Abstract
Exposing an oocyte to CO2/HCO3− causes intracellular pH (pHi) to decline and extracellular-surface pH (pHS) to rise to a peak and decay. The two companion papers showed that oocytes injected with cytosolic carbonic anhydrase II (CA II) or expressing surface CA IV exhibit increased maximal rate of pHi change (dpHi/dt)max, increased maximal pHS changes (ΔpHS), and decreased time constants for pHi decline and pHS decay. Here we investigate these results using refinements of an earlier mathematical model of CO2 influx into a spherical cell. Refinements include 1) reduced cytosolic water content, 2) reduced cytosolic diffusion constants, 3) refined CA II activity, 4) layer of intracellular vesicles, 5) reduced membrane CO2 permeability, 6) microvilli, 7) refined CA IV activity, 8) a vitelline membrane, and 9) a new simulation protocol for delivering and removing the bulk extracellular CO2/HCO3− solution. We show how these features affect the simulated pHi and pHS transients and use the refined model with the experimental data for 1.5% CO2/10 mM HCO3− (pHo = 7.5) to find parameter values that approximate ΔpHS, the time to peak pHS, the time delay to the start of the pHi change, (dpHi/dt)max, and the change in steady-state pHi. We validate the revised model against data collected as we vary levels of CO2/HCO3− or of extracellular HEPES buffer. The model confirms the hypothesis that CA II and CA IV enhance transmembrane CO2 fluxes by maximizing CO2 gradients across the plasma membrane, and it predicts that the pH effects of simultaneously implementing intracellular and extracellular-surface CA are supra-additive.
Keywords: reaction-diffusion, tortuosity factors, intracellular pH, surface pH, competing equilibria, buffers
in the two companion papers, we studied the effects of the cytosolic enzyme carbonic anhydrase II (CA II) and of the GPI-linked extracellular CA IV on enhancing CO2 fluxes across the plasma membrane (14, 15). We assessed these fluxes by using pH microelectrodes to measure simultaneously intracellular (pHi) and surface pH (pHS) as we added or removed extracellular CO2/HCO3−. For pHi measurements, the two key quantities that reflect the CO2 flux were the maximal rate of pHi change (dpHi/dt)max—negative for CO2 influx and positive for CO2 efflux—and the time constant for pHi decline (τpHi). For pHS measurements, the two key quantities that reflect the CO2 flux were the maximal change in pHS (ΔpHS)—the pHS spike, positive for CO2 influx and negative for CO2 efflux—and the time constant for the decay of pHS from its most extreme value (τpHS). We found that both CA II and CA IV increase the flux of CO2 across the plasma membrane by maximizing transmembrane CO2 gradients. We reasoned that (dpHi/dt)max and ΔpHS provide intuitive information on the effects of each of these enzymes on CO2 fluxes only when the measurements are “trans” to (i.e., on the opposite side of the membrane from) the added enzyme. When we assess cytosolic CA II, ΔpHS (i.e., measured “trans” to CA II) can reveal the effects of CA II in enhancing CO2 fluxes, whereas (dpHi/dt)max would reflect changes in CA II activity even without a change in CO2 flux. However, we found that the GPI-linked CA IV is present not only on the extracellular surface but also in an equivalent of a cytosol-accessible compartment. Thus, for CA IV as expressed in oocytes, both pHi and pHS electrodes are “cis” (i.e., on the same side of the membrane as the CA IV) and “trans” to the CA IV. Nevertheless, we reasoned that decreases in τpHi and τpHS provide intuitive information on the effects of CA IV on CO2 fluxes.
In the preceding two papers, we used mathematical simulations to help us understand how CA II (14) or CA IV (15) influences CO2-induced pHi and pHS transients when we alter the experimental protocol. These protocol changes included raising levels of CO2 in the bulk extracellular fluid (BECF), increasing the depth of penetration of the pHi microelectrode, and varying the concentration of the extracellular HEPES buffer. The purpose of the present paper is to describe underpinnings of the mathematical model that we exploited in the two companion papers.1
Several investigators have used mathematical models to study CO2 movements across the cell membrane. In 1976, Boron and De Weer developed a compartmental model that predicted for the first time the pHi time course2 caused by the passive flux of CO2, and the passive flux of HCO3− as well as the active extrusion of H+, across the plasma membrane (5). In the same year, Gros and coworkers (11) developed a model of facilitated CO2 diffusion in phosphate solutions. They used the model to predict the steady-state fluxes and concentrations of the solutes of interest, concluding that CA promotes the diffusion of HCO3− and protonated phosphate in the same direction as CO2, as well as the diffusion of deprotonated phosphate in the opposite direction, thereby facilitating CO2 diffusion (11). In 2000, Geers and Gros (10) employed a compartmental model to describe the diffusion of CO2 and lactic acid from skeletal muscle to erythrocytes as blood moves along a capillary. Spitzer and coworkers (23) studied the role of intracellular CA in regulating H+ diffusion in rabbit ventricular myocytes with the aid of a two-dimensional model of diffusion of intracellular H+ from a constant source. They employed the model to estimate the apparent intracellular H+ diffusion coefficient, finding that CA increases the mobility of H+ by a factor of ∼6. More recently, Swietach and colleagues (24, 25) developed reaction-diffusion spatio-temporal models to study the role of the cancer-associated extracellular CA IX enzyme in three-dimensional tumor cell growths. These authors concluded that CA IX plays a key role in spatially regulating pHi and extracellular pH by facilitating CO2 removal. Note that none of these models considers 1) the diffusion of CO2 through the cytosol or 2) a multitude of buffers.
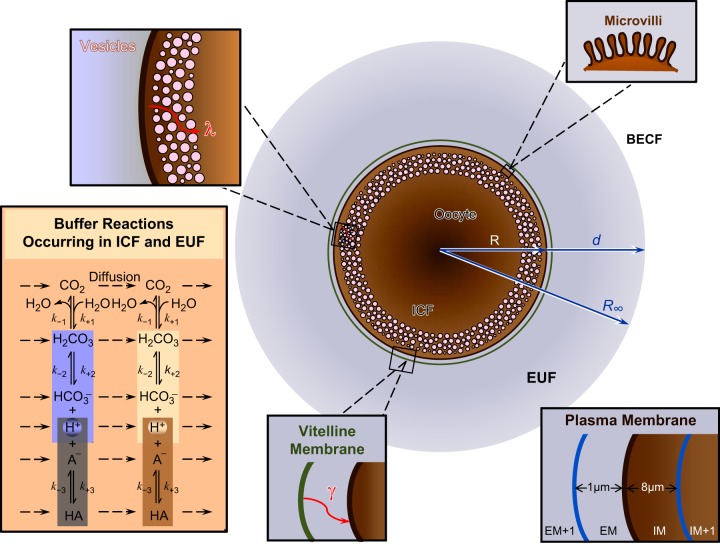
In this third and final paper of this series, we extend the reaction-diffusion mathematical model of CO2 influx into a Xenopus oocyte, introduced in Somersalo et al. (22). The model assumes that the oocyte is a perfectly symmetric sphere, surrounded by the extracellular unconvected fluid (EUF), which in turn is surrounded by the BECF. In both EUF and intracellular fluid (ICF), the model includes the CO2/HCO3− buffer pair as well as a multitude of non-CO2/HCO3− buffer pairs. The model (as presently implemented) permits only the movement of CO2 across the cell membrane. Although valuable and capable of making important theoretical predictions, this model, in its original state, cannot quantitatively reproduce some of the salient features of the measured pHi and pHS transients in the two accompanying papers on CA II and CA IV. Therefore, our goal in the present study is to refine the model by Somersalo et al. to create an enhanced version (Fig. 1) that is better able to simulate our oocyte experiments. We introduce nine new features (identified as 1–9 in the legend to Fig. 1), which we explain in detail in materials and methods.
Fig. 1.
Main features of the revised mathematical model. Following the approach of Somersalo et al. (22), we assume that the oocyte is a perfectly symmetric sphere of radius R, surrounded by a thin layer of extracellular unconvected fluid (EUF) of thickness d. The EUF is in turn surrounded by the bulk extracellular fluid (BECF), which is an infinite reservoir for all solutes. Within the BECF, convection could occur (though it is not included in the present model), but no reaction or diffusion. Solutes can diffuse between the BECF and EUF. In both the intracellular fluid (ICF) and EUF, reaction and diffusion occur, but no convection. The buffer reactions occurring in the ICF and EUF (illustrated in the inset in the bottom left corner) are: the CO2/HCO3− buffer reactions (including the slow conversion of CO2 into H2CO3 and vice versa) and a single non-CO2/HCO3− buffer (HA/A−). New features of the model include (starting from the inside of the cell and moving outward): 1) reduced water content of cytosol, 2) reduced cytosolic diffusion constants, 3) refined CA II activity, 4) layer of intracellular vesicles (magnification in top left corner), 5) reduced membrane CO2 permeability, 6) microvilli (magnification in top right corner), 7) refined CA IV activity, 8) vitelline membrane (magnification on lower edge), and 9) a new simulation protocol for the delivery and removal of the bulk extracellular CO2/HCO3− solution. The inset in the bottom right corner explains the spatial discretization for the numerical solution near the plasma membrane (PM). EM, extracellular surface of the PM; IM, intracellular surface of the PM.
In the present paper, we first show how introducing these nine new features affects the pHi and pHS trajectories caused by the application (or withdrawal) of 1.5% CO2/10 mM HCO3− to an oocyte. We then use this more refined model of the oocyte and of its surrounding environment, with all nine new features, to illustrate how we arrive at parameter values that can approximate the measured ΔpHS, the time to peak pHS, the experimentally observed time delay to the start of the pHi change, (dpHi/dt)max, and the steady-state change in pHi (ΔpHi) caused by the equilibration of CO2 across the cell membrane. We validate the revised model against physiological data as we work with increased CO2/HCO3− levels, or expose oocytes to CO2/HCO3− in the presence of varying levels of extracellular HEPES buffer. The model confirms the hypothesis that CA II and CA IV enhance CO2 fluxes across the plasma membrane by increasing transmembrane CO2 gradients. Finally, the model predicts that the effects on (dpHi/dt)max and ΔpHS of simultaneously implementing intracellular CA and extracellular-surface CA are supra-additive. That is, the pH effects of the two CA activities are substantially larger than the sum of the two individual effects.
MATERIALS AND METHODS
Assumptions Used in Creating the Model
Here we briefly summarize the main features and assumptions of the mathematical model without reporting the details, which are extensively explained in ref. 22, on its numerical implementation. The key components of the model are summarized in Fig. 1. Following the approach of Somersalo et al. (22), we assume that the oocyte is a perfectly symmetric sphere of radius R, fixed at the center of a spherical volume of radius R∞. The oocyte is surrounded by a thin layer of unconvected (i.e., unstirred) fluid of thickness d (d = R∞ − R), the EUF, which in turn is surrounded by the bulk extracellular fluid, the BECF. In both the ICF and EUF, reactions and diffusion occur. The BECF, which represents the bath solution that flows around an oocyte in a physiological experiment, is an infinite reservoir of uniform, preequilibrated solution. Because it is uniform, net diffusion does not occur. Because the BECF is preequilibrated, net reactions do not occur. However, diffusion does occur at the interface between the BECF and EUF.
The buffer reactions considered in this model are 1) the CO2/HCO3− buffer reactions, including the slow conversion of CO2 into H2CO3 and vice versa; and 2) a single mobile non-CO2/HCO3− buffer, indicated by HA/A− in the bottom left inset in Fig. 1. Thus, HA/A− represents the HEPES buffer (H-HEPES ⇌ H+ + HEPES−) that we use in the physiological experiments described in the two companion papers. The intracellular non-CO2/HCO3− buffer mimics the sum of all intrinsic buffers (i.e., intracellular buffers that do not readily cross the plasma membrane; refs. 2, 6) that are present in the ICF of the oocyte. The properties of this hypothetical single intrinsic buffer, intrinsic buffering power (βI), pK, and total concentration [TA], are chosen following ref. 6 and will be described in more detail in the results.
Because, in the physiological experiments, CO2 is the only solute that moves across the plasma membrane (i.e., the oocyte behaves as an open system only for CO2), the model allows only CO2 to cross the plasma membrane. Because CO2 is electrically neutral (and thus expected to be insensitive to membrane potential, Vm), we do not implement Vm in the present model.
The new features of the present model are:
1) Water content.
We now assume that the aqueous portion of the oocyte (i.e., the volume fraction, Vf) occupies not 100% but only 40% of the total oocyte volume (29).
2) Cytoplasm.
We now assume that the viscosity and steric hindrance of the cytoplasm reduce diffusion coefficient of all solutes, specifically by a factor of two (26, 29).
3) Cytosolic CA activity.
We explore a range of values to approximate the electrophysiological data.
4) A layer of intracellular vesicles beneath the plasma membrane (Fig. 1).
This layer can explain in part the observed time delay between the initiation of the pHS spike and the decline of the pHi. We implement the presence of intracellular vesicles (12), which impose geometrical constraints on the diffusion path of each solute, by reducing the mobility of each solute in that layer by the same tortuosity factor λ. That is, we introduce an effective diffusion coefficient D* = D/λ2 for each solute in the layer (17, 18).
5) A reduction in the diffusion constant of the cell membrane for CO2 (DM,CO2).
We now assume that the oocyte plasma membrane offers resistances to the movement of CO2. That is, unlike the original implementation in ref. 22, we no longer assume that the plasma membrane behaves as a thin film of pure water with a high DM,CO2.
6) Folds and microvilli at the plasma membrane (Fig. 1).
We implement these features by multiplying the CO2 membrane permeability by a surface amplification factor (28).
7) Extracellular-surface CA activity.
We explore a range of values to approximate the electrophysiological data.
8) The vitelline membrane, which surrounds the plasma membrane (Fig. 1), and is not removed during the physiological experiments in the accompanying papers.
Introducing this membrane helps to approximate ΔpHS. We implement the vitelline membrane, which represents an additional barrier to the diffusion of all solutes, by reducing the mobility of each solute in the region (see Fig. 1, bottom right inset) immediately adjacent to the extracellular surface of the membrane (EM) by the same tortuosity factor γ. That is, we introduce an effective diffusion coefficient D* = D/γ2 in the EM region.
9) Simulation protocol to mimic delivery of CO2/HCO3− to and removal from the BECF.
This protocol can explain the time that it takes for the pHS transient to reach its peak (tp). To mimic the change of solutions in electrophysiological experiments, we implement parallel, exponential changes in [CO2], [H2CO3], and [HCO3−].
Buffer Reactions, Chemical Equilibria, and Carbonic- Anhydrase Activity
The buffer reactions with the corresponding equilibrium constants (Kℓ, ℓ = 1, 2, 3) and rate constants (kℓ, ℓ = ± 1, 2, 3) are:
1) The CO2 hydration/dehydration reaction:
| (1) |
2) The carbonic acid dissociation reaction:
| (2) |
and
3) The dissociation reaction for the single non-CO2/HCO3− buffer:
| (3) |
Reaction 1 is very slow unless bypassed by a CA enzyme. We simulate the catalytic action of a CA enzyme—CA-like activity—by multiplying the rate constants of reaction 1, k+1 and k−1, by the same acceleration factor A, which could vary in space and time. In the present study, we do not allow A to vary with time. We assume that Ai (the factor A for intracellular/cytosolic CA activity that mimics CA II) amplifies the rate constants k+1 and k−1 everywhere inside the oocyte. However, we assume that AS (the factor A for surface CA activity that mimics the subset of CA IV present on the outer surface of the cell) amplifies the rate constants k+1 and k−1 only in the EM region.
Reactions 2 and 3 are extremely rapid, limited only by diffusion. We ensure instantaneous equilibrium of reactions 2 and 3 by choosing the rate constants in such a way that, in the time scale of diffusion and reaction, each reaction occurs instantaneously (22).
The values of the equilibrium constants and rate constants used in the simulations are those reported in Table 1 of ref. 22.
Governing Equations
With the assumption of spherical radial symmetry, the concentration C of each solute s, Cs, depends only on the radial distance r from the center of the oocyte. Because the two processes affecting Cs are chemical reactions and diffusion, we model the change of Cs in time and space according to the reaction-diffusion equation (22):
 |
The first term on the right-hand side describes diffusion according to Fick's second law of diffusion, whereas the second term describes the chemical reactions among the solutes. Ds(r) is the diffusion coefficient of solute s as a function of r; Ss,ℓ are the stoichiometry coefficients that can take the value +1, −1, or 0, depending on whether the ℓ-th reaction produces, consumes, or does not involve solute s; and Φℓ are the reaction fluxes modeled according to the law of mass action (22). For the reactions occurring in the ICF, the reaction term is divided by Vf, to reflect that, in the ICF, solutes are restricted to a smaller water volume than the entire oocyte volume (18, 27).
In addition to Eq. 4, we postulate a set of boundary and initial conditions. At the center of the oocyte we posit a Neumann boundary condition:
| (5) |
which states that the flux of solute s at the center of the oocyte is zero.
At the plasma membrane, we assign the transmission boundary conditions:
| (6) |
which establish continuity of Fick's diffusive fluxes of solute s across the plasma membrane, and
| (7) |
which is a statement of Fick's law for diffusion across the plasma membrane.
In Eqs. 6 and 7, Cs(t, R±) denotes the concentration of solute s in the aqueous phase adjacent to the intracellular (−) or extracellular (+) side of the plasma membrane. SA denotes the surface amplification factor, accounting for the presence of folds and microvilli and PM,s is the true permeability of the membrane to solute s (7, 22).
At R = R∞ we assign the Dirichlet boundary condition:
| (8) |
which specifies the concentration of solute s in the BECF, Cs,BECF. Cs,BECF can be constant or time dependent. More specifically,
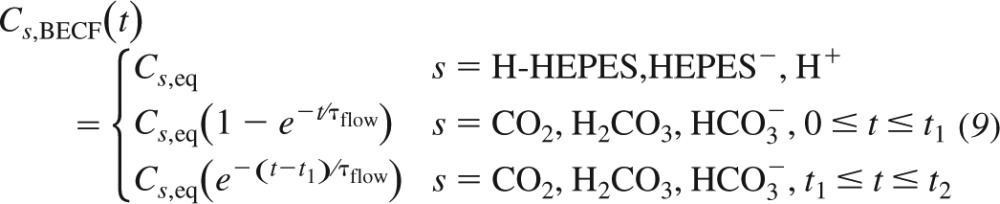 |
Here Cs,eq is the concentration of solute s in a solution equilibrated at pH = 7.50, τflow is the time constant of the exponential function that we use to simulate the delivery, from time t = 0 to time t = t1, and removal, from time t = t1 to time t = t2, of a solution containing CO2/HCO3− (pH = 7.50). In the results, we will discuss how the new choice of Cs,BECF affects the results of our numerical experiments.
Finally, we assign the initial condition Cs(0, r) = Cs(r), which specifies the radially dependent concentration of solute s at time zero, that is, when we start the experiment.
Numerical Approach
We solve the resulting system of reaction-diffusion equations using the method of lines (MOL). The MOL consists of two steps: first we discretize in the radial direction using an appropriate finite-difference scheme and then we use a stiff ode solver (the MATLAB ode15s, in our case) to find the numerical solution of the resulting time-dependent system of ordinary differential equations. The details of the numerical implementation, which we perform in MATLAB, are described in ref. 22.
RESULTS
Key Quantities for Describing pHi and pHS Transients in Physiological experiments
Exposing an oocyte to a solution containing 1.5% CO2/10 mM HCO3− causes pHi to fall monotonically and pHS to rise to a peak and then decay. Figure 2 schematically illustrates such transients of pHi (in red) and pHS (in green) and some of the experimentally measurable quantities that characterize these transients. For pHi, these quantities include:
td: the time delay between the initiation of the pHS spike and the initiation of the pHi change
(dpHi/dt)max: the maximal rate of intracellular acidification caused by the CO2 influx (a negative number)
τpHi: the time constant of the pHi decline
ΔpHi: the difference between the initial pHi (in the absence of CO2) and the final pHi (after complete equilibration of CO2)
For pHS, the key quantities include:
tp: the time to peak (time that it takes for pHS to reach its maximal value)
ΔpHS: the difference between the maximal height of the pHS trajectory (in the presence of CO2) and the initial pHS (in the absence of CO2), which in physiological experiments is very similar to pHBECF
τpHS: the time constant of the decay phase of pHS
We can use the same quantities, but with opposite signs, to describe pHi and pHS transients during CO2 removal.
Fig. 2.
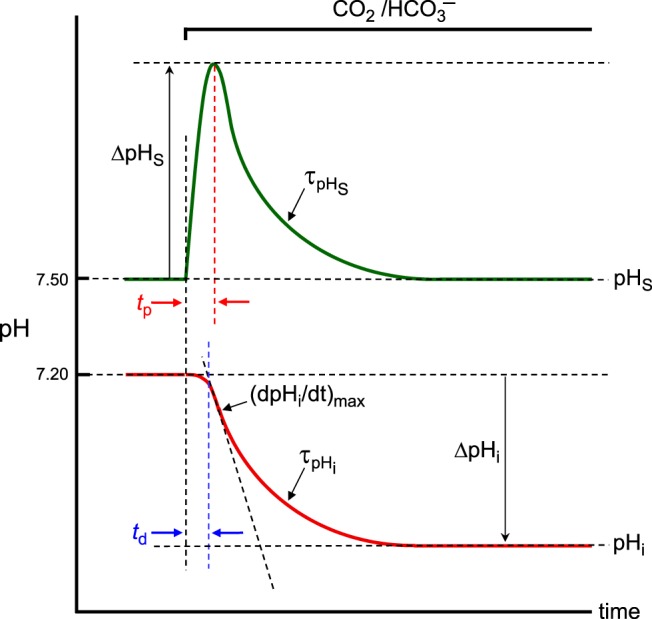
Summary of the key quantities of interest for describing intracellular-pH (pHi) and surface pH (pHS) transients in our physiological experiments during the addition of CO2/HCO3−. This figure schematically illustrates the transients of pHi (in red) and pHS (in green) and some of the experimentally measurable key quantities as we expose an oocyte to a CO2/HCO3− solution. For pHi, key quantities are time delay (td), the maximal rate of pHi descent [(dpHi/dt)max], time constant for pHi decay (τpHi), and the difference between initial and final pHi (ΔpHi). For pHS, key quantities are time to peak (tp), maximal height of the pHS trajectory (ΔpHS), and the time constant for pHS decay (τpHS).
In the two companion papers we observed that injecting oocytes with recombinant human CA II increases the magnitudes of (dpHi/dt)max and ΔpHS caused by switching from a BECF lacking CO2/HCO3− to one containing CO2/HCO3−, or vice versa. Moreover, CA II causes τpHi and τpHS to become smaller (i.e., faster). Following a similar pattern, expressing CA IV, which appears on the outer surface of the plasma membrane but also in an equivalent of a cytosol-accessible compartment, increases the magnitude of (dpHi/dt)max and ΔpHS and causes τpHi and τpHS to become smaller (faster) as we switch the BECF to one (or from one) containing CO2/HCO3−. Moreover, we observed that the expression of CA IV produces more extreme changes in these key quantities than does the injection of CA II.
In the following two sections we explain how we choose parameter values—τflow, tortuosity factors (λ and γ), and acceleration factors (Ai and AS)—to approximate the physiological data (mean value ± SD), as described by the above key quantities, when we switch the BECF to one containing 1.5% CO2/10 mM HCO3−. In the next section, we explore the dependence of (dpHi/dt)max and ΔpHS on Ai and AS. In the last section, we validate the model by exploring the effects of altering the [CO2]o/[HCO3−]o or [HEPES]o.
Standard Features of an In Silico Oocyte Experiment
In our numerical experiments we consider three types of oocytes: “control”, “CA II,” and “CA IV” oocytes.
A “control” oocyte is one not injected with, or expressing, a heterologous protein. Thus, such an oocyte would have been injected with Tris (rather than CA II + Tris) in the first paper in this series (14) or H2O (rather than cRNA encoding CA IV + H2O) in the second paper (15). In our numerical experiments, we will not distinguish between Tris- and H2O-injected oocytes. Indeed, in the previous two papers, we observed that Tris and H2O-injected oocytes behave similarly; the quantities shown in Fig. 2 are not significantly different between the two types of oocytes. For consistency, we will use the physiological data from Tris oocytes, from the first paper (14), to set up the properties of our in silico “control” oocyte in all of our simulations.
A “CA II” oocyte in our physiological experiments is one injected with recombinant human CA II protein and, from a modeling prospective, has the same features as a “control” oocyte (see below) but higher CA-like activity (Ai) throughout the ICF.
A “CA IV” oocyte in our physiological experiments is one expressing human CA IV on the outer surface of the plasma membrane as well as in an equivalent of a cytosol-accessible compartment. From a modeling prospective, a “CA IV” oocyte has the same features as a “control” oocyte (see below) but higher CA-like activity (AS) in the EM region (Fig. 1, bottom right inset), and a slightly higher CA-like activity (Ai) throughout the ICF.
Features of a “typical” oocyte.
Based on the assumptions of our model, each oocyte type (“control”, “CA II”, “CA IV”) has the following features:
1) Only 40% of the oocyte total volume (we assume that an average oocyte has a radius R = 0.13/2 = 0.065 cm) is water (29). For implementation of this feature, see Governing Equations in materials and methods. The model predicts that as Vf decreases, reaction rates and the magnitudes of both (dpHi/dt)max and ΔpHS modestly increase. For example, starting with an in silico control oocyte, decreasing Vf from 60% to 40% (the control value) to 20% causes the predicted (dpHi/dt)max to increase from 0.0011 to 0.0012 to 0.0015 pH s−1 and causes ΔpHS to increase from 0.027 to 0.031 to 0.036.
2) The ICF contains a single non-CO2/HCO3− buffer (HA/A−), the properties of which we choose so that the mathematical model predicts a fall in ΔpHi that matches the overall mean values for the physiological data.
3) The mobility of each solute (CO2, H2CO3, HCO3−, H+, HA, A−) in the ICF is ½ the mobility in pure water (26, 29).3
4) Intracellular CA-like activity (Ai) is present everywhere inside the oocyte. For a “control” oocyte, Ai = 5, and this value is higher in “CA II” and “CA IV” oocytes.
5) A layer of intracellular vesicles begins ∼10 μm beneath the plasma membrane and extends an additional ∼25 μm into the oocyte (12). As we will explain later, we choose the tortuosity factor, λ, to be ∼3.16 (i.e., 1/ λ2 = 0.10) in this layer of the cytosol.
6) The true permeability of the plasma membrane to CO2 is PM,CO2 = Pwater,CO2/10,000 = 34.2/10,000 = 0.00342 cm/s. That is, we assume that the diffusion coefficient of CO2 within the plasma membrane, DM,CO2, is 1/10,000 of the diffusion coefficient of CO2 within a film of water of the same thickness, hM = 5 nm, as the plasma membrane (1). We choose the value 10,000 based on the conclusion that the oocyte PM,CO2 would have to be ∼1/1,000 of the value of ∼3 cm/s reported for the PM,CO2 of artificial bilayer in Missner et al. experiments (4, 13).
7) The plasma membrane has folds and microvilli that amplify its surface by ninefold (28).
8) Extracellular CA-like activity (AS) is present in the EM region. For a “control” oocyte, AS = 150, and this value is higher in “CA IV” oocytes.
9) A vitelline membrane surrounds the oocyte. As we will explain later, we choose the tortuosity factor, γ, to be ∼5.77 (i.e., 1/γ2 = 0.03) in the EM region (Fig. 1, bottom right inset).
Simulation of a physiological experiment.
To simulate the peculiarities of our experimental system, we assume that
1) The EUF has a thickness d of 10 μm (9).
2) The EUF and BECF have the properties of pure water. Thus each solute (CO2, H2CO3, HCO3−, H+, H+-HEPES, HEPES−) has the same mobility as in pure water.3
3) Before the beginning of an experiment
The BECF and EUF both contain H+-HEPES/HEPES− (pK = 7.50) at a total concentration of 5 mM in a standard experiment (pHBECF = 7.50). The concentrations of CO2, H2CO3, and HCO3− are all zero. This assumption mimics the composition of the ND96 solution (which contains other solutes not pertinent to the present model). In our simulations, we will refer to this simplified ND96 solution as “ND96”.
In the ICF, the concentrations of CO2, H2CO3, and HCO3− are all zero. The initial pHi corresponds to the mean value of the initial pHi for the physiological data for each oocyte type (i.e., “control”, “CA II”, “CA IV”) and experimental protocol (i.e., 1.5% CO2/10 mM HCO3− vs. 5% CO2/33 mM HCO3− vs. 10% CO2/66 mM HCO3−).4,5,6
4) As the experiment begins at time t = 0, we simulate the delivery of CO2/HCO3− at pH 7.50 by raising exponentially, with a time constant τflow of 4 s, the concentrations of CO2, H2CO3, and HCO3− in the BECF from zero to the values in an equilibrated CO2/HCO3− solution (see Eq. 9).
Parameter Estimation Using the Physiological Data on the Addition of 1.5% CO2/10 mM HCO3−
We approached the estimation of parameters in an iterative process. Here we present these parameters in an order that reflects, first, the relative certainty of our knowledge (i.e., intracellular buffering power, time course of switching BECF composition) and, second, the importance of the parameters for establishing realistic time courses for pHi and pHS (i.e., layer of intracellular vesicles, vitelline membrane, CA activities).
ΔpHi: choosing the intracellular buffering parameters.
The magnitude of the change in steady-state pHi (ΔpHi) produced by exposing a cell to a solution containing equilibrated CO2/HCO3− depends on the initial pHi, [CO2]o, and intrinsic buffering power, βI (2, 19). Using the mean values for the initial (pHi,1) and final pHi (pHi,2) measured in the physiological experiments for each combination of oocyte type and [CO2]o, and the concepts described previously (6), we determine the properties of the single intracellular non-CO2/HCO3− so that the mathematical model correctly predicts a ΔpHi = (pHi,2 − pHi,1). Specifically, 1) we assume that the pK of the HA/A− buffer is halfway between pHi,1 and pHi,2; 2) we calculate βI over the interval (pHi,1, pHi,2) using the Davenport diagram (3, 8); and 3) knowing pK and βI, we calculate the total amount of intracellular non-CO2/HCO3− buffer, [TA]i, using the equation introduced in ref. 22:
where
Indeed, when we use the computed value of [TA]i in simulations, the model predicts ΔpHi = (pHi,2 − pHi,1) values that agree with the physiological data.
Time to peak pHS: choosing the protocol for simulating the switch in BECF composition.
In our physiological experiments at 1.5% CO2/10 mM HCO3−/pH 7.50, the time to peak is ∼11.2 ± 3.7 s (means ± SD) for “control” oocytes (see Table 3 in the first paper in this series; ref. 14), ∼7.6 ± 2.7 s for “CA II” oocytes (see Table 3 in the first paper; ref. 14), and ∼6.5 ± 0.8 s for “CA IV” oocytes (not reported in the second paper; ref. 15).
We observe that, to simulate a pHS trajectory with a tp close to the physiological data, we had to simulate the initiation of the experiment using an approach substantially different from that used by Somersalo et al. (22), in which: 1) At the outset of the simulation, the BECF and EUF both contain 1.5% CO2/10 mM HCO3−/pH 7.50 plus 5 mM HEPES at a pH of 7.50. 2) The simulation starts at time t = 0, as the CO2 begins to diffuse into the cell. Figure 3A shows results of initiating the simulations using the protocol of Somersalo et al. with a “control” oocyte. We observe that the pHS trajectory has a nonrealistic shape, with a tp (∼0.4 s) that is more than an order of magnitude smaller that the physiologically observed value. Moreover, after pHS reaches its maximum, it initially falls off quite rapidly before continuing with a slower decline.
Fig. 3.
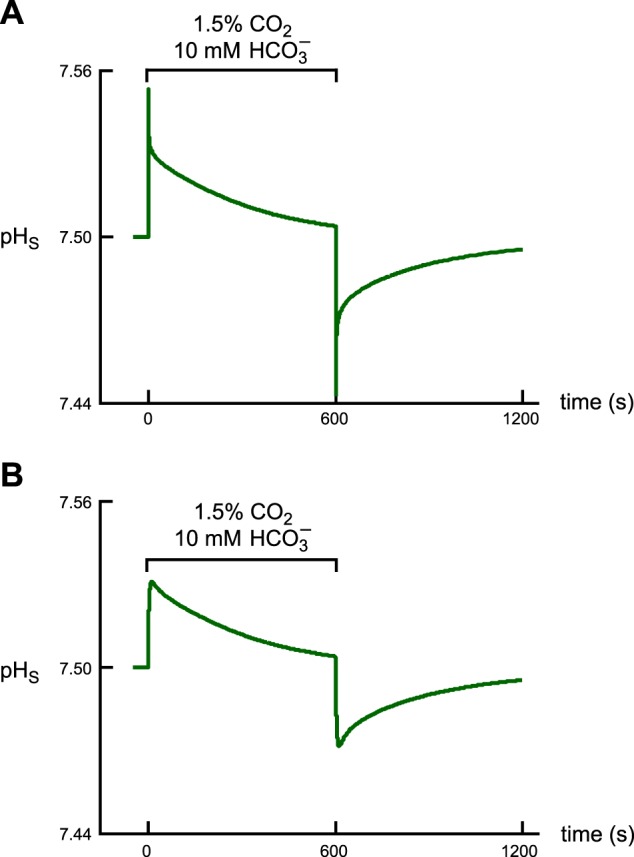
Effect of the new experimental protocol on the shape of the pHS trajectory and on the time to peak. A: pHS trajectory predicted by the model when using the protocol of Somersalo et al. (22). In using this original protocol, we assume that, at the outset of the simulation, the CO2/HCO3− is already at the plasma membrane. B: pHS trajectory predicted by the model when using the revised protocol. Here we assume that the oocyte is initially superfused with the CO2/HCO3−-free ND96 solution until we switch the bulk solution to one containing CO2/HCO3− and allow the CO2 and HCO3− to diffuse to the cell surface.
Figure 3B shows results of initiating simulations using our revised protocol, which is mathematically described by Eq. 9. In this new approach: 1) Before the beginning of a simulation, the BECF and EUF both contain 5 mM HEPES (pHBECF = 7.50) but no CO2, H2CO3, or HCO3−. This condition reflects the situation in a physiological experiment, in which the oocyte is exposed to the “ND96” solution until the switch to bulk solution containing CO2/HCO3−. 2) As the simulation begins, the concentrations of CO2, H2CO3, and HCO3− in the BECF rise exponentially. It is only after the CO2 diffuses through the EUF that the CO2 can begin diffusing through the cell membrane and enter the ICF. Figure 3B shows that our revised protocol, with τflow = 4 s, produces a pHS trajectory that is far more realistic, with a tp of ∼12.6 s. This value is very close to the physiological mean ± SD (∼11.2 ± 3.7). Regarding “CA II” and “CA IV” oocytes exposed to 1.5% CO2/10 mM HCO3−, the model predicts a tp of ∼10 s for “CA II” oocytes (physiological mean ± SD ∼ 7.6 ± 2.7), and ∼8 s for “CA IV” oocytes (physiological mean ± SD ∼6.5 ± 0.8). These simulated tp values are systematically somewhat larger than the physiologically observed mean values. On the other hand, if we reduce τflow to match the tp values better, other aspects of the simulation (e.g., time delay) are negatively impacted.
Time delay and (dpHi/dt)max: simulating the effect of intracellular vesicles.
In the physiological experiments reported in the first paper of this series (14), we observed a pHi time delay (averaged for CA II and Tris experiments) of ∼9 s for CO2 addition. We explained this delay as reflecting: 1) the depth of impalement of the pHi electrode and 2) the resistance offered to CO2 diffusion by a relatively superficial portion of the oocyte's cytoplasm that contains vesicles (12). In the first paper (see Figs. 15–17 in ref. 14) we used our revised mathematical model to illustrate how the time delay increases with the depth of impalement. In the present paper, we show how the time delay increases as we implement a layer of intracellular vesicles, beginning ∼10 μm beneath the plasma membrane and extending an additional ∼25 μm into the oocyte (12), with increasingly larger values of the tortuosity factor λ.
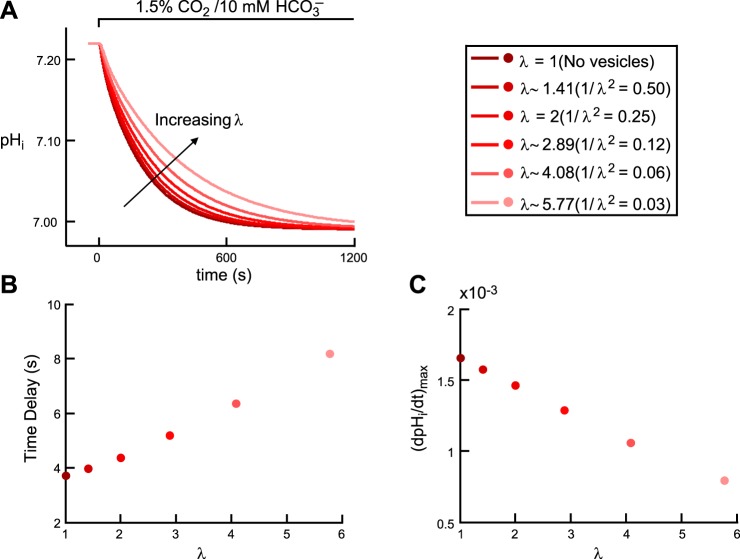
Figure 4 shows the effects of implementing such a layer of vesicles on the simulated pHi time course (Fig. 4A), time delay (Fig. 4B), and (dpHi/dt)max (Fig. 4C).
Fig. 4.
Effect of intracellular vesicles on the pHi transient during the addition of CO2/HCO3−. A: pHi trajectories, at a depth of ∼50 μm. The six simulations correspond to six increasing values of the tortuosity factor λ. B: time delay as a function of λ. The six colored dots correspond to the six simulations in A. C: maximal rate of pHi change (negative direction) as a function of λ. The six colored dots correspond to the six simulations reported in A.
As the tortuosity factor λ increases from 1 (i.e., no vesicles) to ∼5.77 (i.e., as 1/λ2 decreases from 1 to 0.03), pHi, computed at a depth of ∼50 μm into the oocyte, declines more slowly (Fig. 4A), the time delay rises from 3.73 to 8.18 s (Fig. 4B), and (dpHi/dt)max falls from 0.0017 to 0.0008 pH U/s (Fig. 4C). The magnitude of (dpHi/dt)max falls because, as λ rises (i.e., we reduce the mobility of solutes in the band corresponding to vesicles), the flux of CO2 across the plasma membrane decreases (CO2 builds up in the regions near the inner side of the plasma membrane), causing CO2 to enter into the cell more slowly, as also indicated by the slower pHi decline (Fig. 4A) and larger time delay (Fig. 4B).
What would the tortuosity factor have to be, in a band 10 to 25 μm below the oocyte surface, to achieve a delay of ∼9 s in simulations of CO2 addition in “control” experiments at a reasonable electrode depth of 50 μm? If we use a λ of ∼6.32 (i.e., multiply the diffusion constants for all solutes by 1/λ2 = 0.025) in the aforementioned vesicle band, we can account for a pHi delay of ∼9 s. However, such a high λ produces excessive slowing of pHi and pHS transients (not shown). In fact, the events inside (and outside) the oocyte become so slow that it becomes impossible to reproduce the features of pHi (faster pHi decay) and pHS (higher ΔpHS) transients when implementing larger intracellular and extracellular values for CA-like activity, that is, to mimic a “CA II” and a “CA IV” oocyte. Therefore, in our “typical” oocyte, we compromise by using a λ of ∼3.16 (i.e., 1/ λ2 = 0.10), which has the effect of reducing the pHi delay from ∼9 s to ∼5.5 s for CO2 addition in a “control” oocyte. As addressed in the second paper of this series (15), we believe that, in addition to vesicles, other factors, including the asymmetry of [CO2]BECF around the oocyte (not part of the present model), contribute to the pHi delay.
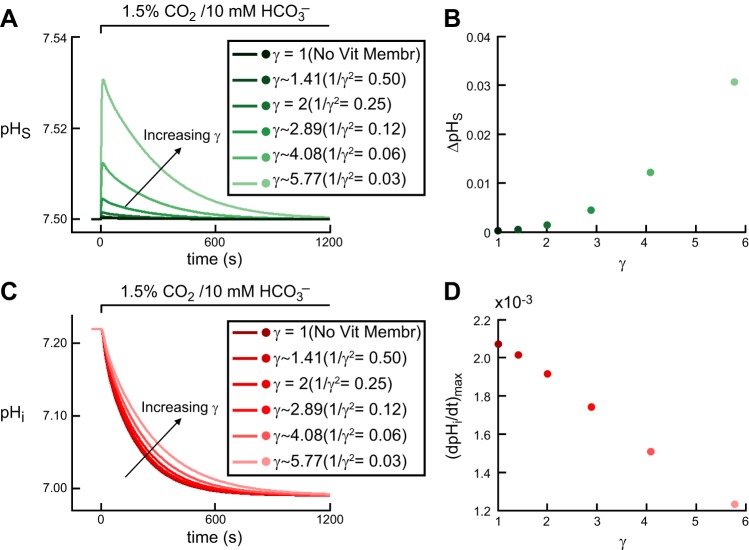
ΔpHS: simulating the effect of vitelline membrane.
In Fig. 5, A and B, we show the effects on pHS of implementing the vitelline membrane in a “control” oocyte, that is, the effects on pHS of reducing the mobility of all solutes in the region immediately adjacent to the extracellular surface of the plasma membrane. The reason for implementing this vitelline membrane is that ΔpHS approaches zero when this membrane is not present (Fig. 5, A and B). The first electrophysiologically meaningful effect on ΔpHS occurs when we increase γ from 2 to ∼2.89 (i.e., reduce 1/γ2 from 0.25 to 0.12). However, a γ of 2.89 predicts a ΔpHS of ∼0.005, which is much less than the physiological value of 0.046 ± 0.016 for a “control” oocyte. Further increases in γ cause further increases in ΔpHS. For γ ∼5.77 (i.e., 1/γ2 = 0.03) the model predicts a ΔpHS close to the physiological data. We also note that as we increase γ, the pHS decay from the peak to the equilibrium state is slower.
Fig. 5.
Effect of vitelline membrane on pHi and pHS transients during the addition of CO2/HCO3−. A: pHS trajectories. The six simulations correspond to six increasing values of the tortuosity factor γ. B: maximal height of the pHS spike (positive direction) as a function of γ. The six colored dots correspond to the six simulations in A. C: pHi trajectories, at a depth of ∼50 μm, corresponding to the simulations in A. D: maximal rate of pHi change (negative direction) as a function of γ. The six colored dots correspond to the six simulations in A.
It is also informative to see the effects of introducing the vitelline membrane on the pHi trajectory. Figure 5, C and D, shows that, as we increase γ, the decay of pHi to the equilibrium state is slower and (dpHi/dt)max is smaller. Consistent with the analysis in Fig. 5,A and B, a γ of ∼5.77 (i.e., 1/γ2 = 0.03) predicts a (dpHi/dt)max of −0.0012 pH U/s, which is in good agreement with the physiological data for a “control” oocyte.
The reason that increasing γ raises the magnitude of ΔpHS (Fig. 5,A and B) is that, by implementing the vitelline membrane, we reduce the contribution of diffusion and emphasize the contribution of reaction in replacing the lost CO2 in the EM region (for chemistry, see Fig. 1 in the first paper in this series; ref. 14).
The reason that increasing γ lowers the magnitude of (dpHi/dt)max is that reducing diffusion in the EM layer causes the flux of CO2 from the EM+1 region to the EM region to fall, thereby reducing [CO2] at the outer surface of the plasma membrane and causing CO2 to enter into the cell more slowly, as also indicated by the slower pHi decline and larger time delay.
“Control”, “CA II”, and “CA IV” oocytes.
The new protocol to simulate the delivery and removal of CO2/HCO3− solution from/to the BECF, the implementation of the intracellular vesicles, and the implementation of the vitelline membrane allow us to find values for τflow, λ, and γ that produce simulations that approximate a “control” oocyte exposed to 1.5% CO2/10 mM HCO3−/pH 7.50. These parameter values are not unique to a “control” oocyte but also apply to “CA II” and “CA IV” oocytes. In other words, these values approximate the intrinsic properties of our in silico oocyte, independently of the injection or expression of a CA enzyme. To reproduce the pHi and pHS transients of “CA II” and “CA IV” oocytes, we need to find appropriate values for Ai and AS for each oocyte type.
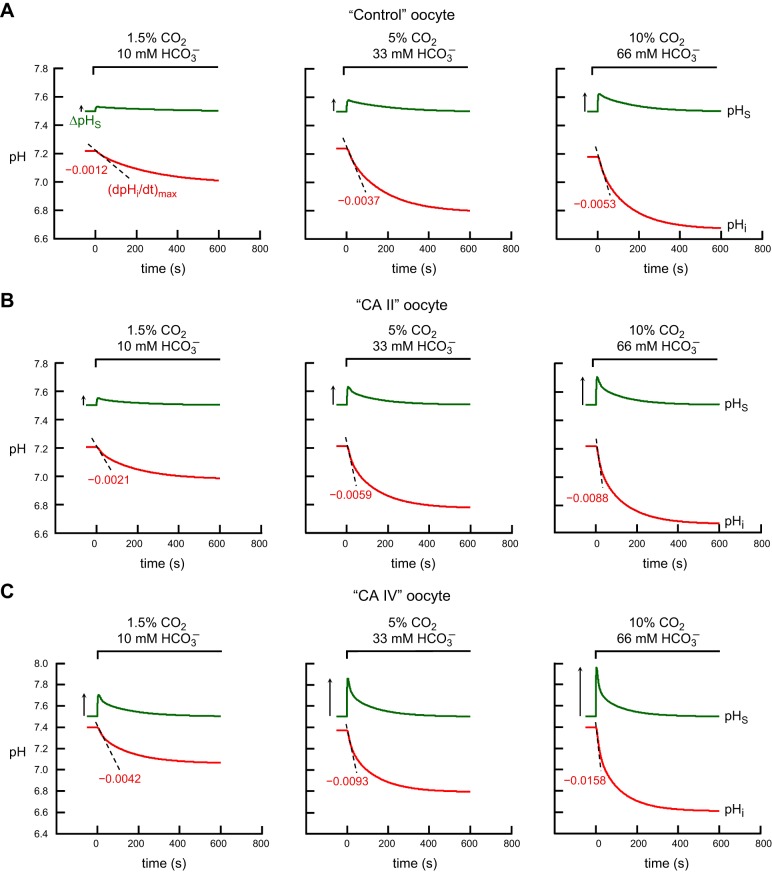
Figure 6 shows the results of simulations that best agree with the physiological data for “control” (Fig. 6A), “CA II” (Fig. 6B), and “CA IV” (Fig. 6C) oocytes. We used:
“Control” oocyte: Ai = 5 and AS = 150
“CA II” oocyte: Ai = 1,000 and AS = 150
“CA IV” oocyte: Ai = 40 and AS = 10,000.
Note that, for a “CA IV” oocyte, we had to increase intracellular CA II activity by a small amount (Ai = 40 vs. Ai = 5) to achieve quantitative agreement with the physiological data (see Fig. 7). The reason is that, for Ai = 5, the model predicts values for (dpHi/dt)max and ΔpHS for CO2 addition [i.e., (dpHi/dt)max = 0.0022 pH U/s and ΔpHS = 0.1015] that are much smaller than the physiological data for “CA IV” oocytes [i.e., (dpHi/dt)max = 0.0042 ± 0.0018 pH U/s and ΔpHS = 0.2488 ± 0.0669 (means ± SD)]. However, it is important to point out that, even when we used an Ai of 5 in a “CA IV” oocyte, an AS of 10,000 is able to produce increases in (dpHi/dt)max and ΔpHS, but not as substantial as when Ai = 40.
Fig. 6.
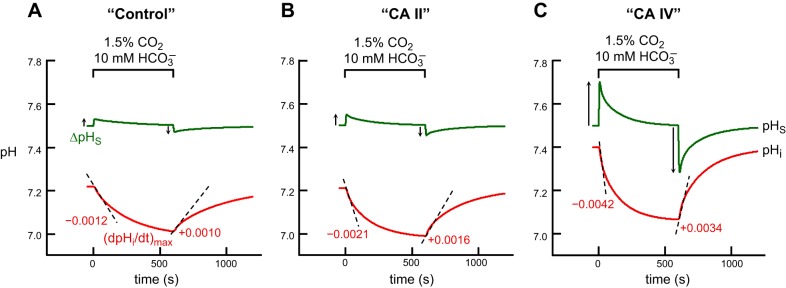
Simulations of pHi and pHS transients for the addition and removal 1.5% CO2/10 mM HCO3− in 5 mM HEPES. A: “control” oocyte“. B: ”CA II“ oocyte. C: ”CA IV“ oocyte. The pHi transients, calculated at a depth of ∼50 μm into the oocyte, are represented by the red lower trajectories, and pHS, by the green upper trajectories. At the indicated time, we switched the extracellular solution from CO2/HCO3−-free ”ND96“ to 1.5% CO2/HCO3− (see Eq. 9). The dashed black lines through the initial portions of the pHi transients for CO2 application and removal represent the maximal rates of pHi change (negative or positive direction), as predicted by the model. The upward and downward arrows near the pHS records represent maximal changes in pHS (positive or negative direction).
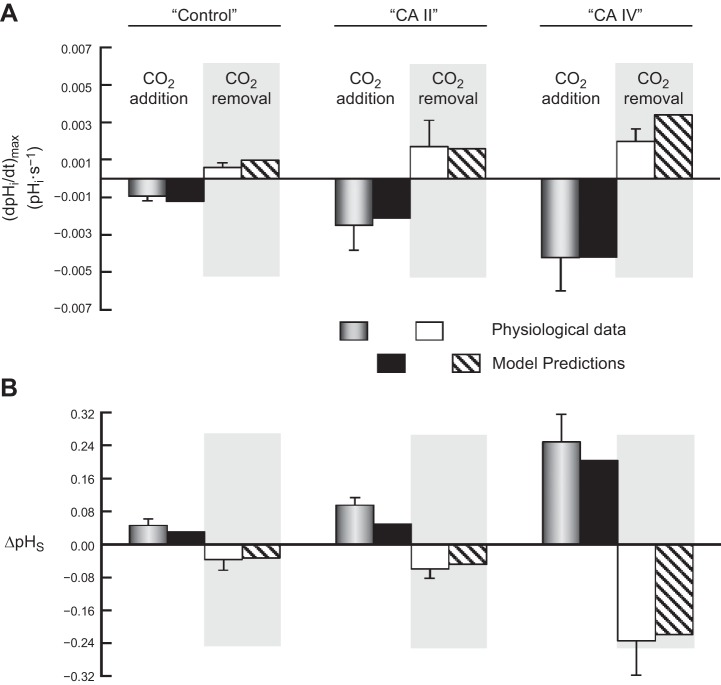
Fig. 7.
Comparison of the physiological data with predictions of the mathematical model for the addition and removal of extracellular 1.5% CO2/10 mM HCO3−. A: maximal rates of pHi change (negative or positive direction) produced by the addition or removal of extracellular CO2/HCO3−. B: maximal changes in pHS (positive or negative direction) caused by the addition or removal of extracellular CO2/HCO3−. The physiological data come from Fig. 2 of the first paper in this series (14) and Fig. 3 of second paper (15). Note that here we report the means ± standard deviation (and not SE, as in the previous two papers). The data for model predictions come from Fig. 6 of the present paper.
Figure 7 shows a comparison between the physiological data (means ± SD) and the predictions of the mathematical model for all three oocyte types, for both CO2 addition and removal.
In agreement with the physiological data, the model predicts that, for both CO2 addition and removal, increasing only cytosolic CA activity (i.e., “CA II” oocyte) increases the magnitude of (dpHi/dt)max and ΔpHS, as well as the time it takes for pHi and pHS to reach equilibration. The model also predicts that increasing extracellular-surface plus cytosolic CA activity (i.e., “CA IV” oocyte) increases these same parameter values. Moreover, consistent with the physiological data, the model predicts that “CA IV” is more effective than “CA II” in enhancing the magnitudes of (dpHi/dt)max and ΔpHS.
Dependence of (dpHi/dt)max and ΔpHS on Ai and AS
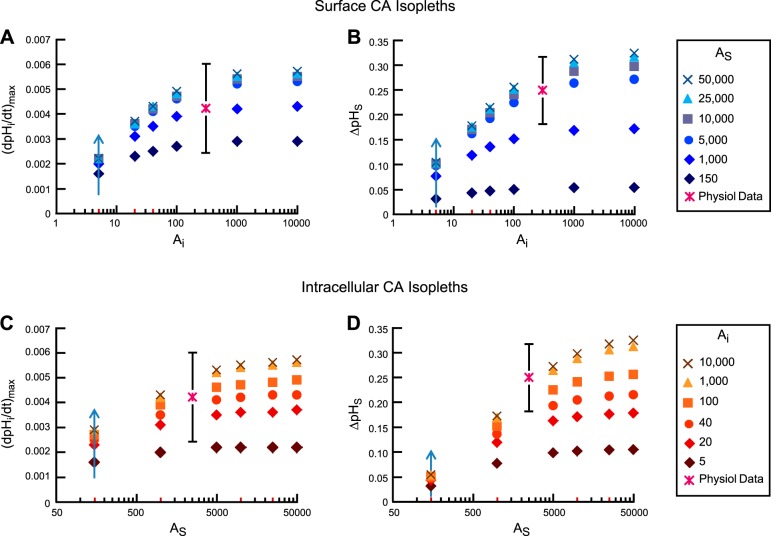
Because Ai and AS play critically important roles in the simulations of the physiological (dpHi/dt)max and ΔpHS data, we investigated how the predicted values for these two pH parameters change as we systematically vary Ai and AS. Here we perform simulations for all possible combinations of Ai values of 5, 20, 40, 100, 1,000, and 10,000 with AS values of 150, 1,000, 5,000, 10,000, 25,000, and 50,000. We assume that the oocyte has the intracellular composition (i.e., initial pHi and ΔpHi) of a “CA IV” oocyte,6 and that we switch from a CO2/HCO3−-free bulk solution to one containing 1.5% CO2/10 mM HCO3−.
Figure 8 shows the values predicted by the model for (dpHi/dt)max (Fig. 8A) and ΔpHS (Fig. 8B) as a function of Ai for six surface CA isopleths (i.e., AS is held constant for each set of symbols). Figure 8, C and D, shows the same predicted values, but as a function of AS for six intracellular CA isopleths. In each panel we identify with a “star” the physiological value of (dpHi/dt)max or ΔpHS as mean ± standard deviation; the position of the “star” along the x-axis is arbitrary.
Fig. 8.
Dependence of (dpHi/dt)max and ΔpHS on Ai and AS. A: dependence of (dpHi/dt)max on Ai, for several fixed values of AS (i.e., each set of symbols is a surface CA isopleth). B: dependence of ΔpHS on Ai, for several fixed values of AS (i.e., surface CA isopleths). C: dependence of (dpHi/dt)max on AS, for several fixed values of Ai (i.e., intracellular CA isopleths). D: dependence of ΔpHS on AS, for several fixed values of Ai (i.e., intracellular CA isopleths). In these simulations, we assume that a hypothetical oocyte with the intracellular composition (i.e., initial pHi and ΔpHi) of a ”CA IV“ oocyte is exposed to a bulk solution containing 1.5% CO2/10 mM HCO3−. The physiological data are plotted on the ordinate as mean ± standard deviation; the position of these points along the abscissa is arbitrary.
Focusing first on the surface CA isopleths (Fig. 8, A and B), we observe that for Ai = 5 (background value for a “control” oocyte), increasing AS from 150 to 50,000 (following the upward arrow at Ai = 5) produces only a 40% increase in (dpHi/dt)max and ∼3.3-fold increase in ΔpHS. Moreover, the (dpHi/dt)max and ΔpHS values predicted by these combinations of Ai (i.e., 5) and AS (i.e., 150–50,000) are far from the mean values measured in our physiological experiments. Thus, as noted above, to simulate a “CA IV” oocyte, we must increase not only AS but also Ai. We choose Ai = 40 because, together with AS = 10,000, it matches the observed (dpHi/dt)max and is only slightly below the observed mean for ΔpHS. Moreover, because our current model does a better job of reproducing the pHi than the pHS data, as also indicated by the t63 values predicted by the model for pHi (see Fig. 13 in the first paper of this series, ref. 14, and Fig. 21 in second paper, ref. 15), we gave more weight to the pHi data in choosing the above parameter values.
Fig. 13.
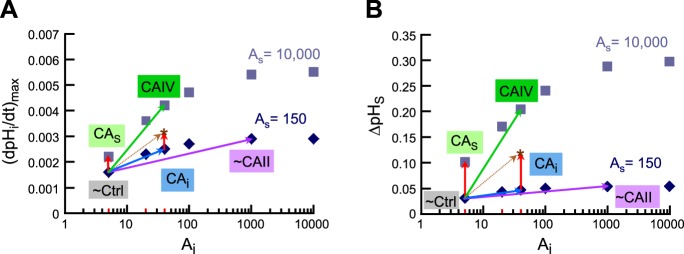
Supra-additivity of simultaneous implementation of intracellular (i.e., cytosolic) and extracellular-surface CA activity. A: dependence of (dpHi/dt)max on Ai, for two fixed values of AS (i.e., surface CA isopleths), a replot of selected data from Fig. 8A. B: dependence of ΔpHS on Ai, for two fixed values of AS (i.e., surface CA isopleths), a replot of selected data from Fig. 8B. In all simulations for this figure, we assume that a hypothetical oocyte with the intracellular composition (i.e., initial pHi and ΔpHi) of a “CA IV” oocyte is exposed to a bulk solution containing 1.5% CO2/10 mM HCO3−. The points labeled “∼Ctrl” represent our hypothetical oocyte but with the CA properties of a “control” oocyte (i.e., Ai = 5, on the 150 AS isopleth). The points labeled “∼CAII” represent our hypothetical oocyte but with the CA properties of a “CA II” oocyte (i.e., Ai = 1,000, on the 150 AS isopleth). These points (lavender vectors) approximate the physiologically observed (dpHi/dt)max and ΔpHS values for oocytes injected with CA II. The points labeled “CAS” represent a hypothetical oocyte but in which we raise AS to 10,000 but leave Ai = 5. These points (red vectors on left side of panels) represent (dpHi/dt)max and ΔpHS values that are far smaller than those physiologically observed with oocytes expressing CA IV. The points labeled “CAi” represent a hypothetical oocyte but in which we raise Ai to 40 but leave AS = 150. These points (blue vectors) represent (dpHi/dt)max and ΔpHS values that are far smaller than those physiologically observed with oocytes expressing CA IV. The sum of the red and blue vectors produce the brown vectors, which indicate the effects of simply adding CAi and CAo. Note that the asterisks (at the head of the brown vectors) represent (dpHi/dt)max and ΔpHS values that are still much smaller than those physiologically observed with oocytes expressing CA IV. Finally, the points labeled “CAIV” represent the combination of simultaneously raising AS from 150 to 10,000 and raising Ai from 5 to 40 in our simulations (green vectors). These points do indeed approximate the physiologically observed (dpHi/dt)max and ΔpHS values of oocytes expressing CA IV.
As Ai increases along the x-axis, each surface CA isopleth, both in the (dpHi/dt)max plot (Fig. 8A) and in the ΔpHS plot (Fig. 8B), at first increases and then tends to plateau at Ai values above ∼1,000. For any AS isopleth, (dpHi/dt)max and ΔpHS rise very little as we increase Ai from 1,000 to 10,000.
Focusing now on the intracellular CA isopleths (Fig. 8, C and D), we observe that for AS = 150 (background value for a “control” oocyte), increasing Ai from 5 to 10,000 (following the upward arrow at AS = 150) causes (dpHi/dt)max and ΔpHS to increase by only ∼2-fold. As AS increases along the x-axis, each intracellular CA isopleth, for both (dpHi/dt)max and ΔpHS, increases but eventually tends to plateau. For any Ai isopleth, (dpHi/dt)max and ΔpHS increase relatively little as we raise AS from 10,000 to 50,000.
In summary, the mathematical model shows that simulating the physiological pH data requires that Ai and AS be sufficiently high, but that the predictions for (dpHi/dt)max and ΔpHS are not very sensitive to increases in Ai beyond ∼1,000 or to AS beyond ∼10,000.
Model Validation
The purpose of these simulations is not to explain the physiological principles, which we have already addressed in the first two papers in this series, but to validate the mathematical model.
Effect of increasing extracellular CO2/HCO3− levels.
Figure 9 shows the predictions of the mathematical model for “control” (Fig. 9A), “CA II” (Fig. 9B), and “CA IV” (Fig. 9C) oocytes as they are each exposed to increasing levels of extracellular CO2/HCO3−−1.5% CO2/10 HCO3− mM, 5% CO2/33 HCO3− mM, and 10% CO2/66 HCO3− mM (virtually identical [CO2]/[HCO3−] ratios to fix pHBECF at ∼7.50). In these simulations we used the same parameter values for τflow, λ, γ, Ai and AS that we used to simulate the pHi and pHS transients illustrated in Fig. 6. Note that the three simulations corresponding to 1.5% CO2/10 HCO3− mM in Fig. 9 (left) are the same as those illustrated in Fig. 6, A–C.
Fig. 9.
Simulations of pHi and pHS transients for the addition of graded levels of extracellular CO2/HCO3− in 5 mM HEPES. A: ”control“ oocyte”. B: “CA II” oocyte. C: “CA IV” oocyte. The pHi transients, calculated at a depth of ∼50 μm, are represented by the red lower trajectories, and pHS, by the green upper trajectories. At the indicated time, we switched the extracellular solution from CO2/HCO3−-free “ND96” to 1) 1.5% CO2/10 mM HCO3− (left), or 2) 5% CO2/33 mM HCO3− (center), or 3) 10% CO2/66 mM HCO3− (right). The dashed black lines through the initial portions of the pHi transients for CO2 application represent the maximal rates of pHi change (negative direction) predicted by the model. The upward arrow near the pHS records represent maximal changes in pHS (positive direction).
Figure 10 compares the predictions of the mathematical model (based on parameter values developed for 1.5% CO2), shown in Fig. 9, with the physiological data for (dpHi/dt)max (Fig. 10A) and ΔpHS (Fig. 10B). The good agreement of the simulations with the physiological data for the 5% CO2 and 10% CO2 data validates the model.
Fig. 10.
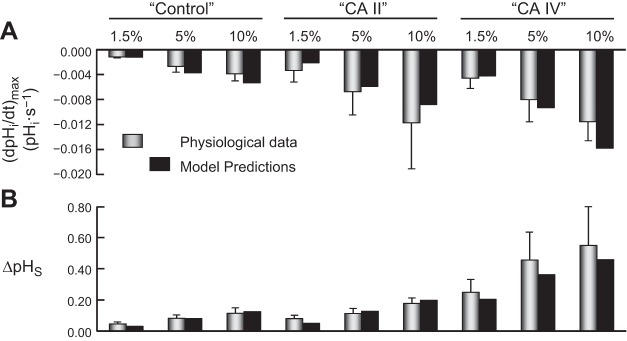
Comparison of the physiological data with predictions of the mathematical model for the addition of graded levels of extracellular CO2/HCO3−. A: maximal rates of pHi change (negative direction) produced by the addition of extracellular CO2/HCO3−. B: maximal changes in pHS (positive direction) caused by the addition of extracellular CO2/HCO3−. The physiological data are taken from Fig. 10 of the first paper in this series (14) and Fig. 10 of the second paper (15). Note that here we report the means ± standard deviation (and not SE, as in the previous two papers). The data for model predictions come from Fig. 9 of the present paper.
For pHi, the model predicts that increasing [CO2]BECF from 1.5% to 5% to 10%, in the ratios 1/∼3.3/∼6.6, causes a progressive increase in the magnitude of (dpHi/dt)max, in agreement with the physiological data (Fig. 10A). Moreover, comparing “control” vs. “CA II” vs. “CA IV” oocytes at a given CO2 level, we see that, at each CO2 level, the simulated (dpHi/dt)max is greatest for “CA IV” oocyte and smallest for “control” oocyte.
For pHS, the model predicts that increasing [CO2]BECF from 1.5% to 5% to 10%, in the ratios 1/∼3.3/∼6.6, causes a progressive increase in ΔpHS in agreement with the physiological data (Fig. 10B). Moreover, comparing “control” vs. “CA II” vs. “CA IV” oocytes at a given CO2 level, we see that ΔpHS is greatest for “CA IV” oocyte and smallest for “control” oocyte.
Effect of increasing extracellular HEPES levels.
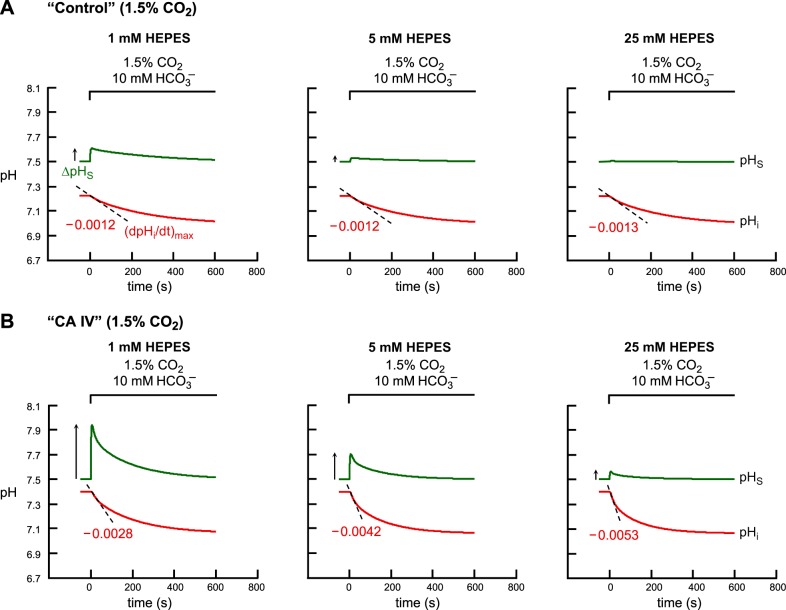
Again, for purposes of model validation, Fig. 11 shows the predictions of the mathematical model for “control” (Fig. 11A) and “CA IV” (Fig. 11B) oocytes as they are exposed to 1) 1.5% CO2/10 mM HCO3− at pH 7.50 + 1 mM HEPES, or 2) CO2/HCO3− + 5 mM HEPES, or 3) CO2/HCO3− + 25 mM HEPES. In these simulations we used the same parameter values for τflow, λ, γ, Ai, and AS that we used to simulate the pHi and pHS transients illustrated in Fig. 6, where [HEPES]o is 5 mM. Here, for model validation, we change the experimental protocol by varying the levels of extracellular HEPES.
Fig. 11.
Simulations of pHi and pHS transients for the addition of extracellular 1.5% CO2/10 mM HCO3−, with graded increases in levels of extracellular HEPES. A: “control” oocyte. B: “CA IV” oocyte. The pHi transients, calculated at a depth of ∼50 μm, are represented by the red lower trajectories, and pHS, by the green upper trajectories. At the indicated time, we switched the extracellular solution from CO2/HCO3−-free “ND96” to: 1) CO2/HCO3− + 1 mM HEPES, or 2) CO2/HCO3− + 5 mM HEPES, or 3) CO2/HCO3− + 25 mM HEPES. The dashed black lines through the initial portions of the pHi transients for CO2 application represent the maximal rates of pHi change (negative direction) predicted by the model. The upward arrows near the pHS records represent maximal changes in pHS (positive direction).
For a “control” oocyte, the model predicts that raising [HEPES]o from 1 to 5 to 25 mM causes minimal changes in (dpHi/dt)max (Fig. 11A in the present paper and Fig. 23D in the second paper in this series, ref. 15). However, as expected because of the increased extracellular buffering power, the graded increase in [HEPES]o causes substantial decreases in ΔpHS (Fig. 12A in the present paper and Fig. 23E in ref. 15). Finally, the model predicts that graded increases in [HEPES]o have little effect on t63 (i.e., the time required to fall by ∼63%) for pHS (Fig. 23F in ref. 15).
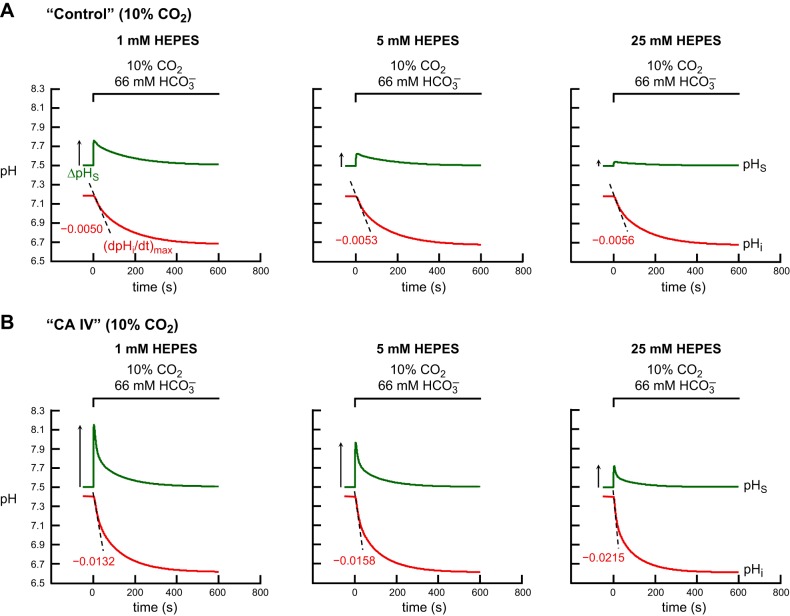
Fig. 12.
Simulations of pHi and pHS transients for the addition of extracellular 10% CO2/66 mM HCO3−, with graded increases in levels of extracellular HEPES. A: “control” oocyte. B: “CA IV” oocyte. This figure differs from Fig. 11 in that, here, the CO2/HCO3− levels are ∼6.6-fold higher. The pHi transients, calculated at a depth of ∼50 μm, are represented by the red lower trajectories, and pHS, by the green upper trajectories. At the indicated time, we switched the extracellular solution from CO2/HCO3−-free “ND96” to 1) CO2/HCO3− + 1 mM HEPES, or 2) CO2/HCO3− + 5 mM HEPES, or 3) CO2/HCO3− + 25 mM HEPES. The dashed black lines through the initial portions of the pHi transients for CO2 application represent the maximal rates of pHi change (negative direction) predicted by the model. The upward arrows near the pHS records represent maximal changes in pHS (positive direction).
For a “CA IV” oocyte, the model predicts that raising [HEPES]o from 1 to 5 to 25 mM causes pHi to decay faster (Fig. 11B in the present paper and Fig. 23A in ref. 15) and greatly reduces ΔpHS (Fig. 11B in the present paper and Fig. 23B in ref. 15). The increase in [HEPES]o also causes t63 to fall modestly (Fig. 23C in ref. 15).
The simulations in Fig. 12 are identical to those in Fig. 11 except that in Fig. 12 we work with 10% CO2/66 mM HCO3−. As we have already seen at 1.5% CO2 for a “control” oocyte (Fig. 11A), the model predicts that, at 10% CO2, raising [HEPES]o from 1 to 5 to 25 mM produces a minimal enhancement of (dpHi/dt)max (Fig. 12A in the present paper and Fig. 24D in ref. 15). The model also predicts that raising [HEPES]o markedly reduces ΔpHS (Fig. 12A in the present paper and Fig. 24E in ref. 15), and has little effect on t63 for pHS (Fig. 24F in ref. 15).
Finally, for a “CA IV” oocyte exposed to 10% CO2/66 mM HCO3−, the model predicts that raising [HEPES]o from 1 to 5 to 25 mM produces very large increases in the magnitude of (dpHi/dt)max (Fig. 12B in the present paper and Fig. 24A in ref. 15) and large decreases in ΔpHS (Fig. 12B in the present paper and Fig. 24B in ref. 15). Moreover, t63 for pHS decreases modestly (Fig. 24C in ref. 15).
Note that the simulation trends that we observe in Figs. 11 and 12 for a range of [HEPES]o values, based on parameter values developed for 5 mM HEPES, parallel the physiological data presented in the second paper in this series (15), and thereby validate the model.
DISCUSSION
Overview
When CO2 is the only acid-base-related solute that moves across the plasma membrane, as is essentially the case in our physiological experiments, the model predicts that a CO2 influx (or efflux) will cause a sustained fall (rise) in pHi, but a transient rise (fall) in pHS, followed by a relaxation. In fact, the predicted time course of the pHS relaxation is approximately proportional to the negative derivative of the time course of pHi. As CO2 moves across the plasma membrane, it perturbs the equilibria of all buffer pairs, both CO2/HCO3− and non-CO2/HCO3− buffers, in both the intra- and extracellular fluids. The perturbations occur because these buffers all compete for a common pool of H+. Aside from the reaction CO2 + H2O ⇌ H2CO3, all others occur virtually instantaneously, being limited only by diffusion of buffer components. Because the CO2/H2CO3 interconversion is slow, the nature of the participation by CO2/HCO3− in buffering depends not just on diffusion but on the activity and location of CAs. In a compartment delimited by a membrane traversable only by CO2, that is, a compartment that behaves as a closed system to all solutes other than CO2, the CO2/HCO3− buffer system will eventually exert its full buffering power, even in the absence of catalysis. Thus, we see that ΔpHi is unaffected by the presence of CAs. However, in the EUF, where buffer components exchange freely with the BECF, and thus are only transiently available, the ability of the CO2/HCO3− buffer pair to influence pH, and thus influence other reactions in the EUF, depends on the diffusion of CO2 and HCO3− as well as the rates of the reactions that allow CO2/HCO3− interconversion.
In the preceding two papers, we used electrophysiological approaches to study the effects of the cytosolic CA II and of the GPI-linked extracellular CA IV on accelerating transmembrane CO2 fluxes. Moreover, we investigated the effects of CA II and CA IV on the CO2-induced pHi and pHS transients when we: 1) raised the concentration of CO2 in the BECF; 2) increased the depth of penetration of the pHi microelectrode; 3) changed the concentration of HEPES in the BECF.
To understand the complex interconnected acid-base events triggered by the movement of CO2 in or out of the cell, in the presence of CA II or CA IV—events so complex that their interpretation is not easily amenable to intuition—we used a previously published mathematical model (22) that we appropriately modified for the present study by introducing the nine new features 1 through 9, as indicated in Fig. 1 and its legend.
The revised mathematical model has proven to be very useful in providing new insights into the complex reaction and diffusion events underlying the pHi and pHS transients measured in physiological experiments in the first two papers in this series.
Major New Features of the Mathematical Model
Here we discuss a subset of the nine new features 1–9 in Fig. 1 legend, in the order that we present them in results: 9, 4, 8, 3, and 7.
Protocol for simulating the switch in BECF composition.
In physiological experiments, exposing an oocyte to a CO2/HCO3− solution initiates an influx of CO2 that causes pHS to rise to a peak and then decay with a trajectory like the one schematically illustrated in Fig. 2. When we initiate a simulation using the protocol of Somersalo et al. (22), which assumes that, at the outset of the simulation, the CO2/HCO3− solution is already at the plasma membrane, the initial portion of the pHS trajectory exhibits a quite unrealistic needle-like shape (Fig. 3A). We reasoned that this inconsistency between the simulation and the physiological data must be related to the assumption that, at the beginning of the simulation, the CO2/HCO3− solution is already in both BECF and EUF, and therefore immediately ready to diffuse into the ICF.
Our revised approach reflects the situation in a physiological experiment, in which the oocyte is initially superfused with the ND96 solution until we switch the bulk solution to one containing CO2/HCO3−. Indeed, Fig. 3B shows that our revised protocol produces a pHS trajectory with a far more realistic shape and a time to peak very close to the physiological data. The results obtained with the revised protocol suggest that tp reflects in part the time required for levels of the CO2, H2CO3, and HCO3− to rise in the BECF, and in part the time required for the buffer components to diffuse throughout the EUF and enter the ICF. In our revised simulation protocol we assumed that the concentrations of CO2, H2CO3, and HCO3− in the BECF rise exponentially, from zero to the values of an equilibrated solution at pH = 7.50, with a time constant τflow = 4 s. This τflow reflects the mixing of old and new solutions in the tubing (between the switches and the chamber) and the turnover of solution in the chamber. Recall that in the first paper in this series (14), we observed that the time constant for switching the solution in the chamber is ∼1.7 s. The difference between the measured τ of ∼1.7 s and the τflow of 4 s required to simulate a realistic tp could reflect: 1) the special properties of the volume beneath the pHS electrode and 2) the asymmetry of [CO2] over the surface of the oocyte. Supporting 2), Fig. 18 in the second paper (15) shows that tp is longer for the electrode positioned at the downstream side of the oocyte than the one positioned at the equator. Note that the present model considers neither 1) nor 2).
Intracellular vesicles.
In the physiological experiments, we consistently observe a time delay between the initiation of the pHS spike and the start of the pHi decline. Therefore, we used our revised mathematical model to investigate the dependence of the time delay on the presence of a layer of intracellular vesicles beneath the plasma membrane by implementing a tortuosity factor λ. The model predicts that increasing λ causes the simulated pHi trajectory to decline more slowly (Fig. 4A), the time delay to increase (Fig. 4B), and the magnitude of (dpHi/dt)max to decrease (Fig. 4C). These results are consistent with the hypothesis that intracellular vesicles are, in part, responsible for the observed time delay. By offering resistance to diffusion of solutes (and in particular to CO2), the vesicle layer presumably causes CO2 to enter into the oocyte more slowly, thereby producing pHi changes that occur later in time.
Vitelline membrane and pHS electrode.
The implementation of the vitelline membrane is important to simulate pHS transients that are large enough to approximate physiological values (Fig. 5A). When CO2 enters (or leaves) the cell, diffusion and reaction are the two processes that compete to replenish (or deplete) CO2 in the EM region. ΔpHS is an index of the amount of protons that are consumed through the reaction HCO3− + H+ → CO2 + H2O (or produced through the opposite reaction, CO2 + H2O → HCO3− + H+) to replenish (or deplete) CO2 during influx (or efflux). An extremely small ΔpHS, which is what our simulation predicts without a vitelline membrane, indicates that the vast majority of the CO2 is replenished (or depleted) via diffusion.
We implement the vitelline membrane by reducing the mobility of all solutes in the EM region by the same tortuosity factor γ. This approach reduces the contribution of diffusion and enhances that of reaction in the EM region. After examining the predictions of the model with increasing values of γ, we decided to use a value of γ ∼5.77 to set up the properties of a “typical” oocyte. However, we do not believe that the vitelline membrane by itself could offer such a high resistance to the diffusion of solutes. Instead, we hypothesize that the reason that the pHS transients are so large is that the presence of the pHS electrode creates a special environment, between the outer surface of the cell membrane and the flat surface of the electrode, where diffusion is largely reduced and reaction is enhanced. Thus, our current implementation of the vitelline membrane is a temporizing maneuver to mimic, as far as the pHS time course is concerned, the presence of the pHS electrode near the oocyte surface.
Because of the assumption of spherical symmetry, the vitelline membrane is implemented everywhere around the oocyte, whereas the pHS electrode covers only a very tiny portion of the oocyte surface. Thus, choosing a large value for γ, while helpful to simulate ΔpHS, tends to slow changes in pHi. In our present implementation, to produce a realistically fast pHi trajectory, we have compensated for the large γ by reducing λ (the resistance due to the layer of vesicles). The result, as noted in the previous section, is an unrealistically short td. Using a future model that could properly account for the volume beneath the pHS electrode, we could reduce γ (resistance due to vitelline membrane) and replace it with an increased λ (resistance to due to layer of vesicles). The result would be more realistic values for both ΔpHS and td.
CA II and CA IV.
In the present paper, our goal was to develop a mathematical model capable of reproducing the salient features of the physiological transients measured for pHi [i.e., td, τpHi, (dpHi/dt)max, ΔpHi] and for pHS (i.e., tp, ΔpHS, τpHS) as we expose each oocyte type (“control”, “CA II,” and “CA IV”) to an extracellular solution containing or lacking CO2/HCO3−.
We began by focusing on the intrinsic properties of our in silico oocyte (i.e., in the absence of added CA). As described in results (see Standard Features of an In Silico Oocyte Experiment), we estimated reasonable parameter values for a “control” oocyte4—τflow = 4 s, λ ∼ 3.16, γ ∼ 5.77, Ai = 5, and AS = 150—using physiological data obtained as we switched oocytes from a CO2/HCO3−-free solution to one containing 1.5% CO2/10 mM HCO3−/pH 7.50. Figure 13, A and B, is a replot of the CA II and CA IV analyses from Fig. 8, A and B, where the starting point for all simulations was a hypothetical oocyte with the composition of a CA IV oocyte.6 The point labeled “∼Ctrl” represents such a hypothetical oocyte with the CA properties of a “control” oocyte (i.e., Ai = 5, on the 150 AS isopleth).
Our next step was to estimate reasonable parameters for “CA II” oocytes. Because injecting an oocyte with CA II ought to affect only the intracellular CA activity, we fixed AS to the “control” value of 150, and estimated that Ai would have to be ∼1,000 to reproduce the pHi and pHS transients for an oocyte exposed to 1.5% CO2/10 mM HCO3−/pH 7.50. In Fig. 13, A and B, we represent this condition by the point labeled “∼CAII” (lavender vector).
Finally, we estimated parameters for an oocyte expressing CA IV. Our first thought was to fix Ai to the “control” value of 5, and then to estimate AS. However, as pointed out in results, it is impossible to achieve reasonable values for (dpHi/dt)max or ΔpHS if we leave Ai at 5. In Fig. 13, A and B, the point labeled “CAS” represents a condition in which we raise AS to 10,000 but leave Ai at 5 (red vector). In fact, inspection of Fig. 8, A and B, reveals that, at a fixed Ai of 5, raising AS to even 50,000 has virtually no additional effect. To achieve reasonable increments in (dpHi/dt)max and ΔpHS, we must also raise Ai by a small amount. As shown by the point labeled “CAIV” in Fig. 13, A and B, the combination of raising AS from 150 to 10,000 (∼67-fold) and raising Ai from 5 to 40 (8-fold, but by an absolute amount that is rather small) is sufficient to approximately replicate, in our simulation, the physiologically observed (dpHi/dt)max and ΔpHS values (green vector).
A question that arises is whether one can simulate the effects of CA IV expression by reversing the fold increases: raising Ai by 67-fold (from 5 to 335) and raising AS by only 8-fold (from 150 to 1,200). Inspection of Fig. 8 reveals that this combination adequately predicts the (dpHi/dt)max data for CA IV oocytes (Fig. 8A) but produces ΔpHS values that fall far below the physiological data (Fig. 8B). In fact, following the 1,000 AS isopleth in Fig. 8B, the ΔpHS effect of Ai saturates at ∼1,000. Thus, although a small increment in Ai is necessary to model the effects of CA IV expression, a much larger increment in AS (e.g., to ∼5,000 in Fig. 8B) is also necessary.
The foregoing analysis reveals an important concept that we will call supra-additivity. If we raise AS from 150 to 10,000 at a fixed Ai of 5, the effect on the two pH parameters is small (red vectors to the left in both Fig. 13, A and B). Conversely, if we raise Ai from 5 to 40 at a fixed AS of 150, the effect on the two pHi parameters is also small (blue vectors in Fig. 13, A and B). However, raising Ai and AS simultaneously produces pH effects (green vectors) that are substantially larger than the sum of the two individual effects (brown vectors)—supra-additivity. The explanation for this effect is that adequate CA activity must be present on both sides of the membrane to optimize the CO2 flux: CA on one side of the membrane helps to replenish CO2 that has departed, and CA on the other side of the membrane helps dispose of newly arriving CO2.
Is it reasonable that the expression of CA IV should increase cytosolic CA activity from 5 to 40? The preliminary immunohistochemistry work of Nakhoul et al. (16) on oocytes expressing CA IV has shown that CA IV is distributed both near the plasma membrane and near the intracellular vesicles. Recently, Schneider et al. (21) used a mass spectrometry approach to measure CA activity in oocytes expressing CA IV, with measurements on intact oocytes representing the extracellular activity of the CA IV, and measurements on lysed oocytes representing total (i.e., extracellular + intracellular) CA activity. These authors concluded that only ∼24% of the total CA activity is on the extracellular surface of the oocyte, and that ∼76% is in intracellular vesicles. Let us consider two scenarios:
If extracellular CA IV activity is confined to a shell 1 μm thick (as it is in our simulations) immediately adjacent to the cell membrane, then this shell would represent ∼1% of the H2O in an oocyte of radius 650 μm (assuming that an oocyte is 40% H2O; ref. 29). Thus, if Ai = 40 and AS = 10,000, then only ∼25% of total CA activity would be in the cytosol. If ∼76% of total oocyte CA IV is located in intracellular vesicles, then, to be consistent with our simulations, only about one-third could be available to catalyze cytosolic reactions.
On the other hand, if extracellular CA IV is confined to a shell only 0.1 μm thick (AS = 10,000), then we calculate that ∼78% of total CA activity would be in the cytosol, a figure virtually identical to the estimate of Schneider et al. (21). In this scenario, to be consistent with our simulations, virtually all vesicular CA IV would have to be available to catalyze cytosolic reactions with ∼100% efficiency.
Regardless of whether we consider the shell width to be 1 μm or 0.1 μm, it is clear that, because of the large discrepancy between cytosolic H2O and the H2O in which surface CA IV is distributed, cytosolic CA IV activity could be extremely low (e.g., Ai = 40) compared with cell-surface CA IV activity (e.g., AS = 10,000) even if the vast majority of total CA IV protein were intracellular.
In summary, our simulations show that a small increase in cytosolic CA activity, representing a large amount of total CA IV protein, is necessary to account for the full-blown effects of CA IV as expressed in oocytes.
Conclusions and Future Directions
We have extended and improved the reaction-diffusion mathematical model of CO2 influx into a Xenopus oocyte, presented in ref. 22. Enhancements to the model include the nine changes 1 through 9 in the legend of Fig. 1, which allow us to produce more realistic simulations of a Xenopus oocyte and of our experimental setup, capable of reproducing the salient features of our physiological experiments in the two companion papers. Because in our physiological experiments we do not have access to such parameters as CO2 concentrations, we can use the output of the model to calculate these parameters (e.g., CO2 fluxes across the plasma membrane for each oocyte type). The model supports the hypothesis that the cytosolic enzyme CA II and the GPI-linked extracellular CA IV enhance CO2 fluxes across the plasma membrane by increasing transmembrane CO2 gradients.
This revised version of the model is a major step forward towards simulating the measured pHi and pHS transients as we expose an oocyte to an extracellular solution containing or lacking CO2/HCO3−. However, as pointed out in the present paper and the two companion papers, the revised model has difficulties quantitatively reproducing certain aspects of the physiological experiments. These discrepancies between the physiological data and the model predictions are due to elements missing in the present reaction-diffusion model: 1) the special environment between the pHS electrode and membrane and 2) the convective flow of BECF around the oocyte. Implementation of the pHS electrode will allow us to reduce the resistance of the vitelline membrane and thereby produce more realistic simulations of both the measured pHi and pHS. Addressing the asymmetry issues will provide better agreement between τpHi and τpHS.
The revised model provides estimates of CA activity on the oocyte surface and in the cytosol. The model also will make it possible to produce rough estimates of the permeability of the oocyte membrane to CO2, which will be invaluable for gas-channel research. Improved models that address the pHS electrode and convective flow would improve each of these estimates, as well as our understanding of the complex acid-base chemistry that occurs on the surface and within living cells.
GRANTS
The work of R. Occhipinti was supported by a fellowship from the American Heart Association (11POST7670015). The work of R. Musa-Aziz was also supported by a fellowship from the American Heart Association (0625891T) and by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP no. 08/128663). W. F. Boron gratefully acknowledges the support of the Myers/Scarpa endowed chair. This work was supported by grants from the Office of Naval Research (N00014-05-1-0345, N00014-08-1-0532, and N00014-11-1-0889) and the National Institutes of Health (NS-18400 and DK-81567) to W. F. Boron.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.O., R.M.A., and W.F.B. conception and design of research; R.O. performed experiments; R.O. analyzed data; R.O., R.M.A., and W.F.B. interpreted results of experiments; R.O. prepared figures; R.O. and W.F.B. drafted manuscript; R.O., R.M.A., and W.F.B. edited and revised manuscript; R.O., R.M.A., and W.F.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nazih Nakhoul and Michael F. Romero for helpful discussions regarding the intracellular effects of expressing CA IV in oocytes. We also acknowledge valuable consultations with Duncan Wong and Dale Huffman.
Footnotes
This article is the topic of an Editorial Focus by Eric Delpire (8a).
In the previous year, Roos had developed a steady-state model describing the passive efflux of lactate, which led to the passive influx—of equal magnitude—of lactic acid (20).
The values for the mobility of each solute in water are those reported in Table 1 of ref. 22.
The “control” oocyte, which mimics a Tris (first paper in this series; ref. 14) or H2O-injected oocyte (second paper; ref. 15), has 1) an initial pHi of 7.22 and a ΔpHi of 0.23 when exposed to 1.5% CO2/10 mM HCO3−; 2) an initial pHi of 7.24 and a ΔpHi of 0.45 when exposed to 5% CO2/33 mM HCO3−; and 3) an initial pHi of 7.18 and a ΔpHi of 0.51 when exposed to 10% CO2/66 mM HCO3−.
The “CA II” oocyte, which mimics an oocyte injected with CA II (first paper in this series; ref. 14), has 1) an initial pHi of 7.21 and a ΔpHi of 0.23 when exposed to 1.5% CO2/10 mM HCO3−; 2) an initial pHi of 7.21 and a ΔpHi of 0.44 when exposed to 5% CO2/33 mM HCO3−; and 3) an initial pHi of 7.21 and a ΔpHi of 0.55 when exposed to 10% CO2/66 mM HCO3−.
The “CA IV” oocyte, which mimics an oocyte expressing CA IV (second paper in this series; ref. 15), has 1) an initial pHi of 7.40 and a ΔpHi of 0.34 when exposed to 1.5% CO2/10 mM HCO3−; 2) an initial pHi of 7.37 and a ΔpHi of 0.58 when exposed to 5% CO2/33 mM HCO3−; and 3) an initial pHi of 7.40 and a ΔpHi of 0.79 when exposed to 10% CO2/66 mM HCO3−.
REFERENCES
- 1.Alessandrini A, Gavazzo P, Picco C, Facci P. Voltage-induced morphological modifications in oocyte membranes containing exogenous K+ channels studied by electrochemical scanning force microscopy. Microsc Res Tech 71: 274–278, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bevensee MO, Boron WF. Control of intracellular pH. In: Seldin and Giebisch's The Kidney: Physiology & Pathophysiology. New York: Academic, 2013, p. 1773–1835 [Google Scholar]
- 3.Boron W. Acid-base physiology. In: Medical Physiology, edited by Boron W, Boulpaep E. Philadelphia, PA: Saunders Elsevier, 2012, p. 652–671 [Google Scholar]
- 4.Boron WF, Endeward V, Gros G, Musa-Aziz R, Pohl P. Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels. Chemphyschem 12: 1017–1019, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67: 91–112, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boron WF. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol Cell Physiol 233: C61–C73, 1977 [DOI] [PubMed] [Google Scholar]
- 7.Boron WF. Sharpey-Schafer lecture: gas channels. Exp Physiol 95: 1107–1130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davenport HW. The ABC of Acid-Base Chemistry (6th ed., rev.). Chicago: Univ. of Chicago Press, 1974 [Google Scholar]
- 8a.Delpire E. How carbonic anhydrases and pH buffers facilitate the movement of carbon dioxide through biological membranes. Focus on “Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes”; “Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes”; and “Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes.” Am J Physiol Cell Physiol (June 25, 2014). 10.1152/ajpcell.00198.2014 [DOI] [PubMed] [Google Scholar]
- 9.Endeward V, Gros G. Extra- and intracellular unstirred layer effects in measurements of CO2 diffusion across membranes–a novel approach applied to the mass spectrometric 18O technique for red blood cells. J Physiol 587: 1153–1167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 80: 681–715, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Gros G, Moll W, Hoppe H, Gros H. Proton transport by phosphate diffusion–a mechanism of facilitated CO2 transfer. J Gen Physiol 67: 773–790, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, Boron WF. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem 281: 19241–19250, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Missner A, Kügler P, Saparov SM, Sommer K, Mathai JC, Zeidel ML, Pohl P. Carbon dioxide transport through membranes. J Biol Chem 283: 25340–25347, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musa-Aziz R, Occhipinti R, Boron WF. Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes. Am J Physiol Cell Physiol (June 25, 2014). 10.1152/ajpcell.00051.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musa-Aziz R, Occhipinti R, Boron WF. Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocytes plasma membranes. Am J Physiol Cell Physiol (June 25, 2014). 10.1152/ajpcell.00050.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakhoul NL, Romero MF, Waheed A, Davis BA, Mullins R, Hageman G, Sly WS, Boron WF. Processing and functional expression of carbonic anhydrase isoforms in Xenopus laevis oocytes (Abstract). FASEB J 10: A88, 1996 [Google Scholar]
- 17.Nicholson C, Phillips JM. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol 321: 225–257, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson C, Syková E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci 21: 207–215, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Roos A, Boron WF. Intracellular pH. Physiol Rev 61: 296–434, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Roos A. Intracellular pH and distribution of weak acids across cell membranes. A study of d- and l-lactate and of DMO in rat diaphragm. J Physiol 249: 1–25, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider HP, Alt MD, Klier M, Spiess A, Andes FT, Waheed A, Sly WS, Becker HM, Deitmer JW. GPI-anchored carbonic anhydrase IV displays both intra- and extracellular activity in cRNA-injected oocytes and in mouse neurons. Proc Natl Acad Sci USA 110: 1494–1499, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somersalo E, Occhipinti R, Boron WF, Calvetti D. A reaction-diffusion model of CO2 influx into an oocyte. J Theor Biol 309: 185–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spitzer KW, Skolnick RL, Peercy BE, Keener JP, Vaughan-Jones RD. Facilitation of intracellular H+ ion mobility by CO2/HCO3− in rabbit ventricular myocytes is regulated by carbonic anhydrase. J Physiol 541: 159–167, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J Biol Chem 284: 20299–20310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swietach P, Wigfield S, Cobden P, Supuran CT, Harris AL, Vaughan-Jones RD. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J Biol Chem 283: 20473–20483, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Swietach P, Zaniboni M, Stewart AK, Rossini A, Spitzer KW, Vaughan-Jones RD. Modelling intracellular H+ ion diffusion. Prog Biophys Mol Biol 83: 69–100, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Syková E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev 88: 1277–1340, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zampighi GA, Kreman M, Boorer KJ, Loo DD, Bezanilla F, Chandy G, Hall JE, Wright EM. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J Membr Biol 148: 65–78, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Zeuthen T, Zeuthen E, Klaerke DA. Mobility of ions, sugar, and water in the cytoplasm of Xenopus oocytes expressing Na+-coupled sugar transporters (SGLT1). J Physiol 542: 71–87, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]