Abstract
Meal-fed (MF) rats with access to food for only 4 consecutive hours during the light cycle learn to eat large meals to maintain energy balance. MF animals develop behavioral and endocrine changes that permit glucose tolerance despite increased meal size. We hypothesized that enhanced activity of the enteroinsular axis mediates glucose homeostasis during MF. Cohorts of rats were allocated to MF or ad libitum (AL) regimens for 2–4 wk. Insulin secretion and glucose tolerance were determined after oral carbohydrate and intraperitoneal (ip) and intravenous (iv) glucose. MF rats ate less than AL in the first week but maintained a comparable weight trajectory thereafter. MF rats had decreased glucose excursions after a liquid mixed meal (AUC: MF 75 ± 7, AL 461 ± 28 mmol·l−1·min, P < 0.001), with left-shifted insulin secretion (AUC0–15: MF 31.0 ± 4.9, AL 9.6 ± 4.4 pM·min, P < 0.02), which peaked before a significant rise in blood glucose. Both groups had comparable fasting glucagon levels, but postprandial responses were lower with MF. However, neither intestinal expression of proGIP and proglucagon mRNA nor plasma incretin levels differed between MF and AL groups. There were no differences in the insulin response to ip or iv glucose between MF and AL rats. These findings demonstrate that MF improves oral glucose tolerance and is associated with significant changes in postprandial islet hormone secretion. Because MF enhanced β-cell function during oral but not parenteral carbohydrate administration, and was not accounted for by changes in circulating incretins, these results support a neural mechanism of adaptive insulin secretion.
Keywords: meal feeding, time-restricted feeding, glucose tolerance, cephalic insulin secretion, insulin sensitivity
blood glucose concentration is tightly regulated in healthy animals, and the complex, multilayered homeostatic system that has evolved indicates that euglycemia is important for health and normal function. Elevated glucose levels are associated with both short-term complications (e.g., hyperosmolarity, fluid shifts, glucosuria) and long-term pathology (vascular and neuropathic damage). Under normal circumstances, the major challenge to glucose homeostasis is nutrient ingestion (38, 39), which can involve the absorption of amounts of carbohydrate that far exceed the basal circulating glucose pool. To avoid hyperglycemia and promote efficient storage and oxidation of meal carbohydrates, a concert of pre- and postprandial hormonal responses tightly controls the postprandial glucose excursion (8, 33, 37).
Meal feeding (MF), an experimental paradigm in which animals receive the entirety of their daily food ration in a fixed window of time, requires the ingestion of very large meals and presents a major challenge to glucose homeostasis (1, 17, 22, 24). Animals require several days of training on a MF schedule before they are able to consume sufficient daily calories in the constrained feeding period (12). In rats, 5–10 days of time-limited food presentation is required before the animals are considered meal fed and can maintain energy intake sufficient to support normal body composition and function. Over the first 2–4 days of MF, rats lose body weight because they are not accustomed to the limited feeding period nor adapted to consume large meals, but in short order, MF rats are able to adapt and reestablish a weight trajectory comparable to that of control animals (12). This process involves the development of behavioral and physiological adaptations to the short period of food presentation. For example, there are hormonal adaptations to the scheduled large meal, including secretion of preprandial ghrelin, glucagon-like peptide-1 (GLP-1), and insulin, so-called cephalic responses, which precede endocrine responses directly stimulated by eating (4, 12, 34, 35). These anticipatory responses are important for animals to assimilate and tolerate large meals (4, 10, 34) and may even be advantageous. Indeed, there is experimental evidence that rodents maintained on a MF regimen have improved glucose tolerance in response to oral and parenteral carbohydrate challenges (24, 31).
Prandial and postprandial adaptations also occur in MF animals. We have observed that MF rats secrete twice the amount of insulin in the 30 min after onset of feeding than ad libitum-fed (AL) rats despite similar plasma glucose levels (12). We hypothesized that enhanced activity of the enteroinsular axis, with increased production and secretion of the incretins GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), contributes to increased insulin secretion and improved glucose tolerance in MF. To address whether the incretin system mediates the enhancement in β-cell function and glucose tolerance during MF, we compared the insulin response to oral, intraperitoneal (ip), and intravenous (iv) glucose administration in MF and AL rats. We predicted that insulin secretion and glucose tolerance would be enhanced during oral compared with parenteral carbohydrate administration.
METHODS
Animals.
Prior to the initiation of experimental procedures, male Long-Evans rats (Harlan, Indianapolis, IN) weighing between 300 and 350 g were housed individually in plastic shoebox cages with ad libitum access to pelleted rat chow (Teklad; Harlan, Madison, WI) and water in a temperature-controlled vivarium (22 ± 2°C) on a 12:12-h light-dark schedule, with lights on at 0600. This research was conducted in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care, and conformed to National Institutes of Health and US Department of Agriculture regulations, with the approval of the University of Cincinnati Internal Animal Care and Use Committee. At the beginning of each experiment, rats assigned to MF or AL groups were matched for body weight. MF rats had access to chow plus water from 1200 to1600 daily and water alone for the remainder of the day; AL controls had free access to food and water at all times unless otherwise specified. As demonstrated previously (12), MF rats learned within a few days to eat all of their daily calories within the allotted 4-h time window, such that they maintained a stable weight after a small loss at the initiation of the feeding paradigm.
Experiment 1: oral glucose tolerance during a liquid meal challenge.
To determine the role of incretins on the glucose and insulin responses to MF, a mixed-meal tolerance test (MTT) was used. After 10 days of the MF/AL paradigm, Ensure Plus was substituted for chow as the sole source of calories for both groups; i.e., MF rats received Ensure Plus for 3 h daily, and AL rats had access to Ensure Plus at all times. The meal feeding window was reduced to 3 h/day because pilot work indicated that MF rats consume the same number of calories as Ensure Plus within 3 h as they as they consume within 4 h when fed chow. After 6 days of training on Ensure, daily intake of the MF animals stabilized at a volume of 42.3 ± 1.6 ml/3 h whereas the AL rats consumed a volume of 76.9 ± 3.4 ml/24 h. On the day prior to the meal challenge, food was removed from both groups at 1600, the time when it was normally removed from the MF rats. At 1200 the next day, 5 ml of Ensure Plus with 5% d-xylose was provided to both groups. Separate cohorts of eight rats each were decapitated at 0, 15, 30, 45, 60, and 120 min after the Ensure Plus was presented, and trunk blood was collected. This method permitted sufficient blood collection for the measurement of multiple hormones and substrates as well as tissue collection for the assessment of incretin gene expression. Fasting and postprandial blood samples were immediately assayed for glucose with a hand-held glucometer (Accu-Chek Advantage; Roche Diagnostics, Indianapolis, IN).
A similar paradigm was repeated in a second group of rats that were allocated to MF or AL intake of pelleted rat chow for 2 wk. The animals were familiarized with Ensure Plus through small daily exposures over 3 days. They were fasted from 1600 on the day before a MTT, similar to the first group. Fasting blood samples were taken from the tail at 1100, and animals were presented with 7.5 ml of Ensure Plus including acetaminophen (100 mg/kg) at 1200. Postprandial blood was sampled serially at 7.5, 15, 30, 45, and 60 min through a nick in the tail for measurement of glucose, insulin, and acetaminophen.
Experiment 2: ip glucose tolerance.
To determine whether the MF regimen alters insulin secretion in the absence of incretin secretion, we administered glucose ip to MF and AL rats. Cohorts of MF and AL rats (10/group) were established as described above. To habituate the rats to ip injections, rats in both groups were injected with saline (1 ml/kg) just before the MF group received their food (i.e., at 1200) starting the first week of MF. Over the course of 7 days, both groups adjusted to the injections and tolerated them well with no obvious signs of stress. On the day before the glucose tolerance test, food was removed from all animals at 1600. At 1130 the next day, a fasting blood sample was taken from the tip of the tail from all animals as described previously (36). At 1200, all rats were injected with 1.5 g/kg glucose ip as a 20% solution of dextrose in water. Subsequent blood samples were collected 15, 30, 45, 60, and 120 min after the glucose challenge for measurement of blood glucose, and plasma was saved for assay of insulin.
Experiment 3: iv glucose tolerance and hyperinsulinemic-euglycemic clamps.
To understand the contribution of insulin sensitivity and the relative β-cell response to the improved glucose tolerance in MF rats, we performed iv glucose tolerance tests (IVGTTs) followed by hyperinsulinemic-euglycemic clamps in a group of rats before and after MF. A cohort of 12 rats had catheters placed in the right jugular vein and the left carotid artery. Both lines were tunneled subcutaneously to exit the skin between the scapulae, where they were closed with stainless steel rods and anchored with sutures. The rats recovered for 1 wk to regain their preoperative body weight, and during that time they were handled on a daily basis to habituate them to contact by investigators. While on the regimen of AL feeding, the rats underwent an IVGTT/hyperinsulinemic-euglycemic clamp as detailed below. This group was then meal fed for 2 wk and the studies were repeated. During both the AL and MF periods, experimental procedures were conducted after a 20-h fast; food was removed at 1600, and the rats were studied the next day at 1200. Plastic tubing was connected to each catheter and connected to a swivel (Instech Laboratories, Plymouth Meeting, PA) such that the rats were able to move freely in their home cages during the experiment. Fasting blood samples were taken at 1130, and glucose was measured as described above. At 1200, an iv glucose bolus of 0.5 g/kg was injected as a 20% solution of dextrose in water into the jugular vein at time 0. Blood samples were collected from the carotid artery at 2, 4, 6, 8, 10, 15, 20, 25, and 30 min. At 60 min, a hyperinsulinemic-euglycemic clamp was started with an infusion of insulin at 4 mU·kg−1·min−1 that continued for the next 2 h. The insulin solution was prepared in a 10-ml syringe containing 0.5 ml of each rat's own blood, isotonic saline with heparin (20 U heparin/ml) and human regular insulin (Novolin; Novo Nordisk, Copenhagen, Denmark), and the mixture was infused at a constant rate of 50 μl/min. A 20% solution of dextrose in water was given iv at a variable rate; blood glucose concentration was measured with a hand-held glucometer every 5 min, and the glucose infusion was adjusted to maintain a glucose target of 6 mM. Larger blood samples for measurement of insulin were taken at 60, 90, 120, 135, 150, 165, and 180 min. Blood samples were centrifuged immediately at 3,000 rpm for 5 min, and the plasma was removed for subsequent measurement of insulin. The red blood cells were carefully immersed in heparinized saline (20 U/ml) and reinfused into the rat to prevent excessive depletion and anemia.
Plasma hormone and glucose analysis.
Trunk blood was collected into tubes on ice containing heparin (2,000 U/ml)-EDTA (50 mM)-aprotinin (500 kallikrein inhibitory units/ml). After immediate centrifugation at 3,000 rpm, plasma was stored at −80°C until assayed. Plasma insulin and total GIP were determined by previously described radioimmunoassays (12, 15). Plasma glucagon and cortisosterone and total GLP-1 levels were measured with commercially available RIA kits (Linco Research, St. Louis, MO, and ICN, Costa Mesa, CA). Plasma d-xylose (32) and acetaminophen (21), included in meals as measures of gastric emptying, were analyzed by spectrophotometric assay.
RNA extraction and analysis.
Segments of jejunum and ileum (∼1 cm) were collected from animals euthanized during experiment 1 at 0, 15, 30, 60, and 120 min after ingestion of the test meal. Contents were removed and tissues frozen in liquid nitrogen. Total RNA was extracted by homogenization in Tri Reagent (Molecular Research Center, Cincinnati, OH). cDNA was synthesized from 500 ng of total RNA by RT using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). An aliquot of 1 μl of a total of 20 μl of cDNA was used in PCR with primer pairs for proGIP (forward, AAG CCA GTT CAC AAA GTC TTG TT; reverse, AAA AGA AGA GGT TGA GTT CCG AT), proglucagon (forward, GAC GTT TGG CAA TGT TGT TC; reverse, GCA ATT ACC TAG ACT CCC GC), or L32 (forward, CAT CGT AGA AAG AGC AGC AC; reverse, GCA CAC AAG CCA TCT ATT CA). PCR amplifications were done in triplicate for 40 cycles with a QuantiTect SYBR Green real-time PCR kit (QIAGEN, Valencia, CA) in a SmartCycler instrument (Cepheid, Sunnyvale, CA).
Statistical analyses.
Results are presented as means ± SE. The glucose and plasma hormone responses to glucose tolerance tests were summed as AUCs above baseline using the trapezoidal rule. For experiment 1, which used individual animals to generate values for single time points, AUCs were generated for glucose, insulin, and glucagon by randomly grouping rats euthanized at different times across the postprandial period to generate separate time-response curves. The glucose disappearance constant (kg) was calculated as the slope of the natural logarithm of the plasma glucose values from 10 to 30 min during the IVGTT. Glucose and hormone responses were compared using t-tests for unpaired samples in the meal tolerance and ip glucose tolerance tests. Paired t-tests were used to compare insulin secretion and insulin sensitivity during the IVGTT/clamp study. Statistical significance was accepted when P values were <0.05.
RESULTS
The body weights and fasting glucose concentrations before glucose tolerance testing are presented in Table 1 for the three cohorts of rats. In the first two cohorts, AL and MF rats had similar body weights before the start of MF; the third cohort was studied before and after MF. As described previously (Fig. 1 and Ref. 12), and, as occurred in the present experiments, MF rats lose weight during the first few days of meal feeding until they learn to consume sufficient calories in the 4 h during which they have access to food. Body weight stabilizes by days 2–4 of MF, and subsequently the body weight trajectory increases and parallels that of AL rats. MF rats did not compensate for the several days of weight loss at the beginning of the experiment, and their body weights remained lower than those of rats in the AL condition in all cohorts. This difference was largest in the first MTT cohort, likely because of increased caloric consumption by the AL group during the week of Ensure feeding.
Table 1.
Body weights and fasting glucose concentrations in rats before oral, ip, and iv tolerance tests
| Body Weight, g | Fasting Glucose, mmol/l | |
|---|---|---|
| MTT cohort | ||
| MF | 323.6 ± 3.1* | 8.1 ± 0.15* |
| AL | 410.5 ± 5.3 | 7.0 ± 0.22 |
| IPGTT cohort | ||
| MF | 350.8 ± 7.6* | 7.5 ± 0.4* |
| AL | 371.5 ± 13.6 | 6.0 ± 0.1 |
| IVGTT cohort | ||
| MF | 302.0 ± 6.0* | 5.6 ± 0.18 |
| AL | 315.6 ± 3.8 | 5.4 ± 0.11 |
Values are means ± SE. MTT, meal tolerance test; IPGTT, intraperitoneal glucose tolerance test; IVGTT, intravenous glucose tolerance test; MF, meal fed; AL, ad libitum fed. For the IV cohort, the values are from the same animals before (AL) and after MF.
P < 0.05 vs. AL control.
Fig. 1.
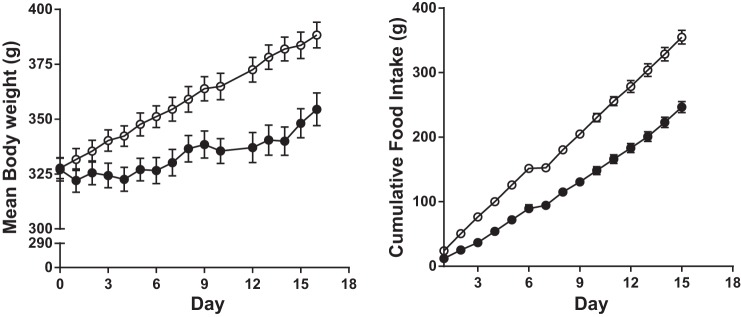
Body weight (left) and cumulative food intake (right) in groups of 10 rats on meal feeding (MF; ○) or ad libitum (AL; ●) feeding regimens for 16 days.
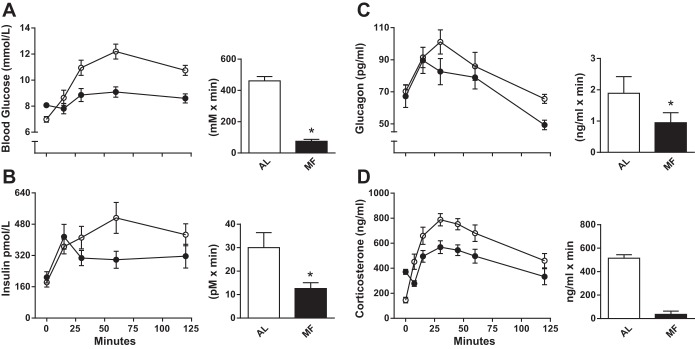
Fasting glucose concentrations were consistently 14–20% lower in AL compared with MF rats in these studies, similar to what has been reported previously (3). In addition, MF rats had higher corticosterone levels prior to food presentation than AL controls (398.8 ± 16.9 and 170.6 ± 46.8 ng/ml, respectively), another finding previously noted (18). However, it seems unlikely that stress contributed to the consistently higher basal glucose levels in the MF groups. In all probability, this was due to different degrees of fasting between the AL and MF animals (3). Both groups had food removed at 1600, 2 h before the onset of the dark, but the MF animals had just finished a large meal at that time. In contrast, AL rats do not typically eat substantial amounts during the light phase and consequently were actually fasted for a longer period. This was confirmed by fasting MF rats for an equivalent period after feeding as AL rats and obtaining comparable basal glucose levels (data not shown). The postprandial corticosterone levels decreased in the MF animals such that they were lower those of than the AL group by the conclusion of the meals (Fig. 2D).
Fig. 2.
Blood glucose (A) and plasma insulin (B), glucagon (C), and corticosterone (D) in AL (○) and MF (●) rats following ingestion of a mixed nutrient liquid meal. The 120-min AUCs above fasting for each measure are shown to the right. Data in A, B, and C were generated from groups of 8 rats euthanized at each time point; data in D are from groups of 8 MF and AL animals sampled serially. *P < 0.05 MF vs. AL.
Experiment 1: glucose tolerance during an oral liquid meal.
During the first MTT, all rats in the AL and MF groups consumed the test meal (5 ml of Ensure Plus) within 5 min. Although fasting glucose levels were ∼15% higher in the MF than the AL group (Table 1), the glucose excursion after the Ensure Plus challenge was substantially attenuated in the MF rats (glucose AUC: MF 75 ± 7, AL 461 ± 28 mmol·l−1·min, P < 0.001; Fig. 2A). It is noteworthy that MF rats had no increase in plasma glucose over the first 15 min after meal consumption. The glucose levels in the AL rats, on the other hand, rose consistently after eating, peaked at 60 min, and were still elevated after 2 h. Meal feeding decreased the postprandial increment in blood glucose by 83% compared with the response in the AL-fed rats.
During the first MTT, the insulin response to oral meal ingestion was left-shifted in the MF rats, with peak levels measured at 15 min, a time when there was still no increase in plasma glucose (Fig. 2B). AL rats, on the other hand, reached their maximum insulin secretion at 60 min, coinciding with the highest plasma glucose level. Despite significantly improved glucose tolerance, the total postprandial insulin secretion calculated as AUC was 58% lower in the meal-fed rats (Fig. 2B; insulin AUC: MF 12,604 ± 1,267, AL 30,034 ± 6,046 pmol·l−1·min, P = 0.023). However, the ratio AUCinsulin/AUCglucose was significantly greater in the MF animals during the meal test (MF 175 ± 36, AL 65 ± 18 pM/mM, P < 0.01). With a blood sampling paradigm in the second MTT that included earlier time points, it was apparent that plasma insulin levels in the MF group were highest in the initial 10 min after food presentation, and the insulin AUC0–15 was more than three times the value in AL animals (MF 31.0 ± 4.9, AL 9.9 ± 4.4 pmol·l−1·min, P < 0.02).
The lower postprandial glucose excursion in the MF rats was not due to a reduction in the rate of gastric emptying. As indicated by the plasma levels of d-xylose (Fig. 3; d-xylose AUC: MF 91 ± 4, AL 36 ± 2 mmol·l−1·min, P < 0.001), gastric emptying was significantly accelerated in MF rats compared with controls (P < 0.05). These findings were corroborated by 1) weighing stomach contents from a group of 6 MF and AL animals 2 h after presentation of chow following a 20-h fast (Fig. 3), and 2) 0- to 60-min plasma acetaminophen levels from MF and AL rats in the second meal tolerance study (Fig. 3).
Fig. 3.
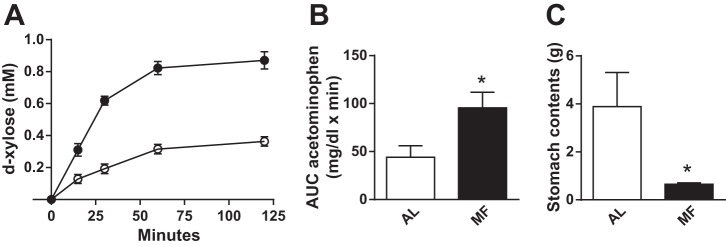
Measures of gastric emptying in MF and AL rats. Plasma d-xylose concentrations (left) and AUC acetaminophen (middle) following consumption of a mixed nutrient liquid meal. Stomach contents in AL and MF rats 2 h after chow refeeding (right). Data in A were generated from groups of 8 rats per time point in the MF and AL groups; data in B were obtained from groups of 8 and those in C from groups of 6 MF and AL rats sampled serially. *P < 0.05 MF vs. AL.
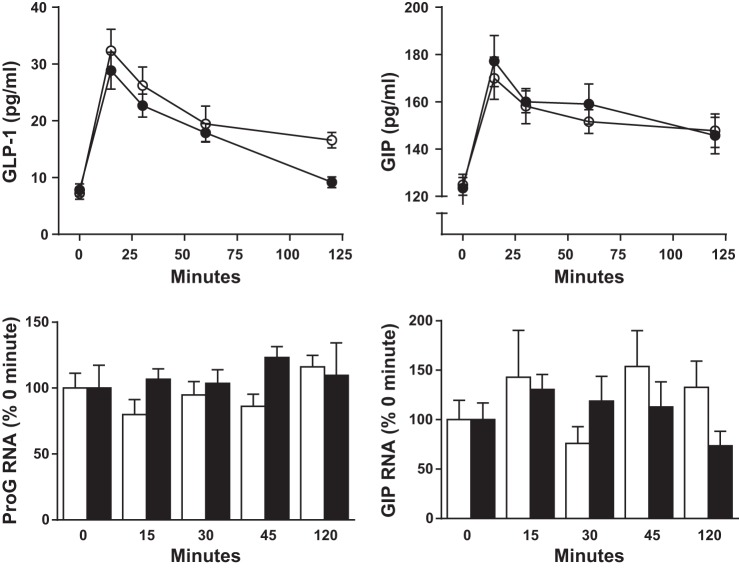
Fasting plasma glucagon levels were similar in MF and control rats, and plasma values peaked at similar levels 15 min after the meal (Fig. 2C). However, there was a relatively greater postprandial suppression of plasma glucagon in MF rats compared with AL rats, with overall responses 40–50% lower (Fig. 4; glucagon AUC: MF 949 ± 319, AL 1,886 ± 528 pg·ml−1·min, P < 0.05). Plasma GLP-1 and GIP concentrations in the MF and AL rats did not differ following the MTT (Fig. 4). Moreover, neither fasting nor postprandial intestinal expression of proglucagon (proG) and proGIP mRNA differed in animals habituated to MF or AL regimens (Fig. 4).
Fig. 4.
Plasma glucagon-like peptide-1 (GLP-1; top left) and (GIP; top right) in AL (○) and MF (●) rats after ingestion of a mixed nutrient liquid meal. Intestinal proglucagon (proG) and proGIP mRNA from rats euthanized sequentially after the meal. All data were generated from groups of 8 MF and AL rats at each time point.
Experiment 2: ip glucose tolerance.
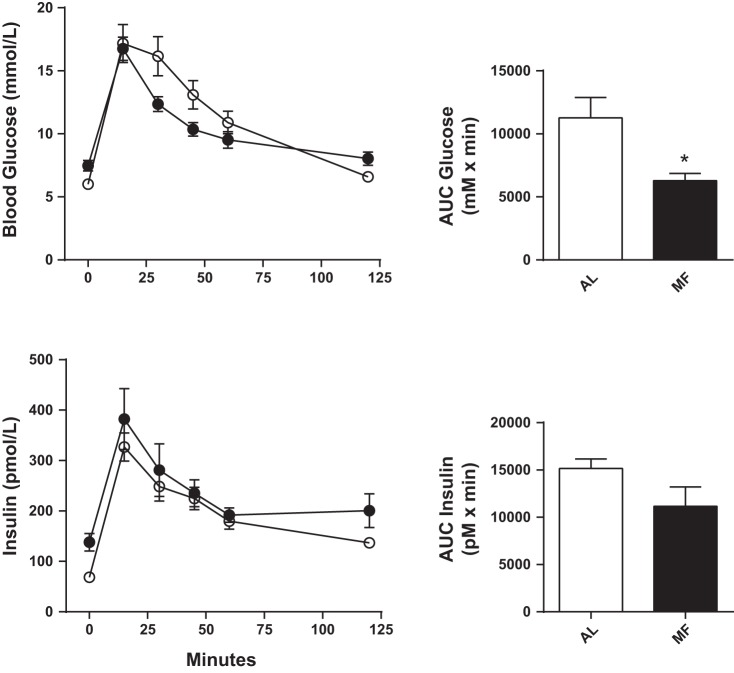
On the day of the IPGTT, MF rats had levels of fasting glucose that were ∼19% greater than those of AL rats (Table 1). However, both groups reached a similar glucose peak 15 min after the ip glucose load (Fig. 5). The MF rats cleared the glucose load from the circulation more rapidly than did the AL controls, with a 54% lower incremental glucose response (Fig. 5; glucose AUC: MF 349 ± 33, AL 626 ± 90 mmol·l−1·min, P < 0.05). Fasting insulin was greater in MF than in AL controls (MF 138 ± 17, AL 68 ± 10 pmol/l, P < 0.01), but insulin levels after the glucose challenge did not differ between the two groups (Fig. 5; insulin AUC: MF 11,165 ± 2,056, AL 15,156 ± 1,006, P = 0.17). Likewise, the ratio AUCinsulin/AUCglucose did not differ between the two groups after the IPGTT (MF 32.8 ± 5.0, AL 27.7 ± 4.5 pM/mM, P = 0.49).
Fig. 5.
Plasma glucose (top left) and insulin (bottom left) in AL (○) and MF (●) rats (n = 10 animals per group). The 120-min glucose AUC was decreased in the MF group (top right) but the insulin AUC did not differ in the 2 groups.
Experiment 3: IVGTT and hyperinsulinemic-euglycemic clamp.
On the days of the IVGTT/hyperinsulinemic clamps, fasting glucose values were similar, 98 ± 2.9 before MF and 101 ± 3.1 afterward, and the glucose excursions following iv glucose administration likewise did not differ. There was no difference in iv glucose tolerance, as reflected in the glucose disappearance constant between the two groups (kg: MF 2.4 ± 0.2, AL 2.4 ± 0.1%/min). Similarly, the acute insulin response to glucose did not differ between the two groups (Fig. 6; insulin AUC0–10: MF 231 ± 45, AL 321 ± 21 pmol/l, P = 0.18). During the hyperinsulinemic clamps, blood glucose levels were maintained near target for both groups (MF 5.5 ± 0.1, AL 5.4 ± 0.1 mmol/l), with mean coefficients of variation of 7.8 and 7.9%, respectively. Plasma insulin during the clamp was also comparable in the two groups (MF 392 ± 15, AL 375 ± 13 pmol/l). Insulin sensitivity, measured as the glucose infusion rate during the steady state of the clamp (150–180 min), was ∼20% higher after meal feeding (Fig. 6; MF 38.6 ± 0.8, AL 31.2 ± 1.7 mcg·kg−1·min−1, P < 0.01). The disposition index, insulin secretion expressed as a function of insulin sensitivity, did not differ between the two groups (Fig. 6; MF 9,460 ± 1,506, AL 10,075 ± 725, P = 0.725).
Fig. 6.
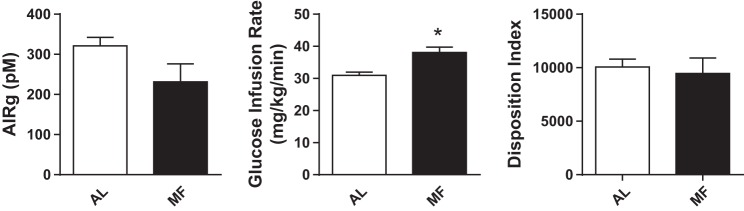
Insulin secretion (left) and insulin sensitivity (middle) from AL and MF rats (n = 10 per group) determined from an iv glucose tolerance test and euglycemic-hyperinsulinemic clamp. The MF group had lower AIRg (acute insulin response to glucose) and was significantly more insulin sensitive, but adjusted insulin secretion, as the disposition index, did not differ between the groups.
DISCUSSION
MF presents two opposing homeostatic challenges to an animal. On the one hand, restriction of food access to a fixed period requires huge meals to maintain sufficient energy intake to support normal function. On the other hand, these large meals present a decided threat to the normal levels of plasma nutrients, since there is an unusually large appearance of these substrates from the gut. In the present studies, we have demonstrated that rats resolve this conflict by making dramatic adaptations to allow more efficient glucose tolerance in response to the food intakes necessary during MF. Central to the adaptation for MF, rats had more rapid insulin responses and a greater suppression of glucagon secretion associated with attenuations in the postprandial glucose excursion. There were no differences in plasma or tissue levels of GLP-1 or GIP in MF rats compared with controls, suggesting that the insulin response was not mediated by greater incretin stimulation. MF rats had less impressive changes in glucose tolerance in response to parenteral administration of glucose and no evidence of enhanced β-cell function during ip or iv glucose administration. These data demonstrate that MF rats respond to the homeostatic challenge of large nutrient loads by adapting islet hormone secretion, and based on the relative differences between oral and parenteral glucose challenges, the enhanced β-cell function is specific to the postprandial state. Overall, these results demonstrate that in healthy animals glucose tolerance is highly adaptable and can be substantially improved in extraordinary circumstances such as MF.
There is a long history of MF as a model to study nutrient homeostasis as well as feeding behavior (17, 20, 23, 24, 29, 31). Our approach provided reproducible adaptation of MF animals, and the cohorts of animals reported here had trajectories of food intake and body weight that were similar to those in our previous work (12). The initial MTT cohort had the largest difference in body weights between MF and AL groups that we have seen, in large part due to unexpected overeating by the AL animals when switched to Ensure feeding; this was mitigated in our second MTT cohort by changing the protocol and giving Ensure exposures while maintaining most of the daily caloric intake as chow. What is unique about the present data set is that during our studies of oral glucose tolerance, animals freely ingested a test meal rather than have the nutrients delivered by gavage. We believe that this more physiological paradigm accentuated the response to MF by allowing meal anticipation and meal presentation to follow the expected course for the animals and thus engage the full spectrum of adaptive responses. Consistent with this, we found in preliminary experiments that the improvement in glucose tolerance was not as great after a gavage of Ensure (data not shown).
In a general sense, our studies conform to the results that Leveille and colleagues reported more than three decades ago (24, 31). They noted that rats with access to food for only 2 h a day cleared ip and intragastric glucose loads faster than rats with ad libitum access to food. In our experiments, MF rats had a pronounced response to meal ingestion, with a more than 80% reduction of the glycemic response that occurred at the time the animals were accustomed to eat. There was a significant but lesser, ∼40%, improvement in the glycemic response to ip glucose. Undoubtedly some of the marked improvement in oral glucose tolerance was due differences in body weight and insulin sensitivity between the MF and AL groups. However, it seems very unlikely that this is the primary explanation for these results. First, there were marked qualitative differences in islet secretion and gastric function during meal absorption that cannot be attributed to differences in body weight. In particular, the rapid insulin response seems key to improved glucose handling, and the amount of insulin secreted following meal ingestion in MF animals was greater than that of the AL group when normalized to blood glucose, a difference not observed with parenteral glucose administration. Second, glucose tolerance was not improved in response to parenteral glucose despite significant, albeit smaller, differences in body weight between the MF and AL rats. Finally, in models of bariatric surgery, the improvement in glucose tolerance between rats with GI surgery or control operations is only 20–40%, much less than what we report here for MF, despite comparable differences in body weight (6, 7, 28). Taken together, these observations support an effect of MF on glucose tolerance that goes beyond changes in body weight. We propose that MF elicits larger differences in insulin secretion during ingested compared with parenteral challenges because of the broad range of regulatory signals initiated by an adapted eating pattern, rather than simply hyperglycemia through ip or iv injection.
It seems very likely that changes in islet hormone secretion were central to the improvement of oral glucose tolerance in MF rats. Insulin secretion occurred earlier in the meal and was increased before significant increases in blood glucose were evident in experiment 1. This left-shifted insulin response is a hallmark of a variety of models of enhanced glucose tolerance (11, 30). In addition, MF rats had reduced postprandial glucagon excursions, a response which would be expected to contribute to a more rapid and complete suppression of hepatic glucose production. The increased β-cell responsiveness to meals could not be accounted for by changes in plasma levels of GIP or GLP-1, as these did not differ between MF and AL rats. We measured only total GIP and GLP-1 levels and cannot exclude the possibility that levels of intact, bioactive peptides differed in the two groups. However, this would have required that MF induce significant differences in the metabolism of the incretins, and to date such systemic changes have only been described in rodents with natural or induced mutations of dipeptidyl peptidase-4 (13). In addition, we cannot rule out the possibility that β-cell sensitivity to stimulation by GLP-1 and GIP increased with MF, but such a mechanism has not been demonstrated previously, making this explanation also only theoretical. Thus, our primary hypothesis that a greater incretin effect was part of the MF adaptation seems an unlikely explanation for the present results. Moreover, the enhanced postprandial insulin secretion cannot be attributed to general hyperfunctioning of β-cells, since the response to ip and iv glucose in the MF rats did not differ from that in the AL controls. Therefore, it is likely that postprandial adaptations in islet function to MF are neurally mediated, especially given the early insulin response that developed in MF rats prior to meal-induced hyperglycemia, a setting in which neither glycemic nor incretin stimulation would be expected. Cephalic or preprandial insulin secretion is well described in both rodents and humans (4, 26, 34) and is enhanced by MF (12). Our results are compatible with postprandial islet function having a strong cephalic component as well, and one that is adaptable to a MF paradigm. We (9) have previously reported important cholinergic mediation of insulin secretion and glucose tolerance in freely feeding nonhuman primates, results supporting important neural regulation of postprandial metabolism. Considered together with the current findings, it seems likely that CNS control of islet function is an important component of normal physiology and is adaptable to homeostatic challenges such as MF.
MF also increased insulin sensitivity, an effect that was modest but significant. The 20% increase in insulin sensitivity after MF might simply be a function of the weight differences observed in our various MF and AL cohorts, although it would require studies with pair-fed cohorts to determine whether the differences in insulin action are purely due to changes in body weight or if there is some effect of MF per se. Increased insulin sensitivity could account for the greater ip glucose tolerance in MF compared with AL animals that had comparable insulin secretion. And although it seems unlikely that greater insulin sensitivity was the principal cause of the dramatic reduction in glucose excursion after the test meal, this change would be expected to complement the adaptations of insulin and glucagon secretion to enhance glucose metabolism.
The increased gastric emptying observed in the MF animals was a surprising and dramatic finding that would seem to run contrary to the improved glucose tolerance in this group. There is a general line of reasoning that delayed gastric emptying of carbohydrate improves glucose tolerance, a concept with both experimental support (9) and application in pharmacology (5, 25). Not only did this fail to occur, but in contrast, we noted a substantial increase in the rates of both liquid and solid meal emptying in MF compared with AL rats. In fact, this finding has been reported previously in MF paradigms such as the one used here in which rats were given sufficient time with food availability to maintain a positive growth trajectory (1). In this previous study, rats on a fixed, MF schedule had increased frequency and magnitude of gastric contractions, a response associated temporally with maximum plasma levels of ghrelin. The plausibility of a neuroendocrine mechanism for increased gastric motility in MF is supported by in vivo and in vitro demonstrations of the effects of ghrelin on gastric contractility (2, 14). While more work will be necessary to establish this mechanism, it is a reproducible response amenable to further study. Regardless, our findings confirm that an increased rate of gastric emptying is an important adaptation to our MF paradigm, probably necessary to allow an increased frequency of meal consumption during the limited feeding time to maintain energy balance. Given the significant enhancement of gastric emptying with MF, the improved glucose tolerance in MF animals is even more remarkable.
Our findings with MF rats suggest several extensions to metabolic physiology more broadly. The first is that regular, anticipated meals may be beneficial for glucose homeostasis. Studies of so-called clock genes, which are central for establishing diurnal rhythms, indicate that regular temporal patterns are essential for normal glucose tolerance (27). A recent study by Hatori et al. (16) suggests that MF leads to a broad and specific pattern of gene expression connecting clock mechanisms with important metabolic pathways; similar mechanisms could be involved in the adaptations of islet function reported here. Second, it is notable that normal glucose tolerance, as reflected in the profiles of our AL rats, can be so dramatically improved. This demonstrates a powerful capacity for physiological enhancement in healthy subjects in response to environmental stressors. Understanding the mechanisms that allow superphysiological glucose tolerance may have useful application to treating states of impaired glucose regulation. Finally, there are aspects of the responses of MF rats that have parallels in clinical medicine. Bariatric surgical procedures, such as gastric bypass and vertical sleeve gastrectomy, are also associated with rapid gastric emptying and substantial improvements of glucose tolerance (19). Whether the responses to weight loss surgery and MF recruit comparable physiological mechanisms is unclear but seems worthy of further study.
In summary, we have demonstrated that MF rats have substantial improvements in glucose tolerance that are most apparent when they actively consume a large carbohydrate-containing meal. The main difference in the prandial response in MF compared with AL rats was a left shift in insulin secretion likely mediated by neural signals, since plasma insulin was elevated before any rise in blood glucose and since incretin levels were not enhanced. These findings point to the adaptability and potency of the systems normally regulating blood glucose and raise the possibility that mechanisms underlying these responses could have novel application in therapeutics.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK-057900, DK-56863, and DK-067550.
DISCLOSURES
T.P.V., B.A.A., D.L.D., E.P.S., Y.U.-L., and S.C.W. have no conflikcts of interest to declare. R.J.S. consults for Amylin Pharmaceuticals, Johnson & Johnson, and Roche; is a scientific advisory board member for Eli Lilly, Johnson & Johnson, Zafgen Inc., and Merck; is on the speaker's bureau for Amylin Pharmaceuticals, Eli Lilly, Novo Nordisk, and Merck; has stock options in Zafgen Inc.; and research grants from Amylin Pharmaceuticals, Johnson & Johnson, Zafgen Inc., Roche, and Mannkind. D.A.D. consults for Lilly, Merck, Novo Nordisk, Roche, and Zealand.
AUTHOR CONTRIBUTIONS
Author contributions: T.P.V., B.A.A., E.P.S., D.L.D., R.J.S., S.C.W., and D.A.D. conception and design of research; T.P.V., B.A.A., E.P.S., D.L.D., and Y.U.-L. performed experiments; T.P.V., B.A.A., E.P.S., D.L.D., Y.U.-L., R.J.S., S.C.W., and D.A.D. analyzed data; T.P.V., B.A.A., E.P.S., D.L.D., Y.U.-L., R.J.S., S.C.W., and D.A.D. interpreted results of experiments; T.P.V. and D.A.D. prepared figures; T.P.V. drafted manuscript; T.P.V., B.A.A., E.P.S., D.L.D., Y.U.-L., R.J.S., S.C.W., and D.A.D. approved final version of manuscript; S.C.W. and D.A.D. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Kay Ellis, Brianne Reedy, Eileen Elfers, and Todd Greer for their careful and skilled technical assistance.
REFERENCES
- 1.Ariga H, Imai K, Chen C, Mantyh C, Pappas TN, Takahashi T. Fixed feeding potentiates interdigestive gastric motor activity in rats: importance of eating habits for maintaining interdigestive MMC. Am J Physiol Gastrointest Liver Physiol 294: G655–G659, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil 19: 675–680, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Batista MR, Ferraz M, Bazotte RB. Are physiological changes in meal-fed rats determined by the amount of food ingested in the last meal or due to feeding schedule? Physiol Behav 62: 249–253, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR, Bereiter DA, Trimble ER, Siegel EG, Jeanrenaud B. Cephalic phase, reflex insulin secretion. Neuroanatomical and physiological characterization. Diabetologia 20 Suppl: 393–401, 1981 [PubMed] [Google Scholar]
- 5.Cervera A, Wajcberg E, Sriwijitkamol A, Fernandez M, Zuo P, Triplitt C, Musi N, DeFronzo RA, Cersosimo E. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 294: E846–E852, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141: 950–958, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, LaSance K, Woods SC, Seeley RJ, D'Alessio DA, Sandoval DA. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab 306: E424–E432, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creutzfeldt W, Nauck M. Gut hormones and diabetes mellitus. Diabetes Metab Rev 8: 149–177, 1992 [DOI] [PubMed] [Google Scholar]
- 9.D'Alessio DA, Kieffer TJ, Taborsky GJ, Jr., Havel PJ. Activation of the parasympathetic nervous system is necessary for normal meal-induced insulin secretion in rhesus macaques. J Clin Endocrinol Metab 86: 1253–1259, 2001 [DOI] [PubMed] [Google Scholar]
- 10.de Souza CJ, Gagen K, Chen W, Dragonas N. Early insulin release effectively improves glucose tolerance: studies in two rodent models of type 2 diabetes mellitus. Diabetes Obes Metab 3: 85–95, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev 17: 164–174, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147: 23–30, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 30: 1335–1343, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol 550: 227–240, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenbaum CJ, Prigeon RL, D'Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes 51: 951–957, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15: 848–860, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollifield G, Parson W. Metabolic adaptations to a “stuff and starve” feeding program. II. Obesity and the persistence of adaptive changes in adipose tissue and liver occurring in rats limited to a short daily feeding period. J Clin Invest 41: 250–253, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honma KI, Honma S, Hiroshige T. Feeding-associated corticosterone peak in rats under various feeding cycles. Am J Physiol Regul Integr Comp Physiol 246: R721–R726, 1984 [DOI] [PubMed] [Google Scholar]
- 19.Horner KM, Byrne NM, Cleghorn GJ, Naslund E, King NA. The effects of weight loss strategies on gastric emptying and appetite control. Obes Rev 12: 935–951, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Kaul L, Berdanier CD. Effect of meal-feeding on the daily variations of insulin, glucose, and NADP-linked dehydrogenases in rats. J Nutr 105: 1132–1140, 1975 [DOI] [PubMed] [Google Scholar]
- 21.Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest 122: 388–402, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leveille GA. Influence of dietary fat level on the enzymatic and lipogenic adaptations in adipose tissue of meal-fed rats. J Nutr 91: 267–274, 1967 [DOI] [PubMed] [Google Scholar]
- 23.Leveille GA, Chakrabarty K. Diurnal variations in tissue glycogen and liver weight of meal-fed rats. J Nutr 93: 546–554, 1967 [DOI] [PubMed] [Google Scholar]
- 24.Leveille GA, Chakrabarty K. In vivo and in vitro studies of gluconeogenesis in meal-fed and nibbling rats. J Nutr 96: 397–402, 1968 [DOI] [PubMed] [Google Scholar]
- 25.Linnebjerg H, Park S, Kothare PA, Trautmann ME, Mace K, Fineman M, Wilding I, Nauck M, Horowitz M. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 151: 123–129, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Louis-Sylvestre J. Relationship between two stages of prandial insulin release in rats. Am J Physiol Endocrinol Metab Gastrointest Physiol 235: E103–E111, 1978 [DOI] [PubMed] [Google Scholar]
- 27.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466: 627–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nausheen S, Shah IH, Pezeshki A, Sigalet DL, Chelikani PK. Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. Am J Physiol Endocrinol Metab 305: E507–E518, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Parks EJ, Schneider TL, Baar RA. Meal-feeding studies in mice: effects of diet on blood lipids and energy expenditure. Comp Med 55: 24–29, 2005 [PubMed] [Google Scholar]
- 30.Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia 44: 929–945, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Romsos DR, Leveille GA. Effect of meal frequency and diet composition on glucose tolerance in the rat. J Nutr 104: 1503–1512, 1974 [DOI] [PubMed] [Google Scholar]
- 32.Salehi M, Vahl TP, D'Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab 93: 4909–4916, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shulman G, Barrett E, Sherwin R. Integrated fuel metabolism. In: Ellenberg and Rifkin's Diabetes Mellitus (6th ed.), edited by Porte D, Sherwin R, Baron A. New York: McGraw-Hill, 2003, p. 1–14 [Google Scholar]
- 34.Teff KL. How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol Behav 103: 44–50, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vahl TP, Drazen DL, Seeley RJ, D'Alessio DA, Woods SC. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology 151: 569–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289: E823–E828, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Vella A, Camilleri M, Rizza RA. The gastrointestinal tract and glucose tolerance. Curr Opin Clin Nutr Metab Care 7: 479–484, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab 9: 489–498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods SC. The eating paradox: how we tolerate food. Psychol Rev 98: 488–505, 1991 [DOI] [PubMed] [Google Scholar]