Abstract
The α-carbonic anhydrases (CAs) are zinc-containing enzymes that catalyze the interconversion of CO2 and HCO3−. Here, we focus on human CA II (CA II), a ubiquitous cytoplasmic enzyme. In the second paper in this series, we examine CA IV at the extracellular surface. After microinjecting recombinant CA II in a Tris solution (or just Tris) into oocytes, we expose oocytes to 1.5% CO2/10 mM HCO3−/pH 7.50 while using microelectrodes to monitor intracellular pH (pHi) and surface pH (pHS). CO2 influx causes the familiar sustained pHi fall as well as a transient pHS rise; CO2 efflux does the opposite. Both during CO2 addition and removal, CA II increases the magnitudes of the maximal rate of pHi change, (dpHi/dt)max, and the maximal change in pHS, ΔpHS. Preincubating oocytes with the inhibitor ethoxzolamide eliminates the effects of CA II. Compared with pHS, pHi begins to change only after a delay of ∼9 s and its relaxation has a larger (i.e., slower) time constant (τpHi > τpHS). Simultaneous measurements with two pHi electrodes, one superficial and one deep, suggest that impalement depth contributes to pHi delay and higher τpHi. Using higher CO2/HCO3− levels, i.e., 5%/33 mM HCO3− or 10%/66 mM HCO3−, increases (dpHi/dt)max and ΔpHS, though not in proportion to the increase in [CO2]. A reaction-diffusion mathematical model (described in the third paper in this series) accounts for the above general features and supports the conclusion that cytosolic CA—consuming entering CO2 or replenishing exiting CO2—increases CO2 fluxes across the cell membrane.
Keywords: ion-sensitive microelectrodes, ethoxzolamide, electrode depth, electrophysiology, mathematical modeling
the α-carbonic anhydrases (CAs) are a family of zinc-containing enzymes that catalyze the interconversion of CO2 and HCO3−. Of the 15 human CAs (32, 37, 68), 12 are known to be active (54) in catalyzing the reaction CO2 + H2O ⇌ HCO3− + H+, thereby bypassing the slow CO2-hydration/dehydration reactions involving H2CO3. Three CAs—CA VIII (34, 64), CA X (50), and CA XI (23)—lack at least one of the three conserved histidine residues that coordinate Zn2+ in other CAs. Because Zn2+ is required for carbonic-anhydrase activity, and because the three His residues are required for high-affinity binding of Zn2+, it is reasonable to suggest that CA VIII, X, and XI are not catalytically active. Indeed, Sjöblom et al. (63) restored the CA activity of CA VIII by making two simultaneous point mutations: converting an Arg residue to the His that is conserved in catalytically active α-CAs, and converting a Glu residue to a conserved Gln.
Although CA VIII, X, and XI do not have clear physiological roles, the catalytically active CAs are major players in a variety of physiological processes that involve CO2 and HCO3− transport across cell membranes. These processes include CO2 carriage by erythrocytes (42), HCO3− transport across epithelia (11, 21, 36, 53, 54), fluid transport across epithelia (2, 18, 35, 40, 53, 62), transient changes in extracellular pH in the brain (12), and the speed of certain intracellular pH transients (58, 71). For a particular animal species, the enzymatically active α-CAs differ in catalytic activity, affinity for CO2, inhibitor sensitivity, and expression pattern (32, 54, 65). Deficiency of CA II is associated with osteopetrosis, renal-tubular acidosis, and cerebral calcification (65). Moreover, CA blockers are used clinically to treat glaucoma (2, 6, 39, 60), metabolic and respiratory alkalosis (3–6), and acute mountain sickness (4, 6, 55, 69, 74), and are also weak diuretics (4–6).
In 1973, Gutknecht and Tosteson (30) explored factors that influence the diffusion of salicylic acid across an artificial lipid bilayer. They concluded that the reaction HA ⇌ H+ + A− (where A− is the salicylate anion) in the unstirred layers near the membrane, as well as the presence of non-salicylate buffers, plays a major role in enhancing HA flux across the membrane. They also predicted that non-CO2/HCO3− buffers would accelerate the flux of CO2. However, in 1977, Gutknecht et al. (29) reported that non-CO2/HCO3− buffers (phosphate, HEPES, Tris) enhance CO2 fluxes across lipid bilayers—measured using 14C-labeled CO2—only in the presence of mobile CA.
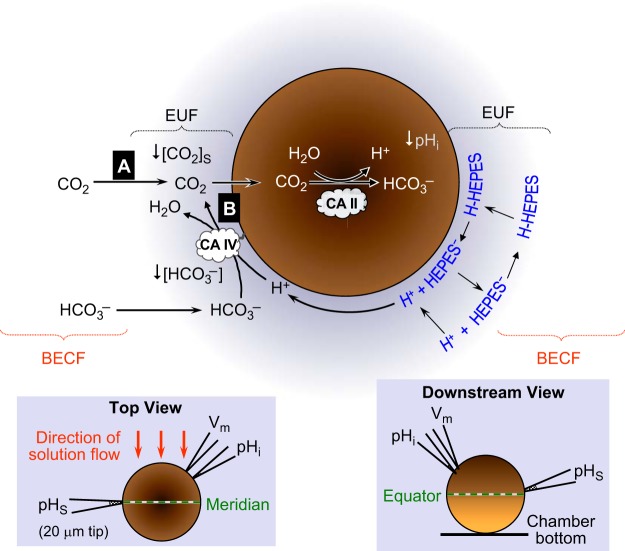
The purpose of the present study, summarized in this paper and its two companions (46, 49), is to elucidate the influence of CAs and non-CO2/HCO3− buffers on CO2 fluxes across the plasma membranes of living cells.1 In 1984, De Hemptinne and Huguenin (31) used a miniaturized electrode, with a glass sensor, to monitor the pH at the extracellular surface (pHS) of rat soleus muscle as they applied CO2/HCO3−. They observed a transient rise in pHS that, as they pointed out, presumably reflected the influx of CO2, which would promote the following reaction at the extracellular surface of the cell: H+ + HCO3− → CO2 + H2O. We and our collaborators used an adaptation of this pHS approach to study the role of aquaporins and rhesus proteins as CO2 channels (14, 22, 24–26, 45). In the present study, we chose to use this adapted pHS approach, as well as simultaneous measurements of intracellular pH (pHi), to assess the CO2 fluxes across the membranes of Xenopus oocytes. In this first paper, we examine the effect of microinjecting oocytes with the soluble enzyme CA II. We chose to work with CA II because it is ubiquitous and has the highest enzymatic activity of all CAs (reviewed in ref. 54). In the second paper (46), we examine the effect of expressing CA IV (GPI-linked to the extracellular cell surface), with and without CA II in the bulk extracellular fluid (BECF). Early exploratory work by Nakhoul et al. (48) on the heterologous expression of CA IV in oocytes motivated portions of this project. In the third paper (49), we extend the three-dimensional reaction-diffusion model of Somersalo et al. (66) to assist in the interpretation of our pHi and pHS data in the other two papers. This model, an an extension of the single-compartment approach developed by Boron and De Weer (9), treats the oocyte as a spherical symmetric cell, with simultaneous reaction and diffusion processes occurring in both the extracellular unconvected fluid (EUF, an extension of the unstirred-layer concept) and the intracellular fluid (ICF). The model accounts for the slow equilibration CO2 + H2O ⇌ H2CO3, effective acceleration of this reaction by CA II and CA IV, and competing equilibria from a multitude of non-CO2/HCO3− buffers in both EUF and ICF.
Our basic experimental protocol was to expose an oocyte—importantly, an oocyte not expressing any HCO3− transporters—to a solution containing 1.5% CO2/10 mM HCO3−/pH 7.50 (Fig. 1). The CO2 influx causes the familiar fall in pHi (for review, see ref. 58), which intracellular CA ought to accelerate, as first observed by Thomas (71). The influx of CO2 should also reduce [CO2]S. The depleted CO2 at the cell surface is replenished by 1) diffusion from the BECF and 2) the reaction HCO3− + H+ → CO2 + H2O at the cell surface. Because this reaction consumes H+, pHS rises, as observed by De Hemptinne and Huguenin (31) and by Saarikoski and Kaila (59). The reaction also depletes HCO3− at the cell surface, and we would expect that the diffusion of H+ and HCO3− from the BECF would replenish the depleted H+ and HCO3− at the cell surface. If an extracellular buffer, such as HEPES, is present, it would undergo the reaction H-HEPES → H+ + HEPES− at the cell surface and thereby reduce the magnitude of the pHS increase. However, according to the experiments of Gutknecht et al. (29), this HEPES reaction should substantially raise [CO2]S (and thus enhance the influx of CO2) in the presence of mobile extracellular CA.2 Over time, as [CO2]i approaches [CO2]S, and as [CO2]S approaches [CO2]BECF, all of these fluxes and reactions slow and the system reaches equilibrium.
Fig. 1.
Model of an oocyte exposed to CO2/HCO3−. The main part of the figure illustrates the diffusion and reaction events as CO2 enters an oocyte. The black arrows with sharp heads indicate solute diffusion in the extracellular unconvected fluid (EUF) and intracellular fluid. The black arrows with dull heads indicate reactions. The left inset is a schematic top view of the oocyte in the chamber, as seen through the microscope. The orange arrows indicate the direction of convective flow of the bulk extracellular fluid (BECF). The right inset is a schematic view of the oocyte along the axis of convective flow, looking downstream. The darker half of the oocyte, oriented upward, is the animal pole. CA II, carbonic anhydrase II; pHi, intracellular pH; pHS, surface pH.
In this first paper, we find that applying 1.5% CO2/10 mM HCO3− (at a fixed pH of 7.50) does indeed cause pHS to rise transiently and then decay with an exponential time course. pHi also falls with an exponential time course, but more slowly, reflecting the depth of the penetration of the pHi electrode into the cell as well as the asymmetry of [CO2]S as BECF flows past the oocyte. Injecting CA II into the oocyte not only accelerates the decline in pHi but also increases the height and the rate of decay of the pHS spike. The CA II inhibitor ethoxzolamide (EZA) blocks all of the effects of CA II. Our pHS data imply that, by speeding the conversion of incoming CO2 to HCO3− + H+, intracellular CA II keeps [CO2]i relatively low and thus enhances the gradient driving CO2 influx. In turn, this increased CO2 influx enhances the fall in [CO2]S, and thus accentuates the pHS spike. A mathematical model, which is discussed in the third paper in this series (49), supports this hypothesis.
MATERIALS AND METHODS
Preparation of Xenopus Oocytes
We isolated stage V-VI oocytes from Xenopus laevis using standard techniques (27). Briefly, we anesthetized animals in 0.2% MS-222 (ethyl 3-aminobenzoate methanesulfonate, Sigma-Aldrich, St. Louis, MO) and removed ovarian lobes, which we placed in a 0-Ca solution (98 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.50). After washing the oocytes 5× in the 0-Ca solution, we dissociated and defolliculated them by enzymatic digestion, using 2 mg/ml type IA collagenase (Sigma-Aldrich) in the 0-Ca solution. We selected stage V-VI oocytes and kept them at 18°C in sterile-filtered OR3 medium, each 2 liters of which contained one pack of powdered Leibovitz L-15 media with l-glutamine (GIBCO-BRL), 100 ml of 10,000 U/ml penicillin, 10,000 U/ml streptomycin solution (Sigma-Aldrich), and 5 mM HEPES titrated to pH 7.50, and osmolality adjusted to 195 mosmol/kgH2O. One day after their isolation, we injected the oocytes with 50 nl of H2O as a control for the injection of cRNA encoding CA IV in the accompanying paper (46). Three days after this H2O injection, we injected the oocytes with 50 nl of fluid, either Tris buffer (50 mM titrated to pH 7.5 with HCl) or Tris buffer containing 6 ng/nl of recombinant human CA II protein (i.e., 300 ng/oocyte ≅ 10 pmol, for a final [CA II]i of ∼30 μM, ∼50% higher than the level in red blood cells). Dr. Peter Piermarini kindly prepared the CA II, as described previously (38, 52), using an approach similar to that described by others (70), and assessing protein purity by polyacrylamide gel electrophoresis (PAGE) and MALDI mass spectrometry (SynPep, Dublin, CA). After the Tris or CA II injection, we incubated the oocytes overnight at 18°C in the sterile-filtered OR3 medium described above. The protocols for housing and handling of Xenopus laevis were approved by the Institutional Animal Care and Use Committee of Case Western Reserve and Yale Universities.
Solutions
Table 1 summarizes the composition of the solutions. The ND96 solution is nominally CO2/HCO3− free. The CO2/HCO3− solutions were bubbled vigorously with the appropriate gas mixture (balance O2) for ∼30 min.
Table 1.
Solutions
| 1.5% CO2/10 mM HCO3− |
5% CO2/33 mM HCO3− |
10% CO2/66 mM HCO3− |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HEPES | HEPES | HEPES | ||||||||
| Component | ND96 | 1 | 5 | 25 | 1 | 5 | 25 | 1 | 5 | 25 |
| Sol. 1 | Sol. 2 | Sol. 3 | Sol. 4 | Sol. 5 | Sol. 6 | Sol. 7 | Sol. 8 | Sol. 9 | Sol. 10 | |
| NaCl, mM* | 96 | 88 | 86 | 76 | 65 | 63 | 53 | 32 | 30 | 20 |
| KCl, mM | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| MgCl2, mM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| CaCl2, mM | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| HEPES, mM* | 5 | 1 | 5 | 25 | 1 | 5 | 25 | 1 | 5 | 25 |
| HCO3−, mM | 0 | 10 | 10 | 10 | 33 | 33 | 33 | 66 | 66 | 66 |
| pH | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Osmolality, mosmol/kgH2O | ≅195 | ≅195 | ≅195 | ≅195 | ≅195 | ≅195 | ≅195 | ≅195 | ≅195 | ≅195 |
We titrated HEPES free acid (pK 7.5) to pH 7.5 with NaOH (so that, theoretically, we have incremented H-HEPES, HEPES−, and Na+ concentrations by equal amounts) and then secondarily adjusted [NaCl], as indicated in this table, to achieve a final osmolality of ∼195 mosmol/kgH2O.
We dissolved the CA II inhibitor EZA (Sigma-Aldrich) in 0.05 N NaOH, to prepare a stock solution with a final concentration of 50 mM. We achieved a final EZA concentration of 400 μM by diluting this stock 1:125 in ND96, and adjusted the pH to 7.50 with 5 N HCl.
Electrophysiological Measurements
Figure 8B in Ref. 44 provides a detailed view of the arrangement of the chamber, oocyte, and electrodes.
Fig. 8.
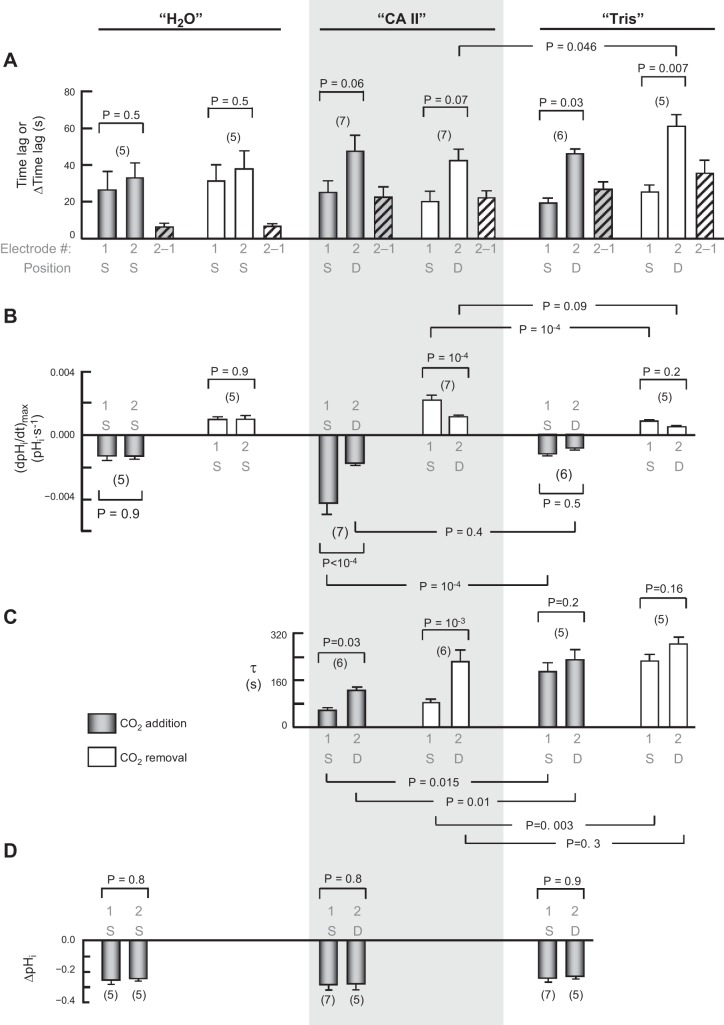
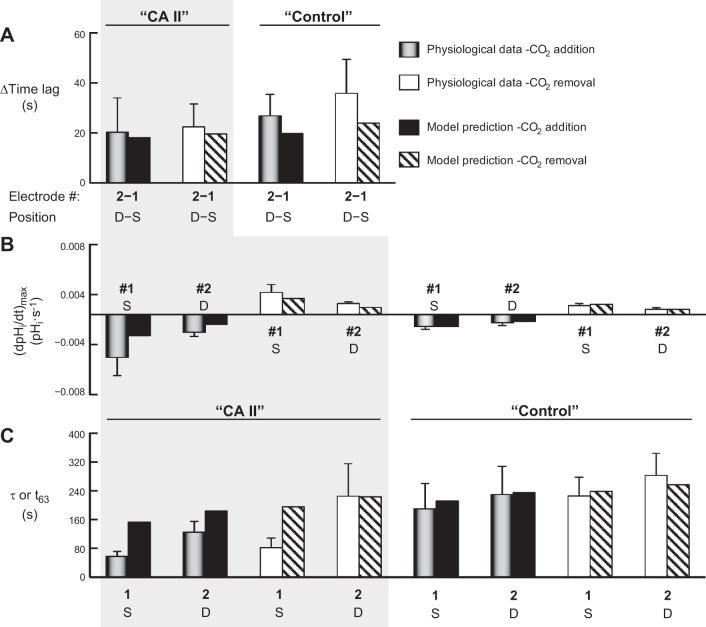
Summary of dual pHi-electrode impalements. This figure summarizes results from a larger number of experiments, such as those in Fig. 7. We injected all oocytes with H2O on day 0. Those for which this was the only injection are labeled “H2O.” We impaled these oocytes superficially (S) with two pHi microelectrodes, electrode no. 1 positioned by a manual manipulator (the one that held pHi electrodes in other protocols), and electrode no. 2 positioned by a motorized manipulator (the one that held pHS electrodes in other protocols). For other oocytes, we followed the injection of water on day 0 with the injection on day 3 with either “CA II” in Tris buffer, or only “Tris.” We impaled these oocytes superficially with pHi microelectrode no. 1, and deep (D) with electrode no. 2. A: time lags between initiation of the transients for pHi electrodes no. 1 and no. 2. The hatched bars represent the oocyte-by-oocyte difference between electrodes no. 2 and no. 1. An ANOVA for the six differences in time lags between electrode no. 2 and electrode no. 1 (six hatched bars) had an overall P value of 0.0003. The two “H2O” values were significantly different from the others. B: maximal rates of pHi change (negative or positive direction) produced by the extracellular solution switch. C: time constants (τ) for pHi changes. D: changes in steady-state pHi induced by the addition of CO2/HCO3−. Values are means ± SE, with nos. of oocytes in parentheses. We performed a one-way ANOVA, followed by a Student-Newman-Keuls (SNK) analysis (P shown for individual comparisons). The initial pHi values for “H2O”, “CA II”, and “Tris” oocytes in the ND96 (i.e., CO2/HCO3−-free) solution were not significantly different from one another, both for electrode no. 1 and electrode no. 2 (overall ANOVA, P = 0.81).
Chamber.
We placed an oocyte in a plastic perfusion chamber with a channel 3 mm wide × 30 mm long. A glass coverslip formed the bottom of the chamber. Solutions were placed in 140-ml plastic syringes (Sherwood Medical, St. Louis, MO) and delivered to the chamber using syringe pumps (Harvard Apparatus, South Natick, MA). Solutions were carried to the chamber via Tygon tubing (Ryan Herco Products, Burbank, CA; Formulation R3603-3; OD 4.8 mm/ID 1.6 mm) and flowed from one end of the 30-mm channel to the other. In initial experiments for the data set summarized below in Fig. 3, the solution flowed at 2 ml/min, and in later experiments, 3 ml/min. As noted in results, it does not appear that this change had a significant effect. For all other data sets in this paper, solutions flowed at 3 ml/min. We switched among solutions with pneumatically operated valves (Clippard Instrument Laboratory, Cincinnati, OH). All experiments were performed at room temperature (∼22°C), and in all experiments, the oocyte was initially superfused with the ND96 solution.
Fig. 3.
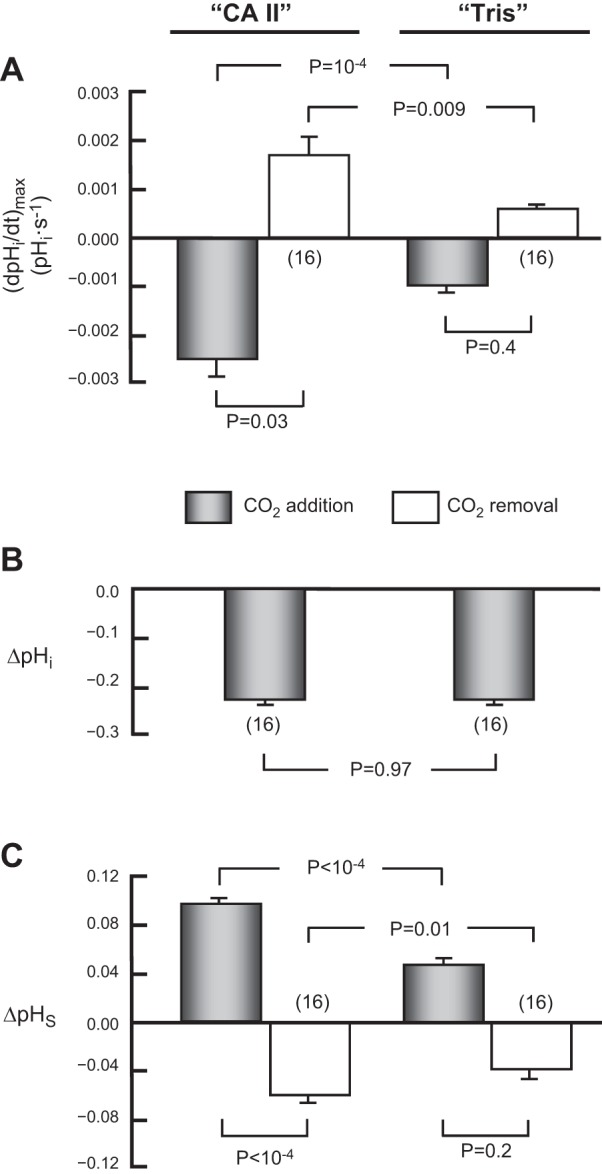
Summary of effects of CA II on pHi and pHS. This figure summarizes results from a larger number of experiments, such as those in Fig. 2, on oocytes injected with CA II in Tris buffer, or only Tris buffer. In each case, we switched the extracellular solution from ND96 to 1.5% CO2/10 mM HCO3− (gray bars) and vice versa (white bars). A: maximal rates of pHi change (negative or positive direction) produced by the extracellular solution switch. B: changes in steady-state pHi, induced by the addition of CO2/HCO3−. C: maximal changes in pHS (positive or negative direction) caused by the extracellular solution switch. This summary includes all oocytes injected with CA II (and exposed to 1.5% CO2 in 5 mM HEPES) in the present study, as well as their day-matched Tris-injected controls. Values are means ± SE, with nos. of oocytes in parentheses. We performed a one-way ANOVA (overall P values: P < 10−4 in A, and P < 10−4 in C), followed by a Student-Newman-Keuls (SNK) analysis (P shown for individual comparisons). We performed a paired t-test in B. The mean initial pHi for “CA II” oocytes in the CO2/HCO3−-free ND96 solution was 7.21 ± 0.05 (n = 16). This mean is not significantly different from that for “Tris” oocytes, 7.22 ± 0.03 (n = 16; P = 0.93).8
Measurement of intracellular pH.
In a typical experiment, we impaled the oocyte (with the dark animal pole facing upward) with two microelectrodes (Fig. 1, left and right insets), one for sensing membrane potential (Vm, amplified by a model OC-725 two-electrode Oocyte Clamp, Warner Instruments, Hamden, CT) and the other for sensing pHi (amplified by a model FD223 high-impedance electrometer, World Precision Instruments, Sarasota, FL). We fabricated and used the electrodes as described previously (56, 57, 61). We used a horizontal microelectrode puller (model P-97, Sutter Instrument, Novato, CA) to produce microelectrodes from thin-walled borosilicate glass (part no. G200TF-4, 2.0 mm OD ×1.56 mm ID, Warner Instruments). We filled the Vm electrodes with 3 M KCl; these had resistances of ∼0.6 MΩ. The pHi electrodes were identical but we filled them with a liquid, pH-sensitive membrane (Hydrogen Ionophore I, mixture B, Fluka Chemical, Ronkonkoma, NY).
The extracellular reference for the Vm electrode was the virtual ground created by the Warner OC-725 Oocyte Clamp. The ISENSE connection was attached to a microelectrode holder (model 64-1010, Warner Instruments), which contained a Ag/AgCl half-cell that served as a bridge to a broken-tipped glass microelectrode filled with 3 M KCl (∼0.1 MΩ), and positioned so that its tip was close to the oocyte. The IOUT connection was attached to a platinum wire that rested in a reservoir at the end of the chamber's channel (i.e., downstream from the oocyte). The circuit ground of the OC-725 (i.e., the virtual ground of the BECF or bath), and indeed the circuit grounds of all electronic components, was connected to a brass plate (i.e., “system ground”), which was in turn connected to earth.
We obtained the voltage due to pHi by electronically subtracting the signal of the Vm electrode from that of the pHi electrode. We obtained Vm by electronically subtracting the system ground of the OC-725 from the signal of the Vm electrode. The device that performed the subtractions (Yale University Subtraction Amplifier, V3.1) also appropriately scaled the voltages for the inputs of an analog-to-digital converter within a Windows-based computer. We simultaneously acquired all electrical data, initially, once every 2,000 ms, later once every 1,000 ms, and finally once every 500 ms, and analyzed it using software written in-house. As noted in the discussion, these differences do not appear to affect our data. We obtained the slope of the intracellular pH electrodes by calibrating pH standards at pH 6.0 and 8.0 (model SB 104-1 and SB 112-1, respectively, Fisher Scientific, Fair Lawn, NJ). An additional single-point calibration was obtained in the ND96 solution (pH 7.50) in the bath before impaling the oocyte. Oocytes had a spontaneous Vm at least as negative as −40 mV.
Measurement of surface pH.
In addition to the electrodes described above, in a typical experiment we measured pHS using a liquid-ion-exchange pH electrode (Fig. 1, left and right insets) with an outer tip diameter of 20 μm (amplified by a FD223 electrometer, WPI). We pulled these pipettes from standard-wall borosilicate tubing (part no. G200F-4, 2.0 mm OD ×1.16 mm ID, Warner Instruments) and used a microforge to break off and fire polish the tips as one would for a giant-patch pipette (33). During experiments, we attach the pHS electrode to an ultra-fine computer-controlled micromanipulator with digital position display (model MPC-200 system, Sutter Instrument).
A typical experiment begins with the pHS electrode tip in the bath—in the “home” position, ∼300 μm from the oocyte surface—to calibrate the electrode at pH 7.50 in ND96 solution (Table 1). After that, we move the flat tip of the pHS electrode to the oocyte's surface—the “zero” position—near the equator (i.e., between the animal and vegetal poles, halfway between top and bottom), and ∼5° behind the meridian perpendicular to the axis of flow (i.e., slightly in the “shadow” of the flowing extracellular solution), as illustrated in the left inset of Fig. 1. Finally, we further advance the electrode tip ∼40 μm until we observe a slight dimple in the membrane. The creation of this small dimple produces a microenvironment between the pHS electrode tip and the oocyte membrane and has two effects: 1) maximizing the pHS change and 2) ensuring a consistent and reproducible electrode placement, in the radial direction, on the oocyte membrane for each experiment, and thus minimizing variability from experiment to experiment. During the experiment, we periodically withdraw the electrode to its “home” position for recalibration in the flowing BECF solution at pH 7.50. In the figures, we indicate these movements of the pHS electrode in the radial direction by “Surface” and “BECF” in the step chart.
The external reference for the pHS electrode was a very long, broken-tipped (∼10 μm ID) glass micropipette that we pulled from the above from thin-walled borosilicate tubing on a rotating micropipette puller (model 51.511, Stoelting, Chicago, IL), filled with 3 M KCl, and bridged with a calomel half-cell to the input of a model 750 electrometer (WPI). pHS was obtained by subtracting the calomel signal from the pHS signal. As for the pHi electrodes, we obtained the slope of the pHS electrodes by calibrating pH standards at pH 6.0 and 8.0. In preliminary experiments, we found that the slope of the electrodes was the same in ND96 vs. CO2/HCO3−.
Dual measurement of intracellular pH.
In some experiments, we replaced the above pHS microelectrode with a second sharp pHi microelectrode (designated electrode no. 2), which we moved using the same Sutter 200 micromanipulator that otherwise carried the pHS electrode.
Rate of bath solution change.
In mock experiments, we used pHi and pHS electrodes (sampling 1 per 500 ms) to monitor the pH of the bath (flowing at 3 ml/min) as we switched from a pH-7.5 to a pH-8.0 solution. We found that the time constant (τ) for the reported time course of pH was 1.76 ± 0.13 s (n = 3) for pHi and 1.70 ± 0.35 s (n = 4) for pHS. Because these τ figures include the response time of the electrodes, ∼1.7 s is the maximum τ for switching the solution in the chamber.
Analysis of pH Data
Intracellular pH.
Applying extracellular CO2/HCO3− causes a fall in the measured pHi that at first begins slowly (presumably reflecting the finite time required for [CO2]BECF to rise to its maximal value, the depth of penetration of the electrode into the cell, and the asymmetry of [CO2]S over the oocyte surface) and then accelerates to the maximal rate of decline—(dpHi/dt)max. The rate of pHi decline then slows and gradually falls to zero. For example, in CA II experiments such as that in Fig. 2A below (summarized in Fig. 3 below), the fall in pHi accelerated for an average of 9 ± 1 s (n = 16) before reaching the period of steepest decline. By inspection, we determined the time interval (which lasted an average of 39 ± 4 s, n = 16) during which pHi declined most steeply and used software written in-house to perform a linear least-square curve fit to compute (dpHi/dt)max.
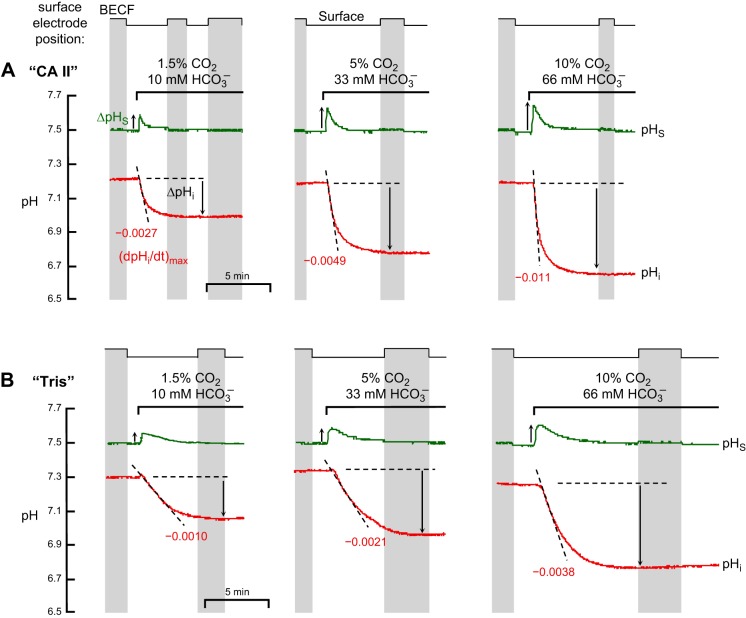
Fig. 2.
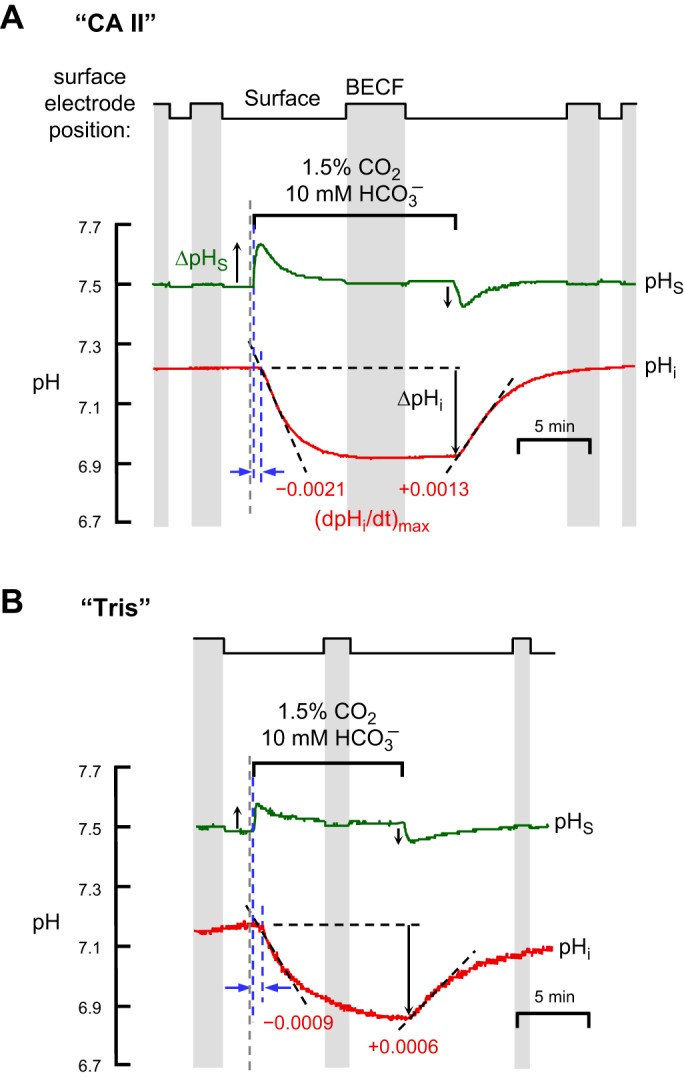
Representative experiments showing effects of CA II on pHi and pHS changes evoked by application and removal of CO2/HCO3−. A: oocytes injected with recombinant human CA II dissolved in Tris buffer. B: oocytes injected only with Tris buffer. Both in “CA II” or “Tris” oocytes, the pHi trace is represented by the red lower record, and pHS, by the green upper record. At the indicated times, we switched the extracellular solution from ND96 to 1.5% CO2/10 mM HCO3−/pH 7.50 (Table 1) and then back again. In this example, the extracellular solution flowed at 2 ml/min and the sampling rate was 1 per 1,000 ms. The vertical gray bands represent periods during which the pHS electrode was withdrawn to the BECF for calibration. At other times the pHS electrode was dimpling ∼40 μm into the oocyte surface. The gray, dashed vertical lines represent the times that the computer switched the valves to initiate a change of solutions. The left and right blue, dashed vertical lines (see results) represent the initiation of the pHS and pHi transients, respectively. The dashed black lines through the initial portions of the pHi records for CO2 application and removal represent best linear fits for maximal rates of pHi change (negative or positive direction). The downward vertical arrows near the pHi records represent the CO2-induced changes in steady-state pHi. The upward and downward arrows near the pHS records represent maximal changes in pHS (positive or negative direction).
In a subset of experiments, we used a nonlinear least-squares approach to obtain the best fit of a single-exponential function to the pHi vs. time record.
Intrinsic intracellular buffering power.
We computed the intrinsic intracellular buffering power [βI, mM·(pH unit)−1] from the magnitude of the change in steady-state pHi produced by exposing the cells to a solution containing 1.5% CO2/10 mM HCO3−. Because the amount of intracellular H+ generated by the influx of CO2 is virtually the same as the resulting rise in intracellular [HCO3−] (see ref. 58),
| (1) |
The initial [HCO3−]i is assumed to be zero, and the final [HCO3−]i is computed from the steady-state pHi and the Henderson-Hasselbalch equation. ΔpHi is the magnitude of the fall in steady-state pHi caused by the exposure to CO2/HCO3−.
Surface pH.
Applying extracellular CO2/HCO3− causes pHS to rise rapidly and then decline. We determined the maximum size of the pHS transient (i.e., the “spike height” or ΔpHS) as follows. The initial pHS, before application of CO2/HCO3− (i.e., with the oocyte in ND96 solution, Table 1), was computed by comparing the “pHS” voltage reading obtained with the electrode tip at the oocyte surface with the “pHS” voltage reading obtained with the electrode tip in the BECF (assumed to have a pH of 7.50). The peak pHS during the CO2/HCO3− exposure was computed by comparing the “pHS” voltage reading obtained with the electrode tip at the oocyte surface (at a time corresponding to the highest pHS) with the “pHS” voltage reading obtained a few minutes later (after pHS declined to ∼7.50) when we temporarily withdrew the electrode tip to the BECF (assumed to have a pH of 7.50). ΔpHS was the difference between the peak pHS in CO2/HCO3− and the pHS measured in ND96, just before the switch to CO2/HCO3−.
We used a similar approach to determine the maximum magnitude of the pHS transient (i.e., an acidification) that occurred when we removed extracellular CO2/HCO3−.
In some experiments, we used a nonlinear least-squares approach to obtain the best fit of a single-exponential function to the pHS vs. time record.
Statistics
Data are reported as means ± SE unless stated otherwise. To compare the difference between two means, Student's t-tests (two tails) was performed. To compare more than two means, one-way ANOVA Multiple Comparison was performed, followed by a Student-Newman-Keuls (SNK) analysis, using KaleidaGraph (version 4, Synergy Software). P < 0.05 was considered significant.
RESULTS
Effect of Injected CA II on pHi and pHS Changes
Protocol.
Figure 2 shows a pair of experiments in which we examined the effects of CA II on the pHi and pHS transients caused by applying and then removing 1.5% CO2/10 mM HCO3− at a constant pHBECF of 7.50. Our protocol to evaluate the effects of injected CA II was the same as previously employed by Lu et al. (38), except that in the present study we monitored pHS in addition to pHi. 1) Our first step was to inject H2O into all Xenopus oocytes (day 0/step a); we did this as a control for experiments in the second paper in this series (46), in which, on day 0, we injected cRNA encoding CA IV. 2) After waiting 3 days, we randomly divided the oocytes into two groups (day 3/step b). We injected each oocyte in one group with 300 ng (or ∼10−11 mol) of recombinant human CA II protein dissolved in Tris buffer (“CA II” group), and injected each oocyte in the other group with just the Tris buffer (“Tris” group). 3) One day later, we did our pH measurements (day 4/step c), such as those shown in Fig. 2.
The step chart at the top of Fig. 2, A and B, shows whether the extracellular pH electrode was in the BECF (gray background) or at the cell surface, dimpling ∼40 μm into the oocyte surface (white background).
pHi changes.
An exposure to 1.5% CO2/10 mM HCO3−, causes pHi to decrease much faster in a “CA II” oocyte (Fig. 2A, lower record) than in a “Tris” oocyte (Fig. 2B, lower record). We would expect these results, even if CA II did not increase the CO2 influx, simply because the enzyme catalyzes the conversion of incoming intracellular CO2 to produce HCO3− plus the intracellular H+ that the pHi electrode senses (see Fig. 1). We define the maximal rate of pHi change as (dpHi/dt)max; it is negative for CO2 influx and positive for CO2 efflux. As indicated by the two gray bars in Fig. 3A, which summarizes data for a larger series of experiments, the CO2-induced maximal intracellular acidification rate, (dpHi/dt)max, is 2.5-fold greater in the presence of CA II than in its absence. However, as shown in Fig. 2 and summarized by Fig. 3B, CA II had no effect on the CO2-induced change in steady-state pHi (ΔpHi). The most straightforward explanation for this result is that CA II affected neither the intrinsic buffering power of the oocyte (βI) nor the total net entry of CO2 into the cytosol.
pHS changes.
The upper records in Fig. 2, A and B, show that applying of CO2/HCO3− causes a transient rise in pHS, as previously reported (22, 31). We define the maximal pHS change as ΔpHS; it is positive for CO2 influx and negative for CO2 efflux. Note that intracellular CA II markedly increases ΔpHS, which, as outlined in Fig. 1, reflects the maximal rate of CO2 entry into the cell. The gray bars in Fig. 3C show that CA II increases the mean ΔpHS by approximately twofold. Thus, these pHS data show that CA II speeds the influx of CO2. CA II presumably produces this effect by keeping [CO2]i—especially at the inner surface of the membrane, but throughout the cytosol—relatively low during the initial part of the experiment.
Reversibility.
As shown in Fig. 2, A and B, and summarized in Fig. 3, A and C, removing CO2/HCO3− causes pHi and pHS changes that are opposite in direction of those evoked by applying CO2/HCO3−. However, at least in the case of “CA II” oocytes, the magnitudes of (dpHi/dt)max and ΔpHS are smaller for CO2 removal than for CO2 addition. We will revisit this subject in Fig. 6 below and consider the basis for the differences, in the context of the mathematical model, in the discussion (see section “Asymmetry of pH Changes Upon Addition vs. Removal of CO2”).
Fig. 6.
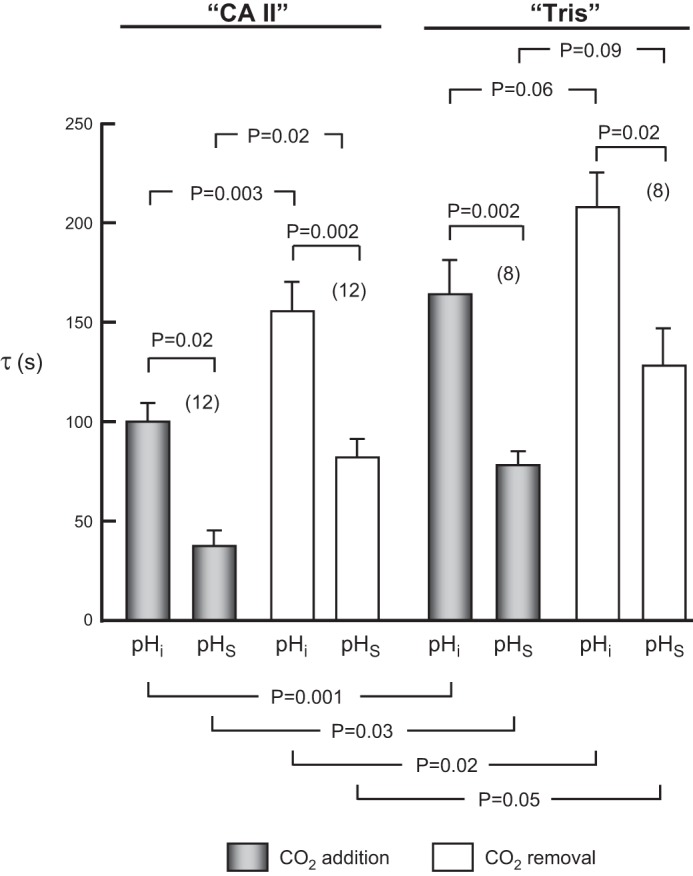
Summary of time constants (τ) for pHi and pHS changes for a larger number of experiments, such as those in Fig. 2, on oocytes injected with CA II in Tris buffer, or only Tris. Values are means ± SE, with nos. of oocytes in parentheses. We performed a one-way ANOVA followed by a Student-Newman-Keuls (SNK) analysis (P shown for individual comparisons).
Effect of Blocking CA II With EZA
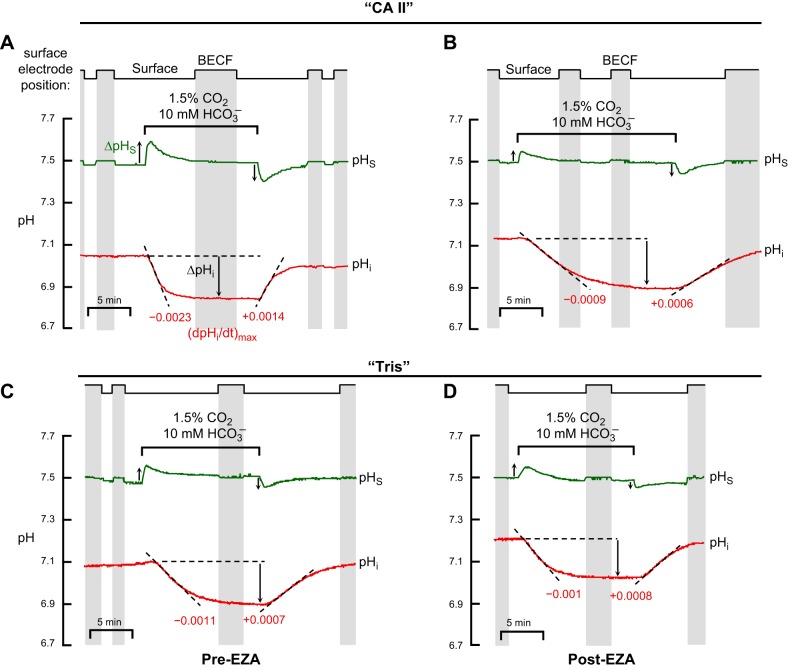
If our hypothesis is correct, then the effects that we observed with CA II ought to be blocked by an inhibitor of CA II. In a subset of the experiments summarized above in Fig. 3, we followed the CO2/HCO3− exposure (as in Fig. 2) by 1) a ∼3-h treatment with 400 μM ethoxzolamide (EZA, a permeant CA II inhibitor) and three washes, and then 2) a second exposure to CO2/HCO3−. Figure 4 shows a representative pair of such experiments, one on a “CA II” oocyte (Fig. 4A/Pre-EZA and Fig. 4B/Post-3h EZA), and the other on a “Tris” oocyte (Fig. 4C/Pre-EZA and Fig. 4D/Post-3 h EZA), similar to the protocol introduced by Lu et al. (38).
Fig. 4.
Representative experiments showing effects of ethoxzolamide (EZA) EZA on pHi and pHS. A: oocyte injected with recombinant human CA II dissolved in Tris buffer, assayed before treatment with EZA (Pre-EZA). B: “CA II” oocyte, assayed after a 3-h treatment with EZA (Post-EZA). C: oocyte injected only with Tris buffer, assayed before treatment with EZA. D: “Tris” oocyte, assayed after a 3-h treatment with EZA. For both CA II and Tris experiments, the Pre-EZA and Post-EZA pHi and pHS records were obtained from the same oocytes. Between the two CO2/HCO3− exposures, the oocytes were exposed for 3 h to the ND96 solution (Table 1) supplemented with 400 μM EZA, followed by 3 washes in ND96, and incubation in ND96 (30 min to 3 h). We noticed no effect of the variable duration of the post-EZA incubation. The extracellular solutions were ND96 and, during the indicated time, 1.5% CO2/10 mM HCO3−/pH 7.50. In both experiments, the solution flowed at 2 ml/min and the sampling rate was 1 per 1,000 ms. The vertical gray bands represent periods during which the pHS electrode was withdrawn to the BECF for calibration. The dashed black lines through the initial portions of the pHi records for CO2 application and removal represent best linear fits for maximal rates of pHi change (negative or positive direction). The downward vertical arrows represent CO2-induced change in steady-state pHi. The upward and downward vertical arrows near the pHS records represent the maximal CO2-induced changes in pHS (positive or negative direction).
pHi changes.
As hypothesized, the CA II blocker EZA markedly slows the CO2-induced maximal acidification rate in the “CA II” oocyte (Fig. 4, A vs. B, lower records), but not in the “Tris” oocyte (Fig. 4, C vs. D, lower records). The four bars in Fig. 5A summarize the (dpHi/dt)max values-corresponding to the application of CO2/HCO3−-for a larger number of experiments like those shown in the lower records of Fig. 4A–D. These data show that, in “CA II” oocytes, EZA reduces the magnitude of (dpHi/dt)max to a value indistinguishable from that in “Tris” oocytes, with or without EZA. As expected, Fig. 5B shows that EZA does not significantly affect the CO2-induced change in steady-state pHi in either “CA II” or “Tris” oocytes.
Fig. 5.
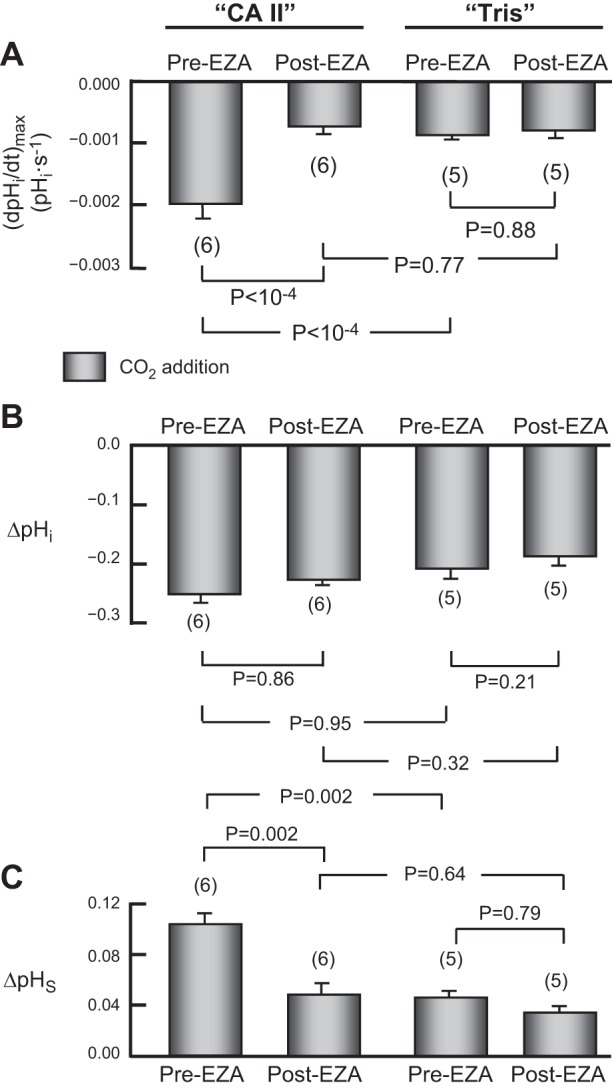
Summary of the effects of EZA on pHi and pHS. This figure summarizes data relating to CO2 addition from a larger number of experiments, such as those in Fig. 4, on oocytes injected with CA II in Tris buffer, or only Tris buffer. After an assay during which we switched the extracellular solution from ND96 to 1.5% CO2/10 mM HCO3−, in the absence of EZA (Pre-EZA), we exposed the same oocyte to 400 μM EZA for 3 h, and then repeated the assay (Post-EZA). A: maximal rates of pHi change (negative direction) produced by the extracellular solution switch. B: changes in steady-state pHi produced by the switch to CO2/HCO3−. C: maximal changes in pHS (positive direction) produced by the extracellular solution switch. Values are means ± SE, with nos. of oocytes in parentheses. We performed a one-way ANOVA (overall P values: P < 10−4 in A, P = 0.38 in B, and P = 0.0012 in C), followed by a Student-Newman- Keuls (SNK) analysis (P shown for individual comparisons). The mean initial pHi for “CA II” oocytes in the CO2/HCO3−-free ND96 solution was 7.13 ± 0.11 (n = 6), which is not significantly different from the mean value for “Tris” oocytes, 7.05 ± 0.07 (n = 5; P = 0.93). For both “CA II” and “Tris” oocytes, EZA had no effect on the initial pHi.
pHS changes.
EZA markedly blunts the pHS transients in the “CA II” oocyte (Fig. 4A vs. B, upper records) but does not reduce the size of the already blunted pHS transients in the “Tris” oocyte (Fig. 4, C vs. D, upper records). Finally, Fig. 5C shows that, in “CA II” oocytes, EZA reduces the CO2-induced ΔpHS to a value not significantly different from that in “Tris” oocytes, with or without EZA.
Figure 5 does not show the data summary for (dpHi/dt)max, ΔpHi, and ΔpHS for the CO2-removal phase of the experiment. However, the effects of EZA on these three parameters during CO2 removal were similar to the effects during CO2 addition. Also not shown are two sham experiments on CA II oocytes and one on a Tris oocyte, in which we followed the first CO2/HCO3− exposure by a treatment in ND96 without EZA, followed by washes and a second exposure to CO2/HCO3−. We saw no obvious difference, in either the pHi or pHS changes, between first and second exposures to CO2/HCO3−. Thus, the differences between pre-EZA and post-EZA values in Fig. 4 and Fig. 5 are due to EZA and not the time course of the experiment.
Analysis of Time Constants for pHi and pHS Changes
According to the model in Fig. 1, after a step increase in [CO2]BECF, the rate of pHi decline should fall over time as the rate of CO2 entry falls. Similarly, after reaching its maximal value, the change in pHS—another manifestation of this same CO2 entry—should fall over time as the rate of CO2 entry falls. Thus, we might expect that the time courses of both the decrease in pHi and the decay in pHS from its peak value should follow approximately exponential time courses with similar time constants. For each experiment summarized in Fig. 3, we used whenever possible a nonlinear least-squares approach to obtain four sets of best-fit parameters for a single-exponential function, one each for pHi and pHS, both for the addition and removal of CO2/HCO3−.3
CA II vs. Tris.
As far as the pHi time course is concerned, because CA II increased the magnitude of (dpHi/dt)max when we applied CO2 (Fig. 3A, gray bars), we expected that it also would decrease the time constant (τ) for the CO2-induced pHi decline. This was indeed the case (compare pHi bars 1 vs. 5 in Fig. 6).
As far as the pHS time course is concerned, the initial rate of CO2 entry is greater for “CA II” than for “Tris” oocytes, as evidenced by the greater ΔpHS in Fig. 3C (gray bars). However, the net number of CO2 molecules entering the cytosol is the same for “CA II” and “Tris” oocytes, as evidenced by the identical magnitudes of the CO2-induced ΔpHi (Fig. 3B, gray bars). Thus, CO2 entry across the plasma membrane should come to completion earlier in “CA II” oocytes, which should therefore have a smaller τ for pHS, as was indeed the case (compare pHS bars 2 vs. 6 in Fig. 6).
With the removal of CO2, we also observed, as expected, that the τ for pHi was smaller (i.e., faster) for “CA II” than for “Tris” oocytes (compare bars 3 vs. 7 in Fig. 6). Similarly, the τ for pHS was smaller (faster) for “CA II” oocytes (compare bars 4 vs. 8 in Fig. 6).
CO2 addition vs. CO2 removal.
For pHi, the τ values for CO2 addition—oocyte by oocyte—were almost invariably smaller (faster) than their counterparts for CO2 removal for “CA II” oocytes (compare bars 1 vs. 3 in Fig. 6) and for “Tris” oocytes (compare bars 5 vs. 7 in Fig. 6). However, in an ANOVA, the differences reached statistical significance only for the CA II data.
For pHS, like pHi, the mean τ values—oocyte by oocyte—were again invariably smaller (faster) for CO2 addition than for CO2 removal for “CA II” (compare bars 2 vs. 4 in Fig. 6) and for “Tris” (compare bars 6 vs. 8 in Fig. 6). Again, the difference reached statistical significance in an ANOVA only for the CA II data. These trends for τ are antiparallel to the trends that we noted above for (dpHi/dt)max (Fig. 3A) and ΔpHS (Fig. 3C). In the discussion, we will examine these issues in the context of the mathematical model.
pHi vs. pHS.
Another unexpected observation was that the τ values for the pHS transients—oocyte by oocyte—were consistently less than those of the corresponding pHi transients, both for “CA II” and “Tris” oocytes, and both for CO2 application and removal (compare bars 1 vs. 2, 3 vs. 4, 5 vs. 6, and 7 vs. 8 in Fig. 6). As we will see in the section after the next, and as confirmed by our mathematical model, the slower time course of pHi vs. pHS probably reflects, at least in part, delays due to the depth of penetration of the pHi electrode. In addition, the asymmetry of [CO2]BECF around the oocyte probably makes a contribution (see discussion).
Analysis of Time Delay For pHi Changes
Time delay is the time difference between the initiation of the CO2-induced acidification rate upswing in pHS (see next section) and the initiation of the fall in pHi. To estimate the time of the initiation of the pHi decline, we obtained two linear fits of pHi time courses: 1) baseline pHi vs. time (i.e., before the addition of CO2/HCO3−) and 2) the maximal rate of pHi decline (see materials and methods). The time of initiation (indicated by the right blue, dashed vertical line in Fig. 2A) is the time at the intersection of the two linear fits.
We observed, with both the application and removal of CO2, and for both “CA II” and “Tris” oocytes, that the initiation of the pHS spike preceded the initiation of the pHi change for every oocyte. Table 2 summarizes the delays between the initiation of the pHS spike and the initiation of the corresponding pHi change (i.e., the time between the two vertical blue, dashed lines in Fig. 2A). This delay is not significantly different among the four conditions in Table 2. As we will see in the next section, this delay reflects, to some extent, the depth of impalement of the pHi electrode. Our mathematical model (see discussion) confirms this depth effect and predicts that other factors, such as the tortuosity factor for the diffusion of intracellular solutes, also could influence the delay.
Table 2.
Delay between initiation of pHS and pHi transients
| Delay, s |
||||
|---|---|---|---|---|
| “CA II” |
“Tris” |
|||
| CO2 Addition | CO2 Removal | CO2 Addition | CO2 Removal | |
| Initiation of pHS spike to initiation of pHi change* | 11.2 ± 1.9 (9) | 12.0 ± 2.6 (9) | 7.2 ± 1.1 (6) | 14.0 ± 1.8 (6) |
Values are means ± SE (no. of experiments).
Differences among values in this row are not significant based on an ANOVA (P = 0.18). pHS, surface pH; pHi, intracellular pH; CA II, carbonic anhydrase II; Tris, control group injected with Tris buffer.
Analysis of Time to Peak For pHS Changes
Time to peak is the time difference between the initiation of the CO2-induced acidification rate upswing in pHS and the peak of the pHS spike. To determine the time of the initiation of the CO2-induced acidification rate upswing, we obtained two linear fits of pHS time courses: 1) baseline pHS vs. time (i.e., before the addition of CO2/HCO3−) and 2) the linear phase of the CO2-induced acidification rate pHS increase. The time of initiation (indicated by the left blue, dashed vertical line in Fig. 2A) is the time at the intersection of the two linear fits. We determined the peak of the pHS spike by inspection of an Excel file. Table 3 summarizes the time to peak for a large number of experiments. Differences among values in the columns are not significant based on an ANOVA either for “CA II” (P = 0.09) or for “Tris” (P = 0.6). On the other hand, differences between “CA II” and “Tris” oocytes were statistically significant for each CO2/HCO3−combination. Our mathematical model (see third paper in this series; ref. 49) suggests that time to peak reflects in part the time required for the CO2/HCO3−buffer components to rise in the BECF, and in part the time required for the buffer components to diffuse throughout the EUF and enter the ICF.
Table 3.
Time to peak pHS in “CA II” or “Tris” oocytes
| Time, s |
|||
|---|---|---|---|
| Condition | “CA II” | “Tris” | P Value |
| 1.5% CO2/10 mM HCO3− | 7.6 ± 0.9 (9) | 11.2 ± 1.5 (6) | P = 0.05 |
| 5% CO2/33 mM HCO3− | 5.3 ± 0.3 (7) | 12.3 ± 2.3 (6) | P = 0.006 |
| 10% CO2/66 mM HCO3− | 7.0 ± 0.6 (7) | 14.0 ± 1.9 (6 | P = 0.007 |
Values are means ± SE (no. of experiments).
Experiments With Dual-pHi Microelectrodes: Depth of Impalement
To elucidate the differences in τ values between pHi and pHS, as well as the delays between the initiation of the pHi and pHS transients, we performed three series of experiments in which we impaled the oocyte with two pHi electrodes. Note that we did not obtain pHS records in these experiments.
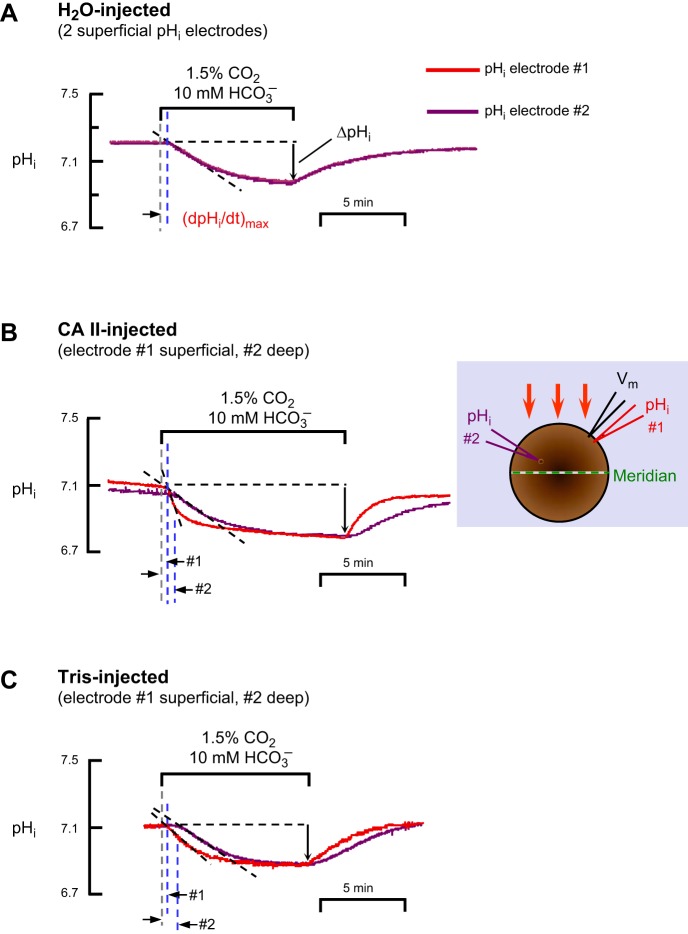
In the first series of experiments, as a control, we inserted both pHi microelectrodes (referred to as electrode no. 1 and electrode no. 2) as superficially as possible into H2O-injected oocytes.4 After impaling with the Vm electrode, we inserted pHi electrode no. no. 1, as we would insert the lone pHi electrode in a standard experiment (Fig. 1): we advanced the pHi microelectrode with a manual manipulator, observed a small dimple on the oocyte surface, tapped the table, and observed the voltage deflection indicating a successful impalement. We mounted pHi electrode #2 on motorized manipulator, which held the pHS electrode in a standard experiment (Fig. 1), and regarded, as “0”, the position where the electrode tip was at the oocyte surface. We advanced electrode no. 2, observed a dimple, and continued to advance the electrode slowly, a total of ∼40 μm from the “0” position, until we observed the appropriate voltage deflection. As shown in Fig. 7A, one of seven similar experiments, when we apply and then remove CO2/HCO3−, the pHi records from the two superficially placed electrodes were virtually identical.
Fig. 7.
Representative experiments with dual pHi-electrode impalements of Xenopus oocytes. A: oocyte injected with H2O on day 0. B: oocyte injected with recombinant human CA II dissolved in Tris buffer on day 3. The inset is a schematic top view of the oocyte, showing the arrangement of microelectrodes; the orange arrows show the direction of convective flow. C: oocyte injected only with Tris buffer on day 3. The extracellular solutions were ND96 (Table 1) and, during the indicated time, 1.5% CO2/10 mM HCO3−/pH 7.50. For all three oocytes, the solution flowed at 3 ml/min and the sampling rate was 1 per 500 ms. The pHi record from electrode no. 1 (superficial) is in red, and the pHi record from electrode no. 2 (deep) is in purple. The gray, dashed vertical lines represent the times that the computer switched the valves to initiate a change of solutions. The left and right blue, dashed vertical lines represent the initiation of the transients for pHi electrodes no. 1 and no. 2, respectively. The dashed black lines through the initial portions of the pHi records for CO2 application represent best linear fits for maximal rates of pHi change (negative direction). The downward vertical arrows represent the CO2-induced change in steady-state pHi.
In the second set of experiments, we performed a superficial impalement with electrode no. 1 and used the motorized manipulator to drive electrode no. 2 increasingly deeper into “CA II” oocytes (Fig. 7B) or “Tris” oocytes (Fig. 7C). In reviewing the initial data on “Tris” oocytes, we noticed that for two impalements to an apparent depth of 250–300 μm beyond the “0” position, the CO2-induced steady-state ΔpHi was indistinguishable from that detected by the superficially placed pHi electrode no. 1 (data not shown). However, for a single impalement to an apparent depth of 540 μm, the ΔpHi value was ∼25% less than that of a simultaneous superficial impalement. Therefore, in all subsequent experiments we chose ∼300 μm beyond the “0” position to be the standard depth of the no. 2 or “deep” electrode.
Figure 7B shows one of seven experiments on “CA II” oocytes in which electrode no. 1 was superficial and electrode no. 2 was at an apparent depth of ∼300 μm. Figure 7C shows one of six such experiments on “Tris” oocytes.
The three vertical regions in Fig. 8, i.e., H2O, CA II, and Tris, summarize the salient features of the three protocols illustrated in Fig. 7, A–C. In Fig. 8A, each cluster of three bars summarizes the data for CO2 addition (gray or gray hatched) or for CO2 removal (white or white hatched). The leftmost bar in each cluster is the time lag5 between the change of the computer-actuated valve (to switch extracellular solutions) and initiation of the change sensed by pHi electrode no. 1. The middle bar is the comparable time lag for pHi electrode no. 2. The rightmost (hatched) bar is the mean difference in the time lags between pHi electrodes no. 2 and no. 1. For H2O-injected oocytes, we observe no statistically significant difference between the time lags detected by superficial electrode no. 1 vs. superficial electrode no. 2. For both the “CA II” oocytes and the “Tris” oocytes, the no. 2–no. 1 differences in time lags are substantially larger than for “H2O” oocytes, reflecting the deeper impalement with electrode no. 2. Note that the no. 2–no. 1 differences are the same for “CA II” vs. “Tris”, and CO2 addition vs. removal.
Figure 8B summarizes the (dpHi/dt)max data. Note that for the “H2O” oocytes, the (dpHi/dt)max values are identical for superficial electrode no. 1 and superficial electrode no. 2. For the “CA II” (but not “Tris”) oocytes, the pHi changes are significantly faster for superficial vs. the deep electrodes. Comparing “CA II” vs. “Tris” oocytes, we see that CA II accelerates the pHi changes recorded by superficial but not by the deep electrodes. The mathematical model confirms this pattern (see discussion).
The (dpHi/dt)max values in Fig. 8B reflect linear fits to the region of steepest CO2-induced descent in the pHi vs. time plot. In parallel, we also used a nonlinear least-squares approach to obtain the best-fit parameters for a single-exponential function, beginning at the point of steepest descent of the pHi vs. time plot. Figure 8C summarizes the mean time τ values from these exponential fits. The pattern of rate constants (i.e., 1/τ values) is similar to the pattern for (dpHi/dt)max data in Fig. 8B: the rate constants for pHi change are significantly faster for superficial vs. the deep electrodes for “CA II” oocytes (but not for “Tris” oocytes).
Figure 8D summarizes the ΔpHi data and shows that neither the presence of CA II nor the depth of impalement (to ∼300 μm) has a significant effect.
Effect of Increasing Extracellular CO2/HCO3− Levels
The preceding data, all obtained with 1.5% CO2, are consistent with the hypothesis that the maximal increase in pHS that occurs during an exposure to CO2/HCO3−—under a particular set of experimental conditions6—is a semiquantitative index of the rate of CO2 entry into the cell. If this hypothesis is true, then the maximal ΔpHS ought to increase when we raise [CO2], [CO2]BECF, and [HCO3−]BECF at a fixed pHBECF. Figure 9 shows experiments in which we pulsed a “CA II” oocyte (Fig. 9A) and a “Tris” oocyte (Fig. 9B) with increasing levels of extracellular CO2/HCO3−—1.5%/10 mM, 5%/33 mM, and 10%/66 mM—always using the same ratio of [CO2]/[HCO3−] to keep pHBECF at 7.50. Figure 10 summarizes a larger series of experiments similar to those in Fig. 9.
Fig. 9.
Representative experiments showing the effect of graded increases in extracellular CO2/HCO3− levels on pHi and pHS. A: oocyte injected with recombinant human CA II in Tris buffer. B: oocyte injected only with Tris buffer. A and B each represent an experiment on single oocytes. The pHi trace is represented by the red lower record, and pHS, by the green upper record. At the indicated times, we switched the extracellular solution (Table 1) from 1) ND96 to 1.5% CO2/10 mM HCO3− (left), or 2) ND96 to 5% CO2/33 mM HCO3− (center), or 3) ND96 to 10% CO2/66 mM HCO3− (right). After the first two CO2/HCO3− exposures, we restored the ND96 solution (not shown). For both oocytes, the solution flowed at 3 ml/min and the sampling rate was 1 per 500 ms. The vertical gray bands represent periods during which the pHS electrodes were withdrawn to the BECF for calibration. The dashed black lines through the initial portions of the pHi records for CO2 application represent best linear fits for maximal rates of pHi change (negative direction). The downward vertical arrows represent CO2-induced changes in steady-state pHi. The upward vertical arrows represent the maximal excursion of pHS (positive direction).
Fig. 10.
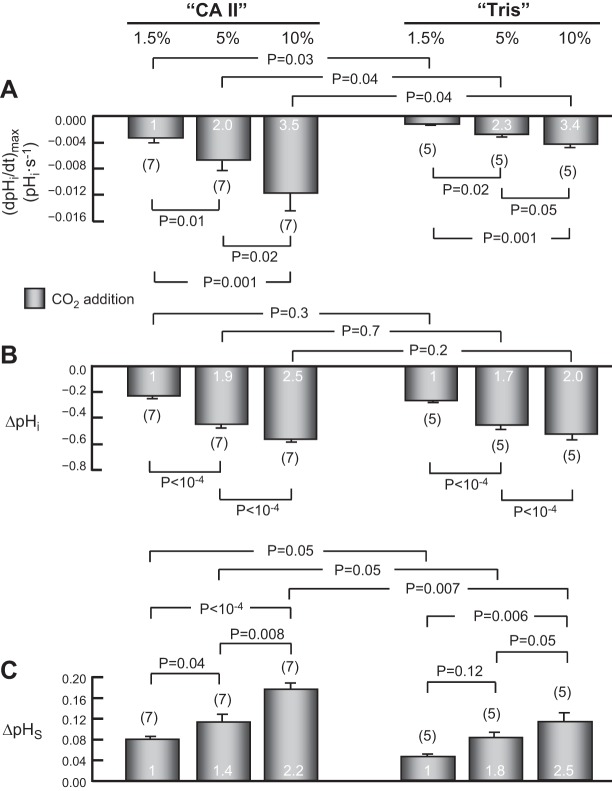
Summary of effects of graded increases of extracellular CO2/HCO3− levels on pHi and pHS. This figure summarizes results from a larger number of experiments, such as those in Fig. 9, on oocytes injected with CA II in Tris buffer, or only Tris buffer. A: maximal rates of pHi change (negative direction) produced by the introduction of extracellular CO2/HCO3−. B: changes in steady-state pHi induced by the addition of CO2/HCO3−. C: maximal changes in pHS (positive direction) caused by the addition of CO2/HCO3−. Values are means ± SE, with nos. of oocytes in parentheses. The white numerals are the ratios of mean values, relative to the value at 1.5% CO2. We performed a one-way ANOVA followed by a Student-Newman-Keuls (SNK) analysis (P shown for individual comparisons). The initial pHi values for “CA II” or “Tris” oocytes in the ND96 (i.e., CO2/HCO3−-free) solution were not significantly different (overall ANOVA, P = 0.84).
pHi changes.
Comparing 1.5% vs. 5% vs. 10% CO2, either for “CA II” or “Tris” oocytes, we see that graded increases in [CO2]BECF cause pHi to decrease faster (Fig. 10A) and to a greater extent (Fig. 10B). Increasing [CO2]BECF from 1.5% to 5% to 10%—in the ratios 1/∼3.3/∼6.6—increases the magnitude of (dpHi/dt)max in the ratios 1/∼2.0/∼3.5 for “CA II” oocytes and in virtually the same ratios (i.e., 1/∼2.3/∼3.4) for “Tris” oocytes (Fig. 10A, white numerals). These results are generally in agreement with the predictions of the mathematical model (see discussion).
Comparing “CA II” vs. “Tris” oocytes at a given CO2 level, we see that even though the steady-state ΔpHi is the same (Fig. 10B), the (dpHi/dt)max is significantly greater in “CA II” oocytes (Fig. 10A). These results are consistent with the 1.5%-CO2 data presented above in Fig. 3, A and B. However, because the pHi electrode is “cis” (i.e., “on the same side”) to the added CA II, and because this enzyme speeds the production of H+ from incoming CO2, these (dpHi/dt)max data per se do not provide intuitive insight into the effect of CA II on the CO2 influx.
From the magnitude of the CO2-induced decreases in steady-state ΔpHi, we can compute the intrinsic intracellular buffering power (βI). As summarized in Table 4, the βI values that we computed were very similar, whether we raised [CO2]BECF to 1.5%, 5%, or to 10%. An ANOVA indicated no significant difference among the values. Thus, βI must be virtually constant over the entire pHi range of our experiments (10). In addition, the ΔpHi data (Fig. 10B) reveal that, as expected, incremental increases in [CO2]BECF lead to incremental increase in the total net influx of CO2 integrated over the period of the CO2 exposure.
Table 4.
Nominal intrinsic intracellular buffering power (β)*
| Buffering Power (β) | “CA II” | “Tris” |
|---|---|---|
| 1.5% CO2/10 mM HCO3− | 14 ± 1 (7) | 14 ± 1 (5) |
| 5% CO2/33 mM HCO3− | 15 ± 1 (7) | 17 ± 3 (5) |
| 10% CO2/66 mM HCO3− | 17 ± 2 (7) | 15 ± 4 (5) |
Values are means ± SE (no. of experiments).
We computed β from the change in steady-state pHi caused by the addition of CO2/HCO3−, in oocytes injected with CA II protein or in others injected just with Tris buffer. Differences among values are not significant based on an ANOVA (P = 0.67).
pHS changes.
Comparing 1.5% vs. 5% vs. 10% CO2, either for “CA II” or “Tris” oocytes, we see that graded increases in [CO2]BECF (in the ratios 1/∼3.3/∼6.6) cause ΔpHS to rise in the ratios 1/∼1.4/∼2.2 for “CA II” oocytes and 1/1.8/2.5 for “Tris” oocytes (Fig. 10C). These results are in general qualitative agreement with the predictions of the mathematical model.
Comparing “CA II” vs. “Tris” oocytes at a given CO2 level, we see that ΔpHS is significantly greater in “CA II” than in “Tris” oocytes, consistent with the 1.5%-CO2 data presented above in Fig. 3C. Because the pHS electrode is “trans” to the added CA II enzyme, we can conclude, on the basis of intuition alone, that the increments in ΔpHS caused by CA II must reflect increases in CO2 influx across the plasma membrane. Figure 10C shows that intracellular CA II, over a wide range of CO2 levels, enhances the influx of CO2.
DISCUSSION
Previous investigators had used extracellular electrodes to monitor transient changes in pH caused by the addition of CO2/HCO3−to the fluid surrounding skeletal muscle fibers (31) or lamprey neurons (15). We have adapted their approach to track pHS as CO2 crosses the cell membrane of Xenopus oocytes. Previously, in the absence of exogenous CA, we used ΔpHS during CO2/HCO3− applications to assess the role of heterologously expressed channel proteins in enhancing the influx of CO2 into oocytes (14, 22, 24–26, 45). According to Fick's law, this increased flux could be due to an increase in CO2 permeability or to an increase in the CO2 transmembrane gradient. Inasmuch as aquaporin 1 (AQP1) (45) and the rhesus proteins (26) have no apparent CA-like activity, they increase ΔpHS by raising CO2 permeability (i.e., acting as gas channels). Nevertheless, even in gas-channel studies, the CO2 gradient is important. Indeed, in the work of Nakhoul et al. (47) on oocytes with intact vitelline membranes, the ability of AQP1 to increase CO2-induced (dpHi/dt)max did not become statistically significant until those authors injected CA II. Cooper and Boron (16) detected a statistically significant effect of AQP1 on CO2-induced (dpHi/dt)max in the absence of injected CA II, but only after removing the vitelline membrane, which reduces the extracellular unconvected layer. Both CA II injection and devitellinization presumably acted by increasing the transmembrane CO2 gradient.
The purpose of the present study was to better understand how transmembrane CO2 gradients, at a fixed permeability, affect CO2 fluxes. Specifically, we ask: 1) how CA II influences CO2 fluxes across the cell membrane (done by monitoring pHS) and 2) how CO2/HCO3− levels influence CO2-induced pHS transients. A key element in our approach was to monitor simultaneously both pHi and pHS. It is very likely that our pHS electrode creates a special environment—an enhancement of the natural extracellular unconvected fluid—between the outer surface of the cell membrane and the flat surface of the electrode. However, this special environment almost certainly communicates by diffusion with the surrounding EUF. Otherwise, we could not detect the pHS transients that we observe with the introduction or removal of extracellular CO2/HCO3−.
We find that cytosolic CA II accelerates the transient CO2 influx produced by raising [CO2]BECF as well as the transient CO2 efflux produced by lowering [CO2]BECF. The evidence for this acceleration is threefold: 1) a marked increase in the magnitude of maximal CO2-induced pHS changes, 2) a marked decrease in the τ for the pHi change following application (or removal) of CO2/HCO3−, and 3) a marked decrease in the τ for the relaxation of pHS from its maximum (or minimum) following application (or removal) of CO2/HCO3−. To our knowledge, ours is the first use of τpHi or τpHS to infer an increase in the flux of an acid-base-related species.
Some of our observations were predictable: 1) increasing the depth of penetration by the pHi electrode lengthens the time lag and increases time constant of measured pHi changes and 2) increasing [CO2]BECF/[HCO3−]BECF levels increases the rate and magnitude of the decrease in pHi and increases the magnitude of pHS trajectories.
In addition, we made three unanticipated observations: 1) the time constants for pHi changes are greater than those for pHS changes, 2) following a change in [CO2]BECF, pHi begins to change many seconds after pHS begins to change, and 3) during removal of CO2/HCO3− from “CA II” oocytes, the magnitudes of both the rate of pHi change and the pHS transient are smaller than those observed during the application of CO2/HCO3−.
To improve our understanding of the above seven issues, we extended a mathematical model of a spherical cell and of its surrounding environment (66) that allows one to simulate the effects of introducing (or removing) CO2/HCO3− to (or from) the BECF. The third paper in this series introduces extensions to the model of Somersalo et al. (66) that make it more realistic for oocytes. In the following sections, we present predictions of the extended model that are germane to the analysis and discussion of the CA II data in the present paper. The parameter values for the simulations are those of the “standard oocyte,” as defined in the third paper (49).
Effect of CA II on CO2 Fluxes
Importance of trans-side pH measurements.
Even if the injection of CA II did not enhance CO2 influx per se, we would expect the enzyme to increase (dpHi/dt)max because CA II, which is “cis” to the pHi electrode, catalyzes the overall intracellular reaction CO2 + H2O → HCO3− + H+. For example, preliminary work of Nakhoul et al. (48) showed that injecting CA II into Xenopus oocytes increases CO2-induced (dpHi/dt)max. However, this result, like the (dpHi/dt)max data in the present study, does not prove that CA II enhances CO2 influx. We can apply the same blanket statement about speeding pHi changes to any process that, in the presence of a CO2/HCO3− buffer, produces or consumes H+ (or OH−). This principle explains at least in part why others, working with cells expressing the Cl-HCO3 exchanger AE1, observed that the extracellular removal or re-addition of Cl− produces larger and faster pHi changes in the presence of active CA II (67). Although (dpHi/dt)max provides little intuitive insight on the effect of CA II on fluxes, a new contribution of the present paper is that the time constant for the pHi trajectory does provide such intuitive insight, as discussed below (section titled “Effects of CA II on time constants for pHi and pHS”).
In principle, we also could use an intracellular CO2 electrode to assess the effect of intracellular CA II on CO2 fluxes. However, a more practical alternative is to monitor pH at the extracellular surface, that is, on the side “trans” to the added CA II.
Effects of CA II on ΔpHS.
The fundamental observation in this paper is that introducing CA II into an oocyte increases the magnitude of ΔpHS, both with CO2 application or withdrawal (Fig. 2 and Fig. 3C). According to both the cartoon in Fig. 1 and the mathematical model, the higher ΔpHS magnitude in oocytes injected with CA II only could have been the result of three effects: 1) a reduced buffering power in the EUF, 2) a greater extracellular CA activity, or 3) a greater maximal CO2 influx (independent of extracellular CA activity). We know of no reason why the injection of CA II should have reduced βEUF. We can rule out an increase in extracellular EZA-sensitive CA activity because loading an oocyte with EZA, which entailed an EZA pretreatment followed by three washes (so that extracellular EZA was not present during the pHS assay), completely reverses the effects of CA II on pHi and pHS (Figs. 4 and 5). Thus, we are left with the third possibility: CA II must increase the ΔpHS magnitude by increasing the maximal CO2 flux, presumably as outlined in Fig. 1 and confirmed by our mathematical model.
The mathematical model predicts that, during the initial phase of CO2 influx7 (Fig. 11A, hatched area), cytosolic CA should decrease [CO2] at the intracellular surface of the membrane ([CO2]IM), thereby providing a larger gradient for CO2 influx, which should secondarily lead to a fall in [CO2] at the extracellular surface ([CO2]EM; Fig. 11B). However, two processes replenish CO2 at the outer membrane surface: 1) diffusion of CO2 from the BECF (which has no effect on pHS) and 2) the reactions HCO3− + H+ → H2CO3 → CO2 + H2O at the outer surface of the membrane (which raise pHS). As a result, ([CO2]EM) is less than it otherwise would have been. Nevertheless, the net effect is that, during the initial phase of CO2 influx, CA II increases the CO2 gradient (Δ[CO2]PM; Fig. 11C), thus causing a greater transient increase in pHS.
Fig. 11.
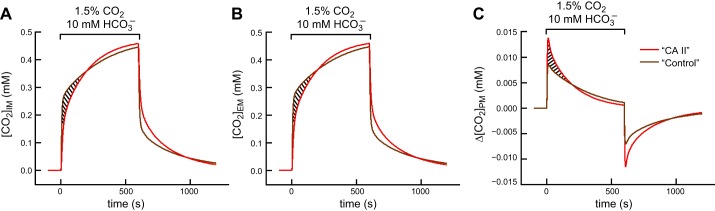
Predictions of the mathematical model for concentration-time profiles of CO2 for a “CA II” oocyte (red curves) and a “Control” or “Tris” oocyte (brown curves). A: [CO2] at the intracellular surface of the plasma membrane (IM). B: [CO2] at the extracellular surface of the plasma membrane (EM). C: gradient of CO2 across the plasma membrane (PM), the result of subtracting values in B from corresponding values in A. The hatched areas in A and B identify the initial phase of CO2 influx, during which the model predicts that cytosolic CA decreases [CO2] at the inner and outer surfaces of the plasma membrane. However, the dominant effect is at the inner membrane, so that CA provides a larger gradient for CO2 influx, as indicated by the hatched area in C. Details of the mathematical model are presented in the third paper in this series (49).
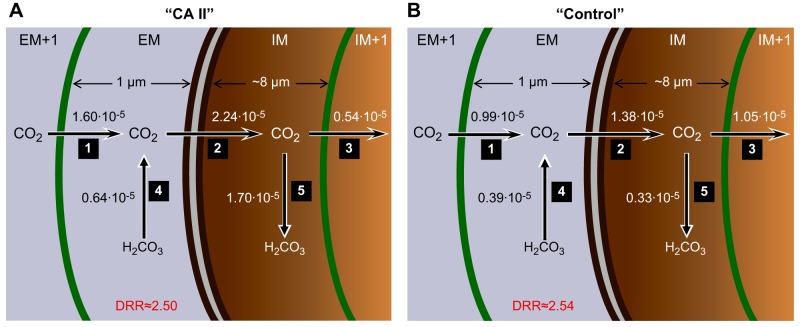
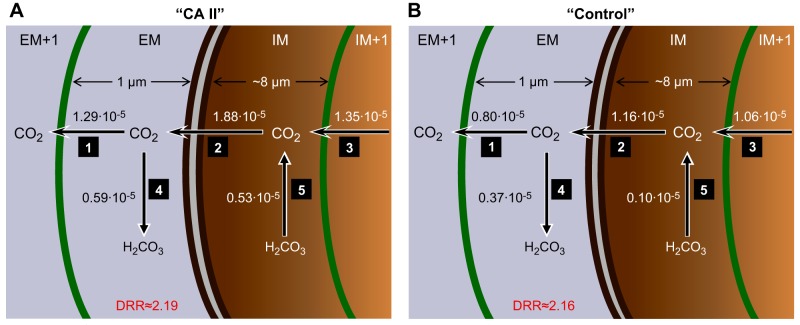
Figure 12 summarizes the effect of CA II on the predicted maximal CO2 diffusion and reaction rates near the membrane. Note that the model predicts that the diffusion/reaction ratio (DRR) for replenishing CO2 at the extracellular surface of the membrane is ∼2.5, regardless of whether cytosolic CA II is present (Fig. 12). As mentioned in the previous paragraph, the model predicts that the maximal transmembrane flux is higher in the presence of cytosolic CA II (compare arrow no. 2 of A vs. B) because the CA II in the IM region rapidly consumes incoming CO2 (compare arrow no. 5 of A vs. B). As a result of this rapid CO2 consumption, [CO2]IM is relatively low, accounting for the paradoxical decrease in CO2 flux from the IM region to the IM+1 region (compare arrow no. 3 of A vs. B).
Fig. 12.
Predictions of the mathematical model for maximal CO2 diffusion and reaction fluxes near the plasma membrane during addition of 1.5% CO2/10 mM HCO3−. A: “CA II” oocyte. B: “Control” or “Tris” oocyte. The parallel dark brown curves identify the plasma membrane (PM). The green curves to the left and right of the PM represent the spatial discretizations for the numerical solutions. The numbers near the arrows 1 through 5 indicate the maximal diffusive fluxes (sharp arrowheads) and reaction fluxes (dull arrowheads) in units of μmol/s. These maximal fluxes occur within ∼1 s of each other. The diffusion/reaction ratio (DRR) is the quotient of flux no. 1/flux no. 4. Details of the mathematical model are presented in the third paper in this series (49).
Effects of CA II on time constants for pHi and pHS.
CA II has no effect on the total net amount of CO2 entering or leaving the cell, as evidenced by the lack of an effect on the CO2-induced fall in steady-state ΔpHi (Fig. 3B). However, as we saw above, because we observe that CA II causes a larger maximal ΔpHS, we can conclude that CA II must increase the maximal CO2 flux. Therefore, CA II should reduce the total time necessary for CO2 to complete its equilibration across the plasma membrane, and thus reduce both the τ for the change in pHi and the τ for the decay of pHS from its peak. Indeed, this is what we observe under all experimental conditions (Fig. 6). Moreover, the mathematical model predicts this same trend for “CA II” vs. “control” (Fig. 13).
Fig. 13.
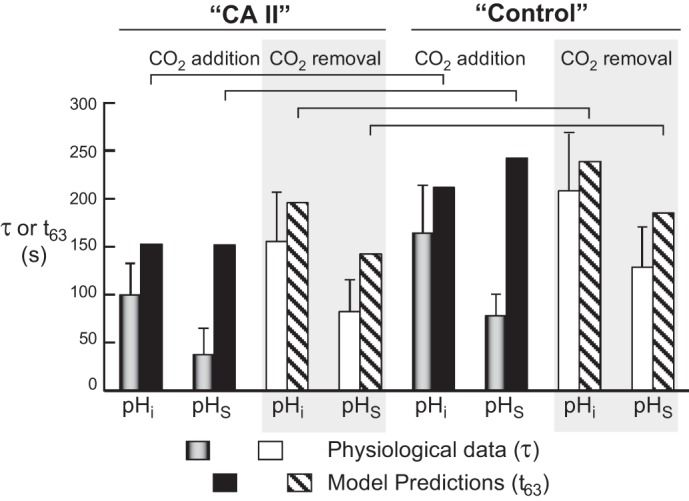
Comparison of physiological τ data with t63 data predicted by the mathematical model. The physiological data are taken from Fig. 6; note that here we report the means ± standard deviation (and not SE, as in Fig. 6). An apparent inconsistency arises when we compare pHS data from physiological experiments (time constant, τ) and simulations (time to 63% of completion, t63) for CO2 addition, under both CA II and control conditions. The inconsistency probably arises, in part, because of the multiexponential decay of the simulated pH trajectory, the special nature of the pHS trajectory beneath the pHS electrode, and the asymmetry of pHS over the oocyte surface. Details of the mathematical model are presented in the third paper in this series (49).
In our physiological experiments, both under “control” (“Tris”) and CA II conditions, we consistently see that the τ that describes the CO2-induced fall in pHi (τpHi) is much larger (i.e., slower) than the τ that describes the decay of pHS (τpHS). As described in the second paper in this series (46), this τpHi–τpHS difference probably reflects an asymmetrical [CO2]EUF over the surface of the oocyte. We suspect that pHS actually decays with more than one exponential, and that our τpHS values reflect only the large, rapid relaxation of pHS, and not the much smaller and slower decay that necessarily parallels the diffusion of CO2 to the center of the cell.
An apparent inconsistency between the model and the physiological data arises when we compare pHi and pHS data during CO2 addition, both under “CA II” and “control” conditions (Fig. 13). Note that we report the modeling data as t63, i.e., the time required for pHS to fall by 1 − (1/e) or ∼63%, to provide a simple measure of pH decay. However, because both computed pH trajectories, particularly pHS, approximate multiexponential decays, the t63 parameter is an imperfect reflection of the simulated pH trajectories. The simulation, of course, would benefit from further refinements. Finally, as noted above, the physiological data are probably influenced by [CO2]EUF asymmetries over the oocyte surface.
Evidence that oocytes have modest endogenous CA-like activity.
As described by Somersalo et al. (66), if oocytes totally lacked CA activity, then the addition of CO2/HCO3− would elicit a rapid rise of pHS, followed by an extremely rapid but partial decay of pHS, and then by a very slow pHS decay to pHBECF. Simulating a more natural pHS trajectory (e.g., Fig. 2A) requires assuming that the oocyte has modest CA activity both on the extracellular surface and in the cytoplasm. However, this endogenous intracellular CA activity must be insensitive to EZA because both Lu et al. (33) and we found that pretreating “Tris” oocytes with 400 μM EZA has no effect on a CO2-induced (dpHi/dt)max (Fig. 4C vs. D, Fig. 5A), even though this pretreatment completely blocks the sizeable effects of injected CA II protein. Note that Dahl et al. (17) report the presence of endogenous CA II protein in Xenopus oocytes and demonstrate that injected acetazolamide (ACZ) causes a slight but statistically significant decrease in the rate of pHi rise elicited by removing extracellular Cl−. Thus, it is possible that modest, endogenous CA II activity—sensitive to ACZ but not EZA-contributed to CO2—induced pHi (and to a lesser extent, pHS) transients in our experiments.
In the second paper in this series (46), we find that ACZ has no effect on ΔpHS in H2O-injected control oocytes. Thus, we can conclude that the modest, endogenous CA activity on the oocyte surface must be ACZ insensitive.
Likely effects of HCO3− transport on pHS.
In his experiments on lamprey neurons, Chesler (15) found that applying CO2/HCO3− did not cause a transient increase in pHS, but a decrease. Importantly, these neurons, unlike the oocytes in our experiments, had a powerful transport mechanism for the uptake of HCO3− (or an equivalent species, such as CO̿3). Such a HCO3−-uptake mechanism, by itself, would have caused pHS to decrease. We suggest that, in Chesler's experiments, the tendency of HCO3− influx to lower pHS outweighed the tendency of CO2 influx to raise pHS.
Effect of Varying CO2/HCO3− Levels
Effect on pHi.
In Figs. 9 and 10, we saw that, as we increase [CO2]BECF from 1.5% to 5% to 10% (ratios: 1/∼3.3/∼6.6), (dpHi/dt)max increases in smaller ratios (1/2.0–2.3/3.4–3.5), regardless of whether CA II is present.
The mathematical model predicts that, as [CO2]BECF increases, the maximal CO2 influx (which occurs at ∼9–12 s into the simulation) across the plasma membrane (arrow no. 2 in Fig. 12) should increase in the ratios 1/∼3.0–3.2/∼5.6–6.2 (Table 5, column “2”), which is only slightly less than the ratios of [CO2]BECF values. This small discrepancy almost certainly reflects the effect of CO2 diffusing across the EUF, inasmuch as the discrepancy nearly vanishes when we preequilibrate CO2/HCO3− throughout the EUF and then begin the simulations by suddenly allowing the CO2 to cross the membrane at time 0.
Table 5.
Parameter ratios from simulations at 1.5%, 5%, and 10% CO2
| Predicted Ratios |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| [CO2]BECF | (dpHi/dt)max | ΔpHS | 1 | 2 | 3 | 4 | 5 | (d[H+]IM/dt)max | (dpHIM/dt)max |
| “CA II” | |||||||||
| 1.5% (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5% (∼3.3) | 2.8 | 2.5 | 3.2 | 3.0 | 3.6 | 2.5 | 2.9 | 3.2 | 2.9 |
| 10% (∼6.6) | 4.2 | 3.9 | 6.3 | 5.6 | 7.5 | 3.9 | 5.2 | 5.1 | 4.3 |
| “Control” (Tris) | |||||||||
| 1.5% (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5% (∼3.3) | 3.1 | 2.6 | 3.5 | 3.2 | 3.2 | 2.6 | 3.2 | 3.2 | 3.0 |
| 10% (∼6.6) | 4.4 | 4.1 | 7.0 | 6.2 | 6.2 | 4.0 | 6.0 | 5.3 | 4.3 |
Ratios predicted by the mathematical model for “CA II” oocyte and “Control” (Tris) oocyte at the three different experimental conditions (i.e., 1.5% CO2, 5% CO2, and 10% CO2). The numerals at the top of the columns refer to the numbered arrows in Fig. 12, A (CA II) and B (Control). BECF, bulk extracellular fluid; (dpHi/dt)max, maximal rate of pHi change; (d[H+]IM/dt)max, maximum rate of change of [H+] in intracellular surface of the membrane.
Returning to the standard mathematical model, in which CO2 and HCO3− must diffuse across the EUF at the beginning of the simulation, we find that the maximal CO2 fluxes into the region adjacent to the extracellular surface of the membrane (EM) from the adjacent extracellular region (EM+1; arrow no. 1 in Fig. 12) increase in the ratios 1/3.2–3.5/6.3–7.0 (Table 5, column “1”). Similarly, the maximal CO2 fluxes from the region adjacent to the intracellular surface of the membrane (IM) to the adjacent intracellular region (IM+1; arrow no. 3 in Fig. 12) increase in similar ratios, 1/3.2–3.6/6.2–7.5 (Table 5, column “3”). Thus, we can conclude that CO2 diffusion near the membrane is not responsible for the relatively small ratios that we observe for (dpHi/dt)max in our physiological experiments.
When we examine the maximal fluxes through the reaction CO2 + H2O → H2CO3 in the IM region (arrow no. 5 in Fig. 12), we see that they increase in the ratios 1/2.9–3.2/5.2–6.0 (Table 5, column “5”). Because the ratios of CO2 diffusive fluxes (arrow nos. 1, 2, and 3) are near ideal, this small decrease in the ratios for the reaction flux (arrow no. 5) must be due to a rise in [H2CO3]IM, which in turn is likely due to an accumulation of H+ in “IM.”
When we examine the maximum rate of change of [H+] in “IM,” we see that the (d[H+]IM/dt)max rises in the ratios 1/3.1–3.2/5.1–5.3 [Table 5, “(d[H+]IM/dt)max” column], indicating a decrease in ratios at 10% CO2. Finally, when we examine (dpHIM/dt)max, the model predicts ratios of 1/2.9–3.0/4.3 [Table 5, “(dpHIM/dt)max” column], indicating an effect of converting rates of [H+] change to rates of pH change at 10% CO2. These (dpHIM/dt)max ratios in the “IM” region are virtually identical to those predicted at a depth of 50 μm, 1/∼2.8–3.1/∼4.2–4.4, regardless of the presence of CA II [see Table 5, “(dpHi/dt)max” column]. Indeed, we find that although the absolute magnitudes of (dpHi/dt)max decrease with increasing depth, the ratios of the (dpHi/dt)max values are not appreciably depth sensitive.
The (dpHi/dt)max values that we observed in our physiological experiments were smaller yet than those predicted by the model for a depth of 50 μm (compare Fig. 10 and Table 5). One explanation for this discrepancy is that we obtained physiological (dpHi/dt)max values in Fig. 10 not precisely at 10 s, but between ∼10 and ∼40 s into the electrophysiological experiments (during which time pHi was falling at an approximately steady rate). Indeed, the model predicts that the ratio of dpHi/dt values at 40 s into the simulation will be ∼10–26% less than those taken at 10 s. In fact, the dpHi/dt ratios predicted by the model at 50 μm and 40 s (not shown) are now rather close to those reported in Fig. 10A.
Effect on pHS.
In Figs. 9 and 10, we saw that, as we increase [CO2]BECF from 1.5% to 5% to 10% (1/∼3.3/∼6.6), ΔpHS increases in smaller ratios (1/1.4–1.8/2.2–2.5), regardless of whether CA II is present. In fact, these ratios are about 1/3 less than the (dpHi/dt)max ratios obtained in physiological experiments, and discussed above.
We have already seen in the previous section that the mathematical model predicts that, as [CO2]BECF increases, the maximal CO2 fluxes from “EM+1” to “EM” to “IM” to “IM+1” increase in nearly the same ratio as [CO2]BECF (Table 5, columns “1”, “2”, and “3”). However, when we examine the fluxes through the reaction H2CO3 → CO2 + H2O in “EM” region (arrow no. 4 in Fig. 12), we see that they increase in the ratios 1/2.5–2.6/3.9–4.0 (Table 5, column “4”), which are virtually identical to those for the predicted ΔpHS values, 1/∼2.5–2.6/∼3.9–4.1.
Even in the case of the mathematical model, the ratios of ΔpHS are somewhat smaller (∼7%) than the ratios of (dpHi/dt)max. However, in the case of the physiological data, the ΔpHS ratios can be as much as 37% smaller than the (dpHi/dt)max ratios. We think that the explanation for this larger discrepancy lies with the pHS electrode and the special environment it creates between the electrode tip and the oocyte membrane. When we introduce CO2/HCO3− into the BECF, CO2 and HCO3− diffuse from the BECF to the EUF that surrounds the electrode tip. As CO2 and HCO3− diffuse into the space between the electrode and membrane, a portion of the CO2 that has entered the space diffuses into the cell, lowering the local [CO2] and thus raising the local [HCO3−]/[CO2] ratio in the electrode/membrane space. As CO2 and HCO3− diffuse even deeper into the space between the electrode and membrane, and as more CO2 enters the cell, the local [HCO3−]/[CO2] ratio increases even further. Thus, on a relative scale, [CO2] should rise more slowly than [HCO3−] near the center of the pHS electrode, promoting the reactions HCO3− + H+ → H2CO3 → CO2 + H2O, and thus tending to amplify the ΔpHS value reported by the pHS electrode. However, as we proportionally raise [CO2]BECF and [HCO3−]BECF, the rate of the preceding reactions does not go up in the same proportions, either because of the special environment beneath the pHS electrode (which is not in our model) or because the native carbonic-anhydrase activity at the extracellular surface of the oocyte (which we did model) begins to saturate (19) with respect to local [HCO3−] (which we did not model). A combination of these factors puts a brake on the formation of CO2 and therefore the consumption of H+. Testing this prediction will require more sophisticated models of the interface between the electrode and cell membrane.
Asymmetry of pH Changes Upon Addition vs. Removal of CO2
In oocytes injected with CA II, CO2 addition, compared with CO2 removal, produced the following: 1) initial (dpHi/dt)max values whose magnitudes were greater and pHi trajectories whose time constants were smaller (faster) and 2) pHS spikes whose magnitudes were greater and that decayed with smaller τ values. In Tris-injected oocytes, we observed similar trends, but the differences did not reach statistical significance.
Effect on pHi.
Figure 3A shows that, in “CA II” oocytes, the magnitude of (dpHi/dt)max is only about two-thirds as great during CO2 removal (white bar) as during CO2 addition (gray bar). Figure 6 shows that the τ values for pHi are similarly larger (slower) for CO2 removal vs. addition. As far as we are aware, previous authors over the past four decades have not explicitly noted such differences in pHi data between CO2 addition and removal. However, in examining pHi-vs.-time records in the literature, both in studies in which one of us was a coauthor (9, 10, 28, 51, 57, 72, 73) as well as studies of others (1, 8, 20, 41, 43, 71), we find many examples in which the magnitude of (dpHi/dt)max was 40–170% greater for CO2 addition vs. CO2 removal.
One reason for the CO2-on/off disparity is clear from the first-generation mathematical model developed by Boron and De Weer (9), which ignores diffusion. That is, when CO2 first enters the cell following addition of extracellular CO2, pHi is relatively high (i.e., further away from the pK), so that a relatively high fraction (“1-α” in the Boron-De Weer model) of incoming CO2 will undergo the reaction CO2 + H2O → HCO3− + H+.
| (2) |
Thus, during the initial moments of the CO2 exposure, when pHi is relatively high (e.g., ∼7.22 in Fig. 2A), the influx of a bolus of CO2 generates a relatively large amount of H+, causing the cell to acidify relatively rapidly. Eventually, CO2 equilibrates between the extra- and intracellular fluid, and, if the effects of pHi regulation have been small, pHi is now lower than before CO2 was added (e.g., ∼6.93 in Fig. 2A). Upon the subsequent removal of extracellular CO2, the exit of CO2 leads to a depletion of cytosolic CO2, which is partially replenished via the net reaction HCO3− + H+ → CO2 + H2O. However, because pHi (and thus “1-α”) is now relatively low, the previous reaction replenishes a relatively small fraction of the exiting CO2, and thus consumes a relatively small amount of H+. Thus, assuming that βI is the same under both conditions, we expect the initial maximal rate of pHi rise with CO2 removal to be less than the initial maximal rate of pHi fall with CO2 addition. In our experiments in which we added 1.5% CO2, the mean initial pHi was ∼7.22 (1-α ≅ 0.929). Just before subsequently removing this 1.5% CO2, the mean steady-state pHi was ∼6.98 (1-α ≅ 0.884). Thus, the Boron-De Weer model predicts a slowing of the initial pHi trajectory for CO2 removal vs. addition of only [(0.929/0.884) − 1] or ∼5%. In fact, our physiological data reveal a slowing of ∼1/3.
Our present mathematical model predicts (dpHi/dt)max magnitudes that are 17–19% less for CO2 removal vs. addition (see Table 6). The model also predicts t63 values for pHi that are larger (slower) for CO2 removal vs. addition, both for “CA II” and “control” oocytes (Fig. 13), in agreement with the experimental results (Fig. 6). As to the mechanism, the model predicts a maximal CO2 efflux during CO2 removal (arrow no. 2 in Fig. 14) that is ∼16% less than the influx during CO2 addition (arrow no. 2 in Fig. 12). The difference in predictions between the Boron-De Weer model (∼5% asymmetry) and the present model must be due to the inherent asymmetry of the oocyte vs. its surroundings (discussed below) and diffusion. Indeed, increasing the width of the EUF from 10 μm to 100 μm causes the discrepancy of (dpHi/dt)max magnitudes between CO2 removal vs. addition to increase by ∼30%. Nevertheless, the present model cannot adequately account for the reduction of (dpHi/dt)max magnitude for CO2 removal vs. addition observed in our physiological experiments, and in the experiments of others. One possibility, discussed in the second paper in this series (46), is that convective flow over the oocyte creates asymmetries of [CO2] at the oocyte surface. Another possibility is that βI could be greater at low pHi (during the first moments of CO2 efflux) than at high pHi (during the first moments of CO2 influx), as has indeed been observed in some cell types (7, 10, 13).
Table 6.
Simulations of CO2 addition vs. removal
| “CA II” |
“Control” (Tris) |
|||
|---|---|---|---|---|
| (dpHi/dt)max | ΔpHS | (dpHi/dt)max | ΔpHS | |
| CO2 Addition | 0.0021 | 0.0501 | 0.0012 | 0.0307 |
| CO2 Removal | 0.0017 | 0.0481 | 0.0010 | 0.0324 |
Predictions of the mathematical model for (dpHi/dt)max and ΔpHS for CO2 addition and removal at 1.5% CO2.
Fig. 14.
Predictions of the mathematical model for maximal CO2 diffusion and reaction fluxes near the plasma membrane during removal of 1.5% CO2/10 mM HCO3−. A: “CA II” oocyte. B: “Control” or “Tris” oocyte. The parallel dark brown curves identify the plasma membrane (PM). The green curves to the left and right of the PM represent the spatial discretizations for the numerical solutions. The numbers near the arrows 1 through 5 indicate the maximal diffusive fluxes (sharp arrowheads) and reaction fluxes (dull arrowheads) in units of μmol/s. These maximal fluxes occur within ∼1 s of each other. The diffusion/reaction ratio (DRR) is the quotient of flux no. 1/flux no. 4. Details of the mathematical model are presented in the third paper in this series (49).
Effect on pHS.
Figure 3C shows that, in the presence of CA II, the magnitude of ΔpHS with CO2 removal (white bar) is only ∼40% as great as that for CO2 addition (gray bar). In the absence of CA II, the magnitudes of ΔpHS are not statistically significant different between CO2 addition vs. removal. Figure 6 shows that the τ values for pHS are smaller (faster) for CO2 addition vs. removal, both in the presence and absence of CA II.
A small part of the explanation for the pHS asymmetries between CO2 removal and addition comes from the mathematical model. The DRR is modestly smaller during CO2 efflux (Fig. 14A) than CO2 influx (Fig. 12A), indicating that the reaction is more important during efflux. However, because the maximal transmembrane CO2 flux is smaller during efflux (arrow no. 2 in Fig. 14A) than influx (arrow no. 2 in Fig. 12A), the overall effect is a decrease in reaction rate. Thus, in the presence of CA II, the reaction CO2 + H2O → H2CO3 during CO2 removal (arrow no. 4 in Fig. 14A) is ∼8% slower than the opposite reaction H2CO3 → CO2 + H2O during CO2 addition (arrow no. 4 in Fig. 12A). As summarized in Table 6, this asymmetry in reaction rates translates to an even smaller asymmetry of ΔpHS magnitude (∼4%) between CO2 efflux vs. influx, only about 1/10th of the observed asymmetry of 40%.
What, then, is the explanation for the remaining ∼90% of the pHS asymmetry with CO2 addition vs. removal? We suggest that two elements are essential: 1) The special environment between the pHS electrode and the oocyte membrane, which our present model does not include. Our present model simulates pH over the vast majority of the oocyte surface, whereas the pHS electrodes reports pHS only beneath the electrode tip. 2) The relatively low CA activity on the outer surface of the native oocyte plasma membrane. Indeed, as reported in the second paper in the series (46), and as confirmed by the model, we find that expressing CA IV on the cell surface eliminates the CO2 off/on asymmetry for ΔpHS.
How do these two elements conspire to produce the large pHS asymmetry? CO2 efflux is not the mirror image of CO2 influx. During the first instants of influx, CO2 diffuses from an infinite reservoir to a cell (a blind alley) that is CO2 free, whereas during the first instants of efflux, CO2 diffuses from the cell into the EM region that still contains considerable CO2. Indeed, we already saw that the maximal efflux (arrow no. 2 in Fig. 14A) is smaller than the maximal influx (arrow no. 2 in Fig. 12A). We suggest that during CO2 efflux, particularly beneath the pHS electrode, CO2 builds up near the outer surface of the cell membrane and reduces the local transmembrane flux, thereby reducing the reaction CO2 + H2O → HCO3− + H+ at the cell surface, and thus the magnitude of the transient fall in pHS. In oocytes expressing CA IV, the enzyme consumes enough of the exiting CO2 to maintain a low [CO2] beneath the electrode, thereby maintaining a gradient for CO2 efflux. Figures 20 and 22 in the second paper of this series (46) support this explanation.
Effect of Electrode Depth on CO2-Induced pHi Changes
Even though we inserted the pHi electrode as superficially as possible, we hypothesize that two unexpected findings in the present study, namely, 1) the delay between the onsets of the pHS and pHi changes (see Table 2) and 2) the larger (slower) τ values for the pHi vs. the pHS changes (see Fig. 6), reflect the depth of impalement of the pHi electrode. To test this hypothesis, we performed experiments with dual pHi electrodes, one superficial and one deep.
Effects on pHi delay.
The delay between an extracellular solution change and a pHi response, which almost certainly occurred in previous oocyte studies from our laboratory and other laboratories, is not easy to recognize without a parallel indicator of the BECF solution change, pHS in the present study.
Figure 7 and Fig. 8A show that, starting from the “0” position (i.e., outer surface of cell), advancing a second pHi microelectrode (electrode no. 2) to a total depth of ∼300 μm deeper into the oocyte increases the time lag (i.e., pHi delay)—with or without CA II, with application or removal of CO2. We do not know the precise depths of the tips of either electrode no. 1 (advanced ∼40 μm beyond the “0” position) or no. 2 because the electrode tips could drag the plasma membrane deeper into the oocyte. Thus, the actual depth beneath the local plasma membrane could be less than the apparent depth. Nevertheless, it is reasonable to conclude from Figs. 7 and 8A that an increased depth of impalement exaggerates delay.
The mathematical model makes the following predictions for the application of CO2: 1) Immediately beneath the cell membrane, pH should begin to fall virtually instantaneously. 2) At progressively greater depths, pH should remain relatively stable for a progressively longer time period before beginning the transition to a near-linear phase of decline, as simulated for several electrode depths in Fig. 15.
Fig. 15.
Predictions of the mathematical model for different depths of impalement, during the application of 1.5% CO2/10 mM HCO3− to a “Control” or “Tris” oocyte. We report pHi trajectories for the first 60 s for six depths (∼50, 100, 150, 200, 250, and 300 μm) for a hypothetical oocyte having a radius of 650 μm. At progressively greater depths, pHi remains relatively stable for progressively longer time periods before beginning the transition to a near-linear phase of decline. Details of the mathematical model are presented in the third paper in this series (49).
How deep would the electrode tip have to be to account for a delay (averaged for CA II and Tris experiments) of ∼9 s for CO2 addition (see Table 2)? In addressing this question using the mathematical simulations, we initially omit the implementation of vesicles beneath the oocyte membrane (present for the simulation in Fig. 15) by reducing the tortuosity factor (λ) beneath the membrane from ∼3.16 (representing our “standard oocyte”) to 1. This implementation of the model (λ = 1) predicts that the electrode tip would have to be ∼150–200 μm beneath the cell membrane. This figure is unreasonably deep, considering that in the experiment of Fig. 7A we advanced pHi electrode no. 2 only ∼40 μm beyond the “0” position. Therefore, we suspected that a relatively superficial portion of the oocyte's cytoplasm offers considerable resistance to the diffusion of CO2 and other solutes. Indeed, light micrographs of oocytes show a layer of vesicles, beginning ∼10 μm below the plasma membrane and extending an additional ∼25 μm into the oocyte (38). In the mathematical model, implementing such a band by reducing the diffusion of all solutes by the same tortuosity factor (λ∼6.32) can by itself account for the observed delay of ∼9 s for CO2 addition at a more realistic depth of 50 μm into the oocyte. In the third paper in this series (49), we will explain how we came to choose a λ of ∼3.16 for our standard oocyte.
Figure 16 (a simulation of Fig. 7) shows the predicted pHi trajectories for “CA II” and “control” oocytes at depths of ∼50 μm and ∼300 μm (assuming a λ of ∼3.16). Figure 17A shows a comparison of the physiological data and mathematical predictions for the difference between pHi time lags at depths of ∼300 μm and ∼50 μm. The model confirms that the time lag (and thus the delay) increases with the depth of impalement—with or without CA II, with application or removal of CO2.
Fig. 16.
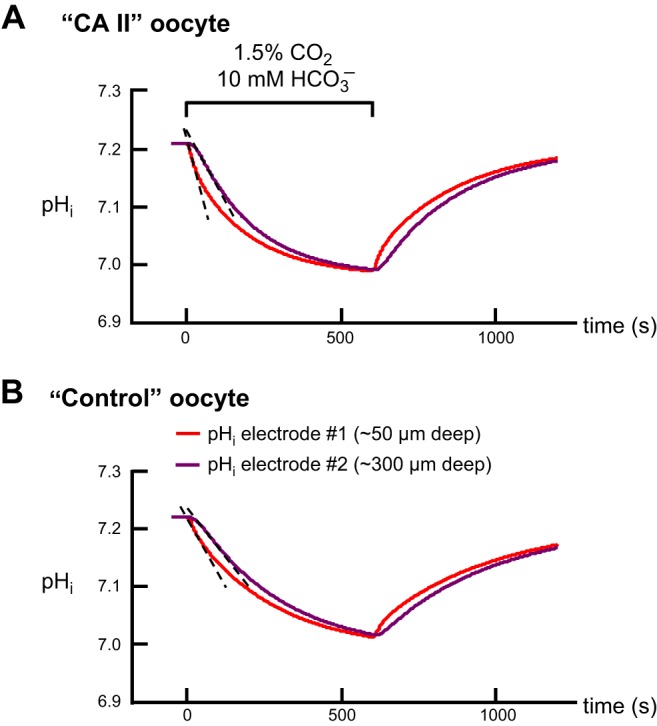
Predictions of the mathematical model for pHi trajectories at depths of ∼50 μm and ∼300 μm. A: simulations for a “CA II” oocyte. B: simulations for a “Control” or “Tris” oocyte. In these simulations, which are comparable to the physiological experiments in Fig. 7, the tortuosity factor (λ) used to simulate a layer of intracellular vesicles is ∼3.16. Details of the mathematical model are presented in the third paper in this series (49).
Fig. 17.
Comparison of predictions of the model, at depths of ∼50 μm (S, Superficial) and ∼300 μm (D, Deep), and the physiological data. A: difference (Deep-Superficial) between pHi time lags. B: (dpHi/dt)max values. C: time constants from physiological experiments or time to 63% of completion (i.e., t63) for simulations. The gray background identifies data for “CA II” oocytes; the absence of background color identifies “Control” or “Tris” oocytes. The physiological data are taken from Fig. 8; note that here we report means ± standard deviation (and not SE, as in Fig. 8).
Effects on (dpHi/dt)max and τ.
The second unexpected finding that, we hypothesize, reflects the depth of impalement is that, both for application and withdrawal of CO2, τ is always larger (slower) for pHi than for pHS (Fig. 6). Compared with electrode no. 1 (∼40 μm beyond the “0” position), electrode no. 2 (∼300 μm beyond the “0” position) reports a significantly smaller (dpHi/dt)max magnitude and larger τ (Fig. 7 and Fig. 8, B and C) in “CA II” oocytes, both for application and removal of CO2. The effects of depth did not reach statistical significance in an ANOVA for “Tris” oocytes. Viewed differently, CA II significantly speeds the CO2-induced pHi changes reported by the superficial electrode, but the effects of CA II did not reach statistical significance for the deeper electrodes.
As summarized in Fig. 17B (comparable to Fig. 8B), the mathematical model is in good agreement with the physiological (dpHi/dt)max data except for CO2 addition in the presence of CA II, where the difference is slightly more than one standard deviation. In fact, for this condition, the model (for an electrode depth of 50 μm) agrees very well with the (dpHi/dt)max data for CO2 addition/CA II averaged over the entire study (Fig. 3A). The reason for the difference in mean values in Fig. 3A vs. Fig. 8B is probably that the superficial electrode no. 1 was less deep for the data summarized in Fig. 8B.
As summarized in Fig. 17C (comparable to Fig. 8C), the mathematical model agrees well with all of the control τ data and with the CA II data for the deep electrode. However, the physiological τ values for the superficial electrode no. 1 in CA II experiments are much smaller (faster) than predicted by the model for an electrode depth of 50 μm. Again, this difference probably reflects a relatively shallow electrode depth for the subset of oocytes summarized in Fig. 8C vs. the overall data summarized in Fig. 6. Indeed, the agreement between the model and the physiological τ data is much better for the data in Fig. 6.
Conclusions
Injected CA II increases the magnitude of the pHS spike—a semiquantitative index of peak, transmembrane CO2 flux—both during CO2 addition and removal, and EZA reverses these effects. The mathematical model predicts that CA II maintains a relatively low [CO2]i during CO2 influx, and a relatively high [CO2]i during efflux, thereby maximizing CO2 gradients, and thus CO2 fluxes and magnitudes of (dpHi/dt)max.
The time constant for the decay of pHi is larger (slower) than that of pHS, reflecting in part the depth of the tip of the pHi electrode, even during “superficial” impalements.
The ΔpHS magnitude increases with [CO2]BECF, but less than proportionally.
As an index of peak CO2 flux, ΔpHS approach offers the practical advantage of requiring a relatively simple and reproducible measurement. Moreover, ΔpHS measurements provide intuitive insights into 1) the effects of cytosolic CA on CO2 fluxes and 2) reaction-diffusion events involving acids and bases at the outer surface of the membrane, near many membrane proteins. Although ΔpHS is presently only a semiquantitative index of the absolute maximal CO2 flux (or permeability), it is nonetheless invaluable for assessing CO2 permeability through channels. More sophisticated mathematical models could in principle extract CO2 permeabilities from ΔpHS data. In principle, one could use pH-sensitive fluorescent dyes, confined to the extra- or intracellular cell surface. We predict that the dye-derived pHS values from isolated cells would be smaller than electrode-derived pHS values because of the special environment created beneath the electrode. Nevertheless, this special environment may provide insight into events in narrow extracellular spaces.
The traditional approach of determining the maximal CO2 flux from the maximal slope of the pHi vs. time record has the disadvantage of requiring that one use a curve-fitting procedure with several (time, pHi) points. The fitted (dpHi/dt)max value depends on one's judgment of the region to be fitted and the depth of pHi-electrode impalement. In the case of pHi measurements based on cytosolic fluorescent dyes, (dpHi/dt)max values represent a mean over the volume of observation. In either case, (dpHi/dt)max provides limited intuitive insight into CA II activity because the measurement is “cis” to the enzyme.
Measurements of pHi are intrinsically valuable for assessing transporter-mediated acid-base fluxes as well as intracellular buffering power. Thus, the combination of pHi and pHS approaches provides a depth of insight that is not possible from either modality alone.
GRANTS
R. Musa-Aziz was supported, for a part of this project, by a fellowship from the American Heart Association (0625891T) and by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP no. 08/128663). R. Occhipinti was supported by a fellowship from the American Heart Association (11POST7670015). W. F. Boron gratefully acknowledges the support of the Myers/Scarpa endowed chair. This work was supported by National Institutes of Health Grants NS-18400 and DK-81567 as well as Office of Naval Research Grants N00014-05-1-0345, N00014-08-1-0532, and N00014-11-1-0889 to W. F. Boron.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.M.A., R.O., and W.F.B. conception and design of research; R.M.A. and R.O. performed experiments; R.M.A. and R.O. analyzed data; R.M.A., R.O., and W.F.B. interpreted results of experiments; R.M.A. and R.O. prepared figures; R.M.A. and W.F.B. drafted manuscript; R.M.A., R.O., and W.F.B. edited and revised manuscript; R.M.A., R.O., and W.F.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Duncan Wong for helpful discussions and computer support; Dr. Bruce Davis for helpful discussions; Victor Pantani for custom design, fabrication, help with troubleshooting, and repair of some of the electronic instruments; Christopher Daily for technical assistance; Dr. Peter Piermarini for providing the CA II protein; Steven Torontali for designing and fabricating the oocyte chamber; Gerald Babcock for technical assistance; Dale Huffman for engineering assistance; as well as Charleen Bertolini and Rosalyn Forster for administrative support. We acknowledge the early preliminary work of Nakhoul et al. (48) and thank two authors of that work, Nazih Nakhoul and Michael Romero, for helpful discussions.
Footnotes
This article is the topic of an Editorial Focus by Eric Delpire (18a).
In the companion paper (46), we tested these predictions concerning the extracellular buffer.
Our algorithm generally produced excellent single-exponential fits, although sometimes we were unsuccessful because 1) the magnitude of the ΔpHS was too small in a “Tris” oocyte, yielding an inadequate signal-to-noise ratio; 2) we had an insufficient number of points to fit either because of an electrical-noise transient or because we moved the pHS electrode away from the surface for calibration. We obtained a full set of four fits (CO2 addition and removal for both pHi and pHS) for a total of 12 (of 16) CA II and 8 (of 16) Tris experiments.
These oocytes were injected with H2O (our usual control that we employ in comparisons with oocytes injected with cRNA) 1 day after the oocytes were isolated.
Note that the time lag (between switching of valve and initiation of pHi transient), which includes the transit time of the solution from the valve to the chamber, is much longer than the time delay (between initiation of the pHS and pHi transients).
Parameters such as the following would have to be invariant: initial pHi and pHBECF; concentrations and pK values of non-CO2/HCO3− buffers in BECF and cytosol; CA activity in BECF, outer cell surface, and cytosol.
The opposite would be true during CO2 efflux.
For the first 5 CA II experiments and first 4 Tris experiments, extracellular solution flowed at 2 ml/min and the data collection period was 1 per 2,000 ms. For the next 5 CA II and 6 Tris experiments, we decreased the data collection period to 1 per 1,000 ms. For the final 6 CA II and 6 Tris experiments (and all other experiments for this paper), we increased flow to 3 ml/min and reduced the data collection period to 1 per 500 ms. ANOVA analyses revealed no significant differences among initial CO2-induced (dpHi/dt)max, CO2-induced ΔpHi, or CO2-induced ΔpHS data sets, for either “CA II” or “Tris” oocytes.
REFERENCES
- 1.Aalkjaer C, Cragoe EJ, Jr. Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J Physiol 402: 391–410, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker B. Decrease in intraocular pressure in man by a carbonic anhydrase inhibitor, diamox; a preliminary report. Am J Ophthalmol 37: 13–15, 1954 [DOI] [PubMed] [Google Scholar]
- 3.Becker EL, Hodler JE, Fishman AP. Effect of carbonic anhydrase inhibitor on arterial-alveolar CO2 gradient in man. Proc Soc Exp Biol Med 84: 193–195, 1953 [DOI] [PubMed] [Google Scholar]
- 4.Beckman WW, Rossmeisl EC, Pettengill RB, Bauer W. A study of the effects of sulfanilamide on acid-base metabolism. J Clin Invest 19: 635–644, 1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell AL, Jr, Smith CN, Andreae E. Effects of the carbonic anhydrase inhibitor “6063” (diamox) on respiration and electrolyte metabolism of patients with respiratory acidosis. Am J Med 18: 536–546, 1955 [DOI] [PubMed] [Google Scholar]
- 6.Berliner RW, Orloff J. Carbonic anhydrase inhibitors. Pharmacol Rev 8: 137–174, 1956 [PubMed] [Google Scholar]
- 7.Bevensee MO, Weed RA, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role of HCO3−. J Gen Physiol 110: 453–465, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton TB, Vaughan-Jones RD. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol 270: 801–833, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67: 91–112, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boron WF. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol Cell Physiol 233: C61–C73, 1977 [DOI] [PubMed] [Google Scholar]
- 11.Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol 17: 2368–2382, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Chen JC, Chesler M. pH transients evoked by excitatory synaptic transmission are increased by inhibition of extracellular carbonic anhydrase. Proc Natl Acad Sci USA 89: 7786–7790, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LK, Boron WF. Acid extrusion in S3 segment of rabbit proximal tubule. I. Effect of bilateral CO2/HCO3−. Am J Physiol Renal Fluid Electrolyte Physiol 268: F179–F192, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Chen LM, Zhao J, Musa-Aziz R, Pelletier MF, Drummond IA, Boron WF. Cloning and characterization of a zebrafish homologue of human AQP1: a bifunctional water and gas channel. Am J Physiol Regul Integr Comp Physiol 299: R1163–R1174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesler M. Regulation of intracellular pH in reticulospinal neurones of the lamprey, Petromyzon marinus. J Physiol 381: 241–261, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper GJ, Boron WF. Effect of pCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol Cell Physiol 275: C1481–C1486, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Dahl NK, Jiang L, Chernova MN, Stuart-Tilley AK, Shmukler BE, Alper SL. Deficient HCO3− transport in an AE1 mutant with normal Cl− transport can be rescued by carbonic anhydrase II presented on an adjacent AE1 protomer. J Biol Chem 278: 44949–44958, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda) 25: 239–249, 2010 [DOI] [PubMed] [Google Scholar]
- 18a.Delpire E. How carbonic anhydrases and pH buffers facilitate the movement of carbon dioxide through biological membranes. Focus on “Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes”; “Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes”; and “Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes.” Am J Physiol Cell Physiol (June 25, 2014). 10.1152/ajpcell.00198.2014 [DOI] [PubMed] [Google Scholar]
- 19.DeVoe H, Kistiakowsky GB. The enzymic kinetics of carbonic anhydrase from bovine and human erythrocytes. J Am Chem Soc 83: 274–280, 1961 [Google Scholar]
- 20.Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279: 52238–52246, 2004 [DOI] [PubMed] [Google Scholar]
- 21.DuBose TD, Jr. Carbonic anhydrase-dependent bicarbonate transport in the kidney. Ann NY Acad Sci 429: 528–537, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Endeward V, Musa-Aziz R, Cooper GJ, Chen LM, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J 20: 1974–1981, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Fujikawa-Adachi K, Nishimori I, Taguchi T, Yuri K, Onishi S. cDNA sequence, mRNA expression, and chromosomal localization of human carbonic anhydrase-related protein, CA-RP XI. Biochim Biophys Acta 1431: 518–524, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Geyer RR, Musa-Aziz R, Enkavi G, Mahinthichaichan P, Tajkhorshid E, Boron WF. Movement of NH3 through the human urea transporter B: a new gas channel. Am J Physiol Renal Physiol 304: F1447–F1457, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geyer RR, Musa-Aziz R, Qin X, Boron WF. Relative CO2/NH3 selectivities of mammalian aquaporins 0–9. Am J Physiol Cell Physiol 304: C985–C994, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Geyer RR, Parker MD, Toye AM, Boron WF, Musa-Aziz R. Relative CO2/NH3 permeabilities of human RhAG, RhBG and RhCG. J Membr Biol 246: 915–926, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol 207: 266–279, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem 276: 8358–8363, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Gutknecht J, Bisson MA, Tosteson FC. Diffusion of carbon dioxide through lipid bilayer membranes: effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol 69: 779–794, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutknecht J, Tosteson DC. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science 182: 1258–1261, 1973 [DOI] [PubMed] [Google Scholar]
- 31.De Hemptinne A, Huguenin F. The influence of muscle respiration and glycolysis on surface and intracellular pH in fibres of the rat soleus. J Physiol 347: 581–592, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imtaiyaz Hassan M, Shajee B, Waheed A, Ahmad F, Sly WS. Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med Chem 21: 1570–1582, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Kang TM, Markin VS, Hilgemann DW. Ion fluxes in giant excised cardiac membrane patches detected and quantified with ion-selective microelectrodes. J Gen Physiol 121: 325–347, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato K. Sequence of a novel carbonic anhydrase-related polypeptide and its exclusive presence in Purkinje cells. FEBS Lett 271: 137–140, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Knickelbein R, Aronson PS, Schron CM, Seifter J, Dobbins JW. Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl-HCO3 exchange and mechanism of coupling. Am J Physiol Gastrointest Liver Physiol 249: G236–G245, 1985 [DOI] [PubMed] [Google Scholar]
- 36.Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev 92: 39–74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindskog S, Nyman PO. Metal-binding properties of human erythrocyte carbonic anhydrases. Biochim Biophys Acta 85: 462–474, 1964 [DOI] [PubMed] [Google Scholar]
- 38.Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, Boron WF. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem 281: 19241–19250, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Maren TH, Jankowska L, Sanyal G, Edelhauser HF. The transcorneal permeability of sulfonamide carbonic anhydrase inhibitors and their effect on aqueous humor secretion. Exp Eye Res 36: 457–479, 1983 [DOI] [PubMed] [Google Scholar]
- 40.Maren TH. The kinetics of HCO3− synthesis related to fluid secretion, pH control, and CO2 elimination. Annu Rev Physiol 50: 695–717, 1988 [DOI] [PubMed] [Google Scholar]
- 41.McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol 127: 639–658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meldrum NU, Roughton FJ. Carbonic anhydrase. Its preparation and properties. J Physiol 80: 113–142, 1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulkey DK, Henderson RA, 3rd, Ritucci NA, Putnam RW, Dean JB. Oxidative stress decreases pHi and Na+/H+ exchange and increases excitability of solitary complex neurons from rat brain slices. Am J Physiol Cell Physiol 286: C940–C951, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Musa-Aziz R, Boron WF, Parker MD. Using fluorometry and ion-sensitive microelectrodes to study the functional expression of heterologously-expressed ion channels and transporters in Xenopus oocytes. Methods 51: 134–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106: 5406–5411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Musa-Aziz R, Occhipinti R, Boron WF. Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes. Am J Physiol Cell Physiol (June 25, 2014). 10.1152/ajpcell.00050.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol Cell Physiol 274: C543–C548, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Nakhoul NL, Romero MF, Waheed A, Davis BA, Mullins R, Hageman G, Sly WS, Boron WF. Processing and functional expression of carbonic anhydrase isoforms in Xenopus laevis oocytes (Abstract). FASEB J 10: A88, 1996 [Google Scholar]
- 49.Occhipinti R, Musa-Aziz R, Boron WF. Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes. Am J Physiol Cell Physiol (June 25, 2014). 10.1152/ajpcell.00049.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okamoto N, Fujikawa-Adachi K, Nishimori I, Taniuchi K, Onishi S. cDNA sequence of human carbonic anhydrase-related protein, CA-RP X: mRNA expressions of CA-RP X and XI in human brain. Biochim Biophys Acta 1518: 311–316, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Piermarini PM, Choi I, Boron WF. Cloning and characterization of an electrogenic Na/HCO3− cotransporter from the squid giant fiber lobe. Am J Physiol Cell Physiol 292: C2032–C2045, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Piermarini PM, Kim EY, Boron WF. Evidence against a direct interaction between intracellular carbonic anhydrase II and pure C-terminal domains of SLC4 bicarbonate transporters. J Biol Chem 282: 1409–1421, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Praetorius J. Na+-coupled bicarbonate transporters in duodenum, collecting ducts and choroid plexus. J Nephrol 23, Suppl 16: S35–S42, 2010 [PubMed] [Google Scholar]
- 54.Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int 71: 103–115, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Rivera-Ch M, Huicho L, Bouchet P, Richalet JP, León-Velarde F. Effect of acetazolamide on ventilatory response in subjects with chronic mountain sickness. Respir Physiol Neurobiol 162: 184–189, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature 387: 409–413, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Roos A, Boron WF. Intracellular pH. Physiol Rev 61: 296–434, 1981 [DOI] [PubMed] [Google Scholar]
- 59.Saarikoski J, Kaila K. Simultaneous measurement of intracellular and extracellular carbonic anhydrase activity in intact muscle fibres. Pflügers Arch 421: 357–363, 1992 [DOI] [PubMed] [Google Scholar]
- 60.Scozzafava A, Supuran CT. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell Biochem 75: 349–359, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Siebens AW, Boron WF. Effect of electroneutral luminal and basolateral lactate transport on intracellular pH in salamander proximal tubules. J Gen Physiol 90: 799–831, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh SK, Binder HJ, Boron WF, Geibel JP. Fluid absorption in isolated perfused colonic crypts. J Clin Invest 96: 2373–2379, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sjöblom B, Elleby B, Wallgren K, Jonsson BH, Lindskog S. Two point mutations convert a catalytically inactive carbonic anhydrase-related protein (CARP) to an active enzyme. FEBS Lett 398: 322–325, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Skaggs LA, Bergenhem NC, Venta PJ, Tashian RE. The deduced amino acid sequence of human carbonic anhydrase-related protein (CARP) is 98% identical to the mouse homologue. Gene 126: 291–292, 1993 [DOI] [PubMed] [Google Scholar]
- 65.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 64: 375–401, 1995 [DOI] [PubMed] [Google Scholar]
- 66.Somersalo E, Occhipinti R, Boron WF, Calvetti D. A reaction-diffusion model of CO2 influx into an oocyte. J Theor Biol 309: 185–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem 276: 47886–47894, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Supuran CT. Carbonic anhydrases–an overview. Curr Pharm Des 14: 603–614, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Swenson ER. Carbonic anhydrase inhibitors and high altitude illnesses. Subcell Biochem 75: 361–386, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Tanhauser SM, Jewell DA, Tu CK, Silverman DN, Laipis PJ. A T7 expression vector optimized for site-directed mutagenesis using oligodeoxyribonucleotide cassettes. Gene 117: 113–117, 1992 [DOI] [PubMed] [Google Scholar]
- 71.Thomas RC. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol 255: 715–735, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Virkki LV, Choi I, Davis BA, Boron WF. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol 285: C771–C780, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO3− cotransporter (NBCe2). Am J Physiol Cell Physiol 282: C1278–C1289, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Wright AD, Bradwell AR, Fletcher RF. Methazolamide and acetazolamide in acute mountain sickness. Aviat Space Environ Med 54: 619–621, 1983 [PubMed] [Google Scholar]