Abstract
Selection of a model organism creates a tension between competing constraints. The recent explosion of modern molecular techniques has revolutionized the analysis of neural systems in organisms that are amenable to genetic techniques. Yet, the non-human primate remains the gold-standard for the analysis of the neural basis of behavior, and as a bridge to the operation of the human brain. The challenge is to generalize across species in a way that exposes the operation of circuits as well as the relationship of circuits to behavior. Eye movements provide an opportunity to cross the bridge from mechanism to behavior through research on diverse species. Here, we review experiments and computational studies on a circuit function called “neural integration” that occurs in the brainstems of larval zebrafish, non-human primates, and species “in between”. We show that analysis of circuit structure using modern molecular and imaging approaches in zebrafish has remarkable explanatory power for the details of the responses of integrator neurons in the monkey. The combination of research from the two species has led to a much stronger hypothesis for the implementation of the neural integrator than could have been achieved using either species alone.
Eye movements as a model system
The eye movement system is one of the most-studied and best-understood sensory-motor systems in neuroscience. We move our eyes for two clearly-defined purposes: to shift the eyes to point them at objects of interest, and to rotate the eyes smoothly so that they remain pointed at objects of interest in face of self-motion or object-motion. Eye movement is a particularly apt movement to understand because of its power as a diagnostic tool for neurological and neuropsychiatric disorders (Klin et al., 2002, Garbutt et al., 2008, Jones et al., 2008). Research on monkeys should provide the “final common path” to understanding human eye movements in health and disease. Yet, the machinery of the eyes and the behaviors have been preserved during evolution so that many animal models can be used to understand the neural circuit basis for eye movements.
Research on humans and non-human primates has made steps in understanding eye motor control that are essential for research on any motor system. First, analysis of the motor behavior has dissected eye movement into its components and categorized different types of movements. We make rapid, saccadic eye movements to reorient the gaze. We use the vestibulo-ocular reflex to stabilize gaze in the face of our own motion. We use smooth pursuit eye movements to keep the fovea pointed at moving objects. Second, recordings of the electrical activity of neurons in the brainstem have revealed the details of the final motor command signals. As a consequence, it is easier to interpret the responses of other neurons in relation to the signals that appear on motoneurons (Fuchs and Luschei, 1970, Robinson, 1970, Robinson and Keller, 1972). Recordings that work backwards from the motor nuclei have revealed the discharge properties of neurons in premotor brainstem nuclei, and have suggested how the premotor circuits might be organized (Robinson, 1981, Sparks, 2002). Third, the relative simplicity of the eye movement system has made it tractable for computational modeling, which provides the language needed to understand any neural system.
Monkeys have provided an excellent animal model for understanding many aspects of how the brain controls eye movements. Causal manipulations, such as inactivation or stimulation of specific groups of neurons, have identified the brain areas that control different kinds of eye movements (Robinson, 1972, Wurtz and Goldberg, 1972a, Schiller et al., 1980, Rambold et al., 2002). Because of the excellent understanding of the final motor pathways and behavior, eye movement has provided an excellent model system for studying higher commands for movement in areas such as superior colliculus (Wurtz and Goldberg, 1972b, Zenon and Krauzlis, 2012), basal ganglia (Hikosaka and Wurtz, 1983, Lau and Glimcher, 2008), cerebellum (Lisberger and Fuchs, 1974, Shidara et al., 1993), and frontal cortex (Bruce and Goldberg, 1985, Gottlieb et al., 1994). Eye movements also have provided the substrate for advancing knowledge about the neural mechanisms of perceptual decisions (Platt and Glimcher, 1999, Shadlen and Newsome, 2001). Overall, there is a remarkable body of work that describes neural activity during oculomotor behavior in monkeys. Nothing comparable to it exists any other species.
Monkey research has been challenged to link function to structure, but there have been some notable successes. These have been based mainly on using electrical stimulation in the brain to identify neurons according to their connections to other neurons, or at least according to their anatomical projections. For example, identification of the neurons in the brainstem that receive monosynaptic inhibition from the floccular complex of the cerebellum has revealed their role in driving smooth eye movements (Lisberger et al., 1994b, Zhang et al., 1995, Ramachandran and Lisberger, 2008, Joshua et al., 2013) and motor learning (Lisberger and Pavelko, 1988, Lisberger et al., 1994a). Antidromic activation has revealed rules for distributing output from the cortex by studying the functional discharge properties during eye movements of the neurons that project from the frontal eye field (FEF) to the reticular formation, the pons and the superior colliculus (Segraves and Goldberg, 1987, Segraves, 1992, Ono and Mustari, 2009). Electrical stimulation has outlined a pathway that transmits an efference copy of the command for saccadic eye movements from the superior colliculus through the thalamus to the FEF (Sommer and Wurtz, 2002).
The explosion of new techniques for studying neural networks has created opportunities for a new kind of analysis of neural circuits and how they work. It now is possible to go beyond the traditional approaches used in monkey research, and to answer questions that were intractable in the past. For example, imaging of calcium signals makes it possible to record from many nearby neurons simultaneously with a temporal resolution that is good enough to capture the relationships between neural and behavioral or stimulus dynamics (Stosiek et al., 2003, Rothschild et al., 2010, Miri et al., 2011). Activation of specific subpopulations of neurons through optogenetics provides a carefully controlled tool for dissection of neural circuits in behaving animals (Han and Boyden, 2007). Genetic manipulations make it possible to eliminate, reversibly inactivate, or activate specific types of neurons (Schonewille et al., 2011). These modern approaches have enormous potential for understanding how neural circuits work, but they are challenging to apply in non-human primates.
Because of the differences in the techniques that can be applied efficiently in different species, analysis of the primate oculomotor system faces a challenge. Primates offer the most impressive, flexible, and repeatable oculomotor behavior along with the ability to study eye movements and the associated neural activity on a millisecond time scale. Yet, advances are stymied because of the challenges of measuring the architecture and electrical activity within defined circuits in monkeys. The measurements needed in monkeys are possible using modern imaging and molecular tools in non-primate model organisms, but these organisms lack the exquisite-control of motor behaviors seen in primates.
We see two ways to bridge the gap between species. One is to apply modern molecular and viral techniques in monkeys, an approach taken by a couple of laboratories (Jazayeri et al., 2012, Adelsberger et al., 2014). The other way is to study the same behavioral phenomena in multiple species, leveraging the advantages of each. The key is to use experimental design and data analyses that are similar enough across species to allow the unified understanding to be greater than the sum of its parts. We have adopted this second approach to understand the implementation of “neural integration” in the oculomotor brainstem. Neural integration is a computation that is common to primates, rodents and fish. While expressed in its purest form in the oculomotor system for converting transient commands for eye movement into sustained signals, neural integration also is important to retain a working memory of a transient event (Goldman-Rakic, 1995), and to accumulate evidence in favor of particular perceptual decisions (Shadlen and Newsome, 2001, Brunton et al., 2013).
The oculomotor neural integrator
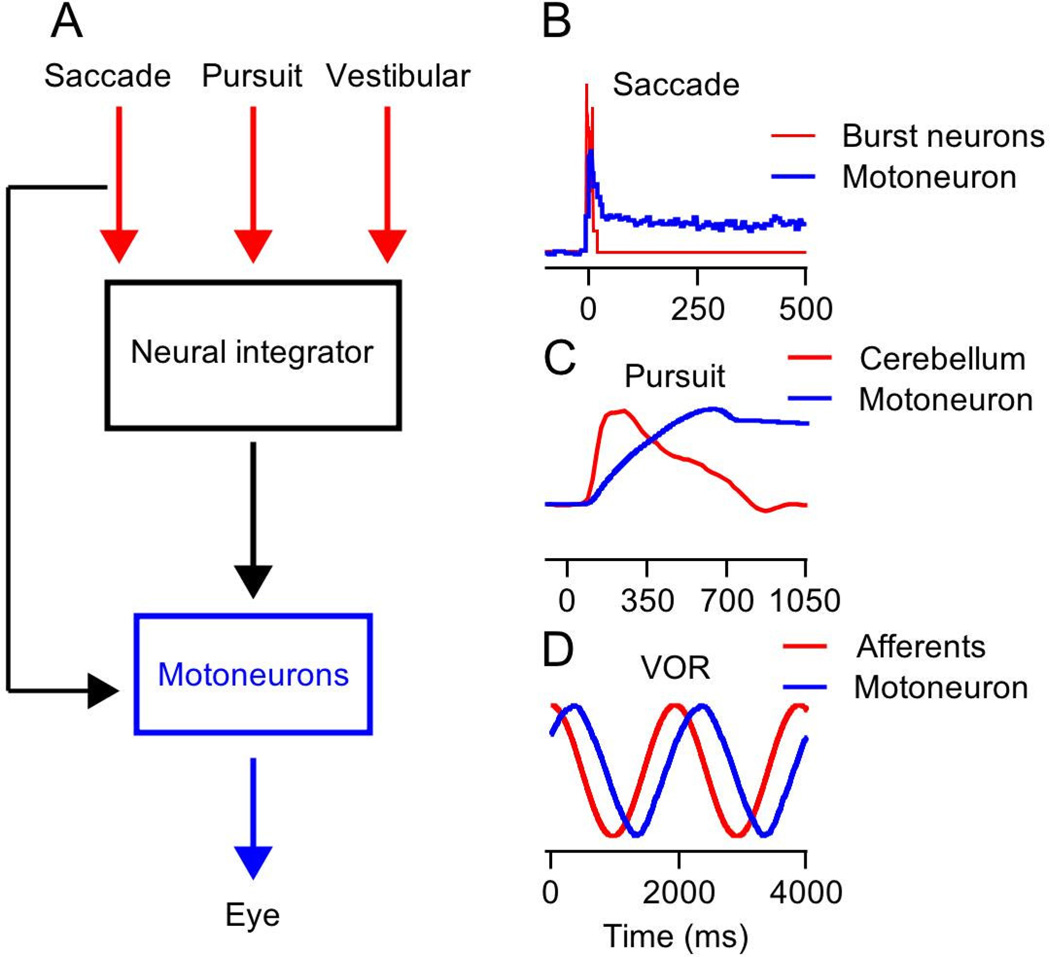
The need for a neural integrator in the oculomotor system (Fig. 1A) arises from the discharge properties of extraocular motoneurons. The output of the oculomotor system is understood very well through measures of the forces generated by the extraocular muscles (Robinson, 1964, Miller et al., 2002, Davis-Lopez de Carrizosa et al., 2011) and recordings from motoneurons that control eye muscles (Fuchs and Luschei, 1970, Keller and Robinson, 1972, Sylvestre and Cullen, 1999). During rapid, saccadic eye movements, motoneurons emit a transient burst of action potentials followed by a change in steady firing rate related to eye position (Fig. 1B, blue line). Muscle force shows a pulse during movement that is followed by sustained force at the end of the eye movement. The appearance of sustained force in the muscles was the first hint of a neural integrator that holds the eye steady at eccentric positions. Integration would explain the fact that motoneurons have sustained activity even in complete darkness, and without a stretch reflex (Keller and Robinson, 1971). Inactivation of premotor neurons in the brainstem and cerebellum have located the neural integrator in the brainstem and suggested the existence of a common integrator (Cannon and Robinson, 1987, Cheron and Godaux, 1987) for saccades, smooth pursuit eye movements, and the vestibulo-ocular reflex (Fig. 1B–D).
Figure 1. Rationale for an oculomotor neural integrator.
A: Schematic representation of the neuron integrator hypothesis. Commands for saccades, smooth pursuit, and the vestibulo-ocular reflex all signal the desired eye velocity, and therefore require neural integration to create the eye position signal that dominates the activity of extraocular motoneurons. The “Neural integrator” converts its input signals into commands for maintaining the eye in an eccentric position. The discharge of “Motoneurons” is assembled as a combination of the inputs and outputs of the integrator, the inputs to move the eye to a new position and the outputs to hold the eye stable in the final position. B–D: Red and blue traces represent the inputs to the integrator and motoneuron activity during saccades (B), smooth pursuit eye movement (C) and the vestibulo-ocular reflex (D). The traces were scaled arbitrarily to allow easier comparison of their temporal dynamics.
Compelling evidence for an oculomotor integrator and its location in the brainstem comes from examination of the relation between inputs to and outputs from the motoneurons during saccades. Premotor cells in the brainstem (Fig. 1B, red line) (Cohen and Henn, 1972) and cells in the superior colliculus (Wurtz and Goldberg, 1971), a major input to the brainstem, discharge transiently in conjunction with saccades but do not show sustained activity in relation to eye position. Motoneurons, on the other hand, show steady firing that is linearly related to steady eye position and that persists long after the transient command has ended (Fig. 1B, blue line) (Fuchs and Luschei, 1970, Robinson, 1970). Transient electrical stimulation in the superior colliculus or paramedian pontine reticular formation (PPRF), even in darkness, evokes saccades followed by steady eye fixation at eccentric positions (Cohen and Komatsuzaki, 1972, Robinson, 1972). Temporal integration is needed to convert transient bursts to steady firing.
Mathematical integration also describes well the transformation from inputs to outputs in the brainstem during smooth pursuit eye movements (Krauzlis and Lisberger, 1994, Joshua et al., 2013) and the vestibulo-ocular reflex (Skavenski and Robinson, 1973). One of the most important command signals for pursuit arises from the floccular complex of the cerebellum. Floccular Purkinje cells have a large transient firing during the initiation of pursuit and steady firing during sustained eye velocity. But, Purkinje cells show little or no sustained activity related to the changed eye position at the end of the movement (Fig. 1C, red line). In contrast, the activity of motoneurons lacks a transient during the initiation of pursuit and instead shows a ramp increase in firing during sustained eye velocity and a steady persistent firing rate after the eye stops moving (Fig. 1C, blue line). A model that performs mathematical integration of floccular output generates trajectories of firing rate that match the activity of Abducens neurons quite well (Krauzlis and Lisberger, 1994, Joshua et al., 2013).
To a first approximation, the same situation occurs during the vestibulo-ocular reflex. During sinusoidal head rotation, vestibular afferents tend to fire in relation to head velocity, which is equivalent to desired eye velocity (Fernandez and Goldberg, 1971, Ramachandran and Lisberger, 2006). The activity of motoneurons, in contrast, is most closely related to eye position (Fig. 1D, blue line) (Fuchs et al., 1988, Lisberger et al., 1994b). The transformation from signals that are close to eye velocity to signals that are closer to eye positions is consistent with processing by a neural integrator that shifts the phase of sine waves by 900 (Skavenski and Robinson, 1973, Ramachandran and Lisberger, 2006).
The architecture of the neural integrator
The first step in the analysis of neural integration, made decades ago, was to identify the need for mathematical integration in the final oculomotor pathways. In the formulation of Marr (1982), this places the analysis of how the brain moves the eyes at the middle, or algorithmic level. However, it has turned out to be a harder problem to get to the third level of “implementation” by unraveling how neural circuits perform integration. Extracellular single unit recordings from monkeys and cats identified neurons in the medial vestibular nucleus and nucleus prepositus that have sustained, steady firing of action potentials in relation to steady eye positions. These neurons encode the output that a neural integrator should have (Delgado-Garcia et al., 1989, Escudero et al., 1992, McFarland and Fuchs, 1992). Thus, one cogent suggestion was that the integrator for horizontal eye movement is implemented in the nucleus prepositus (Escudero et al., 1992, Fukushima et al., 1992).
In parallel to the experimental effort to identify the location and neural components of the integrator, computational studies asked about the neuron and neural circuit mechanisms that could lead to neural integration. Different possible implementations of integration have different theoretical advantages and disadvantages. In principle, integration can be implemented in a simple recurrent network that operates as a line attractor (Seung, 1996), or even in a single neuron with appropriate recurrent connections to itself (Seung et al., 2000). In practice, biological facts such as neuron physiology, actual anatomical connections, and the dynamics of the firing rate during eye movements needed to constrain the recurrent networks that might mimic the oculomotor integrator (Cannon et al., 1983, Galiana and Outerbridge, 1984, Cannon and Robinson, 1985, Seung, 1996, Miri et al., 2011, Joshua et al., 2013).
The theoretical studies have been very useful in illuminating how the connectivity in a circuit and the intrinsic properties of its constituent neurons could interact to implement an integrator (Seung, 1996, Koulakov et al., 2002, Goldman, 2009). However, the next step of discovering how the brain implements integration is much more challenging. Both precise circuit connectivity and/or cell physiology are currently extremely hard to deduce in monkeys. Thus, even the combination of the excellent anatomical localization of the oculomotor integrator and the precise control over eye movement behavior in monkeys has not allowed traditional approaches in non-human primates to determine how a neural circuit actually performs mathematical integration.
At the same time as research on non-human primates stalled in revealing the neural mechanisms of integration, experiments on the neural integrator in goldfish and zebrafish have opened some new horizons. As in monkeys, the oculomotor integrator in fish resides in specific neurons in the medulla (Pastor et al., 1994, Aksay et al., 2000, Aksay et al., 2001). In vivo intracellular recordings from integrator neurons in the goldfish demonstrated the necessity of synaptic inputs for integration (Aksay et al., 2001): at least part of neural integration must depend on neural connections. Focal lesions and simultaneous recordings from multiple neurons revealed the functional relation between bilateral integrator circuits (Aksay et al., 2007). Most recently, calcium imaging in behaving zebrafish demonstrated the relationship between the spatial location of individual neurons and their temporal response profiles (Miri et al., 2011), and suggested principles of a circuit architecture for neural integration. Thus, the new challenge became to bridge across two disparate species. This involves finding a way to use findings obtained with modern invasive technology on behaving fish to shed light on the organization of the integrator circuit in monkeys, and vice versa.
Advantages of monkeys and zebrafish
The monkey and the zebrafish share the common need for mathematical integration in the oculomotor system in spite of some differences in their eye movement capabilities. Monkeys have very fast saccades, hold eccentric position almost perfectly so that eye position drifts back toward straight ahead with a time constant longer than 20 seconds (Cannon and Robinson, 1987), and can produce smooth pursuit eye movements to track a small moving target. Larval zebrafish have slower saccades, and lower gains of the vestibulo-ocular reflex and the optokinetic response (Easter and Nicola, 1997, Beck et al., 2004). In many of the published examples gaze drifts back towards straight-ahead gaze slowly (Fig. 2E) (Miri et al., 2011). In general, zebrafish show smooth tracking only when the whole world moves (Portugues et al., 2014). Yet, the eye movements of both species share the property that eye position would drift back to straight-ahead gaze within less than one second if the motor innervation did not include steady force to hold the eyes eccentric. The integrator is needed in both species to convert pre-motor commands for eye velocity into eye position signals on motoneurons.
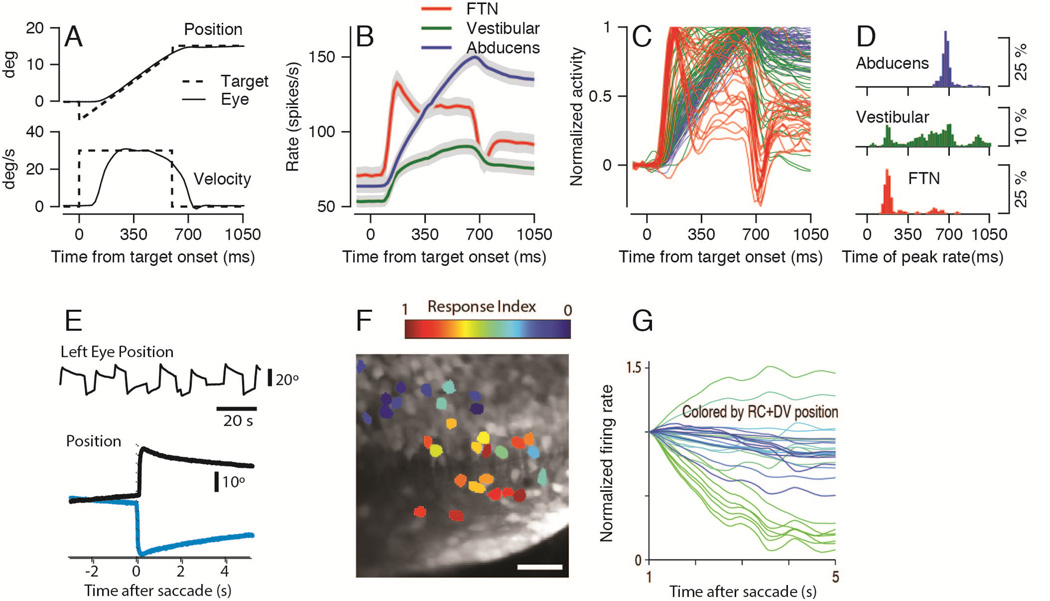
Figure 2. Eye movements and time-varying waveforms of neural integrator activity in zebrafish and monkeys.
A: Top and bottom superimposed traces show horizontal eye position (top) and velocity (bottom) during smooth pursuit eye movements. Solid and dashed lines show eye and target motion parameters. B–C: Each trace shows the time-varying firing rate in the monkey brainstem during the pursuit behavior illustrated in A. Panel B shows averages across functionally identified neuron types and panel C shows firing rate averages from individual neurons. Red, green, and blue traces denote the activity of FTNs, other, non-FTN neurons in the vestibular nucleus, and Abducens neurons. D: Histograms plot the time of peak firing rate using the same color code as in B and C. E: Eye movements of a larva zebrafish. Top, an eye position trace from 100 seconds of spontaneous movements. Bottom, representative eye position trajectories before and after saccades that take eye position toward (black) or away from (green) the side of the integrator under study. F: Image of 29 identified integrator neurons in the brainstem of the larval zebrafish. Neurons are color coded according to how strongly the calcium responses of pairs of neurons co-varied with eye position versus eye velocity. Red versus blue coloring indicates neurons that co-varied strongly with eye position versus eye velocity. G. Each trace shows the estimate of the time varying firing rate of an individual neuron in the zebrafish neural integrator from 1 to 5 seconds after a saccade. Traces are colored according to the location of the cell, which was defined as the sum of the rostrocaudal (RC) and dorsoventral (DV) coordinates. Panels B–D are adapted with permission from Joshua et al. (2013). Panels E–G are adapted with permission from Miri et al. (2011).
Each species has its own, unique, advantages for analysis of neural integration. Monkeys are “hands-down” winners in terms of precisely controlling behavior while studying properties of neural activity on a time scale of milliseconds. After training, they will fixate and track stationary or moving targets, and they will emit the same crisp and accurate eye movement many times during an experimental session. The accuracy and precision of the behavior make it easy to manipulate target parameters so that we can relate the neural response to behavior. For example, we can ask how are neurons tuned to the movement parameters by changing target speed or direction (Stone and Lisberger, 1990, Tanaka and Lisberger, 2002). We can use the same sensory stimulus to study movement-by-movement variation in the operation of the sensory-motor system (Medina and Lisberger, 2007, Schoppik et al., 2008, Hohl et al., 2013, Joshua and Lisberger, 2014). Zebrafish are ideal for the application of modern techniques to study neural circuits during reasonable, if sub-optimal, saccades and fixations. In larval zebrafish, the body is transparent so that many neurons can be imaged at the same time during behavior. Molecular techniques can be used to label specific neurons, and to allow manipulation of the activity of specific neurons with optical stimulation. For circuit-breaking, the zebrafish is ideal in many ways.
System principles link integrator implementation across species
In both monkey and fish, integration is needed and the integrator resides in homologous structures. This allowed an approach that merges knowledge from monkeys and zebrafish, capitalizing on the advantages of each in a way that obviates the technical disadvantages of each.
The new experimental evidence that links monkeys and zebrafish at the level of implementation of integration comes from parallels in the diversity of the time-varying firing rate of neurons in the neural integrator in the two species. In monkeys, we calculated the time-varying firing rate of brainstem neurons during the initiation and steady-state component of smooth pursuit eye movements (Joshua et al., 2013). For each neuron, we recorded spike trains while the monkey tracked many repetitions of “step-ramp” target motion (Figure 2A). The tracking target initially was stationary at straight-ahead gaze. It then underwent a small displacement and a ramp at constant speed. The specifics of the displacement’s amplitude and the ramp’s speed allowed the monkey to initiate crisp pursuit without a saccadic eye movement. Finally, the target stopped and remained stationary at a new position.
The pursuit behavior we used endures only 1.5 seconds, but still provides quite stringent constraints on the operation of the neural integrator. It requires a millisecond-by-millisecond mathematical integration to create the motoneuron firing that controls eye position during pursuit. The paradigm also requires a sustained component of motoneuron firing to maintain the final eye position at the end of each target motion. The properties of the required neural integration are the same during pursuit and to hold eye position at the end of pursuit; we think that a single integrator supports both phases of the movement. Other papers agree about the existence of a single neural integrator for horizontal eye movement (Cannon and Robinson, 1987, Cheron and Godaux, 1987).
The simplest description of the data from monkeys is that the time-varying trajectory of firing rate varied considerably among brainstem neurons. We found structure in the variation by sorting the neurons according to the details of their relationship to the parameters of eye movement, their anatomical location, and their identity as interneurons that receive monosynaptic inhibition from the floccular complex of the cerebellum, or “FTNs”. As a general rule, FTNs inherited the transient response also seen in floccular Purkinje cells during the initiation of pursuit (Fig. 2B, C red traces), but showed only small changes in steady firing rate during the sustained eye position at the end of the pursuit movement. Vestibular nucleus neurons that lacked monosynaptic inhibition from the floccular complex had very different responses. Few showed a transient response during the initiation of pursuit. In general, they showed a steady increase in firing rate during steady-state pursuit training, but with a wide range of rates of rise and diversity in the steady firing rate at the end of the pursuit movement (Fig. 2B, C green traces).
The diversity in the responses of brainstem neurons is in sharp contrast to the homogeneity of the time-varying firing rates of motoneurons that move the eyes. A group of neurons that seems to include FTNs and some of the non-FTN vestibular neurons makes synapses on the motoneurons in the Abducens nucleus (Sato et al., 1988, Scudder and Fuchs, 1992, Lisberger et al., 1994a, Shin et al., 2011), but the pattern of activity was different in motoneurons versus vestibular last-order interneurons. The firing rate of the motoneurons increased gradually during the eye movement with no sign of a transient during pursuit initiation (Fig. 1C and 2B, blue trace). Motoneuron firing rate remained steady when the eye stopped at an eccentric position at the end of the trial, and showed large changes between the initial and final position. The firing pattern of Abducens neurons showed remarkable uniformity across all members of the population (Fig. 2C, see also Luschei and Fuchs, 1972).
To emphasize the differences among the three populations, we measured the time to the peak of firing rate for each neuron. We plotted distributions of time-to-peak firing separately for Abducens neurons, FTNs, and the non-FTN vestibular neurons defined as “position-vestibular-pause” neurons (Tomlinson and Robinson, 1984) or “EHV” neurons (Scudder and Fuchs, 1992). The distributions (Figure 2D) peaked at early and late times for the FTNs and Abducens neurons, but covered the entire range of the pursuit eye movement for the non-FTN vestibular neurons. An additional group of non-FTN vestibular neurons was related solely to the eye movement and reached peak firing at times in line with the Abducens neurons (Joshua et al., 2013).
Calcium imaging in awake, behaving larval zebrafish revealed similarly diverse temporal responses in neurons that are part of this species’ neural integrator (Miri et al., 2011). During the drift of the eye towards the central position after saccades, some neurons showed very rapid decreases in calcium activity, while others showed activity that remained high for the entire five-second duration of the recordings. Further, it was possible to take advantage of the transparent larval zebrafish to discover that neurons that tended to cluster together had many similarities. They had similar decay rates, they correlated similarly with eye velocity and position, and they had higher pairwise correlations (Fig. 2E, F). The co-localization of neurons with similar decay rates and higher correlations suggests that the physiological connections are strongest between integrator neurons that are near neighbors, and are weaker between integrator neurons that are more distant from each other.
Thus, research in monkeys and fish revealed homologous neural circuits that perform the same computation. In both species, integrator neurons show a wide diversity in time-varying responses. Zebrafish allowed analysis of circuit structure, and the recordings revealed a structural feature of the circuit that is correlated with the diversity of calcium responses. Monkeys allowed identification of neurons that receive input from the cerebellum, as well as neurons at the intermediate and output levels of the circuit. The recordings revealed that knowing the functional level in the integrator explains much of the response diversity. Future experiments in zebrafish might be able to use optogenetic approaches to identify neurons according to their level in the integrator hierarchy, much as we have in monkey. Still, there are enough similarities between the results from the two species that it should be valid to combine the data from the two species. The different levels of analysis in the two species offer an opportunity to combine their results in a way that will bridge from network structure to function.
Bridging from zebrafish to monkeys
Computational models provide a unifying “language” (Carandini, 2012) that allows us to connect disparate experimental approaches to the same neural computation by the same neural circuitry in monkey and fish. Miri et al. (2011) found that the diversity of responses could be reproduced in simulations of hierarchical networks that perform integration gradually. For example, a network with strong feedforward and weak feedback connections (Fig. 3B, C) mimics the diverse responses they found in neurons (Fig. 3E). The model uses connection strengths that are graded according to the distance between elements, matching the evidence in their data for stronger near-neighbor correlations. We show next that the model proposed by Miri et al. (2011) on the basis of their calcium imaging in zebrafish had impressive explanatory power for our electrical recordings from the monkey’s brainstem.
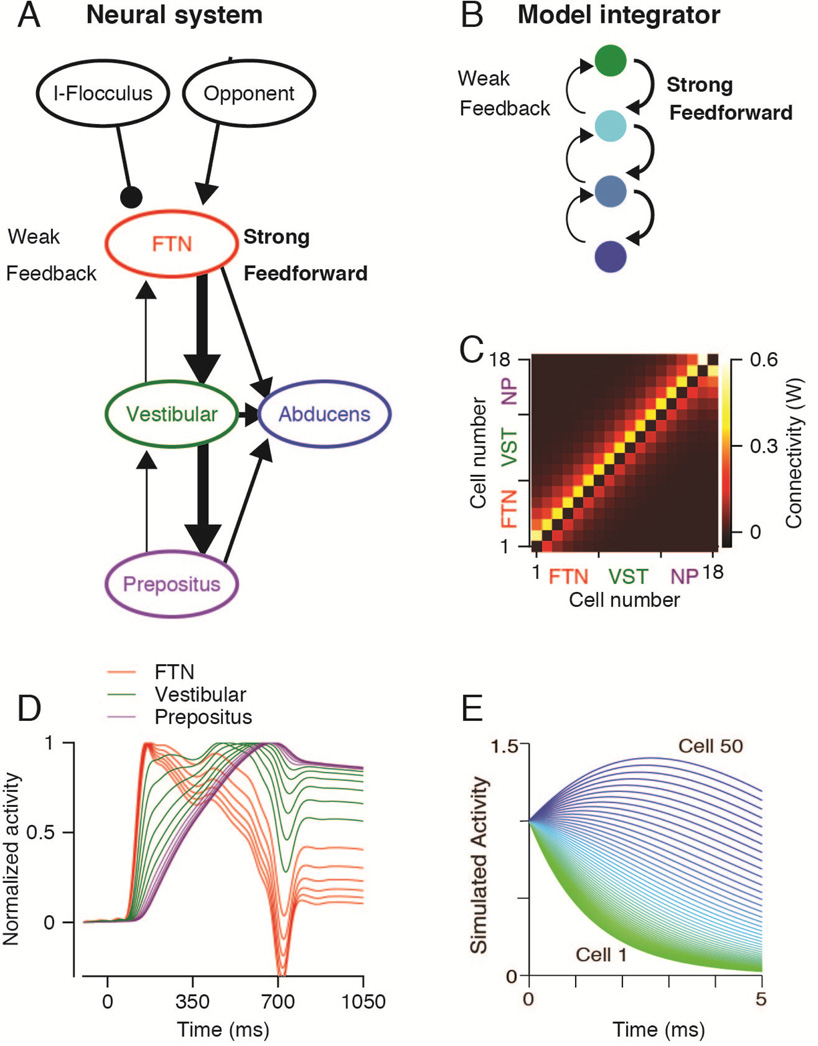
Figure 3. A network model that integrates and reproduces the time-varying firing rates of neurons in the oculomotor neural integrator of monkeys and zebrafish.
A: Schematic representation of the brainstem pursuit network as defined by recordings from functionally identified neurons in monkeys. Each ellipse represents a population of neurons, arrows are excitatory connections, and lines ending with a circle represent inhibitory connections. Thick and thin arrows indicate strong versus weak connections. B. The architecture of a neural integrator model. Each circle represents a single neuron or group of neurons, and the arrows represent connections. Each neuron is connected strongly the next neuron and weakly to the previous neuron. C: The connectivity matrix used for simulation of monkey brainstem. The colors indicate the connection weights in the connection matrix (W) between neurons for a network with stronger feed-forward versus feedback connections. D–E. Each trace shows the time-varying firing rate of an individual model neuron in the neural networks used by the two laboratories to simulate neural integration in monkey (D) and zebrafish (E). In panel D, the colors of the traces indicate the functional group assigned to the three groups of 6 neurons in the model integrator. Panels A–D are adapted with permission from Joshua et al. (2013). Panel E is adapted with permission from Miri et al. (2011).
We implemented a model (Figure 3A) that started from the principles expressed in the model of Miri et al. (2011). Our model contained 18 model neurons with strong feedforward connections from one model neuron to the next, and weak feedback connections (see connection matrix in Figure 3C). From the start to the end of the chain of model neurons, we think of the first six as FTNs, the next six as non-FTN vestibular neurons, and the last six as prepositus neurons. This hierarchy is justified by the facts that FTNs receive the pursuit command from the cerebellum (Lisberger et al., 1994b) and that prepositus neurons receive much of their input from the vestibular nucleus (Baker and Berthoz, 1975). All 18 of the model “integrator” neurons projected to a model Abducens motoneuron. As an input to drive the model, we computed the “opponent” output from the cerebellar floccular complex. Opponent firing rate was defined as the mean simple-spike firing rate for pursuit towards the side of recording minus that for pursuit away from the side of recording. Prior research has demonstrated that the opponent floccular output needs to be subjected to integration to create motoneuron firing (Krauzlis and Lisberger, 1994). The model we have used bears a number of similarities to the cascade model proposed by Delgado-Garcia et al. (1992).
The responses of the model neurons captured many of the properties that we found in the data from monkeys (Fig. 2C), including the diversity of response profiles. Model FTNs, which receive direct input from the cerebellum, have transient responses during the initiation of pursuit and sustained responses during steady-state tracking (Fig. 3D, red traces). Their time-varying firing rates show very little evidence of integration, because they are “clamped” at the trajectory of their inputs by the strong cerebellar input they receive. Model non-FTN vestibular neurons show a diversity of response profiles that indicates many stages along the process of total integration. They mostly lacked transient responses during pursuit initiation (Fig. 3D, green traces). Model prepositus neurons (Figure 3D, purple traces) show a strong eye position component and relatively little diversity of response profile, in good agreement with recordings by other laboratories (Escudero et al., 1992, McFarland and Fuchs, 1992). Studies of the saccadic part of the brainstem provide data that agree with ours and would support a model of integration similar to the one we have used. Prepositus neurons that project to the Abducens nucleus tend to discharge more strongly in relation to eye position, while other neurons in the prepositus and vestibular nucleus discharge in relation to different combinations of saccadic eye velocity and position (Delgado-Garcia et al., 1989, Escudero et al., 1992).
What makes our integrator model work, and what breaks it? We analyzed this question in Figure 8 in Joshua et al. (2013). Briefly, when the model used uniform, random connection weights between units, the diversity of neural responses was lost and all model neurons showed the same, integrated, time-varying firing rate. In this network configuration, all model neurons operate as equals. The uniformity of the weights effectively clamps all model units together so that they all show the same time-varying response patterns. When we created symmetry in the feed-forward and feedback connection weights, much of the diversity of time-varying neural firing rates was lost. Also, the early transient response in the responses of real FTNs did not appear in the simulations. In this symmetrical network configuration, the integrated output of the network is fed back strongly to the input layers and overwrites the time-varying input pattern.
A number of alternate models have the same performance as the soft feed-forward architecture we have used. They integrate, and also reproduce the diversity of time-varying firing rate found in brainstem neurons. For example, a network with feed-forward connections from unit to unit and a single feedback connection from one unit to the whole network works as well as the network we have used. Also, we could reproduce the model’s performance with internal weights that are random and a hierarchy of intrinsic time constants that progresses from very short for the model FTNs to longer for other neurons in the integrator. Neural responses with diverse time constants could result from a network of model neurons with uniform intrinsic time constants and self-inhibitory connections of different strengths. Thus, inhibition might also be important for generating diverse responses.
Once we discovered that the model derived from the larval zebrafish had surprising explanatory power for the data acquired in monkeys, we realized that we might have uncovered a design principle for neural integration that was common across a wide range of species. Without the data and models of Miri et al. (2011), one might have regarded the variety of response properties in brainstem neurons as a curiosity that we did not understand. With the data and models of Miri et al. (2011), noise becomes understandable as signal. Thus, system level principles that were learned in fish appear to be relevant to primates. This success in bridging across model organisms suggests a common ground for future research. The link between animals allows data-driven assumptions from one model organism to assist in interpreting results from another model organism. In this instance, application of principles derived in the larval zebrafish has led to conclusions that may be relevant to the organization of the human neural integrator, as well as those of the various model organisms used in reductionist research.
The comparison between integrator data in fish and monkeys might be improved by analyzing more similar eye movement behaviors. We have studied a 1.5 second pursuit eye movement that includes a change in eye position that is held for 400 ms at the end of the movement. Miri et al. (2011) used the spontaneous saccades and much longer, but sometimes leaky fixations of larval zebrafish. Our understanding of the existence of a single, common integrator for all horizontal eye movements (Cannon and Robinson, 1987, Cheron and Godaux, 1987) suggests that the comparison is valid in spite of differences in the eye movement behavior. Further, the proposed intrinsic constants of integrator neurons may be quite different in the two species, so that the temporal demands are actually greater for an integrator circuit in monkeys. In zebrafish, the time constant is modeled as 1 second to produce 5 seconds of neural responses, meaning that the network must extend the integration time constant 5-fold. In monkeys, the time constant of integrator neurons needs to be short (<10 ms) to allow the rapid changes in firing rate recorded at the initiation of pursuit or during saccades. We have studied integration over a time scale of 1.5 seconds, so that the integrator circuit must extend the integration time constant 150-fold. Still, it would be valuable in the future to conduct a similar analysis with long fixations in monkeys, or using brief epochs of optokinetic tracking in zebrafish (Portugues et al., 2014), or both. It also would be valuable to study the integrator circuit in mature zebrafish, to verify that the architecture revealed by (Miri et al. 2011) is a computational feature of integration rather than an intermediate stage in neural development.
Known unknowns in neural integration
The data from zebrafish have allowed us to advance towards an answer to an old question that was first posed in primate research almost 50 years ago (Robinson, 1964, Skavenski and Robinson, 1973). Our research and that of (Miri et al. 2011) together suggest a circuit architecture that accounts for a wide range of data. But important issues remain unanswered. Many details of the circuit remain under-constrained.
We still do not know whether the intrinsic properties of single neurons or the connectivity within the circuit is more important for integration. The long time constants of decay in a neural integrator could result from recurrent connections of neurons with traditional short time constants, or partly from longer intrinsic time constants of decays in the neurons. Long cellular time constants could arise from intrinsic mechanisms such as dendritic calcium dynamics (Goldman et al., 2003, Loewenstein and Sompolinsky, 2003) or through slow synaptic currents (Seung, 1996, Wang et al., 2013). We do not know how much the intrinsic properties of the neurons contribute to neural integration (Major and Tank, 2004, Fisher et al., 2013).
The properties of the neurons in the integrator might be different in fish and monkey. The model of (Miri et al. 2011) assumed that the neurons in the integrator had intrinsic time constants of about 1 second in the absence of recurrent connections. Long time constants allowed the time-varying responses of the different model neurons to show the diversity recorded in larval zebrafish (Joshua et al., 2013). In contrast, the model that was successful at reproducing the recordings from monkeys used a very short intrinsic time constant and relied on recurrent connections to perform integration. Neurons in the monkey brainstem show a tight relationship between the instantaneous activity and eye movement (Joshua and Lisberger, 2014). If intrinsic time constants were longer, then neurons in the model could not follow the dynamics of the movement reliably. We do not think this subtle difference between the most successful models detracts from the appeal of the similar computational explanations for the two sets of data in the two model organisms.
We do not know whether neurons with different functional discharge properties during eye movement also have different intrinsic cellular properties. Available evidence suggests that they may. Neurons in the brainstem of mice that receive strong input from the flocculus (i.e. FTNs) are unique in their physiology. The gain of their responses to injection of current is larger than other cells in the vestibular nucleus, and they show a stronger rebound depolarization after strong inhibitory currents (Sekirnjak et al., 2003, Shin et al., 2011). In monkeys, FTNs were the cells with the fastest dynamics during pursuit (Fig 2C). Future work might bridge the gap between cell physiology studied in vitro and neural dynamics recorded in vivo during movements, and might help to establish a unified framework based on studies in monkeys and mice.
We do not know the role of inhibition in neural integration, or whether it is the same across species. Inhibition has an important role in brainstem processing generally (Shimazu and Precht, 1966, Mettens et al., 1994, Gittis and du Lac, 2007), and has been an important feature of some models of the neural integrator (Cannon and Robinson, 1985, Boerlin et al., 2013, Lim and Goldman, 2013). In goldfish, most connections between the two sides of the brainstem seem to be inhibitory (Aksay et al., 2003), but cutting the brainstem commissures does not change the time constant of the integration (Debowy and Baker, 2011). Further, silencing the integrator circuit on one side of the brainstem does not cause a deficit in integrator function when the eye is pointing in the direction opposite to the side of silencing (Aksay et al., 2007). Thus, inhibitory networks seem to be important for coordination between the two sides of the brainstem in fish, but they do not provide positive feedback that is part of the implementation of integration (Aksay et al., 2007).
Inhibition may play a more important role in neural integration in monkeys. Unilateral lesions to the neural integrator cause centripetal eye drifts from all starting positions (Straube et al., 1991, Arnold et al., 1999) suggesting that commissural connections are important for integration in monkeys. In addition, electrical stimulation or lesions close to the midline cause leaky integration (Arnold and Robinson, 1997). Thus inhibitory commissures may be essential for neural integration (Galiana et al., 1984, Arnold and Robinson, 1997) in monkeys but not in fish. We might expect some differences in the coupling of the two sides of the brainstem in fish versus monkeys, given that monkeys have frontal eyes that operate in close coordination to align both foveae while fish have lateral eyes with little overlap of the visual fields. Differences in the role of inhibition could be a correlate of differences in the degree of frontal versus lateral eyes. As an alternative, the apparent species differences in the effects of lesions could be due to misinterpretation of the data (Arnold and Robinson, 1997, Debowy and Baker, 2011). Electrical stimulations and lesions have a low spatial resolution, and may involve larger or smaller groups of neurons than intended by experimenters. For example, midline lesions may damage more than commissural connections; they may damage a population of midline neurons that encodes vertical eye position (Nakamagoe et al., 2000).
Finally, even though we have suggested a circuit architecture that reproduces our data, the actual connections within the neural integrator are not known in either species. Modern approaches open the possibility of establishing the wiring diagram for the integrator circuit in the zebrafish. For example, this might be a situation where a “connectome” could have immediate functional significance. In addition, it may be possible to probe circuit organization more directly through combining ablation of single neurons with electrical recordings or calcium imaging of the rest of the circuit. Perhaps the circuit can be analyzed in the primate as well, through correlations of the spike timing of pairs of neurons that are identified according to their connections from the cerebellum and functional discharge in relation to eye movements.
Studying systems across animal models
Research done on simple and experimentally-convenient animal models has been very important in understanding many features of the organization and function of the nervous system. For example, the classical work on the giant axon of the squid revealed the basic ionic mechanisms of the action potential in all species (Hodgkin and Huxley, 1952). Molecular mechanisms for learning and memory were first understood in Aplysia (Kandel, 2001). Simple animal models were successful because the experiments studied neural mechanisms that are so basic that they are preserved across species. Hence, the studies were able to derive important principles that generalized to more complex animal models.
The extreme complexity of the human brain might raise concerns about a reduced utility of understanding the brains of animals that are phylogenetically distant from us. Thus, we must look deeply for homologies that allow us to use each species to answer the questions it is best suited for. For example, we can study homologous brain structures that may have unified functions across modalities even when the behavior they mediate or the sensor they use is very different. The electric fish uses cerebellum-like pathways to cancel the electric field that is generated by it’s own movement (Bell et al., 1997, Sawtell and Bell, 2008). This neural mechanism allows the fish to distinguish its own movements from the movements of others. The electric fish is an excellent system for studying how the circuit works because the computation is well understood. But the work takes on greater impact because the brain structure that cancels the self-generated signal is similar to the cerebellum of mammals.
Our approach has highlighted the advantage of bridging across model organisms using nearly identical behaviors that involve the same motor effectors. The seeming similarity of the computations that move the eyes of fish and monkeys suggest analogies that go beyond “functional similarities”. They suggest a link between species at the levels of the computation and its implementation. We realize that the neural integrator may be a special case, but we expect it is possible to find other neural computations where it will be productive to bridge from a species that is amenable to modern molecular approaches to one that is ideal for traditional behavioral systems neuroscience.
Bridging across disparate species provides a parallel approach to the challenges of deploying modern molecular approaches in non-human primates. In our research on the neural integrator, the data from zebrafish provided a hypothesis about the organization in monkeys that was based on structural data and could lead to testable predictions. Without the research on zebrafish, we would have lacked important details of the system because we cannot probe network architecture with the same precision in monkeys. With the research on the zebrafish, we were able to draw conclusions about the potential organization of neural integrators, which are important for many functions in monkeys and humans. At the same time, the relevance of the fish study is greatly enhanced by our ability to link its predictions to data from monkeys.
Highlights.
Neural integration converts transient to sustained activity in many systems
The oculomotor neural integrator reveals neural implementation of integration
Similar diversity of responses in oculomotor integrator neurons of monkeys and fish
Combination of data from diverse animal organisms is a powerful research tool
Acknowledgements
Research supported by the Howard Hughes Medical Institute, NIH grant EY017210, and the Human Frontiers Science Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adelsberger H, Zainos A, Alvarez M, Romo R, Konnerth A. Local domains of motor cortical activity revealed by fiber-optic calcium recordings in behaving nonhuman primates. Proc Natl Acad Sci U S A. 2014;111:463–468. doi: 10.1073/pnas.1321612111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksay E, Baker R, Seung HS, Tank DW. Anatomy and discharge properties of pre-motor neurons in the goldfish medulla that have eye-position signals during fixations. J Neurophysiol. 2000;84:1035–1049. doi: 10.1152/jn.2000.84.2.1035. [DOI] [PubMed] [Google Scholar]
- Aksay E, Baker R, Seung HS, Tank DW. Correlated discharge among cell pairs within the oculomotor horizontal velocity-to-position integrator. J Neurosci. 2003;23:10852–10858. doi: 10.1523/JNEUROSCI.23-34-10852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci. 2001;4:184–193. doi: 10.1038/84023. [DOI] [PubMed] [Google Scholar]
- Aksay E, Olasagasti I, Mensh BD, Baker R, Goldman MS, Tank DW. Functional dissection of circuitry in a neural integrator. Nat Neurosci. 2007;10:494–504. doi: 10.1038/nn1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DB, Robinson DA. The oculomotor integrator: testing of a neural network model. Exp Brain Res. 1997;113:57–74. doi: 10.1007/BF02454142. [DOI] [PubMed] [Google Scholar]
- Arnold DB, Robinson DA, Leigh RJ. Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkey. Vision Res. 1999;39:4286–4295. doi: 10.1016/s0042-6989(99)00142-x. [DOI] [PubMed] [Google Scholar]
- Baker R, Berthoz A. Is the prepositus hypoglossi nucleus the source of another vestibulo-ocular pathway? Brain Res. 1975;86:121–127. doi: 10.1016/0006-8993(75)90643-5. [DOI] [PubMed] [Google Scholar]
- Beck JC, Gilland E, Tank DW, Baker R. Quantifying the ontogeny of optokinetic and vestibuloocular behaviors in zebrafish, medaka, and goldfish. J Neurophysiol. 2004;92:3546–3561. doi: 10.1152/jn.00311.2004. [DOI] [PubMed] [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- Boerlin M, Machens CK, Deneve S. Predictive coding of dynamical variables in balanced spiking networks. PLoS Comput Biol. 2013;9:e1003258. doi: 10.1371/journal.pcbi.1003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Brunton BW, Botvinick MM, Brody CD. Rats and humans can optimally accumulate evidence for decision-making. Science. 2013;340:95–98. doi: 10.1126/science.1233912. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Robinson DA. An improved neural-network model for the neural integrator of the oculomotor system: more realistic neuron behavior. Biol Cybern. 1985;53:93–108. doi: 10.1007/BF00337026. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Robinson DA. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J Neurophysiol. 1987;57:1383–1409. doi: 10.1152/jn.1987.57.5.1383. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Robinson DA, Shamma S. A proposed neural network for the integrator of the oculomotor system. Biol Cybern. 1983;49:127–136. doi: 10.1007/BF00320393. [DOI] [PubMed] [Google Scholar]
- Carandini M. From circuits to behavior: a bridge too far? Nat Neurosci. 2012;15:507–509. doi: 10.1038/nn.3043. [DOI] [PubMed] [Google Scholar]
- Cheron G, Godaux E. Disabling of the oculomotor neural integrator by kainic acid injections in the prepositus-vestibular complex of the cat. J Physiol. 1987;394:267–290. doi: 10.1113/jphysiol.1987.sp016870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Henn V. Unit activity in the pontine reticular formation associated with eye movements. Brain Res. 1972;46:403–410. doi: 10.1016/0006-8993(72)90030-3. [DOI] [PubMed] [Google Scholar]
- Cohen B, Komatsuzaki A. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp Neurol. 1972;36:101–117. doi: 10.1016/0014-4886(72)90139-2. [DOI] [PubMed] [Google Scholar]
- Davis-Lopez de Carrizosa MA, Morado-Diaz CJ, Miller JM, de la Cruz RR, Pastor AM. Dual encoding of muscle tension and eye position by abducens motoneurons. J Neurosci. 2011;31:2271–2279. doi: 10.1523/JNEUROSCI.5416-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debowy O, Baker R. Encoding of eye position in the goldfish horizontal oculomotor neural integrator. J Neurophysiol. 2011;105:896–909. doi: 10.1152/jn.00313.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Garcia JM, Vidal PP, Gomez C, Berthoz A. A neurophysiological study of prepositus hypoglossi neurons projecting to oculomotor and preoculomotor nuclei in the alert cat. Neuroscience. 1989;29:291–307. doi: 10.1016/0306-4522(89)90058-4. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Nicola GN. The development of eye movements in the zebrafish (Danio rerio) Developmental psychobiology. 1997;31:267–276. doi: 10.1002/(sici)1098-2302(199712)31:4<267::aid-dev4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Escudero M, de la Cruz RR, Delgado-Garcia JM. A physiological study of vestibular and prepositus hypoglossi neurones projecting to the abducens nucleus in the alert cat. J Physiol. 1992;458:539–560. doi: 10.1113/jphysiol.1992.sp019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971;34:661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Fisher D, Olasagasti I, Tank DW, Aksay ER, Goldman MS. A modeling framework for deriving the structural and functional architecture of a short-term memory microcircuit. Neuron. 2013;79:987–1000. doi: 10.1016/j.neuron.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Luschei ES. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol. 1970;33:382–392. doi: 10.1152/jn.1970.33.3.382. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Scudder CA, Kaneko CR. Discharge patterns and recruitment order of identified motoneurons and internuclear neurons in the monkey abducens nucleus. J Neurophysiol. 1988;60:1874–1895. doi: 10.1152/jn.1988.60.6.1874. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Kaneko CR, Fuchs AF. The neuronal substrate of integration in the oculomotor system. Prog Neurobiol. 1992;39:609–639. doi: 10.1016/0301-0082(92)90016-8. [DOI] [PubMed] [Google Scholar]
- Galiana HL, Flohr H, Jones GM. A reevaluation of intervestibular nuclear coupling: its role in vestibular compensation. J Neurophysiol. 1984;51:242–259. doi: 10.1152/jn.1984.51.2.242. [DOI] [PubMed] [Google Scholar]
- Galiana HL, Outerbridge JS. A bilateral model for central neural pathways in vestibuloocular reflex. J Neurophysiol. 1984;51:210–241. doi: 10.1152/jn.1984.51.2.210. [DOI] [PubMed] [Google Scholar]
- Garbutt S, Matlin A, Hellmuth J, Schenk AK, Johnson JK, Rosen H, Dean D, Kramer J, Neuhaus J, Miller BL, Lisberger SG, Boxer AL. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain. 2008;131:1268–1281. doi: 10.1093/brain/awn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Firing properties of GABAergic versus non-GABAergic vestibular nucleus neurons conferred by a differential balance of potassium currents. J Neurophysiol. 2007;97:3986–3996. doi: 10.1152/jn.00141.2007. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Memory without feedback in a neural network. Neuron. 2009;61:621–634. doi: 10.1016/j.neuron.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MS, Levine JH, Major G, Tank DW, Seung HS. Robust persistent neural activity in a model integrator with multiple hysteretic dendrites per neuron. Cereb Cortex. 2003;13:1185–1195. doi: 10.1093/cercor/bhg095. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, MacAvoy MG, Bruce CJ. Neural responses related to smooth-pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J Neurophysiol. 1994;72:1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl SS, Chaisanguanthum KS, Lisberger SG. Sensory population decoding for visually-guided movement. Neuron. 2013 doi: 10.1016/j.neuron.2013.05.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, Lindbloom-Brown Z, Horwitz GD. Saccadic eye movements evoked by optogenetic activation of primate V1. Nat Neurosci. 2012;15:1368–1370. doi: 10.1038/nn.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of general psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- Joshua M, Lisberger SG. A framework for using signal, noise, and variation to determine whether the brain controls movement synergies or single muscles. J Neurophysiol. 2014 doi: 10.1152/jn.00510.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Medina JF, Lisberger SG. Diversity of Neural Responses in the Brainstem during Smooth Pursuit Eye Movements Constrains the Circuit Mechanisms of Neural Integration. J Neurosci. 2013;33:6633–6647. doi: 10.1523/JNEUROSCI.3732-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Bioscience reports. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Keller EL, Robinson DA. Absence of a stretch reflex in extraocular muscles of the monkey. J Neurophysiol. 1971;34:908–919. doi: 10.1152/jn.1971.34.5.908. [DOI] [PubMed] [Google Scholar]
- Keller EL, Robinson DA. Abducens unit behavior in the monkey during vergence movements. Vision Res. 1972;12:369–382. doi: 10.1016/0042-6989(72)90082-x. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of general psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Koulakov AA, Raghavachari S, Kepecs A, Lisman JE. Model for a robust neural integrator. Nat Neurosci. 2002;5:775–782. doi: 10.1038/nn893. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. Simple spike responses of gaze velocity Purkinje cells in the floccular lobe of the monkey during the onset and offset of pursuit eye movements. J Neurophysiol. 1994;72:2045–2050. doi: 10.1152/jn.1994.72.4.2045. [DOI] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron. 2008;58:451–463. doi: 10.1016/j.neuron.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Goldman MS. Balanced cortical microcircuitry for maintaining information in working memory. Nat Neurosci. 2013;16:1306–1314. doi: 10.1038/nn.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Fuchs AF. Response of flocculus Purkinje cells to adequate vestibular stimulation in the alert monkey: fixation vs. compensatory eye movements. Brain Res. 1974;69:347–353. doi: 10.1016/0006-8993(74)90013-4. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA. Brain stem neurons in modified pathways for motor learning in the primate vestibulo-ocular reflex. Science. 1988;242:771–773. doi: 10.1126/science.3142040. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Broussard DM. Neural basis for motor learning in the vestibuloocular reflex of primates. I. Changes in the responses of brain stem neurons. J Neurophysiol. 1994a;72:928–953. doi: 10.1152/jn.1994.72.2.928. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Broussard DM. Responses during eye movements of brain stem neurons that receive monosynaptic inhibition from the flocculus and ventral paraflocculus in monkeys. J Neurophysiol. 1994b;72:909–927. doi: 10.1152/jn.1994.72.2.909. [DOI] [PubMed] [Google Scholar]
- Loewenstein Y, Sompolinsky H. Temporal integration by calcium dynamics in a model neuron. Nat Neurosci. 2003;6:961–967. doi: 10.1038/nn1109. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Fuchs AF. Activity of brain stem neurons during eye movements of alert monkeys. J Neurophysiol. 1972;35:445–461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol. 2004;14:675–684. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Marr D. Vision : a computational investigation into the human representation and processing of visual information. San Francisco: W.H. Freeman; 1982. [Google Scholar]
- McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. J Neurophysiol. 1992;68:319–332. doi: 10.1152/jn.1992.68.1.319. [DOI] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth-pursuit eye movements. J Neurosci. 2007;27:6832–6842. doi: 10.1523/JNEUROSCI.1323-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettens P, Godaux E, Cheron G, Galiana HL. Effect of muscimol microinjections into the prepositus hypoglossi and the medial vestibular nuclei on cat eye movements. J Neurophysiol. 1994;72:785–802. doi: 10.1152/jn.1994.72.2.785. [DOI] [PubMed] [Google Scholar]
- Miller JM, Bockisch CJ, Pavlovski DS. Missing lateral rectus force and absence of medial rectus co-contraction in ocular convergence. J Neurophysiol. 2002;87:2421–2433. doi: 10.1152/jn.00566.2001. [DOI] [PubMed] [Google Scholar]
- Miri A, Daie K, Arrenberg AB, Baier H, Aksay E, Tank DW. Spatial gradients and multidimensional dynamics in a neural integrator circuit. Nat Neurosci. 2011;14:1150–1159. doi: 10.1038/nn.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamagoe K, Iwamoto Y, Yoshida K. Evidence for brainstem structures participating in oculomotor integration. Science. 2000;288:857–859. doi: 10.1126/science.288.5467.857. [DOI] [PubMed] [Google Scholar]
- Ono S, Mustari MJ. Smooth pursuit-related information processing in frontal eye field neurons that project to the NRTP. Cereb Cortex. 2009;19:1186–1197. doi: 10.1093/cercor/bhn166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor AM, De la Cruz RR, Baker R. Eye position and eye velocity integrators reside in separate brainstem nuclei. Proc Natl Acad Sci U S A. 1994;91:807–811. doi: 10.1073/pnas.91.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Portugues R, Feierstein CE, Engert F, Orger MB. Whole-brain activity maps reveal stereotyped, distributed networks for visuomotor behavior. Neuron. 2014;81:1328–1343. doi: 10.1016/j.neuron.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Lisberger SG. Transformation of vestibular signals into motor commands in the vestibuloocular reflex pathways of monkeys. J Neurophysiol. 2006;96:1061–1074. doi: 10.1152/jn.00281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Lisberger SG. Neural substrate of modified and unmodified pathways for learning in monkey vestibuloocular reflex. J Neurophysiol. 2008;100:1868–1878. doi: 10.1152/jn.90498.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold H, Churchland A, Selig Y, Jasmin L, Lisberger SG. Partial ablations of the flocculus and ventral paraflocculus in monkeys cause linked deficits in smooth pursuit eye movements and adaptive modification of the VOR. J Neurophysiol. 2002;87:912–924. doi: 10.1152/jn.00768.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. The Mechanics of Human Saccadic Eye Movement. J Physiol. 1964;174:245–264. doi: 10.1113/jphysiol.1964.sp007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor unit behavior in the monkey. J Neurophysiol. 1970;33:393–403. doi: 10.1152/jn.1970.33.3.393. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Robinson DA. The use of control systems analysis in the neurophysiology of eye movements. Annu Rev Neurosci. 1981;4:463–503. doi: 10.1146/annurev.ne.04.030181.002335. [DOI] [PubMed] [Google Scholar]
- Robinson DA, Keller EL. The behavior of eye movement motoneurons in the alert monkey. Bibl Ophthalmol. 1972;82:7–16. [PubMed] [Google Scholar]
- Rothschild G, Nelken I, Mizrahi A. Functional organization and population dynamics in the mouse primary auditory cortex. Nat Neurosci. 2010;13:353–360. doi: 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kanda K, Kawasaki T. Target neurons of floccular middle zone inhibition in medial vestibular nucleus. Brain Res. 1988;446:225–235. doi: 10.1016/0006-8993(88)90881-5. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Bell CC. Adaptive processing in electrosensory systems: links to cerebellar plasticity and learning. J Physiol Paris. 2008;102:223–232. doi: 10.1016/j.jphysparis.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol. 1980;44:1175–1189. doi: 10.1152/jn.1980.44.6.1175. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Gao Z, Boele HJ, Veloz MF, Amerika WE, Simek AA, De Jeu MT, Steinberg JP, Takamiya K, Hoebeek FE, Linden DJ, Huganir RL, De Zeeuw CI. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70:43–50. doi: 10.1016/j.neuron.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppik D, Nagel KI, Lisberger SG. Cortical mechanisms of smooth eye movements revealed by dynamic covariations of neural and behavioral responses. Neuron. 2008;58:248–260. doi: 10.1016/j.neuron.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol. 1992;68:244–264. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- Segraves MA. Activity of monkey frontal eye field neurons projecting to oculomotor regions of the pons. J Neurophysiol. 1992;68:1967–1985. doi: 10.1152/jn.1992.68.6.1967. [DOI] [PubMed] [Google Scholar]
- Segraves MA, Goldberg ME. Functional properties of corticotectal neurons in the monkey's frontal eye field. J Neurophysiol. 1987;58:1387–1419. doi: 10.1152/jn.1987.58.6.1387. [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, Vissel B, Bollinger J, Faulstich M, du Lac S. Purkinje cell synapses target physiologically unique brainstem neurons. J Neurosci. 2003;23:6392–6398. doi: 10.1523/JNEUROSCI.23-15-06392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung HS. How the brain keeps the eyes still. Proc Nat Acad Sci. 1996;93:13339–13344. doi: 10.1073/pnas.93.23.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung HS, Lee DD, Reis BY, Tank DW. The autapse: a simple illustration of short-term analog memory storage by tuned synaptic feedback. J Comput Neurosci. 2000;9:171–185. doi: 10.1023/a:1008971908649. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Shidara M, Kawano K, Gomi H, Kawato M. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature. 1993;365:50–52. doi: 10.1038/365050a0. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Precht W. Inhibition of central vestibular neurons from the contralateral labyrinth and its mediating pathway. J Neurophysiol. 1966;29:467–492. doi: 10.1152/jn.1966.29.3.467. [DOI] [PubMed] [Google Scholar]
- Shin M, Moghadam SH, Sekirnjak C, Bagnall MW, Kolkman KE, Jacobs R, Faulstich M, du Lac S. Multiple types of cerebellar target neurons and their circuitry in the vestibulo-ocular reflex. J Neurosci. 2011;31:10776–10786. doi: 10.1523/JNEUROSCI.0768-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skavenski AA, Robinson DA. Role of abducens neurons in vestibuloocular reflex. J Neurophysiol. 1973;36:724–738. doi: 10.1152/jn.1973.36.4.724. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci. 2002;3:952–964. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol. 1990;63:1241–1261. doi: 10.1152/jn.1990.63.5.1241. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube A, Kurzan R, Buttner U. Differential effects of bicuculline and muscimol microinjections into the vestibular nuclei on simian eye movements. Exp Brain Res. 1991;86:347–358. doi: 10.1007/BF00228958. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J Neurophysiol. 1999;82:2612–2632. doi: 10.1152/jn.1999.82.5.2612. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. I. Basic response properties to retinal image motion and position. J Neurophysiol. 2002;87:2684–2699. doi: 10.1152/jn.2002.87.6.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson RD, Robinson DA. Signals in vestibular nucleus mediating vertical eye movements in the monkey. J Neurophysiol. 1984;51:1121–1136. doi: 10.1152/jn.1984.51.6.1121. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Superior colliculus cell responses related to eye movements in awake monkeys. Science. 1971;171:82–84. doi: 10.1126/science.171.3966.82. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. IV. Effects of lesions on eye movements. J Neurophysiol. 1972a;35:587–596. doi: 10.1152/jn.1972.35.4.587. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. The primate superior colliculus and the shift of visual attention. Investigative ophthalmology. 1972b;11:441–450. [PubMed] [Google Scholar]
- Zenon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–437. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Partsalis AM, Highstein SM. Properties of superior vestibular nucleus flocculus target neurons in the squirrel monkey. I. General properties in comparison with flocculus projecting neurons. J Neurophysiol. 1995;73:2261–2278. doi: 10.1152/jn.1995.73.6.2261. [DOI] [PubMed] [Google Scholar]