Abstract
Myocardial infarction (MI), secondary to atherosclerotic plaque rupture and occlusive thrombi, triggers acute margination of inflammatory neutrophils and monocyte phagocyte subsets to the damaged heart, the latter of which may give rise briefly to differentiated macrophage-like or dendritic-like cells. Within the injured myocardium, a primary function of these phagocytic cells is to remove damaged extracellular matrix, necrotic and apoptotic cardiac cells, as well as immune cells that turn over. Recognition of dying cellular targets by phagocytes triggers intracellular signaling, particularly in macrophages, wherein cytokines and lipid mediators are generated to promote inflammation resolution, fibrotic scarring, angiogenesis, and compensatory organ remodeling. These actions cooperate in an effort to preserve myocardial contractility and prevent heart failure. Immune cell function is modulated by local tissue factors that include secreted protease activity, oxidative stress during clinical reperfusion, and hypoxia. Importantly, experimental evidence suggests that monocyte function and phagocytosis efficiency is compromised in the setting of MI risk factors, including hyperlipidemia and ageing, however underlying mechanisms remain unclear. Herein we review seminal phagocyte and cardiac molecular factors that lead to, and culminate in, the recognition and removal of dying injured myocardium, the effects of hypoxia, and their relationship to cardiac infarct size and heart healing.
Keywords: monocyte, cardiomyocyte, myocardial infarction
Physiological relevance of the cardiac innate immune response post myocardial infarction (MI) and the importance of phagocytosis to heart repair
Heart failure after MI is a leading cause of morbidity and mortality in the industrialized world (Lloyd-Jones, Adams et al. 2010). MI often occurs secondary to atherosclerotic plaque destabilization, the precursor to atherothrombosis (Tabas 2005). Infarction triggers inflammatory cell recruitment, which is a critical component of healing after tissue injury (Frangogiannis, Smith et al. 2002). A diverse population of bone marrow and spleen-derived immune cells are recruited to the heart after ischemia (Swirski, Nahrendorf et al. 2009) and function to promote clearance of damaged cardiac myocytes and repair of damaged myocardium (Thorp 2012). In contrast to inflammation during atherosclerosis (Libby 2012), immune cell mobilization after a heart attack is relatively acute and resolving. Although the initial inflammatory response may last just a few weeks, the activation state of recruited myocardial immune cells, molded by MI risk factors, may in turn modify cardiac infarct size and subsequently, the extent of cardiac remodeling and heart function (Panizzi, Swirski et al. 2010).
Myocardial phagocytosis
A central function of recruited leukocytes to sites of sterile injury is the degradation and phagocytosis of degraded extracellular matrix and dying and necrotic cells. This in turn promotes fibrogenic, and potentially angiogenic, responses that contribute to filling the void of lost and non-regenerative cardiac myocytes. Recent data collectively and directly link efferocytosis by inflammatory immune cells (Vandivier, Henson et al. 2006), i.e., the phagocytosis of apoptotic cells, to wound healing in the myocardium and in turn implicate phagocytosis receptors on monocytes and macrophages as a significant link between acute inflammation resolution and organ function (Wan, Yeap et al. 2013). Importantly and in the elderly, sub-optimal dying-cell clearance may lead to maladaptive cardiac remodeling and tissue repair, thereby accelerating the transition to heart failure (Chen and Frangogiannis 2010). Below, we focus on basic cellular and molecular mechanisms of inflammation and its resolution in the myocardium post infarction, with a focus on phagocyte-mediated interactions with dying tissue. These concepts form a testable working model (Figure 1. Working Model of Phagocyte and Cardiac Myocyte Interactions) that predict relationships between phagocyte-mediated dying-cell recognition, efferocytosis, infarct size, tissue-reparative signaling, and myocardial remodeling in the hypoxic heart.
Figure 1. Working Model of How the Cell Biology of Inflammation post Myocardial Infarction regulates Heart Failure.
Advanced atherosclerosis promotes atherothrombotic myocardial infarction (MI), the latter of which is characterized by recruitment of neutrophils and monocyte subsets that can differentiate into macrophages or dendritic-like cells. Phagocytes promote clearance of dying cardiac and immune cells. However, inherent inefficiency or MI associated risk factors promote inefficient dying-cell clearance, leading to secondary necrosis and further loss of non-regenerative cardiomyocytes. These acute events can affect later cardiac remodeling and inflammation that may lead to heart failure.
Immune cell mobilization to the heart
Although the extent of resident phagocytes in the human heart is unclear, the healthy murine heart harbors a population of resident macrophages (Pinto, Paolicelli et al. 2012). At the time of this Review’s publication, Epelman et al., provided evidence for both embryonic and adult-derived resident cardiac macrophages (Epelman, Lavine et al. 2014), that auto-renew similar to other tissues (Hashimoto, Chow et al. 2013), independent of recruitment from circulating monocyte pools. Cardiac injury elicits additional myeloid cells, largely a consequence of CC, CXC, and CX3C chemotactic factor release, many of which are found elevated in the bloodstream and lymph post MI (Frangogiannis, Smith et al. 2002). Chemokine release promotes influx of neutrophils, monocytes, and to a lesser extent, lymphocytes, into the heart, where they become tissue macrophages. Many chemotactic factors are recognized similarly by both neutrophils and monocytes, however, selective trafficking can be partially explained by differential expression or post-translational sialylation of G-protein-linked transmembrane chemokine receptors (Frommhold, Ludwig et al. 2008). Upon cardiac injury, release of damage associated molecular patterns from dying cardiomyocytes triggers chemokine synthesis by endothelial and resident cardiac cells. This process is later reinforced by newly recruited immune cells that additionally secrete chemokines as a consequence of pattern recognition receptor signaling. Evidence suggests that CXCL8 (IL-8) is induced by both endothelial cells and fibroblasts and in the myocardium, within the first hour of clinical reperfusion. Endothelial CXCL8 activates neutrophils through recognition of neutrophil CXCR1 and CXCR2 (Kukielka, Youker et al. 1995). CXCR2 recognizes CXCL2 (macrophage inflammatory protein 2-α, or MIP2-α) as well, and CXCL2 production by macrophages is also chemotactic for neutrophils (Belperio, Keane et al. 2002). Another important chemokine, CCL2, or monocyte chemo-attractant protein (MCP)-1, promotes recruitment of inflammatory monocytes through the CCR2 receptor. CCR2 null mice exhibit diminished acute cardiac macrophage accumulation and attenuated ventricular dilation after permanent coronary occlusion (Kaikita, Hayasaki et al. 2004). CCR2 also has affinity for CCL5 (RANTES) and CCL5 antagonists reduce myocardial reperfusion injury in hyper-lipidemic mice, yet this is not the case in Ccr5−/− mice, potentially due to enhanced expression of compensatory chemokines (Braunersreuther, Pellieux et al. 2010) or effects on myeloid progenitors in the bone marrow and circulation (Ergen, Boles et al. 2012).
Besides myeloid effects, a member of the CXC subfamily, Stromal cell-Derived Factor (SDF)-1 (CXCL12), both suppresses apoptosis in cardiomyocytes and promotes chemotaxis of endothelial progenitors to assist in angiogenesis (Imtiyaz, Williams et al. 2010). Furthermore, non-hematopoietic-derived cells are also targeted. For example, the CXC chemokine Interferon-gamma-inducible Protein (IP)-10 (CXCL10) is markedly induced in myocardial infarcts and exerts anti-fibrotic actions, inhibiting fibroblast migration to promote wound contraction and attenuate adverse remodeling (Bujak, Dobaczewski et al. 2009).
In addition to prototypical chemokines, angiotesin II (ANG II) is key for splenic monocyte mobilization post MI (Swirski, Nahrendorf et al. 2009). Utilizing congenic markers to track origins of cardiac monocytes from transplanted spleens, Swirski et al. calculated that the spleen contributes ~40% of monocytes to the ischemic myocardium (Swirski, Nahrendorf et al. 2009). Deficiency of the angiotensin receptor Atgr1α, as well as angiotensin-converting enzyme (ACE) inhibitor therapy, led to failure of splenic monocyte release after MI and reduced monocytic cells within the ischemic myocardium (Leuschner, Panizzi et al. 2010). In vitro, angiotensin II receptor engagement in monocytes promoted cytoskeletal rearrangement and monocyte migration. In vivo, these related pathways were augmented after activation of the sympathetic nervous system. That is, heightened sympathetic nervous system activity after cardiac injury promoted hematopoietic stem and progenitor cell (HSPC) release from bone marrow, as well as amplified extramedullary monocytopoiesis after experimental MI (Dutta, Courties et al. 2012). Enhanced monocyte mobilization required β3-adrenoceptor signaling on monocytes as infarcted mice treated with a β3- antagonist exhibited decreased HSPCs in the blood, as well as increased stem cell retention factors, compared to non-treated MI mice (Dutta, Courties et al. 2012). Importantly, recruitment of immune cells may continue after the acute inflammatory stage in the heart as chronic heart failure patients exhibit significantly elevated levels of CXC cytokines (Dahl, Husberg et al. 2009).
Cardiac “find me” vs “keep out” signals
After margination to post capillary venules, immune cells transmigrate past endothelial cells and chemotax towards the site of infarction. Directed migration to the ischemic core of an MI requires trafficking through a gradient of reducing oxygen tension. Apoptotic cells, primarily located in the zone bordering the infarct (Whelan, Kaplinskiy et al. 2010), likely secrete local so-called find-me signals, which is the first essential step for phagocyte recruitment in tissue. Find-me molecules are soluble chemo-attractants released by dying cells to establish a chemotactic gradient to attract phagocytes (Ravichandran 2011). Many of these signaling pathways act on RhoGTPases, which regulate cytoskeleton rearrangement to promote cellular migration (Singer, Tian et al. 2005). Known find-me signals include lipids, such as lyso-phosphatidyl-choline (LPC) and sphingosine-1-phosphate (S1P). LPC, one of the better-characterized find-me signals, is externalized and excreted during apoptosis (Lauber, Bohn et al. 2003). Secreted LPC interacts with G-protein-coupled receptor G2A, stimulating macrophage chemotaxis towards apoptotic cells (Peter, Waibel et al. 2008). LPC accumulates during ischemia in myocardium via thrombin activation of Ca2+-independent phospholipases (Daleau 1999), consistent with its role as a find-me signal in the damaged heart. S1P, another lipid find-me signal is produced by sphingosine kinase 1 (SPHK1) for recognition by S1P receptors on distal cells. Apoptotic stress induces SPHK1 activation, which can then promote S1P secretion (Matloubian, Lo et al. 2004). In addition to lipid find-me signals, proteinaceous tissue recruitment factors include cytokines and chemokines, including fractalkine (CX3CL1), which is cleaved by caspase-3 during apoptosis. In turn, the released fractalkine extracellular domain interacts with CX3CR1 on macrophages for cell recruitment (Truman, Ford et al. 2008). Nucleotides, including ATP and UTP, originate from both apoptotic and necrotic cells, and also likely act as find-me signals in the myocardium. In apoptotic cells, the plasma membrane channel pannexin 1 (PANX1) may serve as a conduit for nucleotide release after cleavage by caspases 3 and 7 (Chekeni, Elliott et al. 2010). During ischemia, cellular stress increases glycosylation of PANX1, resulting in enhanced ATP release from myocytes to promote fibroblast transformation (Dolmatova, Spagnol et al. 2012). Also, ATP can guide neutrophil chemotaxis via purinergic P2Y2 and A3 adenosine receptors in vitro and in vivo (Ayata, Ganal et al. 2012). Knockdown of P2y2 inhibits migration (Chen, Corriden et al. 2006), all consistent with the possibility that ATP released from PANX1 may act as a find-me signal in the heart.
Keep-out/Keep-away signals
Local find me signals are balanced by local keep-out/keep-away signals. In some instances, apoptotic cells selectively recruit monocytes as opposed to neutrophils. For example, monocytes in contrast to neutrophils are selectively recruited after injecting apoptotic cell supernatants into an air-pouch model of inflammation (Elliott, Harriman et al. 2009). Additionally, lactoferrin from apoptotic cell supernatants “kept out” neutrophils but not monocytes (Bournazou, Pound et al. 2009). Interestingly, the apo-form of lactoferrin can function as a mimetic of hypoxia by stabilizing the hypoxia inducible factor HIF-1α (Zakharova, Korneeva et al. 2012). Consistent with this, lactoferrin increased in patients during ischemia, just prior to reperfusion (Fiane, Videm et al. 2003). Growth differentiation factor-15 (GDF-15), a TGF-β-related cytokine, is also a keep-out signal. For example, GDF-15 is induced in the infarcted heart and Gdf15 deficient mice exhibit enhanced recruitment of neutrophils to the infarcted myocardium. GDF-15 activates the small Rho GTPase CDC42, inhibits activation of another small GTPase RAP1, and furthermore counteracts chemokine-triggered conformational activation and clustering of adhesive β2 integrins (Kempf, Zarbock et al. 2011). Though other inflammatory signals from the surrounding parenchymal pattern recognition receptor response also strongly influence inflammatory cell recruitment (Singh, Swaminathan et al. 2012), the ratio of find-me signals to keep-away may be important in regulating local responses of phagocytes in close proximity to dying cells.
Cardiac neutrophils
Release of bone marrow-retained circulating polymorphonuclear granulocytes (PMNs), or neutrophils, is one of the earliest and most robust coordinated steps of leukocyte mobilization to the heart. After experimental coronary artery ligation in rodents, PMNs are recruited during the first few hours following blockage of blood flow, with levels peaking as early as one day after injury and further heightened after clinically relevant reperfusion (Yan, Anzai et al. 2013). Much of our in vivo insight into PMN contributions to inflammation has been achieved after targeting PMN or endothelial adhesion molecules. PMNs bind endothelial adhesion molecules such as L- and P- selectins, integrins, and intercellular adhesion molecules (ICAMs), and monoclonal anti-L- and P-selectin antibodies reduce myocardial necrosis and enhance coronary endothelial function in association with reduced PMN margination (Ma, Weyrich et al. 1993). Conversely, impaired PMN trafficking associated with combined P-selectin and ICAM-1 deficiency exhibited no significant difference in infarct size after ischemia followed by reperfusion (Briaud, Ding et al. 2001). The selectins are involved in early PMN attachment and rolling, however firm adhesion and transmigration past the endothelial layer requires PMN integrin leukocyte function associated antigen-1 (LFA-1 or CD11a/CD18)-dependent activation of endothelial integrins. Inhibiting LFA-1 has been shown to reduce infarct size in primates (Aversano, Zhou et al. 1995), however decreased PMN recruitment in mice treated with vascular cell adhesion molecule (VCAM-1) antibodies was not associated with decreased myocardial injury (Bowden, Ding et al. 2002). However it is important to note that both LFA-1 and VCAM are expressed by other immune cells during MI (Meisel, Shapiro et al. 1998) and therefore PMN-specific effects of these molecules will require conditional blockade approaches. PMNs also express PECAM (platelet endothelial cell adhesion molecule), and to a lesser extent CD99, both adhesion molecules necessary for transmigration across the endothelium, independent of adhesion (Muller 1995). Combined antibody blocking of PEACAM and CD99 had an additive effect to reduce transmigration, suggesting these two molecules regulate distinct steps in PMN extravasation (Lou, Alcaide et al. 2007). How PECAM/CD99-dependent recruitment of PMNs to the heart and their influence to cardiac pathology is unclear.
“Bad” cardiac PMNs
In a prospective observational study, patients with chest pain in the highest tertile of blood PMN-count were at increased risk for non-fatal acute MI and death (Meissner, Irfan et al. 2011). Similarly, STEMI-classified (ST segment elevation MI) patients were less likely to survive during a 30 day follow-up if they had a higher baseline PMN count (O’Donoghue, Morrow et al. 2008). Patients with neutrophilia 4 days post-MI were more likely to develop congestive heart failure (Kyne, Hausdorff et al. 2000). These findings highlight PMNs as potential therapeutic targets for recovery following MI (Akpek, Kaya et al. 2012), although it is difficult to delineate whether heightened PMNs could also simply reflect more severe cardiac damage. Consistent with this, anti-PMN antibodies reduce ischemic injury in canine models of reperfusion following ischemia (Romson, Hook et al. 1983). Some of the untoward effects of PMNs are associated with their potent secretion of inflammatory mediators. Gelatinase degranulation in PMNs follows transendothelial migration, in turn releasing matrix metallo-proteinases MMP-8 and -9. Azurophilic granules release myeloperoxidase (MPO) as well as serine proteases (Soehnlein, Weber et al. 2009). MPO (Rudolph, Goldmann et al. 2011), lipocalin-2 (Yndestad, Landro et al. 2009) and MMPs, are associated with worsened cardiac outcome following MI. MPO generates cytotoxic fomaldehyde, acrolein, and chlorinating species in the infarct zone, which is adversely associated with LV remodeling and function in mice (Vasilyev, Williams et al. 2005). MMP-8 is responsible for the degradation of fibrillar collagen, promoting PMN migration (Lin, Jackson et al. 2008). MMP-9 is up-regulated within 24 hours post injury and secreted mostly by recruited PMNs and macrophages (Lindsey, Gannon et al. 2002; Tao, Cavasin et al. 2004). Patients suffering from ischemic, chronic heart failure were more likely to have higher serum levels of PMN-associated lipocalin than control patients (Yndestad, Landro et al. 2009). PMNs can also directly promote cardiac myocyte damage and potentially infarct size through the release of reactive oxygen species (ROS) through the NADPH oxidative burst (Ciz, Denev et al. 2012). ROS may be generated after PMNs interact with platelets through the binding of Triggering Receptor Expressed on Myeloid Cells (TREM-1). Finally, ROS production can incite further leukocyte extravasation through elevated P-selectin expression (Griendling and FitzGerald 2003) and complement activation (Shingu, Nonaka et al. 1992).
Beneficial PMN functions in heart
In humans, PMN depletion strategies have caused reduced infarct size and scar formation (Cavanagh, Gough et al. 1998), consistent with specific PMN functions contributing beneficially to cardiac wound healing. For example, PMN-derived and activated MMPs degrade pre-existing extracellular matrix, allowing extravasated leukocytes to migrate to the infarct tissue for tissue repair (Cleutjens, Blankesteijn et al. 1999). Also, PMNs are phagocytic and to a lesser extent efferocytic and contribute to removal of necrotic cardiac infarction debris. To a lesser extent, PMNs may also promote clearance of apoptotic cells, however, PMN efferocytic function is less studied than in macrophages. Efferocytosis by PMNs requires lipoprotein-receptor related protein (LRP) through recognition of calreticulin on target cells (Park, Liu et al. 2008). Apoptotic cell recognition tempers PMN oxidative burst, which is heightened after reperfusion, and reduces production of TNF-α and CXCL10 (Esmann, Idel et al. 2010). As PMNs themselves become apoptotic, they produce lactoferrin to “keep out” further PMN infiltration (Curran, Demick et al. 2006). Lactoferrin also decreases PMN activation by impairing degranulation and reducing β2 integrin expression, the latter of which suppresses cellular motility (Bournazou, Pound et al. 2009). In fact, during homeostatic conditions, cyclic flux of PMN release from bone marrow followed by elimination of aged PMNs serves as a feedback mechanism to modulate the hematopoietic niche (Casanova-Acebes, Pitaval et al. 2013). Finally, PMNs chaperone recruitment of monocytes from the blood stream. PMN-derived cathelicidin accumulates on endothelial proteoglycans to bind monocyte formyl-peptide receptor 2 (Swirski and Robbins 2013). In turn, this increases binding of integrins MAC1 and VLA-4 to cell adhesion molecules (Wantha, Alard et al. 2013).
Blood Ly6cHI monocytes and Ly6cLO monocytes and cardiac tissue Ly6cLO macrophages
The mononuclear phagocyte system (MPS) of monocytes, macrophages, and dendritic, collectively scavenge damaged matrix, microparticles, dead cells, and regulate inflammation. In the heart, monocyte residence time has been approximated to 20 hours. Sustained levels post MI are supplied by extramedullary splenic hematopoiesis, as well as from bone marrow sources (Leuschner, Rauch et al. 2012). There are two monocyte/macrophage subsets in the heart identified by expression of surface markers and characterized by inflammatory phenotype: Ly6CHI monocytic (analogous to CD14+ CD16− in humans) and Ly6CLO monocytic/macrophage cells (CD14+ CD16+ in humans) (Shantsila and Lip 2009; Shantsila, Wrigley et al. 2011; Tapp, Shantsila et al. 2011; Nahrendorf and Swirski 2013). In human MI patients, individuals with prolonged prevalence of proinflammatory CD14+/CD16− cells have decreased myocardial salvage, a measure of the amount of healthy tissue in the infarct (Tsujioka, Imanishi et al. 2009). Ly6CHI cells exhibit a pro-inflammatory phenotype and are found early in the infarct after occlusion, peaking in mice ~3 days post permanent occlusion of the murine left anterior descending artery. These cells also promote removal of necrotic debris (Nahrendorf, Swirski et al. 2007). Ly6cHI monocytes express high levels of CCR2, respond to MCP-1, and produce TNFα and proteolytic enzymes. Ly6cHI monocytes typically are not associated with high efficiency of apoptotic cell clearance, however, recent studies suggest that cross-talk with macrophages may be partially responsible. For example, 12/15 lipooxygenase (LO), expressed by alternatively activated macrophages (described below), generate phospholipid oxidation motifs on the macrophage that sequester soluble molecules that bridge apoptotic cell receptors and apoptotic targets. This in turn reduces monocyte efferocytosis efficiency (Uderhardt, Herrmann et al. 2012). Ly6cLO, CX3CR1HI, CCR2LO monocyte/macrophages emerge in the myocardium soon after Ly6cHI subsets and are critical for myocardial repair, where they secrete pro-fibrotic and angiogenic cytokines. Ly6cLO pro-reparative functions are tied to the Ly6cHI response, as clodronate-mediated depletion during the predominantly Ly6cHI monocyte phase delays healing. Ly6CLO cells “patrol” endothelial capillaries during homeostasis, and therefore are positioned to respond immediately after MI (Auffray, Fogg et al. 2007). Alternatively, Ly6cLO cells need not extravasate into tissue to promote wound healing, as Carlin et al. (Carlin, Stamatiades et al. 2013) reported that Ly6cLO cells can be interestingly retained by endothelial cells in the kidney vasculature to recruit PMNs. In this example, recruited PMNs promoted lysis of compromised endothelium, which was subsequently cleared up by monocytes. In the heart, and in terms of absolute numbers, the vast majority of Ly6CLO cells accumulate in the cardiac wound after Ly6cHI monocytes peak, eventually outnumbering Ly6CHI cells in the later stages of the cardiac inflammatory response (Nahrendorf, Swirski et al. 2007). Interestingly, the apoptotic cell receptor MERTK is expressed predominantly by Ly6CLO cells post MI, suggesting distinct clearance mechanisms utilized by monocyte subsets (Wan, Yeap et al. 2013).
Cardiac macrophages and dendritic-like cells
Monocytes differentiate into macrophage-like cells and proliferate in the presence of Macrophage-Colony Stimulating Factor (M-CSF), which is elevated in canine infarcts (Frangogiannis, Mendoza et al. 2003). Monocytosis is associated with higher numbers of mature macrophages in the infarct on day 5 (Panizzi, Swirski et al. 2010). Within the myocardium, the early macrophage phenotype is similar to pro-inflammatory/activated M1-like macrophages (F4/80+, CD86+), which is followed by a phenotypically similar anti-inflammatory M2-like macrophage profile (F4/80+, CD206+). Furthermore, increasing the M2/M1 ratio after mesenchymal stem cell therapy was associated with improved regional function at the mid-anterior infarct zone, an effect that was abrogated upon clodronate depletion of phagocytic cells (Ben-Mordechai, Holbova et al. 2013). Dendritic-like cells peak at day 7 in experimental models of MI. Following dendritic cell ablation, mice exhibited enhanced inflammation and extracellular matrix degradation in the infarcted myocardium, leading to wall thinning, impaired neo-angiogenesis, and increased infiltration of Ly6CHI monocytes. This suggested that at least immature CD11c+ dendritic-like cells may play a protective role in post-MI repair (Anzai, Anzai et al. 2012). Other cell lineages classically associated with the adaptive immune arm have been discovered in injured myocardium, including IL-10 and TGF-β producing regulatory T cells, and B-cells (Zouggari, Ait-Oufella et al. 2013), both of which may interact with phagocytes to control inflammation and potentially other aspects of chronic heart healing.
Macrophage-mediated efferocytosis and inflammation resolution in the heart
Whereas efficient efferocytosis activates pro-resolving/anti-inflammatory pathways in the phagocyte (Serhan and Savill 2005; Birge and Ucker 2008), defective efferocytosis leads to secondary post-apoptotic necrosis and expansion of tissue necrosis (Vandivier, Henson et al. 2006). Previous studies have linked defective apoptotic cell clearance to diseases of chronic non-resolving inflammation such as atherosclerosis and lupus (Tabas and Glass 2013). In contrast, the extent to which efferocytosis efficiency during acute resolving inflammation may affect long-lasting organ function is much less clear. In particular, inefficient removal of dead cardiac tissue in aged hearts has been linked to progression of heart failure (Bujak, Kweon et al. 2008). Clearance of apoptotic PMNs by macrophages initiates the resolution phase of inflammation, inducing the production of IL-10, TGF-β, lipoxins, and resolvins. IL-10 appears late in the infarcted myocardium and contributes to the stabilization of the matrix by promoting macrophage production of tissue inhibitor of metallo-proteinases (Frangogiannis 2013). IL-10 knockout animals display an increased inflammatory response (Krishnamurthy, Rajasingh et al. 2009), including heightened TNF-α and MCP-1 mRNA and increased mortality rates during ischemia-reperfusion (Yang, Zingarelli et al. 2000). Lipoxins and resolvins, derived from poly-unsaturated fatty acids, are protective for cardiomyocyte reperfusion injury (Keyes, Ye et al. 2010) and promote efferocytosis of PMNs by macrophages (Schwab, Chiang et al. 2007) while reducing vascular permeability (Takano, Clish et al. 1998) and PMN infiltration (Serhan, Maddox et al. 1995). Enhanced clearance of PMNs may feed-back through an IL-23 pathway to affect granulopoiesis and PMN production (Stark, Huo et al. 2005). In a mouse model of acute kidney ischemia reperfusion injury, PMN-associated production of IL-17/IL-23 was shown to be required for further PMN infiltration and IFN-γ production (Li, Huang et al. 2010). Finally, If PMNs are cleared by macrophages, the question arises: What cells are responsible for clearing macrophages that turnover in the heart? Dying macrophages may emigrate to spleen or lymph or alternatively be removed by new resident cardiac macrophages or other resident cardiac cells.
Phagocyte directed cardiac repair
Efferocytosis induces TGF-β (Fadok, Bratton et al. 1998), which also plays an important role in tissue remodeling and post-MI inflammation resolution. TGF-β activates fibroblasts and induces collagen and fibronectin production (Bassols and Massague 1988). This cytokine also reduces adhesion molecule expression and promotes the differentiation of regulatory T cells (Bujak and Frangogiannis 2007). After activation by IFNγ, LPS, lactate, or hypoxia, macrophages may produce pro-angiogenic factors, including nitric oxide and VEGF (Vascular Endothelial Growth Factor), or. During hypoxia, VEGF is up-regulated through the action of hypoxia transcription factors and through increased mRNA stability (Xiong, Elson et al. 1998). A subset of pro-angiogenic macrophages have been described, termed myeloid angiogenic cells (MACS), which are similar in function to alternatively active/M2 phagocytes, but also express endothelial cell markers including TIE2 (Tunica Interna Endothelial Cell Kinase, or TEK tyrosine kinase) and VEGFR. These cells promote angiogenesis via paracrine signaling, producing MCP-1, MMP9 and IL-8, which act on endothelial cells to activate VEGFR (Medina, O’Neill et al. 2011). Interestingly, when these cells were administered intravenously in a rat model of MI, they were found to localize to ischemic areas, causing reduced scarring and improved ventricular function (Kawamoto, Gwon et al. 2001). Whether these cells are capable of differentiating into endothelial cells or maintain their myeloid phenotype once within tissue, however, remains unclear (Chambers, O’Neill et al. 2013). Of further interest, when CD14+ monocytes were delivered to ischemic sites of oxygen-induced retinopathy, the result was an enhanced pro-angiogenic M2 macrophage phenotype that improved vascularization and reduced retinopathy-associated inflammation (Marchetti, Yanes et al. 2011). Furthermore, in a hind-limb ischemia model, deletion of one allele of hypoxia transcription factor-suppressor PHD2 (prolyl hydroxylase domain protein 2), in turn skewed macrophage polarization towards a pro-arteriogenic phenotype, thereby preventing tissue necrosis and preserving limb perfusion (Takeda, Costa et al. 2011). Finally, Hochreiter-Hufford showed that through recognition of externalized phosphatidylserine on neighboring apoptotic cells, BAI1 (brain-specific angiogenesis inhibitor), a member of the adhesion type-G protein coupled receptor family, can signal through the EMLO-DOCK180-Rac1 pathway and enhance myoblast fusion during muscle development, regeneration and repair (Hochreiter-Hufford, Lee et al. 2013; Novak, Weinheimer-Haus et al. 2014).
Hypoxic effcts on phagocytes
A significant aspect of the post-MI or wound injury phagocyte response is an ischemic environment, with oxygen tensions is healing wounds reported as low as 0–3 kPa (Hunt, Twomey et al. 1967). In this context, it’s worth noting that most mechanistic studies of immune cell function are not modeled under reduced oxygen tension, although interestingly, over-confluent tissue culture conditions can reduce oxygen saturation in tissue culture media (Thompson, Binham et al. 2013). In PMNs, Hypoxia Inducible Factor 1α (HIF-1α) extends PMN lifespan during hypoxia (Hannah, Mecklenburgh et al. 1995) and this in part may be promoted by HIF-enhanced glycolysis (Walmsley, Chilvers et al. 2011). Prolonged PMN survival in the heart could extent the direct deleterious action of PMNs and further delay monocyte/macrophage-mediated anti-inflammatory signaling, secondary to efferocytosis of apoptotic PMNs. In monocytes, responses to hypoxia have only initially begun to be investigated: Knockdown of monocyte Hif-1α supressed CCR2 expression in Ly6cHI monocytes, resulting in decreased monocyte entry into the infarct and increased cardiac ejection fraction (Dong, Khalil et al. 2010). Low oxygen importantly also modulates angiogenic and inflammatory gene expression in monocytes through increased expression of VEGF (Bosco, Puppo et al. 2008), as eluded to above. Myeloid expression of Hif-1α was required for adequate skeletal muscle regeneration. In mice with a myeloid-specific deletion of Hif-1α, there was delayed invasion of F4/80+ macrophage-like cells, suppression of myoblast proliferation in parallel with decreased cyclooxygenase-2 activity, impaired removal of necrotic cell debris, and reduced regeneration of endothelial cell structures following myoblast injury (Scheerer, Dehne et al. 2013). Interestingly, these effects were shown to be independent of phagocyte polarization phenotype between control and Hif-deficient mice. Much less has been studied regarding Ly6CLO phagocytes and their adaptation to low oxygen levels. Future work in this area is needed to resolve the effects of hypoxia on monocyte subset levels, recruitment, clearance capacity and a possible inter-conversion between subsets during MI. Also, many questions remain regarding the involvement of each monocytic subset in post-ischemic neovascularization. While TIE2-expressing Ly6CLO cells have been shown to promote tumor angiogenesis, in contrast, in mice with hind limb ischemic injury and adoptive transfer of bone marrow derived Ly6CHI monocytes, there was significant improvement of blood flow recovery, an effect not seen after transfer of Ly6CLO cells (Capoccia, Gregory et al. 2008). Similarly, CCL2/CCR2 signaling was required for adequate neo-vascularization following hind limb ischemia, as blood flow recovery was promoted by adoptive transfer of Ly6CHI, but not Ly6CLO phagocytes. A differential angiogenic capacity of each subset may be influenced by increased MMP-9 expression by Ly6CHI cells, which promotes capillary branching and revascularization (Cochain, Rodero et al. 2010). HIFs are also required for myeloid cell aggregation, invasion, and motility (Kong, Scully et al. 2007), as well as during macrophage differentiation (Oda, Hirota et al. 2006). HIFs also mediate macrophage motility and energy homeostasis through the glycolytic maintenance of the cellular ATP pool, which was decreased in Hif-1α knockout cells (Cramer, Yamanishi et al. 2003). Additionally, macrophage migration in response to nitric oxide is dependent on HIF-1α signaling through small GTPase CDC42 and RAC-1 (Zhou, Dehne et al. 2009). Much of the investigation of HIF regulation in macrophages has focused on Tumor Associated Macrophages (TAMs), as hypoxia is a major component of the tumor microenvironment. In this context, Hif-1α knockout TAMs had impaired inflammatory TLR4 responses and decreased cytotoxicity to tumor-spheroid cells. Taken together these results indicate that HIF-1α is required for the maintenance of M1 polarization (Werno, Menrad et al. 2010). Duration of hypoxic exposure can also determine macrophage response. Death-resistant macrophages induced by long-term (48hrs) exposure to 1% O2 exhibited decreased expression of Heat Shock Protein70, and increased TNFα (Degrossoli and Giorgio 2007). In macrophages, interestingly, HIF-1α does not always require low oxygen for activation. Case in point, normoxic (21% oxygen) supernatants from apoptotic cells have been shown to transcriptionally activate Hif-1α expression, in a NFAT (nuclear factor of activated T cells)-dependent manner. One of the key factors that promoted Hif-1α expression was S1P, or sphingosine 1 phosphate (Herr, Zhou et al. 2009), the find-me signal described above.
Phagocyte: Myocyote interactions: “Eat me” vs “don’t eat me” ligands
The molecular pathways responsible for phagocyte interactions with cardiomyocytes remain largely unknown. A key purpose of the aforementioned recruitment signals and recruited leukocytes is to promote interactions with dying or necrotic cardiac tissue. Phagocytes distinguish viable from non-viable cells through the aid of so-called self eat-me and don’t-eat-me signals, which are presented on the target cell surface and aided by binding of bridging molecules that interface between the target cell and phagocyte (Figure 2). Eat-me signals can be externalized phospholipids, proteins, alterations in cell-surface charge or glycosylation patterns, and nucleotides (Hochreiter-Hufford and Ravichandran 2013). Externalization of phosphatidyl-serine (PS) is one of the most conserved apoptotic markers, however, we are still just learning about the mechanism of PS externalization. Just this past year, Nagata and colleagues published that Xk-Related Protein 8 (XKR8) is required for PS externalization under apoptotic stimuli. Cells deficient for Xkr-8 failed to expose PS during apoptosis and were inefficiently engulfed by phagocytes. Interestingly, both cancer cells and terminally differentiated cardiomyocytes were found to express low levels of Xkr-8 (at the mRNA level), consistent with recent data showing reduced efferocytosis efficiency of cardiac myocytes by macrophages in vitro (data not published). In the context of the infarcted heart, eat-me signals in vivo may be affected by low oxygen levels. For example, acute hypoxia alters PS content in erythrocytes, potentially through modulation of phospholipid scramblases, aminophospholipid translocases and ATP-dependent floppases (Nie, Tian et al. 2011). In addition to PS, annexin 1, a calcium and phospholipid binding protein in the annexin superfamily, is an endogenous eat-me signal for macrophages (Swathi Arur et al.). Annexin 1 is cleaved by ADAM10 (A Disintegrin And Metalloproteinase) during cell necrosis, also contributing as a monocytic chemotactic signal (Blume, Soeroes et al. 2012). This pathway may be especially important after MI considering the extensive level of cellular necrosis. Another newly defined eat-me signal is the ficolin1-PTX3 heterocomplex, which can interact with late apoptotic or necrotic cells and enhance their clearance (Ma, Doni et al. 2013).
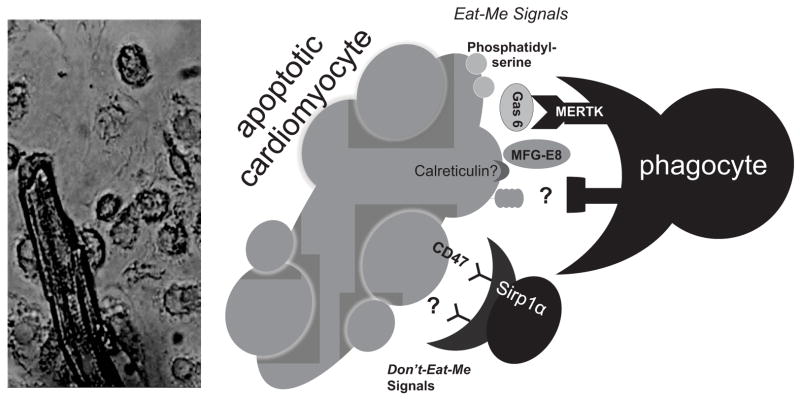
Figure 2. Phagocyte-Myocyte interactions.
To the left is a micrograph of the elongated cardiomyocyte juxtaposed next to multiple circular macrophages. To the right is a schematic exhibiting the unknown spectrum of interacting ligands between macrophages and cardiomyocytes, including putative eat-me signals on the apoptotic cardiomyocyte, such as phosphatidylserine and calreticulin, as well as potential molecules that bridge cardiomyocytes and macrophages, such as Gas6 (growth arrest specific) or MFG-E8 (milk fat globule). Don’t eat me signals may include CD47 through interactions with Sirp1α. MERTK is important on the phagocyte side for efferocytosis of cardiomyocyte apoptotic bodies. Other putative cardiac recognition ligands and macrophage receptors are yet to be described.
“Don’t-eat-me” signals in the heart
Don’t-eat-me signals, such as CD31 and PAI-I (Plasminogen activator inhibitor), can also help prevent viable cells from being engulfed by phagocytes. The most widely studied don’t-eat-me signal is CD47, which is a membrane protein expressed on the surface of most cells. CD47 interacts with SIRPα on phagocytes, recruits phosphatases, and inhibits downstream activation of the phagocyte actin cytoskeleton, thereby preventing engulfment (Tsai and Discher 2008). It has been shown that CD47 is expressed in abundance on apoptotic neonatal cardiocytes (Izmirly, Saxena et al. 2011). However, nothing more has been studied in the heart. By showing thrombospondin-2 (a CD47 ligand) knockout mice have higher mortality and dilated cardiomyopathy, it was concluded that TSP-2 protects age-related dilated cardiomyopathy (Swinnen, Vanhoutte et al. 2009). However, whether this phenomenon requires CD47, or how CD47 may be directly involved in removal of apoptotic cells in the heart, is unknown. Furthermore, the TSP1-CD47 axis is induced in renal tubular epithelial cells (RTEC) under hypoxia (Rogers, Yao et al. 2012). Thus, it will be interesting to see if such an axis is also induced between cardiomyocytes and macrophages within the hypoxic environment of the ischemic heart.
Therapeutic implications and future directions
Though pharmacological advances have significantly reduced mortality, the residual risk of post MI-induced heart failure remains high. This necessitates the development of complementary approaches to preserve heart function. It stands to reason that clearance of dying cells after MI by recruited phagocytes may be inherently inefficient as evolutionary pressure has not selected for optimal monocyte interactions with cardiac myocytes during diseases of aging. It is tempting to speculate that enhancing efferocytosis in the heart might help wound healing after heart attack. Efficient clearance of dying cells, both in a timely manner and of a significant quantity, is a pre-requisite for resolution of inflammation and downstream reparative processes after tissue clearance. In addition to clearance efficiency, the downstream signaling responses of phagocytes post engulfment are critical to inflammation resolution. Imbalances between pro- and anti-inflammatory stimuli may contribute to clearance inefficiency. The extent of cell death in the acute inflammatory phase of MI is a critical determinant of the degree of adverse remodeling leading to heart failure (Whelan, Kaplinskiy et al. 2010). Therefore, strategies that enhance efficient resolution of inflammation and prevent unnecessary further cell death of terminally differentiated heart myocytes (cardiomyocytes) may be useful in slowing the progression to heart failure and potential autoimmune reactions. Additional MI risk factors, such as hyperlipidemia may further reduce efferocytosis efficiency and repair in the heart.
Though many potential molecular reasons may explain inefficient effferocytosis of cardiomycoytes, one candidate worth noting is at the level of apoptotic cell receptors, which could be rendered naturally dysfunctional in the setting of disease or genetic risk factors. For example, polymorphisms in the apoptotic cell receptor Mertk are associated with increased autoimmune inflammation in diseases such as Lupus (Cheong, Lee et al. 2007). In addition, ADAM metallopeptidase 17, which cleaves MERTK into a soluble inhibitory receptor (Sather, Kenyon et al. 2007; Thorp, Vaisar et al. 2011), is increased during MI (Akatsu, Nakamura et al. 2003). This may explain the identification of solMER in murine extracts post MI (Wan, Yeap et al. 2013) and further provide the impetus for investigation of human MI specimens or blood. MERTK activity may also be limited by availability of its ligand Gas6, which is required for binding to apoptotic cells (Munoz, Sumoy et al. 2004). Future therapeutic approaches must strike a balance as, although the innate immune response has helpful activity in the healing heart, maladaptive inflammation can also be detrimental. Therefore, selective approaches that target specific immune subsets or cellular pathways harbor the most potential, as broad immunosuppressive therapy post MI can be detrimental in mice and humans (Hammerman, Kloner et al. 1983; Getts, Terry et al. 2014). Targeting of Ly6cHI monocytes after reperfusion have shown promise in (Majmudar, Keliher et al. 2013). Also, stimulation of so called pro-resolving pathways downstream of efferocytosis and phosphatidylserine recognition have proven beneficial in animal models (Harel-Adar T et al.).
Highlights.
The molecular mediators of phagocyte-myocyte interactions are just beginning to be studied
We review critical phagocytes populations in the heart during injury
We discuss critical myocyte interactions with phagocytes
We discuss how hypoxia and hypoxia-inducible factors may affect phagocyte-myocyte interactions
We provide a working model of phagocyte-myocyte biology and physiological significance
Acknowledgments
Funding from NIH 4R00HL097021-03 grant from the NHLBI (to ET).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akatsu T, Nakamura M, et al. Increased mRNA expression of tumour necrosis factor-alpha and its converting enzyme in circulating leucocytes of patients with acute myocardial infarction. Clin Sci (Lond) 2003;105(1):39–44. doi: 10.1042/CS20020367. [DOI] [PubMed] [Google Scholar]
- Akpek M, Kaya MG, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110(5):621–627. doi: 10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Anzai A, Anzai T, et al. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation. 2012;125(10):1234–1245. doi: 10.1161/CIRCULATIONAHA.111.052126. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Aversano T, Zhou W, et al. A chimeric IgG4 monoclonal antibody directed against CD18 reduces infarct size in a primate model of myocardial ischemia and reperfusion. J Am Coll Cardiol. 1995;25(3):781–788. doi: 10.1016/0735-1097(94)00443-T. [DOI] [PubMed] [Google Scholar]
- Ayata CK, Ganal SC, et al. Purinergic P2Y(2) receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology. 2012;143(6):1620–1629. doi: 10.1053/j.gastro.2012.08.049. [DOI] [PubMed] [Google Scholar]
- Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988;263(6):3039–3045. [PubMed] [Google Scholar]
- Belperio JA, Keane MP, et al. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest. 2002;110(11):1703–1716. doi: 10.1172/JCI15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Mordechai T, Holbova R, et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62(20):1890–1901. doi: 10.1016/j.jacc.2013.07.057. [DOI] [PubMed] [Google Scholar]
- Birge RB, Ucker DS. Innate apoptotic immunity: the calming touch of death. Cell Death Differ. 2008;15(7):1096–1102. doi: 10.1038/cdd.2008.58. [DOI] [PubMed] [Google Scholar]
- Blume KE, Soeroes S, et al. “Cleavage of annexin A1 by ADAM10 during secondary necrosis generates a monocytic find-me” signal. J Immunol. 2012;188(1):135–145. doi: 10.4049/jimmunol.1004073. [DOI] [PubMed] [Google Scholar]
- Bosco MC, Puppo M, et al. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology. 2008;213(9–10):733–749. doi: 10.1016/j.imbio.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Bournazou I, Pound JD, et al. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119(1):20–32. doi: 10.1172/JCI36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden RA, Ding ZM, et al. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ Res. 2002;90(5):562–569. doi: 10.1161/01.res.0000013835.53611.97. [DOI] [PubMed] [Google Scholar]
- Braunersreuther V, Pellieux C, et al. Chemokine CCL5/RANTES inhibition reduces myocardial reperfusion injury in atherosclerotic mice. J Mol Cell Cardiol. 2010;48(4):789–798. doi: 10.1016/j.yjmcc.2009.07.029. [DOI] [PubMed] [Google Scholar]
- Briaud SA, Ding ZM, et al. Leukocyte trafficking and myocardial reperfusion injury in ICAM-1/P-selectin-knockout mice. Am J Physiol Heart Circ Physiol. 2001;280(1):H60–67. doi: 10.1152/ajpheart.2001.280.1.H60. [DOI] [PubMed] [Google Scholar]
- Bujak M, Dobaczewski M, et al. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105(10):973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74(2):184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujak M, Kweon HJ, et al. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51(14):1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capoccia BJ, Gregory AD, et al. Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J Leukoc Biol. 2008;84(3):760–768. doi: 10.1189/jlb.1107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin LM, Stamatiades EG, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153(2):362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Acebes M, Pitaval C, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh SP, Gough MJ, et al. The role of the neutrophil in ischaemia-reperfusion injury: potential therapeutic interventions. Cardiovasc Surg. 1998;6(2):112–118. doi: 10.1016/s0967-2109(97)00133-6. [DOI] [PubMed] [Google Scholar]
- Chambers SE, O’Neill CL, et al. The role of immune-related myeloid cells in angiogenesis. Immunobiology. 2013;218(11):1370–1375. doi: 10.1016/j.imbio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467(7317):863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Frangogiannis NG. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev. 2010;15(5):415–422. doi: 10.1007/s10741-010-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Corriden R, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Cheong HS, Lee SO, et al. MERTK polymorphisms associated with risk of haematological disorders among Korean SLE patients. Rheumatology. 2007;46(2):209–214. doi: 10.1093/rheumatology/kel182. [DOI] [PubMed] [Google Scholar]
- Ciz M, Denev P, et al. Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxid Med Cell Longev. 2012;2012:181295. doi: 10.1155/2012/181295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleutjens JP, Blankesteijn WM, et al. The infarcted myocardium: simply dead tissue, or a lively target for therapeutic interventions. Cardiovasc Res. 1999;44(2):232–241. doi: 10.1016/s0008-6363(99)00212-6. [DOI] [PubMed] [Google Scholar]
- Cochain C, Rodero MP, et al. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischaemic neovascularization. Cardiovasc Res. 2010;88(1):186–195. doi: 10.1093/cvr/cvq153. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran CS, Demick KP, et al. Lactoferrin activates macrophages via TLR4-dependent and-independent signaling pathways. Cell Immunol. 2006;242(1):23–30. doi: 10.1016/j.cellimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Dahl CP, Husberg C, et al. Increased production of CXCL16 in experimental and clinical heart failure: a possible role in extracellular matrix remodeling. Circulation Heart failure. 2009;2(6):624–632. doi: 10.1161/CIRCHEARTFAILURE.108.821074. [DOI] [PubMed] [Google Scholar]
- Daleau P. Lysophosphatidylcholine, a metabolite which accumulates early in myocardium during ischemia, reduces gap junctional coupling in cardiac cells. J Mol Cell Cardiol. 1999;31(7):1391–1401. doi: 10.1006/jmcc.1999.0973. [DOI] [PubMed] [Google Scholar]
- Degrossoli A, Giorgio S. Functional alterations in macrophages after hypoxia selection. Exp Biol Med (Maywood) 2007;232(1):88–95. [PubMed] [Google Scholar]
- Dolmatova E, Spagnol G, et al. Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol. 2012;303(10):14. doi: 10.1152/ajpheart.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Khalil M, et al. Critical role for leukocyte hypoxia inducible factor-1alpha expression in post-myocardial infarction left ventricular remodeling. Circ Res. 2010;106(3):601–610. doi: 10.1161/CIRCRESAHA.109.208967. [DOI] [PubMed] [Google Scholar]
- Dutta P, Courties G, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott KJ, Harriman A, et al. A porphyrin-polyoxometallate bio-inspired mimic for artificial photosynthesis. Phys Chem Chem Phys. 2009;11(39):8767–8773. doi: 10.1039/b905548g. [DOI] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergen AV, Boles NC, et al. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119(11):2500–2509. doi: 10.1182/blood-2011-11-391730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmann L, Idel C, et al. Phagocytosis of apoptotic cells by neutrophil granulocytes: diminished proinflammatory neutrophil functions in the presence of apoptotic cells. J Immunol. 2010;184(1):391–400. doi: 10.4049/jimmunol.0900564. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiane AE, Videm V, et al. Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation. 2003;108(7):849–856. doi: 10.1161/01.CIR.0000084550.16565.01. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol. 2013 doi: 10.1097/FJC.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG, Mendoza LH, et al. MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am J Physiol Heart Circ Physiol. 2003;285(2):H483–492. doi: 10.1152/ajpheart.01016.2002. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG, Smith CW, et al. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- Frommhold D, Ludwig A, et al. Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation. J Exp Med. 2008;205(6):1435–1446. doi: 10.1084/jem.20070846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Terry RL, et al. Therapeutic Inflammatory Monocyte Modulation Using Immune-Modifying Microparticles. Science Translational Medicine. 2014;6(219):219ra217. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108(16):1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- Hammerman H, Kloner RA, et al. Dose-dependent effects of short-term methylprednisolone on myocardial infarct extent, scar formation, and ventricular function. Circulation. 1983;68(2):446–452. doi: 10.1161/01.cir.68.2.446. [DOI] [PubMed] [Google Scholar]
- Hannah S, Mecklenburgh K, et al. Hypoxia prolongs neutrophil survival in vitro. FEBS Lett. 1995;372(2–3):233–237. doi: 10.1016/0014-5793(95)00986-j. [DOI] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr B, Zhou J, et al. The supernatant of apoptotic cells causes transcriptional activation of hypoxia-inducible factor-1alpha in macrophages via sphingosine-1-phosphate and transforming growth factor-beta. Blood. 2009;114(10):2140–2148. doi: 10.1182/blood-2009-01-201889. [DOI] [PubMed] [Google Scholar]
- Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1) doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter-Hufford AE, Lee CS, et al. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497(7448):263–267. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TK, Twomey P, et al. Respiratory gas tensions and pH in healing wounds. Am J Surg. 1967;114(2):302–307. doi: 10.1016/0002-9610(67)90388-1. [DOI] [PubMed] [Google Scholar]
- Imtiyaz HZ, Williams EP, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120(8):2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmirly PM, Saxena A, et al. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124(18):1927–1935. doi: 10.1161/CIRCULATIONAHA.111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikita K, Hayasaki T, et al. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165(2):439–447. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103(5):634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- Kempf T, Zarbock A, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17(5):581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- Keyes KT, Ye Y, et al. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299(1):H153–164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- Kong T, Scully M, et al. Identification of Pur alpha as a new hypoxia response factor responsible for coordinated induction of the beta 2 integrin family. J Immunol. 2007;179(3):1934–1941. doi: 10.4049/jimmunol.179.3.1934. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Rajasingh J, et al. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res. 2009;104(2):18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukielka GL, Youker KA, et al. Role of early reperfusion in the induction of adhesion molecules and cytokines in previously ischemic myocardium. Mol Cell Biochem. 1995;147(1–2):5–12. doi: 10.1007/BF00944777. [DOI] [PubMed] [Google Scholar]
- Kyne L, Hausdorff JM, et al. Neutrophilia and congestive heart failure after acute myocardial infarction. Am Heart J. 2000;139(1 Pt 1):94–100. doi: 10.1016/s0002-8703(00)90314-4. [DOI] [PubMed] [Google Scholar]
- Lauber K, Bohn E, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Leuschner F, Panizzi P, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circulation research. 2010;107(11):1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner F, Rauch PJ, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209(1):123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Huang L, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120(1):331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Jackson P, et al. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. Am J Pathol. 2008;173(1):144–153. doi: 10.2353/ajpath.2008.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey ML, Gannon J, et al. Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation. 2002;105(6):753–758. doi: 10.1161/hc0602.103674. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- Lou O, Alcaide P, et al. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol. 2007;178(2):1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- Ma XL, Weyrich AS, et al. Monoclonal antibody to L-selectin attenuates neutrophil accumulation and protects ischemic reperfused cat myocardium. Circulation. 1993;88(2):649–658. doi: 10.1161/01.cir.88.2.649. [DOI] [PubMed] [Google Scholar]
- Ma YJ, Doni A, et al. Ficolin-1-PTX3 complex formation promotes clearance of altered self-cells and modulates IL-8 production. J Immunol. 2013;191(3):1324–1333. doi: 10.4049/jimmunol.1300382. [DOI] [PubMed] [Google Scholar]
- Majmudar MD, Keliher EJ, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127(20):2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti V, Yanes O, et al. Differential macrophage polarization promotes tissue remodeling and repair in a model of ischemic retinopathy. Sci Rep. 2011;1:76. doi: 10.1038/srep00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Medina RJ, O’Neill CL, et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med. 2011;17(9–10):1045–1055. doi: 10.2119/molmed.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel SR, Shapiro H, et al. Increased expression of neutrophil and monocyte adhesion molecules LFA-1 and Mac-1 and their ligand ICAM-1 and VLA-4 throughout the acute phase of myocardial infarction: possible implications for leukocyte aggregation and microvascular plugging. J Am Coll Cardiol. 1998;31(1):120–125. doi: 10.1016/s0735-1097(97)00424-5. [DOI] [PubMed] [Google Scholar]
- Meissner J, Irfan A, et al. Use of neutrophil count in early diagnosis and risk stratification of AMI. Am J Med. 2011;124(6):534–542. doi: 10.1016/j.amjmed.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Muller WA. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J Leukoc Biol. 1995;57(4):523–528. doi: 10.1002/jlb.57.4.523. [DOI] [PubMed] [Google Scholar]
- Munoz X, Sumoy L, et al. Human vitamin K-dependent GAS6: gene structure, allelic variation, and association with stroke. Hum Mutat. 2004;23(5):506–512. doi: 10.1002/humu.20025. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112(12):1624–1633. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of experimental medicine. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie HJ, Tian YM, et al. Changes of erythrocyte deformability in rats acclimatized to hypoxia and its molemechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2011;27(1):23–28. [PubMed] [Google Scholar]
- Novak ML, Weinheimer-Haus EM, et al. Macrophage activation and skeletal muscle healing following traumatic injury. The Journal of pathology. 2014;232(3):344–355. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue M, Morrow DA, et al. Association between baseline neutrophil count, clopidogrel therapy, and clinical and angiographic outcomes in patients with ST-elevation myocardial infarction receiving fibrinolytic therapy. Eur Heart J. 2008;29(8):984–991. doi: 10.1093/eurheartj/ehn112. [DOI] [PubMed] [Google Scholar]
- Oda T, Hirota K, et al. Activation of hypoxia-inducible factor 1 during macrophage differentiation. Am J Physiol Cell Physiol. 2006;291(1):C104–113. doi: 10.1152/ajpcell.00614.2005. [DOI] [PubMed] [Google Scholar]
- Panizzi P, Swirski FK, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55(15):1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzi P, Swirski FK, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. Journal of the American College of Cardiology. 2010;55(15):1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Liu G, et al. PAI-1 inhibits neutrophil efferocytosis. Proc Natl Acad Sci U S A. 2008;105(33):11784–11789. doi: 10.1073/pnas.0801394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter C, Waibel M, et al. “Migration to apoptotic find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283(9):5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- Pinto AR, Paolicelli R, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PloS one. 2012;7(5):10. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35(4):445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NM, Yao M, et al. Activated CD47 regulates multiple vascular and stress responses: implications for acute kidney injury and its management. Am J Physiol Renal Physiol. 2012;303(8):F1117–1125. doi: 10.1152/ajprenal.00359.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romson JL, Hook BG, et al. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rudolph V, Goldmann BU, et al. Diagnostic value of MPO plasma levels in patients admitted for suspected myocardial infarction. Int J Cardiol. 2011;153(3):267–271. doi: 10.1016/j.ijcard.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Sather S, Kenyon KD, et al. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109(3):1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer N, Dehne N, et al. Myeloid hypoxia-inducible factor-1alpha is essential for skeletal muscle regeneration in mice. J Immunol. 2013;191(1):407–414. doi: 10.4049/jimmunol.1103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, et al. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Maddox JF, et al. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34(44):14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Shantsila E, Lip GY. Monocyte diversity in myocardial infarction. J Am Coll Cardiol. 2009;54(2):139–142. doi: 10.1016/j.jacc.2009.03.047. [DOI] [PubMed] [Google Scholar]
- Shantsila E, Wrigley B, et al. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. Journal of thrombosis and haemostasis: JTH. 2011;9(5):1056–1066. doi: 10.1111/j.1538-7836.2011.04244.x. [DOI] [PubMed] [Google Scholar]
- Shingu M, Nonaka S, et al. Activation of complement in normal serum by hydrogen peroxide and hydrogen peroxide-related oxygen radicals produced by activated neutrophils. Clin Exp Immunol. 1992;90(1):72–78. doi: 10.1111/j.1365-2249.1992.tb05834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, Tian M, et al. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol. 2005;175(11):7151–7161. doi: 10.4049/jimmunol.175.11.7151. [DOI] [PubMed] [Google Scholar]
- Singh MV, Swaminathan PD, et al. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol. 2012;52(5):1135–1144. doi: 10.1016/j.yjmcc.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnlein O, Weber C, et al. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30(11):538–546. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Stark MA, Huo Y, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Swinnen M, Vanhoutte D, et al. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120(16):1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Robbins CS. Neutrophils usher monocytes into sites of inflammation. Circ Res. 2013;112(5):744–745. doi: 10.1161/CIRCRESAHA.113.300867. [DOI] [PubMed] [Google Scholar]
- Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25(11):2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Clish CB, et al. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101(4):819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Costa S, et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479(7371):122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao ZY, Cavasin MA, et al. Temporal changes in matrix metalloproteinase expression and inflammatory response associated with cardiac rupture after myocardial infarction in mice. Life Sci. 2004;74(12):1561–1572. doi: 10.1016/j.lfs.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Tapp LD, Shantsila E, et al. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. Journal of thrombosis and haemostasis: JTH. 2011 doi: 10.1111/j.1538-7836.2011.04603.x. [DOI] [PubMed] [Google Scholar]
- Thompson AA, Binham J, et al. Hypoxia, the HIF pathway and neutrophilic inflammatory responses. Biol Chem. 2013;394(4):471–477. doi: 10.1515/hsz-2012-0335. [DOI] [PubMed] [Google Scholar]
- Thorp E, Vaisar T, et al. Shedding of the MER tyrosine kinase receptor is mediated by ADAM17 through a pathway involving reactive oxygen species, protein kinase {delta}, and P38 map kinase. J Biol Chem. 2011 doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp EB. Contrasting Inflammation Resolution during Atherosclerosis and Post Myocardial Infarction at the Level of Monocyte/Macrophage Phagocytic Clearance. Front Immunol. 2012;3:39. doi: 10.3389/fimmu.2012.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman LA, Ford CA, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112(13):5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- Tsai RK, Discher DE. “Inhibition of self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180(5):989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujioka H, Imanishi T, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54(2):130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Uderhardt S, Herrmann M, et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36(5):834–846. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Vandivier RW, Henson PM, et al. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129(6):1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- Vasilyev N, Williams T, et al. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112(18):2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- Walmsley SR, Chilvers ER, et al. Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Invest. 2011;121(3):1053–1063. doi: 10.1172/JCI43273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan E, Yeap XY, et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113(8):1004–1012. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wantha S, Alard JE, et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res. 2013;112(5):792–801. doi: 10.1161/CIRCRESAHA.112.300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werno C, Menrad H, et al. Knockout of HIF-1alpha in tumor-associated macrophages enhances M2 polarization and attenuates their pro-angiogenic responses. Carcinogenesis. 2010;31(10):1863–1872. doi: 10.1093/carcin/bgq088. [DOI] [PubMed] [Google Scholar]
- Whelan RS, Kaplinskiy V, et al. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- Xiong M, Elson G, et al. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153(2):587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Anzai A, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zingarelli B, et al. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation. 2000;101(9):1019–1026. doi: 10.1161/01.cir.101.9.1019. [DOI] [PubMed] [Google Scholar]
- Yndestad A, Landro L, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30(10):1229–1236. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- Zakharova EE, V, Korneeva A, et al. Psychrophilic sulfur-reducing bacterium from aerobic Black Sea waters. Mikrobiologiia. 2012;81(6):812–814. [PubMed] [Google Scholar]
- Zhou J, Dehne N, et al. Nitric oxide causes macrophage migration via the HIF-1-stimulated small GTPases Cdc42 and Rac1. Free Radic Biol Med. 2009;47(6):741–749. doi: 10.1016/j.freeradbiomed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Zouggari Y, Ait-Oufella H, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19(10):1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]