Abstract
Objectives
Bacterial carriage in the upper respiratory tract is usually asymptomatic but can lead to respiratory tract infection (RTI), meningitis and septicaemia. We aimed to provide a baseline measure of Streptococcus pneumoniae, Moraxella catarrhalis, Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae and Neisseria meningitidis carriage within the community. Self-swabbing and healthcare professional (HCP) swabbing were compared.
Design
Cross-sectional study.
Setting
Individuals registered at 20 general practitioner practices within the Wessex Primary Care Research Network South West, UK.
Participants
10 448 individuals were invited to participate; 5394 within a self-swabbing group and 5054 within a HCP swabbing group. Self-swabbing invitees included 2405 individuals aged 0–4 years and 3349 individuals aged ≥5 years. HCP swabbing invitees included 1908 individuals aged 0–4 years and 3146 individuals aged ≥5 years.
Results
1574 (15.1%) individuals participated, 1260 (23.4%, 95% CI 22.3% to 24.5%) undertaking self-swabbing and 314 (6.2%, 95% CI 5.5% to 6.9%) undertaking HCP-led swabbing. Participation was lower in young children and more deprived practice locations. Swab positivity rates were 34.8% (95% CI 32.2% to 37.4%) for self-taken nose swabs (NS), 19% (95% CI 16.8% to 21.2%) for self-taken whole mouth swabs (WMS), 25.2% (95% CI 20.4% to 30%) for nasopharyngeal swabs (NPS) and 33.4% (95% CI 28.2% to 38.6%) for HCP-taken WMS. Carriage rates of S. aureus were highest in NS (21.3%). S. pneumoniae carriage was highest in NS (11%) and NPS (7.4%). M. catarrhalis carriage was highest in HCP-taken WMS (28.8%). H. influenzae and P. aeruginosa carriage were similar between swab types. N. meningitidis was not detected in any swab. Age and recent RTI affected carriage of S. pneumoniae and H. influenzae. Participant costs were lower for self-swabbing (£41.21) versus HCP swabbing (£69.66).
Conclusions
Higher participation and lower costs of self-swabbing as well as sensitivity of self-swabbing favour this method for use in large population-based respiratory carriage studies.
Keywords: Epidemiology < INFECTIOUS DISEASES, MICROBIOLOGY, Respiratory infections < THORACIC MEDICINE, PRIMARY CARE
Strengths and limitations of this study.
This study is the largest community-based swabbing study to date to compare carriage rates of multiple bacterial species simultaneously between self-swabbing and healthcare professional swabbing methods.
This study provides important evidence for the use of nose swabs for detection of Streptococcus pneumoniae and other respiratory pathogens.
Non-response bias needs to be considered within both self-swabbing and healthcare professional swabbing groups.
Introduction
The respiratory tract is host to a wide variety of commensal and pathogenic microorganisms, with approximately 250 species colonising the nasopharynx alone.1 Asymptomatic carriage in the upper respiratory tract (URT) is the first stage in the process of respiratory tract infection (RTI), meningitis and sepsis. Carriage often occurs without disease but may also lead to serious invasive illness.2 3 In 2010, approximately 4.4 million deaths worldwide resulted from an RTI, most commonly in young children.4
Collecting samples from the URT enables the estimation of carriage rates of pathogenic organisms. The determination of carriage rates is essential for assessing circulating respiratory microbes which may go on to cause disease. A number of sites within the URT have been used to assess carriage, including the nasopharynx, oropharynx, nose and throat. Methods for assessing carriage have included swabbing, nose blowing and nasopharyngeal aspiration.5–12 However, no single study has evaluated the use of different swabbing methods using a large population-based sample. Streptococcus pneumoniae remains the only bacterial species for which a WHO standard method has been established for detecting carriage.13 It is currently recommended to take a nasopharyngeal swab despite other sites being equally as effective, if not more sensitive, in assessing carriage of this organism.7 10 Self-swabbing has also been shown to be effective in assessing nasal carriage of Staphylococcus aureus and viruses and offers a cheaper alternative to more traditional healthcare professional (HCP) swabbing.12 14
Most carriage studies have focused on a particular organism and participant age group. However, many microorganisms are thought to play a role in RTI development and carriage in all age groups is important in terms of understanding disease transmission and immunity against specific pathogens.15 Moreover, in the current vaccine era, we are likely to see an explosion of new vaccines during the coming decade that will affect the respiratory tract microbiota.16–20 This highlights the need for large population-based studies that include all age groups and aim to detect as many relevant microbial species as possible.
Our study aimed to provide a baseline measure for understanding multispecies bacterial carriage in the respiratory tract within the general population of one geographical area of the UK. The objectives were to assess the optimal sample collection method and site by comparing self-taken nose and mouth swabs with HCP-taken nasopharyngeal and mouth swabs; to gain an estimate of participant consent rates in both study groups and to test the feasibility of conducting a larger multisite investigation. Finally, the study aimed to estimate carriage rates of relevant URT bacterial species. This would help inform samples sizes for multicentre studies, particularly for use in prevaccine and postvaccine studies, as well as to aid in understanding the effects of demographic factors and deprivation on carriage.
Methods
Sample size
This was a pilot study and not designed to have the power to detect non-inferiority of estimating carriage rates by HCP-administered versus self-administered swabs. Data from this study was predicted to inform sample sizes required for future large carriage studies. The sample size for this pilot study was based on the precision with which we can estimate true carriage rates. A 25% response rate among self-swabbing participants was assumed based on results from a previous staphylococcal carriage study.12 A 25% response rate was also assumed for HCP-swabbing.
We estimated that by inviting 2020 children (101 from each general practitioner (GP) practice) aged 0–4 years and 3200 older children and adults (160 from each GP practice) to participate within each swabbing group, this would result in 505 children and 800 older children and adult responders within each swabbing group, accounting for predicted lower carriage rates in older children and adults. A predicted carriage rate of 30% in 505 participating children would enable the determination of true carriage to within ±4% (95% confidence).21 A predicted carriage rate of 20% in 800 participating older children and adults would enable the determination of true carriage to within ±2.8% (95% confidence).9
Participant recruitment
Participants were selected from 20 GP practices within the Wessex Primary Care Research Network (PCRN) South West (East hub) area, in Southern England. GP practices were chosen to reflect a mix of urban/rural locations, practice sizes and area deprivation levels. Each GP practice produced a list of their entire patient cohort. Any individual deemed unfit for participation at the discretion of their GP, for example due to terminal illness or serious mental health problems, was removed from the list. From each GP list, 202 individuals aged 0–4 years and 320 individuals aged ≥5 years were randomly selected and allocated to one of two study groups using the ralloc command in Stata V.12. This resulted in approximately 101 individuals aged 0–4 years and 160 individuals aged ≥5 years within each swabbing group per GP practice.
The HCP group involved participants being invited, via letter, to organise a swabbing appointment at their GP practice where nasopharyngeal (NPS) and whole mouth (WMS) swabs were taken by a registered HCP. Appointments were within normal surgery opening hours and at the individuals’ GP practice (local to each participant). The self-swabbing group involved participants being sent a self-swabbing pack containing nose (NS) and WMS swabs by Danvers International (London, UK). Participants were not sent reminders. All swab heads were viscose (rayon). Nose and both WMS shafts were polystyrene whereas NP swab shafts were aluminium. Once taken, swabs were placed in polypropylene tubes containing amies transport medium with charcoal. HCP-taken swabs were returned for analysis on the day of swabbing by taxi or within 1–2 days by pre-existing National Health Service (NHS) delivery service. Self-taken swabs were returned by first-class freepost return (1–2 days).
Each participant was given an age-appropriate information sheet explaining the study aims, which aimed to motivate individuals to participate. Participants were asked to complete a consent form and questionnaire, provided either at their swabbing appointment or within their self-swabbing pack. The study questionnaire was identical for both study groups and requested the following details pertinent to bacterial carriage: participant age, recent use of antibiotics (within the past month), recent RTI (cold, flu, ear infection or chest infection within the past month) and vaccination status. Age was split into the following groups for analysis: 0–4, 5–17, 18–64 and 65 years and older due to the relevance of each of these age groups in carriage of the different bacterial species. Recent use of antibiotics and recent RTI were split into the following groups for analysis: yes, no and do not know/missing. Vaccination status was split into the following groups for analysis: up-to-date, not up-to-date and do not know/missing. UK Index of Multiple Deprivation (IMD) 2010 scores were obtained for each GP practice based on the lower layer super output area (LSOA) it was located in and was used as a proxy for deprivation of each practices’ patient population.22 UK IMD 2010 Score includes seven features of deprivation: income, education, employment, health, housing, crime and living environment. More deprived areas have lower levels of these seven features whereas less deprived areas have higher levels for the same seven features. This would enable the relationship between carriage and deprivation to be assessed, as in disease studies.23 A total of 10 448 individuals were invited to participate in the study.
Sample collection and analysis
Self-swabbing packs were sent out to individuals between the 15 May and 23 July 2012 and samples were received between the 18 May and 31 August 2012. HCP swabbing appointments took place between 7 June and 28 August and samples were received between the 7 June and 31 August. On receipt, swabs were immersed in skim milk, tryptone, glucose and glycerine (STGG) storage media, vigorously rubbed against the side of the tube and vortexed to ensure transfer of bacteria into the STGG. Standard microbiology culture and identification techniques were used to analyse the swab contents for the presence of S. pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, S. aureus, Pseudomonas aeruginosa and Neisseria meningitidis. This was performed by transferring 10 μL STGG onto Columbia blood agar with horse blood (Oxoid, PB0124), Columbia blood agar with colistin and nalidixic acid (Oxoid, PB0308), Columbia blood agar with chocolated horse blood (Oxoid, PB0124), Columbia blood agar with chocolated horse blood and bacitracin (Oxoid, PB0220), Pseudomonas selective agar (Oxoid, PB0291) and lysed GC selective agar (Oxoid, PB0962). Identification of each bacterial species was undertaken according to methodology described in online supplementary table S1. After plating, the remaining swab content in STGG was then frozen for future use at −70°C.
Statistical analysis
Culture data and participant questionnaire information were tabulated into SPSS (V.20) for analysis. Missing or incomplete data was classed as missing within the SPSS variables window. Participation rates, the proportion of participants relative to total number of individuals invited, were calculated for each GP practice and age group. UK IMD 2010 scores for each GP practice area were examined in relation to participation rates using Pearson's Correlation. Swab positivity rates, the proportion of swabs that isolated any of the target bacteria relative to total swab numbers, were calculated for each swab type. 95% CIs were calculated to assess reliability of participation and positivity rates.
Carriage rates, the proportion of a specific bacterial species relative to total number of swabs, were calculated according to swab type, age, recent RTI, recent antibiotic use, vaccination status, geographical location and deprivation. χ2 and Fisher's Exact tests were used to determine any associations between carriage and these variables. Geographical mapping of carriage rates was performed using ArcGIS (ESRI, V.10.1).24 Practices were grouped into geographical areas for statistical analysis based on proximity to one another. Finally, co-carriage rates, the proportion of samples containing multiple bacterial species relative to total number of swabs, were calculated according to swab type, age, recent RTI, recent antibiotic use, vaccination status and geographical location.
Study costs
Total costs associated with each swabbing method were calculated to allow cost comparisons between methods. Costs were calculated as total costs within a single swabbing group divided by the total number of responders from that swabbing group. This included swab packs sent out to individuals but not used. Costs were separated into laboratory consumables, printing, swabs, NHS Service Support Costs (additional healthcare costs due to the research taking place), transport and postage.
Results
Participation rates
Eighteen of the 20 GP practices participated in both self-swabbing and HCP-swabbing, one participated in self-swabbing only and one dropped out of the study. Participant characteristics are shown in table 1. Overall participation rates were higher in the self-swabbing group at 23.4% (n=1260; N=5395; 95% CI 22.3% to 24.5%) compared with the HCP group at 6.2% (n=314; N=5054; 95% CI 5.5% to 6.9%). Self-swabbing participation rates varied from 9.3% (n=27; N=290) to 33.1% (n=96; N=290) between practices whereas HCP participation rates varied from 1% (n=3; N=290) to 12.3% (n=34; N=277). Ten practices had participation rates ≥25% in the self-swabbing group, which was the anticipated level of participation. There was a negative correlation between participation rate and IMD score in the self-swabbing group (r=−0.473, p=0.041) and the HCP group (r=−0.417, p=0.085), which was only significant in the former. Participation was higher in individuals aged ≥5 years at 27.8% (n=931; N=3349; 95% CI 26.8% to 29.3%) in the self-swabbing group and 8.2% (n=258; N=3146; 95% CI 7.2% to 9.2%) in the HCP group versus 0–4 years at 16.1% (n=329; N=2045; 95% CI 14.5% to 17.7%) in the self-swabbing group and 2.9% (n=56; N=1908; 95% CI 2.2% to 3.7%) in the HCP group. The greatest number of responses received was from individuals aged 50–80 years, comprising 41.7% (n=656, N=1574) of total participants.
Table 1.
Participant characteristics and study costs (in British Pounds) for self-swabbing and HCP swabbing
| Participant characteristics n(%) and costs per participant (£) |
||
|---|---|---|
| Self-swabbing | HCP swabbing | |
| Age (years) | ||
| Mean | 37.42 | 50.09 |
| Minimum | 0 | 0 |
| Maximum | 94 | 88 |
| 0–4 | 329 (26.1) | 56 (17.8) |
| 5–17 | 137 (10.9) | 24 (7.6) |
| 18–64 | 465 (36.9) | 89 (28.3) |
| 65+ | 311 (24.7) | 145 (46.2) |
| Missing | 18 (1.4) | 0 (0.0) |
| Recent antibiotic treatment | ||
| Yes | 101 (8.0) | 26 (8.3) |
| No | 1124 (89.2) | 286 (91.1) |
| Unknown/missing | 35 (2.8) | 2 (0.6) |
| Recent respiratory infection | ||
| Yes | 365 (29.0) | 61 (19.4) |
| No | 860 (68.3) | 250 (79.6) |
| Unknown/missing | 35 (2.8) | 3 (1.0) |
| Vaccination status | ||
| Up-to-date | 1022 (81.1) | 270 (86.0) |
| Not up-to-date | 40 (3.2) | 10 (3.2) |
| Unknown/missing | 198 (15.7) | 34 (10.8) |
| Costs per participant (£) | ||
| Laboratory consumables | 8.06 | 8.47 |
| Printing | 2.14 | 7.23 |
| Swabs and swab packs | 17.08 | 9.65 |
| Service support costs (SSC) | 2.81 | 39.52 |
| Transport (by taxi or internal mail) | 0.00 | 4.78 |
| Postage | 11.12 | 0.00 |
| Total | 41.21 | 69.66 |
Costs (British Pounds) are per participant taking into account wastage of swabs and swab packs.
HCP, healthcare professional.
Swab positivity rates
Out of 1260 self-swabbing participants, 1254 returned both swabs with labels distinguishing nose from WMS but six individuals failed to label their swabs and thus were excluded from analyses. Out of 314 HCP swabbing participants, 309 had both swabs returned by their GP but five individuals were incorrectly swabbed by their GP and thus were excluded from analyses. Swab positivity rates were 35% (n=439; N=1254; 95% CI 32.4% to 37.6%) for NS, 19.1% (n=239; N=1254; 95% CI 16.9% to 21.3%) for self-taken WMS, 25.6% (n=79; N=309; 95% CI 20.7% to 30.5%) for NPS and 34% (n=105; N=309; 95% CI 28.7% to 39.3%) for HCP-taken WMS (see online supplementary figure S1). The NS and HCP-taken WMS were most effective in detecting carriage of the target organisms. Positivity rates of NS were significantly higher than NPS (χ2=9.974, df=1, p=0.002). Positivity rates of HCP-taken WMS were significantly higher than self-taken WMS (χ2=32.157, df=1, p<0.001).
Bacterial carriage rates
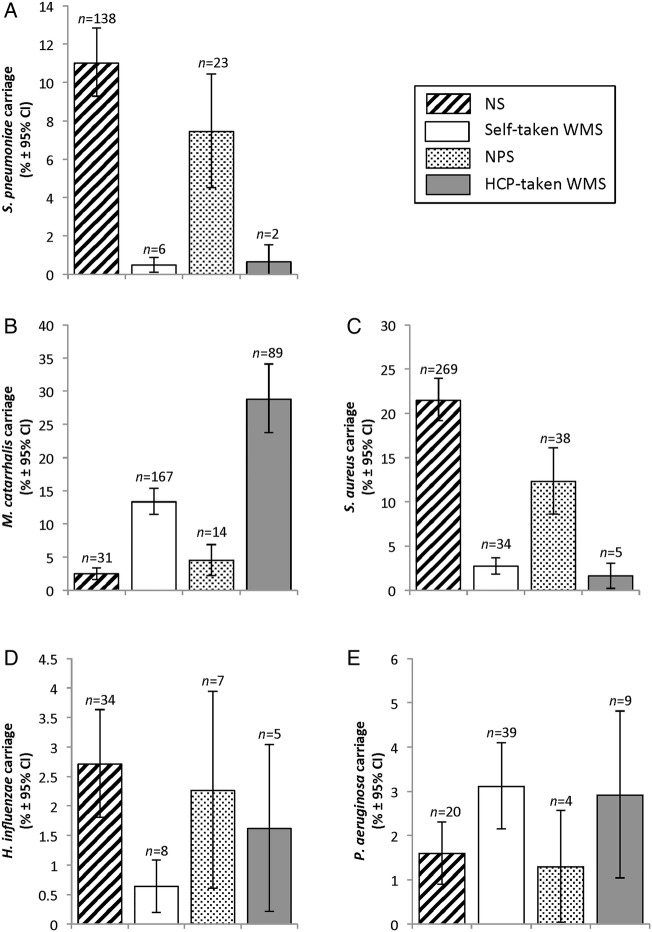
Carriage rates within each swab type (figure 1) show few significant differences between self-swabbing and HCP swabbing. S. pneumoniae carriage was similar between NS and NPS (χ2=3.403, df=1, p=0.075) and between self-taken and HCP-taken WMS (test value=0.139, df=1, p=0.661). M. catarrhalis carriage was similar between NS and NPS (χ2=3.757, df=1, p=0.058) but significantly higher in HCP-taken WMS compared to self-taken WMS (χ2=43.404, df=1, p<0.001). S. aureus carriage was significantly higher in NS than NPS (χ2=13.161, df=1, p<0.001) but was similarly low in self-taken and HCP-taken WMS (χ2=1.218, df=1, p=0.315). H. influenzae carriage was similarly low in NS and NPS (χ2=0.193, df=1, p=0.700) as well as in self-taken and HCP-taken WMS (test value=2.888, df=1, p=0.151). P. aeruginosa carriage was similar in NS and NPS (test value=0.148, df=1, p=1.000) as well as in self-taken and HCP-taken WMS (χ2=0.032, df=1, p=1.000) N. meningitidis was not detected in any swab type used in this study.
Figure 1.
Bacterial Carriage Rates (%) of (A) Streptococcus pneumoniae (B) Moraxella catarrhalis (C) Staphylococcus aureus (D) Haemophilus influenzae (E) Pseudomonas aeruginosa by Swab Method and Site. Graphs are bar charts representing carriage frequencies as percentages. Error bars represent 95% CIs.
Cocarriage rates
Overall cocarriage rates were 3.9% (n=49; N=1219; 95% CI 2.8% to 5%) in NS, 1.1% (n=13; N=1219; 95% CI 0.5% to 1.7%) in self-taken WMS, 2.3% (n=7; N=307; 95% CI 0.6% to 4%) in NPS and 1.6% (n=5; N=307; 95% CI 0.2% to 3%) in HCP-taken WMS. In NS and NPS, cocarriage rates were significantly higher in individuals aged 0–4 years (NS (9.1%; n=30; N=329; 95% CI 6% to 12.2%) and NPS (8.9%; n=5; N=56, 95% CI 1.4% to 16.4%)) versus ≥5 years (NS (2.1%; n=19; N=907; 95% CI 1.2% to 3%) and NPS (1.8%; n=2; N=253, 95% CI 0.2% to 3.4%)). Nose cocarriage decreased with age, with 8% (n=11; N=137, 95% CI 3.5% to 12.5%) in individuals aged 5–17 years, 1.1% (n=5; N=464; 95% CI 0.2% to 2.1%) in individuals aged 18–64 years and 1% (n=3; N=306; 95% CI −0.1% to 2.1%) in those aged ≥65 years. The most common cocarriage relationship in nose swabs was between S. pneumoniae and H. influenzae (50% (n=15; N=30) in 0–4 years, 26.3% (n=5, N=19) in ≥5 years).
Association between demographics and carriage
Participant age
Bacterial carriage was highly variable with age, in particular carriage of S. pneumoniae, H. influenzae M. catarrhalis and S. aureus (tables 2 and 3). Carriage rates of S. pneumoniae and H. influenzae in both NS and NPS decreased with age, with 0–4-year-olds experiencing the highest carriage rates. S. pneumoniae carriage dropped off significantly after 5 years of age with >2× difference in NS and >3× difference in NPS between those aged 0–4 years and those aged 5–17 years. S. pneumoniae carriage in self-taken WMS also showed higher carriage in the young (0–4 years and 5–17 years age groups) compared with adults. H. influenzae nasal carriage decreased more steadily with age. M. catarrhalis nose carriage was also highest in those aged 0–4 years but remained at lower levels in the other age groups. S. aureus nose carriage increased sharply after the age of 5 years but remained high in older children and adults. S. aureus nose carriage was >3× higher in participants aged 5–17 years when compared with participants 0–4 years. P. aeruginosa did not vary between the age groups in any swab type.
Table 2.
Bacterial nose and nasopharyngeal carriage rates of Streptococcus pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, Haemophilus influenzae and Pseudomonas aeruginosa by participant age group, recent RTI, recent antibiotic treatment and vaccination status
| Carriage of bacterial species within nose and nasopharyngeal swabs in different participant categories % (n) (95% CI) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (N) |
S. pneumoniae |
H. influenzae |
M. catarrhalis |
S. aureus |
P. aeruginosa |
|||||||
| Category | SS | HCP | Nose | NP | Nose | NP | Nose | NP | Nose | NP | Nose | NP |
| Age (years) | ||||||||||||
| 0–4 | 329 | 56 | 32.8 (108) (27.7 to 37.9) |
33.9 (19) (21.5 to 46.3) |
7.3 (24) (4.5 to 10.1) |
10.7 (6) (2.6 to 18.8) |
5.8 (19) (3.3 to 8.3) |
10.7 (6) (2.6 to 18.8) |
9.7 (32) (6.5 to 12.9) |
5.4 (3) (−0.5 to 11.3) |
2.7 (9) (1.0 to 4.5) |
1.8 (1) (−1.7 to 5.3) |
| 5–17 | 137 | 22 | 13.1 (18) (7.5 to 18.8) |
9.1 (2) (−2.9 to 21.1) |
5.1 (7) (1.4 to 8.8) |
0.0 (0) NA |
0.7 (1) (−0.7 to 2.1) |
4.5 (1) (−4.1 to 13.2) |
35.0 (48) (27.0 to 43.0) |
13.6 (3) (−0.7 to 27.9) |
0.7 (1) (−0.7 to 2.1) |
0.0 (0) NA |
| 18–64 | 464 | 88 | 1.1 (5) (0.2 to 2.1) |
0.0 (0) NA |
0.2 (1) (−0.2 to 0.6) |
1.1 (1) (−1.1 to 3.3) |
1.5 (7) (0.4 to 2.6) |
3.4 (3) (−0.4 to 7.2) |
24.8 (115) (20.9 to 28.7) |
11.4 (10) (4.8 to 18.0) |
1.3 (6) (0.3 to 2.3) |
1.1 (1) (−1.1 to 3.3) |
| 65+ | 306 | 143 | 2.0 (6) (0.4 to 3.6) |
1.4 (2) (−0.5 to 3.3) |
0.7 (2) (−0.2 to 1.6) |
0.0 (0) NA |
1.3 (4) (0.0 to 2.6) |
2.8 (4) (0.1 to 5.5) |
23.2 (71) (18.5 to 27.9) |
15.4 (22) (9.5 to 21.3) |
1.0 (3) (−0.1 to 2.1) |
1.4 (2) (−0.5 to 3.3) |
| p Value | <0.001* | <0.001 | <0.001 | <0.001 | 0.001 | 0.100 | <0.001* | 0.263 | 0.288 | 1.000 | ||
| Recent respiratory tract infection | ||||||||||||
| Yes | 363 | 59 | 22.3 (81) (18.0 to 26.6) |
15.3 (9) (6.1 to 24.5) |
5.2 (19) (2.9 to 7.5) |
6.8 (4) (0.4 to 13.2) |
3.6 (13) (1.7 to 5.5) |
3.4 (2) (−1.2 to 8.0) |
19.3 (70) (15.2 to 23.4) |
6.8 (4) (0.4 to 13.2) |
2.2 (8) (0.7 to 3.7) |
3.4 (2) (−1.2 to 8.0) |
| No | 856 | 247 | 6.3 (54) (4.7 to 7.9) |
5.7 (14) (2.8 to 8.6) |
1.6 (14) (0.8 to 2.4) |
1.2 (3) (−0.2 to 2.6) |
2.1 (18) (1.1 to 3.1) |
4.9 (12) (2.2 to 7.6) |
22.3 (191) (19.5 to 25.1) |
13.8 (34) (9.5 to 18.1) |
1.3 (11) (0.5 to 2.1) |
0.8 (2) (−0.3 to 1.9) |
| p Value | <0.001* | 0.023 | 0.001* | 0.028 | 0.163* | 1.000 | 0.253* | 0.188* | 0.310* | 0.169 | ||
| Recent use of antibiotics | ||||||||||||
| Yes | 101 | 26 | 5.9 (6) (1.3 to 10.5) |
3.8 (1) (−3.6 to 11.2) |
1.0 (1) (−0.9 to 2.9) |
0.0 (0) NA |
1.0 (1) (−0.9 to 2.9) |
3.8 (1) (−3.6 to 11.2) |
15.8 (16) (8.7 to 22.9) |
0.0 (0) NA |
1.0 (1) (−0.9 to 2.9) |
7.7 (2) (−2.6 to 18.0) |
| No | 1118 | 281 | 11.5 (129) (9.6 to 13.4) |
7.8 (22) (4.7 to 10.9) |
2.9 (32) (1.9 to 3.9) |
2.5 (7) (0.7 to 4.3) |
2.7 (30) (1.8 to 3.7) |
4.6 (13) (2.2 to 7.1) |
21.7 (243) (19.3 to 24.1) |
13.5 (38) (9.5 to 17.5) |
1.5 (17) (0.8 to 2.2) |
0.7 (2) (−0.3 to 1.7) |
| p Value | 0.097* | 0.706 | 0.515 | 1.000 | 0.508 | 1.000 | 0.203* | 0.056 | 1.000 | 0.037 | ||
| Vaccinations up-to-date | ||||||||||||
| Yes | 1017 | 265 | 12.8 (130) (10.8 to 14.9) |
8.7 (23) (5.3 to 12.1) |
3.0 (31) (2.0 to 4.1) |
2.6 (7) (0.7 to 4.5) |
2.8 (28) (1.8 to 3.8) |
4.5 (12) (2.0 to 7.0) |
20.4 (207) (17.9 to 22.9) |
13.2 (35) (9.1 to 17.3) |
1.6 (16) (0.8 to 2.4) |
1.5 (4) (0.0 to 3.0) |
| No | 40 | 10 | 5.0 (2) (−1.8 to 11.8) |
0.0 (0) NA |
2.5 (1) (−2.3 to 7.3) |
0.0 (0) NA |
2.5 (1) (−2.3 to 7.3) |
10.0 (1) (−8.6 to 28.6) |
25.0 (10) (11.6 to 38.4) |
0.0 (0) NA |
2.5 (1) (−2.3 to 7.3) |
0.0 (0) NA |
| p Value | 0.219 | 1.000 | 1.000 | 1.000 | 1.000 | 0.389 | 0.548* | 0.621 | 0.484 | 1.000 | ||
χ2 (indicated by *) and Fisher's exact tests for independence were used to determine significant differences between bacterial carriage rates in different age groups, with/without recent RTI, with/without recent antibiotic treatment and with/without an up-to-date vaccination status. p Values are 2-tailed. 95% CI are written as (upper CI to lower CI).
NP, nasopharyngeal swab; NA, not applicable.
Table 3.
Bacterial self-taken and HCP-taken whole mouth swab carriage rates of Streptococcus pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, Haemophilus influenzae and Pseudomonas aeruginosa by participant age group, recent RTI, recent antibiotic treatment and vaccination status
| Carriage of bacterial species within mouth swabs in different participant categories % (n) (95% CI) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (N) |
S. pneumoniae |
H. influenzae |
M. catarrhalis |
S. aureus |
P. aeruginosa |
|||||||
| Category | SS | HCP | Self-taken WMS | HCP-taken WMS | Self-taken WMS | HCP-taken WMS | Self-taken WMS | HCP-taken WMS | Self-taken WMS | HCP-taken WMS | Self-taken WMS | HCP-taken WMS |
| Age (years) | ||||||||||||
| 0–4 | 329 | 56 | 1.2 (4) (0.0 to 2.4) |
3.6 (2) (−1.3 to 8.5) |
1.2 (4) (0.0 to 2.4) |
5.4 (3) (−0.5 to 11.3) |
11.9 (39) (8.4 to 15.4) |
35.7 (20) (23.2 to 48.3) |
2.4 (8) (0.8 to 4.1) |
0.0 (0) NA |
4.9 (16) (2.6 to 7.2) |
3.6 (2) (−1.3 to 8.5) |
| 5–17 | 137 | 22 | 1.5 (2) (−0.5 to 3.5) |
0.0 (0) NA |
0.0 (0) NA |
0.0 (0) NA |
11.7 (16) (6.3 to 17.1) |
27.3 (6) (8.7 to 45.9) |
4.4 (6) (1.0 to 7.8) |
4.5 (1) (−4.2 to 13.2) |
3.6 (5) (0.5 to 6.7) |
0.0 (0) NA |
| 18–64 | 464 | 88 | 0.0 (0) NA |
0.0 (0) NA |
0.9 (4) (0.0 to 1.8) |
1.1 (1) (−1.1 to 3.3) |
15.3 (71) (12.0 to 18.6) |
22.7 (20) (14.0 to 31.5) |
3.0 (14) (1.5 to 4.6) |
2.3 (2) (−0.8 to 5.4) |
1.7 (8) (0.5 to 2.9) |
2.3 (2) (−0.8 to 5.4) |
| 65+ | 306 | 143 | 0.0 (0) NA |
0.0 (0) NA |
0.0 (0) NA |
0.7 (1) (−0.7 to 2.1) |
13.1 (40) (9.3 to 16.9) |
30.1 (43) (22.6 to 37.6) |
1.6 (5) (0.2 to 3.0) |
1.4 (2) (−0.5 to 3.3) |
2.9 (9) (1.0 to 4.8) |
3.5 (5) (0.5 to 6.5) |
| p Value | 0.006 | 0.063 | 0.204 | 0.159 | 0.476* | 0.390* | 0.361 | 0.377 | 0.079 | 0.910 | ||
| Recent respiratory tract infection | ||||||||||||
| Yes | 363 | 59 | 0.8 (3) (−0.1 to 1.7) |
1.7 (1) (−1.6 to 5.0) |
0.8 (3) (−0.1 to 1.7) |
1.7 (1) (−1.6 to 5.0) |
10.5 (38) (7.4 to 13.7) |
25.4 (15) (14.3 to 36.5) |
2.5 (9) (0.9 to 4.1) |
0.0 (0) NA |
3.9 (14) (1.9 to 5.9) |
5.1 (3) (−0.5 to 10.7) |
| No | 856 | 247 | 0.4 (3) (0.0–0.8) |
0.4 (1) (−0.4 to 1.2) |
0.6 (5) (0.1 to 1.1) |
1.6 (4) (0.0 to 3.2) |
14.7 (126) (12.3 to 17.1) |
29.6 (73) (23.9 to 35.3) |
2.7 (23) (1.6 to 3.8) |
1.6 (4) (0.0 to 3.2) |
2.8 (24) (1.7 to 3.9) |
2.4 (6) (0.5 to 4.3) |
| p Value | 0.370 | 0.349 | 0.701 | 1.000 | 0.054* | 0.632* | 0.850* | 1.000 | 0.368* | 0.382 | ||
| Recent use of antibiotics | ||||||||||||
| Yes | 101 | 26 | 0.0 (0) NA |
0.0 (0) NA |
0.0 (0) NA |
0.0 (0) NA |
14.9 (15) (8.0 to 21.8) |
23.1 (6) (6.9 to 39.3) |
3.0 (3) (−0.3 to 6.3) |
3.8 (1) (−3.6 to 11.2) |
2.0 (2) (−0.7 to 4.7) |
3.8 (1) (−3.6 to 11.2) |
| No | 1118 | 281 | 0.5 (6) (0.1 to 0.9) |
0.7 (2) (−0.3 to 1.7) |
0.7 (8) (0.2 to 1.2) |
1.8 (5) (0.3 to 3.4) |
13.4 (150) (11.4 to 15.4) |
29.2 (82) (23.9 to 34.5) |
2.6 (29) (1.7 to 3.5) |
1.4 (4) (0.0 to 2.8) |
3.2 (36) (2.2 to 4.2) |
2.8 (8) (0.9 to 4.7) |
| p Value | 1.000 | 1.000 | 1.000 | 1.000 | 0.761* | 0.652* | 0.744 | 0.360 | 0.764 | 0.554 | ||
| Vaccinations up-to-date | ||||||||||||
| Yes | 1017 | 265 | 0.6 (6) (0.1 to 1.1) |
0.8 (2) (−0.3 to 1.9) |
0.6 (6) (0.1 to 1.1) |
1.9 (5) (0.3 to 3.5) |
13.7 (139) (11.6 to 15.8) |
29.4 (78) (23.9 to 34.9) |
2.8 (28) (1.8 to 3.8) |
1.5 (4) (0.0 to 3.0) |
3.2 (33) (2.1 to 4.3) |
3.0 (8) (1.0 to 5.1) |
| No | 40 | 10 | 0.0 (0) NA |
0.0 (0) NA |
2.5 (1) (−2.3 to 7.3) |
0.0 (0) NA |
5.0 (2) (−1.8 to 11.8) |
50.0 (5) (19.0 to 81.0) |
2.5 (1) (−2.3 to 7.3) |
0.0 (0) NA |
2.5 (1) (−2.3 to 7.3) |
0.0 (0) NA |
| p Value | 1.000 | 1.000 | 0.237 | 1.000 | 0.153* | 0.175 | 1.000 | 1.000 | 1.000 | 1.000 | ||
χ2 (indicated by *) and Fisher's exact tests for independence were used to determine significant differences between bacterial carriage rates in different age groups, with/without recent RTI, with/without recent antibiotic treatment and with/without an up-to-date vaccination status. p Values are 2-tailed. 95% CI are written as (upper CI to lower CI).
NA, not applicable; WMS, whole mouth swab.
Participant questionnaire information
Higher nasal and NP carriage rates of S. pneumoniae and H. influenzae were observed in participants who had experienced a recent RTI. S. pneumoniae nose carriage was >3× higher in those with recent RTI versus those without recent RTI, using χ2 (χ2=66.408, df=1, p<0.001). H. influenzae nose carriage was also >2× higher in those with recent RTI versus those without recent RTI, using the χ2 test (χ2=12.533, df=1, p=0.001). Recent antibiotic treatment was only significant in P. aeruginosa NP carriage, where recent antibiotics use was associated with increased carriage of this bacterium (test value=9.018, df=1, p=0.037). Vaccination status was not associated with significant changes in carriage of any of the target bacteria. Full results are shown in tables 2 and 3. In NS, recent RTI was also associated with higher co-carriage rates at 8% (n=29) when compared with no recent RTI at 2.2% (n=19). Recent antibiotic use, vaccination status and geographical location did not appear to affect co-carriage rates.
Geographical location
Carriage rates of the target bacterial species showed some differences according to practice location (see online supplementary figure S2). Overall bacterial carriage was significantly different by geographical area in NS (χ2=11.609, df=5, p=0.04) and self-taken WMS (χ2=13.900, df=5, p=0.02) but not in either HCP swab. However, individual bacteria carriage rates were not significantly different between geographical areas.
Deprivation
Participants attending practices in less deprived locations had slightly higher bacterial carriage rates, except for P. aeruginosa, suggesting a possible negative relationship between deprivation score and bacterial carriage. However, the differences observed were not statistically significant.
Study costs
Overall, total costs per participant were over a third lower in the self-swabbing group at £41.21 ($67.92) versus the HCP group at £69.66 ($114.82; table 1). NHS service support costs made up a large proportion of the difference between the two study groups, representing 56.7% (£39.52/person) of costs in the HCP group but only 6.8% (£2.81/person) of costs in the self-swabbing group.
Discussion
Our study demonstrates that self-swabbing is as effective in detecting bacterial pathogens in the respiratory tract as HCP swabbing and that nose swabs could be used more routinely to detect the presence of bacterial pathogens S. pneumoniae, H. influenzae, S. aureus and P. aeruginosa. WMS, on the other hand, are the most sensitive swab for detection of M. catarrhalis. The swabs used in this study were not sensitive for detection of N. meningitidis.
Higher participation rates within the self-swabbing group compared with the HCP group highlight the willingness of individuals to participate in such studies when the process is facilitated. The very low participation rate of the HCP group would render this method invalid for large-scale studies. While the responsiveness of the self-swabbing group was higher, it was still less than the anticipated 25%, meaning there will always be a problem of non-response bias. However, similar carriage rates were observed in our study when compared with previous swabbing studies, demonstrating that our sample size is large enough to overcome differences that may result from non-response bias. Barriers to participation in the HCP group might include the amount of time required for organising and attending swabbing appointments and the slight discomfort experienced during nasopharyngeal swabbing. Self-swabbing overcame many of these barriers by offering a relatively straightforward, rapid and easy alternative. High participation rates in elderly participants might be a result of their increased availability for participation and their increased chance of exposure to RTI allowing them to relate to the study aims. Parents may also be reluctant to swab their children if they are very young. The negative correlation between participation rates and deprivation highlights certain barriers associated with high levels of deprivation, which have been observed in other studies.25
Swab positivity rates and bacterial carriage rates indicate that self-swabbing is as effective as HCP swabbing in sampling microbial species within the airways of the general population within our large population-based study. Higher positivity rates in NS versus NPS and higher carriage of S. aureus within NS versus NPS demonstrate the potential for using a self-taken NS rather than HCP-taken NPS to detect respiratory pathogens. Higher positivity rates in HCP-taken WMS versus self-taken WMS and higher carriage of M. catarrhalis within HCP-taken WMS demonstrate the sensitivity of HCP-swabbing. However, lower participation rates with fewer children and more elderly participants within HCP swabbing have most probably resulted in reduced carriage rates within NPS. Self-swabbing allowed the recruitment of a greater spread of age groups, which is essential for obtaining a true estimate of carriage. Very low participation in the HCP group is problematic for assessing carriage within the general population as fewer numbers of samples can be obtained and the cost of obtaining them is high. In order to obtain the same spread of ages as the self-swabbing group, a much larger number of individuals would need to be invited. The high costs of HCP swabbing are mainly due to the operation of swabbing clinics. In order to increase participation, healthcare providers could undertake verbal encouragement or study advertisement in practice. WMS were efficient in isolating M. catarrhalis and P. aeruginosa, however, large amounts of background flora within this site and low isolation levels for the other bacteria render this swab less efficient on the whole. The lack of isolation of N. meningitidis may be due to the type of swabs used, as oropharyngeal swabs are often preferred.26 Low response rates from teenagers, the most frequent carriers of N. meningitidis, may also have caused the lack of isolation of this species.27
Carriage rates of five out of the six target organisms follow previously observed patterns with S. pneumoniae and H. influenzae being carried predominantly in young children and S. aureus being carried more in older children and adults.12 28 29 M. catarrhalis and P. aeruginosa carriage rates were constant across all age groups demonstrating that carriage of these organisms is unaffected by age. N. meningitidis carriage did not follow previously observed patterns as no isolates were detected. However, the number of participants in the study may not have been large enough to detect any isolates with 95% confidence. Furthermore, swab types used and turn-around times from swabbing to sample processing may not be optimal for N. meningitidis recovery. The effect of recent RTI on carriage of S. pneumoniae and H. influenzae is one that might be expected as colds and flu weaken host immunity allowing for carriage by these organisms.30 The lack of an apparent effect of vaccination status is potentially due to herd immunity, as unvaccinated people benefit from protection from disease as a result of a largely vaccinated population.31 However, further details of vaccines received via access to individual participant immunisation records in future studies might enable improved assessment of the effects of immunisation on carriage of target and non-target bacteria.
This pilot study has also enabled all aspects of study set-up through to completion to be tried and tested, which will be essential for setting up larger swabbing studies. Study documentation, study protocol, ethics application and sample size calculations have been trialled and alterations can now be performed on further studies in order to improve outcomes and efficiency. Limitations, including numbers of non-responses, can be improved in further studies in order to increase confidence in study outcomes. The results from this pilot study have allowed the comparison of swabbing methodologies for determining carriage of the targeted bacterial species within the respiratory tract. The advantages of self-swabbing are evident with higher responsiveness and lower costs than HCP swabbing. Further assessment will determine whether our findings are applicable to other geographical locations, over time and to a wider array of bacterial species. Such assessment would help to refine methodologies, which will be key to obtaining a precise understanding of bacterial carriage in the respiratory tract.
Supplementary Material
Acknowledgments
The authors thank Shabana Hussain and Karen Cox for technical assistance throughout the study. The authors also thank the Bupa Foundation for providing the funding to SCC in order to undertake the study and the Rosetrees Trust for their funding contribution for ALC's PhD studentship. The authors thank the Southampton NIHR Wellcome Trust Clinical Research Facility for part-funding SNF. The authors also thank the National Institute for Health Research Comprehensive Local Research Network (NIHR CLRN), NHS Service Support Costs, Solent Primary Care Trust (PCT), South West (East Hub) Primary Care Research Network (PCRN) and Danvers International for their contributions.
Footnotes
Contributors: ALC was involved in study set-up, data collection, data analysis and writing. RNW was involved in study set-up, data collection and proofreading of manuscript. NB and RA were involved in data collection, proof-reading of manuscript. AT was involved in study design, data collection and proof-reading of manuscript. SNF, JMJ, HMY, PJR, MAM and MVM were involved in study design, data analysis, proofreading of manuscript. SCC was involved in study design, data collection, data analysis and proof-reading of manuscript.
Funding: This work was supported by the Rosetrees Trust [M141 to SCC] and the Bupa Foundation [TBF-M11-019 to SCC].
Competing interests: SNF receives support from the National Institute for Health Research funding via the Southampton NIHR Wellcome Trust Clinical Research Facility and the Southampton NIHR Respiratory Biomedical Research Unit. JMJ has received consulting fees from GlaxoSmithKline. SNF acts as principal investigator for clinical trials conducted on behalf of University Hospital Southampton NHS Foundation Trust/University of Southampton that are sponsored by vaccine manufacturers but receives no personal payments from them. SNF has participated in advisory boards for vaccine manufacturers but receives no personal payments for this work. SCC currently receives unrestricted research funding from Pfizer Vaccines (previously Wyeth Vaccines) and has participated in advisory boards and expert panels for GSK, Pfizer and Novartis. SCC is an investigator on studies conducted on behalf of University Hospital Southampton NHS Foundation Trust/University of Southampton/Public Health England that are sponsored by vaccine manufacturers but receives no personal payments from them. SNF, SCC and JMJ have received financial assistance from vaccine manufacturers to attend conferences. All grants and honoraria are paid into accounts within the respective NHS Trusts or Universities, or to independent charities. All other authors have no conflicts of interest.
Ethics approval: National Research Ethics Service (NRES).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Bogaert D, Keijser B, Huse S, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS ONE 2011;6:e17035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faden H, Duffy L, Wasielewski R, et al. Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis 1997;175:1440–5 [DOI] [PubMed] [Google Scholar]
- 3.Brueggemann AB, Griffiths DT, Meats E, et al. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis 2003;187:1424–32 [DOI] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akmatov MK, Krebs S, Preusse M, et al. E-mail-based symptomatic surveillance combined with self-collection of nasal swabs: a new tool for acute respiratory infection epidemiology. Int J Infect Dis 2011;15:e799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach AJ, Stubbs E, Hare K, et al. Comparison of nasal swabs with nose blowing for community-based pneumococcal surveillance of healthy children. J Clin Microbiol 2008;46:2081–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberman D, Shleyfer E, Castel H, et al. Nasopharyngeal versus oropharyngeal sampling for isolation of potential respiratory pathogens in adults. J Clin Microbiol 2006;44:525–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trotter CL, Gay NJ. Analysis of longitudinal bacterial carriage studies accounting for sensitivity of swabbing: an application to Neisseria meningitidis. Epidemiol Infect 2003;130:201–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt JP, O'Brien KL, Katz S, et al. Nasopharyngeal versus oropharyngeal sampling for detection of pneumococcal carriage in adults. J Clin Microbiol 2004;42:4974–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapola S, Salo E, Kiiski P, et al. Comparison of four different sampling methods for detecting pharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae in children. J Clin Microbiol 1997;35:1077–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg D, Broides A, Blancovich I, et al. Relative importance of nasopharyngeal versus oropharyngeal sampling for isolation of Streptococcus pneumoniae and Haemophilus influenzae from healthy and sick individuals varies with age. J Clin Microbiol 2004;42:4604–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamblin J, Jefferies JM, Harris S, et al. Nasal self-swabbing for estimating the prevalence of Staphylococcus aureus in the community. J Med Microbiol 2013;62:437–40 [DOI] [PubMed] [Google Scholar]
- 13.Satzke C, Turner P, Virolainen-Julkunen A, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013;32:165–79 [DOI] [PubMed] [Google Scholar]
- 14.Akmatov MK, Gatzemeier A, Schughart K, et al. Equivalence of self- and staff-collected nasal swabs for the detection of viral respiratory pathogens. PLoS ONE 2012;7:e48508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Roux A, Ewig S, Garcia E, et al. Mixed community-acquired pneumonia in hospitalised patients. Eur Respir J 2006;27:795–800 [DOI] [PubMed] [Google Scholar]
- 16.Harro CD, Betts RF, Hartzel JS, et al. The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): results of two Phase I studies. Vaccine 2012;30:1729–36 [DOI] [PubMed] [Google Scholar]
- 17.Moore R, Kyd JM, Carzino R, et al. Mucosal and systemic antibody responses to potential Pseudomonas aeruginosa vaccine protein antigens in young children with cystic fibrosis following colonization and infection. Hum Vaccin Immunother 2012;9:506–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serruto D, Bottomley MJ, Ram S, et al. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 2012;30:B87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheldon E, Schwartz H, Jiang Q, et al. A Phase 1, randomized, open-label, active-controlled trial to assess the safety of a meningococcal serogroup B bivalent rLP2086 vaccine in healthy adults. Hum Vaccin Immunother 2012;8:888–95 [DOI] [PubMed] [Google Scholar]
- 20.Skinner JM, Indrawati L, Cannon J, et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine 2011;29:8870–6 [DOI] [PubMed] [Google Scholar]
- 21.Tocheva AS, Jefferies JM, Rubery H, et al. Declining serotype coverage of new pneumococcal conjugate vaccines relating to the carriage of Streptococcus pneumoniae in young children. Vaccine 2011;29:4400–4 [DOI] [PubMed] [Google Scholar]
- 22.UK Department for Communities and Local Government. English indices of deprivation 2010 [Docment on the Internet]. London: Department for Communities and Local Government, 2011. [cited 2014 August 22]. https://www.gov.uk/government/publications/english-indices-of-deprivation-2010 [Google Scholar]
- 23.Chapman KE, Wilson D, Gorton R. Invasive pneumococcal disease and socioeconomic deprivation: a population study from the North East of England. J Public Health 2013;35:558–69 [DOI] [PubMed] [Google Scholar]
- 24.ESRI. ArcGiS 10.1. Redlands, California: ESRI, 2012 [Google Scholar]
- 25.Sheron N, Moore M, O'Brien W, et al. Feasibility of detection and intervention for alcohol-related liver disease in the community: the Alcohol and Liver Disease Detection study (ALDDeS). Br J Gen Pract 2013;63:e698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olcen P, Kjellander J, Danielsson D, et al. Culture diagnosis of meningococcal carriers: yield from different sites and influence of storage in transport medium. J Clin Pathol 1979;32:1222–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartwright KA, Stuart JM, Jones DM, et al. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect 1987;99:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray BM, Converse GM, Dillon HC. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 1980;142:923–33 [DOI] [PubMed] [Google Scholar]
- 29.Peltola H, Kilpi T, Anttila M. Rapid disappearance of Haemophilus influenzae type b meningitis after routine childhood immunisation with conjugate vaccines. Lancet 1992;340:592–4 [DOI] [PubMed] [Google Scholar]
- 30.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis 2006;6:303–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ 2003;326:365–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.