Abstract
Fertility awareness based methods (FABMs) can be used to ameliorate the likelihood to conceive. A literature search was performed to evaluate the relationship of cervical mucus monitoring (CMM) and the day-specific pregnancy rate, in case of subfertility. A MEDLINE search revealed a total of 3331 articles. After excluding articles based on their relevance, 10 studies and were selected. The observed studies demonstrated that the cervical mucus monitoring (CMM) can identify the days with the highest pregnancy rate. According to the literature, the quality of the vaginal discharge correlates well with the cycle-specific probability of pregnancy in normally fertile couples but less in subfertile couples. The results indicate an urgent need for more prospective randomised trials and prospective cohort studies on CMM in a subfertile population to evaluate the effectiveness of CMM in the subfertile couple.
Keywords: Billings method, cervical mucus, conception, Creighton model, fertility awareness, infertility, natural family planning, subfertility, symptothermal method
Introduction
Only 2% of finally successful couples conceived after 12 cycles with unprotected intercourse. After six unsuccessful cycles, subfertility has to be assumed in 50% of all couples (Gnoth et al., 2003; Wang et al., 2003; Sozou and Hartshorne, 2012). Besides surgical and medical treatment for those couples, there is a tremendous uprising trend to useassisted reproductive technologies (ART). ART comprises all treatments or procedures used to establish a pregnancy in which there is in vitro handling of human oocytes, sperm and/or embryo. The development of these techniques surely has led to overtreatment in fertility care. However, there are other less-known measures to ameliorate the likelihood to conceive, the so-called fertility awareness-based methods (FABMs).
FABMs use physical signs and symptoms that change along with hormone fluctuations throughout the different phases of a woman’s menstrual cycle to predict and monitor the fertile and infertile days (Pallone and Bergus, 2009). This knowledge, referred to as ‘fertility awareness’, can be used either to plan or to avoid a pregnancy. The key variables which FABMs rely on are the reliable identification of the fertile window (FW) and modification of sexual behaviour (Frank-Herrmann et al., 2007). Different modifications of FABMs are known, cervical mucus based methods, temperature methods, combinations of both variables and calculation methods. The most extensively studied method is Sensiplan®, the symptothermal method of Natural Family Planning (NFP), mucus, temperature and calculation rules (Frank-Herrmann et al., 2007).
There is a compelling need to educate women about their fertility awareness. Primary care providers need to integrate fertility health literacy into health promotion of women of reproductive age. The guideline currently recommended by many physicians is that women who wish to become pregnant should have frequent random intercourse, optimally every other day. With application of this guideline, some acts of intercourse will occur in the FW (ASRM, 2008).
The use of FABMs could increase the pregnancy rate when used properly. Our systematic review will focus on cervical mucus and its function to become a useful FABM. Previous studies showed that more oestrogen-type mucus is present in the fertile days of the menstrual cycle, but also an increasing trend in the amount of mucus secretions can be noticed. This rise in volume is associated with a change in vaginal discharge, which women can monitor based on different characteristics (Hilgers and Prebil, 1979). In addition, this awareness can be used to evaluate the ovarian function (Moghissi et al., 1972). It also provides information about the fertility status of the different days in the cycle, because fertile type mucus ensures a facilitated transport of sperm cells to the ovary through the fallopian tubes (Billings et al., 1972; Katz et al., 1997).
The main objective of this review was to determine the effectiveness of cervical mucus as a predictor for the FW. Furthermore, the probability of whether or not cervical mucus monitoring is associated with increased cycle-specific pregnancy rates will be specifically examined for the subfertile couple.
Different methods for fertility awareness assessment
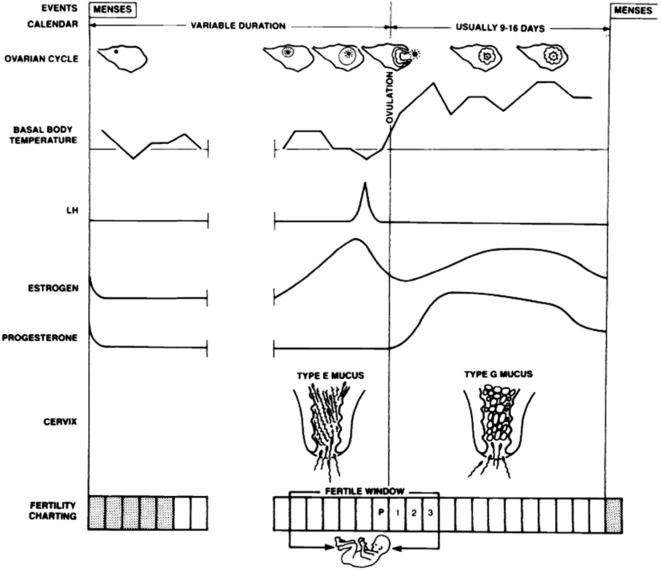
All FABMs are based on the detection of ovulation and the related fertile window. Retrospectively, ovulation occurred approximately 14 days before the onset of the next menstrual cycle (Wilcox et al., 2000). Throughout the woman’s menstrual cycle, there is a six day fertile interval during which conception more likely occurs if intercourse takes place (Bilian et al., 2010). This FW comprises the five days before ovulation and the day of ovulation itself. There is only a limited period of time during which fertilisation can take place due to the limited duration of viability of the ovum and sperm which accounts for the different probabilities of conception for days of the menstrual cycle (Colombo and Masarotto, 2000). After ovulation has occurred, the ovum will lose its ability to become fertilised after 10 to 24 hours (Stanford et al., 2002). The life span of sperm within the female reproductive tract is more variable. Spermatozoa have a life span of 24-48 hours if hostile mucus is absent. When there is proper oestrogenic cervical mucus, the fertilising capacity of sperm can last 3-7 days in the periovulatory period (Pallone and Bergus, 2009).
There has been a search for simple and reliable methods for both predicting and confirming ovulation. The golden standard of monitoring ovarian function in women has been exact ovulation detection through the use of daily sonography and specific hormone testing (estradiol, luteinizing hormone, progesterone) (Albertson and Zinaman, 1987). These clinical methods for cycle monitoring are expensive and time-consuming. FABMs show the same reliable results for determining the FW and predicting the time of ovulation. The methods are based on a detection of the FW and use symptoms the women are able to observe themselves, such as bleeding rhythm, cervical mucus, measurement of the basal body temperature, auto-palpation of the cervix, etc. (Gnoth et al., 1996; Frank-Herrmann et al., 2005). The rhythm method or calendar-based method requires to calculate the fertile days according to the length of the menstrual cycles. This is possible because the duration of the luteal phase is relatively stable (Evans-Hoeker et al., 2013). The basal body temperature (BBT) method uses a temperature elevation to determine the day of ovulation. This rise (0.3° to 0.6° C) is due to the progesterone surge (Pallone and Bergus, 2009). After ovulation, the BBT remains elevated due to increased progesterone levels after ovulation until next menstruation. Therefore, the temperature rise identifies the end, rather than the onset of the fertile period. This implies that this method can only be used to retrospectively identify ovulation. Furthermore, there are other factors that limit the accuracy of a BBT method used solely. Temperature measurements may be disturbed by a variety of factors, some women ovulate without a clear rise in BBT and the shift may vary up to one day before and three days after actual ovulation (Pallone and Bergus, 2009).
On the other hand, cervical mucus patterns, which reflect rising estradiol, are shown to be accurate markers of the onset of the fertile and infertile phases of a woman’s menstrual cycle (Hume, 1991). Cervical mucus is an aqueous or gel mixture of proteins and mucopolysaccharids, ions and compounds, and cells, primarily produced by the endocervical epithelium (Fordney-Settlage, 1981). Estradiol and progesterone levels are responsible for the changes in characteristics of cervical secretions during the menstrual cycle. Mucus characteristics have been tried to be typed. The secretion of the oestrogenic mucus (E mucus) is stimulated by the rise in oestrogen produced by the dominant follicle five to six days before ovulation. E mucus is clear, wet, stretchy and slippery, which makes it ideal to facilitate the transport and the survival of the spermatozoa in the cervix. In addition, it leads to functional maturation of sperm (capacitation) so that the fertilisation potential of the ovum is increased. This mucus is present in the fertile phase of the menstruation cycle. Following ovulation there is a second type of mucus, the progesterone mucus (G mucus). This type is produced by the progesterone release from the corpus luteum. Progesterone stimulates the cervix to produce G mucus, which inhibits sperm capacitation and motility and blocks the passage of sperm. This cervical secretion is considered infertile due to the unchanging and generally dry, sticky, cloudy and not stretching characteristics (Pallone and Bergus, 2009; Stanford et al., 2002).
Cervical mucus monitoring (CMM) is performed by observing these mucus secretions, whereby internal checking of the vagina or cervix is not necessary (Evans-Hoeker et al., 2013). The goal is to identify the onset of the production of any type of fertile mucus. Even a change of feeling (dry to humid or wet) may be indicative of impending fertility. The peak day can be identified as the last day of any vaginal discharge that has type E characteristics (Fig. 1) (Stanford et al., 2002). There are different methods that use cervical mucus as a predictor of the FW. Each method uses a different way to identify the fertile phase, but the main focus relies on observing the absence or presence of cervical mucus secretions of dry/humid feeling. In some elaborated mucus methods women are asked to describe the colour, texture and stretch of the cervical discharge (Pallone and Bergus, 2009). Table I shows four mucus categories ranging from absence of discharge or dry mucus characteristics (score 1) to transparent, stretchy and slippery mucus (score 4) which is the commonly used typing. A high mucus score is consistent with the presence of fertile-type E mucus. Important conception studies are based on this typology of mucus (Colombo and Masarotto, 2000; Dunson et al., 2001; Bigelow et al., 2004).
Fig. 1. Physiologic parameters of the menstrual cycle that can be used to identify days during which intercourse may result in pregnancy, i.e. the fertile window. LH: luteinizing hormone; P: peak day (Adapted from Stanford et al., 2002).
Table I. Classification of mucus symptoms from vaginal discharge.
| Mucus score | Feeling | Appearance | Secretions |
|---|---|---|---|
| 1 | Dry, rough and itchy or nothing felt | Nothing seen | No secretions |
| 2 | Damp | Nothing seen | Secretions |
| 3 | Damp | Mucus is thick, creamy, whitish, yellowish, or sticky | Secretions |
| 4 | Wet, slippery, smooth | Mucus is transparent, like raw egg white, stretchy/elastic , liquid, watery, or reddish | Secretions |
(Adapted from Colombo and Masarotto (2000)).
The first method described is the Billings Ovulation Method (BOM), in which women record the mucus secretions ‘in their own words’ with a focus on changes in cervical characteristics (Bhargava et al., 1996; Stanford et al., 1999). Another more standardised method, named the Creighton Model (CrM), characterises cervical secretions by pictures and precise words (Howard and Stanford, 1999; Pallone and Bergus, 2009). In addition to the methods above, the TwoDays Method (TDM) is the simplest method, which focuses on the presence or absence of cervical mucus and not on the characteristics of the secretions (Dunson et al., 2001). A woman is considered fertile on a given day if she notices secretions on that specific day or the previous day (Jennings et al., 2011).
The Symptothermal Method (Sensiplan®) uses a combination of predictors, namely the BBT, recording of cervical secretions and, important, calculation rules (Frank-Herrmann et al., 2007; Pallone and Bergus, 2009). The opening of the FW is calculated from previous cycles (min. 12) but detection of any fertile type of mucus marks the abrupt onset of impending fertility (“what comes first”). The change of cervical mucus together with the rise in BBT indicates the end of the fertile phase (“what comes last”). Therefore, this method can be used prospectively as well as retrospectively to identify the periovulatory period (Gross, 1987). This method has been proven to be very effective in prospective studies (Frank-Herrmann et al., 2007) because it is based on a double check mechanism: It can be used by women with short, long or irregular cycles (Frank-Herrmann et al., 2007) (Pallone and Bergus, 2009). Table II provides a short overview of the different FABMs.
Table II. Overview of the different FABMs.
| Methods | Mechanism |
|---|---|
| Rhythm (calendar-based) method | Calculation of the fertile days according to the length of a woman’s previous menstrual cycles |
| The basal body temperature method | Charting the BBT to detect ovulation day |
| Billings ovulation method | Identification of the changes in vaginal discharge in a woman’s own words |
| Creighton Model | Identification of the changes in vaginal discharge with use of pictures and suggested words |
| TwoDays Method | Focus on the presence or absence of cervical mucus |
| Symptothermal Method | Identification of the FW through use of the BBT and cervical secretions |
Materials and Methods
Search strategy
We made use of a computerised literature search executed to search for studies examining the value of CMM, specifically applied to the subfertile couple. The database used was MEDLINE, and the following search terms were considered: fertility awareness, self-assessment, cervical mucus, infertility, subfertility, natural family planning, conception, Symptothermal Method, Billings Ovulation Method, Creighton Model, in combination with ‘not contraception’. These keywords were used in different combinations which led to 3331 hits. No time limitation or other filters such as language restriction were entered in the search. Subsequently, the reports were screened by two readers regarding the relevance of the title, resulting in 315 remaining articles. After reading the abstracts in the following stage, 39 articles were retained. Having read the 39 papers carefully, the following articles were excluded: studies mainly focusing on avoiding pregnancy or natural family planning used for contraceptive procedures and articles with reference to cancer, breastfeeding or sex pre-selection. Studies where cervical mucus was used for the determination of the FW were included. These also comprise methods like the Creighton Model, the Billings Ovulation Method, the TwoDays Method and the Symptothermal Method. Ultimately, 10 relevant studies were selected. Examining the reference lists of the selected articles yielded no new hits. A schematic overview of the search strategy can be seen in Figure 2.
Fig. 2. Schematic overview of the search strategy.
Results
Multiple studies have been performed to obtain information about the day-specific probability of conception through observation of the vaginal discharge, used as a marker of ovulation. This systematic review compares the results of ten different studies and examines the relevance and outcome of each study. Nine out of the ten selected articles were prospective cohort studies and one was a retrospective cohort study (Table V).
Table V. Table V. — Summarisationof the examined studies.
| Author | Year | Country | Study design | Population | N° of couples | N° of cycles | N° of pregnancys | |
| Colombo, et al. | 2000 | Europe | Prospective | Fertile | NA | 3265 | 434 | Highest probability of conception is two days before the peak mucus day. |
| Dunson, et al. | 2001 | Europe | Prospective | Fertile | 660 | 2832 | 434 | The days estimated by the TwoDay Algorithm as fertile were the days with the highest fecundability. There is twice as much chance to achieve a pregnancy when intercourse finds place on a day covered by the TwoDay Algorithm. |
| Stanford, et al. | 2003 | USA | Retrospective | Fertile | 309 | 1681 | 81 | Observation of the vaginal mucus discharge can identify the days with the highest pregnancy rate from intercourse in normal fertility and subfertility. |
| Subfertile | 117 | 373 | 30 | There is a significant effect of the quality of mucus discharge on the cycle--specific probability on conception by fertile couples, this relationship couldn’t be found the subfertile couple. | ||||
| Bigelow, et al. | 2004 | Europe | Prospective | Fertile | NA | 1473 | 353 | Increasing trend in the day-specific probabilities of pregnancy with increases in the mucus score. |
| Frank-Herrmann, et al. | 2005 | Germany | Prospective | Fertile | NA | 62 | NA | Cervical mucus symptoms in combination with BBT have a good correlation with ovulation. |
| 346 | NA | NA | Women can monitor their cervical mucus changes to increase their probability of pregnancy. FABMs seem to shorten the time to pregnancy, and can be used in the management of subfertility. | |||||
| Colombo, et al. | 2006 | Italy | Prospective | Fertile | NA | 963 | 142 | Highest probability of conception: peak mucus day (day 0). A relationship between the presence of the mucus symptom and the pregnancy rate could be established. |
| Scarpa, et al. | 2006 | Italy | Prospective | Fertile | 191 | 2536 | 161 | Conception probability is negligible on days with no noticeable mucus secretions and approximately 30% for days with most fertile--type mucus detected by woman. |
| Scarpa, et al. | 2007 | Italy | Prospective | Fertile | 191 | 2536 | 161 | TTP can be shortened when intercourse takes place on days with the highest mucus score. |
| Evans-Hoeker, et al. | 2013 | USA | Prospective | Fertile | 331 | NA | NA | Fecundability increases with increasing consistency of CMM. The time to pregnancy can be reduced through use of CMM. |
| Mu, et al. | 2014 | USA | Prospective | Fertile | 124 | 469 | NA | Intercourse on high or peak days increases the pregnancy probability. |
BBT: basal body temperature, CMM: cervical mucus monitoring, NA: not available, TTP: time to pregnancy.
Stanford et al. (2003) retrospectively evaluateddata extracted from the Creighton Model Fertility Care System in four different cities. Fertile and subfertile couples were identified and, after selection, 1681 cycles from 309 fertile couples resulting in 80 pregnancies and 373 cycles from 117 subfertile couples which resulted in 30 pregnancies, were incorporated in the study. The highest probability of conception could be seen on the mucus peak day (identified as ovulation), both for fertile and subfertile couples. For the fertile couples, the probability to become pregnant on the mucus peak day was 0,38 and for the subfertile couples 0,14. The mucus peak day was appointed day 0. The probability of conception was greater than 0,5 for day -3 to day +2 for the fertile couples and for day -1 to day +1 for the subfertile couples.
Frank-Herrmann et al. (2005) reviewed the main results of recent European cycle databases (WHO database, German Long-term Cycle database, I European Cycle Database and II European Cycle Database) on ovulation detection and determination of the FW performed by women themselves. Results showed that although ovulation detection can be adequately determined with the peak mucus symptom and the BBT, the combination gave a significant better correlation for a correct identification of ovulation. Furthermore, the study investigated the role of the changing quality of the cervical mucus in relation to the probability of conception. This technique was used by 346 women and resulted in a cumulative probability of conception of 81% after 6 months and 92% after 12 months.
Evans-Hoeker et al. (2013), investigated the use and consistency of cervical mucus monitoring (CMM) to determine the FW in women who wanted to get pregnant. The second goal was to examine whether monitoring mucus was associated with an increased cycle-specific probability of conception independent of intercourse frequency or use of urinary luteinizing hormone monitoring kits. This time-to-pregnancy study examined a cohort of 331 women between 30 and 44 years of age who had been trying to conceive for three months or less and had no known issues related to fertility. If women checked their cervical mucus on a particular day, they had to make a choice which type they observed (Table I).The FW could be estimated through the use of calendar based calculations. Cycles in which women made consistent use of CMM were more likely to result in conception. The results of this study showed that fecundability increased with increasing use of CMM.
A large multinational prospective cohort study, the European Study of Daily Fecundability (ESDF, database: Fertili), was conducted by Colombo and Masarotto (2000) to determine the daily probability of conception (Table IV) among healthy women and to compare different statistical models on the matter. Inclusion criteria for the ESDF are listed in Table III. Women were instructed to keep daily records of their BBT, cervical mucus symptoms (Table I) and coitus. The results found by Colombo and Masarotto (2000) are described in the next section together with their results of a more recent study (Colombo et al., 2006).
Table IV. Daily probabilities of conception referenced to the day of temperature rise (3175 natural cycles with 434 pregnancies) according to (Colombo and Masarotto, 2000).
| - 8 | - 7 | - 6 | - 5 | - 4 | - 3 | - 2 | - 1 | Temp.rise | +1 | +2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0,3% | 1,4% | 2,7% | 6,8% | 17,6% | 23,7% | 25,5% | 21,2% | 10,3% | 0,8% | 0,35% |
Table III. Overview of the inclusion criteria for the ESDF.
| Women are experienced in use of a Natural Family Planning method |
| Married or in a stable relationship |
| Age: 18-40 |
| Having at least one menses after cessation of breastfeeding or after delivery |
| No use of drugs or hormonal medications that could affect fertility |
| Couples have no history of fertility problems or disorders that might cause subfertility |
| Not mixing unprotected and protected intercourse |
Dunson et al. (2001) used the data from the ESDF to evaluate the theoretical effectiveness of the TwoDay Algorithm. Out of the 2832 cycles studied, 434 resulted in a pregnancy. Data suggested that, for most women, the TwoDay Algorithm was useful in identifying the most fertile days of the menstrual cycle. They showed that the pregnancy probability was highest in the six day interval, beginning five days before ovulation and ending on the estimated ovulation day, with the peak day being two days prior to the estimated ovulation. Additionally, the pregnancy rate increased by 50% when women had cervical mucus secretions for two days.
Also Bigelow et al. (2004) made use of the ESDF database. From the database, 1473 cycles remained after exclusion, resulting in 353 pregnancies. The aim was to determine the day-specific probabilities of pregnancy according to the timing of intercourse relative to ovulation and/or the mucus characteristics. The outcome proved that the day of lowest fertility is five days before ovulation and the day of highest fertility is three days before ovulation, as detected by BBT. However, the differences in dailyfecundability are more attributable to a rise in mucus score than the timing of intercourse relative to ovulation.
The next three prospective multicentre studies (Colombo et al., 2006; Scarpa et al., 2006; Scarpa et al., 2007) used another database which included 193 couples from four Italian centres using the Billings Ovulation method. Inclusion criteria and mucus scoring were similar to the ESDF study (Table III and Table I respectively).
The first study was performed by Colombo et al. (2006) aiming to determine the effect of cervical mucus symptoms on the daily fecundability. The earlier study performed by Colombo and Masarotto in 2000, had the objective to determine the relationship between the intercourse patterns and the fecundability (Table IV). In 2000, outcome measures included 3255 cycles, 435 of which resulted in a pregnancy; in 2006 respectively 963 cycles and 142 pregnancies (Colombo and Masarotto, 2000; Colombo et al., 2006).
The mucus reference day, identified as day 0, is defined as being the last day with the best quality or peak mucus in a specific cycle, with fluid mucus and/or a wet-slippery sensation. Results showed that the highest pregnancy rate can be found on day 0 with a probability of 0,429 in 2006, respectively on day -2 with a probability of 0,203 in 2000.
The second study (Scarpa et al., 2006) investigated the relationship between the self-observed characteristics of cervical mucus on the day of intercourse and the day-specific probability of conception across the menstrual cycle. The most fertile type mucus (mucus score 4) was registered for six days on average. In general, mucus score 4 had a peak on day 13 of the cycle, in contradiction to mucus score 1 which was unlikely to be seen midcycle. Moreover, results showed that the conception probabilities varied among the mucus scores, increasing from mucus score 1 with a probability of 0,003 to a mucus score 4 with a probability of 0,29. In between, there was a conception probability of 0,013 for days with mucus score 2 and 0,025 for days with mucus score 3.
The third study (Scarpa et al., 2007) determined adequate rules for timing of coitus to achieve conception, based on calendar and cervical mucus. Results showed that, in a midcycle interval beginning on day 7 and ending on day 20, the probability of conception increased with a rise in mucus score. On days with the highest mucus score, there was a 40 times higher conception probability than on days when no mucus score was noticed. Outside this midcycle interval, conception probabilities were negligible. Rules were established, based on the increase in frequency of coitus on days within a midcycle interval which had a mucus score at or above a threshold value on a scale from 1 to 4.
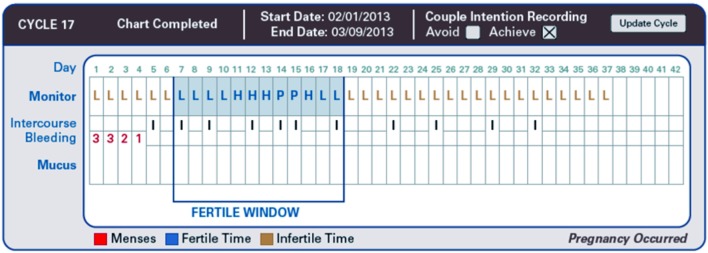
The purpose of the final examined study (Mu and Fehring, 2014) was to determine and compare pregnancy rates when women were having intercourse during the FW in which there was a differentiation between self-estimated high, peak and low fertile days. Figure 3 shows a cycle with correct use of intercourse patterns on high and peak fertility rated days, which resulted in a pregnancy. A 12-month prospective, observational cohort design was used to study 124 women seeking to become pregnant with the use of this natural family planning method. To determine their fertile days the women utilised either cervical mucus monitoring or electronic hormonal fertility monitoring (EHFM) or both. The analysis showed a pregnancy rate of 0,87 at 12 months of trying when intercourse happened on either high or peak days during the fertile window and a pregnancy rate of 0,5 when intercourse occurred only on days with a low mucus score.
Fig. 3. Pregnancy cycle with correct use intercourse pattern on high and peakfertility rated days. (Adapted from Mu and Fehring (2014)).
Discussion
Fertility awareness based methods (FABMs) are another measure a couple can take to ameliorate their likelihood to conceive. In order to evaluate the effectiveness of cervical mucus monitoring (CMM) in shortening the time to pregnancy, especially for the subfertile couple, a literature search was performed. After selection, a total of 10 articles remained to be included in this systematic review.
The highest probability of conception is one or two days before ovulation (Dunson et al., 1999; Colombo and Masarotto, 2000; Dunson et al., 2001) calculated from models solely using BBT signals. But for clinical usage the mucus symptom is superior. Dunson et al. (2001) identified the relationship between cervical secretions and day-specific fecundability, providing evidence that cervical mucus monitoring can be offered to couples who are trying to conceive. By timing intercourse on days with noticeable secretions, couples can significantly increase their chance of achieving pregnancy (Bigelow et al., 2004) and Stanford et al. (2003) showed that the highest pregnancy rate can be seen on the mucus peak day (identified as ovulation) both for fertile and subfertile couples. Furthermore, the quality of the vaginal discharge correlates well with the cycle-specific probability of pregnancy in normally fertile couples but not in subfertile couples (Stanford et al., 2003; Frank-Herrmann et al., 2005; Scarpa et al., 2006). However, several weaknesses of the study by Stanford et al. (2003) should be taken into account: (i) there was no independent marker of ovulation and (ii) the retrospective design of the study.
Scarpa et al. (2006) compared their results with a study performed by Wilcox et al. (2001). The probability of conception on the peak day described by Wilcox et al. (2001) is substantially lower than the findings reported by Scarpa et al. (2006). This difference might be explained because, in the Wilcox study, couples who wished to conceive were less fertile than the couples selected for the Scarpa study, who had more regular menstrual cycles. The difference in fecundability on the peak mucus day may be caused by including women who had already given birth, which yielded a higher probability to become pregnant. Despite this difference in the probability of conception on the peak day by Scarpa et al. (2006) and Wilcox et al. (2001), the number of days with the most fertile-type mucus symptom was six days in both studies.The pattern of the occurrences of the different types of mucus throughout the cycle and the peak day on day 13, corresponds with previously described outcomes (Wilcox et al., 2000).
To efficiently shorten the time to pregnancy, a couple could rely on timing coitus during self-estimated high and peak fertile days in the FW, based on days with the most fertile-type mucus symptom (Scarpa et al., 2007; Evans-Hoeker et al., 2013; Mu and Fehring, 2014). Scarpa et al. (2007) suggested rules for timing intercourse to have a higher probability of conception. However, among women, there are differences in age, hormone secretions, length of the menstrual cycles and numbers of cycles attempting. This indicates that some recommendationsmight work for some couples, but not for everyone due to woman-specific characteristics.
Another important issue is the relevance of coital patterns. The ASRM recommends sexual intercourse at least every other day to optimize natural fertility (ASRM, 2008). However, in case of subfertility, there is no place to recommend just an increase of coital frequency probably causing an increase of psychological stress only. In contrary, couples should be informed about the fact that a single episode of intercourse on a day of highly fertile mucus gives nearly the same chance of pregnancy then multiple acts (Bilian et al., 2010).
Until now, most studies used a small sample size and there is a need for prospective studies including couples planning to become pregnant. Most studies examined either excluded couples with fertility problems or did not take them into account. We urgently need a prospective study comparing fertile and subfertile groups of couples with a duration of infertility for at least 6 months and without an obvious reason for infertility, such as tubal factor or a very poor sperm quality.
Our findings indicate that natural family planning methods can be used as a diagnostic tool to identify subfertility and its possible causes, but also provide a better understanding of the menstrual cycle (Gnoth et al., 2003). However, we still lack research onsubfertile couples who use CMM and evaluate the effect on conception probability and time to pregnancy.
Most studies that rely on mucus observations are classified according to a rough 1 to 4 scale. This scheme is non-invasive and easy to implement after a minimal amount of training. It could be interesting to better quantify these mucus characteristics and to remove the potential subjectivity in this classification. A few studies (Scarpa et al., 2006; Evans-Hoeker et al., 2013) suggested to assess the utility of urinary LH monitoring with CMM for couples attempting pregnancy. A randomised trial could provide information on the precise relationships that exist between hormones and mucus for women varying in their fecundability.
Until now, there are no evidence-based guidelines for couples to shorten their time to pregnancy by timing intercourse. The only recommendation, not evidence-based, is to have frequent intercourse every other day. It is important for women to know that CMM can decrease the time to pregnancy if used properly. This method is easily taught with the help of nurses/physicians and online education (Sensiplan®). The combination of these two sources of education and the use of an online fertility charting system, can provide an efficient system to shorten the time to pregnancy in a selected group of patients with no obvious cause for infertility (Mu and Fehring, 2014). However, evidence showed that most physicians do not give information about this method and underestimate the value of this approach (Stanford et al., 1999). All people involved in infertility care should be informed about the existence and effectiveness of the FABM, in order to properly educate women about it and to decrease the number of couples that will eventually need assisted reproductive technologies.
Conclusion
Based on the results of the selected studies after literature search, we can conclude that cervical mucus secretions can be used as a good predictor of impending fertility. All evaluated studies demonstrated that observing the cervical mucus can identify the days with the highest pregnancy probability. When intercourse takes place on a day in the fertile interval with the highest mucus score, the time to pregnancy can be shortened significantly. According to the literature, the quality of the vaginal discharge correlates well with the cycle-specific probability of pregnancy in fertile couples but less in subfertile couples. Most of the results are based on studies with couples with unknown fertility status. However, there is some evidence that cervical mucus monitoring can become a very useful approach for women with unexplained subfertility. There is an urgent need for further research to confirm the effectiveness of the CMM method to increase the probability of pregnancy in subfertile couples. If these methods prove to be effective for the subfertile couple, more patients will become pregnant without the need of assisted reproductive technologies (ART). When comparing cumulative pregnancy rates after ART with cumulative pregnancy rates in natural cycles (Gnoth et al., 2011) we find congruent curves which is beautifully in line with some simulation models (Stanford et al., 2010). This suggests that ART may reach natural fertility rates but cannot exceed them and patients will not benefit from a rush into ART.
These thoughts may help in offering a more patient-friendly approach within infertility centres and probably will also reduce ART-related costs for subfertile couples and social systems.
References
- Albertson BD, Zinaman MJ. The prediction of ovulation and monitoring of the fertile period. Adv Contracept. 1987;3:263–290. doi: 10.1007/BF01849284. [DOI] [PubMed] [Google Scholar]
- ASRM. Optimizing natural fertility. Fertil Steril. 2008;90:S1–6. doi: 10.1016/j.fertnstert.2008.08.122. [DOI] [PubMed] [Google Scholar]
- Bhargava H, Bhatia JC, Ramachandran L, et al. Field trial of billings ovulation method of natural family planning. Contraception. 1996;53 doi: 10.1016/0010-7824(95)00269-3. [DOI] [PubMed] [Google Scholar]
- Bigelow JL, Dunson DB, Stanford JB, et al. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod. 2004;19:889–892. doi: 10.1093/humrep/deh173. [DOI] [PubMed] [Google Scholar]
- Bilian X, Heng Z, Shang-chun W, et al. Conception probabilities at different days of menstrual cycle in Chinese women. Fertil Steril. 2010;94:1208–1211. doi: 10.1016/j.fertnstert.2009.05.054. [DOI] [PubMed] [Google Scholar]
- Billings EL, Brown JB, Billings JJ, et al. Symptoms and hormonal changes accompanying ovulation. Lancet. 1972;1:282–284. doi: 10.1016/s0140-6736(72)90291-7. [DOI] [PubMed] [Google Scholar]
- Colombo B, Masarotto G. Daily fecundability: first results from a new data base. Demogr Res. 2000;3:39. [PubMed] [Google Scholar]
- Colombo B, Mion A, Passarin K, et al. Cervical mucus symptom and daily fecundability: first results from a new database. Stat Methods Med Res. 2006;15:161–180. doi: 10.1191/0962280206sm437oa. [DOI] [PubMed] [Google Scholar]
- Dunson DB, Baird DD, Wilcox AJ, et al. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum Reprod. 1999;14:1835–1839. doi: 10.1093/humrep/14.7.1835. [DOI] [PubMed] [Google Scholar]
- Dunson DB, Sinai I, Colombo B. The relationship between cervical secretions and the daily probabilities of pregnancy: effectiveness of the TwoDay Algorithm. Hum Reprod. 2001;16:2278–2282. doi: 10.1093/humrep/16.11.2278. [DOI] [PubMed] [Google Scholar]
- Evans-Hoeker E, Pritchard DA, Long DL, et al. Cervical mucus monitoring prevalence and associated fecundability in women trying to conceive. Fertil Steril. 2013;100:1033–1038. doi: 10.1016/j.fertnstert.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordney-Settlage D. A review of cervical mucus and sperm interactions in humans. Int J Fertil. 1981;26:161–169. [PubMed] [Google Scholar]
- Frank-Herrmann P, Gnoth C, Baur S, et al. Determination of the fertile window: reproductive competence of women – European cycle databases. Gynecol Endocrinol. 2005;20:305–312. doi: 10.1080/09513590500097507. [DOI] [PubMed] [Google Scholar]
- Frank-Herrmann P, Heil J, Gnoth C, et al. The effectiveness of a fertility awareness based method to avoid pregnancy in relation to a couple’s sexual behaviour during the fertile time: a prospective longitudinal study. Hum Reprod. 2007;22:1310–1319. doi: 10.1093/humrep/dem003. [DOI] [PubMed] [Google Scholar]
- Gnoth C, Frank-Herrmann P, Bremme M, et al. How do self-observed cycle symptoms correlate with ovulation? Zentralbl Gynakol. 1996;118:650–654. [PubMed] [Google Scholar]
- Gnoth C, Godehardt D, Godehardt E, et al. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod. 2003;18:1959–1966. doi: 10.1093/humrep/deg366. [DOI] [PubMed] [Google Scholar]
- Gnoth C, Maxrath B, Skonieczny T, et al. Final ART success rates: a 10 years survey. Hum Reprod. 2011;26:2239–2246. doi: 10.1093/humrep/der178. [DOI] [PubMed] [Google Scholar]
- Gross BA. Natural family planning indicators of ovulation. Clin Reprod Fertil. 1987;5:91–117. [PubMed] [Google Scholar]
- Hilgers TW, Prebil AM. The ovulation method – vulvar observations as an index of fertility/infertility. Obstet Gynecol. 1979;53:12–22. [PubMed] [Google Scholar]
- Howard MP, Stanford JB. Pregnancy probabilities during use of the Creighton Model Fertility Care System. Arch Fam Med. 1999;8:391–402. doi: 10.1001/archfami.8.5.391. [DOI] [PubMed] [Google Scholar]
- Hume K. Fertility awareness in the 1990s – the Billings Ovulation Method of natural family planning, its scientific basis, practical application and effectiveness. Adv Contracept. 1991;7:301–311. doi: 10.1007/BF01849421. [DOI] [PubMed] [Google Scholar]
- Jennings V, Sinai I, Sacieta L, et al. TwoDay Method: a quick-start approach. Contraception. 2011;84:144–149. doi: 10.1016/j.contraception.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Katz DF, Slade DA, Nakajima ST. Analysis of pre-ovulatory changes in cervical mucus hydration and sperm penetrability. Adv Contracept. 1997;13:143–151. doi: 10.1023/a:1006543719401. [DOI] [PubMed] [Google Scholar]
- Moghissi KS, Syner FN, Evans TN. A composite picture of the menstrual cycle. Am J Obstet Gynecol. 1972;114:405–418. doi: 10.1016/0002-9378(72)90617-5. [DOI] [PubMed] [Google Scholar]
- Mu Q, Fehring RJ. MCN Am J Matern Child Nurs. 2014;39:35–40. doi: 10.1097/NMC.0b013e3182a76b88. [DOI] [PubMed] [Google Scholar]
- Pallone SR, Bergus GR. Fertility awareness-based methods: another option for family planning. 2009;22:147–157. doi: 10.3122/jabfm.2009.02.080038. [DOI] [PubMed] [Google Scholar]
- Scarpa B, Dunson DB, Colombo B. Cervical mucus secretions on the day of intercourse: an accurate marker of highly fertile days. Eur J Obstet Gynecol Reprod Biol. 2006;125:72–78. doi: 10.1016/j.ejogrb.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Scarpa B, Dunson DB, Giacchi E. Bayesian selection of optimal rules for timing intercourse to conceive by using calendar and mucus. Fertil Steril. 2007;88:915–924. doi: 10.1016/j.fertnstert.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Sozou PD, Hartshorne GM. Time to pregnancy: a computational method for using the duration of non-conception for predicting conception. PLoS One. 2012;7:e46544. doi: 10.1371/journal.pone.0046544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JB, Thurman PB, Lemaire JC. Physicians’ knowledge and practices regarding natural family planning. Obstet Gynecol. 1999;94:672–678. doi: 10.1016/s0029-7844(99)00388-9. [DOI] [PubMed] [Google Scholar]
- Stanford JB, White GL, Hatasaka H. Timing intercourse to achieve pregnancy: current evidence. Obstet Gynecol. 2002;100:1333–1341. doi: 10.1016/s0029-7844(02)02382-7. [DOI] [PubMed] [Google Scholar]
- Stanford JB, Smith KR, Dunson DB. Vulvar mucus observations and the probability of pregnancy. Obstet Gynecol. 2003;101:1285–1293. doi: 10.1016/s0029-7844(03)00358-2. [DOI] [PubMed] [Google Scholar]
- Stanford JB, Mikolajczyk RT, Lynch CD, et al. Cumulative pregnancy probabilities among couples with subfertility: effects of varying treatments. Fertil Steril. 2010;93:2175–2181. doi: 10.1016/j.fertnstert.2009.01.080. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen C, Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577–784. doi: 10.1016/s0015-0282(02)04694-0. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Dunson D, Baird DD. The timing of the “fertile window” in the menstrual cycle: day specific estimates from a prospective study. BMJ. 2000;321:1259–1262. doi: 10.1136/bmj.321.7271.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Dunson DB, Weinberg CR, et al. Likelihood of conception with a single act of intercourse: providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001;63:211–215. doi: 10.1016/s0010-7824(01)00191-3. [DOI] [PubMed] [Google Scholar]