Abstract
Background
Recent studies demonstrate that chronic pelvic pain is associated with altered afferent sensory input resulting in maladaptive changes in the neural circuitry of pain. To better understand the central changes associated with chronic pelvic pain we investigated the contributions of critical pain-related neural circuits using single-voxel proton magnetic resonance spectroscopy (MRS) and transcranial direct current stimulation (tDCS).
Methods
We measured concentrations of neural metabolites in 4 regions of interest (thalamus, anterior cingulate cortex, primary motor, and occipital cortex [control]) at baseline and after 10 days of active or sham tDCS in patients with chronic pelvic pain.. We then compared our results to those observed in healthy controls, matched by age and gender.
Results
We observed a significant increase in pain thresholds after active tDCS compared to sham conditions. There was a correlation between metabolite concentrations at baseline and quantitative sensory assessments. Chronic pelvic pain patients had significantly lower levels of NAA/Cr in the primary motor cortex compared to healthy patients.
Conclusions
tDCS increases pain thresholds in patients with chronic pelvic pain. Biochemical changes in pain-related neural circuits are associated with pain levels as measured by objective pain testing. These findings support the further investigation of targeted cortical neuromodulatory interventions for chronic pelvic pain.
Keywords: chronic pelvic pain, brain stimulation, transcranial direct current stimulation, tDCS, magnetic resonance spectroscopy
Introduction
Chronic pelvic pain can have detrimental impacts on daily life activities and general well being, often leading to symptoms such as anxiety and depression [1, 2]. This condition may result from a variety of etiologies, including endometriosis, cystitis, and prostatitis. Chronic pelvic pain is often refractory to conventional treatments and therefore, there is a great need for novel approaches to treating it.
The phenomenon known as central sensitization plays a significant role in the development of chronic pelvic pain [3]. While the heightened sensitivity of peripheral pain receptors following local trauma or infection usually resolves with time, in chronic pain this hypersensitivity is sustained and amplified by an extensive central neural network that includes the spinal dorsal horn, limbic system, and cortical structures, referred to as central sensitization. The limbic and sensory systems essentially mediate a cycle of hyper-vigilance for sensory stimuli from pelvic organs, leading to the chronic pain state [4]. In this context, targeted techniques that can modulate central nervous system activity may be useful for pain control [5, 6].
One proposed technique for modulating central nervous system activity in chronic pain states is non-invasive brain stimulation. Transcranial direct current stimulation (tDCS), a specific form of non-invasive brain stimulation, has been shown to improve chronic pain relating to spinal cord injury [7], fibromyalgia [8], and pelvic pain [5, 9]. Although these initial results are encouraging, the precise mechanisms by which tDCS influences chronic pain remain unclear. The leading hypothesis is that modulation of activity in the primary motor cortex using tDCS leads to secondary modulation of neural regions related to pain such as thalamic nuclei [8] [7].
In the present study, we aimed to investigate the contributions of critical pain-related neural circuits to chronic pelvic pain using magnetic resonance spectroscopy and tDCS. We assessed the roles of the thalamus, anterior cingulate cortex, (ACC) and primary motor cortex (M1) (due its primary effects as a modulator of pain) [6] [7]. In patients with chronic pelvic pain, we measured concentrations of neural metabolites in these regions of interest both at baseline and after 10 days of active or sham tDCS. We then compared our results to those observed in healthy controls, matched by age and gender.
Methods
Participants
The experimental group in the study included women and men with chronic pelvic pain who were enrolled from June 2010 through September 2013 at the Spaulding Rehabilitation Hospital (Boston, MA).. Participants were recruited based on referrals from attending physicians or responses to advertisements. Participant inclusion criteria included age 18–64y and having symptoms of pelvic pain for more than 6 months with an average of at least 3 on a 0–10 VAS scale. Participants were excluded if they had a history of any of the following: genitourinary disease (oncologic or infectious); neurogenic bladder dysfunction; alcohol or drug abuse within the past 6 months as self reported; carbamazepine use; severe depression (with a score of >30 in the Beck Depression Inventory); neurological disorders; unexplained fainting spells as self reported, head injury resulting in more than a momentary loss of consciousness. Participants were also excluded if they were currently pregnant or ineligible for MRI or tDCS. Healthy participants serving as controls had the same inclusion/exclusion criteria described above except that they must also not have had symptoms of chronic pain in the previous 6 months.
The local institutional review board of Spaulding Rehabilitation Hospital approved this protocol. Subjects provided written informed consent to participate in this study.
Procedures
Chronic pelvic pain subjects were randomized to receive active or sham tDCS treatment through a randomization approach using random blocks of 4. Patients received a total of 10 consecutive sessions of either active or sham tDCS over a 2-week period (weekdays only). During each session, the anode electrode was placed on the scalp overlying the primary motor cortex (contralateral to the more painful side or the side where the symptoms began) and the cathode was placed over the contralateral supra-orbital area (electrodes were each 35 cm2 in size). This electrode montage has been used with good results in prior tDCS trials for treating chronic pain [7, 8]. The primary motor cortex was localized using the 10/20 EEG system (C3 or C4), which has shown to be a reliable method of localization for this technique of tDCS [7]. For the active tDCS condition, 2 mA of transcranial direct current stimulation was applied for 20 minutes. For the sham tDCS condition, the same montage was applied, but the current was applied for only the first 30 seconds of the 20-minute session. Studies have shown that less than 3 minutes of tDCS has no effects on cortical excitability (Nitsche et al., 2000) and that the above method of sham stimulation is a reliable method of blinding [10]. In the present study, we compared data from the chronic pelvic pain patients with data from healthy controls, who were matched by age and gender and were reported on in more detail elsewhere [11]. Our comparisons included MRS and quantitative sensory assessment measured at baseline.
Assessments
Magnetic Resonance Spectroscopy (MRS)
Subjects with chronic pelvic pain underwent 2 MRS exams. The first exam took place on a visit prior to the first tDCS stimulation session and the second exam took place after the final tDCS stimulation session. Solvent suppressed proton spectra were obtained using the PRESS sequence as implemented in the GE PROBE-P application. The version of PRESS that we employ has very selective spatial (VSS) saturation bands that are automatically placed around the voxel that is selected graphically from a reference image. This feature minimizes artifacts and distortions arising from spectral contributions from regions outside of the voxel selected. Solvent suppression is achieved using the 3 chemical shift selective (CHESS) pulse shown above. The automated optimization of solvent suppression varied the amplitude of the third CHESS pulse until optimal suppression was achieved. The sequence parameters include the following: spectral bandwidth 5000 Hz; TR 2000 ms; TE 35 ms; 2048 complex points; 8 step phase cycle; and 128 acquisitions. We selected voxels of interest of 20×20×20 mm in the primary motor cortex (corresponding to the area of stimulation), thalamic nuclei [12] [13] [14], anterior cingulate cortex (Brodmann’s area 24, which has been shown in studies to be involved in chronic pain) [15], and mid-occipital cortex (to serve as a control area). Outer voxel suppression pulses were employed and the PRESS encoding order employed selection along the S/I direction first to minimize contamination from tissue near the bones and air spaces beneath the temporal lobes. The spectral acquisition time was 4 minutes and 20 seconds for each voxel. Including time for setup, the acquisition of 4 voxels required approximately 20 minutes.
We analyzed metabolite concentration using LC-Model (Stephen Provencher Inc., Oakville, Ontario, Canada). Levels of N-acetylaspartate (NAA), N-acetylaspartateglutamate (NAAG), NAA+NAAG (total NAA), glutamate (Glu), glutamine (Gln), myo-inositol (MI), creatinephosphocreatine (Cr), and choline were analyzed by fitting a linear combination of a basis set of metabolite model spectra to the data (Figure 1). The analyzing spectrum was set from 3.8ppm down to 0.2ppm with no eddy-current correction and water scaling. We expressed metabolite concentrations as mM and ratios relative to Cr peak. We determined metabolite concentrations and metabolite-to-creatine (Cr) ratios in these three spectra for each subject. The same methodology was used for the healthy subjects. Additionally, for both patients and controls, MRS measurements were performed using the same equipment, technician, radiologist, and analytical techniques.
Figure 1.
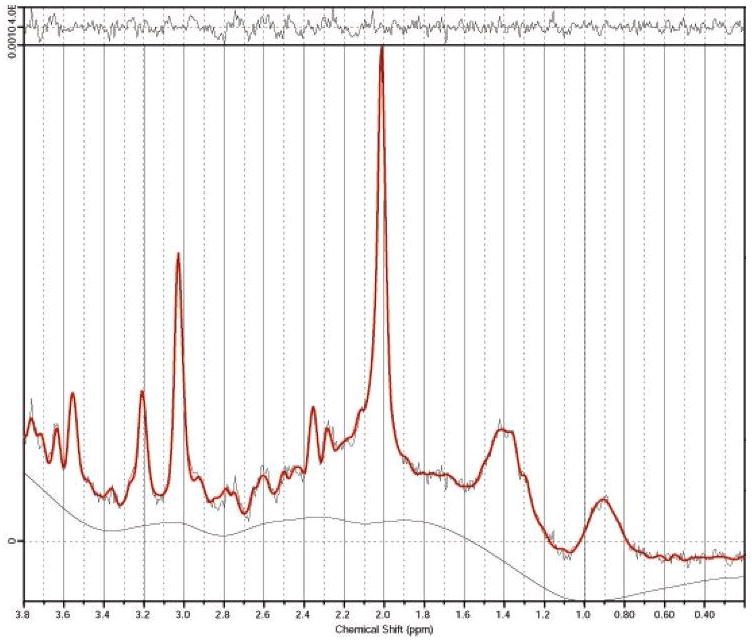
MRS spectra from the primary motor cortex of a pelvic pain patient. Chemical shifts are indicated in parts per million (ppm).
Sensory and Behavioral Assessments
We recorded demographic and clinical characteristics at baseline, including the number of days of pelvic pain experienced prior to the intervention. Pelvic pain intensity was measured at baseline and after intervention using the Visual Analogue Scale (VAS). General sensory perception thresholds and general pain thresholds were measured using von Frey monofilament assessment and algometric pressure-pain assessment on the thenar region of the hand and T12-L1 dermatome of the abdomen (Pressure Pain Threshold (PPT)). We also conducted the PPT during cold-water immersion to evaluate the degree to which pain perception is modulated by diffuse noxious inhibitory control (DNIC). We measured the subjects’ quality of life using a Quality of Life Questionnaire (MSQ, Version 2.1), clinician global impression (CGI), and patient global assessment (PGA). To monitor for possible changes in mood and cognition, we administered the Beck Depression Inventory (BDI) and Mini-mental status exam (MMSE) at baseline and after intervention. Finally, adverse effects were monitored with a Side Effects Questionnaire and success of blinding was assessed with a Blinding Questionnaire after the first day of stimulation for each subject.
Data Analysis
Statistical analyses were conducted using STATA statistical software (version 12.0). Due to the small sample size, we chose to use non-parametric tests. We used Mann-Whitney tests in which the dependent variable was behavioral or MRS findings and the independent variable was the condition of stimulation (active tDCS, sham tDCS). We also computed Spearman correlation coefficients to assess the relationship between neural metabolite concentrations and pain outcomes. These secondary analyses were considered exploratory and we did not apply Bonferroni adjustments (significance level set at p < 0.05).
Results
Of 15 subjects initially enrolled, 4 patients dropped out after the first visit and before MRS assessment. Eleven subjects completed the baseline assessment, including MRS assessment (mean age of 35.18 ± 11.13 years, mean ± SD, 9 females). Two more subjects dropped out after baseline assessment and before stimulation intervention. All 9 subjects who continued in the study completed all treatments and assessments (mean age of 35.56 ± 12.41 years, mean ± SD, 8 females). Five patients received active tDCS and 4 patients received sham tDCS. Subjects tolerated treatment well and there were no serious adverse effects. The most common adverse effect was tingling. Adverse effects are reported in Table 1. This frequency of mild adverse effects is similar to that reported in previous studies [16].
Table 1.
Adverse effects.
| ADVERSE EFFECT | ACTIVE | SHAM |
|---|---|---|
| FREQUENCY | FREQUENCY | |
| Headache | 1/5 (20%) | 1/4 (25%) |
| Neck Pain | 0 | 1/4 (25%) |
| Scalp Pain | 1/5 (20%) | 0 |
| Scalp Burning Sensation | 2/5 (20%) | 0 |
| Scalp Tingling | 5/5 (100%) | 3/4 (75%) |
| Skin Redness | 4/5 (80%) | 2/4 (50%) |
| Sleepiness | 3/5 (60%) | 1/4 (25%) |
| Trouble concentrating | 1/5 (20%) | 0 |
| Acute mood change | 1/5 (20%) | 0 |
| Nausea | 1/5 (20%) | 0 |
| Dizziness | 0 | 2/4 (50%) |
| Itching | 1/5 (20%) | 0 |
| Pelvic Tingling | 1/5 (20%) | 0 |
Our analysis of the von Frey sensory and pain assessments at baseline and after intervention revealed significant differences between the active and sham tDCS groups. After 10-day treatment with tDCS, the active tDCS group had a larger increase in sensory thresholds (p = 0.028) and pain thresholds (p = 0.026) compared to the sham tDCS group. No significant differences were observed between active and sham tDCS groups when analyzing data from the other pain assessments (VAS pain and pressure-pain threshold, p > 0.05) and the behavioral assessments (BDI, anxiety and quality of life scores, p > 0.05).
With respect to the MRS results, we observed a number of significant findings in the thalamus, anterior cingulate cortex, and primary motor cortex (specific p value and rho reported in Table 2 and 3).
Table 2.
Correlation of Pain Threshold with Metabolite Concentration by Region of Interest in Chronic Pelvic Pain patients and Healthy subjects
| Pain Threshold | Pelvic Pain Patients | Healthy Subject | |||
|---|---|---|---|---|---|
| ACC | Thal | M1 | |||
| Naa/Cr | Gln/Cr | Glu/Cr | Gln/GluCr | (Gln/Glu)/Cr | |
| VonP-Th-R | X | X | x | rho = 0.7699; Prob > |t| = 0.0152 | rho = 0.4830; Prob > |t| = 0.0310 |
| VonP-Th-L | X | X | x | x | rho = 0.4573; Prob > |t| = 0.0426 |
| VonP-T12-R | rho = 0.7782; Prob > |t| = 0.0135 | rho = 0.8034; Prob > |t| = 0.0091 | x | X | rho = 0.7850; Prob > |t| < 0.0001 |
| VonP-T12-L | X | X | x | x | rho = 0.5836; Prob > |t| = 0.0069 |
| PPT-Th-R | x | rho = 0.8167; Prob > |t| = 0.0072 | x | rho = 0.7333; Prob > |t| = 0.0246 | X |
| PPT-Th-L | X | rho = 0.7833; Prob > |t| = 0.0125 | x | rho = 0.8000; Prob > |t| = 0.0096 | X |
| PPT-T12-R | X | X | rho = −0.7167; Prob > |t| = 0.0298 | rho = 0.7333; Prob > |t| = 0.0246 | X |
| PPT-T12-L | X | X | rho = −0.7333; Prob > |t| = 0.0246 | rho = 0.6833; Prob > |t| = 0.0424 | X |
| DNIC-Th-R | rho = 0.9000; Prob > |t| = 0.0009 | rho = 0.7333; Prob > |t| = 0.0246 | x | X | X |
| DNIC-Th-L | rho = 0.7333; Prob > |t| = 0.0246 | rho = 0.8167; Prob > |t| = 0.0072 | x | rho = 0.7167; Prob > |t| = 0.0298 | X |
| DNIC-T12-R | X | X | rho = −0.7333; Prob > |t| = 0.0246 | rho = 0.6833; Prob > |t| = 0.0424 | x |
| DNIC-T12-L | x | x | rho = −0.7000; Prob > |t| = 0.0358 | rho = 0.6667; Prob > |t| = 0.0499 | X |
Note: von Frey monofilaments pain threshold (VonP); Pressure-pain thresholds (PPT); Pressure-pain thresholds during Cold Water Immersion (DNIC); Thenar region (Th); T12-L1 dermatome of the abdomen (T12); Right side (R); Left side (L).
Table 3.
Correlation of baseline pain level with MRS.
| Thal | M1 | |||
|---|---|---|---|---|
| Mi/Cr | Gln/Cr | Mi/Cr | (Gln/Glu)/Cr | |
| Pain Level | rho = 0.7534; Prob > |t| = 0.0074 | rho = −0.6256; Prob > |t| = 0.0395 | rho = 0.7489; Prob > |t| = 0.0080 | rho = −0.6438; Prob > |t| = 0.0325 |
In the thalamus, there was a significant positive correlation between baseline Gln/Cr concentrations and pain thresholds, as indexed by von Frey in the abdominal area and pressure-pain thresholds in the thenar region (p < 0.05 for all the analyses). There was also a significant positive correlation between baseline (Gln/Glu)/Cr and pain thresholds, as indexed by von Frey and pressure-pain thresholds in the thenar and abdominal regions (p < 0.05 for all the analyses).
Furthermore, there was a significant positive correlation between baseline MI/Cr and pelvic pain levels (p < 0.05). A significant negative correlation was observed between baseline Glu/Cr concentrations and pain thresholds, as indexed by pressure-pain thresholds in the abdominal area (p < 0.05).
In the anterior cingulate cortex, there was a significant positive correlation between baseline NAA/Cr concentration and pain thresholds, as indexed by von Frey and DNIC in the abdominal thenar region (p < 0.05 for all the analyses).
In the primary motor cortex, there was a significant positive correlation between baseline MI/Cr concentrations and pain VAS levels and a negative correlation between Gln/Cr and pain levels (p < 0.05 for both analyses). Additionally, the pain levels were negatively correlated with (Gln/Glu)/Cr level (p < 0.05).
Finally, we compared MRS data from the chronic pelvic pain subjects to MRS data from the healthy controls that were matched by age and gender. We found that chronic pelvic pain patients had significantly lower levels of NAA/Cr in the primary motor cortex compared to healthy patients. Interestingly, a significant positive correlation between baseline (Gln/Glu)/Cr concentrations in the primary motor cortex and baseline pain threshold levels measured by Von Frey filament was observed in the healthy control group.
The occipital area was analyzed as a control region. In comparison to the significant findings from Thal, M1 and ACC from other areas, in the occipital area we did not observe any significant changes in metabolite concentrations (p > 0.05).
Discussion
In the present study we investigated whether active tDCS in subjects with chronic pelvic pain can significantly decrease pelvic pain (or increase general pain thresholds) when compared to sham conditions. While we did not observe significant changes in reported pelvic pain VAS levels following active tDCS, we did find significant increases in sensory thresholds and pain thresholds (as indexed by von Frey) following a 2-week course of active tDCS compared to sham tDCS.
Furthermore, we aimed to evaluate the contributions of three critical brain regions thought to be involved in mediating the effects of tDCS on chronic pain, the thalamus, anterior cingulate cortex (ACC), and primary motor cortex (M1). We found that pain thresholds (as indexed by a variety of measures) positively correlated with Gln/Cr and (Gln/Glu)/Cr in the thalamus and NAA/Cr in the ACC. Pain thresholds negatively correlated with Glu/Cr in the thalamus. Pelvic pain levels correlated positively with MI/Cr in the thalamus and M1. When comparing chronic pelvic pain and healthy subjects, we found that chronic pelvic pain subjects had significantly lower concentrations of NAA/Cr in M1 compared to healthy subjects. As expected, results from the mid-occipital cortex (our control region) were not significant.
Understanding biochemical changes in chronic pelvic pain
Since Grachev and Pattany first described changes in brain metabolites of patients with chronic back pain and neuropathic pain following spinal cord injury, there has been a great increase in accessibility to and development of MRS, which has led to a rapid expansion of studies employing MRS and a better understanding of the pathophysiology of chronic pain [17] [13]. Below we will relate findings from the present study to those observed in prior studies.
Glutamate and Glutamine
In the present study, we observed that pain thresholds positively correlated with Gln/Cr and (Gln/Glu)/Cr in the thalamus and negatively correlated with Glu/Cr in the thalamus. We found a negative correlation between pain levels and the level of Gln/Cr and (Gln/Glu)/Cr in M1
It is thought that pain stimulation results in an increase in glutamate activity in brain areas associated with pain processing. Studies involving fibromyalgia patients support this hypothesis, demonstrating higher concentrations of Glu or Glx (Glu+Gln) in areas such as left thalamus, right amygdala, posterior gyrus, VLPFC, posterior cingulate cortex, and right posterior insular cortex [18] [12] [19] [20] [21]. Furthermore, it has been shown that painful stimuli lead to increases in Glu or Glx (Glu+Gln) concentration in the anterior insular area and ACC [22, 23].
In the present study, we observed negative correlations between pain thresholds and Glu levels in thalamus. Although the correlation between pain level and pain threshold is not well established [24], these findings fit with data from other chronic pain syndromes. To our knowledge, this is the first time these results have been demonstrated to apply in chronic pelvic pain.
We did not, however, find increases in Glu in the ACC. Gussew et al. (2011) found a decrease of Glu in the ACC of patients with chronic low back pain [25]. Despite the fact that the region of interest (ACC) and the type of pain (chronic low back pain) were different from those in previous papers, Gussew et al. argued that their apparent contradictory finding may be explained by the fact that prolonged chronic pain may result in the decrease of the glutamatergic cells [25].
Finally we found a negative correlation between pain levels and the level of Gln/Cr in M1, which may at first glance seem to be contradictory to some previous studies involving others cerebral areas. This, however, may represent differences in assessment. In other words, when the motor cortex is assessed at resting state, there may be a decrease in overall excitability in patients with chronic pain syndromes such as pelvic pain.
We also tested the Gln/Glu ratio in the thalamus and found that (Gln/Glu)/Cr positively correlated with pain thresholds in more tested areas than the correlation with Glu or Gln alone. Thus, the ratio Gln/Glu more consistently demonstrated a relationship between pain threshold and glutamatergic activity. We suggest that the role of glutamate in pain may not be explained solely by changes in levels measure by MRS, but by the change in homeostasis of glutamate activity, which may also explain the positive correlation observed between pain thresholds and Gln/Cr in the thalamus. In fact, we also found a negative correlation between pain levels and the level of (Gln/Glu)/Cr in M1.
N-Acetylaspartate
As presented above, we found that the concentration of NAA/Cr in M1 was significantly lower in the chronic pelvic pain subjects compared to the healthy subjects. This finding aligns well with previous understandings of NAA.
NAA has been viewed by many as a possible marker of neuronal integrity [26]. Fayed suggested that a lower level of NAA in the hippocampus is a sign of neuronal metabolic dysfunction. Sharma showed that low levels of NAA can result from neuronal mitochondrial depression that is caused by long-term nociceptive input (at least in the somatosensory cortex) [27]. Thus, high levels of NAA may suggest well-functioning neurons supported by strong metabolic activity.
Similar to our findings, many authors have demonstrated a link between chronic pain conditions and decreased concentrations of NAA in varying regions of the brain. Low levels of NAA or NAA+NAAG have been demonstrated in the left hippocampus of fibromyalgia patients (Fayed, 2010), thalamus of patients with neuropathic pain [14], and the ACC, anterior insula, dorsolateral prefrontal cortex, and somatosensory cortex of chronic low back pain patients [17, 25, 27]. Similarly, Sorensen and colleagues found significantly lower amounts of NAA in the thalamus of diabetics experiencing chronic pain compared to diabetics with no pain [28]. It is important to note that it is not possible to define causality when describing the relationship between NAA and chronic pain, since the level of NAA might only be an epiphenomenon of pain.
Our findings suggest that low levels of NAA/Cr may also be found in M1 of patients with chronic pain. Furthermore, we found a previously unreported positive correlation between levels of NAA/Cr in the ACC and pain thresholds. This is an important addition to the previous literature, which found negative correlations between NAA/Cr in the somatosensory cortex and duration of pain in chronic low back pain patients [27] and between NAA/Cr and pain intensity [13].
Myo-inositol
The precise mechanism linking increased pain levels to increased MI concentrations remains unclear. Increased levels of MI have been considered a marker of glial proliferation. MI is a sugar-like molecule found in glial cells of the brain that can function as an osmolyte. The osmotic balance in the tissue is preserved by regulation of MI transport across the plasma membrane [29].
We found that baseline pelvic pain levels positively correlated with MI/Cr concentrations in the thalamus and M1. These findings are similar to those reported in previous studies. Similar positive correlations have been demonstrated between VAS pain levels of fibromyalgia patients and MI concentrations in the right ventrolateral prefrontal cortex [12], and between pain intensity of patients with chronic neuropathic pain after spinal cord injury and MI concentrations in the thalamus [13]. In contrast, Gussew and colleagues showed lower concentrations of MI in the Thalamus and ACC of patients with chronic low back pain when compared to the control group, and Fayed showed similar decreases in the hippocampus and the somatosensory cortex of fibromyalgia patients [18, 25].
Myo-inositol likely does not directly mediate pain, but may be viewed as a marker of pain. Further studies are needed to better understand its relationship to pain in patients with chronic pain syndromes.
The role of motor cortex modulation in chronic pain modulation
Increases in motor cortex excitability following invasive and non-invasive brain stimulation can have significant analgesic effects [30]. In 1991, Tsubokawa and colleagues first demonstrated these effects using invasive stimulation and, in 2001, Lefaucheur and colleagues demonstrated similar effects using transcranial magnetic stimulation, a non-invasive brain-stimulation technique [31, 32]. Following from these studies, tDCS of the motor cortex has been tested and also shown to have analgesic effects, although the specific mechanisms of action remain unclear [33].
Previous neuroimaging studies have shown that motor cortex activity modulation using invasive and non-invasive brain stimulation has secondary effects on pain-related neural structures such as the thalamus and cingulate cortex [34–37]. Indirect activation of the thalamus can result in modulation of activity in other brain areas involved in pain such as the anterior cingulate, periaqueductal gray, and spinal cord [35, 38].
To our knowledge, previous MRS studies have not investigated changes in M1 in patients with chronic pain. Our findings provide evidence that M1 is deeply involved in pain circuitry. For instance, (Gln/Glu)/Cr and Gln/Cr concentrations in M1 negatively correlated with baseline pain levels, and baseline MI/Cr in M1 positively correlated with pelvic pain levels. Furthermore, pelvic pain patients had significantly lower levels of NAA/Cr in M1 compared to healthy patients. Our results suggest that not only is M1 involved with pain levels in pelvic pain patients, but also in healthy subjects, since we found that baseline (Gln/Glu)/Cr concentrations in M1 positively correlated with baseline pain thresholds in healthy controls.
Similar to previous studies in healthy subjects, we showed that tDCS increases pain thresholds in pelvic pain patients [11]. We did not, however, demonstrate improvement in actual pain level after tDCS. This may be a type II error related to the small sample size of the present study. The correlation between pain threshold and actual pain levels is not clear [24], and the specific effects of tDCS on these variables should be studied further.
Despite the lack of significant improvement in pain levels, our results provide additional support for investigating the use of tDCS in treating chronic pain. They also lend support to the notion that M1 modulation has secondary impacts on areas associated with pain processing.
Limitations
A limitation of this study is the small sample size and thus an increased potential for type II error. The negative results in this trial may therefore result from the small sample size. We did not emphasize the negative findings in this study to avoid over-interpretation of potential false negative results. In addition, due to the number of comparisons in this study, our results need to be viewed as exploratory. Another limitation is the heterogeneity in the etiology of our patients’ pelvic pain. This study aimed to explore chronic pain originating in the pelvic region due to a variety of possible etiologies. While there is no evidence that different central changes result from varying etiologies of chronic pain, it is possible that this source of heterogeneity was responsible for some of the negative results observed in this study.
The MRS technique also has limitations, since the spectroscopic voxels contain mixtures of different types of cells and CSF, which do not allow for the study of brain metabolites in very specific contexts. For instance, the voxel resolution (20×20×20 mm) did not allow us to exclusively examine particular thalamic nuclei involved in sensory processing. Additionally, it is difficult to detect Glu and Gln separately due to the overlap of both metabolites. We chose to interpret them separately because of the plausibility of this analysis and the nature of our statistical results.
Conclusions
In the present study, we aimed to investigate the contributions of critical pain-related neural circuits to chronic pelvic pain using MRS and tDCS. We have demonstrated that biochemical changes in pain-related neural circuits are associated with pain levels as measured by quantitative objective pain testing.. This supports the need for further testing of targeted cortical neuromodulatory interventions for chronic pelvic pain.
Acknowledgments
This study was funded by a grant number R21 7R21DK081773 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) - National Institutes of Health. Authors are grateful to Normando Guedes and Luisa Mello Barreto for assistance with database analysis and Joao Daud Amadera for assistance with data collection.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDDK.
The authors report no conflicts of interest.
References
- 1.Savidge CJ, Slade P. Psychological aspects of chronic pelvic pain. J Psychosom Res. 1997;42(5):433–44. doi: 10.1016/s0022-3999(96)00300-5. [DOI] [PubMed] [Google Scholar]
- 2.Grace V, Zondervan K. Chronic pelvic pain in women in New Zealand: comparative well-being, comorbidity, and impact on work and other activities. Health Care Women Int. 2006;27(7):585–99. doi: 10.1080/07399330600803725. [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology. 2007;106(4):864–7. doi: 10.1097/01.anes.0000264769.87038.55. [DOI] [PubMed] [Google Scholar]
- 4.Fenton BW. Limbic associated pelvic pain: a hypothesis to explain the diagnostic relationships and features of patients with chronic pelvic pain. Med Hypotheses. 2007;69(2):282–6. doi: 10.1016/j.mehy.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Fenton BW, et al. A preliminary study of transcranial direct current stimulation for the treatment of refractory chronic pelvic pain. Brain Stimul. 2009;2(2):103–7. doi: 10.1016/j.brs.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol. 2007;6(2):188–91. doi: 10.1016/S1474-4422(07)70032-7. [DOI] [PubMed] [Google Scholar]
- 7.Fregni F, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122(1–2):197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Fregni F, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54(12):3988–98. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 9.Cecilio SB, et al. Exploring a novel therapeutic approach with noninvasive cortical stimulation for vulvodynia. Am J Obstet Gynecol. 2008;199(6):e6–7. doi: 10.1016/j.ajog.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Reidler JS, et al. Effects of motor cortex modulation and descending inhibitory systems on pain thresholds in healthy subjects. J Pain. 2012;13(5):450–8. doi: 10.1016/j.jpain.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Feraco P, et al. Metabolic abnormalities in pain-processing regions of patients with fibromyalgia: a 3T MR spectroscopy study. AJNR Am J Neuroradiol. 2011;32(9):1585–90. doi: 10.3174/ajnr.A2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pattany PM, et al. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23(6):901–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Fukui S, et al. N-Acetylaspartate concentrations in the thalami of neuropathic pain patients and healthy comparison subjects measured with (1)H-MRS. Magn Reson Imaging. 2006;24(1):75–9. doi: 10.1016/j.mri.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Peyron R, et al. Parietal and cingulate processes in central pain. A combined positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) study of an unusual case. Pain. 2000;84(1):77–87. doi: 10.1016/S0304-3959(99)00190-6. [DOI] [PubMed] [Google Scholar]
- 16.Brunoni AR, et al. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14(8):1133–45. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 17.Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89(1):7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 18.Fayed N, et al. Localized 1H-NMR spectroscopy in patients with fibromyalgia: a controlled study of changes in cerebral glutamate/glutamine, inositol, choline, and N-acetylaspartate. Arthritis Res Ther. 2010;12(4):R134. doi: 10.1186/ar3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris RE, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60(10):3146–52. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valdes M, et al. Increased glutamate/glutamine compounds in the brains of patients with fibromyalgia: a magnetic resonance spectroscopy study. Arthritis Rheum. 2010;62(6):1829–36. doi: 10.1002/art.27430. [DOI] [PubMed] [Google Scholar]
- 21.Fayed N, et al. Brain dysfunction in fibromyalgia and somatization disorder using proton magnetic resonance spectroscopy: a controlled study. Acta Psychiatr Scand. 2012;126(2):115–25. doi: 10.1111/j.1600-0447.2011.01820.x. [DOI] [PubMed] [Google Scholar]
- 22.Mullins PG, et al. A novel technique to study the brain’s response to pain: proton magnetic resonance spectroscopy. Neuroimage. 2005;26(2):642–6. doi: 10.1016/j.neuroimage.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Gussew A, et al. Time-resolved functional 1H MR spectroscopic detection of glutamate concentration changes in the brain during acute heat pain stimulation. Neuroimage. 2010;49(2):1895–902. doi: 10.1016/j.neuroimage.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Staud R, et al. Mechanical and heat hyperalgesia highly predict clinical pain intensity in patients with chronic musculoskeletal pain syndromes. J Pain. 2012;13(8):725–35. doi: 10.1016/j.jpain.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gussew A, et al. 1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain. Neuroimage. 2011;54(2):1315–23. doi: 10.1016/j.neuroimage.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 26.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Sharma NK, et al. Primary somatosensory cortex in chronic low back pain - a H-MRS study. J Pain Res. 2011;4:143–50. doi: 10.2147/JPR.S19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorensen L, et al. Differences in metabolites in pain-processing brain regions in patients with diabetes and painful neuropathy. Diabetes Care. 2008;31(5):980–1.35. doi: 10.2337/dc07-2088. [DOI] [PubMed] [Google Scholar]
- 29.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15(3–5):289–98. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 30.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70(24):2329–37. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- 31.Lefaucheur JP, Drouot X, Nguyen JP. Interventional neurophysiology for pain control: duration of pain relief following repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin. 2001;31(4):247–52. doi: 10.1016/s0987-7053(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 32.Tsubokawa T, et al. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–9. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- 33.Knotkova H, Nitsche MA, Cruciani RA. Putative physiological mechanisms underlying tDCS analgesic effects. Front Hum Neurosci. 2013;7:628. doi: 10.3389/fnhum.2013.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peyron R, et al. Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain. 1995;62(3):275–86. doi: 10.1016/0304-3959(94)00211-V. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Larrea L, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83(2):259–73. doi: 10.1016/s0304-3959(99)00114-1. [DOI] [PubMed] [Google Scholar]
- 36.Strafella AP, Vanderwerf Y, Sadikot AF. Transcranial magnetic stimulation of the human motor cortex influences the neuronal activity of subthalamic nucleus. Eur J Neurosci. 2004;20(8):2245–9. doi: 10.1111/j.1460-9568.2004.03669.x. [DOI] [PubMed] [Google Scholar]
- 37.Lang N, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22(2):495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Larrea L, et al. Positron emission tomography during motor cortex stimulation for pain control. Stereotact Funct Neurosurg. 1997;68(1–4 Pt 1):141–8. doi: 10.1159/000099915. [DOI] [PubMed] [Google Scholar]


