Abstract
Bordetella pertussis toxin (PTx), also known as islet-activating protein, induces insulin secretion by ADP-ribosylation of inhibitory G proteins. PTx-induced insulin secretion may result either from inactivation of Gαo proteins or from combined inactivation of Gαo, Gαi1, Gαi2, and Gαi3 isoforms. However, the specific role of Gαi2 in pancreatic β-cells still remains unknown. In global (Gαi2−/−) and β-cell-specific (Gαi2βcko) gene-targeted Gαi2 mouse models, we studied glucose homeostasis and islet functions. Insulin secretion experiments and intracellular Ca2+ measurements were used to characterize Gαi2 function in vitro. Gαi2−/− and Gαi2βcko mice showed an unexpected metabolic phenotype, i.e., significantly lower plasma insulin levels upon intraperitoneal glucose challenge in Gαi2−/− and Gαi2βcko mice, whereas plasma glucose concentrations were unchanged in Gαi2−/− but significantly increased in Gαi2βcko mice. These findings indicate a novel albeit unexpected role for Gαi2 in the expression, turnover, and/or release of insulin from islets. Detection of insulin secretion in isolated islets did not show differences in response to high (16 mM) glucose concentrations between control and β-cell-specific Gαi2-deficient mice. In contrast, the two- to threefold increase in insulin secretion evoked by l-arginine or l-ornithine (in the presence of 16 mM glucose) was significantly reduced in islets lacking Gαi2. In accord with a reduced level of insulin secretion, intracellular calcium concentrations induced by the agonistic amino acid l-arginine did not reach control levels in β-cells. The presented analysis of gene-targeted mice provides novel insights in the role of β-cell Gαi2 showing that amino acid-induced insulin-release depends on Gαi2.
Keywords: Gαi2, insulin secretion, β-cell, l-arginine, GPCR
glucose is the principal stimulator of insulin secretion from pancreatic β-cells. Together with regulators such as other nutrients or hormones, it adjusts insulin secretion according to physiological demands. A disruption of this tightly controlled process can lead to diabetes mellitus and its comorbidities. Physiological regulators of insulin release include not only glucose but also other nutrients such as free fatty acids or amino acids on the one hand, and hormones including glucagon and norepinephrine on the other hand. They all have in common that they signal via seven-transmembrane G protein-coupled receptors (GPCR) present on the surface of pancreatic β-cells (1, 28, 33). Upon ligand binding, GPCRs activate heterotrimeric G proteins, thereby modulating the activity of cellular effectors. It is widely accepted that insulin release from pancreatic β-cells can be triggered via Gαs- and/or Gq/G11-dependent mechanisms (2, 41). Studies in isolated systems and in animals, using pertussis toxin (PTx), also known as islet-activating protein, suggest Gαi/Gαo-dependent signaling as being important for inhibition of insulin secretion (11, 14, 15). PTx specifically catalyzes the ADP-ribosylation of a cysteine residue located four residues from the carboxyl terminus of the α-subunits of Gαi/Gαo proteins. Thus, PTx treatment disrupts GPCR stimulation of the G protein and thereby disconnects it from signal transduction. The α-subunits of the PTx-sensitive G proteins include three Gαi (Gαi1, Gαi2, and Gαi3) proteins, which are the product of three different genes, and two Gαo splice variants i.e., Gαo1 and Gαo2 (3). Gαi/Gαo proteins show a sequence identity of up to 95% with overall sequence homology being more pronounced among the three Gαi isoforms (37). Due to this high homology, it has been suggested that all Gαi and Gαo proteins serve the same functions; namely, they are activated by the same or a similar set of GPCRs and appear to signal to a fully overlapping set of effectors. However, combined analysis of global and islet cell-specific Gαo1- and/or Gαo2-deficient mouse lines and PTx treatment identified Gαo2 as the main inhibitory G protein related to insulin release (32, 34, 42). Glucose-induced insulin secretion (34) and insulin containing vesicle docking (42) were increased in the absence of Gαo2. Moreover, PTx treatment of Gαo-deficient mice had a negligible effect on the already disarranged insulin secretion (42). These findings indicate that Gαo proteins are the major isoform responsible for PTx-sensitive inhibition of insulin secretion. Additional evidence suggests that Gαo2 mediates the somatostatin (SST)- and galanin-induced receptor-to-effector signal transduction in β-cells (32). Together, these findings support a prominent role for Gαo2 as the major regulator in GPCR-induced inhibition of insulin secretion. It is presently largely unclear whether the PTx-sensitive Gαi2 isoform expressed in islets or elsewhere, such as intestine, skeletal muscle, or adipose tissue is of any significance for insulin secretion in vitro and/or glucose homeostasis in vivo. To elucidate the biological role and gain insight into the cellular mechanisms of Gαi2 protein function in pancreatic β-cells, we examined global and β-cell-specific Gαi2-deficient mice. We show that Gαi2 mediates l-arginine-induced insulin secretion by modulation of corresponding changes in [Ca2+]i that trigger exocytosis. Decreased plasma insulin levels in Gαi2-targeted animals leading to an impaired glucose tolerance supported these in vitro findings. These data strongly promote and unexpectedly stress a stimulatory role for Gαi2 in insulin secretion, showing that although PTx inhibits Gαo and Gαi2 proteins these two isoforms have distinct and opposite roles in the regulation of insulin secretion. Whereas Gαo inhibits, Gαi2 stimulates insulin secretion from β-cells.
MATERIALS AND METHODS
Experimental animals.
The generation of the global Gαi2-deficient mice (Gαi2−/−) has been described previously (29). To prolong life expectancy of the Gαi2−/− animals, mice were bred and kept in individually ventilated cages (IVC) under specific-pathogen-free conditions (39) and had free access to water and standard chow. β-Cell-specific deletion of Gαi2 (Gαi2βcko) was achieved by crossing the floxed Gαi2 mouse line (27) and the Rip-Cre+/tg mouse line (12), both on a C57BL/6N background. The Rip-Cre+/tg mouse line was a kind gift from Pedro Luis Herrera, Geneva. Gαi2βcko and their littermate controls (ctrl) were kept under specific-pathogen-free conditions with free access to water and standard chow. For all experiments, gene-targeted Gαi2 animals were compared with their littermate wild types (WT) or controls (genotype: Gαi2+/+;Rip-Cre+/tg or Gαi2+/fl;Rip-Cre+/tg) on a C57BL/6N background (age 6–24 wk). All animals analyzed within this study were maintained and bred in the animal facility of the Institute of Experimental and Clinical Pharmacology and Toxicology, Eberhard Karls University, Tübingen. All experimental procedures were approved by the local government's Committee on Animal Care and Welfare Tübingen.
Isolation of pancreatic islets.
Islets were isolated according to procedure in Ref. 20. Upon a retrograde injection of 2 ml of collagenase solution (1.9 U/ml collagenase; Sigma Aldrich, Munich, Germany) in 1× HBSS (Life Technologies, Darmstadt, Germany) via the bile duct, fully perfused pancreata were incubated for 10 min at 37°C. Islets were picked using a 200-μl pipette during several washing steps.
Whole pancreatic insulin content.
Pancreata were removed and weighed. After a homogenization step in 7 ml of ice-cold 1.5% HCl in 70% ethanol, pancreata were incubated in acid ethanol for 24 h at 4°C. Insulin content was determined in the supernatant with the commercially available ultrasensitive insulin ELISA method (Mercodia, Uppsala, Sweden). Pancreatic insulin content was normalized to the weight of the pancreas.
Islet morphology.
Whole pancreatic tissue was fixed in 4% formaldehyde for 24 h and embedded in paraffin at a stretch according to standard procedures, and longitudinal 6-μm serial sections were performed. Every 250 μm, pancreata were stained with hematoxylin and eosin, and every single islet within the stained pancreatic slice was quantified. Average islet and cell size was determined by hand, whereas the number of nuclei were calculated with the AxioVision software (Zeiss, Jena, Germany). Mean values derived from each individual mouse were used to calculate means ± SE of three mice in each group.
In vitro experiments with isolated islets.
The effects of treatments on insulin secretion were assessed using static incubation experiments (20). Overnight-cultured islets [RPMI 1640 (GE Healthcare Europe, Freiburg, Germany), 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin] were grouped as batches of 10 islets and incubated in Krebs-buffered HEPES saline (120 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1 mM KH2PO4, 1.2 mM MgSO4, 10 mM HEPES, 20 mM NaHCO3, 0.5 mg/ml BSA, pH 7.4) containing 3 mM glucose in humidified 5% CO2 at 37°C for 30 min. Subsequently, the supernatant was discarded, and fresh Krebs-buffered HEPES saline was added supplemented with either 3 or 16 mM glucose with or without l-arginine (10 mM), l-ornithine (10 mM), l-leucine (100 μM), palmitate (0.2 mM), clonidine (1 μM), SST (0.1 μM), or KCl (30 mM). The islets were incubated in humidified 5% CO2 air at 37°C for 1 h. Afterward, islets were treated with ice-cold acid ethanol (1.5% HCl, 70% ethanol) for 1 h. For each condition, aforementioned above 3 and 16 mM glucose was used to test the quality of islet preparation. The amount of secreted insulin in the supernatant as well as the whole insulin content was determined with a commercially available ultrasensitive insulin ELISA method (DRG Diagnostics, Marburg, Germany). Islet DNA was isolated with the Isolate II Genomic DNA kit (Bioline, Luckenwalde, Germany), and the islet DNA content was measured (30). Based on the identical DNA and whole insulin contents of Gαi2βcko and ctrl islets, the amounts of secreted insulin were indicated % of insulin content.
Immunoblot analysis.
Freshly isolated islets were homogenized in 0.8 μl of protein lysis buffer per islet. The protein extractions were performed on pooled islets isolated from a single animal. To achieve electrophoretical separation of Gαi isoforms, separation was performed in gels containing 6 M urea. The proteins were visualized by immunodetection using the following primary antibodies described elsewhere (7, 10, 21, 39): rabbit anti-Gαi2 (1:8,000 islets, 1:20,000 hypothalamus), rabbit anti-Gαi3 (1:5,000), rabbit anti-Gαo1/o2 (1:2,000), and rabbit anti-Gβcommon (1:9,000). Antibodies against Gαs(short)/s(long) (1:1,000; SC-383) and Gαq (1:1,000; SC-393) were purchased from Cell signaling. Equal loading was verified with antibodies against mouse anti-α-tubulin (1:4,000, Sigma-Aldrich) or mouse anti-β-actin (1:4,000 islets, 1:40,000 hypothalamus; Sigma-Aldrich). The protein levels of Gαi2, Gαi3, Gαo, Gαq, Gαs, Gβ1, and Gβ2 were quantified using densitometric analysis software (Image Lab; Bio-Rad, Gräfelfing, Germany) and were normalized to the α-tubulin or β-actin levels of the same samples.
Immunofluorescence.
Immunodetection was performed on serial cryosections (8 μm) of pancreata fixed in 4% paraformaldehyde. For antigen retrieval, slices were treated with 0.3% Triton X-100-PBS for 30 min. To avoid unspecific antibody binding, slices were incubated in 5% normal goat serum (NGS)-PBS prior to incubations in primary antibody dilutions. Primary antibodies used were specific for rabbit anti-Gαi2 (1:1,500), mouse anti-glucagon (1:5,000; Sigma-Aldrich, Hamburg, Germany), and guinea pig anti-insulin (1:5,000; DAKO, Hamburg, Germany) and diluted in 1.5% NGS-PBS. Binding of primary antibodies was performed at 4°C overnight. The complexes were detected with secondary antibodies conjugated to fluorescent dyes (1:200 dilution in PBS, Invitrogen). Slices were embedded in Roti-Mount-Fluoro-Dapi (Carl Roth, Karlsruhe, Germany). Fluorescence was visualized using either the Zeiss laser scanning microscope LSM 510 or the Zeiss Axio Image.M2 microscope (Zeiss, Jena, Germany).
Glucose tolerance tests.
Mice were fasted overnight, followed by an intraperitoneal (ip) injection of glucose (2 mg/g body wt). Blood samples were collected via the tail vein, and blood glucose levels were measured using a Contour glucometer (Bayer, Leverkusen, Germany).
l-Ornithine test.
Mice were fasted overnight but supplemented with 1 mM l-ornithine in their drinking water to ensure sufficient high basal l-ornithine levels in the plasma. Mice received 100 μl of a 1.32 mg/ml l-ornithine solution by ip injection, and blood samples were collected via tail vein before administration and after 1 min to determine plasma insulin levels with the commercially available ultrasensitive insulin ELISA method (see above).
Insulin tolerance test.
Mice were fasted for 4 h, followed by an ip injection of insulin (1 mU/g body wt). Blood glucose levels were measured using a Contour glucometer (Bayer).
Plasma analysis.
Plasma levels of insulin were measured using a commercially available insulin ELISA method (see above), whereas plasma C-peptide levels were determined with the 96-well fluorescent Milliplex immunoassay (Millipore, Darmstadt, Germany).
Ca2+ imaging.
Intracellular calcium measurements were performed as previously described (16). Isolated islets were dispersed by trypsin-EDTA digestion, plated on glass coverslides, and allowed to recover for 4 h in humidified 5% CO2 air at in 37°C. Cells were loaded with Fura 2-AM (5 μM) 30 min prior to calcium measurements. Changes of intracellular calcium concentrations [Ca2+]i were recorded with a Till Photonics Oligochrome V (FEI, Gräfeling, Germany). After 4 min of baseline recordings in the presence of 5 mM glucose, dispersed islet cells were treated with 16 mM glucose, 10 mM l-arginine, 10 mM d-arginine, or a combination of 16 mM glucose and l-arginine for 10 min. Maximum depolarization of β-cells was evaluated by the use of 30 mM KCl. Emitted fluorescence was excited at 340 and 380 nm and measured at 520 nm, and the ratio 340/380 was calculated. Cells were identified as β-cells, when being inactive at 5 mM glucose, with stable baseline recordings, and an obvious response to the applied stimuli (e.g., 16 mM glucose, 10 mM l-arginine). Cells without Ca2+ responses to the respective stimuli were excluded from the measurements and analysis.
Statistical analysis.
Data are presented as means ± SE of individual data points. Data were analyzed using Student's t-test for unpaired groups. P < 0.05 was considered statistically significant.
RESULTS
Characterization of β-cell-specific Gαi2-deficient mice.
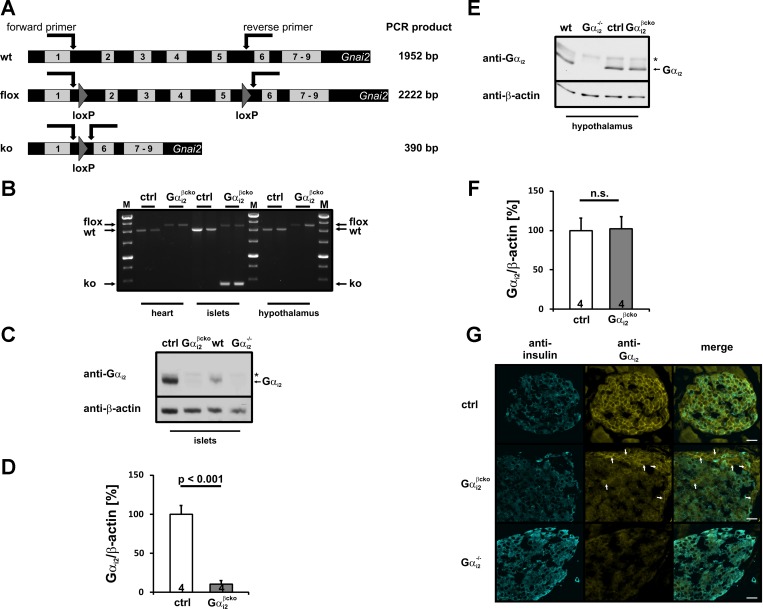
The generation and characterization of global Gαi2-deficient (Gαi2−/−) mice has been described (27, 29, 39). The β-cell-specific Gαi2-deficient mice, termed Gαi2βcko (genotype: Gαi2fl/fl;Rip-Cre+/tg), were generated by crossing Rip-Cre mice (12) with mice carrying floxed Gαi2 alleles (27). The resulting progeny, i.e., the different genotypes, were born at expected Mendelian ratios and were viable and fertile. Since the expression of the Rip-Cre transgene has been reported to have metabolic effects per se (19), we used Rip-Cre-positive littermates throughout this study as controls (ctrl genotype: Gαi2+/+;Rip-Cre+/tg or Gαi2+/fl;Rip-Cre+/tg) for experiments including Gαi2βcko mice after having confirmed that heterozygous mice, e.g., Gαi2+/fl;Rip-Cre+/tg mice were indistinguishable from Gαi2+/+;Rip-Cre+/tg animals. Recombination was validated by PCR analysis of genomic DNA (Fig. 1, A and B). Whereas DNA isolated from hearts and islets from ctrl animals as well as cardiac DNA of the Gαi2βcko mice remained in the premutant state, islet DNA of Gαi2βcko mice showed clear recombination by appearance of an additional 390-bp band (Fig. 1, A and B). Since strong hypothalamic recombination has been reported in different Rip-Cre mouse lines (38), we analyzed DNA isolated from ctrl and Gαi2βcko hypothalami. The absence of the 390-bp band indicative for recombination argues against significant recombination events in the hypothalamic brain region of our mouse line (Fig. 1B). The expression pattern of Gαi2 protein in pancreatic islets was studied by immunoblot analysis. Using specific antibodies against the COOH-terminal sequence of Gαi2, we detected the Gαi2 protein in islet homogenates of WT and ctrl mice, whereas Gαi2 was strongly reduced by ∼90% in Gαi2βcko animals (Fig. 1, C and D), and absent in Gαi2−/− islet lysates (Fig. 1C). Remaining Gαi2 immunoreactivity in Gαi2βcko likely results from the expression of Gαi2 in other endocrine cell types i.e., α-, δ-, ε-, or PP-cells (see below). To further verify the cell-specific ablation of Gαi2 in Gαi2βcko tissues, immunoblot analysis of hypothalamic lysates from ctrl and Gαi2βcko animals were performed. Gαi2 protein was absent in hypothalamic protein extracts from Gαi2−/− mice (Fig. 1E), whereas the Gαi2 protein levels of Gαi2βcko were unaffected, i.e., at control levels (Fig. 1, E and F). The combined analysis of Gαi2βcko tissues by genomic PCRs and immunoblot make it very unlikely that the studied physiological effects resulted from cells other than the pancreatic β-cells.
Fig. 1.
Gαi2 expression analysis in β-cell-specific (Gαi2βcko) and globally Gαi2-deficient (Gαi2−/−) mice A: schematic overview of the Gnai2 gene and PCR strategy for recombination analyses. B: genomic DNA analysis of heart, islets, and hypothalamus isolated from control (ctrl) and Gαi2βcko animals. Recombination is restricted to Gαi2βcko islets. C: representative immunoblots of islet homogenates isolated from ctrl, Gαi2βcko, wild-type (wt), and Gαi2−/− mice. Gαi2 protein is almost absent in Gαi2βcko islets and completely absent in Gαi2−/− islets. The weak top band (*) may have resulted from Gαi1 expression in islets. Equal loading was confirmed by β-actin detection. D: statistical analysis of Gαi2 expression levels in ctrl and Gαi2βcko islets. Islets from 4 animals were analyzed in ≥3 independent experiments. E: representative immunoblot analysis of hypothalamic homogenates isolated from ctrl and Gαi2βcko mice. As a negative control, hypothalamic homogenates from Gαi2−/− mice were loaded. The top band (*) might result from Gαi1 expression in the brain. Equal loading was confirmed by β-actin detection. F: statistical analysis of Gαi2 expression levels in hypothalami of ctrl and Gαi2βcko mice. Hypothalamic tissue from 4 animals was analyzed in ≥3 independent experiments. G: representative immunostainings of pancreatic cryosections stained against Gαi2 (yellow) and insulin (blue). Gαi2 shows proximity to the plasma membrane, whereas insulin is mainly found in the cytoplasm. In ctrl islets, Gαi2 staining is detectable in both insulin-positive β-cells and insulin-negative cells. Gαi2 protein is absent in Gαi2βcko β-cells but can be observed in insulin-negative cells. Gαi2 staining is completely absent in all Gαi2−/− islet cells. Diffuse yellow staining is background staining. Scale bars, 20 μm.
Examination of Rip-Cre-mediated recombination was performed by staining of Gαi2 protein in pancreatic cryosections on the cellular level. β-Cells were visualized by insulin-specific antibodies (Fig. 1G). A diffuse yellow background staining was detectable; however, the specific Gαi2 staining was restricted in proximity to the plasma membrane. Insulin (blue) was found mainly in the cytoplasm in ctrl, Gαi2βcko, and Gαi2−/− islets (left). In ctrl islets, Gαi2 staining mainly localized to the plasma membrane (top middle) and was detectable in insulin-positive and insulin-negative cells (top right). In contrast, only a few primarily outer cells of the Gαi2βcko islets showed the specific membrane-close Gαi2 staining (middle, arrows). These Gαi2-positive cells were all identified as insulin negative and therefore non-β-cells (middle right). In Gαi2−/− pancreata, the membrane-close Gαi2 staining was absent in all islet cells, insulin-negative and insulin-positive ones (bottom middle and bottom right). These results validated that Gαi2 and insulin could be found only in ctrl β-cells, whereas Gαi2βcko β-cells lacked the Gαi2 protein. Therefore, a successful recombination in Gαi2βcko islets was restricted to β-cells and demonstrated on a cellular level.
Analysis of G protein subunit levels in Gαi2−/− and Gαi2βcko islets of Langerhans.
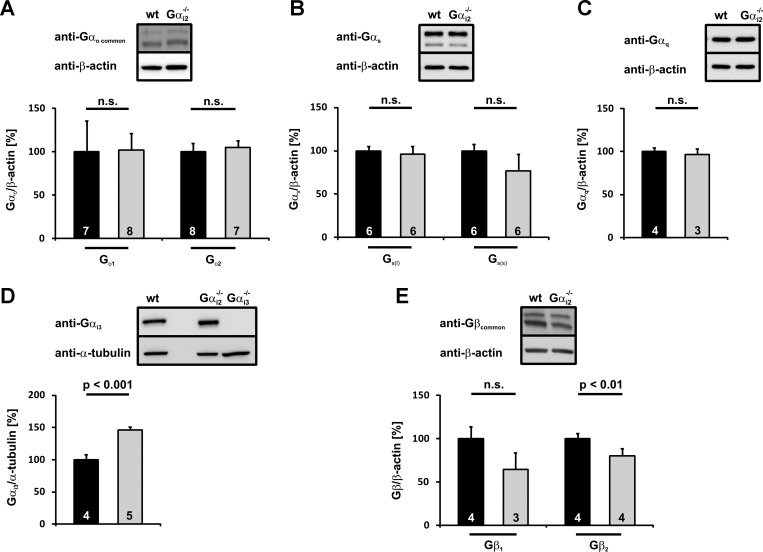
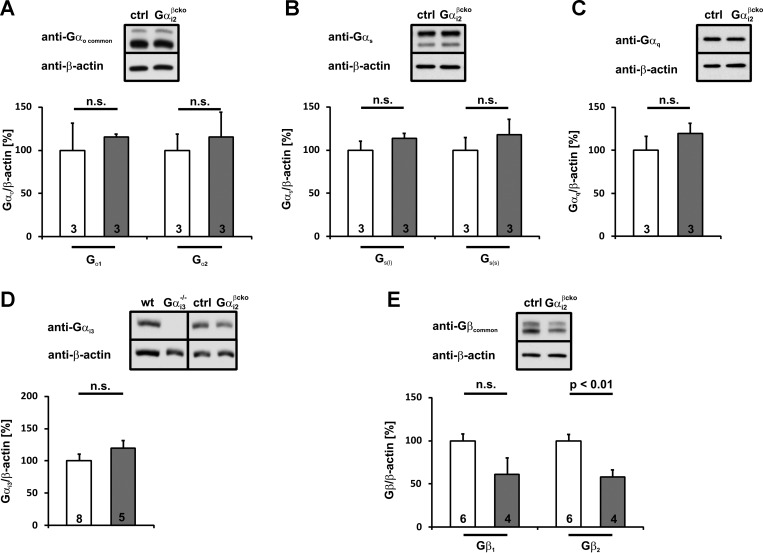
Compensatory upregulation of closely related proteins is a threat of many studies using gene-deficient animals. The expression of almost all Gαi/o isoforms in pancreatic islets as well as Gαq, and Gαs has been described. All of them perform major functions in the insulin secretion machinery. To get insight into possible upregulation and compensation in Gαi2 deficiency by other G protein family members, Gαi2−/− (Fig. 2) and Gαi2βcko (Fig. 3) islet homogenates were immunoblotted for putative changes in the expression of Gαi3, Gαo1, Gαo2, Gαq, Gαs(short), and Gαs(long). The expression levels of the Gαo1, Gαo2, Gαq, and Gαs isoforms remained at the control values in Gαi2−/− (Fig. 2, A–C) and Gαi2βcko islets (Fig. 3, A–C). In contrast, the highly homologous isoform Gαi3 was significantly upregulated in Gαi2−/− islets (Fig. 2D) (146.3 ± 14.3% of wt, P < 0.001). Importantly, in Gαi2βcko islets, Gαi3 protein levels remained unchanged (119.7 ± 12.2% of control, P = 0.22; Fig. 3D).
Fig. 2.
Statistical analysis of different Gα- and Gβ-subunit expression patterns in Gαi2−/− islets. Gαo1, Gαo2 (A), Gαs(long), Gαs(short) (B), and Gαq (C) expression levels are not affected by Gαi2 deletion in Gαi2−/− islets. D: Gαi3 protein upregulation is detectable in Gαi2−/− islets. To verify Gαi3 antibody specificity, Gαi3−/− islets were loaded. E: Gβ1 and Gβ2 protein levels are downregulated in Gαi2−/− islets. Gβ2 downregulation gains statistical significance. Insets: representative immunoblots of islet homogenates from wt and Gαi2−/−. Equal loading was confirmed by β-actin or α-tubulin detection.
Fig. 3.
Statistical analysis of different Gα- and Gβ-subunit expression patterns in Gαi2βcko islets. Gαo1, Gαo2 (A), Gαs(long), Gαs(short) (B), Gαq (C), and Gαi3 (D) protein expression levels are not influenced by Gαi2-deletion in Gαi2βcko islets. Gαi3 antibody specificity was shown by loading of Gαi3−/− islets. E: downregulation of Gβ1 and Gβ2 expression levels in Gαi2βcko islets. Gβ2 downregulation gains statistical significance. Insets: representative immunoblots of islet homogenates from wt and Gαi3−/− or ctrl and Gαi2βcko animals. Equal loading was confirmed by β-actin detection.
G proteins are heterotrimeric proteins consisting of an α-subunit and a βγ-complex. Upon ligand activation of a GPCR, the α-subunit dissociates from the receptor and from the βγ-complex. Both the α-subunit and the βγ-complex modulate the activity of a variety of effectors, including ion channels and enzymes. Due to high Gαi expression levels, the receptor-dependent activation of Gαi results in the release of significant amounts of βγ-complexes (4). Moreover, it has been shown that stabilization of GDP-bound Gα by Gβγ is likely a major mechanism for the maintenance of stoichiometry between Gα and Gβγ. (9, 18). Therefore, an important point was to investigate whether expression levels of the β-isoforms were altered in Gαi2−/− and Gαi2βcko islets. Unaltered Gβ-protein levels, but simultaneously reduced Gα protein levels, might result in permanently free βγ-complexes and increased βγ-mediated effects. This could mask the physiological consequences of Gαi2 deletion. At least five different Gβ subunits (Gβ1–Gβ5) have been described (13), with β1 and β2 being predominantly and ubiquitously expressed. In both mutant mouse lines, lower expression of Gβ1 (Gαi2−/− 64.3 ± 9.6% of wt, P = 0.06; Gαi2βcko 61.1 ± 19.5% of ctrl, P = 0.1) and Gβ2 (Gαi2−/− 80.3 ± 4.3% of wt, P < 0.01; Gαi2βcko 58.2 ± 8.2% of ctrl, P < 0.01) were noted in islet homogenates compared with the respective controls (Figs. 2E and 3E). However, the reduction of Gβ2 levels was more prominent in Gαi2βcko islets than in Gαi2−/− islets, most likely due to increased Gαi3 protein levels (145%) in Gαi2−/− islets. The reduced Gβ protein levels let it seem rather unlikely that effects observed in the gene-targeted mice resulted from increased Gβγ complex activity.
Metabolic evaluation of Gαi2−/− and Gαi2βcko mice.
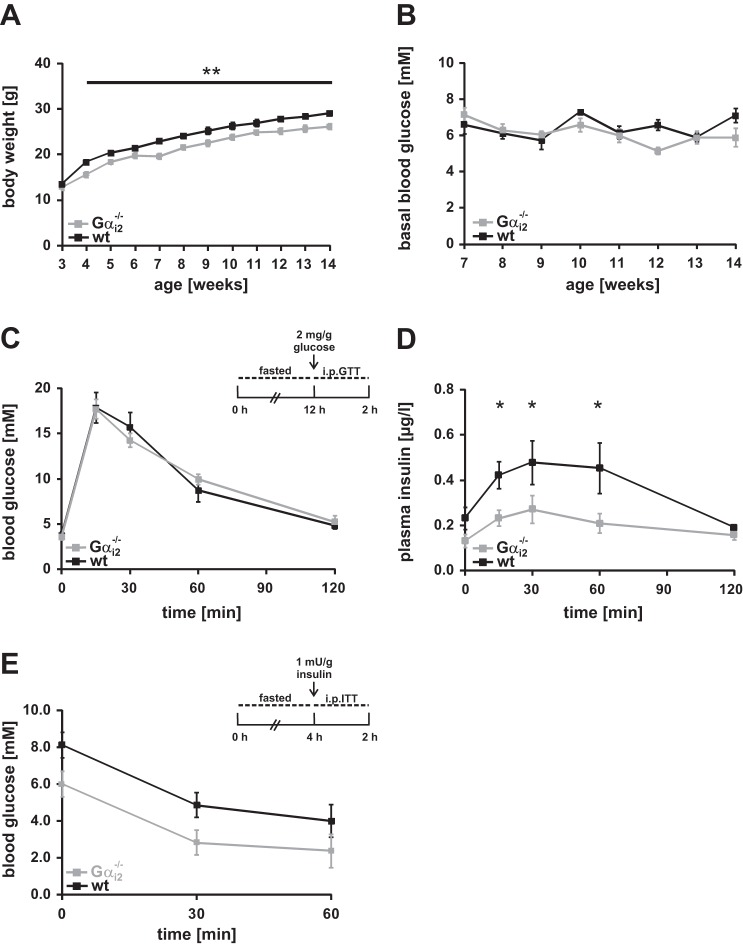
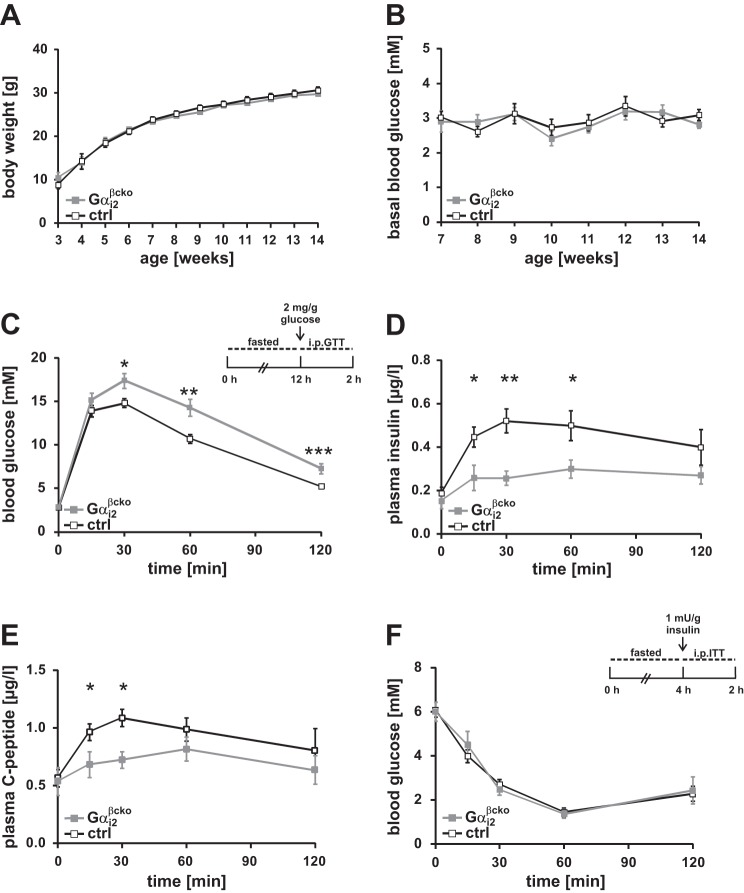
To study the consequences of Gαi2 deletion on the metabolism, we performed metabolic analysis of WT vs. Gαi2−/− (Fig. 4) and ctrl vs. Gαi2βcko (Fig. 5) mice. Global Gαi2 deficiency is associated with a reduced body weight gain (22, 29), which was still observed when animals were kept under specific pathogen-free conditions (SPF) in IVCs (Fig. 4A). Differences in ad libitum-fed blood glucose levels were not obvious (Fig. 4B). Next an ip glucose tolerance test after overnight fasting was performed to analyze the impact of Gαi2 deletion on the glucose homeostasis of these animals. Global deletion of Gαi2 did not affect blood glucose levels during the glucose challenge (Fig. 4C). Interestingly, significantly lower insulin levels were measured in Gαi2−/− mice at 15, 30, and 60 min after glucose injection compared with WT mice (Fig. 4D). In addition, Gαi2−/− animals displayed an improvement in insulin tolerance (Fig. 4E). To assess whether β-cell-specific deletion of Gαi2 influences the body weight of Gαi2βcko in a similar way as observed in Gαi2−/− animals, we recorded body weight and fasted blood glucose levels over time in Gαi2βcko and ctrl animals (Fig. 5, A and B). Continuous measurements did not reveal any differences between these two groups. Again, we monitored blood glucose levels following an ip injection of glucose after an overnight fasting period in the Gαi2βcko and ctrl mice (Fig. 5C). β-Cell-specific Gαi2 deletion caused significantly increased blood glucose levels in the Gαi2βcko mice 30, 60, and 120 min after the glucose challenge, pointing to an impaired glucose tolerance. The parallel analysis of plasma insulin levels during the glucose tolerance test revealed significantly lower plasma insulin levels at 15, 30, and 60 min in Gαi2βcko mice vs. the littermate controls (Fig. 5D). Consistent with the reduced plasma insulin, plasma C-peptide levels were significantly decreased at 15 and 30 min after the glucose bolus in Gαi2βcko mice (Fig. 5E). Insulin sensitivity, tested by the injection of insulin to 4-h-fasted Gαi2βcko and ctrl mice, revealed no differences between the two groups (Fig. 5F). These findings indicate that Gαi2 is involved in the expression, turnover, or release of insulin from pancreatic islets. Higher plasma insulin levels would have been expected from previous PTx-based studies. Surprisingly, Gαi2 deletion restricted to β-cells lowered plasma insulin levels. Collectively, these results indicate that deletion of Gαi2 in β-cells impairs glucose tolerance by decreasing insulin secretion, suggesting that Gαi2 is a positive regulator of insulin secretion.
Fig. 4.
Metabolic phenotyping of Gαi2−/− animals. A: body weight gain of wt and Gαi2−/− mice. Body weight of Gαi2−/− mice is significantly reduced. B: blood glucose levels determined in wt and Gαi2−/− mice over time, which are comparable between the 2 genotypes (n = 16 animals per genotype). C: blood glucose levels in 8- to 10-wk-old wt (■) and Gαi2−/− ( ) mice following ip application of glucose after overnight fasting are equal (n = 8 animals per group). D: plasma insulin levels during ip glucose tolerance testing in wt and Gαi2−/− animals (n = 8 animals per group). Plasma insulin levels are significantly reduced at 15, 30, and 60 min. E: insulin tolerance testing in 4-h-fasted wt and Gαi2−/− mice (n = 6 per genotype). *P < 0.05.
) mice following ip application of glucose after overnight fasting are equal (n = 8 animals per group). D: plasma insulin levels during ip glucose tolerance testing in wt and Gαi2−/− animals (n = 8 animals per group). Plasma insulin levels are significantly reduced at 15, 30, and 60 min. E: insulin tolerance testing in 4-h-fasted wt and Gαi2−/− mice (n = 6 per genotype). *P < 0.05.
Fig. 5.
Metabolic phenotyping of Gαi2βcko mice. A: body weight determined in ctrl and Gαi2βcko mice over time. Body weight gain was similar in ctrl and Gαi2βcko mice (n = 8 animals per genotype). B: fasted blood glucose levels monitored in ctrl (□) and Gαi2βcko ( ) mice over time. C: blood glucose levels of 8- to 10-wk-old ctrl and Gαi2βcko mice following ip application of glucose after overnight fasting (n = 16 animals per group). Gαi2βcko mice have impaired glucose tolerance. Plasma insulin (D) and plasma C-peptide (E) levels during ip glucose tolerance testing were significantly reduced in Gαi2βcko mice (n = 5–8 animals per group). F: insulin tolerance test in 4-h-fasted ctrl and Gαi2βcko mice (n = 8 animals per group). *P < 0.05, **P < 0.01, ***P < 0.001.
) mice over time. C: blood glucose levels of 8- to 10-wk-old ctrl and Gαi2βcko mice following ip application of glucose after overnight fasting (n = 16 animals per group). Gαi2βcko mice have impaired glucose tolerance. Plasma insulin (D) and plasma C-peptide (E) levels during ip glucose tolerance testing were significantly reduced in Gαi2βcko mice (n = 5–8 animals per group). F: insulin tolerance test in 4-h-fasted ctrl and Gαi2βcko mice (n = 8 animals per group). *P < 0.05, **P < 0.01, ***P < 0.001.
Islet function in vitro.
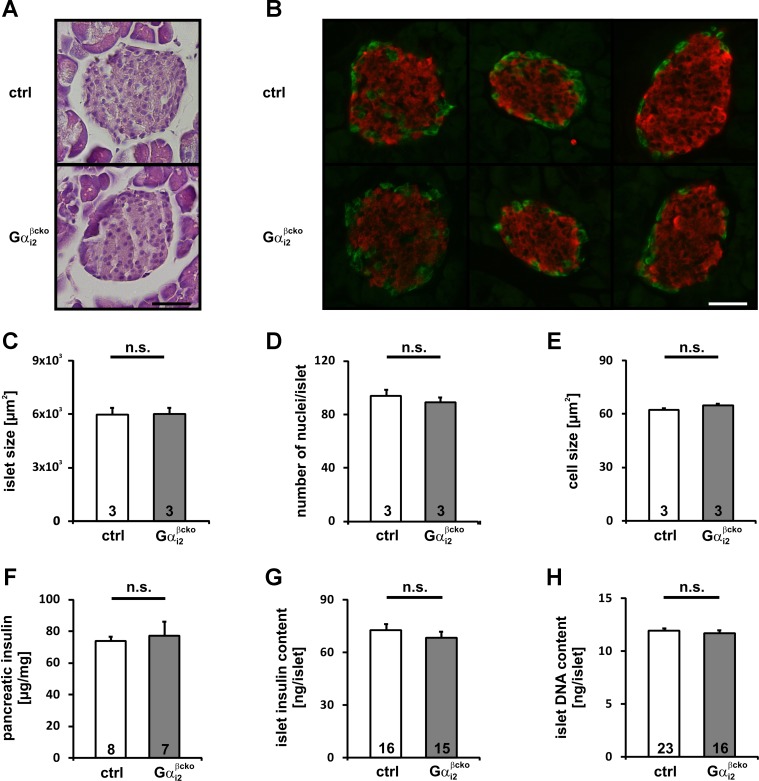
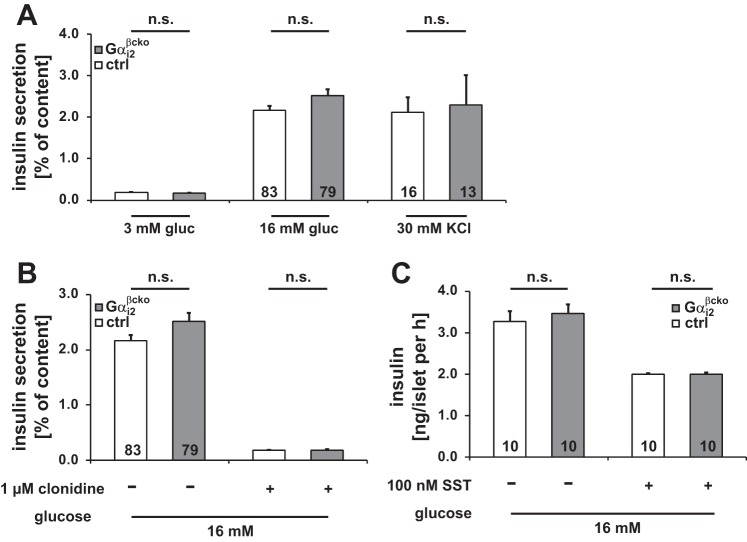
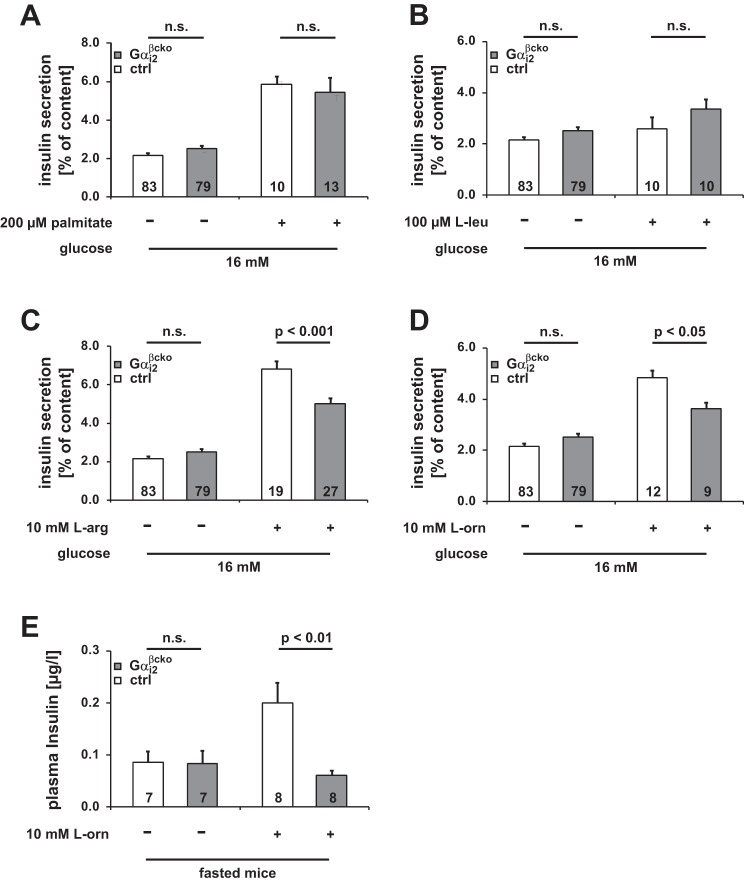
In Gαi2βcko mice, Gαi2 deletion is restricted to β-cells, Gαi3 is not upregulated in this conditional gene-targeted mouse line, and body weight is not affected by β-cell Gαi2 deletion. Thus, these mice are an appropriate model for studying the molecular mechanism of Gαi2 signaling in isolated islets independently of systemic effects. No abnormalities were detected in morphometric analysis of Gαi2βcko islets. Islet area, number of nuclei and islet size (Fig. 6, A–E), pancreas and islet insulin content, and whole islet DNA content were indistinguishable between ctrl and Gαi2βcko islets (Fig. 6, F–H). We performed insulin secretion studies on islets isolated from Gαi2βcko and ctrl mice. Insulin release was studied either under low (3 mM) or high (16 mM) glucose concentrations. All Gαi2βcko and ctrl islets produced a robust insulin-secretory response to high glucose concentrations, whereas insulin release was almost absent at low glucose concentrations (Fig. 7A and Table 1). Insulin secretion evoked by high K+ concentration, which bypasses glucose metabolism, was also not affected by the deletion of Gαi2 in Gαi2βcko islets (Fig. 7A). Since a large body of PTx-based studies suggested that Gαi2 proteins inhibit insulin secretion (11, 15, 28), we tested clonidine- and somatostatin (SST)-induced inhibition of insulin secretion. The inhibiting effects of clonidine (1 μM) and SST (100 nM) were unaffected by the absence of Gαi2 in Gαi2βcko islets (Figs. 7, B and C). This argues against an inhibitory role of Gαi2 in the regulation of insulin secretion from β-cells. In contrast, significantly reduced in vivo plasma insulin levels in Gαi2βcko mice suggest that Gαi2 modulates glucose-induced insulin release by an unknown parameter, for instance, a factor present in the serum, i.e., fatty acids and/or amino acids, which signals via islet GPCRs (37). To identify the stimulus, we analyzed the effect of the free fatty acid palmitate on insulin secretion in Gαi2βcko islets. Simultaneous stimulation of insulin with high glucose and palmitate (200 μM) resulted in increased insulin secretion in ctrl and Gαi2βcko islets (Fig. 8A). Next, we studied amino acids. Application of l-leucine (100 μM) did not alter the insulin response either in ctrl or Gαi2βcko islets (Fig. 8B). However, application of l-arginine and l-ornithine surprisingly produced significantly differing insulin secretion responses in ctrl and Gαi2βcko islets. Costimulation of l-arginine (10 mM) together with glucose (16 mM) showed decreased insulin release from Gαi2βcko islets compared with ctrl islets (Fig. 8C). To test whether l-arginine signals via nitric oxide (NO), we used l-ornithine. In combination with glucose, l-ornithine (10 mM) was able to mimic the effect observed with l-arginine (Fig. 8D). None of the tested inhibitors or stimulators used within this setup was able to induce significant insulin secretion from isolated islets in the presence of 3 mM glucose (Table 1). To test whether our findings of significantly reduced l-arginine- and l-ornithine-induced insulin secretion from Gαi2βcko islets are of any physiological relevance, we performed an in vivo l-ornithine challenge. l-Ornithine was used instead of the more potent stimulus l-arginine to exclude interferences by NO-driven pathways. We studied whether a single dose of l-ornithine (1.32 mg/ml l-ornithine ip) affects plasma insulin levels in vivo in male mice. Whereas plasma insulin levels of control mice immediately increased after the l-ornithine application, plasma insulin levels of Gαi2βcko animals remained at basal levels (Fig. 8E). Taken together, the reduced l-ornithine-induced insulin secretion in vitro and in vivo strengthen the assumption that the PTx-sensitive Gαi2 isoform is not an inhibitor but a stimulator of insulin secretion.
Fig. 6.
Morphometric analyses of Gαi2βcko islets. A: representative H&E-stained ctrl and Gαi2βcko islets. B: representative sections of pancreata from ctrl and Gαi2βcko mice visualized by immunofluorescence with anti-insulin (red) and anti-glucagon (green) antibodies. Scale bar, 40 μm. Islet size (C), number of nuclei per islet (D), and mean cell size (E) of ctrl and Gαi2βcko islets were equal. Morphometric analyses were performed in 6-wk-old male mice. In total, 381 control and 455 Gαi2βcko islets derived from 3 mice per genotype were quantified for this evaluation. Whole pancreas insulin content (F), islet insulin (G), and islet DNA content (H) of ctrl and Gαi2βcko mice did not differ between genotypes. Islet insulin or islet DNA content was determined following static incubation experiments (n, number of triplicates).
Fig. 7.
Insulin secretion experiments in ctrl and Gαi2βcko islets. A: glucose-induced insulin secretion measured at 3 and 16 mM glucose and 30 mM KCl (3 mM glucose: ctrl n = 60; Gαi2βcko n = 59; n, number of incubations). Inhibitory effect of clonidine (B) and SST (C) on glucose-induced insulin secretion (1 μM clonidine: ctrl n = 15; Gαi2βcko n = 14) was not affected by deletion of Gαi2 in β-cells. All conditions were performed in islet preparations derived from ≥3 animals per genotype.
Table 1.
Insulin secretion evoked by 3 mM glucose concentration and in the presence of the respective substances
| Ctrl |
Gαi2βcko |
|||||||
|---|---|---|---|---|---|---|---|---|
| Secreted insulin |
Secreted insulin |
|||||||
| Condition |
%Insulin content | SE | n | %Insulin content | SE | n | P | |
| 3 mM glucose | 0.189 | ± 0.02 | 28 | 0.174 | ± 0.02 | 27 | n.s. | |
| 3 mM glucose | 1 μM clonidine | 0.153 | ± 0.05 | 5 | 0.064 | ± 0.01 | 4 | n.s. |
| 100 nM SST | 0.176 | ± 0.05 | 4 | 0.141 | ± 0.04 | 3 | n.s. | |
| 200 mM palmitate | 0.324 | ± 0.04 | 2 | 0.470 | ± 0.02 | 2 | n.s. | |
| 100 μM l-leucine | 0.292 | ± 0.05 | 3 | 0.329 | ± 0.06 | 3 | n.s. | |
| 10 mM l-arginine | 0.075 | ± 0.02 | 3 | 0.143 | ± 0.03 | 4 | n.s. | |
| 10 mM l-ornithine | 0.150 | ± 0.02 | 6 | 0.039 | ± 0.01 | 5 | n.s. | |
n.s., not significant; n, set of triplicates.
Fig. 8.
Effect of different stimuli on insulin secretion experiments in ctrl and Gαi2βcko islets. Stimulatory effect of palmitic acid (A), and l-leucine (B) was not affected by deletion of Gαi2 in β-cells. Potentiating effect of l-arginine (C) and l-ornithine (D) on insulin secretion was significantly reduced in Gαi2βcko (n, number of incubations). All conditions were performed in islet preparations derived from ≥3 animals per genotype. E: plasma insulin levels before and 1 min after ip injection of l-ornithine.
[Ca2+]i measurements in islets.
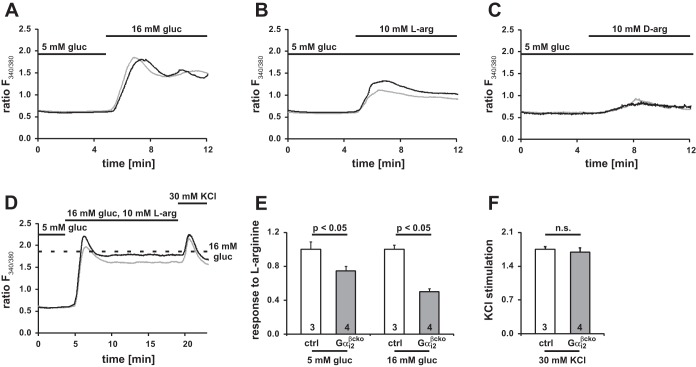
To gain detailed insight into the signaling pathway, we aimed to link our in vitro findings to an intracellular correlate. It is commonly accepted that insulin secretion is a result of an increase in [Ca2+]i. This can be determined in single islet cells and is therefore a cell-based correlate for β-cell insulin secretion. Measurements of intracellular calcium changes link the Gαi-dependent signaling to insulin secretion at a cellular level. Changes in [Ca2+]i signals for individual β-cells were recorded in Fura 2-loaded single islet cells. For basal conditions, β-cells were kept at 5 mM glucose before treatment with 16 mM glucose. Control and Gαi2βcko single islet cells showed a similar increase in [Ca2+]i in response to the application of 16 mM glucose (Fig. 9A). The Ca2+ response was significantly reduced in Gαi2βcko islet cells when stimulated with l-arginine (10 mM) in the presence of basal glucose levels (5 mM) (Fig. 9B). Being aware that a simple depolarization event could be caused by the positive charge of the basic amino acid, we tested d-arginine (10 mM), the enantiomer of l-arginine. In contrast to l-arginine, d-arginine did not induce a calcium response either in Gαi2βcko or in ctrl single islet cells (Fig. 9C). In contrast, the l-arginine-induced response was reduced by 25% (Fig. 9E, left). The reduced response to l-arginine of Gαi2βcko cells was even more evident when β-cells were coexposed to high glucose concentrations and l-arginine (Fig. 9D). The l-arginine-induced increase in [Ca2+]i was calculated as the difference between the maxima obtained with glucose plus l-arginine (Fig. 9D) minus the maxima obtained with glucose alone (Fig. 9A). The cotreatment resulted in a robust increase in [Ca2+]i in control islets, whereas the response of Gαi2βcko islet cells was significantly reduced by 44% (Fig. 9E, right). The Ca2+ responses evoked by high K+ were similar in ctrl and Gαi2βcko β-cells (Fig. 9, D and F). These analyses identify β-cell Gαi2 as necessary for the stimulation of insulin secretion by l-arginine in a calcium-dependent manner but independent of a depolarization that may be caused by the positive charge of the amino acid.
Fig. 9.
Calcium measurements in dispersed ctrl and Gαi2βcko β-cells. Representative [Ca2+]i ratios (ctrl, black line; Gαi2βcko, gray line) elicited by 16 mM glucose (A), 10 mM l-arginine at 5 mM glucose (B), or 10 mM d-arginine at 5 mM glucose (C) (A, ctrl n = 3 mice, 465 cells; Gαi2βcko n = 4 mice, 298 cells; B, ctrl n = 7 mice, 606 cells; Gαi2βcko n = 5 mice, 687 cells; C, n = 3 mice, 447 cells; Gαi2βcko n = 3 mice, 413 cells). D: representative [Ca2+]i response to 16 mM glucose in combination with 10 mM l-arginine and final application of KCl (ctrl n = 3 mice, 585 cells; Gαi2βcko n = 4 mice, 687 cells). Dotted line indicates maximum response to 16 mM glucose shown in A. E: maximum response to l-arginine was significantly reduced by 25% at 5 mM and 44% at 16 mM glucose concentrations in Gαi2βcko β-cells compared with ctrl β-cells, whereas the response is the difference between the maxima obtained with glucose plus l-arginine (D) minus the maxima obtained with glucose alone (A); n = 3–4 animals per group. F: maximum depolarization evoked by 30 mM KCl (n = 3–4 animals per group). All recordings were started at 5 mM glucose with additions as shown on the figure.
DISCUSSION
The present study analyzed the physiological role of Gαi2 in insulin-secreting β-cells by using global and β-cell-specific Gαi2 mutants. Significantly lower plasma insulin levels were observed upon glucose challenge in both the Gαi2βcko and the Gαi2−/− animal models. A normal glucose-induced insulin secretion in vitro per se accompanied these in vivo findings. Importantly, the selective and specific stimulatory effects of amino acids such as l-arginine and l-ornithine on insulin secretion were significantly diminished in Gαi2-deficient β-cells. This was not due to alterations in islet morphology, since islet size, the number of nuclei per islet, and islet cell size were unaffected by the Gαi2 deficiency. This study provides causal evidence for the involvement of Gαi2 in the positive regulation of plasma insulin levels and glucose tolerance in vivo and islet insulin secretion in vitro. Although PTx-based studies in mice and isolated islets assumed an inhibitory role for all Gαi and Gαo proteins, our data surprisingly indicate that Gαi2 proteins rather stimulate than inhibit the secretion of insulin by regulating l-arginine- and l-ornithine-induced insulin secretion in vitro and in vivo.
Currently, the upstream regulator of Gαi2 is unknown. Two recent studies describe mouse models lacking the nutrient receptor GPCR family C group 6 member A (GPRC6A), which is a receptor for l-amino acids (35), in particular those with basic side chains (24, 36). Whereas Smajilovic et al. (31) found evidence for the expression of GPRC6A in pancreatic islets but found no evidence that the l-arginine-induced insulin secretion was affected by GPRC6A deletion, Pi et al. (25, 26) reported that GPRC6A mediates osteocalcin- and l-arginine-induced insulin secretion from β-cells in global and β-cell-specific GPRC6A-deficient mouse models. Our data from global and β-cell-specific Gαi2-deficient mice showing significantly decreased plasma insulin levels following glucose challenge are consistent with those from global and β-cell-deficient GPRC6A mice (25). Future studies will have to clarify whether the concordant findings in the GPRC6A-deficient and Gαi2-deficient mice are mechanistically connected.
Gαi2βcko islets secreted significantly less insulin upon costimulation with glucose and l-ornithine or l-arginine. These findings are complementary to recent studies analyzing global and β-cell-specific gene-targeted Gαo mice (42). Those authors suggest that Gαo is a strong inhibitor of insulin secretion, since its ablation produced a strong stimulatory effect on insulin release. Gαo has been implicated in regulating insulin vesicle docking on β-cell membranes (42) and in inhibiting insulin release via signaling through galanin- and SST-activated GPCRs (32, 34). In line with these findings, herein we find significant inhibition of insulin secretion by SST in both control and Gαi2βcko islets. Of course, we are aware of the fact that these findings conflict with the expected functions for Gαi2 based on PTx experiments, a pan inhibitor of Gαi and Gαo proteins and therefore a strong stimulator of insulin secretion. However, clonidine, an α2-adrenoreceptor agonist, completely inhibited insulin release in control and Gαi2βcko islets. Thus, our data set provides additional evidence that Gαi2 is not involved in the α2-adrenoreceptor-mediated signaling. The inhibition of the inhibitory Gαo-driven pathway by PTx may be the major cause of PTx-induced insulin release from islets.
Moreover, the PTx effect was negligible in β-cell-specific Gαo-deficient animals (42). This suggests that, despite the fact that Gαi proteins being expressed in islet cells and ADP-ribosylated by PTX, Gαo is the major target for PTx stimulating insulin secretion, thereby making an inhibitory role of Gαi2 very unlikely (42). A single study shows an inhibitory role for Gαi2 in ghrelin-dependent inhibition of insulin secretion based on an antisense oligonucleotide approach (5). Our data demonstrate that deletion of Gαi2 resulted in normal glucose-induced insulin secretion per se, but stimulatory effects of amino acids like l-arginine and l-ornithine on insulin secretion were diminished in Gαi2βcko islets. Moreover, Gαi2βcko mice exhibit impaired glucose tolerance caused by significantly decreased plasma insulin levels. This is also in contrast to data obtained from PTx-based studies, strengthening again the fact that Gαi2 is an important stimulator of insulin secretion. This view is also supported by the failure of in vivo-applied l-ornithine to provoke a rise in insulin plasma levels in β-cell-specific Gαi2-deficient animals, which was seen in littermate controls.
Our detailed analysis of G protein levels in the Gαi2 gene-targeted mouse lines corroborates significant upregulation of Gαi3 (about 45%) in the global Gαi2-deficient mice, which has also been described for other tissues and organs (6, 17, 39, 40). In contrast to the global Gαi2-deficient islets, the Gαi3 protein levels were unchanged in Gαi2βcko islets. Since plasma insulin levels are reduced in both gene-targeted mouse lines, Gαi3 is obviously not able to compensate for the lack of Gαi2. This confirms previous reports on specific and distinct functions of Gαi2 and Gαi3 (8, 10, 17). Reduced Gβ expression levels parallel deletion of Gαi2. Heterotrimeric G proteins are stoichiometrically composed of Gα and Gβγ, which dissociate selectively during the activation cycle. The stabilization of Gα and Gβγ in the trimeric complex is likely a major mechanism for the maintenance of the stoichiometric equivalence between Gα and Gβγ in the cell (9, 18). We found Gβ levels significantly reduced in both global and β-cell-specific Gαi2-deficient mouse lines, with a more pronounced reduction of Gβ levels in Gαi2βcko islets (see Fig. 3E) than in Gαi2−/− islets (see Fig. 2E). It is reasonable to assume that the differences in the extent of reduction between Gαi2−/− and Gαi2βcko islets result from the selective upregulation of Gαi3 protein in Gαi2−/− animals only, as Gαi3 also requires Gβ as counterpart for the stabilization. This is in line with results from approaches using shRNA to downregulate Gβ isoforms in HeLa cells, which was accompanied with parallel reduced Gα protein levels in this cell line. Vice versa, several studies show that shRNA against Gαi2 resulted in decreased Gβ1, Gβ2, and Gβ4 protein levels (18, 39, 40). It has to be noted that Gβ2 is the predominant isoform in islets, which has been reported only for human placenta (23). Signaling by the Gαi family of “inhibitory” proteins is complex. Gαi proteins can activate or inhibit many other effectors through either the GTP-bound Gαi or the released Gβγ dimer upon activation. Therefore, it is important to note that both the abolished signaling of Gαi2 via direct interaction partners and the signaling via Gβγ complexes may equally contribute to the observed phenotypes of our gene-targeted mice.
The reduced insulin levels during glucose tolerance tests were observed in global and β-cell-specific Gαi2-deficient mice. Differences in glucose were visible only in Gαi2βcko animals, not in mice globally lacking Gαi2. Wang et al. (34) also analyzed blood glucose levels of global Gαi2-deficient mice, which were, similar to our studies, unaltered upon glucose challenge. However, those authors did not study β-cell-specific Gαi2 mutants, and they did not report the plasma insulin levels during the glucose challenge. A reduced body weight of Gαi2−/− mice has been reported (29) and is also observed in our mouse line. A lean phenotype improves glucose tolerance per se, which might explain similar blood glucose levels but simultaneously decreased plasma insulin levels in global Gαi2 mutants. In line with this, Gαi2-deficient mice showed improved insulin tolerance, arguing for the improved peripheral insulin sensitivity. The lean phenotype was not observed in β-cell-specific Gαi2 mutants. Control and mutant animals showed similar weight gain. However, Gαi2βcko animals showed impaired glucose tolerance caused by impairment of the β-cells' ability to secrete the required amount of insulin. The differences in blood glucose levels between global and β-cell-specific Gαi2 mice imply that the metabolic functions of Gαi2 in the body are broader than previously thought. Gαi2 signaling might play a role for glucose homeostasis in liver, white adipose tissue, or skeletal muscle as well, but Gαi2 signaling is also important for efficient pancreatic β-cell function. Thus, we propose that the diminished plasma insulin levels indeed result from islet Gαi2 dysfunction, caused by an impaired potentiating effect of l-arginine and l-ornithine in pancreatic β-cells. In addition, the reduced increases of intracellular calcium levels upon l-arginine treatment in Gαi2βcko β-cells strengthen the finding that Gαi2 stimulates insulin secretion in a calcium-dependent manner. However, it remains elusive whether regulation of intracellular calcium release and/or extracellular calcium influx is disturbed by the Gαi2 deletion.
In conclusion, the experiments presented identify Gαi2 as an endogenous positive modulator of the l-arginine- and l-ornithine-induced stimulation of insulin secretion, which may be an important feedback mechanism for fine-tuning amino acid plasma levels and insulin secretion. Interestingly, the PTx-sensitive Gαi2 isoform was identified as a stimulator of insulin secretion. This strengthens the hypothesis that Gαo and Gαi proteins have distinct, nonredundant, and importantly opposite roles in pancreatic β-cells: 1) Gαo isoforms function as inhibitors of insulin secretion and are the main isoform being responsible for the dysregulated insulin release upon PTx treatment; and 2) Gαi2 isoforms stimulate insulin secretion upon l-arginine and l-ornithine treatment.
GRANTS
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) with grants to B. Nürnberg and V. Leiss, and GRK 1302 to B. Nürnberg, by the Intramural Program of the National Institutes of Health to L. Birnbaumer (Project Z01 ES-101684), and by the German Ministry of Education and Research (BMBF: DZD, 01GI0922) to A. Schürmann.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.L., A. Schürmann, C.H., and B.N. conception and design of research; V.L., K.F., A.N., M.R., and A. Schönsiegel performed experiments; V.L., K.F., M.R., and A. Schönsiegel analyzed data; V.L., K.F., L.B., A. Schürmann, C.H., and B.N. interpreted results of experiments; V.L. and K.F. prepared figures; V.L., C.H., and B.N. drafted manuscript; V.L., K.F., A.N., L.B., A. Schürmann, C.H., and B.N. edited and revised manuscript; V.L., K.F., A.N., M.R., A. Schönsiegel, L.B., A. Schürmann, C.H., and B.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We kindly thank Pedro Herrera, University of Geneva, for providing us with the Rip-Cre mouse line. We express our appreciation to Hans-Ulrich Häring and Susanne Ullrich for critical discussion of results and manuscript. We thank Lennart Fiedler, Anna-Floriane Hennig, and Anna-Elisa Glaser for genotyping the mice. We acknowledge the support of U. Schmidt, and B. Schreiner for assistance at the BioPlex 200 System. We thank Bianca Walter for performing morphological analysis.
REFERENCES
- 1.Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 8: 369–385, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft FM, Proks P, Smith PA, Ammala C, Bokvist K, Rorsman P. Stimulus-secretion coupling in pancreatic β-cells. J Cell Biochem 55 Suppl: 54–65, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Birnbaumer L. G proteins in signal transduction. Annu Rev Pharmacol Toxicol 30: 675–705, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Clapham DE, Neer EJ. G protein βγ subunits. Annu Rev Pharmacol Toxicol 37: 167–203, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Dezaki K, Kakei M, Yada T. Ghrelin uses Gαi2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes 56: 2319–2327, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Dizayee S, Kaestner S, Kuck F, Hein P, Klein C, Piekorz RP, Meszaros J, Matthes J, Nürnberg B, Herzig S. Gαi2- and Gαi3-specific regulation of voltage-dependent l-type calcium channels in cardiomyocytes. PloS one 6: e24979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Exner T, Jensen ON, Mann M, Kleuss C, Nürnberg B. Posttranslational modification of Gao1 generates Gao3, an abundant G protein in brain. Proc Natl Acad Sci USA 96: 1327–1332, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezan J, Lasvaux L, Gezer A, Novakovic A, May-Simera H, Belotti E, Lhoumeau AC, Birnbaumer L, Beer-Hammer S, Borg JP, Le Bivic A, Nürnberg B, Sans N, Montcouquiol M. Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nat Cell Biol 15: 1107–1115, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Gohla A, Klement K, Nürnberg B. The heterotrimeric G protein Gi3 regulates hepatic autophagy downstream of the insulin receptor. Autophagy 3: 393–395, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Gulbenkian A, Schobert L, Nixon, Tabachnick II. Metabolic effects of pertussis sensitization in mice and rats. Endocrinology 83: 885–892, 1968 [DOI] [PubMed] [Google Scholar]
- 12.Herrera PL, Orci L, Vassalli JD. Two transgenic approaches to define the cell lineages in endocrine pancreas development. Mol Cell Endocrinol 140: 45–50, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H. Genomic characterization of the human heterotrimeric G protein α, β, and γ subunit genes. DNA Res 7: 111–120, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Katada T, Ui M. Effect of in vivo pretreatment of rats with a new protein purified from Bordetella pertussis on in vitro secretion of insulin: role of calcium. Endocrinology 104: 1822–1827, 1979 [DOI] [PubMed] [Google Scholar]
- 15.Katada T, Ui M. In vitro effects of islet-activating protein on cultured rat pancreatic islets. Enhancement of insulin secretion, adenosine 3′:5′-monophosphate accumulation and 45Ca flux. J Biochem 89: 979–990, 1981 [PubMed] [Google Scholar]
- 16.Klose C, Straub I, Riehle M, Ranta F, Krautwurst D, Ullrich S, Meyerhof W, Harteneck C. Fenamates as TRP channel blockers: mefenamic acid selectively blocks TRPM3. Br J Pharmacol 162: 1757–1769, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Köhler D, Devanathan V, Bernardo de Oliveira Franz C, Eldh T, Novakovic A, Roth JM, Granja T, Birnbaumer L, Rosenberger P, Beer-Hammer S, Nürnberg B. Gαi2- and Gαi3-deficient mice display opposite severity of myocardial ischemia reperfusion injury. PloS one 9: e98325, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krumins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J Biol Chem 281: 10250–10262, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic β-cell function. J Biol Chem 281: 2649–2653, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Leiss V, Friebe A, Welling A, Hofmann F, Lukowski R. Cyclic GMP kinase I modulates glucagon release from pancreatic α-cells. Diabetes 60: 148–156, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leopoldt D, Harteneck C, Nürnberg B. G proteins endogenously expressed in Sf 9 cells: interactions with mammalian histamine receptors. Naunyn Schmiedebergs Arch Pharmacol 356: 216–224, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Minetti GC, Feige JN, Bombard F, Heier A, Morvan F, Nürnberg B, Leiss V, Birnbaumer L, Glass DJ, Fornaro M. Gαi2 signaling is required for skeletal muscle growth, regeneration, and satellite cell proliferation and differentiation. Mol Cell Biol 34: 619–630, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nürnberg B, Harhammer R, Exner T, Schulze RA, Wieland T. Species- and tissue-dependent diversity of G-protein β-subunit phosphorylation: evidence for a cofactor. Biochem J 318: 717–722, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, Christenson L, Li B, Zhang J, Jackson PD, Faber P, Brunden KR, Harrington JJ, Quarles LD. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PloS one 3: e3858, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pi M, Wu Y, Lenchik NI, Gerling I, Quarles LD. GPRC6A mediates the effects of l-arginine on insulin secretion in mouse pancreatic islets. Endocrinology 153: 4608–4615, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. J Bone Miner Res 26: 1680–1683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plummer NW, Spicher K, Malphurs J, Akiyama H, Abramowitz J, Nürnberg B, Birnbaumer L. Development of the mammalian axial skeleton requires signaling through the Gαi subfamily of heterotrimeric G proteins. Proc Natl Acad Sci USA 109: 21366–21371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, Scanlan TS, Hebrok M, Coughlin SR. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest 117: 4034–4043, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in Gαi2-deficient mice. Nat Genet 10: 143–150, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Schulz N, Kluth O, Jastroch M, Schürmann A. Minor role of mitochondrial respiration for fatty-acid induced insulin secretion. Int J Mol Sci 14: 18989–18998, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smajilovic S, Clemmensen C, Johansen LD, Wellendorph P, Holst JJ, Thams PG, Ogo E, Brauner-Osborne H. The l-α-amino acid receptor GPRC6A is expressed in the islets of Langerhans but is not involved in l-arginine-induced insulin release. Amino Acids 44: 383–390, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Tang G, Wang Y, Park S, Bajpayee NS, Vi D, Nagaoka Y, Birnbaumer L, Jiang M. Go2 G protein mediates galanin inhibitory effects on insulin release from pancreatic β-cells. Proc Natl Acad Sci USA 109: 2636–2641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tonack S, Tang C, Offermanns S. Endogenous metabolites as ligands for G protein-coupled receptors modulating risk factors for metabolic and cardiovascular disease. Am J Physiol Heart Circ Physiol 304: H501–H513, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Park S, Bajpayee NS, Nagaoka Y, Boulay G, Birnbaumer L, Jiang M. Augmented glucose-induced insulin release in mice lacking Go2, but not Go1 or Gi proteins. Proc Natl Acad Sci USA 108: 1693–1698, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wellendorph P, Brauner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335: 37–46, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Wellendorph P, Johansen LD, Jensen AA, Casanova E, Gassmann M, Deprez P, Clement-Lacroix P, Bettler B, Brauner-Osborne H. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol 42: 215–223, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev 85: 1159–1204, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Wicksteed B, Brissova M, Yan W, Opland DM, Plank JL, Reinert RB, Dickson LM, Tamarina NA, Philipson LH, Shostak A, Bernal-Mizrachi E, Elghazi L, Roe MW, Labosky PA, Myers MG, Jr., Gannon M, Powers AC, Dempsey PJ. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 59: 3090–3098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiege K, Ali SR, Gewecke B, Novakovic A, Konrad FM, Pexa K, Beer-Hammer S, Reutershan J, Piekorz RP, Schmidt RE, Nürnberg B, Gessner JE. Gαi2 is the essential Gαi protein in immune complex-induced lung disease. J Immunol 190: 324–333, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Wiege K, Le DD, Syed SN, Ali SR, Novakovic A, Beer-Hammer S, Piekorz RP, Schmidt RE, Nürnberg B, Gessner JE. Defective macrophage migration in Gαi2- but not Gαi3-deficient mice. J Immunol 189: 980–987, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Zawalich WS, Zawalich KC. Regulation of insulin secretion by phospholipase C. Am J Physiol Endocrinol Metab 271: E409–E416, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Zhao A, Ohara-Imaizumi M, Brissova M, Benninger RK, Xu Y, Hao Y, Abramowitz J, Boulay G, Powers AC, Piston D, Jiang M, Nagamatsu S, Birnbaumer L, Gu G. Gαo represses insulin secretion by reducing vesicular docking in pancreatic beta-cells. Diabetes 59: 2522–2529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]