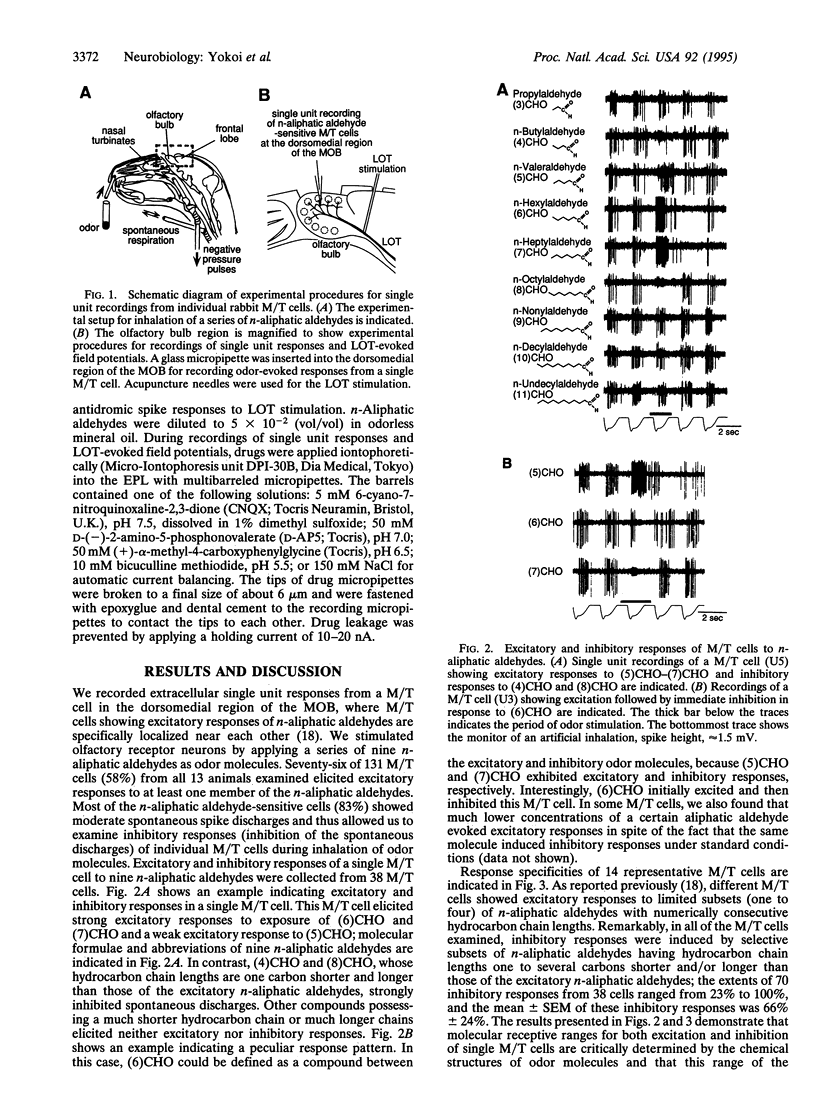
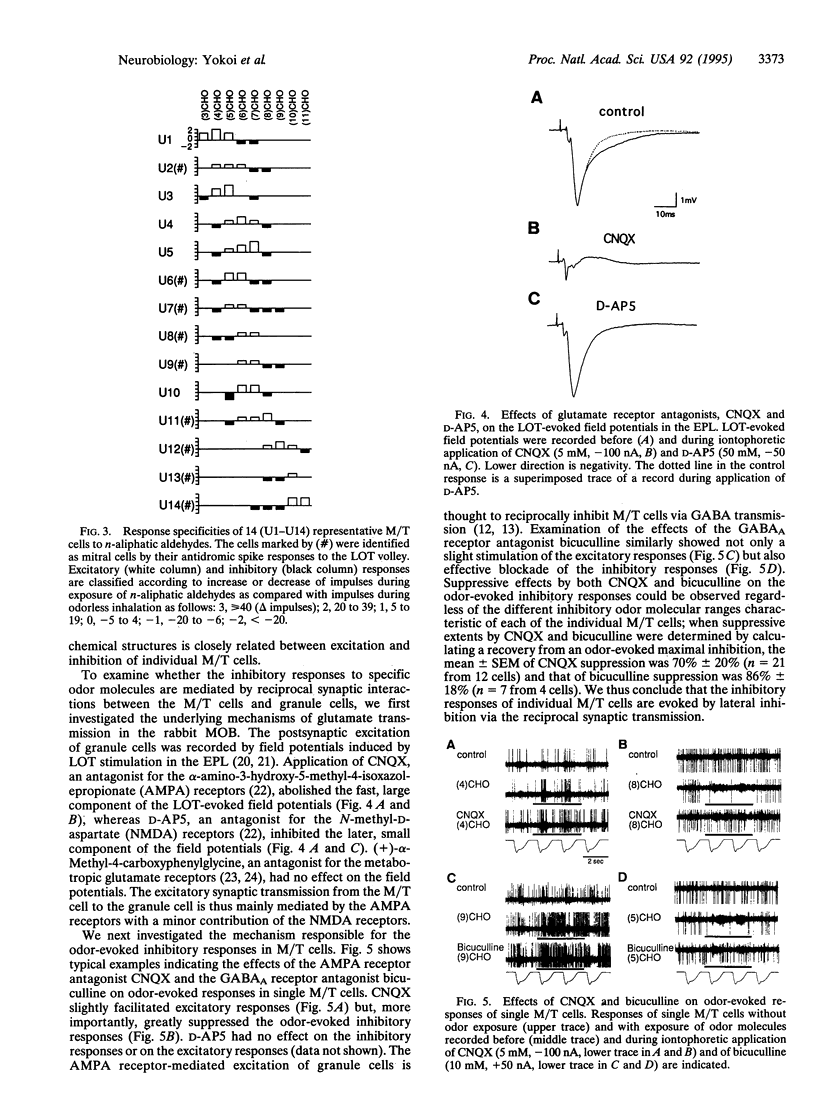
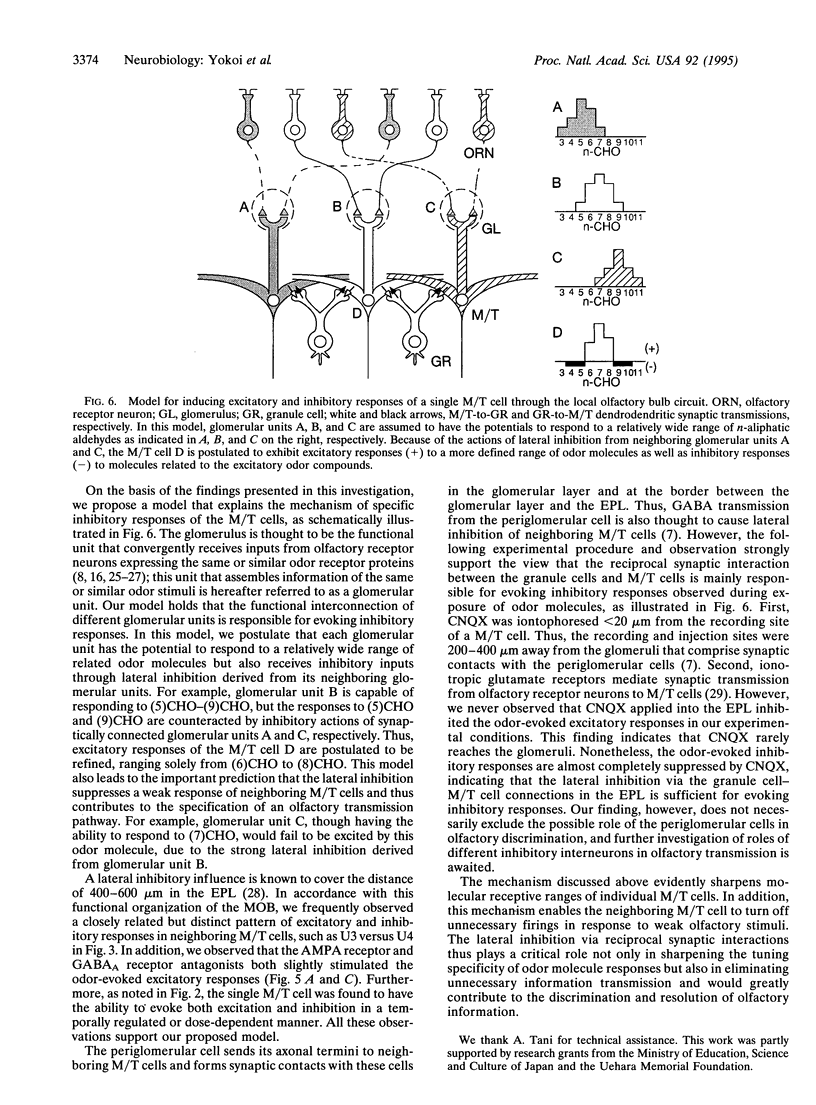
Abstract
Mitral/tufted cells (M/T cells) and granule cells form reciprocal dendrodendritic synapses in the main olfactory bulb; the granule cell is excited by glutamate from the M/T cell and in turn inhibits M/T cells by gamma-aminobutyrate. The trans-synaptically excited granule cell is thought to induce lateral inhibition in neighboring M/T cells and to refine olfactory information. It remains, however, elusive how significantly and specifically this synaptic regulation contributes to the discrimination of different olfactory stimuli. This investigation concerns the mechanism of olfactory discrimination by single unit recordings of responses to a series of normal aliphatic aldehydes from individual rabbit M/T cells. This analysis revealed that inhibitory responses are evoked in a M/T cell by a defined subset of odor molecules with structures closely related to the excitatory odor molecules. Furthermore, blockade of the reciprocal synaptic transmission by the glutamate receptor antagonist or the gamma-aminobutyrate receptor antagonist markedly suppressed the odor-evoked inhibition, indicating that the inhibitory responses are evoked by lateral inhibition via the reciprocal synaptic transmission. The synaptic regulation in the olfactory bulb thus greatly enhances the tuning specificity of odor responses and would contribute to discrimination of olfactory information.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowicz D. A., Trombley P. Q., Shepherd G. M. Evidence for glutamate as the olfactory receptor cell neurotransmitter. J Neurophysiol. 1994 Jun;71(6):2557–2561. doi: 10.1152/jn.1994.71.6.2557. [DOI] [PubMed] [Google Scholar]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991 Apr 5;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Eaton S. A., Jane D. E., Jones P. L., Porter R. H., Pook P. C., Sunter D. C., Udvarhelyi P. M., Roberts P. J., Salt T. E., Watkins J. C. Competitive antagonism at metabotropic glutamate receptors by (S)-4-carboxyphenylglycine and (RS)-alpha-methyl-4-carboxyphenylglycine. Eur J Pharmacol. 1993 Jan 15;244(2):195–197. doi: 10.1016/0922-4106(93)90028-8. [DOI] [PubMed] [Google Scholar]
- Guthrie K. M., Anderson A. J., Leon M., Gall C. Odor-induced increases in c-fos mRNA expression reveal an anatomical "unit" for odor processing in olfactory bulb. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3329–3333. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Sekiyama N., Nakanishi S., Jane D. E., Sunter D. C., Birse E. F., Udvarhelyi P. M., Watkins J. C. Analysis of agonist and antagonist activities of phenylglycine derivatives for different cloned metabotropic glutamate receptor subtypes. J Neurosci. 1994 May;14(5 Pt 2):3370–3377. doi: 10.1523/JNEUROSCI.14-05-03370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Mataga N., Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. I. Aliphatic compounds. J Neurophysiol. 1992 Dec;68(6):1986–2002. doi: 10.1152/jn.1992.68.6.1986. [DOI] [PubMed] [Google Scholar]
- Jacobson I., Hamberger A. Effects of kynurenic acid on evoked extracellular field potentials in the rat olfactory bulb in vivo. Brain Res. 1986 Oct 29;386(1-2):389–392. doi: 10.1016/0006-8993(86)90177-0. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Nicoll R. A. An intracellular analysis of dendrodendritic inhibition in the turtle in vitro olfactory bulb. J Physiol. 1982 May;326:213–234. doi: 10.1113/jphysiol.1982.sp014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Koshimoto H., Tani A., Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. II. Aromatic compounds. J Neurophysiol. 1993 Nov;70(5):2161–2175. doi: 10.1152/jn.1993.70.5.2161. [DOI] [PubMed] [Google Scholar]
- Kauer J. S., Cinelli A. R. Are there structural and functional modules in the vertebrate olfactory bulb? Microsc Res Tech. 1993 Feb 1;24(2):157–167. doi: 10.1002/jemt.1070240207. [DOI] [PubMed] [Google Scholar]
- Meredith M. Patterned response to odor in mammalian olfactory bulb: the influence of intensity. J Neurophysiol. 1986 Sep;56(3):572–597. doi: 10.1152/jn.1986.56.3.572. [DOI] [PubMed] [Google Scholar]
- Mori K., Mataga N., Imamura K. Differential specificities of single mitral cells in rabbit olfactory bulb for a homologous series of fatty acid odor molecules. J Neurophysiol. 1992 Mar;67(3):786–789. doi: 10.1152/jn.1992.67.3.786. [DOI] [PubMed] [Google Scholar]
- Mori K. Membrane and synaptic properties of identified neurons in the olfactory bulb. Prog Neurobiol. 1987;29(3):275–320. doi: 10.1016/0301-0082(87)90024-4. [DOI] [PubMed] [Google Scholar]
- Mori K. Molecular and cellular properties of mammalian primary olfactory axons. Microsc Res Tech. 1993 Feb 1;24(2):131–141. doi: 10.1002/jemt.1070240205. [DOI] [PubMed] [Google Scholar]
- Mori K., Shepherd G. M. Emerging principles of molecular signal processing by mitral/tufted cells in the olfactory bulb. Semin Cell Biol. 1994 Feb;5(1):65–74. doi: 10.1006/scel.1994.1009. [DOI] [PubMed] [Google Scholar]
- Ngai J., Chess A., Dowling M. M., Necles N., Macagno E. R., Axel R. Coding of olfactory information: topography of odorant receptor expression in the catfish olfactory epithelium. Cell. 1993 Mar 12;72(5):667–680. doi: 10.1016/0092-8674(93)90396-8. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Mori K., Shepherd G. M. GABAergic mechanisms of dendrodendritic synapses in isolated turtle olfactory bulb. J Neurophysiol. 1981 Sep;46(3):639–648. doi: 10.1152/jn.1981.46.3.639. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., POWELL T. P., SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO STIMULATION OF THE LATERAL OLFACTORY TRACT IN THE RABBIT. J Physiol. 1963 Aug;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M., Reese T. S., Brightman M. W. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966 Jan;14(1):44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Raming K., Krieger J., Strotmann J., Boekhoff I., Kubick S., Baumstark C., Breer H. Cloning and expression of odorant receptors. Nature. 1993 Jan 28;361(6410):353–356. doi: 10.1038/361353a0. [DOI] [PubMed] [Google Scholar]
- Ressler K. J., Sullivan S. L., Buck L. B. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993 May 7;73(3):597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Sallaz M., Jourdan F. C-fos expression and 2-deoxyglucose uptake in the olfactory bulb of odour-stimulated awake rats. Neuroreport. 1993 Jan;4(1):55–58. doi: 10.1097/00001756-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Scott J. W., Wellis D. P., Riggott M. J., Buonviso N. Functional organization of the main olfactory bulb. Microsc Res Tech. 1993 Feb 1;24(2):142–156. doi: 10.1002/jemt.1070240206. [DOI] [PubMed] [Google Scholar]
- Trombley P. Q., Shepherd G. M. Noradrenergic inhibition of synaptic transmission between mitral and granule cells in mammalian olfactory bulb cultures. J Neurosci. 1992 Oct;12(10):3985–3991. doi: 10.1523/JNEUROSCI.12-10-03985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley P. Q., Shepherd G. M. Synaptic transmission and modulation in the olfactory bulb. Curr Opin Neurobiol. 1993 Aug;3(4):540–547. doi: 10.1016/0959-4388(93)90053-2. [DOI] [PubMed] [Google Scholar]
- Vassar R., Ngai J., Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993 Jul 30;74(2):309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]




