Abstract
The purpose of the study was to investigate if the number of goblet cells expanded ex vivo from a conjunctival explant is affected by the biopsy harvesting site on the conjunctiva and the distance from the explant. Conjunctival explants from six regions: superior and inferior bulbus, fornix, and tarsus of male Sprague–Dawley rats were grown in RPMI 1640 with 10% fetal bovine serum on coverslips for eight days. Histochemical and immunofluorescent staining of goblet (CK-7/UEA-1/1MUC5AC), stratified squamous, non-goblet (CK-4), proliferating (PCNA) and progenitor (ABCG2) cells were analyzed by epifluorescence and laser confocal microscopy. Outgrowth was measured with NIH ImageJ. For statistical analysis the Mann–Whitney test and Spearman’s rank–order correlation test were used. Cultures from superior and inferior fornix contained the most goblet cells as indicated by the presence of CK-7+, UEA-1+ and MUC5AC+ cells. Superior and inferior forniceal cultures displayed 60.8% ± 9.2% and 64.7% ± 6.7% CK-7+ cells, respectively, compared to the superior tarsal (26.6% ± 8.4%; P < 0.05), superior bulbar (31.0% ± 4.0%; P < 0.05), inferior bulbar (38.5% ± 9.3%; P < 0.05) and inferior tarsal cultures (27.7% ± 8.3%; P < 0.05). While 28.4% ± 6.3% of CK-7+ goblet cells co-labeled with PCNA, only 7.4% ± 1.6% of UEA-1 + goblet cells did (P < 0.01). CK-7+ goblet cells were located at a lower concentration close to the explant (39.8% ± 3.1%) compared to near the leading edge (58.2% ± 4.5%; P < 0.05). Both markers for goblet cell secretory product (UEA-1 and MUC5AC), however, displayed the opposite pattern with a higher percentage of positive cells close to the explant than near the leading edge (P < 0.05). The percentage of CK-4+ cells was higher near the explant compared to near the leading edge (P < 0.01). The percentage of CK-7+ goblet cells in the cultures did not correlate with the outgrowth size (rs = −0.086; P = 0.435). The percentage of UEA-1 + goblet cells correlated negatively with outgrowth size (rs = −0.347; P < 0.01), whereas the percentage of CK-4+ cells correlated positively with the outgrowth size (rs = 0.473; P < 0.05). We conclude that forniceal explants yield the highest number of goblet cells ex vivo and thereby seem to be optimal for goblet cell transplantation. We also suggest that CK-7+/UEA-1− cells represent highly proliferative immature goblet cells. These cells could be important during conjunctival migration as they are mostly located close to the leading edge and their density does not decrease with increasing outgrowth size.
Keywords: conjunctival epithelium, goblet cells, cell culture, transplantation
1. Introduction
Conjunctival epithelium, which covers the posterior surface of the lids and the anterior surface of the globe, consists of two cell types: stratified, squamous, non-goblet epithelium and goblet cells. The latter cells produce the main part of the soluble mucin component in the tear film (Shapiro et al., 1981). In cases of ocular surface disease, the protective and supportive role of the conjunctiva may be restored by transplantation of ex vivo expanded conjunctival epithelial cells (Ang et al., 2005). However, in order for a conjunctival substitute to be successful, it must contain goblet cells (Schrader et al., 2009). A decreased number of goblet cells is associated with tear film instability and symptoms of dry eye (Cennamo et al., 2008). Moreover, a decreased number of goblet cells can be observed in Stevens Johnson syndrome, ocular mucous membrane pemphigoid, and graft versus host-disease (Pflugfelder et al., 1997). Despite the importance of goblet cells in conjunctival regeneration the effect of biopsy location on the number of goblet cells has not been explored.
In the human fetus goblet cells first appear in the fornix and then move towards the tarsal and bulbar regions at nine weeks (Miyashita et al., 1992). Like intestinal and respiratory epithelial stem cells, conjunctival stem cells are bipotent, i.e. capable of committing to both non-goblet and goblet cell lineage (Pellegrini et al., 1999). The differentiation of goblet cells depends on numerous factors, including the substrate (Tsai and Tseng, 1988), number of cell doublings (Pellegrini et al., 1999), cell environment (Meller et al., 2002) and growth factors (Li et al., 2010). Limbal epithelial progenitor cells have been reported to differentiate with increasing distance from the explant (Kolli et al., 2008). However, whether the distance from the explant also affects the differentiation of conjunctival goblet cells has not been investigated.
Several studies have demonstrated that fornix contains the highest number of goblet cells (Goller and Weyrauch, 1993; Huang et al., 1988; Lavker et al., 1998; Moore et al., 1987). Nevertheless, to our knowledge, only two groups have reportedly looked for goblet cell associated proteins in cultures originating from different conjunctival regions (Nizam et al., 2008; Wei et al., 1993). Wei et al. found cytokeratin (CK) 18 (which is expressed in goblet cells (Kasper, 1991)) in cultures from bulbar, forniceal and palpebral conjunctiva by western blot (Wei et al., 1993). However, the AE1 antibody used for detection of CK-18 in that study, is not specific for CK-18, but also detects CK-19, which is present in non-goblet conjunctival epithelial cells (Kasper et al., 1988). Nizam et al. found cells expressing the goblet cell marker MUC5AC by immunocytochemistry in cultures from bulbar, forniceal and palpebral conjunctiva (Nizam et al., 2008). Still, neither groups quantified the amount of goblet cells obtained in culture, and nor did they compare the percentages of goblet cells between the cultured conjunctival regions. Thus, the optimal harvesting site for the culture of goblet cells is yet to be determined.
We recently reported optimal outgrowth ex vivo when using forniceal conjunctival explants, in contrast to tarsal and bulbar explants (Eidet et al., 2012a). In the present study, we sought to determine the best conjunctival harvesting site for the culture of goblet cells and whether the number of goblet cells in culture depends on the distance from the explant. We found that forniceal explants yield the highest number of goblet cells ex vivo and thereby should to be optimal for goblet cell transplantation. Moreover, we suggest that CK-7+/UEA-1− cells represent highly proliferative immature goblet cells. The CK-7+/UEA-1− cells could also be of importance during conjunctival migration as they are mostly located close to the leading edge and their density does not decrease with increasing outgrowth size.
2. Materials and methods
2.1. Explant culture
All removal of tissue and subsequent manipulations of animals used in this study conformed to the guidelines established by the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the Schepens Eye Research Institute Animal Care and Use Committee. Conjunctiva removed from left eyes of male Sprague–Dawley rats (Shatos et al., 2001) was used for culture. Conjunctival tissue from six regions, the superior and inferior bulbar, forniceal and tarsal membranes, were harvested and cultured on glass coverslips in RPMI 1640 medium (BioWhittaker, Walkersville, MD) supplemented with 10% heat-inactivated FBS (HyClone Laboratories, Logan, UT), 2 mM l-glutamine (BioWhittaker), and 100 µg/ml penicillin–streptomycin (BioWhittaker) for eight days, as previously described (Eidet et al., 2012a). There were no significant differences in the average size of the explants (range: 0.85–1.11 mm2) cultured from the six conjunctival regions when analyzed by ImageJ as described in Section 2.8 for the outgrowth measurements (Fig. 1).
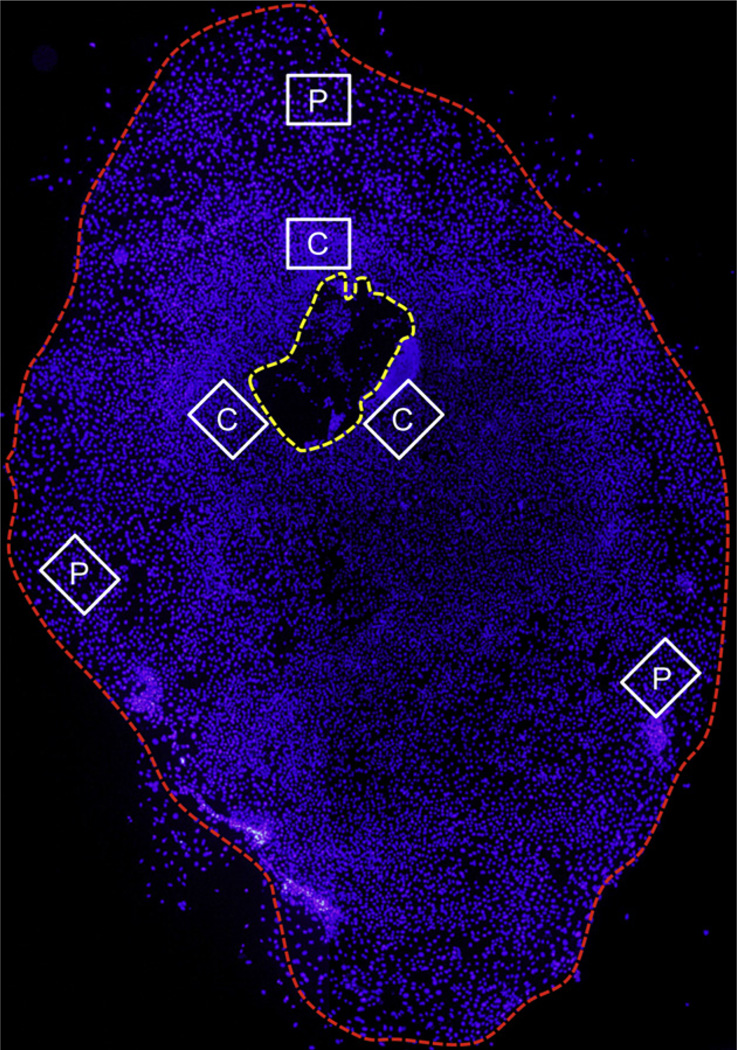
Fig. 1.
Schematic photomicrograph of a conjunctival epithelial primary culture where the cell nuclei have been stained with DAPI (blue). The yellow dotted line indicates the explant, while the red dotted line marks the leading edge of the outgrowth. Six micrographs (×630) for phenotypic analysis were captured centrally in the culture 1–3 cell lengths from the explant (C) and peripherally 1–3 cell lengths from the leading edge (P). Magnification: ×40.
2.2. Preparation of rat conjunctival sections
Right eyes with eyelids were surgically removed and fixed in 4% formaldehyde in PBS overnight at 4 °C. The eyes were then cut vertically in the midline into two equal halves. The two specimens were washed in PBS and dehydrated by graded ethanol solutions (70%–100%) and subsequently embedded in paraffin. Semi-thin (6 µm) sections from the central part of the eye where the lens diameter was the largest were cut and placed on gelatin-coated slides (Nunc, Inc., Naperville, IL) for use in histochemical and immunohistochemical experiments.
2.3. Histochemistry
Sections were deparaffinized, rehydrated and subjected to antigen retrieval before histochemistry and immunohistochemistry. The antigen retrieval step was added as formaldehyde fixation is known to mask antigens due to cross-linking of proteins (Abcam). Antigen retrieval was performed by exposing the sections to 95– 100 °C for 20 min submersed in a buffer solution consisting of 10 mM Tris, 1 mM EDTA and 0.05% Tween-20.
For lectin histochemistry, sections were incubated in blocking buffer for one hour at room temperature, followed by incubation for one hour at room temperature with UEA-1 lectin (Vector Laboratories, Burlingame, CA), a marker for goblet cell selective high molecular weight glycoconjugates (Table 1), conjugated directly to fluorescein isothiocyanate (FITC) diluted 1:3000 in PBS.
Table 1.
Characterization of rat conjunctival epithelial cells.
| Markers | Specificity | Investigator criteria for positive staining | Investigator A and B agreement |
|
|---|---|---|---|---|
| Correlation between investigators |
95% CI of difference between investigators |
|||
| CK-7 | Goblet cells (cytokeratin) | Stained fibrils encircling a nucleus | rs = 0.949; P < 0.001 | −2.2% to +3.2% |
| MUC5AC | Goblet cells (peptide core of secreted soluble mucins) | Stained vesicles encircling a nucleus | rs = 0.926; P < 0.001 | −3.0% to −0.8% |
| UEA-1 lectin | Goblet cells (high molecular weight glycoconjugates) | Stained vesicles encircling a nucleus | rs = 0.985; P< 0.001 | 0% to +2.4% |
| CK-4 | Stratified, squamous, non-goblet epithelial cells (cytokeratin) | Stained fibrils encircling a nucleus | rs = 0.911; P< 0.001 | −2.2% to −1.0% |
| PCNA | Proliferating cells (cell cycle associated protein) | Stained nucleus | rs = 0.929; P < 0.001 | +0.2% to +5.2% |
| ABCG2 | Undifferentiated cells (cell membrane transport protein) | Stained cell membrane covering a nucleus | * | * |
CI = confidence interval; rs = Spearman’s correlation coefficient; * = not quantified.
Explant cultures grown on glass coverslips were rinsed in PBS, and fixed in 100% methanol for 15 min at room temperature before they were returned to fresh PBS. Fixed cells were incubated in blocking buffer that consisted of 1% bovine serum albumin (BSA) and 0.2% Triton X-100 in PBS for one hour at room temperature. Cells then were incubated for one hour at room temperature with UEA-1 conjugated directly to FITC diluted 1:1000 in PBS.
2.4. Immunohistochemistry
Sections were incubated for one hour at room temperature in blocking buffer. Cells were then incubated overnight at 4 °C with antibodies for markers of goblet cells and non-goblet ocular surface epithelial cells (Table 1): CK-7 (RCK105) (Santa Cruz Biotechnology), which recognizes a goblet cell-specific keratin (Kasper, 1991) (1:200); MUC5AC (45M1) (Thermo Fischer Scientific, Fremont, CA), specific for mucin produced by goblet cells (Argueso and Gipson, 2001) (1:100); CK-4 (6B10) (Abcam Inc., Cambridge, MA), specific for stratified, squamous, non-goblet epithelial cells (Kasper, 1991) (1:200); ABCG2 (M-70) (Santa Cruz Biotechnology, Santa Cruz, CA), a putative stem cell marker (1:200); and PCNA (FL-261) (Santa Cruz Biotechnology), a marker for proliferative capacity (1:200). The anti-mouse or rabbit IgG secondary antibodies (Jackson Immuno Research Laboratories, Inc., West Grove, PA), conjugated to either Cy2 or Cy3, were diluted 1:100 and 1:300, respectively, in PBS and incubated for one hour at room temperature. Slides were washed three times in PBS, after which coverslips were mounted on the slides with mounting media containing 100 mM Tris (pH 8.5), 25% glycerol, 10% polyvinyl alcohol, and 2.5% l,4-diazobicyclo-[2.2.2]-octane.
Coverslips with methanol-fixed cells were incubated for one hour at room temperature in blocking buffer. Cells were then incubated with the following primary antibodies diluted in PBS overnight at 4 °C: CK-7 (1:200), MUC5AC (1:100), CK-4 (1:200), ABCG2 (1:200) and PCNA (1:200). The secondary antibodies, conjugated to either Cy2 or Cy3, were diluted 1:100 and 1:300, respectively, in PBS and incubated for one hour at room temperature. Coverslips were washed three times in PBS, after which coverslips were mounted on microscope slides with mounting media.
Cell cultures and sections adherent to glass coverslips and microscope slides were visualized with a fluorescence microscope (Eclipse E 800; Nikon, Tokyo, Japan) or laser confocal microscopy (Leica TCS-SP5; Leica Microsystems, Wetzlar, Germany). Negative controls consisted of substituting PBS for the primary antibody. Positive controls included fixed sections of whole rat eyes with eyelids containing structures with known positive staining for each of the antibodies used. Semi-quantitative immunohistochemical analysis of the sections was carried out at a magnification of 630 × and scored as previously described (Eidet et al., 2012b). Positive staining in the sections was graded as 0 (undetectable), + (detectable in <l/4 of the cells), ++ (detectable in 1/4–1/2 of the cells), +++ (detectable in 1/2–3/4 of the cells) and ++++ (detectable in >3/4 of the cells).
2.5. Calculation of phenotypic data from rat conjunctival cultures
Expression of the various markers was assessed at a magnification of 630 ×, and 100 cells in six fields were counted in each culture (n = 6; Fig. 1). The number of positive cells/total number of cells × 100% was calculated. To assess the reliability of the data, scores from two independent investigators were compared to compute observer agreement (Table 1).
2.6. Comparison of goblet cell proliferative capacity
The proliferative profile of goblet cells was assessed independently of harvesting site by co-labeling antibody to PCNA with CK-7 and UEA-1. The percentage of CK-7+ and UEA-1 + cells, respectively, that co-expressed PCNA+ in primary culture after eight days was assessed at a magnification of 630 × by two independent investigators (n = 6). In order to uncover possible co-expression of markers for undifferentiated cells and goblet cells, antibody to ABCG2 was co-labeled with antibody to CK-7 and UEA-1 in primary culture after eight and 14 days.
2.7. Assessment of goblet cells at different locations in culture
The number of goblet cells and stratified, squamous, non-goblet epithelial cells at different locations in the cultures was characterized independently of harvesting site. The phenotypic data from the three random ‘central’ fields near the explant (at the 12, 4 and 8 o’clock positions; Fig. 1) were compared with data from the three ‘peripheral’ fields near the leading edge (at the 12, 4 and 8 o’clock positions) for each marker (n = 6).
2.8. Comparison of outgrowth size with the number of goblet cells
Nuclei of epithelial cells in eight-day old primary cultures from six conjunctival regions were stained with DAPI and outgrowth was visualized with a fluorescence microscope (Eclipse E 800; Nikon, Tokyo, Japan) at a magnification of 40×. Occasional fibroblasts were recognized by morphology and excluded. Outgrowth size was then quantified using ImageJ software (National Institutes of Health, Bethesda, MD) and correlated with phenotypic data from six random fields from each primary culture (Fig. 1).
2.9. Statistical analysis
The Mann–Whitney test was used to compare phenotypic data between all six conjunctival regions as well as between cells near the explant and close to the leading edge. To analyze the relationship between outgrowth size and phenotypic data, the Spearman’s rank–order correlation test was used. Spearman’s rank–order correlation test and a paired sample T-test were employed to assess observer agreement (Section 2.5). Data were expressed as mean ± SEM. A significance level of 5% was used throughout the study (SPSS ver. 18.0).
3. Results
3.1. Location of goblet cells in sections of conjunctival epithelia
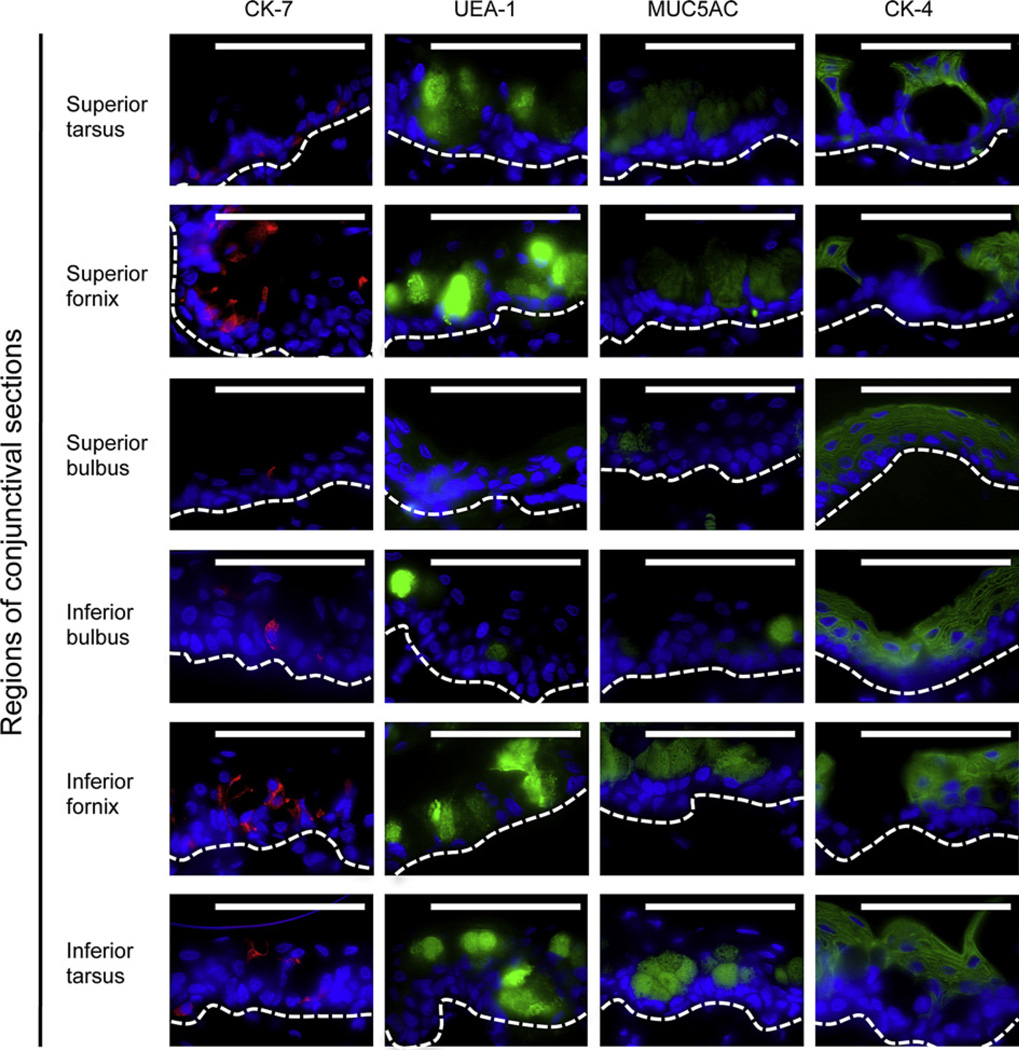
To determine the location of goblet cells, sections of rat conjunctiva were stained with anti-CK-7 and -MUC5AC antibodies, and the lectin UEA-1 (Fig. 2). All three goblet cell markers showed fewest goblet cells in the superior bulbar region, followed by the inferior bulbus, while the highest concentrations were found in the forniceal and tarsal regions (Table 2). Distribution of the CK-4+ stratified, squamous non-goblet epithelial cells was mainly opposite that of the goblet cells, with higher concentrations in the bulbar regions than in the forniceal regions (Fig. 2 and Table 2).
Fig. 2.
Immunostaining of the goblet cell markers anti-CK-7 (red), UEA-1 lectin (green) and MUC5AC (green), and the marker for stratified squamous non-goblet cells anti-CK-4 (green) in sections from six conjunctival regions. Micrographs are representative of six animals. Nuclei were stained with DAPI (blue). The white dotted line indicates the approximate position of the basal membrane. Magnification: ×630. Scale bars: 100 µm.
Table 2.
Semi-quantitative immunohistochemical analysis of rat conjunctival epithelial sections.
| Conjunctival region | CK-7 | UEA-1 | MUC5AC | CK-4 |
|---|---|---|---|---|
| Superior tarsus | + | +++ | +++ | ++ |
| Superior fornix | ++ | +++ | +++ | ++ |
| Superior bulbus | + | + | + | +++ |
| Inferior bulbus | + | ++ | ++ | +++ |
| Inferior fornix | ++ | +++ | +++ | ++ |
| Inferior tarsus | + | +++ | +++ | +++ |
The immunoreactivity was graded as 0 (undetectable), + (detectable positivity in <l/4 of the cells), ++ (detectable positivity in 1/4–1/2 of the cells), +++ (detectable positivity in 1/2–3/4 of the cells) and ++++ (detectable positivity in >3/4 of the cells).
3.2. Number of goblet cells in cultures from six conjunctival regions
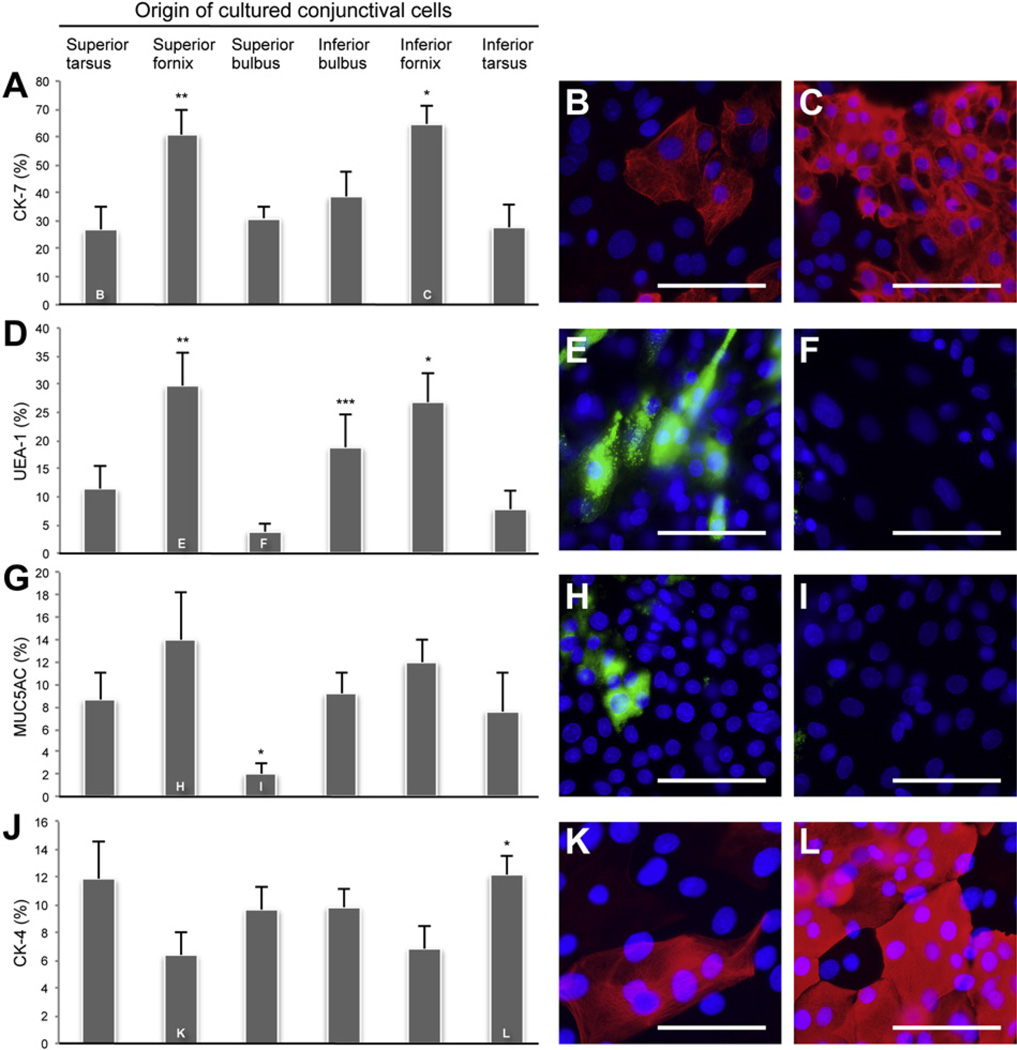
To identify goblet cells, explant cultures from all six conjunctival regions were stained with anti-CK-7 and -MUC5AC antibodies, and the lectin UEA-1. The percentage of CK-7+ cells was greater in cultures originating from the superior fornix (60.8% ± 9.2%; Fig. 3A) than in superior tarsal (P < 0.05), superior bulbar (P < 0.05), and inferior tarsal cultures (P < 0.05). CK-7+ cells were also more abundant in cultures from the inferior forniceal region (64.7% ± 6.7%) than from the superior tarsal (P < 0.05), superior bulbar (P < 0.05), inferior bulbar (P < 0.05), and inferior tarsal regions (P < 0.05). Fig. 3B and C are representative photomicrographs from the superior tarsal and inferior forniceal cultures, which contained the least and the most CK-7+ goblet cells, respectively. There was an increased percentage of UEA-1+ cells in cultures from the superior fornix (29.6% ± 6.0%; Fig. 3D) compared to the superior bulbus (P < 0.05) and inferior tarsus (P < 0.05). The inferior bulbar cultures (18.8% ± 5.9%) displayed an increased percentage of UEA-1+ cells compared to the superior bulbar cultures (P < 0.05). Cultures from the inferior fornix (26.8% ± 5.0%) also showed a greater percentage of UEA-1+ cells than the superior tarsus (P < 0.05), superior bulbus (P < 0.05), and inferior tarsus (P < 0.05). Fig. 3E and F show superior forniceal and superior bulbar cultures, which contained the most and the least UEA-1 + goblet cells, respectively. The percentage of MUC5AC+ cells was less in cultures from superior bulbus (2.0% ± 0.9%; Fig. 3G – I) than superior tarsus (P < 0.05), superior fornix (P < 0.05), inferior bulbus (P < 0.05), inferior fornix (P < 0.05), and inferior tarsus (P < 0.05). Photomicrographs in Fig. 3H and I are representative of superior forniceal and superior bulbar cultures, which contained the most and the least MUC5AC+ goblet cells, respectively. All three goblet cell markers showed the highest incidence of goblet cells in the two forniceal regions. The two markers of goblet cell secretory product (MUC5AC and UEA-1) had an almost identical distribution with the lowest percentage in superior bulbar cultures. The distribution of goblet cells cultured ex vivo was similar to that seen in vivo with the highest percentages of goblet cells found in cultures originating from the forniceal regions and the lowest percentage in the superior bulbar cultures.
Fig. 3.
Comparison of mean ± SEM (n = 6) percentage of CK-7+, UEA-1+, MUC5AC+ and CK-4+ cells in primary culture from six conjunctival regions. (A) *P < 0.05 compared with superior tarsus, superior bulbus, inferior tarsus, and inferior bulbus. **P < 0.05 compared with superior tarsus, superior bulbus and inferior tarsus. Immunostaining of anti-CK-7 (red) in primary culture from superior tarsus (B) and inferior fornix (C). (D) *P < 0.05 compared with superior tarsus, superior bulbus and inferior tarsus. **P < 0.05 compared with superior bulbus and inferior tarsus. ***P < 0.05 compared with superior bulbus. Staining with UEA-1 lectin (green) in primary culture from superior fornix (E) and superior bulbus (F). (G) *P < 0.05 compared with superior tarsus, superior fornix, inferior bulbus, inferior fornix, and inferior tarsus. Immunostaining of anti-MUC5AC (green) in primary culture from superior fornix (H) and superior bulbus (I). (J) *P < 0.05 compared with superior fornix and inferior fornix. Immunostaining of anti-CK-4 (red) in primary culture from superior fornix (K) and inferior tarsus (L). Nuclei were stained with DAPI (blue). All micrographs are representative of six animals. Magnification:×630. Scale bars: 100 µm.
The percentage of the stratified, squamous, non-goblet CK-4+ cells was higher in cultures from the inferior tarsus (12.2% ± 1.4%; Fig. 3J) than the superior fornix (P < 0.05) and inferior fornix (P < 0.05). Fig. 3K and L are representative of the superior forniceal and inferior tarsal cultures, which contained the least and the most CK-4+ cells, respectively. Thus, as expected, the CK-4+ stratified, squamous cells were less numerous in the goblet cell rich forniceal cultures.
3.3. Proliferative capacity of goblet cells
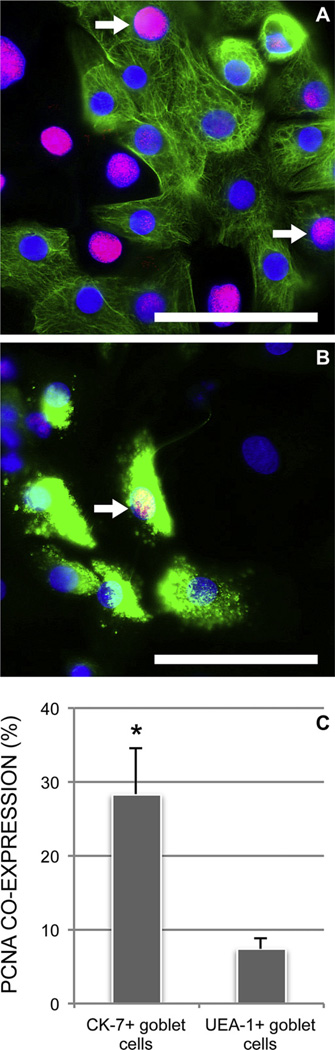
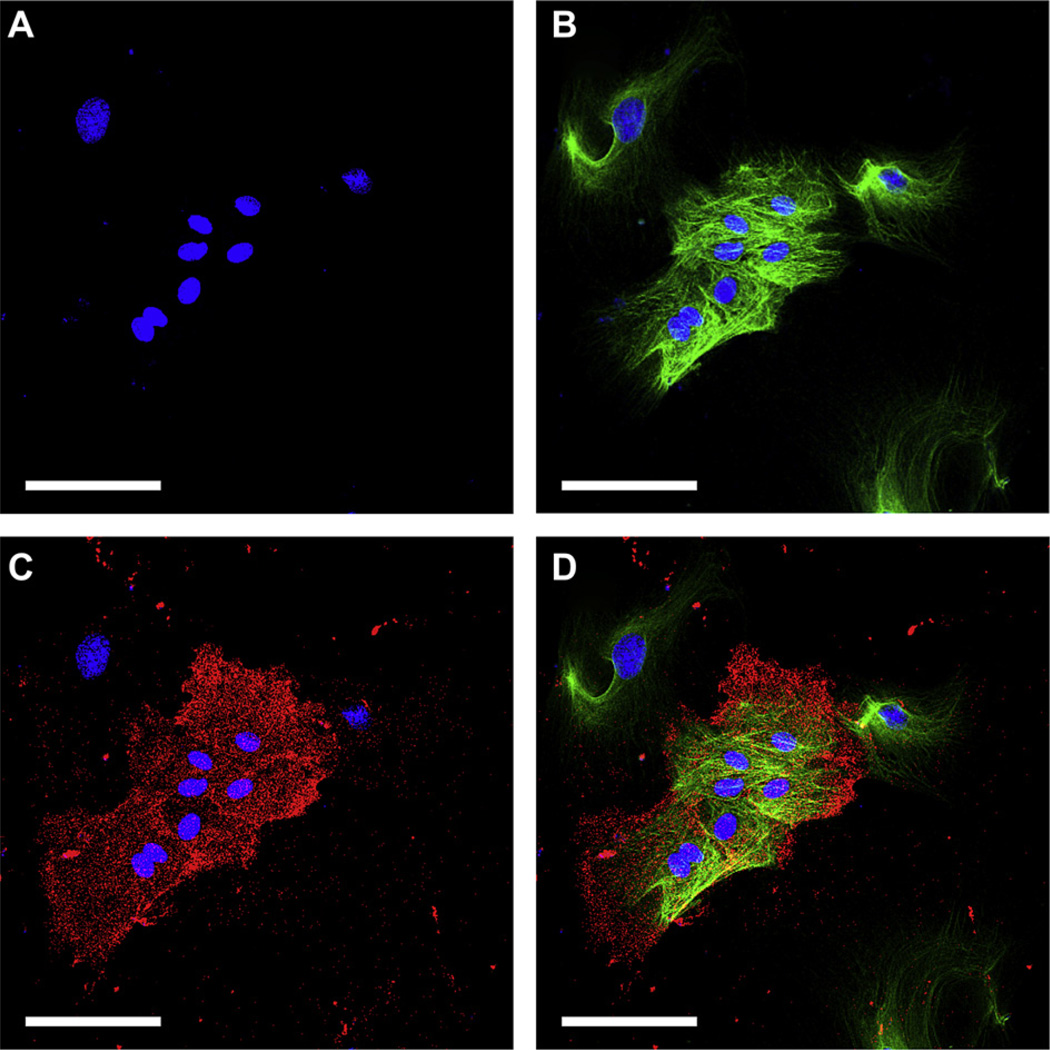
We then investigated if cultured goblet cells, independently of harvesting site, can be sub-divided with respect to proliferative capacity and degree of differentiation. The marker for the goblet cell specific cytokeratin 7 (CK-7) and the marker for goblet cell selective high molecular weight glycoconjugates (UEA-1) were co-labeled in eight-day old rat conjunctival primary cultures (n = 6; data not shown). The CK-7+ cells were either UEA-1 + or UEA-1−, whereas all UEA-1 + cells appeared to be CK-7+. We then co-labeled CK-7 and UEA-1, respectively, with the proliferation marker PCNA and the putative stem cell marker ABCG2. Immunoreactivity against PCNA in primary cultures of rat conjunctival epithelium grown for eight days was higher in CK-7+ cells (28.4% ± 6.3%; Fig. 4) than in UEA-1+ cells (7.4% ± 1.6%; P < 0.01). Assuming that all UEA-1 + cells are CK-7+ this means that the UEA-1 − subgroup of CK-7+ goblet cells is more proliferative than the CK-7+/UEA-1 + goblet cells.
Fig. 4.
The goblet cell markers CK-7 (A, green) and UEA-1 (B, green) were co-labeled with PCNA (red) in primary cultures of rat conjunctival cells independent of harvesting site. Nuclei were stained with DAPI (blue). Both CK-7+ (A, arrows) and UEA-1+ (B, arrow) goblet cells co-expressed PCNA after eight days in culture, indicating cell proliferative activity. Magnification, ×630. Scale bars: 100 µm. Mean ± SEM (n = 6) co-expression of PCNA with CK-7 and UEA-1, respectively, was compared (C). *P < 0.01.
To uncover possible co-expression of goblet cell and undifferentiated cell markers, a primary culture was grown for 14 days and immunostained with anti-ABCG2, CK-7 and UEA-1 lectin. At 14 days in culture the stratified cells had detached and the once coherent sheet of cultured cells was replaced by scattered small colonies that continued to proliferate. The small colonies mainly consisted of ABCG2+ cells that co-expressed CK-7 (Fig. 5), but not UEA-1 (data not shown). These results suggest that CK-7+ cells are less differentiated than UEA-1 + cells and that the CK-7+/UEA-1-cells could represent highly proliferative immature goblet cells.
Fig. 5.
Laser confocal microscopy images obtained after 14 days of rat conjunctival primary culture showing co-expression of the putative stem cell marker ABCG2 and goblet cell marker CK-7. Nuclei were stained with DAPI (A, blue) and cells immunostained with anti-CK-7 (B, green) and ABCG2 (C, red). All cells are CK-7+ with a group of smaller cells co-expressing ABCG2 (D). Magnification: ×400. Scale bars: 100 µm.
3.4. Phenotypic characterization of cells at different locations in the cultures
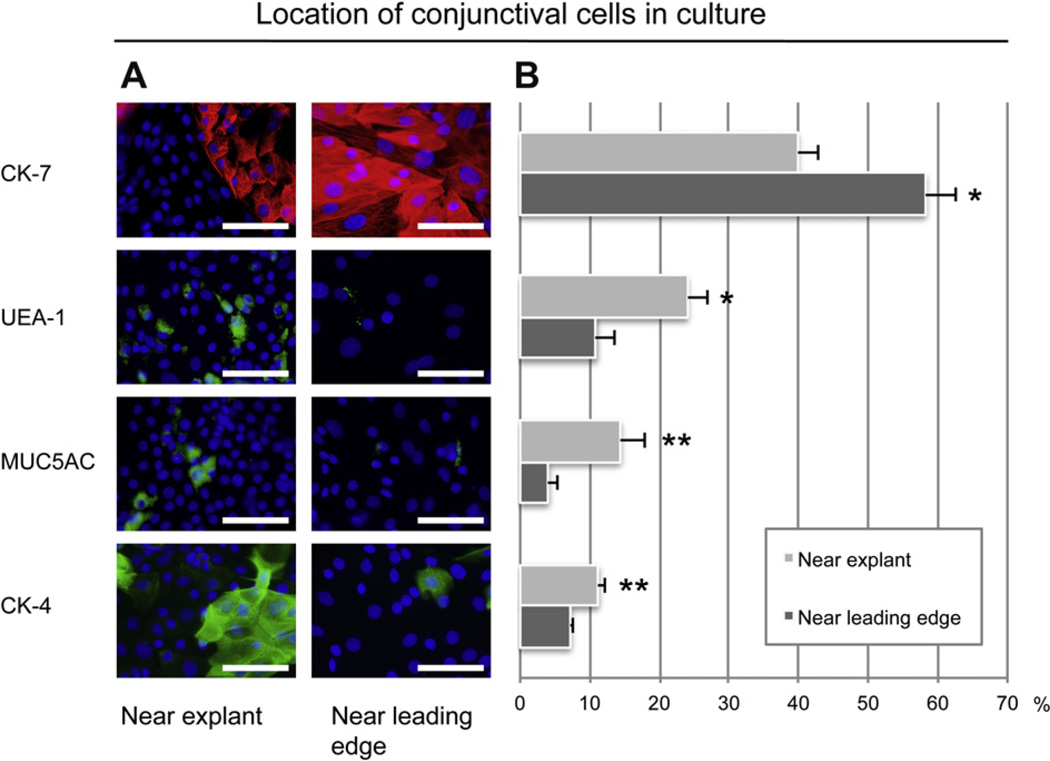
As the CK-7+/UEA-1− and CK-7+/UEA-1+ goblet cells showed different proliferative capacity, we next investigated whether this would affect the location of these cells in the conjunctival primary cultures after eight days. Explant cultures from all six conjunctival regions were combined and percentage of CK-7+, UEA-1+, MUC5AC+ and CK-4+ cells close to the explant were compared with that close to the leading edge using the method shown in Fig. 1. CK-7+ (cell body marker) goblet cells were located at a lower concentration close to the explant (39.8% ± 3.1%; Fig. 6) compared to near the leading edge (58.2% ± 4.5%; P < 0.05). UEA-1 + (secretory product marker) goblet cells however, displayed the opposite pattern with a higher percentage of positive cells close to the explant (24.0% ± 2.9%) than near the leading edge (11.0% ± 2.6%; P < 0.05). The MUC5AC (secretory product marker) immunostaining showed the same pattern as UEA-1 in that most MUC5AC+ cells resided close to the explant (14.4% ± 3.5%) instead of near the leading edge (4.0% ± 1.3%; P < 0.01). These results showed that in primary cultures after eight days, the mucin-filled CK-7+/UEA-1+/MUC5AC+ goblet cells reside mostly around the explant while CK-7+/UEA-1−/MUC5AC− goblet cells are largely located at the leading edge.
Fig. 6.
Independent of harvesting site, the cell phenotype was characterized at different locations in the eight-day old rat primary cultures. (A) Immunostaining of anti-CK-7 (red), UEA-1 lectin (green), MUC5AC (green) and CK-4 (green) was assessed both close to the explant and near the leading edge of the culture. Micrographs are representative of cultures from six animals. Nuclei were stained with DAPI (blue). Magnification: ×630. Scale bars: 100 µm. (B) Comparison of mean ± SEM (n = 6) percentage of CK-7+, UEA-1+, MUC5AC+ and CK-4+ cells near the explant and near the leading edge of the culture. For each antibody, mean ± SEM percentage near the explant and near the leading edge was compared. *P < 0.05. **P < 0.01.
The percentage of CK-4+ cells (stratified, squamous, non-goblet epithelial cells) was higher near the explant (11.2% ± 1.0%; Fig. 6) compared to near the leading edge (7.2% ± 0.5%; P < 0.01). This reflected the observation that the primary cultures were more stratified near the explant compared to near the leading edge.
3.5. Outgrowth size compared to phenotype
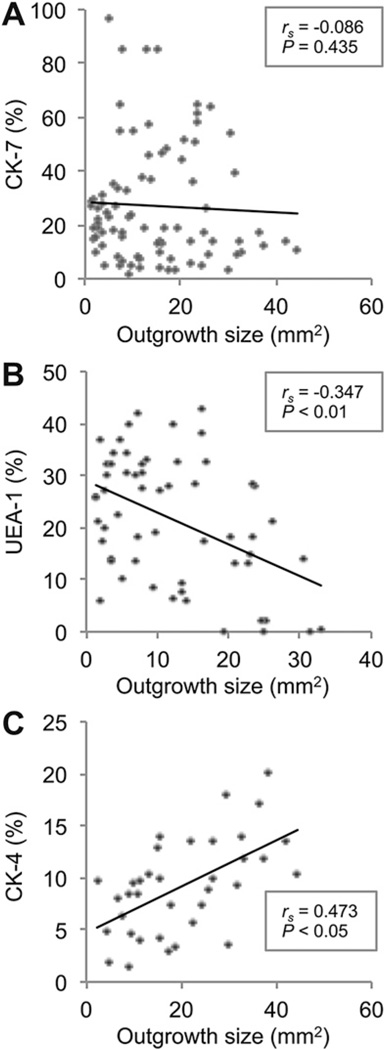
After observing that phenotype of the conjunctival cells partly depended on their location in the primary cultures, we then investigated the effect of the absolute size of the cultures after eight days on phenotype. Primary culture outgrowth measurements from all six conjunctival regions were correlated with the percentage of CK-7+, UEA-1 + and CK-4+ cells in six random fields in each culture using the method shown in Fig. 1. The outgrowth size showed no significant correlation with the percentage of CK-7+ goblet cells in the cultures (rs = −0.086; P = 0.435; 84 paired observations; Fig. 7A). Percentage of UEA-1+ goblet cells correlated negatively with outgrowth size (rs = −0.347; P < 0.01; 58 paired observations; Fig. 7B). In contrast, percentage of CK-4+ stratified, squamous, non-goblet epithelial cells correlated positively with the outgrowth size (rs = 0.473; P < 0.05; 27 paired observations; Fig. 7C). These data show that small cultures contain the highest percentage of mucin-filled UEA-1+ goblet cells at day eight in primary culture, while large cultures contain the highest percentage of stratified, squamous, non-goblet epithelial cells (CK-4+). In contrast, the percentage of CK-7+ goblet cells was independent of culture size, suggesting that CK-7+ cells, compared to UEA-1+ goblet cells, show the largest proliferative and migratory capacity.
Fig. 7.
The relationships among outgrowth size and phenotype in rat primary cultures after eight days were analyzed. (A) Bivariate scattergram illustrating no apparent correlation between outgrowth size and percentage of CK-7+ cells in the culture (84 paired observations). (B) Bivariate scattergram illustrating the negative correlation between outgrowth size and percentage of UEA-1 + cells in the culture (58 paired observations). (C) Bivariate scattergram illustrating the positive correlation between outgrowth size and percentage of CK-4+ cells in the culture (27 paired observations). The Spearman’s correlation test was used. rs = Spearman’s correlation coefficient.
4. Discussion
We recently reported that forniceal conjunctival explants yield the most growth ex vivo, as opposed to tarsal and bulbar explants (Eidet et al., 2012a). We now demonstrate that the forniceal regions also contain the most goblet cells in vivo and give rise to the most proliferative goblet cells ex vivo, as determined by epifluorescence microscopy. These findings further promote fornix as the best harvesting site for culturing conjunctival equivalents. Goblet cells have traditionally been identified through their secretory product using markers including the UEA-1 lectin, anti-MUC5AC antibody and PAS reagent that target the goblet cell gel-forming mucins (Argueso and Gipson, 2001; Kawano et al., 1984). In the clinic, identification of mucin-filled goblet cells on the cornea by impression cytology is one of the diagnostic hallmarks of limbal stem cell deficiency (Puangsricharern and Tseng, 1995). The absence of mucin-filled goblet cells, however, may not exclude the absence of goblet cells altogether. For instance, mucin-filled goblet cells are rarely seen during the initial outgrowth and migration of conjunctival cells even though they reappear later on (Geggel et al., 1984). Our study demonstrates that CK-7+ identifies relatively undifferentiated cells with retained proliferative capacity that are committed to becoming goblet cells, but are not synthesizing or storing secretory product. The feasibility to identify and harvest these cells could improve the production, and subsequently, possibly the survival of goblet cell transplants.
Consistent with previous studies (Goller and Weyrauch, 1993; Huang et al., 1988; Lavker et al., 1998; Moore et al., 1987), the sections of whole rat eyes in our study showed higher concentration of goblet cells, as indicated by CK-7, UEA-1 and MUC5AC, in the forniceal regions than in the bulbar regions. In addition, the superior bulbar region had fewer goblet cells than the inferior bulbar region. A tendency for higher goblet cell concentration in the inferior conjunctiva compared to the superior (Moore et al., 1987) was reported for the first time by Virchow in humans in 1910. The presence of a higher concentration of goblet cells in the inferior conjunctiva has been explained by the level of surface hydration (Kessing, 1968), which is increased in the lower lid sac due to gravitation of the aqueous tear fluid (Moore et al., 1987).
In the present study the number of CK-7+ cells in the cultures was higher than the number of mucin-filled goblet cells. The number of CK-7+ cells has also been demonstrated to be higher than that of mucin-filled goblet cells in vivo (Moore et al., 2011). In the sections of the current study, however, the percentage of CK-7+ cells was lower than that of the mucin-filled goblet cells. The relatively low percentage of CK-7+ cells in the sections may be attributed to three factors. First, Moore et al. used ethanol as cell fixative. In our study, the whole rat eyes were fixed in formaldehyde and embedded in paraffin prior to sectioning, whereas the cultures were fixed in methanol without paraffin embedding. Formaldehyde, in particular, can mask specific antigens due to cross-linking of proteins (Abcam). As the CK-7 antibody, but not the markers for UEA-1 and MUC5AC, initially attached poorly to the CK-7 antigen in the formaldehyde-fixed sections, we performed heat-induced epitope retrieval (HIER), which is often necessary to expose antigens after formaldehyde fixation (Abcam). CK-7 staining was then improved to some degree, although not as strong as in the methanol-fixed cultures, indicating the CK-7 antigens were still partly masked. Second, intracellular mucin, when present in large amounts in a single CK-7+ cell, displaces the CK-7+ filaments, thereby decreasing the CK-7 fluorescence from the cell (not shown). As the mucin content in each goblet cell was generally much higher in the sections than in the cultures, the fluorescence intensity of the CK-7+ filaments in each cell was lower, and consequently less visible in the sections. Third, when goblet cells with large intracellular mucin granules are sectioned, as opposed to stained ‘en block’, as in the cultures, the CK-7+ peripheral cell body is only visualized as a thin line around the large mucin-filled core of the goblet cell body. This makes the CK-7 staining even harder to notice. As a result of these three factors the investigators scored a lower percentage of CK-7+ cells than mucin-filled goblet cells in the sections.
As the rat primary cultures grown in the present study were generally small, yielding little amount of tissue, immunocytochemistry, which in contrast to western blotting necessitates only a small number of cells, was chosen to assess differences in expressed proteins between the groups. Detection of co-expressed proteins in single cells also requires the use of immunocytochemistry compared to western blotting and PCR analyses. Assessment of the agreement between the two independent investigators confirmed high reliability of the phenotypic data. Thus, although a subjective method for quantifying proteins, immunocytochemistry was indispensable for the current study and produced reliable results.
In accordance with Nizam et al., (2008), goblet cells in our study were detected in cultures from explants taken from all conjunctival regions. In addition, we found that the densities of goblet cells in ex vivo cultures from specific locations in the conjunctiva corresponded with the location in sections from conjunctiva removed in vivo, with significantly more goblet cells in the forniceal cultures and least in superior bulbar cultures. Conjunctival transient amplifying cells are capable of differentiating into stratified epithelium and goblet cells (Pellegrini et al., 1999), which may suggest that forniceal explants contain the most progenitor cells committed to the goblet cell lineage. Some goblet cells have been shown to be label-retaining (Wei et al., 1995), indicating proliferative capacity of goblet cells in vivo. Moreover, it is well documented that goblet cells are able to proliferate in ex vivo culture (Shatos et al., 2008, 2003, 2001). Thus, the higher percentage of goblet cells in forniceal cultures may also result from ex vivo proliferation of the larger quantity of goblet cells already present in the forniceal explants.
Kolli et al. (2008) reported a progressively more differentiated limbal epithelium with increasing distance from the explant. In contrast, in limbal cell cultures grown in the presence of feeder layer cells, undifferentiated cells were mostly concentrated at the leading edge, while differentiated cells were found at the stratified center of the colonies (Meyer-Blazejewska et al., 2010). The latter study is in agreement with our study showing stratified squamous epithelial cells (CK-4+) present close to the explants, but absent close to the leading edge.
CK-7 is a secondary cytokeratin of simple epithelia, which can be upregulated in reactive conditions, including various types of injury such as inflammation or atrophy (Moll et al., 2008). Upregulation of CK-7 is also seen in the stratified oral mucosal epithelium ex vivo, even though this epithelium is devoid of CK-7 in vivo (Garzon et al., 2009). Thus, the CK-7+/UEA-1− cells at the leading edge in the primary cultures could represent cells with reactive upregulation of CK-7 rather than true goblet cells. The density of CK-7+ cells at the leading edge in our study, on the other hand, showed significant positive correlation with the percentage of UEA-1 + goblet cells close to the explant in the same culture (data not shown). This suggests that the CK-7+ cells at the leading edge were in fact related to the mucin-filled goblet cells. In cases of limbal stem cell deficiency with few goblet cells, detection of CK-7+ cells can reportedly confirm conjunctival overgrowth of the cornea (Jirsova et al., 2011). That study, though, used a different anti-CK-7 antibody clone (OV-TL 12/30), which stained all conjunctival cells and not specifically the goblet cells. Although the migration pattern of stratified epithelia is not completely understood, corneal leading edge cells have been shown to become displaced to the apical epithelial layer as migration moves forward (Danjo and Gipson, 2002). If this migration pattern also applies for conjunctival epithelium then CK-7+ cells at the leading edge are expected to be displaced to the apical layer during migration, thereafter possibly differentiating into mucin-filled goblet cells. Our finding that CK-7+/UEA-1− cells, but not the CK-7+/UEA-1+ cells, occasionally co-express the putative stem cell marker ABCG2 could support this speculation.
The percentage of CK-7+ cells ex vivo was similar in both large and small cultures, while the concentration of UEA-1 + cells was lowest in the large cultures. This could mean that the CK-7+ goblet cells are more proliferative than the mucin-filled (UEA-1+) goblet cells or that mucin-production ceases during goblet cell migration from the explant. One could argue that the CK-7+/UEA-1− cells growing from the explant have simply secreted their mucin granules and therefore are not less developed than the CK-7+/UEA-1 + goblet cells. Still, the former cells would then not be expected to be more proliferative than the latter, as reported in our study. The maintenance of a differentiated state is linked to cell cycle arrest and when stimulating post-mitotic ocular cells to proliferate, e.g. by exposing them to culture conditions with a medium containing FBS, the cells may lose some of their differentiated characteristics (Valtink and Engelmann, 2009). Transitory cell dedifferentiation initially during primary culture is typical of differentiated cells (MacDonald, 1994). In the current study we used the same culture protocol as in previous reports that demonstrated mucin-producing goblet cells in both primary (Hayashi et al., 2012; Shatos et al., 2003, 2001) and passaged cell culture (Hayashi et al., 2012; Shatos et al., 2001). Knowing that mucin-production can re-establish when passaging the cells using our culture protocol, we hypothesize that the loss of mucin-production during migration may represent temporary goblet cell dedifferentiation. Tracheal epithelial cells have been shown to briefly lose their mucin granules during the first phase of culture, apparently signalizing a transitory phase of dedifferentiation during cell proliferation (Wasano et al., 1988). The loss of mucin-filled goblet cells during conjunctival cell migration has similarly been reported in studies on conjunctival wound healing (Friedenwald, 1951; Geggel et al., 1984; Shapiro et al., 1981). Collectively, these reports indicate that goblet cell mucin-production is temporarily halted during the phase of migration/proliferation. The CK-7+/UEA-1 − cells might therefore represent immature goblet cells, i.e. goblet cells that can further develop into fully differentiated mucin-filled goblet cells. This finding may explain why proliferating goblet cells identified by their secretory product are difficult to detect in vivo.
The higher percentage of mucin-filled goblet cells (UEA-1 + and MUC5AC+) close to the explant compared to near the leading edge may signify effects of different gradients of soluble or insoluble extracellular matrix (ECM) proteins. Meller et al. (2002) showed that although mucin-filled (MUC5AC+) goblet cells were undetected in rabbit conjunctival epithelial cells cultured on amniotic membranes, strong PAS-positive materials presumably representing mucin-filled goblet cells were found eleven days subsequent to transplantation into Balb/c athymic mice. The authors proposed that the production of goblet cell mucins was dependent on a permissive stromal environment. The composition of the basement membrane (BM) and the ECM varies throughout the ocular surface (Schlotzer-Schrehardt et al., 2007). As we used explant culture in the current study, dissimilarities in the BM and ECM in each conjunctival harvesting site could have influenced the ex vivo phenotype. There were, however, no significant differences in the size of the explants obtained from the six conjunctival regions. Still, the relative distribution of subconjunctival tissue and epithelial cells in the explants was nevertheless unknown, and variation in this distribution could also have affected the phenotype of the cultured conjunctival cells. In vitro simulation of the conjunctival epithelial niche using co-cultures with human bulbar subconjunctival fibroblasts reportedly supported more conjunctival epithelial progenitor cells than co-cultures with conventional 3T3 fibroblasts (Schrader et al., 2010). In another study, co-cultures with conjunctival fibroblasts apparently induced the development of more goblet cells than co-cultures with 3T3 fibroblasts (Tsai et al., 1994). Moreover, fibroblast growth factor 10 (FGF10) has been shown to induce the production of the mucins MUC1, MUC4 and MUC5, as well as stimulate growth, of conjunctival epithelial cells (Ma et al., 2011). Hence, in the present study, the differentiation of goblet cells ex vivo could be partly related to the amount of subconjunctival tissue, and the specific composition of the BM/ECM, in the explants.
In conclusion, the present study demonstrates that forniceal explants yield the highest number of goblet cells ex vivo and thereby seem to be optimal for goblet cell transplantation. In addition, we suggest that CK-7+/UEA-1‒ cells represent highly proliferative immature goblet cells that might have the capacity to develop into mucin-filled goblet cells. The CK-7+/UEA-1− cells may be of importance during conjunctival migration as they are mostly located close to the leading edge and their density does not decrease with increasing outgrowth size.
Acknowledgments
The authors thank Robin R. Hodges and Donald Pottle at the Schepens Eye Research Institute, Harvard Medical School, Boston, MA; Torstein Lyberg and Leiv Sandvik at the Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway, and Astrid Østerud at the Department of Ophthalmology, Oslo University Hospital, Oslo, Norway for excellent assistance and support. This work was supported by National Institutes of Health Grant EY009057 and EY019470, in addition to the Norwegian Research Council and Eastern Norway Regional Health Authority.
References
- Abcam. [Last accessed 23.08.12.]; http://www.abcam.com/index.html?pageconfig=resource&rid=11488.
- Ang LP, Tan DT, Cajucom-Uy H, Beuerman RW. Autologous cultivated conjunctival transplantation for pterygium surgery. American Journal of Ophthalmology. 2005;139:611–619. doi: 10.1016/j.ajo.2004.10.056. [DOI] [PubMed] [Google Scholar]
- Argueso P, Gipson IK. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Experimental Eye Research. 2001;73:281–289. doi: 10.1006/exer.2001.1045. [DOI] [PubMed] [Google Scholar]
- Cennamo GL, Del Prete A, Forte R, Cafiero G, Del Prete S, Marasco D. Impression cytology with scanning electron microscopy: a new method in the study of conjunctival microvilli. Eye (London) 2008;22:138–143. doi: 10.1038/sj.eye.6702873. [DOI] [PubMed] [Google Scholar]
- Danjo Y, Gipson IK. Specific transduction of the leading edge cells of migrating epithelia demonstrates that they are replaced during healing. Experimental Eye Research. 2002;74:199–204. doi: 10.1006/exer.2001.1115. [DOI] [PubMed] [Google Scholar]
- Eidet JR, Fostad IG, Shatos MA, Utheim TP, Utheim OA, Raeder S, Dartt DA. Effect of biopsy location and size on proliferative capacity of ex vivo expanded conjunctival tissue. Investigative Ophthalmology & Visual Science. 2012a;53:2897–2903. doi: 10.1167/iovs.11-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidet JR, Utheim OA, Raeder S, Dartt DA, Lyberg T, Carreras E, Huynh TT, Messelt EB, Louch WE, Roald B, Utheim TP. Effects of serum-free storage on morphology, phenotype, and viability of ex vivo cultured human conjunctival epithelium. Experimental Eye Research. 2012b;94:109–116. doi: 10.1016/j.exer.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Friedenwald JS. Growth pressure and metaplasia of conjunctival and corneal epithelium. Documenta Ophthalmologica. 1951;5–6:184–192. doi: 10.1007/BF00143661. [DOI] [PubMed] [Google Scholar]
- Garzon I, Sanchez-Quevedo MC, Moreu G, Gonzalez-Jaranay M, Gonzalez-Andrades M, Montalvo A, Campos A, Alaminos M. In vitro and in vivo cytokeratin patterns of expression in bioengineered human periodontal mucosa. Journal of Periodontal Research. 2009;44:588–597. doi: 10.1111/j.1600-0765.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- Geggel HS, Friend J, Thoft RA. Conjunctival epithelial wound healing. Investigative Ophthalmology & Visual Science. 1984;25:860–863. [PubMed] [Google Scholar]
- Goller T, Weyrauch KD. The conjunctival epithelium of dogs. Light and electron microscopic investigations. Annals of Anatomy. 1993;175:127–134. [PubMed] [Google Scholar]
- Hayashi D, Li D, Hayashi C, Shatos M, Hodges RR, Dartt DA. Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. Investigative Ophthalmology & Visual Science. 2012;53:2993–3003. doi: 10.1167/iovs.11-8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AJ, Tseng SC, Kenyon KR. Morphogenesis of rat conjunctival goblet cells. Investigative Ophthalmology & Visual Science. 1988;29:969–975. [PubMed] [Google Scholar]
- Jirsova K, Dudakova L, Kalasova S, Vesela V, Merjava S. The OV-TL 12/30 clone of anti-cytokeratin 7 antibody as a new marker of corneal conjunctivalization in patients with limbal stem cell deficiency. Investigative Ophthalmology & Visual Science. 2011;52:5892–5898. doi: 10.1167/iovs.10-6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper M, Moll R, Stosiek P, Karsten U. Patterns of cytokeratin and vimentin expression in the human eye. Histochemistry. 1988;89:369–377. doi: 10.1007/BF00500639. [DOI] [PubMed] [Google Scholar]
- Kasper M. Heterogeneity in the immunolocalization of cytokeratin specific monoclonal antibodies in the rat eye: evaluation of unusual epithelial tissue entities. Histochemistry. 1991;95:613–620. doi: 10.1007/BF00266749. [DOI] [PubMed] [Google Scholar]
- Kawano K, Uehara F, Sameshima M, Ohba N. Application of lectins for detection of goblet cell carbohydrates of the human conjunctiva. Experimental Eye Research. 1984;38:439–447. doi: 10.1016/0014-4835(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Kessing SV. Mucous gland system of the conjunctiva. A quantitative normal anatomical study. Acta Ophthalmologica (Copenhagen) 1968;46(S95):91–131. [PubMed] [Google Scholar]
- Kolli S, Lako M, Figueiredo F, Mudhar H, Ahmad S. Loss of corneal epithelial stem cell properties in outgrowths from human limbal explants cultured on intact amniotic membrane. Regenerative Medicine. 2008;3:329–342. doi: 10.2217/17460751.3.3.329. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Wei ZG, Sun TT. Phorbol ester preferentially stimulates mouse fornical conjunctival and limbal epithelial cells to proliferate in vivo. Investigative Ophthalmology & Visual Science. 1998;39:301–307. [PubMed] [Google Scholar]
- Li W, Sun X, Wang Z, Li R, Li L. The effect of nerve growth factor on differentiation of corneal limbal epithelial cells to conjunctival goblet cells in vitro. Molecular Vision. 2010;16:2739–2744. [PMC free article] [PubMed] [Google Scholar]
- Ma M, Zhang Z, Niu W, Zheng W, Kelimu J, Ke B. Fibroblast growth factor 10 upregulates the expression of mucins in rat conjunctival epithelial cells. Molecular Vision. 2011;17:2789–2797. [PMC free article] [PubMed] [Google Scholar]
- MacDonald . Primary culture and the establishment of cell lines. In: Davis JM, editor. Basic Cell Culture. A Practical Approach IRL. New York: Press, Oxford; 1994. pp. 149–180. [Google Scholar]
- Meller D, Dabul V, Tseng SC. Expansion of conjunctival epithelial progenitor cells on amniotic membrane. Experimental Eye Research. 2002;74:537–545. doi: 10.1006/exer.2001.1163. [DOI] [PubMed] [Google Scholar]
- Meyer-Blazejewska EA, Kruse FE, Bitterer K, Meyer C, Hofmann-Rummelt C, Wunsch PH, Schlotzer-Schrehardt U. Preservation of the limbal stem cell phenotype by appropriate culture techniques. Investigative Ophthalmology & Visual Science. 2010;51:765–774. doi: 10.1167/iovs.09-4109. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Azuma N, Hida T. Morphological and histochemical studies of goblet cells in developing human conjunctiva. Japanese Journal of Ophthalmology. 1992;36:169–174. [PubMed] [Google Scholar]
- Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochemistry and Cell Biology. 2008;129:705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CP, Wilsman NJ, Nordheim EV, Majors LJ, Collier LL. Density and distribution of canine conjunctival goblet cells. Investigative Ophthalmology & Visual Science. 1987;28:1925–1932. [PubMed] [Google Scholar]
- Moore JE, Vasey GT, Dartt DA, McGilligan VE, Atkinson SD, Grills C, Lamey PJ, Leccisotti A, Frazer DG, Moore TC. Effect of tear hyper-osmolarity and signs of clinical ocular surface pathology upon conjunctival goblet cell function in the human ocular surface. Investigative Ophthalmology & Visual Science. 2011;52:6174–6180. doi: 10.1167/iovs.10-7022. [DOI] [PubMed] [Google Scholar]
- Nizam MH, Ruszymah BH, Chua KH, Ghafar NA, Hamzah JC. Ex vivo growth of rabbit bulbar, fornix and palpebral conjunctival epithelia in a serum-free and feeder layer-free culture system. Medical Journal of Malaysia. 2008;63(Suppl. A):111–112. [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. The Journal of Cell Biology. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Tseng SC, Yoshino K, Monroy D, Felix C, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104:223–235. doi: 10.1016/s0161-6420(97)30330-3. [DOI] [PubMed] [Google Scholar]
- Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, Kruse FE. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Experimental Eye Research. 2007;85:845–860. doi: 10.1016/j.exer.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Schrader S, Notara M, Beaconsfield M, Tuft SJ, Daniels JT, Geerling G. Tissue engineering for conjunctival reconstruction: established methods and future outlooks. Current Eye Research. 2009;34:913–924. doi: 10.3109/02713680903198045. [DOI] [PubMed] [Google Scholar]
- Schrader S, Notara M, Tuft SJ, Beaconsfield M, Geerling G, Daniels JT. Simulation of an in vitro niche environment that preserves conjunctival progenitor cells. Regenerative Medicine. 2010;5:877–889. doi: 10.2217/rme.10.73. [DOI] [PubMed] [Google Scholar]
- Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Investigative Ophthalmology & Visual Science. 1981;21:135–142. [PubMed] [Google Scholar]
- Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Investigative Ophthalmology & Visual Science. 2001;42:1455–1464. [PubMed] [Google Scholar]
- Shatos MA, Rios JD, Horikawa Y, Hodges RR, Chang EL, Bernardino CR, Rubin PA, Dartt DA. Isolation and characterization of cultured human conjunctival goblet cells. Investigative Ophthalmology & Visual Science. 2003;44:2477–2486. doi: 10.1167/iovs.02-0550. [DOI] [PubMed] [Google Scholar]
- Shatos MA, Gu J, Hodges RR, Lashkari K, Dartt DA. ERK/p44p42 mitogen-activated protein kinase mediates EGF-stimulated proliferation of conjunctival goblet cells in culture. Investigative Ophthalmology & Visual Science. 2008;49:3351–3359. doi: 10.1167/iovs.08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RJ, Tseng SC. Substrate modulation of cultured rabbit conjunctival epithelial cell differentiation and morphology. Investigative Ophthalmology & Visual Science. 1988;29:1565–1576. [PubMed] [Google Scholar]
- Tsai RJ, Ho YS, Chen JK. The effects of fibroblasts on the growth and differentiation of human bulbar conjunctival epithelial cells in an in vitro conjunctival equivalent. Investigative Ophthalmology & Visual Science. 1994;35:2865–2875. [PubMed] [Google Scholar]
- Valtink M, Engelmann K. Culturing of retinal pigment epithelium cells. Developments in Ophthalmology. 2009;43:109–119. doi: 10.1159/000223844. [DOI] [PubMed] [Google Scholar]
- Wasano K, Kim KC, Niles RM, Brody JS. Membrane differentiation markers of airway epithelial secretory cells. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 1988;36:167–178. doi: 10.1177/36.2.3335774. [DOI] [PubMed] [Google Scholar]
- Wei ZG, Wu RL, Lavker RM, Sun TT. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Investigative Ophthalmology & Visual Science. 1993;34:1814–1828. [PubMed] [Google Scholar]
- Wei ZG, Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Investigative Ophthalmology & Visual Science. 1995;36:236–246. [PubMed] [Google Scholar]