 and used to infect LTR-SEAP-CEMx174 cells. (A) SEAP activity was measured by use of a Phosphalight kit according to the manufacturer's recommendations at 3 days postinfection. (B) SEAP activity normalized to the amount of p27.
In order to study the relationship between receptor and coreceptor levels and the infectivities of viruses with various levels of envelope density, we used two T-Rex/CCR5 CD4 cell lines that express CCR5 under the control of a tetracycline-regulated promoter, making it possible to vary CCR5 expression levels by incubating cells with different concentrations of doxycycline. One of the cell lines expresses high levels of CD4 (MFI, 13.5 ± 1.4 [mean ± standard deviation]) and the other one expresses low levels of CD4 (MFI, 4.2 ± 0.7). The expression of CCR5 was induced by adding 0.1 (low CCR5) or 1 (high CCR5) ng of deoxycycline/ml to the culture medium. CCR5 expression was also induced in a T-Rex/CCR5 cell line without CD4. The expression of CD4 and CCR5 was monitored by flow cytometry (Fig. 8A). Some expression of CCR5 was observed in uninduced controls for both the T-Rex/CCR5 CD4hi and T-Rex/CCR5 CD4lo cell lines. Cells expressing low or high levels of CCR5 and low or high levels of CD4, as judged by flow cytometry analysis (Fig. 8A), were infected with SIV239ΔnefEGFP and with the corresponding E767stop mutant. 293T cells and a T-Rex/CCR5 cell line with no CD4 and high levels of induced CCR5 were also infected as controls. Infectivity was monitored at day 2 postinfection by measuring the GFP activity by flow cytometry (Fig. 8B). The infectivity observed for cells with high levels of CD4 expression was higher than that observed for cells with low levels of CD4. No significant differences in infectivity were observed between the two viruses in cells with a low level of CD4 expression and different levels of CCR5 expression. A small increase in infectivity was observed for the E767stop mutant in cells with a high level of CD4 expression, independent of the level of CCR5 expression (Fig. 8B). Thus, the marked increase in Env content in E767stop virions translated into extremely modest increases in infectivity, even when the levels of CD4 and CCR5 were markedly varied.
FIG.8.
Effects of E767stop mutation on viral infectivity in cells with variable receptor and coreceptor densities. (A) CD4 and CCR5 expression as determined by flow cytometry. The SIVΔnefGFP virus and the corresponding E767stop mutant were obtained by transfecting 293T cells. Stocks were normalized for the amount of p27 and used to infect CD4 T-Rex/CCR5 cells. (B) Percentages of GFP-positive cells as determined by flow cytometry.
Viral growth curves.
We also compared the growth curves of the different mutants in two cell lines, the human B-cell/T-cell hybrid line CEMx174 (Fig. 9A) and the rhesus immortalized T-cell line Rh221-89 (Fig. 9B). Normalized amounts of mutant and parental virus stocks produced from transient transfections in 293T cells were used to analyze viral replication in both cell lines. Cell supernatants were harvested on the indicated days, and the relative levels of p27 were quantitated by antigen capture assays. All of the mutants were similarly replication competent in both cell lines. In CEMx174 cells, the Y721G/Q738stop/Q739stop mutant appeared to replicate to lower levels, with slightly delayed kinetics. In the rhesus Rh221-89 cells, peak levels of virus production appeared to be somewhat higher for the Q738stop/Q739stop and Y721G/Q738stop/Q739stop mutants.
FIG. 9.
Replication kinetics of viruses containing mutations in gp41 cytoplasmic domain. (A) CEMx174 cells; (B) rhesus immortalized T-cell line Rh221-89. Viruses were produced by the transfection of 293T cells. p27 was quantified by an antigen capture assay using clarified supernatants. Cells (2 × 106) were infected with 10 ng of p27.
DISCUSSION
Early studies showed that SIVmac produced on human cell lines frequently has a truncating stop codon corresponding to Env residue 738 or 739 in the SIV239 sequence (9, 22, 29). Subsequent studies showed that such truncating mutations in SIVmac can increase its fusogenicity (40, 47, 52, 59), its infectivity (35, 59), and envelope incorporation into virions (25, 35, 50, 59). Our present findings confirm, refine, and extend the results of these previously published studies. In particular, we examined the effects of four different mutations, including both truncation and Y721G point mutations, on these parameters. We have shown that the Env content in virions correlates strictly with the levels of Env expression at the cell surface. We have quantitated the magnitude of the change resulting from these mutations in terms of spikes per virion and have defined a particular change, E767stop, that was not studied previously but produces the highest magnitude of increase. Finally, we have shown that dramatic increases in virion Env content resulting from truncation, or smaller changes resulting from the Y721G point mutation, do not result in corresponding changes in infectivity in the SIV239 background.
All four mutant forms that we studied increased the levels of Env expression on the surfaces of cells and increased the Env content of virions. What forces are likely to be responsible for these increases? The general agreement between the levels of gp120 and TM in virions suggests that gp120 shedding from virions is not a major factor, consistent with a conclusion from a previous report (10). For SIV, the tyrosine at Env residue 721 has been clearly documented to be part of an endocytosis signal (3, 30, 49). It seems highly likely that the increase in surface expression caused by the mutation at residue 721 results directly from decreased endocytosis. However, the largest increases in surface expression and Env content in virions arose from the truncating mutations at residues 738 and particularly 767. Cytoplasmic domain sequences downstream of residue 721 in TM contain additional, putative endocytosis signals (YXXφ and LL) (Fig. 1). It seems reasonable to speculate that the loss of these signals results in decreased endocytosis and increased surface expression. In fact, significant decreases in the endocytosis rate were observed when truncations were introduced into the cytoplasmic domain of SIV TM downstream of Y721 (6). However, the E767stop mutation resulted in considerably larger increases in surface expression and virion Env content than the Q738stop mutation. This suggests that sequences between residues 738 and 767 contain positive signals for the level of Env surface expression.
Manrique et al. were unable to detect increases in Env expression on the surfaces of cells that resulted from SIV truncation mutations that increased both the virion Env content and infectivity (35). Our results clearly demonstrate a close correspondence of the level of Env expression on the surfaces of cells with the level of Env incorporated into virions. This correspondence may have important implications for the mechanism of Env assembly into virions. If the effects of the mutations on these parameters are indeed largely the result of decreased endocytosis, as discussed above, the results suggest that the Env association with cores during the process of virion assembly occurs at the cell surface, not internally. If Env must sit on the surface of an infected cell in order to be subsequently incorporated into a virion, regulated levels of Env surface expression in the face of ongoing immune responses are likely to be critically important for the virus. These observations apply to the traditional mode of retroviral budding from the plasma membrane, not to the intracellular pathway of viral budding that can sometimes occur in some cell types, particularly macrophages.
It is somewhat surprising how little is known about the Gag and Env contents of virions of SIV and HIV. A variety of technical approaches, including electron microscopic and biochemical techniques, have been used to estimate that there are 1,200 to 2,500 Gag molecules per virion (32, 43, 54). However, a recent study determined the masses of assembled immature HIV-1 particles in vitro and suggested that there may be over 4,000 Gag molecules per HIV-1 virion (Marc C. Johnson and Volker M. Vogt, personal communication). The Env spikes on virions of SIV and HIV-1 have unambiguously been demonstrated to be trimers (7, 8). Early electron microscopic studies suggested that immature, budding HIV-1 virions contain about 72 spikes (19, 42). However, Hockley noted that surface spikes were rarely seen on immature or mature HIV-1 particles but were much more obvious on SIV virions (23). Abundant surface spikes have been noted on SIV by electron microscopy performed by others as well (20, 23), but the interpretation of these analyses is complicated by the probable use of truncated-TM versions of SIV of an undefined nature. Chertova et al. (10) used a high-performance liquid chromatography analysis of proteins from purified SIV239 virions to estimate a ratio of Gag to gp120 Env molecules of 60:1. Our analyses, based on totally different methodologies from those used by Chertova et al., gave a very similar molar ratio of Gag to gp120 Env of 54:1 for SIV239. Assuming that there are 1,200 to 2,500 Gag molecules per virion, this corresponds to 7 to 16 trimer spikes on average per SIV239 virion. Subsequent to the submission of our study, Zhu et al. reported the results of an electron tomography analysis of envelope spikes on SIV and HIV (58). They found 7 to 14 trimer spikes on SIV239 and 50 to 133 trimer spikes on tail-truncated SIV239. Thus, the numbers reported here that assume 1,200 to 2,500 Gag molecules per virion are in very good agreement with the sophisticated spike counts of Zhu et al. (58).
Dramatic increases in Env content per virion did not translate to corresponding increases in infectivity in CEMx174 cells. We investigated the hypothesis that more dramatic increases in infectivity might be observed under some conditions in which the concentration of CD4 or the CCR5 coreceptor is limiting. Although the E767stop mutant did exhibit a somewhat higher infectivity than the parental virus at higher levels of CD4 expression, the effect was again extremely modest. One possible explanation is that the truncation of TM affects the ability of Env spikes to function in viral fusion and entry. In this scenario, an increased Env content in virions may be somewhat counterbalanced by a decreased inherent infectivity per virion spike.
It should be noted that the effects of Env content on infectivity are likely to vary with the SIV strain and with the cell line and coreceptor used. Zingler and Littman (59) noted 25- to 100-fold increased infectivities of a truncated form of SIV strain 1A11, but the effect of truncation of SIV239 was only an approximately 3-fold increase. Truncation of the cytoplasmic domain of SIV239 TM appears to increase its infectivity for HUT-78 cells to a considerably larger extent than for CEMx174 cells or U87 cells (25, 59). Thus, under conditions of highly efficient entry by SIV239 into CEMx174 cells with the BOB coreceptor or into cells with the natural CCR5 coreceptor, dramatic increases in Env content in virions may not increase infectivity appreciably or correspondingly. For this scenario, highly inefficient entry conditions may be needed in order to see more dramatic effects of increased virion Env content on infectivity.
The results described in this report help to lay the groundwork for future studies on the biological relevance of variations in virion Env content. Some possible questions include the following. To what extent does the Env spike density influence virion sensitivity to antibody-mediated neutralization? To what extent does the Env spike density influence the ability of the virus to elicit anti-Env antibodies with neutralizing activity? What is the maximal packing density of envelope spikes for wild-type HIV and SIV virions? Does HIV or SIV naturally regulate spike density, and if so, under what selective pressures? Does a full-length cytoplasmic domain of TM limit the Env packing density in virions?
Acknowledgments
We thank Melissa Laird for help with engineered target cell lines and W. E. Johnson for helpful discussions. The following reagents were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: 2F12 from Kai Krohn and Vladimir Ovod and SIVmac239 gp130 from DAIDs, NIAID (Bio-Molecular Technology Inc.).
This work was supported by U.S. Public Health Service grants AI50421 and AI35365 (R.C.D.), RR00168 (New England Primate Research Center), and R01 40880 (R.W.D.). J.D.R. is supported by an AmFAR fellowship.
REFERENCES
- 1.Alexander, L., Z. Du, M. Rosenzweig, J. U. Jung, and R. C. Desrosiers. 1997. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J. Virol. 71:6094-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, L., R. S. Veazey, S. Czajak, M. DeMaria, M. Rosenzweig, A. A. Lackner, R. C. Desrosiers, and V. G. Sasseville. 1999. Recombinant simian immunodeficiency virus expressing green fluorescent protein identifies infected cells in rhesus monkeys. AIDS Res. Hum. Retrovir. 15:11-21. [DOI] [PubMed] [Google Scholar]
- 3.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blot, G., K. Janvier, S. Le Panse, R. Benarous, and C. Berlioz-Torrent. 2003. Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for env incorporation into virions and infectivity. J. Virol. 77:6931-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. [DOI] [PubMed] [Google Scholar]
- 6.Bowers, K., A. Pelchen-Matthews, S. Honing, P. J. Vance, L. Creary, B. S. Haggarty, J. Romano, W. Ballensiefen, J. A. Hoxie, and M. Marsh. 2000. The simian immunodeficiency virus envelope glycoprotein contains multiple signals that regulate its cell surface expression and endocytosis. Traffic 1:661-674. [DOI] [PubMed] [Google Scholar]
- 7.Center, R. J., R. D. Leapman, J. Lebowitz, L. O. Arthur, P. L. Earl, and B. Moss. 2002. Oligomeric structure of the human immunodeficiency virus type 1 envelope protein on the virion surface. J. Virol. 76:7863-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Center, R. J., P. Schuck, R. D. Leapman, L. O. Arthur, P. L. Earl, B. Moss, and J. Lebowitz. 2001. Oligomeric structure of virion-associated and soluble forms of the simian immunodeficiency virus envelope protein in the prefusion activated conformation. Proc. Natl. Acad. Sci. USA 98:14877-14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakrabarti, L., M. Guyader, M. Alizon, M. D. Daniel, R. C. Desrosiers, P. Tiollais, and P. Sonigo. 1987. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature 328:543-547. [DOI] [PubMed] [Google Scholar]
- 10.Chertova, E., J. W. Bess, Jr., B. J. Crise, R. C. Sowder II, T. M. Schaden, J. M. Hilburn, J. A. Hoxie, R. E. Benveniste, J. D. Lifson, L. E. Henderson, and L. O. Arthur. 2002. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J. Virol. 76:5315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole, K. S., M. Alvarez, D. H. Elliott, H. Lam, E. Martin, T. Chau, K. Micken, J. L. Rowles, J. E. Clements, M. Murphey-Corb, R. C. Montelaro, and J. E. Robinson. 2001. Characterization of neutralization epitopes of simian immunodeficiency virus (SIV) recognized by rhesus monoclonal antibodies derived from monkeys infected with an attenuated SIV strain. Virology 290:59-73. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers, R. C. 2001. Nonhuman lentiviruses, p. 2095-2122. In D. M. Knipe et al. (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 13.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, D. T., K. C. Tillman, and R. C. Desrosiers. 2002. Envelope glycoprotein cytoplasmic domains from diverse lentiviruses interact with the prenylated Rab acceptor. J. Virol. 76:327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freed, E. O., and M. A. Martin. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freed, E. O., and M. A. Martin. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 69:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fultz, P. N., P. J. Vance, M. J. Endres, B. Tao, J. D. Dvorin, I. C. Davis, J. D. Lifson, D. C. Montefiori, M. Marsh, M. H. Malim, and J. A. Hoxie. 2001. In vivo attenuation of simian immunodeficiency virus by disruption of a tyrosine-dependent sorting signal in the envelope glycoprotein cytoplasmic tail. J. Virol. 75:278-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabuzda, D. H., A. Lever, E. Terwilliger, and J. Sodroski. 1992. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 66:3306-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelderblom, H. R. 1991. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS 5:617-637. [PubMed] [Google Scholar]
- 20.Grief, C., D. J. Hockley, C. E. Fromholc, and P. A. Kitchin. 1989. The morphology of simian immunodeficiency virus as shown by negative staining electron microscopy. J. Gen. Virol. 70:2215-2219. [DOI] [PubMed] [Google Scholar]
- 21.Higgins, J. R., S. Sutjipto, P. A. Marx, and N. C. Pedersen. 1992. Shared antigenic epitopes of the major core proteins of human and simian immunodeficiency virus isolates. J. Med. Primatol. 21:265-269. [PubMed] [Google Scholar]
- 22.Hirsch, V. M., P. Edmondson, M. Murphey-Corb, B. Arbeille, P. R. Johnson, and J. I. Mullins. 1989. SIV adaptation to human cells. Nature 341:573-574. [DOI] [PubMed] [Google Scholar]
- 23.Hockley, D. J. 1988. Electron microscopy of human immunodeficiency virus. J. Gen. Virol. 69:2455-2469. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, W. E., J. D. Lifson, S. M. Lang, R. P. Johnson, and R. C. Desrosiers. 2003. Importance of B-cell responses for immunological control of variant strains of simian immunodeficiency virus. J. Virol. 77:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston, P. B., J. W. Dubay, and E. Hunter. 1993. Truncations of the simian immunodeficiency virus transmembrane protein confer expanded virus host range by removing a block to virus entry into cells. J. Virol. 67:3077-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kent, K. A., E. Rud, T. Corcoran, C. Powell, C. Thiriart, C. Collignon, and E. J. Stott. 1992. Identification of two neutralizing and 8 non-neutralizing epitopes on simian immunodeficiency virus envelope using monoclonal antibodies. AIDS Res. Hum. Retrovir. 8:1147-1151. [DOI] [PubMed] [Google Scholar]
- 27.Kestler, H., T. Kodama, D. Ringler, M. Marthas, N. Pedersen, A. Lackner, D. Regier, P. Sehgal, M. Daniel, N. King, et al. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109-1112. [DOI] [PubMed] [Google Scholar]
- 28.Kim, E. M., K. H. Lee, and J. W. Kim. 1999. The cytoplasmic domain of HIV-1 gp41 interacts with the carboxyl-terminal region of a-catenin. Mol. Cell 9:281-285. [PubMed] [Google Scholar]
- 29.Kodama, T., D. P. Wooley, Y. M. Naidu, H. W. Kestler, III, M. D. Daniel, Y. Li, and R. C. Desrosiers. 1989. Significance of premature stop codons in env of simian immunodeficiency virus. J. Virol. 63:4709-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaBranche, C. C., M. M. Sauter, B. S. Haggarty, P. J. Vance, J. Romano, T. K. Hart, P. J. Bugelski, M. Marsh, and J. A. Hoxie. 1995. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J. Virol. 69:5217-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 32.Layne, S. P., M. J. Merges, M. Dembo, J. L. Spouge, S. R. Conley, J. P. Moore, J. L. Raina, H. Renz, H. R. Gelderblom, and P. L. Nara. 1992. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189:695-714. [DOI] [PubMed] [Google Scholar]
- 33.Luciw, P. A., K. E. Shaw, B. L. Shacklett, and M. L. Marthas. 1998. Importance of the intracytoplasmic domain of the simian immunodeficiency virus (SIV) envelope glycoprotein for pathogenesis. Virology 252:9-16. [DOI] [PubMed] [Google Scholar]
- 34.Mammano, F., E. Kondo, J. Sodroski, A. Bukovsky, and H. G. Gottlinger. 1995. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J. Virol. 69:3824-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manrique, J. M., C. C. Celma, J. L. Affranchino, E. Hunter, and S. A. Gonzalez. 2001. Small variations in the length of the cytoplasmic domain of the simian immunodeficiency virus transmembrane protein drastically affect envelope incorporation and virus entry. AIDS Res. Hum. Retrovir. 17:1615-1624. [DOI] [PubMed] [Google Scholar]
- 36.Marcon, L., and J. Sodroski. 1997. High degree of sensitivity of the simian immunodeficiency virus (SIVmac) envelope glycoprotein subunit association to amino acid changes in the glycoprotein 41 ectodomain. AIDS Res. Hum. Retrovir. 13:441-447. [DOI] [PubMed] [Google Scholar]
- 37.Means, R. E., T. Greenough, and R. C. Desrosiers. 1997. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J. Virol. 71:7895-7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori, K., D. J. Ringler, T. Kodama, and R. C. Desrosiers. 1992. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J. Virol. 66:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison, H. G., F. Kirchhoff, and R. C. Desrosiers. 1993. Evidence for the cooperation of gp120 amino acids 322 and 448 in SIVmac entry. Virology 195:167-174. [DOI] [PubMed] [Google Scholar]
- 40.Mulligan, M. J., G. V. Yamshchikov, G. D. Ritter, Jr., F. Gao, M. J. Jin, C. D. Nail, C. P. Spies, B. H. Hahn, and R. W. Compans. 1992. Cytoplasmic domain truncation enhances fusion activity by the exterior glycoprotein complex of human immunodeficiency virus type 2 in selected cell types. J. Virol. 66:3971-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohno, H., R. C. Aguilar, M. C. Fournier, S. Hennecke, P. Cosson, and J. S. Bonifacino. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238:305-315. [DOI] [PubMed] [Google Scholar]
- 42.Ozel, M., G. Pauli, and H. R. Gelderblom. 1988. The organization of the envelope projections on the surface of HIV. Arch. Virol. 100:255-266. [DOI] [PubMed] [Google Scholar]
- 43.Piatak, M., Jr., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 44.Piller, S. C., J. W. Dubay, C. A. Derdeyn, and E. Hunter. 2000. Mutational analysis of conserved domains within the cytoplasmic tail of gp41 from human immunodeficiency virus type 1: effects on glycoprotein incorporation and infectivity. J. Virol. 74:11717-11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reeves, J. D., S. A. Gallo, N. Ahmad, J. L. Miamidian, P. E. Harvey, M. Sharron, S. Pohlmann, J. N. Sfakianos, C. A. Derdeyn, R. Blumenthal, E. Hunter, and R. W. Doms. 2002. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. USA 99:16249-16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regier, D. A., and R. C. Desrosiers. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 6:1221-1231. [DOI] [PubMed] [Google Scholar]
- 47.Ritter, G. D., Jr., M. J. Mulligan, S. L. Lydy, and R. W. Compans. 1993. Cell fusion activity of the simian immunodeficiency virus envelope protein is modulated by the intracytoplasmic domain. Virology 197:255-264. [DOI] [PubMed] [Google Scholar]
- 48.Rowell, J. F., P. E. Stanhope, and R. F. Siliciano. 1995. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J. Immunol. 155:473-488. [PubMed] [Google Scholar]
- 49.Sauter, M. M., A. Pelchen-Matthews, R. Bron, M. Marsh, C. C. LaBranche, P. J. Vance, J. Romano, B. S. Haggarty, T. K. Hart, W. M. Lee, and J. A. Hoxie. 1996. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J. Cell Biol. 132:795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shacklett, B. L., C. Denesvre, B. Boson, and P. Sonigo. 1998. Features of the SIVmac transmembrane glycoprotein cytoplasmic domain that are important for Env functions. AIDS Res. Hum. Retrovir. 14:373-383. [DOI] [PubMed] [Google Scholar]
- 51.Shacklett, B. L., C. J. Weber, K. E. Shaw, E. M. Keddie, M. B. Gardner, P. Sonigo, and P. A. Luciw. 2000. The intracytoplasmic domain of the Env transmembrane protein is a locus for attenuation of simian immunodeficiency virus SIVmac in rhesus macaques. J. Virol. 74:5836-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spies, C. P., and R. W. Compans. 1994. Effects of cytoplasmic domain length on cell surface expression and syncytium-forming capacity of the simian immunodeficiency virus envelope glycoprotein. Virology 203:8-19. [DOI] [PubMed] [Google Scholar]
- 53.Srinivas, S. K., R. V. Srinivas, G. M. Anantharamaiah, R. W. Compans, and J. P. Segrest. 1993. Cytosolic domain of the human immunodeficiency virus envelope glycoprotein binds to calmodulin and inhibits calmodulin-regulated proteins. J. Biol. Chem. 268:22895-22899. [PubMed] [Google Scholar]
- 54.Vogt, V. M., and M. N. Simon. 1999. Mass determination of Rous sarcoma virus virions by scanning transmission electron microscopy. J. Virol. 73:7050-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West, J. T., S. K. Weldon, S. Wyss, X. Lin, Q. Yu, M. Thali, and E. Hunter. 2002. Mutation of the dominant endocytosis motif in human immunodeficiency virus type 1 gp41 can complement matrix mutations without increasing Env incorporation. J. Virol. 76:3338-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilk, T., T. Pfeiffer, and V. Bosch. 1992. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology 189:167-177. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, H., L. Wang, S. Kao, I. P. Whitehead, M. J. Hart, B. Liu, K. Duus, K. Burridge, C. J. Der, and L. Su. 1999. Functional interaction between the cytoplasmic leucine-zipper domain of HIV-1 gp41 and p115-RhoGEF. Curr. Biol. 9:1271-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu, P., E. Chertova, J. Bess, Jr., J. D. Lifson, L. O. Arthur, J. Liu, K. A. Taylor, and K. H. Roux. 2003. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc. Natl. Acad. Sci. USA 100:15812-15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zingler, K., and D. R. Littman. 1993. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases env incorporation into particles and fusogenicity and infectivity. J. Virol. 67:2824-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]


