Abstract
The human polyomavirus, JC virus (JCV), encodes two regulatory proteins at the early (T antigen) and the late (agnoprotein) phases of viral infection whose activities are important for the production of the viral capsid proteins and the dysregulation of several host factors and their functions. For this study, we designed and utilized an RNA interference strategy via small interfering RNAs (siRNAs) that targeted the expression of T antigen and agnoprotein in human astrocytic cells. The treatment of cells with specific siRNA oligonucleotides targeting a conserved region of T antigen, nucleotides (nt) 4256 to 4276 (Mad-1 strain), caused a >50% decline in the level of T antigen and in its transcriptional activity upon the viral capsid genes as well as a significant reduction in viral DNA replication in infected cells. Similarly, a single siRNA that aimed at nt 324 to 342 of agnoprotein noticeably reduced early and late viral protein production. A combined treatment of the infected cells with both T-antigen and agnoprotein siRNAs completely abolished viral capsid protein production, indicative of the ability of the siRNAs to effectively halt multiplication of the virus in infected cells. These observations provide a new avenue for possible treatments of patients with the JCV-induced demyelinating disease progressive multifocal leukoencephalopathy.
Progressive multifocal leukoencephalopathy (PML) is a fatal demyelinating disease of the central nervous system which results from reactivation of the latent polyomavirus JC virus (JCV) and its productive replication in glial cells of the human brain (2, 5). Once a rare disease primarily seen in patients with impaired immune systems due to lymphoproliferative and myeloproliferative disorders, PML has become one of the major neurologic problems among patients with AIDS (4). It has been reported that between 4 and 8% of AIDS patients exhibit signs of PML, and JCV has been detected in the cerebrospinal fluid of affected patients, suggesting that there is active replication of the virus in the brain (2, 5). The histological hallmarks of PML include multifocal demyelinated lesions with enlarged eosinophilic nuclei in oligodendrocytes and enlarged bizarre astrocytes with lobulated hyperchromatic nuclei within white matter tracts of the brain (4, 26), although in some instances atypical features that include a unifocal pattern of demyelination and involvement of the gray matter have been reported (for reviews, see references 4 and 25). Earlier observations from in vitro cell culture studies and an in vivo evaluation of JCV in clinical samples led to early assumptions that oligodendrocytes and astrocytes are the only cells which support productive viral infections (9, 12). Accordingly, molecular studies have provided evidence for cell-type-specific transcription of the viral early genome in cells derived from the central nervous system (21). However, subsequent studies have shown low, but detectable, levels of JCV gene expression in nonneural cells, including B cells, and noticeably high levels of production of the viral early protein in several neural and nonneural tumor cells in humans (12, 16).
Like the other polyomaviruses, JCV is a small DNA virus whose genome can be divided into three regions that encompass the transcription control region; the genes responsible for the expression of the viral early protein, T antigen; and the genes encoding the viral late proteins, VP1, VP2, and VP3. In addition, the late genome is also responsible for production of an auxiliary viral protein, agnoprotein. T-antigen expression is pivotal for initiation of the viral lytic cycle, as this protein stimulates transcription of the late genes and induces the process of viral DNA replication (9). Recent studies have ascribed an important role for agnoprotein in the transcription and replication of JCV, as inhibition of its production significantly reduced viral gene expression and replication (M. Safak et al., unpublished observations). Furthermore, the agnoprotein dysregulates the cell cycle by altering the expression of several cyclins and their associated kinases (6).
Thus far, there are no effective therapies for the suppression of JCV replication and the treatment of PML. Cytosine arabinoside (AraC) has been tested for the treatment of PML patients, and the outcome in some instances revealed a remission of JCV-associated demyelination (for a review, see reference 1 and references within). Reports from the AIDS Clinical Trial Group Organized Trial 243, however, have suggested that there is no difference in the survival of human immunodeficiency virus type 1 (HIV-1)-infected patients with PML and that of the control population (13), although in other reports it has been suggested that the failure of AraC in the AIDS Clinical Trial Group trial may have been due to insufficient delivery of the AraC via the intravenous and intrathecal routes (18). Based on in vitro studies showing the ability of inhibitors of topoisomerase to suppress JCV DNA replication (15), the topoisomerase inhibitor topotecan was used for the treatment of AIDS-PML patients, and the results suggested that topotecan treatment may be associated with a decreased lesion size and prolonged survival (22).
Since the effective inhibition of JCV gene expression and replication is the first and most critical step in the treatment of PML, we utilized RNA interference to target the expression of the viral regulatory proteins expressed by the early (T antigen) and late (agnoprotein) genome. Our results show that a combined treatment of the infected cells with small interfering RNAs (siRNAs) targeting T antigen and agnoprotein completely abolishes the production of the viral capsid proteins in glial cells.
In the first series of experiments, we assessed the ability of our designed siRNA to suppress the expression of JCV T antigen. Human primary fetal astrocytes were transfected with a plasmid expressing JCV T antigen (pCMV-T-antigen) and were subsequently transfected with siRNA oligonucleotides targeting the JCV T antigen. As shown in Fig. 1A, the treatment of cells with JCV T-antigen siRNA decreased the level of T antigen but not the production of the unrelated cellular protein Grb-2. Note that the siRNA designed to target the simian virus 40 (SV40) T antigen, which has a 2-bp mismatch with the JCV-specific siRNA, had no effect on JCV T-antigen expression. To further demonstrate the suppression of T antigen by the siRNA, we performed a functional assay in which the level of JCV late promoter (JCVL) activation by the T antigen was tested in astrocytes upon treatment of the cells with JCV-specific and nonspecific T-antigen siRNAs. The results showed a >50% decrease in the level of JCVL transcriptional activation by T antigen, but no effect with the SV40 T-antigen siRNA, indicating that the observed reduction in the level of JCV T antigen by the JCV-specific siRNA has a functional consequence on its ability to stimulate expression of the viral late genome (Fig. 1B). To examine the ability of JCV T-antigen siRNA to suppress viral gene expression during the course of infection, we infected primary human fetal astrocytes with the Mad-4 strain of JCV, and at days 1, 5, and 10 postinfection, transfected the cells with JCV T-antigen siRNA oligonucleotides. The results from the analysis of viral proteins at 15 days postinfection showed a drastic suppression of T antigen and noticeable decreases in the levels of agnoprotein and the viral capsid protein VP1 in the siRNA-treated cells in comparison with control cells (Fig. 1C). Neither viral infection nor treatment with nonspecific siRNAs alone influenced the level of Grb-2 production.
FIG. 1.
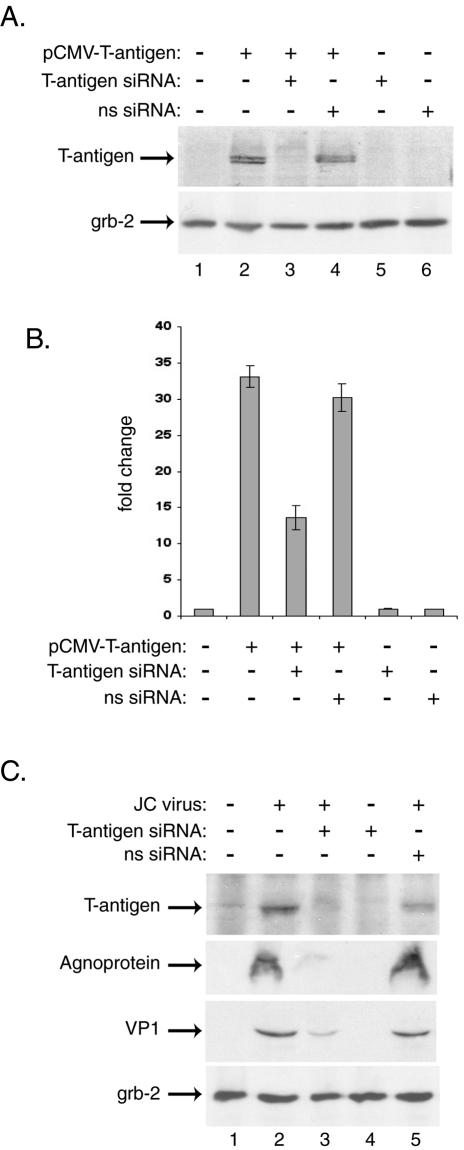
JCV T-antigen siRNA decreases expression of JCV proteins in transiently transfected and infected primary human astrocytes. Primary human fetal astrocytes were prepared as described previously and were seeded into six-well plates at a density of 500,000 cells/well (20). For transient transfections, cells were transfected by using FuGENE 6 with plasmid expressing JCV T antigen (15). The following day, the cells were transfected with double-stranded 21-bp siRNA for JCV T antigen targeting nt 4256 to 4276 of the Mad-1 isolate of JCV (sense strand, 5′-AAGUCUUUAGGGUCUUCUACCUdTdT-3′), while a nonspecific RNA (ns siRNA) targeted nt 4406 to 4426 of the reference strain 776 of SV40 (sense strand, 5′AAGUCCUUGGGGUCUUCUACCUdTdT-3′). The two base pair mismatches between the JCV and SV40 T antigens are underlined. The siRNAs were prepared as double-stranded, 2′-deprotected, and desalted oligonucleotides and were utilized according to the manufacturer's directions (Dharmacon). For the transfection of siRNAs, 100 pmol of siRNA was mixed with 3 μl of Oligofectamine (Invitrogen), diluted in OptiMEM (Invitrogen), and incubated with the cell cultures for 4 h at 37°C under serum- and antibiotic-free conditions. After transfection, the cells were fed with serum-containing medium without removing the siRNA transfection mixture. (A) Whole-cell extracts prepared from transfected astrocytes 24 h after siRNA treatment were analyzed by Western blotting for the presence of T antigen (pAb416; Oncogene Science) and the unrelated protein Grb-2 (upper and lower panels, respectively). (B) In parallel, samples transfected with 1.0 μg of JCV T-antigen expression plasmid along with 0.5 μg of a luciferase reporter construct containing the JCV late promoter (Mad-1 strain) were harvested 24 h after siRNA treatment, and luciferase activity was measured according to the manufacturer's directions (Promega luciferase assay system). Activities are presented as fold changes from the background activity of the JCV late promoter, arbitrarily set as 1. Data are means from four experiments, and standard deviations are indicated by error bars. (C) Primary astrocytes were infected with the JCV Mad-4 strain at a multiplicity of infection of 1 in serum-free medium for 3 h at 37°C. Uninfected and infected cells were then transfected with T-antigen siRNA at days 1, 5, and 10 postinfection and were harvested at day 15. Western blotting was performed on whole-cell extracts for the presence of JCV early and late proteins T antigen (pAb416; Oncogene Research Products), agnoprotein (7), and VP1 (pAb597; kindly provided by Walter Atwood, Brown University) as well as the cellular protein Grb-2 (pAb81; BD Biosciences). Proteins were visualized by using horseradish peroxidase-conjugated secondary antibodies and the ECL-Plus system (Amersham).
Since our strategy for targeting T antigen by siRNA did not completely abolish the production of agnoprotein and the major capsid protein, VP1, as a second approach we designed and employed siRNAs targeting the expression of JCV agnoprotein. As before, the efficacy of JCV agnoprotein siRNA in silencing the expression of agnoprotein was first tested by transfection assay. As seen in Fig. 2A, the transfection of cells expressing YFP-agnoprotein with JCV agnoprotein siRNA oligonucleotides drastically suppressed the production of agnoprotein in the cells. Interestingly, the siRNA designed for the agnoprotein from the polyomavirus BKV, which shares a two-nucleotide mismatch with the analogous region of the JCV genome, failed to suppress expression of JCV agnoprotein in the transfected cells, verifying the high level of specificity of the designed siRNAs. Similarly, JCV agnoprotein siRNA, but not BKV agnoprotein siRNA, drastically suppressed the production of JCV agnoprotein in JCV-infected human fetal astrocytes (Fig. 2B). It was particularly interesting that the extinction of agnoprotein by the siRNA affected production of T antigen. This observation suggests that agnoprotein promotes the expression of T antigen. Also, a substantial decrease in the level of VP1 was detected in cells treated with JCV agnoprotein siRNA.
FIG. 2.
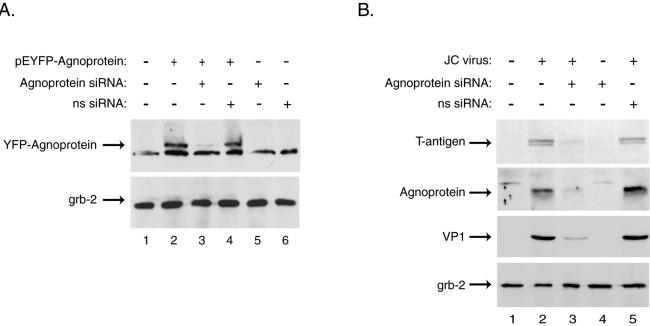
JCV agnoprotein siRNA decreases agnoprotein expression as well as that of other viral proteins in primary human astrocytes. Primary human fetal astrocyte preparations, transient transfections, siRNA treatments, and Western blotting were performed as described in the text and the legend to Fig. 1. The cells were transfected with a plasmid containing JCV agnoprotein fused to YFP (6). The JCV agnoprotein siRNA targeted nt 324 to 342 of the Mad-1 isolate of JCV (sense strand, 5′-AACCUGGAGUGGAACUAAAdTdT-3′), while a nonspecific siRNA (ns siRNA) targeted nt 435 to 453 of the Dunlop strain of BKV (sense strand, 5′-AACCUGGACUGGAACAAAAdTdT-3′). The two base pair mismatches between JCV and BKV agnoprotein sequences are underlined. (A) Whole-cell extracts prepared from transfected astrocytes 24 h after treatment with specific or nonspecific siRNA were analyzed by Western blotting for the presence of agnoprotein and the unrelated cellular factor Grb-2 (upper and lower panels, respectively). (B) Primary astrocytes that were uninfected or infected with the JCV Mad-4 strain were then transfected with JCV agnoprotein or nonspecific BKV agnoprotein siRNA at days 1, 5, and 10 postinfection and were harvested at day 15. Western blotting was performed on whole-cell extracts for presence of the JCV T antigen, agnoprotein, and VP1 as well as the cellular protein Grb-2.
In the next set of experiments, we utilized both JCV-specific T-antigen and agnoprotein siRNAs in combination to block expression of the viral early and late regulatory proteins and to test the expression level of the major viral capsid protein. The results for the cotransfection of cells with plasmids expressing JCV T antigen and YFP-agnoprotein indicated that cotreatment of cells with both JCV-specific siRNAs drastically reduced the levels of both T antigen and agnoprotein in the cells, as shown in Fig. 3. In JCV-infected cells, cotreatment with siRNAs completely abolished the expression of T antigen and VP1 proteins, as no bands corresponding to either protein were detected by Western blot analysis of the protein extracts from infected and treated cells. The level of agnoprotein was substantially decreased in the infected cells upon cotreatment of cells with both siRNAs (Fig. 3B). The complete suppression of T antigen and VP1 proteins by T-antigen and agnoprotein siRNAs indicated the effectiveness of the combined treatment in blocking viral gene expression and protein products.
FIG. 3.
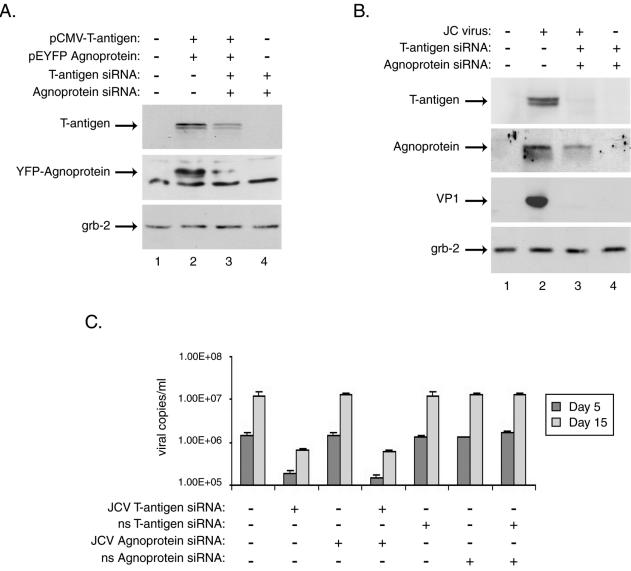
Treatment with siRNAs targeting JCV T antigen and agnoprotein abolishes their expression, as well as that of the JCV late protein VP1, in primary human astrocytes and affects viral replication in infected cells. Primary human fetal astrocyte preparations, transient transfections, siRNA treatments, and Western blotting were performed as described in the text and the legend to Fig. 1. (A) Whole-cell extracts from astrocytes transfected with expression plasmids for JCV T antigen, YFP-agnoprotein, or both were prepared from cultures 24 h after siRNA treatment and were analyzed by Western blotting for the presence of T antigen and agnoprotein as well as for the unrelated protein Grb-2. (B) Astrocyte cultures were infected with the JCV Mad-4 strain and were then transfected with siRNAs targeting JCV T antigen, agnoprotein, or both at days 1, 5, and 10 postinfection. Western blotting was performed on whole-cell extracts harvested at day 15 postinfection for the presence of JCV T antigen and agnoprotein as well as the viral late protein, VP1, and the cellular protein Grb-2. Supernatants collected from infected and siRNA-treated cells at days 5 and 15 postinfection were analyzed by quantitative real-time PCR for the presence of replicated JCV DNA essentially as described previously (17). The PCRs included JCV-specific forward and reverse primers (200 and 400 nM) representing nt 2393 to 2412 and 2468 to 2486 of the Mad-1 strain of JCV plus 200 nM JCV-specific probe (nt 2428 to 2458) fluorescently labeled at the 5′ and 3′ ends with FAM and BHQ1, respectively. Five microliters of cell culture supernatant was directly analyzed in triplicate in 50-μl reaction mixtures containing the above primers and probe in 1× TaqMan Universal Master Mix (Perkin-Elmer). Plasmid DNA containing the JCV genome was used to generate a standard curve against which the samples were analyzed using iCycler software (Bio-Rad).
In parallel, quantitative real-time PCRs were performed with supernatants collected from infected cells at 5 and 15 days postinfection to determine the effect of the siRNAs on viral replication. As shown in Fig. 3C, treatment with JCV-specific T-antigen siRNA reduced viral DNA replication by several logs over the course of the infection. However, although JCV agnoprotein-specific siRNA treatment significantly reduced viral protein levels, the siRNA did not show any effect on viral DNA replication as measured by quantitative PCR. Furthermore, the treatment with both T-antigen and agnoprotein siRNAs showed a reduction in viral DNA levels similar to that from treatment with JCV T-antigen siRNA alone.
Finally, in an alternative approach, we employed immunocytochemistry to assess the level and subcellular location of viral proteins in the infected cells upon treatment with siRNAs targeting one or both viral regulatory proteins. As shown in Fig. 4, high levels of T antigen and VP1 were expressed and were appropriately localized in the nuclei of JCV-infected primary human fetal astrocytic cells. Also, in accord with previous reports (8, 19, 20), high levels of agnoprotein with cytoplasmic perinuclear accumulation were observed in the JCV-infected cells. Treatment of the cells with JCV T-antigen siRNA caused a drastic decrease in the expression of both T antigen and VP1 but had a lesser effect on agnoprotein production. Treatment of the cells with agnoprotein siRNA resulted in a major decline in the levels of agnoprotein and VP1 and in the suppression of T-antigen appearance in the nuclei of some, but not all, infected cells. The observed events were specific, as under similar conditions, BKV agnoprotein siRNA had no effect on the production of T antigen, VP1, or agnoprotein. The cotreatment of infected cells with siRNAs targeting JCV T antigen and agnoprotein resulted in silencing of both viral early and late gene expression, indicating that cotreatment of the infected cells with siRNAs for the two viral regulatory proteins can effectively block viral gene expression in JCV-infected human astrocytes.
FIG. 4.
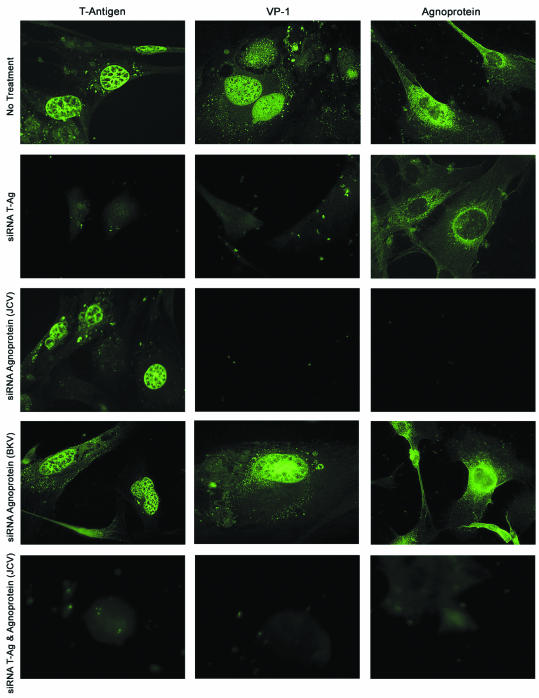
Immunocytochemistry of primary astrocytes infected with JCV reveals alterations in JCV protein levels upon JCV T-antigen and agnoprotein siRNA treatment. Cells were infected with JCV and treated at days 1, 5, and 10 postinfection with JCV T-antigen siRNA, JCV agnoprotein siRNA, or both. The cells were also treated with nonspecific agnoprotein siRNA. The cells were subcultured and plated onto poly-l-lysine-coated chamber slides (Falcon) on day 13 and were fixed on day 15 postinfection with ice-cold acetone for 3 min. Viral proteins were detected by immunocytochemistry as described previously (20), using the same primary antibodies as for the Western blot analysis (see the legend to Fig. 1). The proteins were visualized with fluorescein-conjugated secondary antibodies.
Intracellular immunization against virus replication in mammalian cells can be accomplished by expression of proteins and/or RNAs that effectively interfere with viral gene expression. In this respect, the use of RNA interference, which prevents the expression of genes by the use of small molecules, such as siRNAs, has recently received special attention due to its ability to affect viral gene expression (3, 11, 14, 27). Once formed, siRNAs can be incorporated into a protein complex that recognizes and cleaves target mRNAs (10). In this study, we directed a 21-nucleotide siRNA duplex against two regions of the JCV genome that correspond to the viral early regulatory protein, T antigen, and the viral late regulatory protein, agnoprotein. Our rationale for targeting the early genome of JCV was based on established information indicating that the expression of T antigen is an essential early event for initiating the viral lytic cycle, which includes viral DNA replication and expression of the viral late genome, which is responsible for capsid proteins. Our results show that while one siRNA duplex for T antigen can decrease the level of T antigen, there is still sufficient T antigen to stimulate, albeit at a decreased level, late gene expression and capsid production. However, T-antigen siRNA exhibited a more robust effect in infected cells and resulted in decreased protein levels as well as a substantial reduction in viral DNA replication. It should be noted that in the transient transfection assay, the siRNA was transfected 24 h after the T-antigen plasmid and therefore may not have been delivered into the identical cells that received the T-antigen expression vector. Furthermore, studies in stable cell lines have shown that this siRNA completely abolishes T-antigen expression (data not shown).
In light of previous results suggesting regulatory functions for agnoprotein at the levels of transcription, replication, capsid translation, and assembly (23, 24), we developed an siRNA to target agnoprotein production. Our results demonstrate that the agnoprotein siRNA can be used to decrease the levels of viral early and late protein production by >50%, although minimal effects were observed on DNA replication in infected cells. The strong effect of T-antigen siRNA on DNA replication reinforces the well-established central role of T antigen in orchestrating the JCV life cycle (9). We have also observed the ability of agnoprotein siRNA to significantly down-regulate T-antigen expression at both the RNA and protein levels (Safak et al., unpublished observations). In addition, both siRNAs, when used in combination, were able to completely abolish production of the JCV capsid protein VP1, although neither siRNA directly targeted this protein. In light of the fact that VP1, the major capsid protein, is essential for production of infectious virions, it is quite interesting that VP1 expression can be blocked via targeting of T antigen and agnoprotein. The reduction in VP1 protein levels, in conjunction with decreased viral DNA replication, indicates that our combined approach affects virus production at two different levels. These data indicate that expression of the JCV genome can be effectively controlled by RNA interference technology. The genomic stability of double-stranded DNA viruses such as JCV and the fact that both T antigen and agnoprotein are essential for viral replication make these two proteins attractive therapeutic targets.
Acknowledgments
We thank past and present members of the Center for Neurovirology and Cancer Biology for their insightful discussions and sharing of ideas and reagents. We also thank C. Schriver for editorial assistance.
This work was made possible by grants awarded by the NIH to K.K.
REFERENCES
- 1.Aksamit, A. 2001. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J. Neurovirol. 7:386-390. [DOI] [PubMed] [Google Scholar]
- 2.Berger, J. R., and M. Concha. 1995. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J. Neurovirol. 1:5-18. [DOI] [PubMed] [Google Scholar]
- 3.Chang, J., and J. M. Taylor. 2003. Susceptibility of human hepatitis delta virus RNAs to small interfering RNA action. J. Virol. 77:9728-9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinque, P., I. J. Koralnik, and D. B. Clifford. 2003. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J. Neurovirol. 9(Suppl. 1):88-92. [DOI] [PubMed] [Google Scholar]
- 5.Clifford, D. B., and E. O. Major. 2001. The biology of JC virus and progressive multifocal leukoencephalopathy. J. Neurovirol. 4:279. [DOI] [PubMed] [Google Scholar]
- 6.Darbinyan, A., N. Darbinian, M. Safak, S. Radhakrishnan, A. Giordano, and K. Khalili. 2002. Evidence for dysregulation of cell cycle by human polyomavirus, JCV, late auxiliary protein. Oncogene 21:5574-5581. [DOI] [PubMed] [Google Scholar]
- 7.Del Valle, L., J. Gordon, S. Enam, S. Delbue, S. Croul, S. Abraham, S. Radhakrishnan, M. Assimakoupoulou, C. D. Katsetos, and K. Khalili. 2001. Expression of human neurotropic polyomavirus JCV late gene product AGNO protein in human medulloblastoma. J. Natl. Cancer Inst. 94:267-273. [DOI] [PubMed] [Google Scholar]
- 8.Del Valle, L., J. Gordon, M. Assimakopoulou, S. Enam, J. F. Geddes, J. N. Varakis, C. D. Katsetos, S. Croul, and K. Khalili. 2001. Detection of JC virus DNA sequences and expression of the viral regulatory protein T-antigen in tumors of the central nervous system. Cancer Res. 61:4287-4293. [PubMed] [Google Scholar]
- 9.Frisque, R. J., and R. A. White III. 1992. The molecular biology of JCV, causative agent of progressive multifocal leukoencephalopathy, p. 25-158. In R. P. Roos (ed.), Molecular neurovirology. Humana Press, Totowa, N.J.
- 10.Gitlin, L., and R. Andino. 2003. Nucleic acid-based immune system: the antiviral potential of mammalian RNA silencing. J. Virol. 77:7159-7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gitlin, L., S. Karelsky, and R. Andino. 2002. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature 418:430-434. [DOI] [PubMed] [Google Scholar]
- 12.Gordon, J., and K. Khalili. 1998. The human polyomavirus, JCV, and neurologic diseases. Int. J. Mol. Med. 1:647-655. [DOI] [PubMed] [Google Scholar]
- 13.Hall, C. D., U. Dafni, D. Simpson, D. Clifford, P. E. Wetherill, B. Cohen, J. McArthur, H. Hollander, C. Yainnoutsos, E. Major, L. Millar, and J. Timpone. 1998. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N. Engl. J. Med. 338:1345-1351. [DOI] [PubMed] [Google Scholar]
- 14.Jacque, J.-M., K. Triques, and M. Stevenson. 2002. Modulation of HIV-1 replication by RNA interference. Nature 418:435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr, D. A., C.-F. Chang, J. Gordon, M.-A. Bjornsti, and K. Khalili. 1993. Inhibition of human neurotropic virus (JCV) DNA replication in glial cells by camptothecin. Virology 196:612-618. [DOI] [PubMed] [Google Scholar]
- 16.Khalili, K., L. Del Valle, J. Otte, M. Weaver, and J. Gordon. 2003. Human neurotropic polyomavirus, JCV, and its role in carcinogenesis. Oncogene 22:5181-5191. [DOI] [PubMed] [Google Scholar]
- 17.Leung, A. Y. H., C. K. M. Suen, A. K. W. Lie, R. H. S. Liang, K. Y. Yuen, and Y. L. Dwong. 2001. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood 98:1971-1978. [DOI] [PubMed] [Google Scholar]
- 18.Levy, R. M., E. Major, M. J. Ali, B. Cohen, and D. Groothius. 2001. Convection-enhanced intraparenchymal delivery (CEID) of cytosine arabinoside (AraC) for the treatment of HIV-related progressive multifocal leukoencephalopathy (PML). J. Neurovirol. 7:382-385. [DOI] [PubMed] [Google Scholar]
- 19.Okada, Y., S. Endo, H. Takahashi, H. Sawa, T. Umemura, and K. Nagashima. 2001. Distribution and function of JCV agnoprotein. J. Neurovirol. 7:302-306. [DOI] [PubMed] [Google Scholar]
- 20.Radhakrishnan, S., J. Otte, S. Enam, L. Del Valle, K. Khalili, and J. Gordon. 2003. JC virus-induced changes in cellular gene expression in primary human astrocytes. J. Virol. 77:10638-10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj, G. V., and K. Khalili. 1995. Transcriptional regulation: lessons from the human neurotropic polyomavirus, JCV. Virology 10:283-291. [DOI] [PubMed] [Google Scholar]
- 22.Royal, W., III, B. Dupont, D. McGuire, L. Chang, K. Gookin, T. Ernst, M. J. Post, D. Fish, G. Pailloux, H. Poncelet, M. Concha, L. Appuzzo, and E. Singer. 2003. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J. Neurovirol. 9:411-419. [DOI] [PubMed] [Google Scholar]
- 23.Safak, M., R. Barucco, A. Darbinyan, Y. Okada, K. Nagashima, and K. Khalili. 2001. Interaction of JC virus agno protein with T antigen modulates transcription and replication of the viral genome in glial cells. J. Virol. 75:1476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shishido-Hara, Y., Y. Hara, T. Larson, K. Yasui, K. Nagashima, and G. L. Stoner. 2000. Analysis of capsid formation of human polyomavirus JC (Tokyo-1 strain) by a eukaryotic expression system: splicing of late RNAs, translation and nuclear transport of major capsid protein VP1, and capsid assembly. J. Virol. 74:1840-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweeney, B. J., H. Manji, R. F. Miller, M. J. Harrison, F. Gray, and F. Scaravilli. 1994. Cortical and subcortical JC virus infection: two unusual cases of AIDS associated progressive multifocal leukoencephalopathy. J. Neurol. Neurosurg. Psychiatry 57:994-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker, D. L., and B. L. Padgett. 1983. The epidemiology of human papovaviruses, p. 99-106. In J. L. Sever and D. L. Madden (ed.), Polyomaviruses and human neurological disease. Alan R. Liss, New York, N.Y.
- 27.Ying, C., E. De Clercq, and J. Neyts. 2003. Selective inhibition of hepatitis B virus replication by RNA interference. Biochem. Biophys. Res. Commun. 309:482-484. [DOI] [PubMed] [Google Scholar]


