Abstract
Prenatal glucocorticoid administration in clinically relevant doses reduces nephron number and renal function in adulthood and is associated with hypertension. Nephron loss in early life may predispose the kidney to other insults later but whether sex influences increases in renal susceptibility is unclear. Therefore, we determined, in male and female adult sheep, whether antenatal glucocorticoid (betamethasone) exposure increased 8-isoprostane (marker of oxidative stress) and protein excretion after acute nephron reduction and intrarenal infusions of angiotensin peptides. We also examined whether renal proximal tubule cells (PTCs) could contribute to alterations in 8-isoprostane excretion in a sex-specific fashion. In vivo, ANG II significantly increased 8-isoprostane excretion by 49% and protein excretion by 44% in male betamethasone- but not in female betamethasone- or vehicle-treated sheep. ANG-(1-7) decreased 8-isoprostane excretion but did not affect protein excretion in either group. In vitro, ANG II stimulated 8-isoprostane release from PTCs of male but not female betamethasone-treated sheep. Male betamethasone-exposed sheep had increased p47 phox abundance in the renal cortex while superoxide dismutase (SOD) activity was increased only in females. We conclude that antenatal glucocorticoid exposure enhances the susceptibility of the kidney to oxidative stress induced by ANG II in a sex-specific fashion and the renal proximal tubule is one target of the sex-specific effects of antenatal steroids. ANG-(1-7) may mitigate the impact of prenatal glucocorticoids on the kidney. P47 phox activation may be responsible for the increased oxidative stress and proteinuria in males. The protection from renal oxidative stress in females is associated with increased SOD activity.
Keywords: intrarenal infusion, fetal programming, kidney, proteinuria, 8-isoprostane
antenatal glucocorticoid administration to women at risk for preterm delivery is standard care because it markedly reduces the incidence and severity of prematurity-related complications such as respiratory distress syndrome. However, antenatal steroids can have unintended consequences in the offspring including reducing nephron number, impairing renal function, altering the intrarenal renin-angiotensin system (RAS), and causing hypertension (20, 47, 74). While the precise relationship between adult hypertension and a reduction in nephron number in the perinatal period has not been established, increasing evidence suggests that loss of nephrons in early life is associated with greater risk of renal damage and the development of adult hypertension in response to other insults (2, 31, 67, 73). This has been termed the “second hit hypothesis” (41). It is not known whether antenatal exposure to a clinically relevant dose of glucocorticoid increases susceptibility to a second insult and whether any increase in susceptibility is sex-specific. Moreover, it is not clear which cell types in the kidney are targets for any such effects. In this study, we tested the basic hypothesis that the impact of acute nephron loss in adulthood would be exacerbated by a programming stimulus in fetal life and this impact would be sex-specific. Therefore, we determined whether antenatal steroid exposure (considered a “first hit”) would increase susceptibility to possible deleterious effects of nephron loss in adulthood as reflected by proteinuria and increased oxidative stress (elevated 8-isoprostane). Thus, we performed unilateral nephrectomy as a “second hit” in adult male and female sheep previously exposed to vehicle or betamethasone at 0.6 gestation (full term 145 days) and measured proteinuria and the excretion of 8-isoprostane as markers of renal dysfunction. Moreover, these markers were assessed under baseline conditions and during intrarenal infusions of angiotensin II (ANG II) or angiotensin-(1-7) [ANG-(1-7)] to determine whether these peptides would alter the excretion of the markers of renal dysfunction. ANG II increases oxidative stress in the kidney (32, 56) while ANG-(1-7) tends to oppose the effects of ANG II (12, 13, 19). To determine the possible mechanism of changes in 8-isoprostane excretion, we measured expression of p47 phox and NOX2, key subunits of NADPH oxidase complexes, as well as the enzyme superoxide dismutase (SOD) that scavenges superoxide to hydrogen peroxide (26). We also determined whether the primary cultures of renal proximal tubule cells (PTCs) exhibited a similar susceptibility to ANG II-induced oxidative stress by alterations in 8-isoprostane release. Our results reveal that antenatal glucocorticoid exposure increases renal susceptibility to a second insult in a sex-specific manner, that the PTC is one target of these effects, and that NADPH oxidase and SOD in PTCs may contribute to the sex-specific alterations in response to ANG II.
MATERIALS AND METHODS
Animal preparation.
All procedures were approved by the Institutional Animal Care and Use Committee of Wake Forest University School of Medicine. Time-dated pregnant ewes were randomly assigned to receive either two 0.17-mg/kg intramuscular injections of a 1:1 mixture of betamethasone acetate and betamethasone phosphate (Celestone Soluspan, Schering, Kenilworth, NJ) or vehicle alone which contained 3.4 mg of monobasic sodium phosphate, 7.1 mg of dibasic sodium phosphate, 0.1 mg of sodium ethylenediaminetetra-acetic acid, and 0.2 mg of benzalkonium chlorine per ml given 24 h apart at days 80 and 81 of gestation. The ewes were allowed to deliver naturally at term (term is ∼145 days in our flock).
Sheep offspring were transferred to the laboratory at 12 to 18 mo of age. The research team was blinded to the sheep allocation. The temperature in the laboratory facilities and in the sheep pens was kept stable at 20 ± 2°C, and the humidity at ∼35–40%. Sheep were fed a standard commercial diet (PMI Rumilab, Brentwood, MO) containing 0.75% NaCl and had ad libitum access to clean tap water. Twelve-hour day, night cycles were kept constantly throughout the experiment.
Surgical procedure.
A midline suprapubic laparotomy was made in the lower abdomen to expose the bladder as previously described (7). A 14 French silicone Foley catheter (Bard Medical, Covington, GA) was placed in the bladder and exteriorized through the left flank. Two polyvinyl catheters were placed in the left carotid artery and two catheters were inserted into the left external jugular vein. These were tunneled subcutaneously and emerged through a left anterior neck incision. All vascular catheters were fastened to their corresponding vessels and the distal vessels were all ligated.
A paravertebral left or right flank incision was made to expose the corresponding renal artery. A modified, Teflon-coated, 22-gauge endovascular catheter 25-mm long and with a 0.9-mm external diameter was placed into the left or right renal artery in a nonocclusive fashion and attached to a Tygon catheter. The Tygon catheter was tunneled a short distance to emerge adjacent to the spine. We then removed the contralateral kidney.
All the animals received daily doses of 1,000 mg ampicillin, 80 mg gentamicin, and 100 mg iv ketoprofen the day of surgery and 2 days thereafter. All vascular catheters were flushed with a heparin solution on a daily basis to confirm patency. During the postoperative period and throughout the experimental period, each pair of animals was housed together in individual carts with free access to food and water.
Experimental protocol.
After allowing at least 5 days for the sheep to recover, they underwent an experiment designed to evaluate the response to a 3-h infusion of ANG II (1 ng·kg−1·min−1; Bachem BioScience, Torrance, CA) or ANG-(1-7) (1 ng·kg−1·min−1; Bachem BioScience). The dose for ANG II was obtained from previous work documenting its effect during intrarenal infusion in nonpregnant sheep (23). The dose for ANG-(1-7) was selected from our previous work (64, 65). Data were obtained from five or seven animals in each group in each experiment but in males only four in each group received all the treatments due to occasional catheter failure.
Blood and urine samples were serially obtained before all infusions were initiated, subsequent to infusions initiated, and over the course of the following 3 h. To guarantee adequate hydration status, 2,000 ml of normal saline were given intravenously on the afternoon before each experiment was begun. After all experiments had concluded, the animals were euthanized with 10 ml iv Beuthanasia D (390 mg pentobarbital sodium and 50 mg phenytoin sodium per ml), after which organ harvesting was performed. To determine whether PTCs might contribute to any alterations in 8-isoprostane excretion observed during the in vivo study, renal PTCs were obtained from another cohort of animals that underwent uninephrectomy and then were housed in stalls for the same period as the animals in the previous in vivo study.
Blood and urine samples.
Timed samples of blood and urine were collected 1 h before any infusion was begun and at the end of the infusions. Urine samples were collected in plain tubes. All blood and urine samples were centrifuged at 3,000 rpm, 4°C, for 10 min immediately after collection, and stored at −20 or −80°C for subsequent analysis. Urine volumes were recorded and averaged ∼150 ml/h.
Urine protein measurement.
Urinary protein concentration was measured using the Bradford technique (Pierce Biotechnology, Rockford, IL). The assay is based on the observation that the absorbance maximum for an acidic solution of Coomassie Brilliant Blue G-250 shifts from 465 to 595 nm when binding to protein occurs. Both hydrophobic and ionic interactions stabilize the anionic form of the dye, causing a visible color change.
8-Isoprostane measurement in urine and culture medium.
8-Isoprostane levels in urine and culture medium were measured with commercial EIA kits (Cayman Chemical, Ann Arbor, MI). Before embarking on a large number of urine sample measurements, we checked for interference according to the manufacturer's instruction. We diluted one test sample to three different dilutions. The three different dilutions of the sample showed good correlation with the final calculated 8-isoprostane concentration. The differences between samples were 14, 9.1, and 4.9%, respectively. Urine purification was not required if the concentrations differ <20%. Culture medium samples were assayed without purification and the 8-isoprostane standards were diluted in culture medium instead of EIA buffer. All samples were collected on ice and stored at −80°C in the presence of 0.005% butylated hydroxyl toluene (BHT; Cayman Chemical).
PTCs isolation.
A portion of the freshly isolated renal cortex was dissected out on ice for isolation of proximal tubules as described previously (61). Briefly, kidney outer cortex was minced into fine pieces and incubated with collagenase (1 mg/ml; CLS 1, Worthington) at 37°C in a water-jacketed flask for 60 min containing 100 ml (in mM) of a Krebs-Henseliet buffer (KHB; 25 HEPES, 118 NaCl, 4.8 KCl, 0.96 KH2PO4, 25 NaHCO3, 0.12 MgSO4, 2.55 CaCl2, pH 7.4) with 100 μl/ml DNase. At the end of the digestion, ice-cold KHB containing 10% fetal calf serum (FCS) was added, and the suspension was filtered through a nylon mesh (70 μm) and centrifuged at 500 g for 5 min at 4°C to pellet the tubules. The pellet was resuspended with 32 ml of ice-cold KHB/5% FCS and gently applied to an isotonic discontinuous Percoll gradient (Pharmacia) of 10–35% (vol/vol) with KHB/5% FCS and centrifuged at 15,000 g for 60 min at 4°C. The cell layer at a density of 1,063 as determined by density beads was washed in 3× KHB to remove the Percoll media. The cells were immediately resuspended with DMEM/F12. The cells were cultured for 5 to 10 days. The culture media were changed to serum-free media after the cells reached 60–70% confluence. When they reached a desired confluence (∼95–100%), the cells were incubated with ANG II (2 nM) or ANG-(1-7) (2 nM) for 24 h in serum and phenol red free medium. The dose and timeframe used were based on pilot dose-response studies. Media were collected with 0.005% BHT (Cayman Chemical) and stored at −80°C. The cells were lysed with RIPA buffer (25 mM Tris·HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS; Thermo Scientific, Rockford, IL) and the lysates were stored at −80°C for further protein measurement. Cells were confirmed to be PTCs by immunocytochemical staining for sodium-glucose transporter 2 protein which is specifically located on PTCs of nephron. Approximately 95% of the cells stained positive for this marker (data not shown).
Protein immunoblotting of kidney cortex.
P47 phox and NOx2/gp91 protein expression were determined by Western blotting using 25 μg of protein from each kidney cortex extraction for electrophoresis and transferring to an Immunobilon membrane. Blots for p47 phox were incubated with the p47 phox primary antibody (1:1,000; Cell Signaling Technology) and then secondary antibody (1:5,000; GE Healthcare). Equal loading of the samples was verified using an antibody to β-actin (1:20,000 dilutions; Abcam). Blots for NOX2/gp91 were incubated with the NOX2/gp91primary antibody (1:1,000; Cell Signaling Technology) and then secondary antibody (1:2,500; GE Healthcare). Equal loading of the samples was verified using an antibody to β-actin at 1:20,000 dilutions (Abcam). Relative p47 phox, NOx2/gp91, and β-actin protein abundance were quantitated using densitometry.
SOD assay.
SOD activity in kidney cortex was measured with commercial kits (Cell Technology, Mountain View, CA). Briefly, tissue samples were weighed, minced, and homogenized in an ice-cold sucrose buffer (50 mM sucrose, 200 mM mannitol, 1 mM EDTA in 10 mM TRIS buffer, pH 7.4, 10 μl/mg tissue) using a Tissue Tearor (model 985730, Biosprec Products) at speed 2 for 45-s periods separated by 15 s. The homogenized sample was centrifuged at 10,000 g for 15 min at 4°C and the supernatant was transferred into a new tube. The processed samples were stored on ice and assayed for SOD immediately.
Immunohistochemical determination for NADPH oxidase subunits p47 phox and SOD.
Sections (5 μm) of kidney cortex were cut from 4% paraformaldehyde-fixed, paraffin-embedded tissue blocks, mounted on polylysine-coated slides. The antigen was retrieved by heating the sections in a 10-mM citrate buffer (90°C, 30 min). For SOD staining, the cooled sections were incubated with 3% H2O2 for 10 min to block endogenous peroxidases. The primary antibody was the rabbit polyclonal anti-superoxide dismutase 1 antibody (Abcam, Cambridge, MA), used at a 1:500 dilution. After being washed with PBS, the tissue sections were incubated with rabbit horseradish peroxidase polymers for 30 min (Biocare Medical, Concord, CA); diaminobenzidine was used for visualization. For p47 phox immunostaining, sections were incubated at 4°C overnight with the rabbit polyclonal anti-p47phox antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:500 dilution in 0.5 M TBST. The secondary antibody was coupled to Alexa fluor 546 (Invitrogen, Carlsbad, CA) used at a 1:200 dilution. Nuclear counterstaining was performed with DAPI at mounting medium (H-1200, VECTOR Lab) Sections were kept in the dark until the fluorescence was studied using a microscope (LSM 510 META Carl Zeiss, Overkochen, Germany). Control slides were incubated with the appropriate IgG in both staining.
Statistical analysis.
The study data were tabulated and graphed using Graph Prism version 6.0 software (GraphPad Software, La Jolla, CA). Statistical analysis was accomplished with the use of two-way ANOVA or t-test where appropriate. Baseline comparisons were made using the average of the baseline values from the same sheep in different experiments. Means between the betamethasone- and vehicle-exposed sheep were compared. A P value of ≤0.05 was considered statistically significant. The data are reported as means ± SE.
RESULTS
The baseline values for 8-isoprostane and protein excretion were compared between the uninephrectomized vehicle- and the betamethasone-exposed sheep (Fig. 1, A and B). Overall, there were no significant differences in the baseline 8-isoprostane and protein excretion between the vehicle- and betamethasone-exposed sheep. However, baseline protein excretion was higher in betamethasone- compared with vehicle-exposed males (1.05 ± 0.2 mg·kg−1·h−1 in beta and 0.70 ± 0.03 mg·kg−1·h−1 in vehicle, P < 0.05, Students t-test).
Fig. 1.
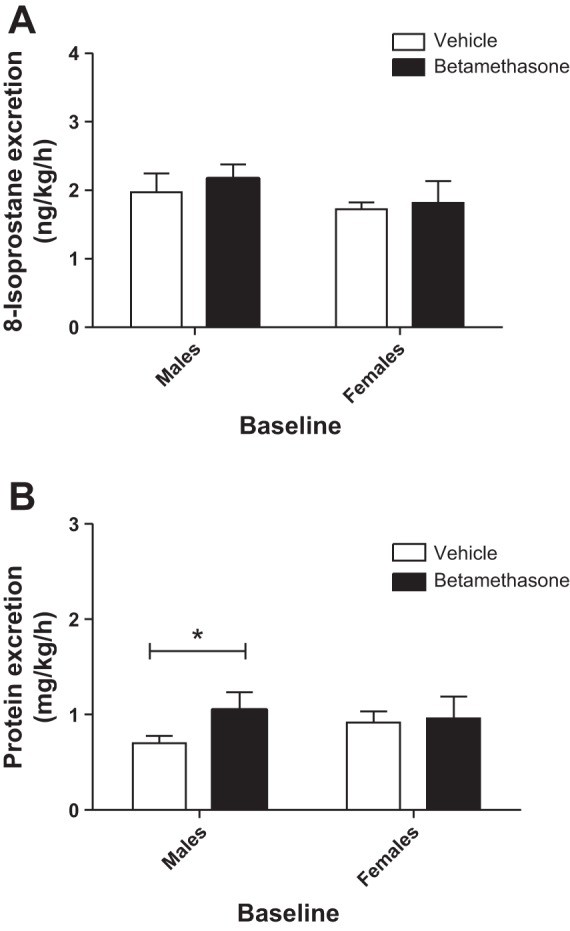
Baseline urinary 8-isoprostane excretion (A) and protein excretion (B) in vehicle- and betamethasone-exposed sheep of males and females. *P < 0.05, male vehicle- vs. male betamethasone-treated sheep. Male vehicle- and betamethasone-treated sheep (n = 9 per group). Female vehicle- and betamethasone-treated sheep (n = 6 per group).
Effect of intrarenal infusion of ANG II on urinary 8-isoprostane and protein excretion.
There was no significant change in 8-isoprostane excretion in vehicle-treated sheep of both sexes during infusion of ANG II (Fig. 2A). In contrast, ANG II infusion increased urinary 8-isoprostane excretion in male but not in female betamethasone-treated sheep at the end of the 3-h infusion. There was a significant effect of sex (F = 4.6, P = 0.04) in 8-isoprostane excretion and a trend for a significant interaction effect of sex and ANG II infusion (F = 3.1, P = 0.09; Fig. 2B) in the betamethasone-exposed animals on 8-isoprostane excretion. Protein excretion did not change in response to ANG II in both male and female vehicle-exposed sheep (Fig. 3A). In contrast, compared with the vehicle-treated males, the ANG II infusion increased protein excretion in the betamethasone-exposed male animals (Fig. 3, A vs. B; F = 7.5, P < 0.02). Furthermore, the ANG II infusion increased protein excretion (F = 6.6, P = 0.03) in male but not in female betamethasone-exposed animals (Fig. 3B).
Fig. 2.
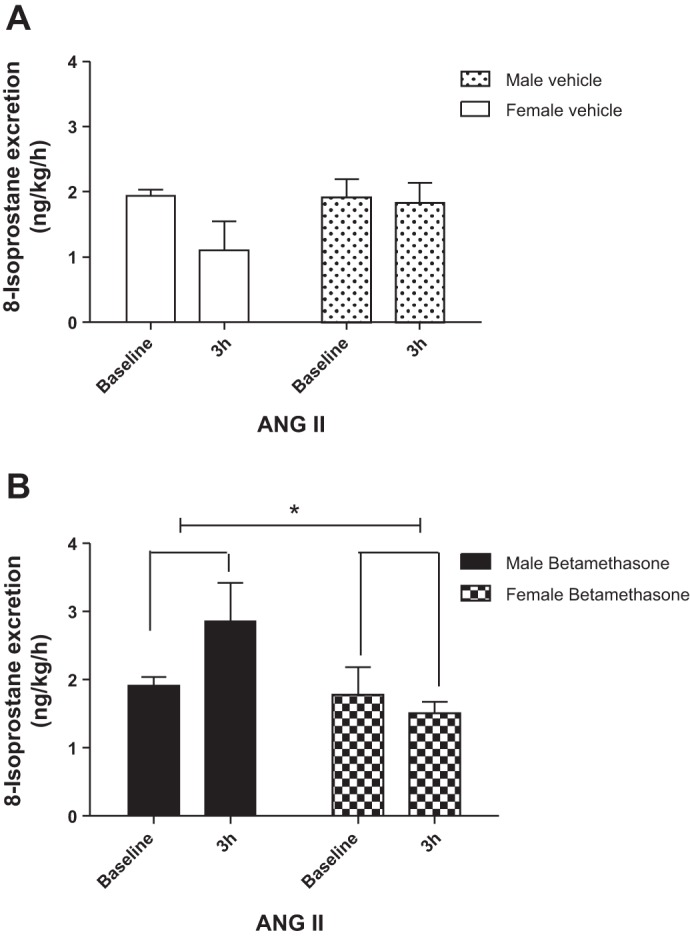
ANG II enhances 8-isoprostane excretion in male betamethasone-exposed sheep. A: urinary 8-isoprostane excretion during intrarenal infusion of ANG II in vehicle-treated sheep. B: urinary 8-isoprostane excretion during intrarenal infusion of ANG II in betamethasone-treated sheep. *F = 4.6, P = 0.04, male vs. female betamethasone-exposed sheep (n = 6 for each group).
Fig. 3.
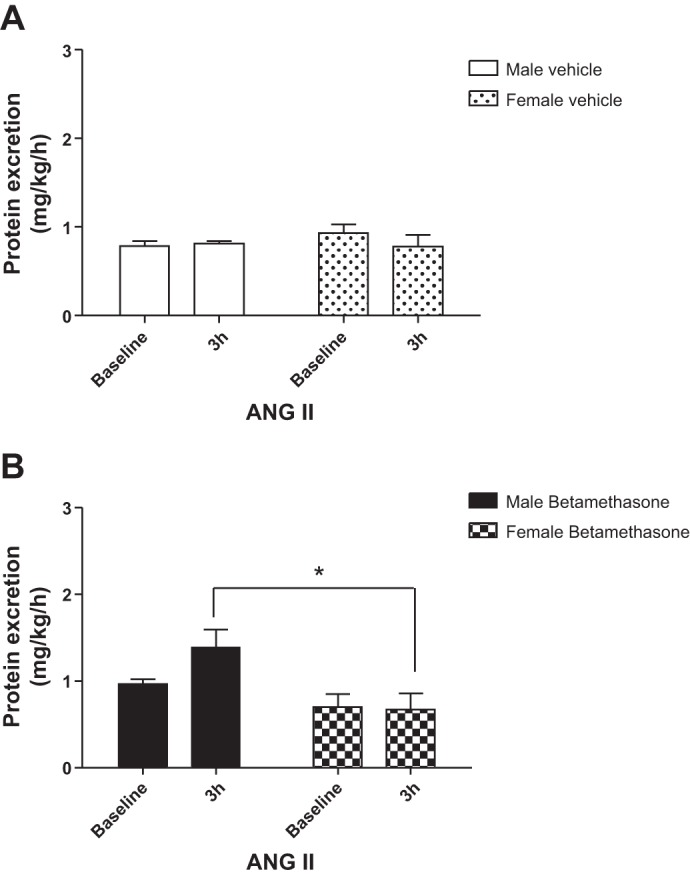
ANG II enhances protein excretion in male betamethasone-exposed sheep. A: urinary protein excretion during intrarenal infusion of ANG II in vehicle-treated sheep. B: urinary protein excretion during intrarenal infusion of ANG II in betamethasone-treated sheep. *F = 6.6, P = 0.03, male vs. female betamethasone-exposed sheep. Male vehicle (n = 6); male betamethasone (n = 7); female vehicle (n = 6); female betamethasone (n = 6).
Effect of ANG II or ANG-(1-7) on 8-isoprostane production in renal PTCs.
ANG II stimulated 8-isoprostane release from PTCs of the kidneys from males but not from females (F = 37.1, P < 0.0001) and the response was greatest in the male betamethasone-treated sheep (Bonferroni posttests, P < 0.05; Fig. 4A). ANG-(1-7) did not significantly change 8-isoprostane release from PTCs of the kidneys in both males and females (Fig. 4B).
Fig. 4.
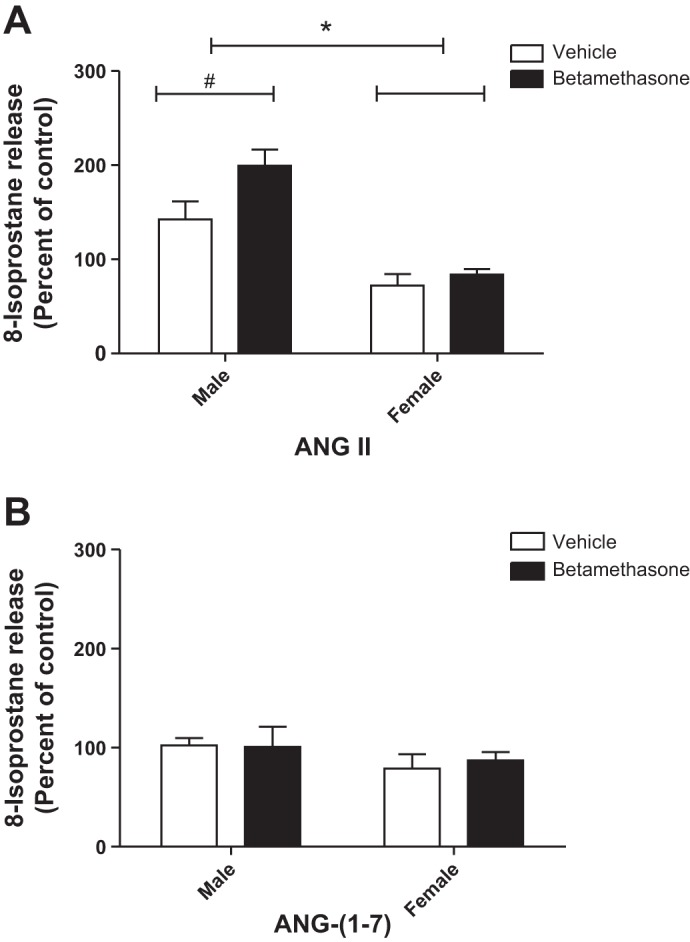
ANG II enhances 8-isoprostane release from proximal tubule cells of male betamethasone-exposed sheep but not females. A: ANG II stimulation, *F = 37.1, P < 0.0001, male vs. female betamethasone-exposed sheep. #P < 0.05, Bonferroni posttests, vehicle- vs. betamethasone-exposed sheep in males. B: ANG-(1-7) stimulation. Male vehicle (n = 6); male betamethasone (n = 5); female vehicle (n = 5); female beta (n = 5).
P47 phox and NOx2/gp91 protein expression in kidney cortex.
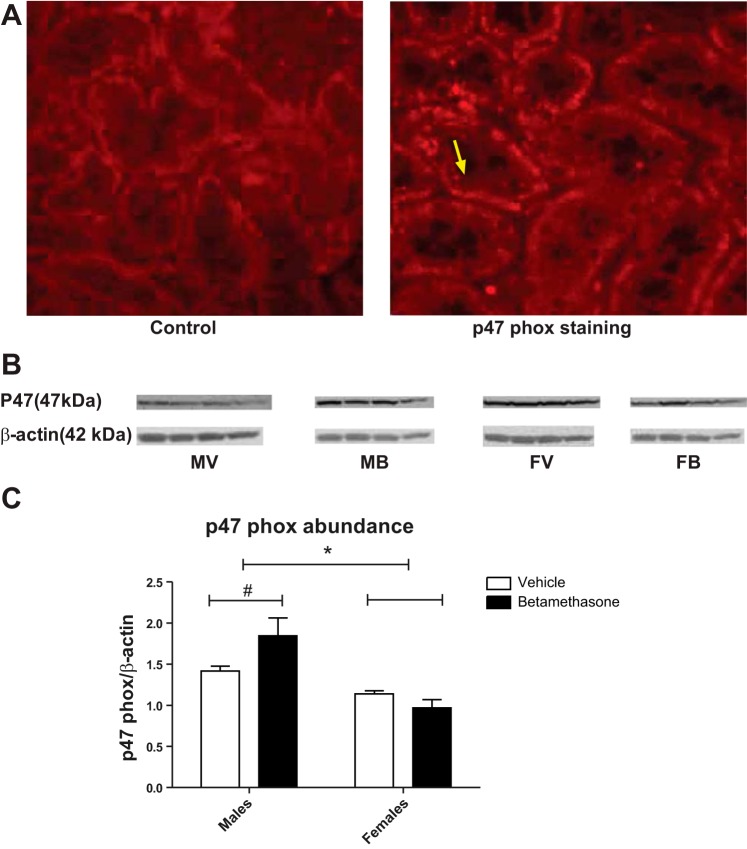
We initially visualized fluorescent p47 phox staining in the renal cortex primarily in the kidney tubules (Fig. 5A). Betamethasone exposure increased p47 phox protein abundance in the kidney cortex from male betamethasone-exposed sheep (P = 0.05; Fig. 5B). There was an overall increase of p47 phox protein abundance in males compared with females (F = 21.6, P = 0.001). Also, there was a significant interaction of sex and betamethasone exposure (F = 5.8, P = 0.03; Fig. 5C) on p47 phox protein expression. There was no difference of NOx2/gp91 protein abundance between the groups (data not shown).
Fig. 5.
A: p47 phox immunofluorescent staining in kidney cortex sections. Negative control (left) and fluorescent p47 staining in renal tubule cells (right, bright red dots, yellow arrow for an example, and ×20 magnifications). B: p47 phox protein abundance levels were analyzed by Western blotting. C: p47 phox protein abundance in kidney cortex in betamethasone-treated and vehicle-treated male and female sheep. *F = 21.6, P = 0.0006, males vs. females. F = 5.8, P = 0.03, interaction of sex and betamethasone exposure. #P = 0.05, male vehicle- vs. male betamethasone-exposed sheep. MV, male vehicle; MB, male betamethasone; FV, female vehicle; FB, female betamethasone.
SOD activity in kidney cortex.
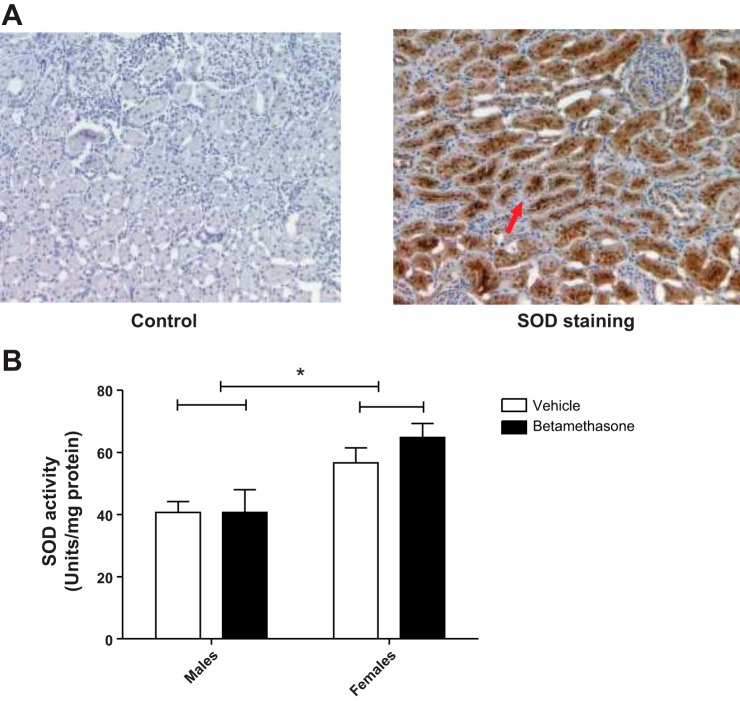
Similar to p47phox, SOD staining in renal sections was primarily localized to the kidney tubules (Fig. 6A). Furthermore, SOD activity measured after uninephrectomy was higher in females than in males (F = 14.7, P = 0.002; Fig. 6B). There was no significant effect of betamethasone exposure.
Fig. 6.
A: representative pictures with superoxide dismutase (SOD) staining in kidney cortex section are shown. Negative control (left) and SOD-positive staining in tubule cells (right, dark brown, red arrow for an example, and ×10 magnifications). B: SOD activity after uninephectomy in vehicle- and betamethasone-exposed sheep from males and females. There was an overall increase of SOD activity after uninephrectomy in females but not in males (F = 14.7, P = 0.002; n = 5 per group).
Effect of intrarenal infusion of ANG-(1-7) on urinary 8-isoprostane and protein excretion.
There was no significant change in 8-isoprostane excretion in vehicle-treated sheep of both sexes during infusion of ANG-(1-7) (Fig. 7A). However, ANG-(1-7) infusion decreased urinary 8-isoprostane excretion in both male and female betamethasone-treated sheep at the end of the 3-h infusion (F = 13.2, P = 0.004; Fig. 7B). Protein excretion was similar in the groups and the ANG-(1-7) infusion did not change protein excretion in males or females (Fig. 8, A and B).
Fig. 7.
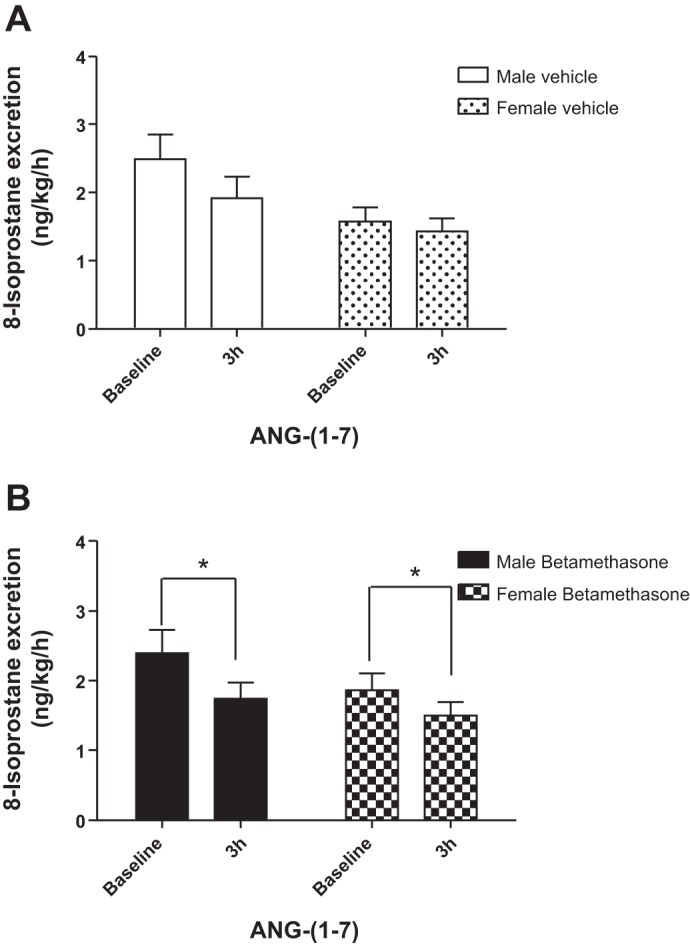
ANG-(1-7) reduces 8-isoprostane excretion in male and female betamethasone-exposed sheep. A: urinary 8-isoprostane excretion during intrarenal infusion of ANG-(1-7) in vehicle-treated sheep. B: urinary 8-isoprostane excretion during intrarenal infusion of ANG-(1-7) in betamethasone-treated sheep. *F = 13.2, P = 0.004, baseline vs. 3-h intrarenal infusion in male and female betamethasone-exposed sheep. MV (n = 7); MB (n = 7); FV (n = 6); FB (n = 6).
Fig. 8.
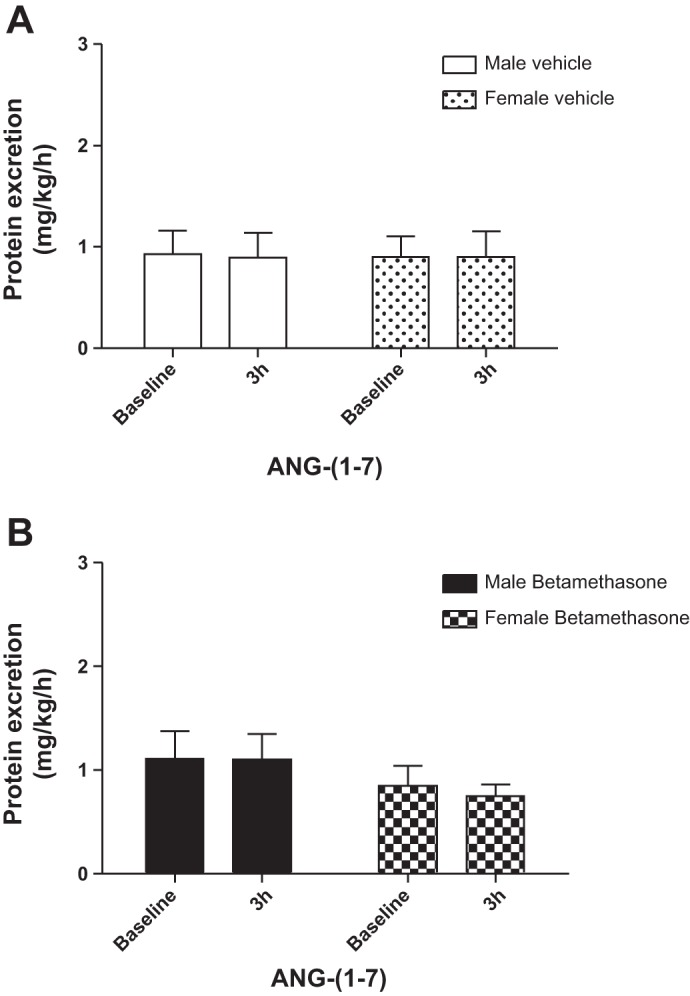
ANG-(1-7) does not alter protein excretion in male or female sheep. A: urinary protein excretion during intrarenal infusion of ANG-(1-7) in vehicle-treated sheep. B: urinary protein excretion during intrarenal infusion of ANG-(1-7) in betamethasone-treated sheep. MV (n = 7); MB (n = 7); FV (n = 6); FB (n = 6).
DISCUSSION
The major new findings of this study are 1) antenatal exposure to a clinically relevant dose of betamethasone enhances renal susceptibility to a “second hit” (nephron loss in adulthood) in a sex-specific fashion as demonstrated by increased excretion of an oxidative stress marker and proteinuria in adult male but not female sheep when infused with ANG II; 2) one site for the sex-specific increase in renal susceptibility is the renal PTC; 3) the antenatal betamethasone-induced increase in susceptibility is associated with a sex-specific increase in expression of a subunit of NADPH oxidase, p47 phox, in adult male sheep; 4) increased SOD activity in the renal cortex of females may provide protection from oxidative stress; and 5) ANG-(1-7) in vivo can mitigate the impact of a second hit (additional loss of nephrons) on a marker of renal oxidative stress in adult betamethasone-exposed sheep. These findings support the concept that antenatal exposure to a clinically relevant dose of betamethasone at the time of peak nephrogenesis has long-term effects on renal function (7, 18, 37, 65), and enhances susceptibility to a second hit in a sex-dependent fashion.
The oxidative marker, 8-isoprostane, belongs to the F2-isoprostane class, which is a stable product resulting from lipid oxidation (22, 38, 40). Increases in 8-isoprostane excretion imply an increased production of reactive oxygen species (ROS) in the kidney and are strongly associated with hypertension and renal damage (3, 56, 71). It is well accepted that ANG II, probably arising from the intrarenal RAS, increases oxidative stress in the kidney and is associated with renal damage in a variety of conditions (43, 56). ANG II activates NADPH oxidase which stimulates the formation of ROS that induce renal injury (27, 70). The NADPH oxidase complex consists of five subunits, the membrane-bound NOX2/p91phox and p22 phox heterodimer, the cytoplasmic complex of p40 phox, p47 phox, and p67 phox (4). Significant reduction of renal mass upregulates expression of p47 phox, p91 phox, and p22 phox in rats (66). Furthermore, there is evidence that p47 phox may be involved in the initial activation of NADPH oxidase (10, 33). We found enhanced expression of p47 phox overall in males compared with females, but only the betameathasone-exposed male sheep increased p47 phox expression after the second hit of uninephrectomy. This suggests the second hit induces higher oxidative activity in those males and is consistent with an observation in a low birth weight model in male rodents (46). Increased NADPH oxidase activity could facilitate the response to ANG II in a sex-specific fashion. In fact, the males subjected to the second hit showed a robust increase in 8-isoprostane excretion following ANG II infusion while the females failed to respond. In rodent models of fetal programming one consequence of higher oxidative activity is increased sodium reabsorption leading to sustained hypertension (48). We observed impaired sodium excretion in betamethasone-exposed adult males while glucocorticoid-treated females readily excrete a sodium load (64, 65). Also, sodium excretion is less in uninephrectomized males exposed prenatally to betamethasone than it is in vehicle-exposed males with a single kidney (7). The present findings would be consistent with the above reports. However, we find antenatal betamethasone increases blood pressure similarly in both sexes which suggests the mechanisms involved in the blood pressure elevation may differ in males and females (74). There was no difference in the expression of NOX2/gp91 phox between groups. This could be a species-related difference in response to nephron loss.
In an attempt to identify a cell type in the kidney that exhibits the increase in susceptibility to a second hit, we studied PTCs in vitro. We chose this cell type because we knew they exhibited alterations in function induced by antenatal betamethasone exposure (18). Renal PTCs were isolated from kidney cortex and 8-isoprostane release was measured before and after stimulation with ANG II. The data in the PTCs (Fig. 4A) agree well with our in vivo observations. Thus, ANG II enhanced 8-isoprostane release from PTCs of the uninephrectomized males exposed to betamethasone prenatally. In contrast, cells from the female animals were protected and did not respond when stimulated with ANG II. This indicates that proximal tubules are one site of the sex-specific increased susceptibility to oxidative stress caused by the interaction of antenatal betamethasone exposure and uninephrectomy. The fact that these cells exhibit this sex-specific effect 1.5 to 2.0 yr after antenatal steroid exposure suggests some epigenetic modifications induced by antenatal betamethasone may account for the different tubular responses in males and females (53).
Proteinuria is also indicative of renal dysfunction/damage (17, 42, 55). In our studies, there was a modest increase in proteinuria in the betamethasone-exposed, uninephrectomized males that was not present in the females under basal conditions, suggesting that even without the additional stimulus of an ANG II infusion there is some functional abnormality present in the kidneys of the males that is absent in the females. We reported that antenatal betamethasone exposure leads to an imbalance in the intrarenal RAS with the ratio of ANG II/AT1 receptor effects enhanced over ANG-(1-7)/Mas receptor actions (29, 60, 62). It is possible that the second hit of additional nephron loss exacerbates this imbalance (18) and this contributes to the proteinuria in the males. Our observation of the proteinuria in males but not females is in concert with the findings of Yagil et al. (72) in a uninephrectomized rat model that suggest that a portion of the mechanisms responsible for proteinuria are sex-specific and that multiple genes and pathways are involved. Similar to others (5, 67–69), we have not observed increased proteinuria in antenatal glucocorticoid-exposed males that have not had an additional insult. This is unlike some data in rodents showing certain programming stimuli can cause proteinuria in the absence of the effects of a second hit (57, 58). It may be this difference is related to the intensity of the stimuli in the different studies or to differences in the age at which the studies were conducted.
The mechanism by which ANG II increases proteinuria in the betamethasone-exposed males is not clear but could involve reduced uptake of proteins by PTCs. While controversies exist (14, 16, 54), it appears that there is uptake of protein from the glomerular filtrate by PTCs which is accomplished by the membrane receptors megalin and cubilin (15, 35, 55). The proteinuria caused by the intrarenal infusion of a low dose of ANG II may result from the downregulation of megalin expression in PTCs by ANG II (30). Why this would be observed only in males is intriguing and requires further investigation.
The major free radical in oxidative stress is superoxide anion (O2·−), which is generated mainly by NADPH oxidase and is rapidly dismutated to hydrogen peroxide (H2O2) by the antioxidant enzyme SOD (26). Therefore, the increased SOD activity found in the renal cortex of the females, but not in the males, suggests that compensatory mechanisms may be upregulated to oppose the programming effects of antenatal betamethasone (25). This could explain the lack of an ANG II-induced response in female betamethasone-treated sheep and why their PTCs did not release 8-isoprostane after ANG II treatment.
The effect of ANG-(1-7) on renal oxidative stress is somewhat controversial (28). However, Benter and colleagues (6) demonstrated that chronic ANG-(1-7) infusion decreased the levels of renal NADPH oxidase (NOX) activity and attenuated oxidative stress in the diabetic spontaneously hypertensive rat kidney. Similar findings have been reported by Mori et al. (39). The ability of ANG-(1-7) to reduce oxidative stress is also supported by in vitro studies (59) in which ANG-(1-7) antagonized the ability of ANG II to induce changes in signaling in endothelial cells. We found that intrarenal infusions of ANG-(1-7) reduced the excretion of 8-isoprostane in betamethasone-exposed male and female sheep, suggesting a reduction in the level of oxidative stress. This is of interest because it suggests that additional nephron loss in adulthood after prenatal betamethasone may induce some level of oxidative stress in the remaining kidney of both males and females in vivo, despite that the females appear protected from the increased susceptibility to ANG II. The fact that ANG-(1-7) did not change glomerular filtration rate (GFR) in males (7) but increased GFR in females (data not shown) may mean that some of the protective effects of ANG-(1-7) are related to different effects on renal hemodynamics in males and females. However, the mechanism for the reduction in 8-isoprostane by ANG-(1-7) within the kidney remains to be established.
ANG-(1-7) did not increase or decrease 8-isoprostane release by PTCs in vitro, suggesting that the level of oxidative stress in the unstimulated cells when in serum-free medium is very low. The effect of ANG-(1-7) to reduce the generation of oxidative stress could be by inhibition of NADPH oxidase (6, 49) or through an increase in the scavenging of ROS in PTCs.
We conclude that antenatal glucocorticoid exposure enhances the susceptibility of the kidney to oxidative stress induced by ANG II in a sex-specific fashion and the proximal tubule is one target of the sex-specific effects of antenatal steroids. ANG-(1-7) may mitigate the impact of prenatal glucocorticoids on the kidney. P47 phox activation may be responsible for the increased oxidative stress and proteinuria in males. The protection from renal oxidative stress in females appears related to increased SOD activity.
Perspectives
The significance of our observations is related to the fact that antenatal glucocorticoids are routinely used in clinical obstetrics when there is a risk that premature delivery may occur. This means that in the United States alone over 100,000 individuals are born each year after antenatal exposure to glucocorticoids (1, 8, 24, 36, 50). Some of the unexpected effects of such exposure are reductions in nephron number, alterations in the intrarenal RAS, hypertension, and impaired renal function in adulthood (5, 13, 44, 69). The data from this study suggest that antenatal glucocorticoid exposure increases renal susceptibility to a second insult in a sex-specific manner and thus the risk for the development of renal disease in males in adulthood. Considering that there is evidence that a final common pathway of many fetal programming stimuli is exposure of the fetus to inappropriate levels of glucocorticoids (11, 21, 51, 52, 63), the likelihood of increased susceptibility may be a significant factor contributing to sex-specific differences in loss of renal function in adults. Thus, early identification of individuals at risk could potentially lead to early intervention and thereby reduce the degree of renal impairment or decrease the development of hypertension in this group as they are exposed to additional insults with age (9, 34, 45).
GRANTS
This work was supported by National Institutes of Health Grants HD-47584 and HD-17644.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.B., M.C.C., and J.C.R. conception and design of research; J.B., S.A.C., K.C., and Y.S. performed experiments; J.B. and J.C.R. analyzed data; J.B., M.C.C., and J.C.R. interpreted results of experiments; J.B. prepared figures; J.B. drafted manuscript; J.B., S.A.C., K.C., J.P.F., M.C.C., and J.C.R. edited and revised manuscript; J.B. and J.C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
David Jones and Eric Lesane provided invaluable support in caring for sheep and assisting with the operation and anesthesia. We thank Katherine Cox for participating in the sample measurements.
REFERENCES
- 1.Authors not listed. NIH Consensus Development Conference 1995. Effects of corticosteroid for fetal maturation on perinatal outcomes. JAMA 273: 413–418, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Abitbol CL, Ingelfinger JR. Nephron mass and cardiovascular and renal disease risks. Semin Nephrol 29: 445–454, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal 20: 74–101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babior BM. NADPH oxidase. Curr Opin Immunol 16: 42–47, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol 298: F235–F247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 28: 25–33, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Bi J, Contag SA, Carey LC, Tang L, Valego NK, Chappell MC, Rose JC. Antenatal betamethasone exposure alters renal responses to angiotensin-(1-7) in uninephrectomized adult male sheep. J Renin Angiotensin Aldosterone Syst 14: 290–298, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonanno C, Wapner RJ. Antenatal corticosteroid treatment: what's happened since Drs Liggins and Howie? Am J Obstet Gynecol 200: 448–457, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Boubred F, Saint-Faust M, Buffat C, Ligi I, Grandvuillemin I, Simeoni U. Developmental origins of chronic renal disease: an integrative hypothesis. Int J Nephrol 2013: 346067, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Chapman K, Holmes M, Seckl J. 11+-Hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93: 1139–1206, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: more than regulation of blood pressure? Hypertension 50: 596–599, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the angiotensin converting enzyme 2-angiotensin (1-7)-Mas receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne) 4: 201, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen EI, Birn H, Rippe B, Maunsbach AB. Controversies in nephrology: renal albumin handling, facts, and artifacts. Kidney Int 72: 1192–1194, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Christensen EI, Birn H, Storm T, Weyer K, Nielsen R. Endocytic receptors in the renal proximal tubule. Physiology (Bethesda) 27: 223–236, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Comper WD, Haraldsson B, Deen WM. Resolved: normal glomeruli filter nephrotic levels of albumin. J Am Soc Nephrol 19: 427–432, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM. Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol 295: F1589–F1600, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Contag SA, Bi J, Chappell MC, Rose JC. Developmental effect of antenatal exposure to betamethasone on renal angiotensin II activity in the young adult sheep. Am J Physiol Renal Physiol 298: F847–F856, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dilauro M, Burns KD. Angiotensin-(1-7) and its effects in the kidney. Scientific World J 9: 522–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 94: 149–155, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 98: 137–142, 2000 [PubMed] [Google Scholar]
- 22.Fam SS, Morrow JD. The isoprostanes: unique products of arachidonic acid oxidation-a review. Curr Med Chem 10: 1723–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Fan L, Mukaddam-Daher S, Javeshghani D, Quillen E, Jr., Gutkowska J. Renal effects of prolonged intrarenal infusions of angiotensin II and atrial natriuretic peptide in sheep. J Cardiovasc Pharmacol 34: 427–433, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 196: 147–148, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Franco MC, Kawamoto EM, Gorjao R, Rastelli VM, Curi R, Scavone C, Sawaya AL, Fortes ZB, Sesso R. Biomarkers of oxidative stress and antioxidant status in children born small for gestational age: evidence of lipid peroxidation. Pediatr Res 62: 204–208, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Fridovich I. Superoxide anion radical (O2·−), superoxide dismutases, and related matters. J Biol Chem 272: 18515–18517, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol 302: 148–158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzales S, Noriega GO, Tomaro ML, Pena C. Angiotensin-(1-7) stimulates oxidative stress in rat kidney. Regul Pept 106: 67–70, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Gwathmey TM, Shaltout HA, Rose JC, Diz DI, Chappell MC. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension 57: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosojima M, Sato H, Yamamoto K, Kaseda R, Soma T, Kobayashi A, Suzuki A, Kabasawa H, Takeyama A, Ikuyama K, Iino N, Nishiyama A, Thekkumkara TJ, Takeda T, Suzuki Y, Gejyo F, Saito A. Regulation of megalin expression in cultured proximal tubule cells by angiotensin II type 1A receptor- and insulin-mediated signaling cross talk. Endocrinology 150: 871–878, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Ingelfinger JR. Disparities in renal endowment: causes and consequences. Adv Chronic Kidney Dis 15: 107–114, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Liu GC, Fang F, Zhou J, Koulajian K, Yang S, Lam L, Reich HN, John R, Herzenberg AM, Giacca A, Oudit GY, Scholey JW. Deletion of p47phox attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouse. Diabetologia 55: 2522–2532, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382: 273–283, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Mahadevappa R, Nielsen R, Christensen EI, Birn H. Megalin in acute kidney injury: foe and friend. Am J Physiol Renal Physiol 306: F147–F154, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Mathews TJ. Birth: final data for 2011. Natl Vital Stat Rep 62: 1–69, 72, 2013 [PubMed] [Google Scholar]
- 37.Massmann GA, Zhang J, Rose JC, Figueroa JP. Acute and long-term effects of clinical doses of antenatal glucocorticoids in the developing fetal sheep kidney. J Soc Gynecol Investig 13: 174–180, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers 10, Suppl 1: S10–S23, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Mori J, Patel VB, Ramprasath T, Alrob OA, DesAulniers J, Scholey JW, Lopaschuk GD, Oudit GY. Angiotensin 1–7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol 306: F812–F821, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Morrow JD. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev 32: 377–385, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Nenov VD, Taal MW, Sakharova OV, Brenner BM. Multi-hit nature of chronic renal disease. Curr Opin Nephrol Hypertens 9: 85–97, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Nielsen R, Christensen EI. Proteinuria and events beyond the slit. Pediatr Nephrol 25: 813–822, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Nistala R, Wei Y, Sowers JR, Whaley-Connell A. Renin-angiotensin-aldosterone system-mediated redox effects in chronic kidney disease. Transl Res 153: 102–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuyt AM. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clin Sci (Lond) 114: 1–17, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Ojeda NB, Intapad S, Alexander BT. Sex differences in the developmental programming of hypertension. Acta Physiol (Oxf) 2013. 10.1111/apha.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojeda NB. Low birth weight increases susceptibility to renal injury in a rat model of mild ischemia-reperfusion. Am J Physiol Renal Physiol 301: F420–F426, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int 59: 1663–1669, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paixao AD, Alexander BT. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol Reprod 89: 144, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Passos-Silva DG, Verano-Braga T, Santos RA. Angiotensin-(1-7): beyond the cardio-renal actions. Clin Sci (Lond) 124: 443–456, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Polyakov A, Cohen S, Baum M, Trickey D, Jolley D, Wallace EM. Patterns of antenatal corticosteroid prescribing 1998–2004. Aust N Z J Obstet Gynaecol 47: 42–45, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Reynolds RM. Corticosteroid-mediated programming and the pathogenesis of obesity and diabetes. J Steroid Biochem Mol Biol 122: 3–9, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Reynolds RM. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis–2012 Curt Richter Award Winner. Psychoneuroendocrinology 38: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Reynolds RM, Jacobsen GH, Drake AJ. What is the evidence in humans that DNA methylation changes link events in utero and later life disease? Clin Endocrinol (Oxf) 78: 814–822, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Russo LM, Sandoval RM, Brown D, Molitoris BA, Comper WD. Controversies in nephrology: response to “renal albumin handling, facts, and artifacts”. Kidney Int 72: 1195–1197, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18: 2439–2446, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Saez F, Castells MT, Zuasti A, Salazar F, Reverte V, Loria A, Salazar FJ. Sex differences in the renal changes elicited by angiotensin II blockade during the nephrogenic period. Hypertension 49: 1429–1435, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Saez F, Reverte V, Salazar F, Castells MT, Llinas MT, Salazar FJ. Hypertension and sex differences in the age-related renal changes when cyclooxygenase-2 activity is reduced during nephrogenesis. Hypertension 53: 331–337, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Sampaio WO, Henrique de CC, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50: 1093–1098, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Shaltout HA, Rose JC, Chappell MC, Diz DI. Angiotensin-(1-7) deficiency and baroreflex impairment precede the antenatal betamethasone exposure-induced elevation in blood pressure. Hypertension 59: 453–458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol 292: F82–F91, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Shaltout HA, Rose JC, Figueroa JP, Chappell MC, Diz DI, Averill DB. Acute AT1-receptor blockade reverses the hemodynamic and baroreflex impairment in adult sheep exposed to antenatal betamethasone. Am J Physiol Heart Circ Physiol 299: H541–H547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh RR, Cuffe JS, Moritz KM. Short- and long-term effects of exposure to natural and synthetic glucocorticoids during development. Clin Exp Pharmacol Physiol 39: 979–989, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Tang L, Bi J, Valego N, Carey L, Figueroa J, Chappell M, Rose JC. Prenatal betamethasone exposure alters renal function in immature sheep: sex differences in effects. Am J Physiol Regul Integr Comp Physiol 299: R793–R803, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 296: R309–R317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaziri ND, Bai Y, Ni Z, Quiroz Y, Pandian R, Rodriguez-Iturbe B. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J Pharmacol Exp Ther 323: 85–93, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Vehaskari VM. Developmental origins of adult hypertension: new insights into the role of the kidney. Pediatr Nephrol 22: 490–495, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Vehaskari VM. Prenatal programming of kidney disease. Curr Opin Pediatr 22: 176–182, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Vehaskari VM, Woods LL. Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol 16: 2545–2556, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Welch WJ. Angiotensin II-dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension 52: 51–56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Yagil Y, Hessner M, Schulz H, Gosele C, Lebedev L, Barkalifa R, Sapojnikov M, Hubner N, Yagil C. Geno-transcriptomic dissection of proteinuria in the uninephrectomized rat uncovers a molecular complexity with sexual dimorphism. Physiological Genomics 42A: 301–316, 2010 [DOI] [PubMed] [Google Scholar]
- 73.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension 47: 502–508, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Massman GA, Rose JC, Figueroa JP. Differential effects of a clinical dose of betamethasone on nephron endowment and glomerular filtration in adult sheep. Repro Sci 17: 186–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]