Abstract
Endothelial-mesenchymal transition (EndoMT) has been recognized as a key determinant of tumor microenvironment in cancer progression and metastasis. Endothelial cells undergoing EndoMT lose their endothelial markers, acquire the mesenchymal phenotype, and become more invasive with increased migratory abilities. Early stages of esophageal adenocarcinoma (EAC) are characterized by strong microvasculature whose impact in tumor progression remains undefined. Our aim was to determine the role of EndoMT in EAC by investigating the impact of tumor cells on normal primary human esophageal microvascular endothelial cells (HEMEC). HEMEC were either cocultured with OE33 adenocarcinoma cells or treated with IL-1β and transforming growth factor-β2 (TGF-β2) for indicated periods and analyzed for EndoMT-associated changes by real-time PCR, Western blotting, immunofluorescence staining, and functional assays. Additionally, human EAC tissues were investigated for detection of EndoMT-like cells. Our results demonstrate an increased expression of mesenchymal markers [fibroblast-specific protein 1 (FSP1), collagen1α2, vimentin, α-smooth muscle actin (α-SMA), and Snail], decreased expression of endothelial markers [CD31, von Willebrand factor VIII (vWF), and VE-cadherin], and elevated migration ability in HEMEC following coculture with OE33 cells. The EndoMT-related changes were inhibited by IL-1β and TGF-β2 gene silencing in OE33 cells. Recombinant IL-1β and TGF-β2 induced EndoMT in HEMEC. Although the level of VEGF expression was elevated in EndoMT cells, the angiogenic property of these cells was diminished. In vivo, by immunostaining EndoMT-like cells were detected at the invasive front of EAC. Our findings underscore a significant role for EndoMT in EAC and provide new insights into the mechanisms and significance of EndoMT in the context of tumor progression.
Keywords: EndoMT, HEMEC, EAC, TGF-β, IL-1
tumor growth is determined not only by tumor cells themselves but also by stromal cells such as fibroblasts, endothelial cells, as well as a variety of infiltrating immune cells including macrophages (32). Interactions between tumor and stromal cells can activate the stromal cells in a way to distinctly differ from the corresponding normal stromal cells, thereby resulting in a unique microenvironment that promotes tumor progression (7, 15). This tumor-mediated specific stroma is mainly characterized by the ingrowths of new blood vessels as well as activated fibroblasts [cancer-associated fibroblasts (CAF)] both of which play critical functions in sustaining cell proliferation, promoting survival, activating invasion and metastasis, and reprogramming energy metabolism of the tumor (3, 32).
Endothelial cells represent a major cellular component of the tumor microenviroment. Their role in pathologic tumor progression has been linked mainly to the formation of new blood vessels from preexisting ones, a process known as angiogenesis (5). Increased expression of the vascular endothelial growth factor (VEGF), the natural stimulus of endothelial cells, contributes to tumor angiogenesis and presumably to tumor growth and hematogenous spread of tumor cells (21). Novel target therapies that aim to inhibit tumor angiogenesis by blocking VEGF and endothelial cells function have enriched the armamentarium of cancer treatment in many solid malignancies by demonstrating significant survival benefits (26). However, recent evidence has supported that endothelial cells demonstrate, apart from their angiogenic properties, a remarkable plasticity within the tumor microenviroment and can also give rise to CAF through a process called endothelial-to-mesenchymal transition (EndoMT) (21).
EndoMT is characterized by 1) endothelial cells loss of cell-cell junctions along with cytoskeletal alterations to induce elongated and spindle-shaped cells; 2) acquisition of invasive and migratory properties; 3) loss of endothelial markers, such platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), von Willebrand factor-VIII (vWF-VIII), and vascular endothelial-cadherin (VE-cadherin); 4) gain of mesenchymal markers, such as fibroblast-specific protein 1 (FSP1), type I collagen, and α-smooth muscle actin (α-SMA); as well as 5) increased expression of transcription factors such as Snail and Slug (21, 29). EndoMT is essential during embryonic development and tissue regeneration, but recent studies point to its role in pathological processes such as tissue fibrosis and cancer (30, 31). With regard to cancer, EndoMT is now recognized as a unique source of CAF, accounting for the up to 40% of CAF found in tumors (8). Nevertheless, the role of EndoMT in tumor progression, in contrast to the well-defined role of epithelial-mesenchymal transition (EMT) in tumor invasion and metastasis (28), remains largely unknown.
Transforming growth factor-β (TGF-β) has been shown to play pivotal role in EndoMT, like in EMT (28). Many studies have pointed to the important role of TGF-β receptor signaling and downstream targets, such as RhoA, Smad, and Snail transcriptional repressor in EndoMT (11, 18). Additionally, interleukin-1β (IL-1β) has been found to also initiate the transition of endothelial cells to mesenchymal cells in corneal endothelial cells (14). Interestingly, a recent report demonstrated strong synergistic induction of EndoMT by IL-1β and TGF-β2 in an NF-κB-dependent manner (17) signifying the complexity of the mechanisms underlying the pathological EndoMT.
Esophageal adenocarcinoma (EAC) is the most rapidly increasing malignancy in the Western world with the mortality rate still exceeding 80%, due to high rates of local recurrence and early lymph node or systemic micrometastases (27). Despite improvements in chemotherapy regimens and the use of multimodal strategies (surgery, neo-adjuvant, and adjuvant), EAC remains a therapeutic challenge for oncologists (27). Its biology is still not well understood and limited data exist on the role of its tumor stroma. Isolated reports have shown that angiogenic properties are acquired in early stages of EAC progression, particularly in the precancerous lesion of Barrett's metaplasia, before invasive carcinoma develops (9), indicating an increased role of microvasculature in esophageal tumorigenesis.
The present study was designed to explore the role of EndoMT in EAC. We show that primary human esophageal microvascular endothelial cells (HEMEC) undergo EndoMT when cocultured with EAC cells in vitro. The synergistic effects of tumor-derived IL-1β and TGF-β2 predominantly mediated this process. Furthermore, we demonstrate that the endothelial cells with fibroblast-like phenotype consist of a source of high amounts of VEGF at the invasive front part of the tumor. Taken together, our findings underlay a significant role of EndoMT in EAC and provide new insights into the mechanisms and significance of EndoMT in the context of tumor progression.
MATERIALS AND METHODS
Reagents.
Fetal bovine serum and RPMI 1640 were obtained from Life Technologies (Grand Island, NY). Collagenase type II was purchased from Worthington Biochem (Lakewood, NJ). Endothelial cell growth supplement, fibronectin, TGF-β1, and TGF-β2 antibodies were obtained from EMD Millipore (Billerica, MA). RNeasy RNA Extraction Kit was purchased from Qiagen (Valencia, CA). iScript cDNA Synthesis Kit, and SYBR Green Master Mix were obtained from Bio-Rad (Hercules, CA). FSP1, α-SMA, and SM22α antibodies and recombinant human TGF-β2 were purchased from Abcam (Cambridge, MA). CD31, α-SMA antibodies, recombinant human IL-1β, TGF-β2, and IL-1β blocking antibodies and ELISA kits for intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), VEGF, and TGF-β2 and Snail antibodies suitable for immunofluorescence staining were obtained from R&D Systems (Minneapolis, MN). VE-cadherin, Snail, cyclooxygenase 2 (COX2), NF-κB p65, and IL-1β antibodies were purchased from Cell Signaling (Danvers, MA). vWF antibody was obtained from Dako (Carpinteria, CA). Collagen1α2 antibody suitable for immunofluorescence staining was obtained from Novus Biologicals (Littleton, CO). Vimentin and β-actin antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Secondary antibodies and endothelial nitric oxide synthase (eNOS) antibody were purchased from Santa Cruz Technology (Santa Cruz, CA). The Cell Contraction Assay Kit was obtained from Cell Biolabs (San Diego, CA). Unless otherwise indicated, all other chemicals were purchased from Sigma-Aldrich.
Tissue and cell culture.
The use of human tissue, cells, and all experiments were approved by the Institutional Review Board of the Medical College of Wisconsin. Primary HEMEC were isolated as described previously (22). Endothelial cultures were recognized by modified lipoprotein uptake (Dil-ac-LDL; Life Technologies, Grand Island, NY) and expression of Factor VIII associated antigen. Primary human intestinal microvascular endothelial cells (HIMEC) were isolated from surgical tissue as described previously (22).
EAC cells (OE33 and OE19) were purchased from Sigma-Aldrich. Human colorectal adenocarcinoma cell line (HT-29) was obtained from ATCC (American Type Culture Collection, Manassas, VA) and grown in DMEM media (GIBCO, Life Technology) containing 2.5% penicillin, streptomycin, fungizone (PSF), d-glucose, l-glutamine, and 1% serum. Endothelial cells were maintained in MCDB-131 media containing 2.5% PSF, 0.003% endothelial growth supplements (platelet-derived endothelial cell growth factor; ECGF1), and 10% FBS. OE33 cells were maintained in RPMI 1640 supplemented with 10% FBS. Slides of human EAC tissue sections (n = 3) were obtained from the Department of Pathology, Medical College of Wisconsin.
OE33 and HEMEC coculture.
HEMEC (1 × 105·ml−1·well−1) were cultured in fibronectin precoated six-well plates, OE33 cells (2 × 104·ml−1·insert−1) were seeded in 8.0-μm cell culture inserts and then carefully placed on top of six-well plates containing MCDB-131 and growth supplement media. During the course of experiments, the growth conditions remained the same between control HEMEC and coculture. Possible toxicity of MCDB-131 media toward OE33 was excluded in optimization experiments. After 3, 6, and 10 days, inserts were removed, and RNA and cell lysate were collected from HEMEC and OE33 for gene and protein analysis. For staining, cells were grown on coverslips and followed the protocol as described above.
Conditioned media.
OE33 (2 × 104·ml−1·well−1) cells were grown in MCDB-131 containing growth supplements and FBS for 24 h. The culture media were removed, filtered, and called conditioned media. Then, HEMEC were cultured in the conditioned media for 6 days. After the indicated time, RNA and protein were extracted for future analysis. For immunofluorescence staining, HEMEC (1 × 103/ml) were seeded on coverslips and grown to 75% confluence in the conditioned media followed the established protocol as above.
HEMEC treatment with IL-1β and TGF-β.
HEMEC were grown as above and then treated with IL-1β (100 U/ml) or TGF-β2 (10 ng/ml) or a combination of both IL-1β and TGF-β2 for the indicated time periods (3, 6, and 10 days). RNA, protein, and culture media were collected for further analysis. Morphological changes were observed by inverted microscopy. Note: the concentrations chosen for IL-1β and TGF-β2 are based on our previous work with HEMEC.
Immunofluorescence staining.
EndoMT markers were determined using primary antibodies against CD31, vWF, VE-cadherin, vimentin, FSP1, α-SMA, COL1A2, and Snail and an Alexa secondary antibody as described previously (20). Paraffin-embedded EAC specimens (5-μm sections) were obtained from the Department of Pathology, Medical College of Wisconsin and stained with hematoxylin-eosin for histological diagnosis. The sections were subjected to immunofluorescence staining using the above antibodies, as described previously (22).
Western blot analysis.
SDS-PAGE and imunoblot analysis were performed as described previously (22), using the antibodies mentioned above.
Real-time PCR.
RNA was isolated using Qiagen's RNeasy Plus Mini Kit according to the manufacturer's instructions. Reverse transcription was done with 1 μg of RNA using the Bio-Rad cDNA Synthesis Kit. Genes expression were analyzed via real-time PCR using SYBR Green Master Mix, 2 μl of cDNA, and 250 nM primer in 25-μl reactions. Cycling parameters were 95°C for 30 s and then 40 cycles of 95°C for 5 s and the annealing temperature for 10 s. The annealing temperatures are included in Table 1. Generation of a single product was confirmed with a melt cycle. Real-time data were analyzed using Bio-Rad CFX software. All primers were obtained from Integrated DNA Technology (IDT, Skokie, IL). Primer sequences are listed in Table 1.
Table 1.
Sequence of primers used for RT-PCR analysis
| Gene/Sequence | Annealing Temperature, °C |
|---|---|
| β-Actin | |
| F 5′-CAC TCT TCC AGC CTT CCT TC-3′ | ∼ |
| R 5′-GGT GTA ACG CAA CTA AGT CAT AG-3′ | |
| GAPDH | |
| F 5′-TGC ACC ACC AAC TGC TTA GC-3′ | ∼ |
| R 5′-GGC ATG GAC TGT GGT CAT GAG-3′ | |
| CD31 | |
| F 5′-TAT CCA AGG TCA GCA GCA TCG TGG-3′ | 61.4 |
| R 5′-GGG TTG TCT TTG AAT ACC GCA G-3′ | |
| VE-cadherin | |
| F 5′-TGT GGG CTC TCT GTT TGT TG-3′ | 56 |
| R 5′-AAT GAC CTG GGC TCT GTT TC-3′ | |
| COL1A2 | |
| F 5′-AGG ACA AGA AAC ACG TCT GG-3′ | 59 |
| R 5′-GGT GAT GTT CTG AGA GGC ATA G-3′ | |
| Snail-1 | |
| F 5′-ACA AGC ACC AAG AGT CCG-3′ | 63.3 |
| R 5′-ATG GCA GTG AGA AGG ATG TG-3′ | |
| FSP1 | |
| F 5′-AGT CAG AAC TAA AGG AGC TGC-3′ | 57 |
| R 5′-GAC ACA GTA CTC TTG GAA GTC C-3′ | |
| Vimentin | |
| F 5′-CGT GAA TAC CAA GAC CTG CTC-3′ | 56 |
| R 5′-GGA AAA GTT TGG AAG AGG CAG-3′ | |
| IL-1β | |
| F 5′-ACG ATG CAC CTG TAC GAT CA-3′ | 56 |
| R 5′-TCT TTC AAC ACG CAG GAC AG-3′ | |
| COX2 | |
| F 5′-CAA ATC CTT GCT GTT CCC ACC CAT-3′ | 57 |
| R 5′-GTG CAC TGT GTT TGG AGT GGG TTT-3′ | |
| ICAM | |
| F 5′-CAA TGT GCT ATT CAA ACT GCC C-3′ | 57 |
| R 5′-CAG CGT AGG GTA AGG TTC TTG-3′ | |
| VCAM | |
| F 5′-TGT TGA GAT CTC CCC TGG AC-3′ | 57 |
| R 5′-CGC TCA GAG GGC TGT CTA TC-3′ | |
| TGF-β2 | |
| F 5′-CCC CAC ATC TCC TGC TAA TG-3′ | 59 |
| R 5′-ATG TAA AGT GGA CGT AGG CAG-3′ | |
| α-SMA | |
| F 5′-AAT GCA GAA GGA GAT CAC GG-3′ | 59 |
| R 5′-TCC TGT TTG CTG ATC CAC ATC-3′ | |
| eNOS | |
| F 5′-GGT ACA TGA GCA CTG AGA TCG-3′ | 61 |
| R 5′-GCC ACG TTG ATT TCC ACT G-3′ |
FSP1, fibroblast-specific protein 1; COX2, cyclooxygenase 2; TGF-β2, transforming growth factor-β2; α-SMA, α-mooth muscle actin; eNOS, endothelial nitric oxide synthase; F, forward; R, reverse.
ELISA assay.
Secretion of TGF-β2 in culture media was assessed by ELISA assay according to the manufacturer's protocol.
Cell proliferation assay.
HEMEC from either coculture or IL-1β- and TGF-β2-treated cells were pulsed with 1 μCi/ml of [3H]thymidine (Perkin Elmer, Waltham, MA) uptake as described earlier (2). Each condition was assessed in triplicate.
Microscopic wounding assay.
To assess HEMEC migration 6 days after coculture and IL-1β- and TGF-β2-treated cells, a microscopic wounding assay was performed as described earlier (2). With the use of an ocular grid, eight random fields were counted in a blinded fashion. Data are expressed as cells per millimeter squared, and each condition was assessed in triplicate.
Matrigel in vitro tube formation assay.
Endothelial tube formation was assessed using Matrigel (BD Biosciences, Bedford, MA) as described previously (6). Tube formation was assessed by inverted phase contrast microscopy after 24 h and photographed. Five high-power fields per condition were examined, and experiments were repeated in three independent cultures.
IL-1β and TGF-β gene silencing.
OE33 cells (2 × 104/ml) either on the insert or plate were grown in RPMI 1460 to 60% confluence and transfected with short interfering RNA (siRNA) targeting human IL-1β and TGF-β2 genes and a negative control (nontargeting siRNA) using DharmaFECT transfection reagent as per manufacturer's protocol (Thermo Fisher Scientific Biosciences, Lafayette, CO). The ratio of siRNA to the DharmaFECT reagent was 50 nM siRNA to 5.0 μl transfection reagent. The efficiency of transfection was verified by real-time PCR (24 h after transfection) and Western blot (48 h after transfection). The knockdown was maintained for up to 6–7 days as determined and proven by RT-PCR.
Coculture of HEMEC with transfected OE33 cells.
Forty-eight-hour transfected OE33 cells on inserts from above were rinsed with PBS. The inserts were placed on top of HEMEC (1 × 105/ml) culture in MCDB-131 plus growth supplement media for 3 days and then replaced with another insert of 48-h transfected OE33 cells for additional 3 days (6 days total). Gene and protein of various markers were determined after 6 days of coculture.
OE33-transfected cell conditioned media.
OE33 cells (2 × 104/ml) were cultured in RPMI 1460 to 60% confluence and then transfected with IL-1β and TGF-β2 siRNA as described above, after 48 h, and transfection media were removed from the wells and cells were rinsed with PBS and incubated in MCDB-131/growth supplements media. Twenty-four hours after incubation, the culture media were collected centrifuged for 5 min at 1,500 rpm to remove debris and dead cells and called condition media in which kept at 4°C until further use.
Inhibition of EndoMT by IL-1β and TGF-β2 knockdown condition media.
HEMEC (1 × 105/ml) were cultured with above conditioned media for 6 days. The media were changed every other day (3× total). Then, transition to EndoMT was assessed by analysis of VE-cadherin and FSP1 expression as described above.
Inhibition of EndoMT by IL-1β and TGF-β2 blocking antibodies.
HEMEC were pretreated with blocking antibodies against the IL-1β (10 ng/ml) and TGF-β2 (1 ng/ml) for 2 h and then cocultured with OE33 for 6 days with or without IL-1β and TGF-β2. EndoMT transition was assessed as above.
Cell contraction assay.
Cell contraction assays were preformed according to the manufacturer's instruction. Briefly, following 6 days of HEMEC-OE33 coculture, the HEMEC were harvested for cell contraction assay. Total of 1.2 × 104 endothelial cells were mixed with type I collagen solution (final concentration to 1 mg/ml of gel) in each well of a 24-well plate. After incubation at 37°C for 30 min, MCDB-131/growth media were added into the wells. Then, using a narrow pipette's tip, the gels were detached from the edges and bottom of the well. The plate was then gently swirled to make sure that the gels were free. The plate was then incubated at 37°C for another 24 h for contraction. Images were taken with a digital camera after the gels were dissociated.
Statistical analysis.
Statistical analysis was performed by ANOVA using StatView for Macintosh (version 4.51; Abacus Concepts, Berkeley, CA). P ≤ 0.05 was considered significant, and data shown are means ± SD.
RESULTS
Coculture with adenocarcinoma OE33 cells promotes EndoMT in HEMEC.
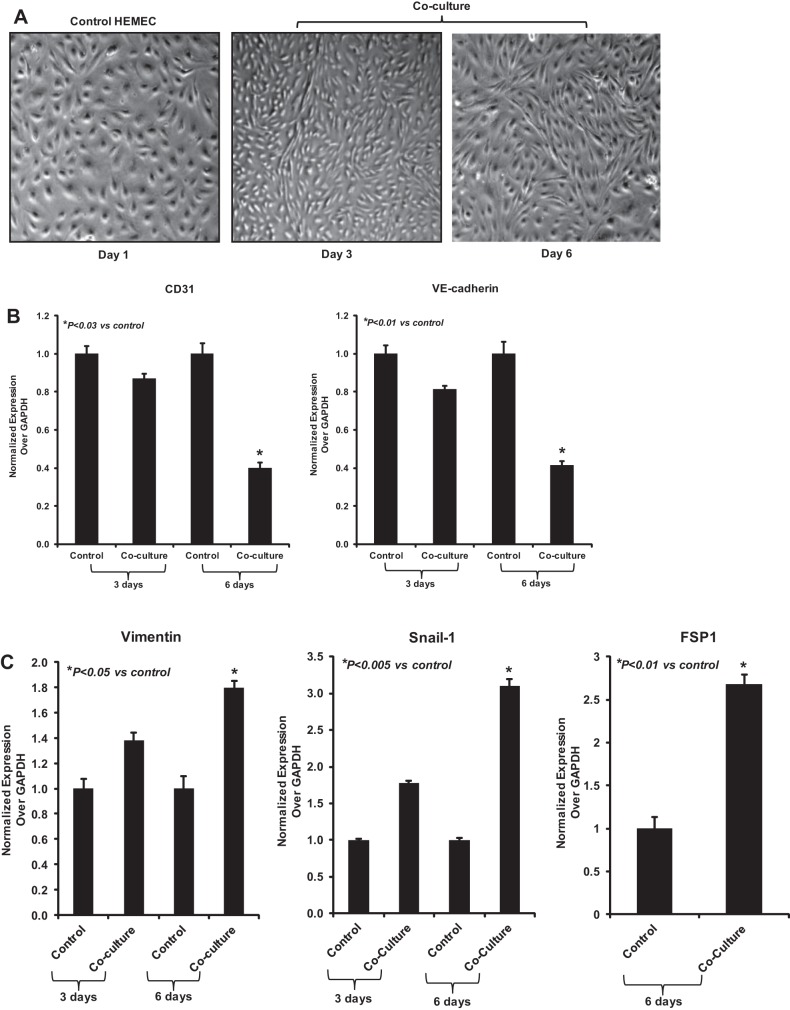
To explore how tumor cells influence endothelial cells in the context of EAC, primary esophageal endothelial cells (HEMEC) were cocultured with OE33 adenocarcinoma cells for indicated days (3, 6, and 10 days) and HEMEC were subsequently examined for phenotypical changes consistent to EndoMT. As shown by inverted microscopy, HEMEC cocultured with OE33 cells progressively changed their cell shape after 3 days by losing their “cobblestone” phenotype and appeared to elongate and to acquire a spindle-like morphology (Fig. 1A). Real-time PCR analysis confirmed a significant, time-dependent decrease in gene expression of the endothelial markers CD31 and VE-cadherin (Fig. 1B) in cocultured HEMEC compared with control cells, whereas the mesenchymal markers vimentin, Snail, and FSP1 gene expression were overexpressed (Fig. 1C). In agreement with the gene expression patterns, immunofluorescence staining demonstrated remarkable downregulation of the endothelial markers CD31 and vWF and upregulation of the mesenchymal markers FSP1 and collagen1α2 in HEMEC following coculture for 6 days (Fig. 1, D and E). Corresponding with gene expression, immunoblots reveled decrease CD31 and VE-cadherin protein expression and increased level of protein expression for α-SMA, vimentin, desmin, and snail in cocultured HEMEC (Fig. 1F).
Fig. 1.
Induction of endothelial-mesenchymal transition (EndoMT) in human esophageal microvascular endothelial cells (HEMEC)-OE33 coculture. A: HEMEC morphological changes. Control HEMEC exhibited a tightly clustered, cobblestone-shaped morphology in culture. Minimum morphological changes were evident by 3 days of HEMEC-OE33 coculture. Significant morphological changes were observed at 6 days; HEMEC exhibited an elongated, spindle-shaped morphology resembling mesenchymal cells. B and C: real-time PCR analysis. Levels of mRNA expression for CD31 and VE-cadherin (endothelial markers) were decreased, where as the vimentin, Snail, and fibroblast-specific protein 1 (FSP1) (mesenchymal markers) were significantly increased at 6 days of HEMEC-OE33 coculture. Data were normalized to GAPDH expression. Values are means ± SD of 3 independent experiments. Asterisks denote significant difference in gene expression between the control and cocultures. D and E: immunofluorescence staining of HEMEC cocultured with OE33 demonstrated the increased expression of mesenchymal markers (FSP1 and collagen1α2) and decreased expression of endothelial markers [CD31 and Von Willebrand factor VIII (vWF)]. F: Western blot analysis. Decreased CD31 and VE-cadherin protein expression and increased protein expression of α-smooth muscle actin (α-SMA), vimentin, desmin, and snail EndoMT cells. Mean densitometric arbitrary units ± SD derived from 3 HEMEC/OE33 cocultures. Asterisks denote significant difference in mean protein band intensity between the control and cocultures. G: HEMEC grown in conditioned media obtained from OE33 cells gone under morphological and phonotypical changes after 6 days. Cells are elongated and expression of CD31 gene was suppressed. H: coculture between HEMEC and OE19 resulted in decrease VE-cadherin and VEGFR2 mRNA expression and increased FSP1 mRNA expression after 6 days. Similarly, coculture between HIMEC and HT29 resulted in decrease VE-cadherin and VEGFR2 mRNA expression and increased Snail-1 mRNA expression after 6 days. Data were normalized to GAPDH expression. Values are means ± SD of 3 independent experiments. Asterisks denote significant difference in gene expression between the control and cocultures.
β-Actin was used as an internal loading control.
More over, when HEMEC were cultured in OE33 cell culture media, the same morphological and molecular changes were observed by 6 days (Fig. 1G), indicating that EndoMT is highly likely induced by mediators already secreted by the tumor cells and not by mediators secreted by tumor cells because of their interaction with HEMEC. Day 6 was chosen because optimum morphological and molecular changes were observed at this time point. At day 3 there were no significant changes in HEMEC and no further changes were observed by 10 days.
To verify that EndoMT changes are not dependent only on the cell line used, we performed the coculture of HEMEC with another EAC cell line (OE19). Our results demonstrated the parallel upregulation of FSP1 gene expression with the subsequent downregulation of VE-cadherin gene expression. Similar EndoMT changes were observed when human intestinal microvascular endothelial cells (HIMEC) were cocultured under same conditions as above with HT29, a human colorectal adenocarcinoma cell line (Fig. 1H).
Taken together, these results indicate that adenocarcinoma cells induce endothelial cells to undergo EndoMT in vitro.
Cell migration, proliferation, and tube formation are altered in HEMEC undergoing tumor-mediated EndoMT.
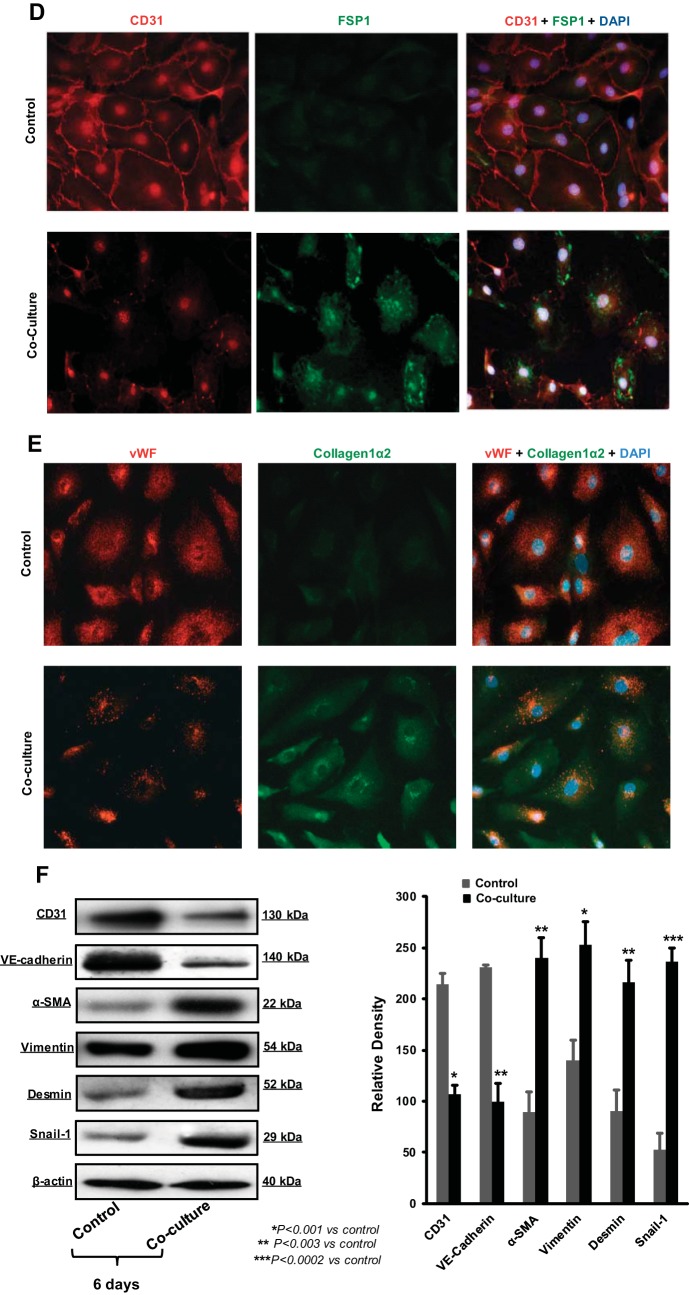
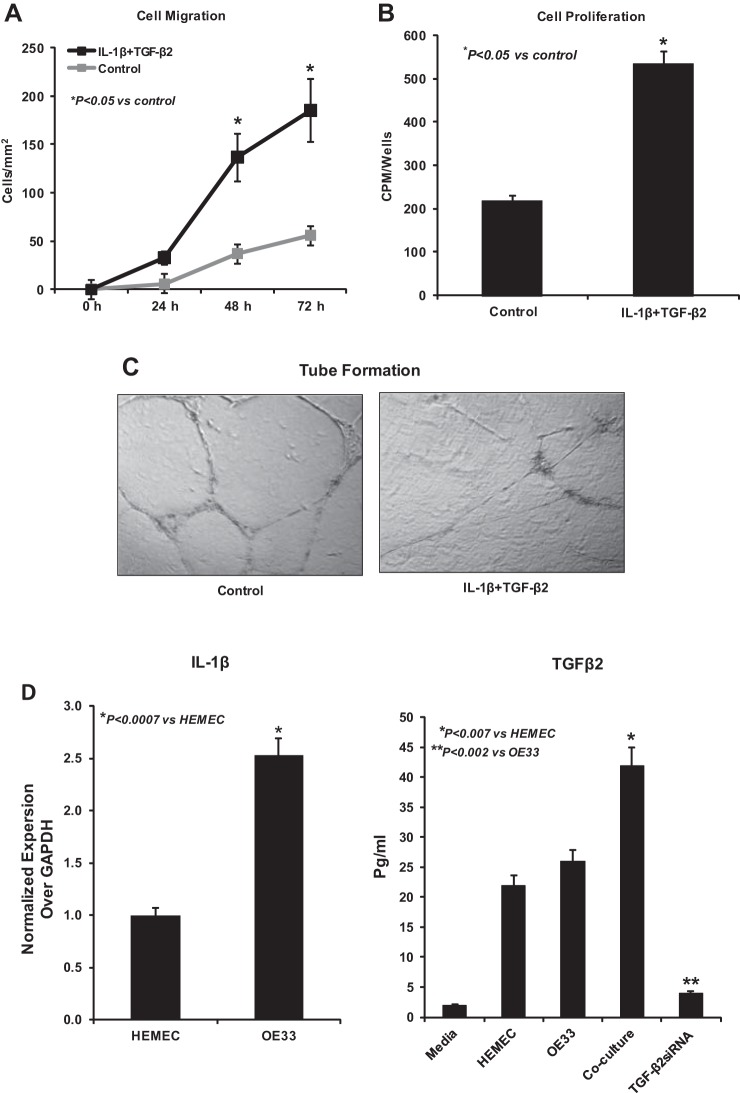
Next, we performed functional assays to further characterize the observed EndoMT in HEMEC. Cell migration and proliferation of HEMEC following 6 days of coculture with OE33 cells were significantly increased compared with control cells as demonstrated by microscopic wounding and [3H]thymidine proliferation assays, respectively (Fig. 2, A and B). However, the ability of HEMEC to form anastomosing capillaries in vitro on Matrigel (in vitro tube formation) was markedly diminished after cocultured with OE33 cells compared with control cells (Fig. 2C). These results indicate that the observed EndoMT-like morphological changes in HEMEC triggered by tumor cells are accompanied by significant functional alterations consistent with the EndoMT phenotype, such as increased migratory capacity and higher proliferation rates, while cells lose their angiogenic ability to form tubes. With respect to the mesenchymal function of the EndoMT cells, cell contraction assay was performed. Twenty-four hours after the contraction initiation, EndoMT cells contracted more matrix compared with control HEMEC (Fig. 2D). Fibroblasts were used as a positive control. The weakened endothelial features of the EndoMT cells were further confirmed by the downregulation of gene and protein expression of eNOS (Fig. 2, E and F).
Fig. 2.
Cell migration, proliferation, and tube formation in EndoMT. A and B: 6 days after OE33-HEMEC coculture, EndoMT cells migrated and proliferated more than control cells. C: however, their ability to form capillary tubes in vitro was decreased. D: collagen contraction assay 24 h after HEMEC-OE33 coculture demonstrate that cells obtained from coculture contracted more collagen than control cells. Representative pictures of collagen matrix contraction are from 3 independent experiments, and fibroblast was used as positive control. E and F: endothelial nitric oxide synthase (eNOS) gene and protein expression was downregulated in coculture compared with control HEMEC. Data are means ± SD of 3 independent experiments. Asterisks denote significant difference in mean gene expression and protein band intensity between the control and cocultures.
OE33 cells promote inflammatory markers in cocultured HEMEC.
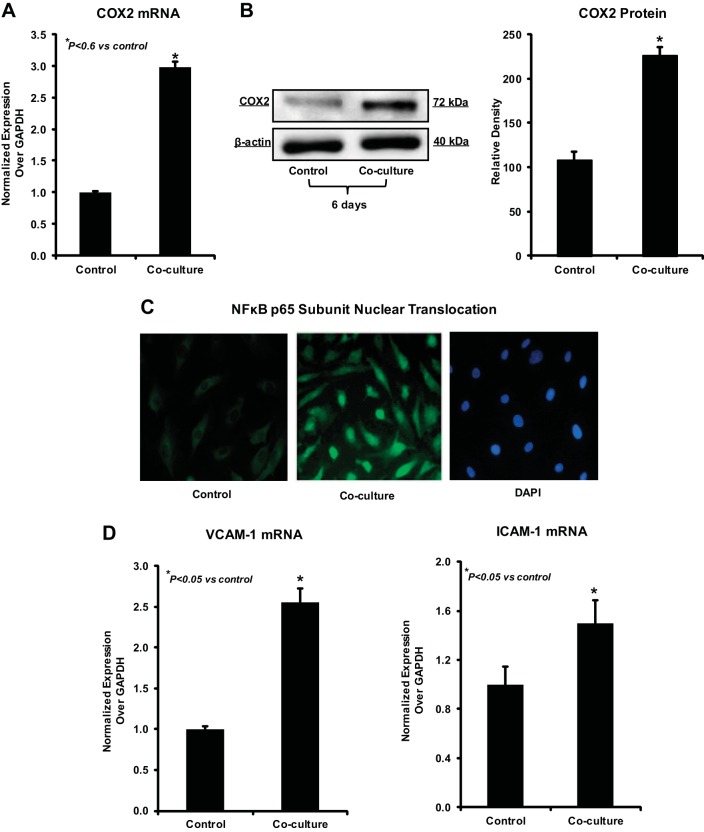
Given that EndoMT is mainly addressed in pathologic processes such as fibrosis and cancer, which are characterized by strong inflammatory tissue responses, we examined next whether tumor-driven EndoMT in HEMEC is accompanied by activation of inflammatory markers. As shown in Fig. 3, A and B, COX2 gene and protein expression was strongly induced in HEMEC cocultured with OE33 by 6 days. Activation of NF-κB in EndoMT-transformed HEMEC was evident by p65 subunit nuclear translocation, as demonstrated by immunofluorescence staining (Fig. 3C). Furthermore, gene expression of endothelial ICAM-1 and VCAM-1, the hallmarks of inflammatory activation of endothelial cells, was found significantly elevated in HEMEC subjected to coculture compared with control cells (Fig. 3D). These findings signify a cross-path between tumor-driven EndoMT and tumor-associated activation of proinflammatory pathways in endothelial cells.
Fig. 3.
Induction of inflammatory markers in EndoMT. A and B: increased cyclooxygenase 2 (COX2) mRNA and protein expression was evident by day 6 in EndoMT cells. Data are means ± SD of 3 independent experiments. Asterisks denote significant difference in mean gene expression and protein band intensity between the control and cocultures. C: immunofluorescence staining demonstrated nuclear translocation of NF-κB p65 subunit in EndoMT cells. D: ICAM-1 and VCAM-1 gene, the hallmarks of inflammatory activation of endothelial cells, were found significantly elevated in EndoMT cells compared with control cells. Data are means ± SD of 3 independent experiments. Asterisks denote significant difference in gene expression between the control and cocultures.
TGF-β2 and IL-1β induce EndoMT in HEMEC.
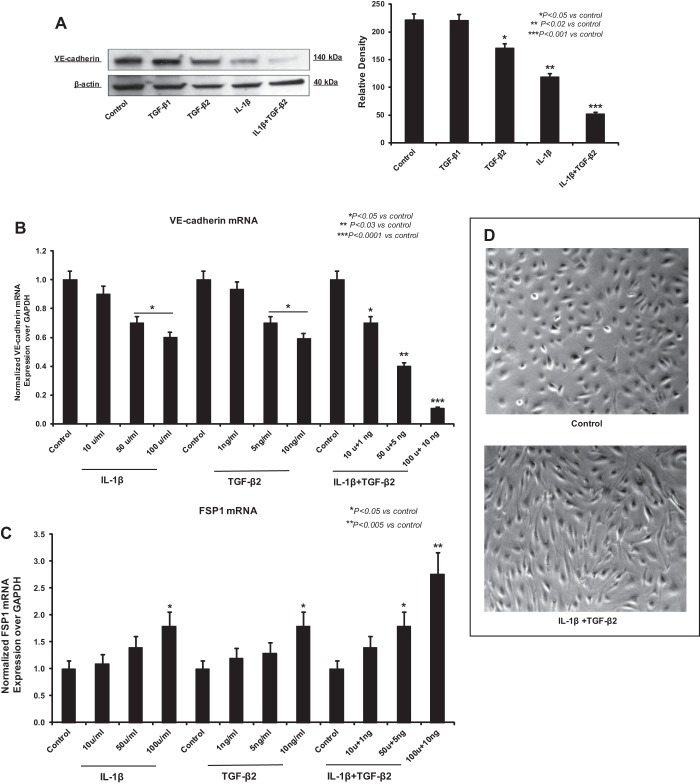
To further determine the role of TGF-β signaling in combination with IL-1β in inducing EndoMT, we treated HEMEC with both isoforms of TGF-β (TGF-β1 and TGF-β2) and IL-1β. Immunoblot analysis revealed that IL-1β and TGF-β2 treatment, but not TGF-β1, caused decrease in VE-cadherin protein expression (Fig. 4A).
Fig. 4.
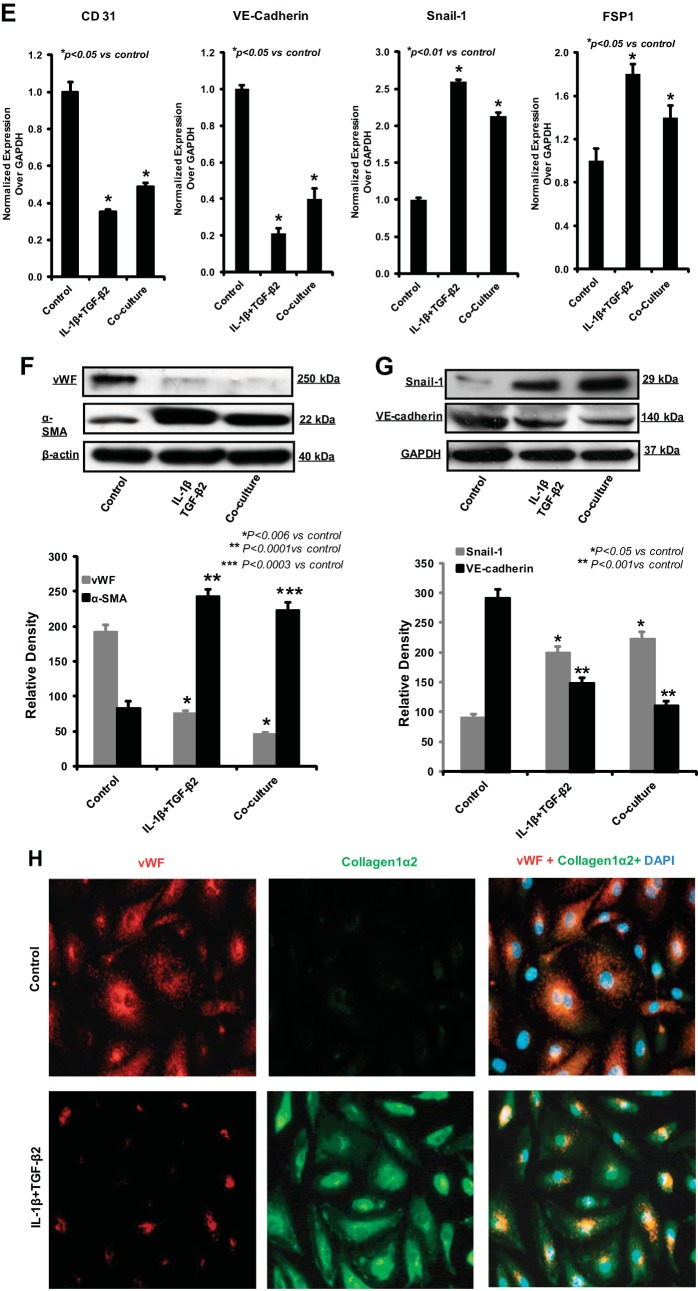
Transforming growth factor-β2 (TGF-β2) and IL-1β Synergistically induce EndoMT. A: immunoblot analysis demonstrated that IL-1β and TGF-β2 treatment, but not TGF-β1, caused decrease in VE-cadherin protein expression in HEMEC. Data are means ± SD of 3 independent experiments. Asterisks denote significant difference in mean protein band intensity between the control and cocultures. B and C: IL-1β/TGF-β2 dose dependently suppressed the VE-cadherin expression and increased FSP1 expression. Data are means ± SD of 3 independent experiments. Asterisks denote significant difference in mRNA expression between the control and treatments. D: treatment of HEMEC with TGF-β2 and IL-1β resulted in morphological alterations similar to coculture, cells become elongated and spindle shaped. E: TGF-β2 and IL-1β treatment significantly decreased the CD31 and VE-cadherin gene expression and upregulated the Snail and FSP1 mRNA expression in EndoMT cells. Data are means ± SD of 3 independent experiments. Asterisks denote significant difference in mRNA expression between the control and treatments. F and G: Western blot analysis of HEMEC treated with TGF-β2 and IL-1β. Increased α-SMA and snail protein expression along with decreased vWF and VE-cadherin protein expression were evident by day 6 of treatment. Data are means ± SD of 3 independent experiments. Asterisks denote significant difference in mean protein band intensity between the control and treatment and between control cells and cocultures. H: immunofluorescence staining of TGF-β2- and IL-1β-treated HEMEC shows the increased expression of mesenchymal markers (collagen1α2) and decreased expression of endothelial markers (vWF).
Remarkably, the combination of TGF-β2 and IL-1β resulted in an even stronger suppression of VE-cadherin protein expression, indicating an enhanced effect of both chemokines in inducing EndoMT (Fig. 4A). IL-1β/TGF-β2 dose dependently suppressed the VE-cadherin expression and increased the FSP1 gene expression (Fig. 4, B and C). Chemokine-driven EndoMT was confirmed by demonstrating similar morphological changes typical of EndoMT with spindle-shaped and elongated cells, after administration of recombinant IL-1β and TGF-β2 in combination (Fig. 4D). EndoMT-related alteration in gene expression upon IL-1β/TGF-β2 treatment was further verified by real-time PCR, in which, combined treatment resulted in decreased levels of the endothelial markers CD31 and VE-cadherin and increased levels of the mesenchymal markers Snail and FSP1 (Fig. 4E). Immunoblot analysis also revealed the loss of vWF and VE-cadherin along with an increase in expression of α-SMA and Snail in HEMEC upon TGF-β2/IL-1β costimulation (Fig. 4, F and G). These results were further complemented by immunofluorescence staining demonstrating vWF downregulation and collagen 1α2 upregulation following HEMEC treatment with combination of TGF-β2 and IL-1β (Fig. 4H).
Effect of TGF-β2 and IL-1β on migration, proliferation, and tube formation of HEMEC.
To confirm that the chemokine-induced EndoMT morphological changes also impact the endothelial functions, HEMEC monolayers were treated with combination of IL-1β and TGF-β2 for 6 days, and then cell migration, proliferation and tube formation were evaluated. In agreement with coculture, cell migration and proliferation were significantly elevated in HEMEC subjected to TGF-β2/IL-1β costimulation compared with untreated control cells (Fig. 5, A and B) while the angiogenic ability of HEMEC to form tubes in vitro was eradicated (Fig. 5C). In all, our findings support that the synergism of TGF-β2 and IL-1β is a strong inducer of EndoMT in esophageal endothelial cells.
Fig. 5.
Effect of TGF-β2 and IL-1β on migration, proliferation, and tube formation. A and B: migratory and proliferative ability of HEMEC treated with combination of IL-1β and TGF-β2 was enhanced by day 6. Values are means ± SD of 3 independent experiments. Asterisks denote significant difference between the control and cocultures. C: their ability to form capillary tubes in vitro was diminished. D: real-time PCR and ELISA assay from OE33 cells and culture media demonstrated that secretions of IL-1β, TGF-β2, and VEGF were higher than HEMEC.
Next, we questioned whether TGF-β2 and IL-1β mediate the EndoMT of HEMEC when cocultured with OE33 adenocarcinoma cells. We confirmed first that OE33 cancer cells express higher levels of IL-1β gene than HEMEC in resting conditions (Fig. 5D), supporting the already reported in vivo data on IL-1β overexpression in EAC tissue (19). In contrast, TGF-β2 secretion from OE33 cells was not markedly different from HEMEC in the resting condition; however, elevated amounts of TGF-β2 were secreted from OE33 following coculture with HEMEC, indicating that in the context of coculture OE33 were induced to express and secrete TGF-β2 (Fig. 5D).
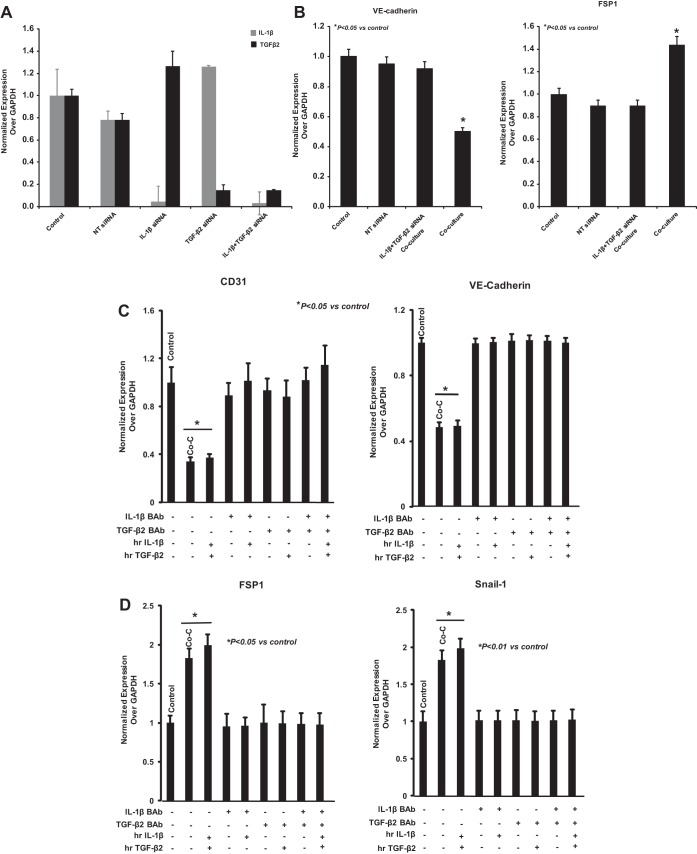
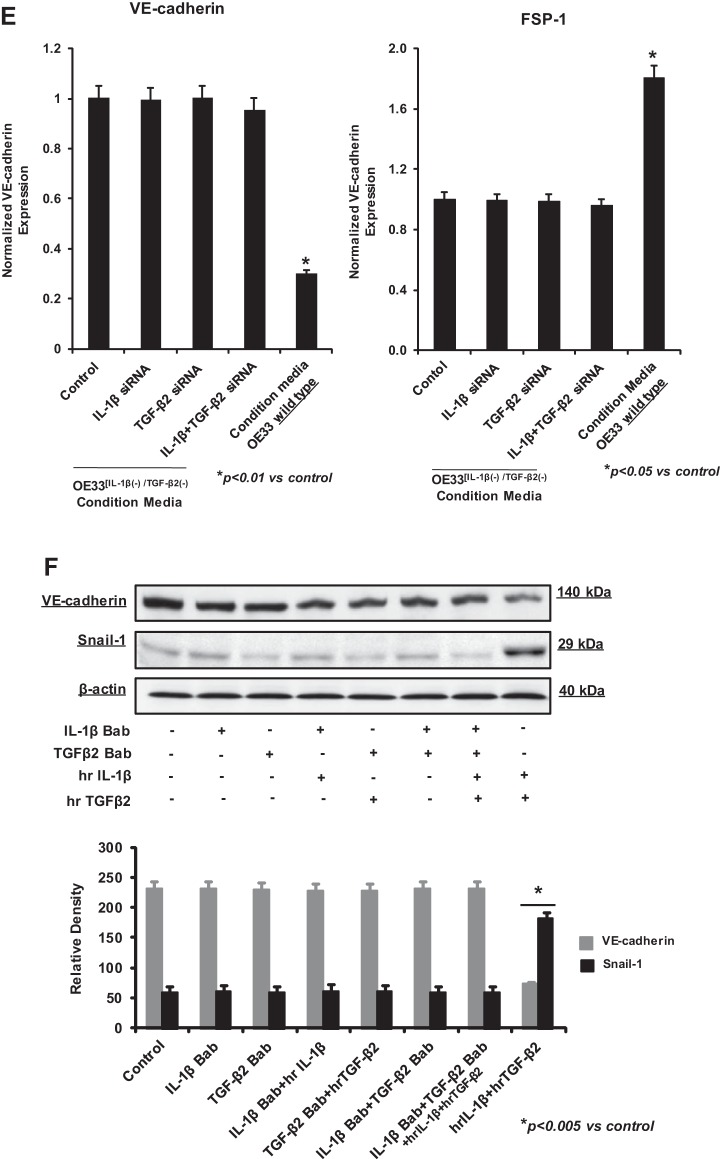
Inhibition of EndoMT by IL-1β and TGF-β2 gene silencing in OE33.
To confirm whether the effect of OE33 on HEMEC is linked to IL-1β and TGF-β2, we performed gene-silencing experiments using transfection with siRNAs specific for IL-1β and TGF-β2 alone and in combination. Using appropriate control (nontargeted siRNA), we demonstrated specific knockdown of the IL-1β and TGF-β2 genes, which was sustained up to 6 days after transfection as revealed by real-time PCR (Fig. 6A). After confirming significant silencing of IL-1β/TGF-β2 expression in OE33 cells, coculture between OE33 [IL-1β(−)/TGF-β2(−)] and HEMEC was performed as described above. As shown by real-time PCR (Fig. 6B), the observed downregulation of VE-cadherin along with the parallel upregulation of FSP1 in HEMEC following coculture with wild-type OE33 cells was not observed in HEMEC cocultured with OE33[IL-1β(−)/TGF-β2(−)]. These data indicate that release of IL-1β and TGF-β2 from OE33 cells in culture media promoted EndoMT in HEMEC. This notion was further supported when specific blocking antibodies against IL-1β and TGF-β2 effectively abolished the effect of recombinant IL-1β and TGF-β2 either on downregulating the endothelial markers CD31 and VE-cadherin (Fig. 6C) or upregulating the mesenchymal markers FSP1 and Snail (Fig. 6D). Moreover, when HEMEC were cultured in condition media obtained from OE33[IL-1β(−)/TGF-β2(−)] cells, transition of HEMEC to EndoMT was blocked as determined by VE-cadherin and FSP1 expression (Fig. 6E). Similar findings were shown at the protein level for VE-cadherin and Snail by immunoblot analysis in HEMEC (Fig. 6F).
Fig. 6.
IL-1β and TGF-β2 siRNA gene silencing in OE33 cells. A: IL-1β and TGF-β2 genes silencing in OE33 by their specific siRNA demonstrated that both genes were effectively inhibited. Nontargeting (NT) siRNA was used as control. B: VE-cadherin and FSP1 gene expression was not altered in HEMEC cocultured with IL-1β and TGF-β2 siRNA-transfected OE33 cells. Values are means ± SD of 3 independent experiments. Asterisks denote significant difference between the control and cocultures. C and D: IL-1β and TGF-β2 specific blocking antibodies effectively blocked the effect of recombinant IL-1β and TGF-β2 on either downregulation of CD31 and VE-cadherin or upregulation of the mesenchymal markers FSP1 and Snail-1. Values are means ± SD of 3 independent experiments. Asterisks denote significant in mRNA expression between the control and cocultures and between control and treatment. E: transition of HEMEC to EndoMT was blocked when HEMEC were cultured in condition media obtained from OE33[IL-1β(−)/TGF-β2(−)] cells comparing with OE33 wild type as determined by VE-cadherin and FSP1 gene expression. Values are means ± SD of 3 independent experiments. Asterisks denote significant difference in mRNA expression between the control and conditioned media from OE33 wild type. F: by immunoblotting similar findings were shown at the protein level for VE-cadherin and Snail-1. Data are means ± SD of 3 independent experiments. Asterisks denote significant difference in mean protein band intensity between the control and cocultures.
Taken together, these findings strongly indicate that tumor-derived IL-1β and TGF-β2 synergistically induce EndoMT in primary esophageal endothelial cells.
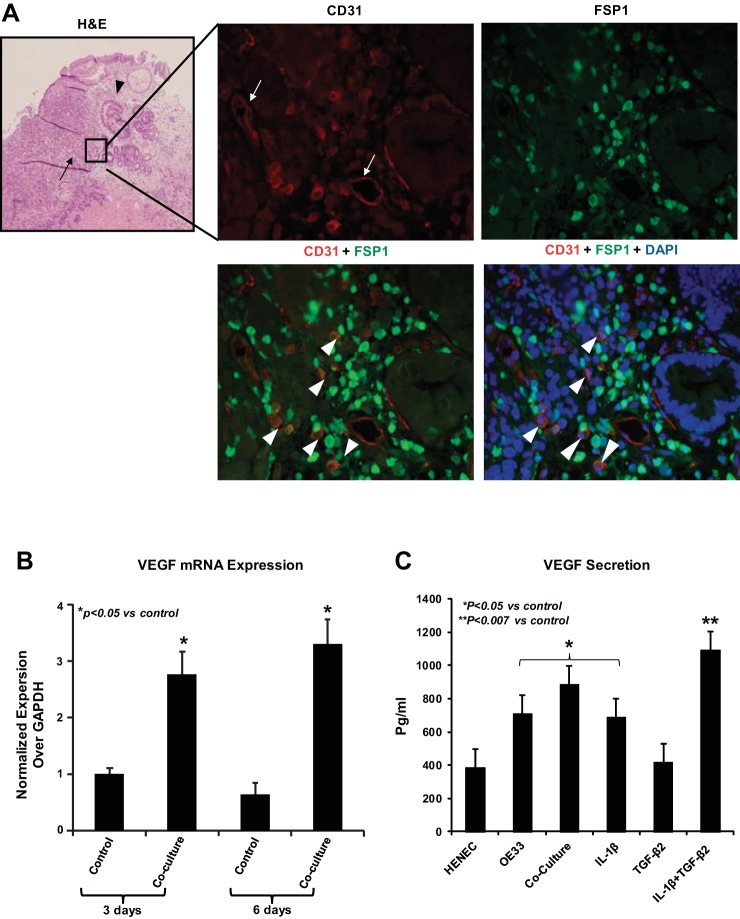
EndoMT as a source of CAF secreting VEGF in EAC.
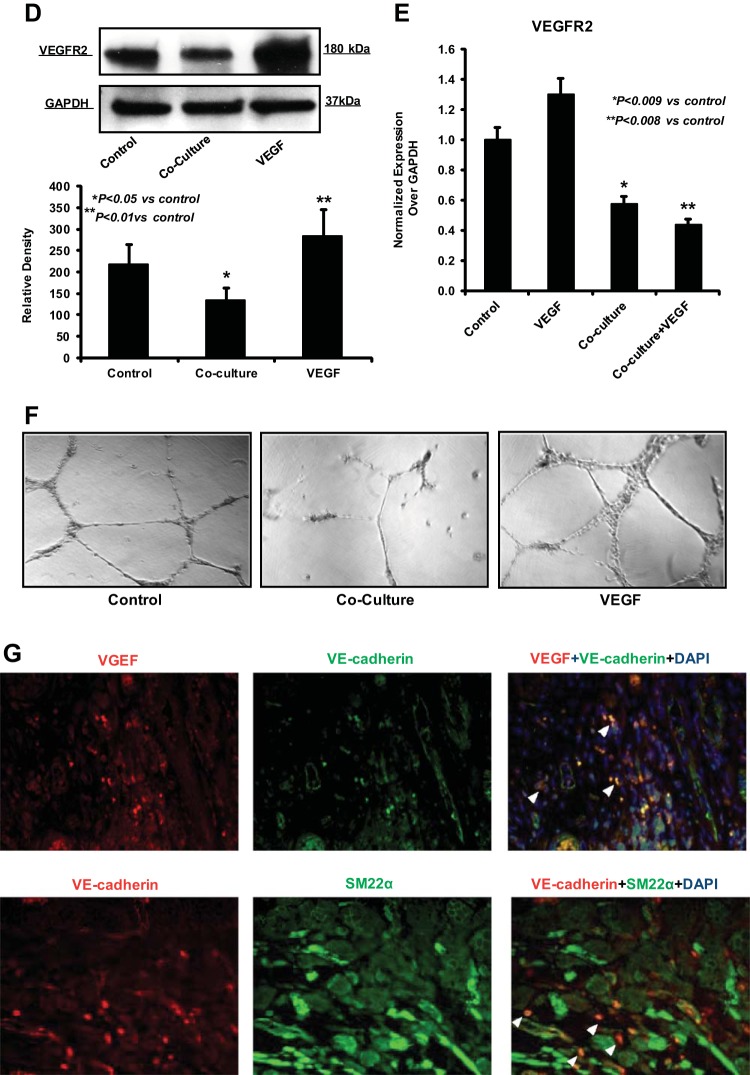
To address the biological relevance of EndoMT in the progression of EAC, we examined whether EndoMT is present in vivo. We analyzed representative EAC tissues by performing simultaneous immunolocalization for CD31 and FSP1. Fluorescence microscopy revealed colocalization of both CD31 and FSP1 in isolated stromal cells, CAF (arrowheads) away from tumor microvessels (arrows), suggesting that these cells have undergone EndoMT (Fig. 7A) Of note, a remarkable large number of these EndoMT-derived CAF was found located close to the invasive front of the tumor (Fig. 7A). This observation prompted us to investigate whether EndoMT-derived CAF could be a separate source of host-derived VEGF promoting tumor growth and angiogenic spouting at the invasive front of EAC. We confirmed that HEMEC undergoing tumor-induced EndoMT following coculture with OE33 cancer cells express higher amounts of VEGF gene than control cells (Fig. 7B). This finding was further confirmed by ELISA, when HEMEC subjected either to coculture or to treatment with TGF-β2 and IL-1β, alone or in combination, and found to secrete significantly higher amounts of VEGF than control cells (Fig. 7C). Interestingly, protein and gene analysis revealed that VEGFR2, the receptor of VEGF, was downregulated in HEMEC cocultured with tumor cells while it was were upregulated following HEMEC stimulation with VEGF alone (Fig. 7, D and E), suggesting that as part of the EndoMT process endothelial cells lose their sensitivity to proangiogenic stimulators such as VEGF. This notion was supported when EndoMT-transformed HEMEC were unable to form tubes on Matrigel in vitro, in contrast to VEGF-stimulated HEMEC which enhanced tube formation more than control cells (Fig. 7F). In vivo, as shown in Fig. 7G the presence of VEGF-producing EndoMT cells within the tumor stroma was validated by two double stainings between VEGF and VE-cadherin (endothelial marker) and VE-cadherin with SM22α (fibroblast marker).
Fig. 7.
EndoMT as a source of cancer-associated fibroblasts (CAF) secreting VEGF in esophageal adenocarcinoma (EAC). A: fluorescence microscopy revealed colocalization of both CD31 and SFP-1 in isolated stromal cells-CAF (arrowheads) away from tumor microvessels (arrows), suggesting that these cells have gone under EndoMT transition. B: HEMEC undergoing tumor-induced EndoMT following coculture with OE33 cancer cells express higher amounts of VEGF gene than control cells. Values are means ± SD of 3 independent experiments. Asterisks denote significant difference in mRNA expression between the control and cocultures. C: HEMEC were subjected either to coculture or to treatment with TGF-β2 and IL-1β, alone or in combination secreted significantly higher amounts of VEGF than control cells as detected by ELISA. Moreover, the level of VEGF secretion from OE33 cells was high. Values are means ± SD of 3 independent experiments. Asterisks denote significant difference in VEGF secretion between the control cells and cocultures and between control and treatment. D: VEGFR2 protein expression was downregulated in HEMEC cocultured with tumor cells at while VEGF stimulation upregulated the expression of VEGFR2 in control HEMEC. Data are means ± SD of 3 independent experiments. Asterisks denote significant difference in mean protein band intensity between the control and coculture and between control and VEGF treatment. E: VEGFR2-mRNA expression was downregulated in HEMEC cocultured with tumor cells and VEGF stimulation of coculture did not upregulate the VEGFR2 in coculture. Values are means ± SD of 3 independent experiments. Asterisks denote significant difference in mRNA expression between the control and cocultures and between control and coculture + VEGF. F: EndoMT-transformed HEMEC were unable to form tubes on Matrigel in contrast to VEGF-stimulated HEMEC. G: immunofluorescence costaining using specific antibodies against VEGF with VE-cadherin and VE-cadherin with SM22α showed VEGF-producing EndoMT cells within the tumor stroma (arrowhead).
These data suggest that EndoMT-transformed esophageal endothelial cells could be a significant source of VEGF in tumor microenviroment working more in a paracrine than autocrine way.
DISCUSSION
In this study, we demonstrate the induction of EndoMT in normal primary HEMEC when cocultured with EAC cells (OE33) in vitro. In response to cancer cells either in coculture or in conditioned media, HEMEC 1) obtained a fibroblast-like spindle cell shape; 2) gained increased expression of the mesenchymal markers FSP1, collagen1α2, vimentin, and Snail; 3) lost expression of the endothelial markers CD31, vWF, and VE-cadherin; and 4) acquired high migratory and proliferative properties. As a result of this transition, the ability of HEMEC to be angiogenic was abolished as depicted by the downregulation of VEGFR2 and the inability to form anastomosing capillaries in vitro. TGF-β2 and IL-1β triggered similar morphological and functional alterations, consistent with EndoMT in HEMEC particularly when both chemokines were combined. Simultaneous inhibition of TGF-β2 and IL-1β in OE33 cells prevented induction of EndoMT in HEMEC subjected to coculture, indicating that tumor-induced EndoMT is mediated by the synergistic effect of both chemokines. Furthermore, HEMEC undergoing EndoMT demonstrated increased expression and secretion of VEGF, while in vivo, EndoMT-like cells were spotted mainly at the invasive front of EAC, underscoring their involvement in tumor growth and invasion. Taken together, our results define EndoMT as an important process in the context of EAC progression and provide more insight into the mechanisms facilitating this process.
As an extreme form of endothelial plasticity, EndoMT is now emerging as a key determinant of the behavior of tumor microenviroment by generating up to 40% of CAF (21, 30), which have been recognized as prominent modifiers of tumor progression (8). Previous studies of EndoMT have mainly focused on embryonic development of the heart (1), while only recently EndoMT has been reported in cancer and fibrosis (21, 30, 31). Although a dynamic interaction between cancer cells and the host microenvironment has been proposed to induce EndoMT (10, 21), solid evidence on the precise inducers of EndoMT in tumor microenvironment is still missing. In this study, we present evidence that the EAC tumor cells directly triggered induction of EndoMT. The fact that EndoMT-phenotype was addressed in HEMEC either in cocultured with OE33 cells or in OE33 media indicates that EndoMT is induced by factors secreted from tumor cells regardless their interaction with HEMEC. This notion highlights an autonomic capacity of tumor cells to dominate and modify the functions of surrounding endothelial cells.
At the molecular level, we associated the tumor-induced EndoMT in HEMEC with the activation of intercellular inflammatory pathways, such as COX2 and NF-κB, and identified the OE33-derived chemokines IL-1β and TGF-β2 to synergistically mediate this process. These findings come in agreement with the recently reported chemokine-driven EndoMT in HIMEC (23) as well as the synergistic induction of EndoMT in an NF-κB-dependent manner by IL-1β and TGF-β2 shown in human umbilical vein endothelial cells (17). Of note, our study showed no role for TGF-β1 isoform in promoting EndoMT in HEMEC verifying similar findings in human umbilical vein endothelial cells (17) but contradicting others on lung endothelial cells (30). This differential response of endothelial cells to TGF-β1 and TGF-β2 isoforms might be explained by their discrete different organ origins suggesting that EndoMT might be mediated by different factors dependent on organ context. The verified pro-EndoMT effect of the TGF-β2 isoform in the context of inflammatory IL-1β costimulation highlights a cross talk between proinflammatory and profibrotic signaling pathways also found in EMT (13) Generally, EndoMT has been proposed to utilize common signaling pathways with EMT as TGF-β, bone morphogenic protein, and Notch signaling pathways appear to play a role in the induction of EndoMT (1, 24, 25). Indeed, the upregulation of the zinc-finger transcription factor Snail in HEMEC undergoing EndoMT as shown here, supports the sharing of similar molecular mechanisms between EndoMT and EMT (24). However, further studies are required to validate this notion, given the key differences between endothelial and epithelial cells.
With regards to the relevance of EndoMT in EAC, we demonstrate a strong presence of EndoMT-like cells at the tumor invasive front in vivo. During EndoMT, resident endothelial cells are expected to delaminate from the organized cell layer due to the acquired mesenchymal phenotype and to invade the underlying tissue (21). In this way, EndoMT generates a specific population of CAF, which can affect the tumor growth either directly through paracrine factors or indirectly by promoting angiogenesis (8). As shown here, HEMEC undergoing EndoMT lost their angiogenic endothelial properties but at the same time became a unique source of VEGF growth factor. These observations strongly support the still speculative role of EndoMT in angiogenic spouting, according to which EndoMT may enable the so-called tip cells, which lead an emerging vascular plexus, to migrate into adjacent tissue (21). As migratory cells with no lumen, a phenotype consistent with EndoMT will allow tips cells to guide angiogenic spouting (4). Notably, the loss of VEGFR2 during EndoMT suggest that the secreted VEGF is working more in a paracrine way towards endothelial, tumor, and stromal cells than in an autocrine way. Since VEGF increases EAC survival and proliferation (16), EndoMT may also contribute directly to tumor cell growth and survival.
Our current findings on EndoMT provide also significant insights on the molecular mechanisms underlying the aggressive phenotype of EAC, as a discrete malignancy. EAC progresses rapidly and demonstrates early metastasis, and tumor cells continue to grow despite strong chemotherapy therapies (27). Due to the increased microvasculature found already at the early stages of EAC progression (9), antiangiogenic therapies appeared attractive to oncologists to handle EAC patients. However, preliminary results of trials with VEGFR inhibitors are, thus far, disappointing in unselected patient cohorts (12). Improved insight into the biologic background of EAC is required to improve patient selection, combine agents, and discover new targets and agents. The involvement of EndoMT in EAC as demonstrated here suggests that modulating EndoMT may serve a promising new therapeutic strategy for EAC treatment. A possible strategy could be the downstream inhibition of the IL-1β and TGF-β2 signaling pathways. The chemokine-driven activation of NF-κB and Snail transcriptional factors demonstrated in our study reveals in part some of the intracellular mechanisms of EndoMT. Precise mechanistic investigation for identification of intracellular pathways involved in IL-1β and TGF-β2 signaling activation is currently under investigation in our laboratory.
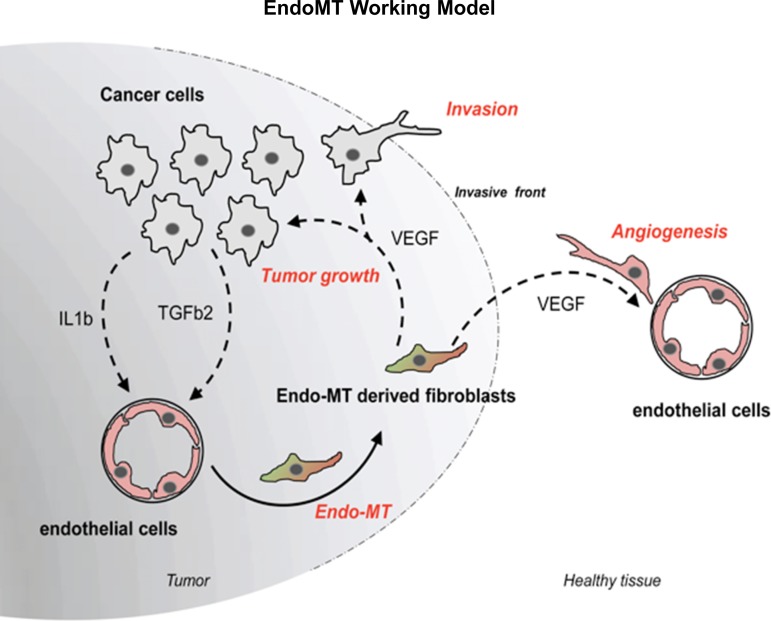
In conclusion, we have shown that primary, organ-specific, HEMEC undergo EndoMT when they directly interact with EAC cells in vitro and highlight the synergistic effect of two tumor-derived chemokines, IL-1β and TGF-β2, to mediate this transition. We also confirmed EndoMT in EAC in vivo and observed increased number of EndoMT-like cells at the invasive tumor front. Remarkably, EndoMT in vitro generated a specific fibroblast-like cell population unable to demonstrate angiogenic properties, thus able to secrete high amounts of VEGF. In this way, these cells are extremely potent inducer of tumor growth and invasion along with promotion of tumor angiogenesis (Fig. 8). Together, these findings define an important role for EndoMT in EAC progression and provide more insight into the mechanisms underlying this process.
Fig. 8.
Proposed model. Schematic illustration of the proposed working model, demonstrating that EndoMT in the context of EAC can influence tumor growth either directly or indirectly by promoting angiogenesis.
GRANTS
This work was supported by Digestive Disease Center, Medical College of Wisconsin and National Center for Advancing Translational Sciences Grant 8UL1TR000055 and National Institutes of Health Grant 5U19-AI067734, Zablocki Veterans Affairs Medical Center research support, and in part by Laura Gralton on behalf of Daniel and Laura Gruber Charitable Lead Trust #3.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.N., O.L., R.M., R.S., and P.R. conception and design of research; L.N., O.L., R.M., N.J., and J.L.S. performed experiments; L.N., R.M., N.J., J.L.S., and P.R. analyzed data; L.N. and P.R. prepared figures; L.N., O.L., R.M., N.J., J.L.S., M.F.O., C.P.J., B.B., R.S., and P.R. approved final version of manuscript; O.L., M.F.O., C.P.J., B.B., R.S., and P.R. interpreted results of experiments; O.L., M.F.O., C.P.J., B.B., R.S., and P.R. edited and revised manuscript; P.R. drafted manuscript.
ACKNOWLEDGMENTS
We thank the Levy family for the generous gift of fluorescence microscope (Gerald Levy, Ellin Levy, Douglas Levy, and Patti Levy).
REFERENCES
- 1.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res 95: 459–470, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binion DG, Otterson MF, Rafiee P. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57: 1509–1517, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86: 353–364, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem 278: 8508–8515, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 9: 239–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 6: 392–401, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Kleespies A, Guba M, Jauch KW, Bruns CJ. Vascular endothelial growth factor in esophageal cancer. J Surg Oncol 87: 95–104, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Klein-Goldberg A, Maman S, Witz IP. The role played by the microenvironment in site-specific metastasis. Cancer Lett 352: 54–58, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci 121: 3317–3324, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Kordes S, Cats A, Meijer SL, van Laarhoven HW. Targeted therapy for advanced esophagogastric adenocarcinoma. Crit Rev Oncol Hematol 90: 68–76, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20: 576–590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JG, Ko MK, Kay EP. Endothelial mesenchymal transformation mediated by IL-1beta-induced FGF-2 in corneal endothelial cells. Exp Eye Res 95: 35–39, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Lorusso G, Ruegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 130: 1091–1103, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Lyros O, Mueller A, Heidel F, Schimanski CC, Gockel I, Galle PR, Lang H, Moehler M. Analysis of anti-proliferative and chemosensitizing effects of sunitinib on human esophagogastric cancer cells: synergistic interaction with vandetanib via inhibition of multi-receptor tyrosine kinase pathways. Int J Cancer 127: 1197–1208, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Maleszewska M, Moonen JR, Huijkman N, van de Sluis B, Krenning G, Harmsen MC. IL-1beta and TGFbeta2 synergistically induce endothelial to mesenchymal transition in an NFkappaB-dependent manner. Immunobiology 218: 443–454, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Mihira H, Suzuki HI, Akatsu Y, Yoshimatsu Y, Igarashi T, Miyazono K, Watabe T. TGF-beta-induced mesenchymal transition of MS-1 endothelial cells requires Smad-dependent cooperative activation of Rho signals and MRTF-A. J Biochem 151: 145–156, 2012 [DOI] [PubMed] [Google Scholar]
- 19.O'Riordan JM, Abdel-latif MM, Ravi N, McNamara D, Byrne PJ, McDonald GS, Keeling PW, Kelleher D, Reynolds JV. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol 100: 1257–1264, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ogawa H, Binion DG, Heidemann J, Theriot M, Fisher PJ, Johnson NA, Otterson MF, Rafiee P. Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells. Am J Physiol Cell Physiol 288: C272–C281, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer 99: 1375–1379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rafiee P, Ogawa H, Heidemann J, Li MS, Aslam M, Lamirand TH, Fisher PJ, Graewin SJ, Dwinell MB, Johnson CP, Shaker R, Binion DG. Isolation and characterization of human esophageal microvascular endothelial cells: mechanisms of inflammatory activation. Am J Physiol Gastrointest Liver Physiol 285: G1277–G1292, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Rieder F, Kessler SP, West GA, Bhilocha S, de la Motte C, Sadler TM, Gopalan B, Stylianou E, Fiocchi C. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol 179: 2660–2673, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 18: 99–115, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonra JR, Hicklin DJ. Targeting the vascular endothelial growth factor pathway in the treatment of human malignancy. Immunol Invest 36: 3–23, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Tougeron D, Richer JP, Silvain C. Management of esophageal adenocarcinoma. J Visc Surg 148: e161–170, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Tse JC, Kalluri R. Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem 101: 816–829, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res 9: 1658–1667, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 67: 10123–10128, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Liu J. Tumor stroma as targets for cancer therapy. Pharmacol Ther 137: 200–215, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]