Abstract
Patients with thrombophilic disorder while undergoing intra-abdominal surgery may develop splanchnic vein thrombosis which can have dire consequences. Here we report a case of a 38-year-old female who developed acute Budd–Chiari syndrome after a laparoscopic cholecystectomy. She had polycythemia vera which was not diagnosed before surgery. In this report we want to highlight presurgical evaluation of routine biochemical tests and ultrasonography suggestive of myeloproliferative disorders were missed which led to the Budd–Chiari syndrome. We recommend a meticulous look at the routine evaluation done prior to cholecystectomy is essential.
Keywords: myeloproliferative disorder, polycythemia vera, cholecystectomy, Budd–Chiari syndrome, JAK 2 mutation
Abbreviations: BCS, Budd–Chiari syndrome; IVC, inferior vena cava; MPD, myeloproliferative disorders; PV, polycythemia vera; ET, essential thrombocythemia; PMF, primary myelofibrosis; PVT, portal venous thrombosis; ABC, acute Budd–Chiari; RUQ, right upper quadrant; USG, ultrasonography; GB, gall bladder; WBC, white blood cell; SAAG, serum ascites albumin gradient; CT, computed tomography; PNH, paroxysmal nocturnal hemoglobinuria; TIPS, transjugular intrahepatic porta caval shunt; LAP, leukocyte alkaline phosphatase score
Budd–Chiari syndrome (BCS) is defined as hepatic venous outflow tract obstruction starting from the level of small hepatic through large hepatic veins and inferior vena cava (IVC) to the junction of IVC and right atrium.1–3 Myeloproliferative disorders (MPD) like polycythemia vera [PV], essential thrombocythemia [ET] and primary myelofibrosis [PMF] are responsible for 50% cases of Budd–Chiari syndrome (BCS) and 35% cases of portal venous thrombosis (PVT) in the Western series.1,4,5 Diagnosis of MPD has always remained difficult in view of latency of clinical and hematological abnormalities. The latent form of MPD lacks the characteristic blood picture and may be classified as idiopathic thrombotic disorder. When patients with underlying hypercoagulable state like overt or latent MPD or congenital or acquired thrombophilia are exposed to provocating factors like abdominal surgery, trauma, cancer, pancreatitis, infection, splenectomy, pregnancy, oral contraceptives and cirrhosis of liver with portal hypertension they may develop abdominal vein thrombosis.6,7 We report a case of an unrecognized PV who developed Acute Budd–Chiari (ABC) syndrome after a laparoscopic cholecystectomy.
Case report
A 38-year-old female was transferred to our hospital for distention of abdomen and breathlessness following 3 days after she underwent a laparoscopic cholecystectomy. The course of her condition till the cholecystectomy is as follows, the patient was apparently alright till she experienced a dull pain in the right upper quadrant (RUQ) of her abdomen for 4 days before cholecystectomy. After 2 days of pain an ultrasonography (USG) was performed this revealed multiple gall bladder (GB) calculi with cholecystitis and splenomegaly. Her pre-operative biochemical work up showed hemoglobin 15.6 g/dL (normal range 12–16 g/dL), white blood cell (WBC) count of 11,330/mm3 (normal range of 5000–10,000/mm3) and platelets 288,000/mm3 (normal range 150,000–500,000/mm3). Serum Bilirubin – 0.7 mg/dL (normal range 0.0–1.0 mg/dL) with direct component of Bilirubin −0.6 mg/dL (normal range 0.0–0.6 mg/dL), SGPT – 31 IU/L (normal range 30–65 IU/L), alkaline phosphatase – 63 IU/L (normal range 40–135 IU/L), serum creatinine – 1 mg/dL (normal range 0.6–1.3 mg/dL). The pre-operative chest X-ray, ECG and 2D echo were within normal limits. At this time she underwent a laparoscopic cholecystectomy. On the second postoperative day she developed a massive distention of abdomen, breathlessness and a drop in the blood pressure requiring a transfer to an intensive care unit. Her biochemical parameter in the intensive care unit were as follows, hemoglobin 16 mg%, WBC 15,374/mm3, platelets 326,000/mm3, serum bilirubin 2.2 mg/dL, direct bilirubin 1.9 mg/dL, SGOT 57 IU/L (normal range 15–37 IU/L), SGPT 42 IU/mL, alkaline phosphatase – 204 IU/mL, serum albumin 2.9 g/dL (normal range 3.4–5.0 g/dL), serum globulin 2.1 g/dL (normal range 2.5–4.0 g/dL), LDH 773 IU/L (normal range 81–234 IU/L), serum creatinine – 1.3 mg/dL, serum amylase – 21 IU/L (normal range 25–115 IU/L) and abdominal ultrasonography revealed hepatosplenomegaly, massive ascites, bilateral pleural effusion and minimal pericardial effusion. Ascitic fluid examination showed total protein of 2.9 g/dL with albumin of 1 g/dL WBC – 16,000/mm3 with 95% polymorphs, Serum Ascites Albumin Gradient (SAAG) −1.9 g% and Adenosine Deaminase-3.0 IU/L (10–33 IU/L). The patient was treated with Meropenem and metronidazole intravenously and an ascitic fluid therapeutic paracentesis was done with albumin infusion. At this stage the patient was transferred to our hospital. Ultrasound Doppler done at our hospital was suggestive of hepatic venous obstruction. A Computed Tomography (CT) scan of the abdomen with a CT angiography was performed because there was high suspicion of ABC syndrome given the high values of patient's ascitic fluid protein levels and SAAG. The CT angiography also gave a good roadmap for further radiological interventions. The results of the CT scan and angiography demonstrated hepatic venous thrombosis with occlusion of all the three veins and a patent portal venous system. A thrombophilia profile (tests for Paroxysmal Nocturnal Hemoglobinuria (PNH), protein C, protein S, anti-thrombin 3, prothrombin gene mutation, APC resistance, anticardiolipin antibodies, factor V mutation and lupus anticoagulants) was done and the patient was started on low molecular weight heparin. Thrombophilia profile and coagulation profile were normal. Patient was being treated with antibiotics, albumin, low-molecular weight heparin and required repeated ascitic fluid paracentesis. Ascitic fluid culture was negative. Her general condition was progressively worsening. Ten days after the antibiotic treatment ascitic fluid cell count dropped to 250/mm3 with predominant lymphocytes. As there was no improvement with anticoagulation it was decided to treat her interventional treatment of hepatic vein thrombosis. On the 17th postoperative day she underwent an IVC gram and hepatic venography. The right hepatic vein being a major vein was chosen for venography. Hepatic venography showed narrowing all the three ostia with extrinsic compression of IVC due to caudate lobe. The patient was diagnosed with ABC syndrome with no hepatic collaterals on digital subtraction angiography (Figure 1). Angioplasty was done for two hepatic veins. There was a decrease in the gradient across the hepatic veins from 11 mm Hg to 3 mm Hg. Post angioplasty she improved partially but by 10th post procedure day she again had an increase in ascites. A repeat hepatic angiography on this day showed narrowing of hepatic veins with an increase in the gradient. She was subjected to a transjugular intrahepatic porta caval shunt (TIPS) with a covered stent placement (Figure 1). Her biochemical profile prior to TIPS was as follows: hemoglobin – 9.1 g/dL, WBC – 13,200/mm3, serum bilirubin − 0.4 mg/dL, SGOT – 16 IU/L, SGPT – 24 IU/L, serum albumin − 2.2 g/mL, serum globulin − 1.1 g/dL, LDH 149 IU/L, serum creatinine 0.6 mg/dL. Post-TIPS she improved remarkably and ascites came under control. Diuretics were withdrawn and she was discharged on 6th post TIPS day on oral anticoagulation. She was on regular follow up. Eight months after the TIPS her hemoglobin was − 14.7 g/dL, WBC – 6200/mm3, platelet 278,000/mm3. Though her liver functions were normal, serum LDH was elevated to 453 IU/L. Diagnosis of PV was suspected and a bone marrow aspiration and biopsy was performed, which showed increased megakaryocytes, myelofibrosis, increased RBC precursors, decreased marrow iron and a negative FISH test. Test for Philadelphia chromosome was negative. The value of the Leukocyte alkaline phosphatase score LAP (A) was 93 and serum erythropoietin level was 4.18 nu/ml. Ultrasound of the abdomen showed splenomegaly, patent TIPS and no ascites. When all her previous records were reviewed 1.5 years prior to cholecystectomy, an ultrasound had revealed a moderate splenomegaly. Her tests back at that time showed hemoglobin − 17.6 g/dL, packed cell volume – 53, WBC – 9700/mm3 and a normal liver and renal function. Based on all these facts a diagnosis of PV was made and she was started on hydroxyurea. Two years after the diagnosis when JAK2 mutation testing became available she was tested for the same and was found to be positive. She continues to be on hydroxyurea, warfarin and aspirin and maintains a hemoglobin of 11 g/dL with a normal liver and renal function and a patent TIPS shunt at 5 years of follow up after diagnosis.
Figure 1.
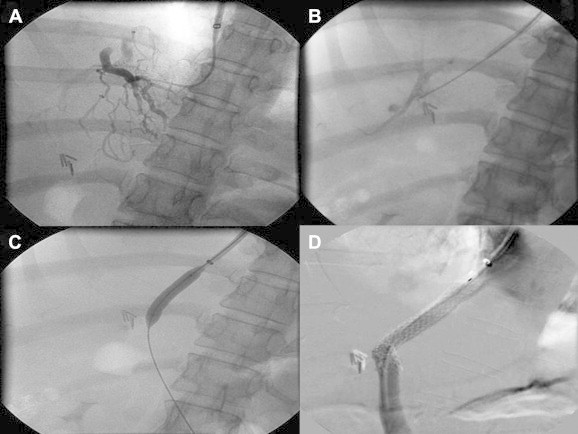
A) Hepatic venogram: Classical spider-w eb appearance of Budd–Chiari syndrome. B) Puncture from IVC to portal vein. C) Tract dilatation. D) Successful TIPS.
Discussion
Philadelphia chromosome negative CMPDs like PV, ET, PMF generally present in the later age group but patients with a typical phenotype i.e. juvenile disorder have a high thrombotic risk and present as abdominal venous thrombosis before they present with an overt MPD.8 Our patient had an unrecognized PV and developed ABC Syndrome postoperatively. Diagnosis of MDP in an acute abdominal venous thrombosis is difficult as peripheral blood picture may show a decrease in the blood count (Hemoglobin, WBC & platelet) due to hemodilution, hypersplenism or concomitant iron deficiency.9 The recent discovery of JAK2 mutation is of great clinical relevance in diagnosis of MPD as JAK2 mutation is specific, can be performed on peripheral blood and even with patients of acute thrombosis and anticoagulation.10 Frequency of JAK2 mutation being present in PV 97%, ET 57% and PMF 50%.11 Screening for JAK2 mutation is suggested in all cases of idiopathic IAVT. Use of JAK2 mutation as an initial diagnostic test can avoid bone marrow study in IAVT in around 40% patients.12 All parameters of the thrombophilia profile can be tested even in an acute episode of BCS. The tests for protein C and protein S levels may be affected in an acute episode but are generally normal with a well preserved liver function. Doppler UGS by an experienced examiner is a reliable tool for diagnosis. CT scan and Magnetic resonance imaging are tests used to confirm the diagnosis. Nuclear medicine calcium colloid liver scan has no role in the diagnosis of BCS in the current era of advanced diagnostic imaging.13
In acute BCS medical treatment like anticoagulation and diuretics may be effective in some patients.2 In our patient medical treatment was not effective. Interventional radiological treatments include HV stenting, IVC stenting and TIPS depending on the anatomy of the obstruction. Surgical treatment includes side-to-side porta caval shunt or peritoneovenous shunt.1–3 Radiological interventions are preferred over surgery and long-term follow up after these procedures have shown good results.14
BCS is a multifactorial disease. Those who have an underlying predisposing thrombophilic condition may develop abdominal venous thrombosis after a stress from surgery, infection, abdominal trauma or dehydration.15 In our patient MPD (polycythemia vera) was unrecognized before cholecystectomy and surgical trauma may have precipitated BCS. The patient was successfully treated with TIPS and anticoagulation with hydroxyurea and is doing well with this treatment at the end of five years of follow up.
In conclusion MPD should be suspected in patients with abdominal vein thrombosis. JAK2 mutation testing can identify latent or overt MPD. Interventional radiological techniques like TIPS are very effective in treating patients with BCS.
Conflicts of interest
All authors have none to declare.
References
- 1.Valla D.-C. The diagnosis and management of the Budd–Chiari syndrome: consensus and controversies. Hepatology. 2003;38:793–803. doi: 10.1053/jhep.2003.50415. [DOI] [PubMed] [Google Scholar]
- 2.Menon K.V.N., Shah V., Kamath P.S. The Budd–Chiari syndrome. N Engl J Med. 2004;350:578–585. doi: 10.1056/NEJMra020282. [DOI] [PubMed] [Google Scholar]
- 3.Janssen H.L.A., Garcia-Pagan J.-C., Elias E., Mentha G., Hadengue A., Valla D.-C. For the European group for the study of vascular disorders of the liver. J Hepatol. 2003;38:364–371. doi: 10.1016/s0168-8278(02)00434-8. [DOI] [PubMed] [Google Scholar]
- 4.Hoekstra J., Janssen H.L.A. Vascular liver disorders (II): portal vein thrombosis. Neth J Med. 2009;67:46–53. [PubMed] [Google Scholar]
- 5.Hoekstra J., Janssen H.L.A. Vascular liver disorders (I): diagnosis, treatment and prognosis of Budd–Chiari syndrome. Neth J Med. 2008;66:334–339. [PubMed] [Google Scholar]
- 6.Zumberg M., Kitchens C.S. Venous thrombosis at unusual sites. In: Kitchens C.S., Alving B.M., Kessler C.M., editors. Consultative Hemostasis and Thrombosis. 2nd ed. Saunders, Elsevier Inc.; USA: 2007. pp. 257–280. [Google Scholar]
- 7.Valla D.C. “Pathophysiologic basis for the therapy of liver diseases” Postgraduate course, American Association for the study of liver disease, Boston, USA. Nov 2007. Prothrombotic disorders and the liver; pp. 139–145. [Google Scholar]
- 8.Barosi G., Buratti A., Costa A. An atypical myeloproliferative disorder with high thrombotic risk and slow disease progression. Cancer. 1991;68:2310–2318. doi: 10.1002/1097-0142(19911115)68:10<2310::aid-cncr2820681034>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Kralovics R., Buser A.S., Teo S.S. Comparison of molecular markers in a cohort of patients with chronic myeloproliferative disorders. Blood. 2003;102:1869–1971. doi: 10.1182/blood-2003-03-0744. [DOI] [PubMed] [Google Scholar]
- 10.Janssen H.L.A., Leebeer F.W. JAK2 mutation: the best diagnostic tool for myeloproliferative disease in splanchnic vein thrombosis? Hepatology. 2006;44:1391–1393. doi: 10.1002/hep.21489. [DOI] [PubMed] [Google Scholar]
- 11.Kilpivara O., Levine R.L. JAK2 and MPL mutations in myeloproliferative neoplasms: discovery and science. Leukemia. 2008;22:1813–1817. doi: 10.1038/leu.2008.229. [DOI] [PubMed] [Google Scholar]
- 12.Kiladjian J.J., Cervantes F., Leebeek F.W.G. The impact of JAK2 and MPL mutations on diagnosis and prognosis of splanchnic vein thrombosis: a report on 241 cases. Blood. 2008;111:4922–4929. doi: 10.1182/blood-2007-11-125328. [DOI] [PubMed] [Google Scholar]
- 13.Plessier A., Rautou P.E., Valla D.C. Management of hepatic vascular diseases. J Hepatol. 2012;56(suppl 1):S25–S38. doi: 10.1016/S0168-8278(12)60004-X. [DOI] [PubMed] [Google Scholar]
- 14.Amarapurkar D.N., Punamiya S.J., Patel N.D. Changing spectrum of Budd–Chiari syndrome in India with special reference to non-surgical treatment. World J Gastroenterol. 2008 Jan 14:278–285. doi: 10.3748/wjg.14.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Primignani M., Mannucci P.M. The role of thrombophilia in splanchnic vein thrombosis. Semin Liver Dis. 2008;28:293–301. doi: 10.1055/s-0028-1085097. [DOI] [PubMed] [Google Scholar]


