Abstract
During maturation, the α- and γ-subunits of the epithelial Na+ channel (ENaC) undergo proteolytic processing by furin. Cleavage of the γ-subunit by furin at the consensus site γRKRR143 and subsequent cleavage by a second protease at a distal site strongly activate the channel. For example, coexpression of prostasin with ENaC increases both channel function and cleavage at the γRKRK186 site. We generated a polyclonal antibody that recognizes the region 144–186 in the γ-subunit (anti-γ43) to determine whether prostasin promotes the release of the intervening tract between the putative furin and γRKRK186 cleavage sites. Anti-γ43 precipitated both full-length (93 kDa) and furin-processed (83 kDa) γ-subunits from extracts obtained from oocytes expressing αβHA-γ-V5 channels, but only the full-length (93 kDa) γ-subunit from oocytes expressing αβHA-γ-V5 channels and either wild-type or a catalytically inactive prostasin. Although both wild-type and catalytically inactive prostasin activated ENaCs in an aprotinin-sensitive manner, only wild-type prostasin bound to aprotinin beads, suggesting that catalytically inactive prostasin facilitates the cleavage of the γ-subunit by an endogenous protease in Xenopus oocytes. As dietary salt restriction increases cleavage of the renal γ-subunit, we assessed release of the 43-mer inhibitory tract on rats fed a low-Na+ diet. We found that a low-Na+ diet increased γ-subunit cleavage detected with the anti-γ antibody and dramatically reduced the fraction precipitated with the anti-γ43 antibody. Our results suggest that the inhibitory tract dissociates from the γ-subunit in kidneys from rats on a low-Na+ diet.
Keywords: epithelial sodium channel, amiloride, prostasin, channel-activating protease 1, proteases
the aldosterone-sensitive distal nephron has a major role in maintaining Na+ and K+ balance and therefore extracellular fluid volume and arterial blood pressure. This segment of the nephron is composed of principal cells, which participate actively in Na+ reabsorption and K+ secretion, and intercalated cells, which contribute to acid-base homeostasis as well as Na+ reabsorption and K+ secretion (14, 21, 26, 28, 51). Epithelial Na+ channels (ENaCs) expressed at the apical membrane of principal cells facilitate the tubular reabsorption of Na+, which generates a favorable electrochemical driving force for K+ secretion. The activity of these channels is modulated by second messengers, specific lipids, laminar fluid flow, extracellular Na+, and proteases (24, 41).
ENaCs are composed of three homologous subunits, termed α, β, and γ (7). Each subunit has two transmembrane domains, a large extracellular region, and intracellular NH2- and COOH-termini (6, 42). The α- and γ-ENaC subunits contain embedded inhibitory tracts in their extracellular loops. Specific proteases cleave these subunits at sites flanking the inhibitory tracts in the biosynthetic pathway during channel maturation and at the plasma membrane (4, 10, 23, 25). Furin, a pro-protein convertase expressed in the trans-Golgi network, cleaves the α-subunit twice, releasing a 26-mer inhibitory tract, and cleaves the γ-subunit at a single site preceding an inhibitory tract (4, 22). Channels lacking proteolytic cleavage by furin have a very low open probability (Po) in the presence of external Na+, whereas channels that have been processed by furin and thereby lack the α-subunit inhibitory tract have an intermediate Po of ∼0.3–0.4 (5, 10, 40). Channels with a γ-subunit that is cleaved only by furin can be further activated by proteases that cleave the γ-subunit at sites distal to the inhibitory tract (4, 25). These “channel-activating” proteases (CAPs) include prostasin (also referred to as CAP1), transmembrane protease serine 4 (TMPRSS4 or CAP2), matriptase (CAP3), elastase, kallikrein, and plasmin (2–4, 32–34, 36, 38, 45, 48). Cleavage of the γ-subunit at the furin cleavage site and at a distal site by a second protease results in full activation of the channel (Po of ∼1) (4, 9).
Prostasin is a glycosylphosphatidylinositol (GPI)-anchored aprotinin-sensitive membrane-associated serine protease that was identified as an ENaC activator by functional expression cloning (48). Coexpression of ENaC and prostasin increases Po of the channel and promotes proteolytic cleavage of the γ-subunit at the RKRK186 site, which is distal to the furin site and embedded inhibitory tract (4). Serine proteases contain a typical His-Asp-Ser catalytic triad that is critical for proteolytic activity (11). Surprisingly, prostasin mutants with substitutions in the catalytic triad activate ENaCs, suggesting that channel activation by prostasin does not require its catalytic activity (3, 4). We (4) have previously reported that the introduction of Gln substitutions in the γ-subunit at the putative prostasin cleavage site (RKRK186 to QQQQ186) prevented ENaC activation by both wild-type and mutant S238A prostasin, consistent with prostasin-dependent cleavage of the channel. However, it was unclear how a prostasin mutant that is presumably catalytically inactive could facilitate channel cleavage and activation.
In the present report, we examined the mechanism by which the catalytically inactive (S238A) mutant prostasin activates ENaC. Our results indicate that the catalytically inactive prostasin mutant promotes γ-subunit cleavage by an endogenous aprotinin-sensitive protease in Xenopus oocytes, facilitating the release of the inhibitory tract between the RKRR143 and RKRK186 sites. To examine the proteolytic release of the inhibitory tract in vivo, we used a polyclonal antibody that recognizes region 144–186 in the γ-subunit. We found that dietary salt restriction promoted cleavage of the γ-subunit and the release of this inhibitory tract. Taken together, these results indicate that dietary salt restriction increases Na+ reabsorption in the distal nephron, at least in part, by promoting the release of an inhibitory tract from the γ-subunit.
EXPERIMENTAL PROCEDURES
Vectors and cell culture.
Wild-type and mutant murine ENaC subunits and prostasin cDNAs were as previously described (4). γ-ENaC was tagged with NH2-terminal hemagglutinin (HA) and COOH-terminal V5 epitopes (HA-γ-V5). Human embryonic kidney (HEK)-293H cells were purchased from Invitrogen (Carlsbad, CA). Cells were grown in DMEM containing 10% FBS, 0.1 mM MEM nonessential amino acids, 50 U/ml penicillin, and 50 μg/ml streptomycin. HEK-293H cells were transiently transfected with cDNAs encoding ENaC and prostasin using 293fectin, as described by the manufacturer (Invitrogen). The total amount of DNA transfected was held constant by cotransfection of humanized recombinant green fluorescent protein (GFP; pIRES-hrGFP II, Stratagene, La Jolla, CA).
Antibodies.
A polyclonal rabbit antibody against residues 131–187 of mouse γ-ENaC (anti-γ43) was raised by Quality Controlled Biochemicals (Hopkinton, MA). A polyclonal rabbit antibody against γ-ENaC was purchased from Stressmarq Bioscience (Victoria, BC, Canada), monoclonal anti-prostasin antibody was purchased from BD Transduction (Minneapolis, MN), monoclonal anti-V5 antibody was purchased from Invitrogen, and agarose-immobilized goat anti-V5 antibody was purchased from Bethyl Laboratories (Montgomery, TX).
Electrophysiology.
Stage 5–6 oocytes were isolated from adult female Xenopus laevis (NASCO, Plant City, FL) using a protocol approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Oocytes were injected with 1 ng cRNA encoding ENaC subunits and 3 ng cRNA encoding wild-type or mutant S238A prostasin. Oocytes were maintained at 18°C in modified Barth's solution (MBS) containing (in mM) 88 NaCl, 1 KCl, 2.4 NaHCO3, 15 HEPES, 0.3 Ca(NO3)2, 0.41 CaCl2, and 0.82 MgSO4 with 10 U/ml penicillin, 10 μg/ml streptomycin, and 100 μg/ml gentamycin sulfate (pH 7.4). Electrophysiological measurements were performed 20–26 h postinjection, as previously described (4, 8–10). The recording solution contained (in mM) 110 NaCl, 2 KCl, 1.54 CaCl2, and 10 HEPES (pH 7.4). Two-electrode voltage clamp was performed with a TEV-200A amplifier (Dagan, Minneapolis, MN). Data were captured with a Digidata 1440A acquisition system and analyzed with pCLAMP 10 (Molecular Devices, Sunnyvale, CA). Oocytes were mounted in an oocyte perfusion chamber and impaled with glass electrodes filled with 3 M KCl. The resistance of the electrodes measured in the recording solution was between 0.2 and 2 MΩ. ENaC-mediated currents were defined as the difference in measured currents in the absence and presence of amiloride (10 μM).
Oocyte biotinylation.
This procedure was performed as previously described (46). To minimize protein endocytosis, experiments were performed at 4°C. Briefly, oocytes injected with αβHA-γ-V5 subunit cRNAs, with or without wild-type or mutant S238A prostasin cRNA, were placed in individual wells of a cell culture cluster 20–26 h postinjection and incubated in MBS without antibiotics on ice for 30 min. Noninjected oocytes served as controls. Groups of 48–50 oocytes were washed twice with biotinylation buffer (10 mM triethanolamine, 150 mM NaCl, and 1 mM CaCl2, pH 8.0) and incubated for 15 min with biotinylation buffer supplemented with 1 mg/ml EZ-link-sulfo-NHS-SS-biotin (Thermo Scientific, Waltham, MA). Oocytes were incubated with quench buffer (MBS + 192 mM glycine) for 5 min with gentle agitation, washed twice with MBS without antibiotics, and then homogenized with an insulin syringe in a buffer containing 100 mM NaCl, 50 mM Tris, and 1% protease inhibitor cocktail III (EMD Millipore, Billerica, MA, pH 7.4). The homogenate was centrifuged twice at 200 g for 10 min, and the supernatant was incubated with solubilization buffer containing 100 mM NaCl, 50 mM Tris, 4% Triton X-100, and 1% protease inhibitor cocktail III (pH 7.4). Insoluble material was sedimented by centrifugation at 14,000 g for 15 min, and the supernatant was used for immunoprecipitation with anti-γ43 or anti-V5 antibodies.
Immunoprecipitation of γ-subunits from oocytes.
Supernatants (300 μl) prepared from oocytes expressing ENaCs were incubated overnight at 4°C with 50 μl protein G-agarose and either the anti-γ43 (12 μl) or anti-V5 (1 μl) antibody. The beads were collected by centrifugation and washed three times in solubilization buffer, and proteins were eluted and incubated overnight with 50 μl of streptavidin-conjugated beads at 4°C. The precipitated proteins were recovered by heating the beads with SDS sample buffer (Bio-Rad, Hercules, CA) containing β-mercaptoethanol (280 mM) for 5 min at 90–100°C.
Immunoprecipitation of γ-subunits from kidney extracts.
Sprague-Dawley rats (180–200 g) of either sex (Charles River Laboratories, Kingston, NY) were maintained for 6–8 days on either a Na+-deficient rat diet (MP Biomedicals, Solon, OH) or a matched diet containing 1% NaCl (control). Rat kidney membranes were prepared as previously described (18) with a protocol approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Membrane extracts (10 μl) were resuspended in (400 μl) buffer containing 62.5 mM EDTA, 50 mM Tris, 0.4% deoxycholate, and 1% Nonidet P-40 (pH 8.4) and incubated overnight at 4°C with 50 μl protein G conjugated to agarose and either the anti-γ43 (4 μl) or anti-γ (5 μl) antibody. The agarose beads were collected by centrifugation and washed three times in solubilization buffer, and proteins were eluted and analyzed by immunoblot analysis.
Coimmunoprecipitation.
HEK-293H cells grown on six-well plastic dishes were transiently transfected with αβHA-γ-V5 ENaC and wild-type or mutant (S238A) prostasin cDNAs. Cells transfected with a vector coding for GFP served as controls. After 3 days in culture, cells were washed twice with Dulbecco's PBS without Ca2+ and Mg2+ (Cellgro, Manassas, VA) and then incubated at room temperature on a rotating shaker with 0.5 ml detergent solution containing 100 mM NaCl, 40 mM KCl, 1 mM EDTA, 20 HEPES, 10% glycerol, 1% Triton X-100, and 1% protease inhibitor cocktail III (pH 7.4) for 20 min to solubilize proteins. Cell extracts were transferred to 1.5-ml tubes and centrifuged at 20,000 g for 7 min at 4°C. Supernatants were moved to a clean 1.5-ml tube, and an aliquot was retained to assess total ENaC and prostasin expression (20 μl). The rest of the sample was incubated end over end overnight at 4°C with either 0.625 μg mouse anti-prostasin antibody (BD Transduction Laboratories) and 50 μl rec-protein G conjugated to sepharose (Invitrogen) or 50 μl agarose-immobilized goat anti-V5 antibody (Bethyl Laboratories). Immunoprecipitated proteins were recovered by centrifugation. The supernatant was removed, and the pellet was washed with 0.5 ml detergent solution and then with 0.5 ml buffer containing 150 mM NaCl and 10 mM HEPES (pH 7.4). At each step, all liquid was removed with a Hamilton syringe. Immunoprecipitated proteins were recovered by heating the beads with 30 μl SDS sample buffer (Bio-Rad) containing β-mercaptoethanol (140 mM) for 2 min at 90–100°C. Aliquots (20 μl) of cell extracts were heated after the addition of 10 μl of the same SDS sample buffer with β-mercaptoethanol.
Immunoblot analyses.
Samples were subjected to SDS-PAGE (Criterion precast gels, Bio-Rad) and then electrophoretically transferred to 0.45-μm pore size nitrocellulose membranes (Millipore). Nitrocellulose blots were blocked with 10% milk in PBS (100 mM NaCl, 80 mM Na2HPO4, and 20 mM NaH2PO4) and then incubated overnight with anti-prostasin (1:1,000, BD Transduction), anti-γ ENaC (1:500, Stressmarq Bioscience), or anti-V5 (1:1,000, Invitrogen) antibodies in 1% milk. Antibody binding was detected with horseradish peroxidase-conjugated secondary antibodies (KPL, Gaithersburg, MD) using Western Lightning Plus-ECL (Perkin-Elmer, Waltham, MA) and film (Kodak, Rochester, NY). The density of the bands was quantified with ImageJ (39).
Prostasin-aprotinin interactions.
HEK-293H cells plated on 10-cm-diameter dishes were transfected with GFP, wild-type prostasin, or mutant S238A prostasin cDNAs. Twenty hours after transfection, the media were replaced with fresh Opti-MEM reduced serum media (Invitrogen). After 48 h, media were collected and centrifuged for 10 min at 200 g. The supernatant was concentrated to ∼200 μl with an Amicon Ultra centrifugal filter device (10,000 molecular weight cutoff, Millipore). Aliquots of supernatants were incubated with 100 μl of aprotinin-conjugated agarose beads (Sigma) for 90 min. The beads were collected by centrifugation, washed three times with PBS supplemented with 1% Triton X-100, and stored at −20°C for immunoblot analyses.
Protease activity.
HEK-293H cells plated on 10-cm-diameter dishes were transfected as described above with cDNAs for GFP, wild-type prostasin, or mutant S238A prostasin. Twenty hours after transfection, media were replaced with fresh Opti-MEM reduced serum media. Media were collected after 48 h and centrifuged for 10 min at 200 g. The supernatant was concentrated to ∼200 μl with an Amicon Ultra centrifugal filter device (10,000 molecular weight cutoff, Millipore). The volume of the samples was adjusted to ∼240 μl with Opti-MEM reduced serum medium. To measure proteolytic activity, 10 μl of concentrated media were incubated with 90 μl of reaction buffer for 80 min at 37°C. The final reaction mixture consisted of 0.2 mM H-d-prolyl-l-phenylalanyl-l-arginine-p-nitroaniline (Chromogenix S-2302, Diapharma, OH), 10 mM Tris, and 0.25% CHAPS (pH 9). The reaction was stopped with 10 μl of 50% acetic acid. Absorbance was measured at 405 nm.
Statistical analysis.
Data are presented as means ± SE. Statistical comparisons between groups were performed with unpaired Student's t-tests unless otherwise indicated. P values of <0.05 were considered statistically different. Statistical analysis was performed with GraphPad 5.0 (GraphPad Software, San Diego, CA).
RESULTS
Prostasin has been recognized as one of the proteases that regulate ENaC activity in epithelia (16, 48). Studies (3, 4) in heterologous expression systems have shown that wild-type prostasin as well as prostasin mutants bearing substitutions in the catalytic triad are capable of activating ENaCs, suggesting that prostasin proteolytic activity is not necessarily required for channel activation. We found that both wild-type and mutant (S238A) prostasin promote γ subunit cleavage at the RKRK186 tract (4), suggesting that wild-type and mutant proteases activate the channel by facilitating the release of the γ-subunit inhibitory tract. To gain further insights into the mechanism of ENaC activation by prostasin, we examined whether wild-type and mutant S238A prostasin promote the release of the inhibitory tract, spanning residues 144–186, from the γ-subunit. For this purpose, we generated a rabbit polyclonal antibody against residues 131–187 of the murine γ-subunit (anti-γ43). We hypothesized that the anti-γ43 antibody would precipitate full-length and furin-processed γ-subunits but would not precipitate subunits cleaved at both the RKRR143 (furin) and RKRK186 (putative prostasin) sites.
αβHA-γ-V5 channels were expressed in Xenopus oocytes alone or with wild-type or mutant (S238A) prostasin. Protein extracts prepared from these oocytes were incubated with either anti-γ43 antibody or anti-V5 antibody and with protein G-conjugated agarose. Precipitated proteins were separated electrophoretically, transferred to nitrocellulose membranes, and immunoblotted with anti-V5 antibody. We found that anti-γ43 antibody immunoprecipitated both full-length (93 kDa) and furin-processed (83 kDa) γ-subunits from extracts obtained from oocytes expressing αβHA-γ-V5 channels (Fig. 1, left). The furin-processed (83 kDa) band was not observed in immunoprecipitates obtained from oocytes expressing both αβHA-γ-V5 channels and either wild-type or mutant prostasin, consistent with the release of the intervening tract between the RKRR143 and RKRK186 sites. In agreement with our previous studies (4, 22), we found that anti-V5 antibody immunoprecipitated both full-length and furin-processed γ-subunits from oocytes expressing wild-type (αβHA-γ-V5) ENaCs (Fig. 1, right). Coexpression of wild-type or mutant prostasin resulted in a shift of the 83-kDa band to a slightly lower molecular mass form (77 kDa) that was detected with the anti-V5 antibody, consistent with proteolytic cleavage at a site distal to the furin cleavage site. Taken together, our results indicate that wild-type and mutant S238A prostasin promote γ-subunit cleavage and release of the fragment between residues 143 and 186.
Fig. 1.
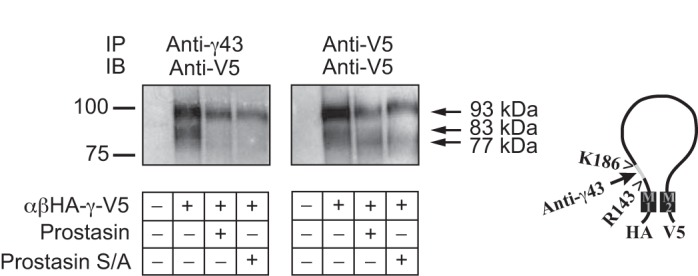
Prostasin expression promotes the release of an inhibitory tract from the γ-subunit. cRNAs encoding epithelial Na+ channel (ENaC) subunits [αβHA-γ-V5] and wild-type or mutant S238A (S/A) prostasin were injected in Xenopus oocytes. A day after injection, surface proteins were biotinylated, oocytes were homogenized, and solubilized proteins were immunoprecipitated with anti-γ43 antibody (left) or anti-V5 antibody (right), as described in experimental procedures. Biotinylated proteins were recovered with streptavidin-conjugated beads, resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-V5 antibody. A representative blot is shown (n = 2). Furin (RKRR143)- and prostasin (RKRK186)-dependent cleavage sites as well as the region flanking these sites that is recognized by anti-γ43 antibody are shown on the right. The mobility of Bio-Rad Precision Plus protein standards is noted on the left, and the relative molecular masses of full-length (93 kDa), furin-cleaved (83 kDa), and twice cleaved (77 kDa) γ-subunits are noted on the right. IP, immunoprecipation; IB, immunoblot analysis.
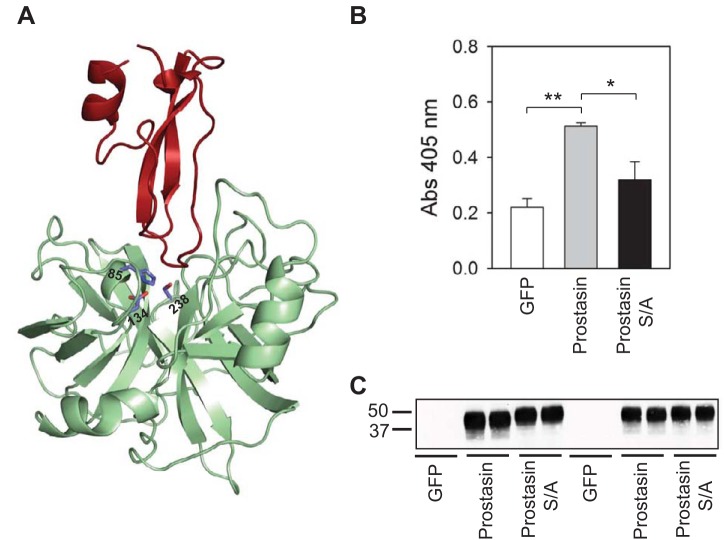
Serine proteases contain three critical residues at their catalytic site: Ser, His, and Asp. Mutations introduced at these sites result in reduced substrate turnover number without major changes in the Michaelis constant (11). The residues that form the catalytic triad in prostasin are His85, Asp134, and Ser238 (Fig. 2A). Although the effects of mutant S238A prostasin on ENaC activity have been previously examined in heterologous expression systems, the catalytic activity of this mutant has not been assessed. To determine whether mutant S238A prostasin is catalytically inactive, we measured amidolytic activity on media collected from cells transfected with cDNAs for GFP, wild-type prostasin, or mutant S238A prostasin (Fig. 2B). We found that amidolytic activity was significantly greater in media collected from HEK-293H cells transfected with prostasin than with either GFP or mutant S238A prostasin (n = 4, P < 0.01 for GFP vs. prostasin and P < 0.05 for prostasin vs. mutant S238A prostasin by ANOVA followed by a Tukey's multiple-comparison test). No statistically significant difference in amidolytic activity was observed between cells transfected with GFP and mutant S238A prostasin. As shown in Fig. 2C, the amount of secreted prostasin was similar in media collected from cells transfected with wild-type or mutant S238A prostasin (n = 4). These results indicate that mutant S238A prostasin is catalytically inactive and is presumably incapable of cleaving the ENaC γ-subunit.
Fig. 2.
Mutant S/A prostasin is catalytically inactive. A: cartoon representation of a prostasin-aprotinin complex (Protein Database code 3GYM). His85, Asp134, and Ser238 in the catalytic triad of prostasin (green) are shown. Aprotinin is colored in red. B: amidolytic activity was assessed in culture media from human embryonic kidney (HEK)-293H cells transfected with green fluorescent protein (GFP), prostasin, or mutant S/A prostasin as described in experimental procedures. Amidolytic activity was significantly greater in media collected from HEK-293H cells transfected with prostasin than media collected from cells expressing GFP or mutant S/A prostasin. Statistically significant differences are indicated as *P < 0.05 and **P < 0.01 (n = 4) by ANOVA followed by Tukey's multiple-comparison test. Amidolytic activity in media collected from HEK-293H cells transfected with GFP was not significantly different than in media collected from cells expressing mutant S/A prostasin. C: prostasin secretion in media collected from HEK-293H cells transfected with GFP, wild-type prostasin, or mutant S/A prostasin. The presence of secreted wild-type and mutant prostasin in the media was assessed by IB. Numbers on the left of the image represent the mobility of Bio-Rad Precision Plus protein standards (in kDa). Data are representative of four independent experiments.
Aprotinin is a competitive serine protease inhibitor derived from the bovine lung that reduces amiloride-sensitive Na+ transport in epithelia (30, 37, 50). In heterologous expression systems, aprotinin prevents ENaC activation by prostasin (1, 13, 48). The structure of a prostasin-aprotinin complex has been recently solved (44). As shown in Fig. 2A, residues in the catalytic triad of prostasin are located in close proximity to the aprotinin-binding site. We examined whether aprotinin interacts with mutant S238A prostasin. Extracts from HEK-293H cells transiently transfected with GFP, wild-type prostasin, or mutant S238A prostasin were incubated with aprotinin-conjugated agarose beads. The beads were then washed, and prostasin binding was assessed by immunoblot analysis. Whereas wild-type prostasin precipitated with aprotinin-agarose beads, mutant S238A prostasin did not (n = 4; Fig. 3A). We next examined whether the activation of ENaC by mutant S238A prostasin was sensitive to aprotinin (Fig. 3B). Oocytes injected with cRNA encoding ENaC alone or ENaC and either wild-type or mutant (S238A) prostasin were incubated in the presence or absence of aprotinin. Amiloride-sensitive currents were determined 20–26 h after injection. Consistent with previous studies (3, 4), we found that amiloride-sensitive currents were significantly greater in oocytes coexpressing ENaC and either wild-type or mutant prostasin than in oocytes expressing ENaC alone (n = 16, P < 0.01 with or without prostasin and P < 0.001 with or without mutant prostasin by Kruskal-Wallis test followed by Dunn's multiple-comparisons test). In contrast, similar levels of amiloride-sensitive currents were seen in oocytes expressing ENaC alone or in oocytes coexpressing ENaC and wild-type or mutant prostasin when oocytes were incubated with aprotinin (n = 15–16, P = not significant by Kruskal-Wallis test followed by Dunn's multiple-comparisons test). Overall, our experiments revealed that aprotinin prevents the activation of ENaC by wild-type and mutant prostasin, even though mutant prostasin does not bind to aprotinin. Taken together, these results suggest that mutant prostasin promotes the cleavage and activation of ENaC by an endogenous aprotinin-sensitive protease in Xenopus oocytes.
Fig. 3.
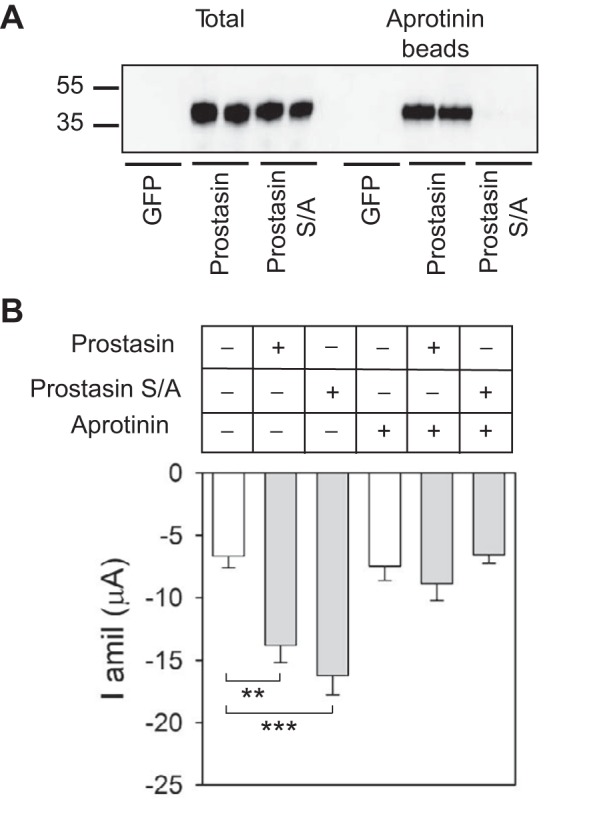
Catalytically inactive prostasin facilitates cleavage of the γ-subunit by an endogenous protease in Xenopus oocytes. A: prostasin-aprotinin interactions. Culture media from HEK-293H cells transfected with GFP, prostasin, or mutant S/A prostasin were collected and concentrated as described in experimental procedures. Proteins eluted from aprotinin-conjugated beads were separated by SDS-PAGE and analyzed by IB with anti-prostasin antibody (right). Prostasin expression in the whole cell lysate was also assessed (left). Numbers on the left of the image represent the mobility of protein standards (in kDa). Blots are representative of four independent experiments. B: aprotinin prevents the activation of ENaC by wild-type and mutant prostasin. Two-electrode voltage clamp was performed in oocytes injected with αβγ-ENaC with or without wild-type prostasin or mutant S/A prostasin cRNAs. After cRNA injection, oocytes were incubated with or without aprotinin (100 μg/ml). Amiloride-sensitive Na+ currents (I amil) were measured with the two-electrode voltage-clamp technique as described in experimental procedures. Statistically significant differences in whole cell currents with coexpression of wild-type or mutant prostasin versus expression of ENaC alone are indicated as **P < 0.01 and ***P < 0.001 (n = 15–16 from 2 batches of oocytes) by Kruskal-Wallis test followed by Dunn's multiple-comparisons test.
Our experiments indicated that the prostasin mutant does not directly cleave ENaC, but it promotes the release of the inhibitory fragment from the γ-subunit by another protease expressed in oocytes. We hypothesized that a physical interaction between the prostasin mutant and ENaC promotes proteolytic processing of the channel by the endogenous protease. To examine whether ENaC binds to wild-type or mutant prostasin, we conducted coimmunoprecipitation experiments with extracts obtained from HEK-293H cells expressing GFP, HA-γ-V5, αβHA-γ-V5, αβHA-γ-V5 and prostasin, and αβHA-γ-V5 and mutant S238A prostasin (Fig. 4). Anti-V5 or anti-prostasin immunoprecipitates were separated electrophoretically, transferred to nitrocellulose membranes, and immunoblotted with antibodies against prostasin or the V5 epitope. As shown in Fig. 4, both wild-type and mutant (S238A) prostasin were recovered with anti-V5 antibody (n = 4). Conversely, ENaC γ-subunits were recovered with anti-prostasin antibody. These results suggest that ENaC and prostasin, either wild-type or mutant, are present in a protein complex.
Fig. 4.
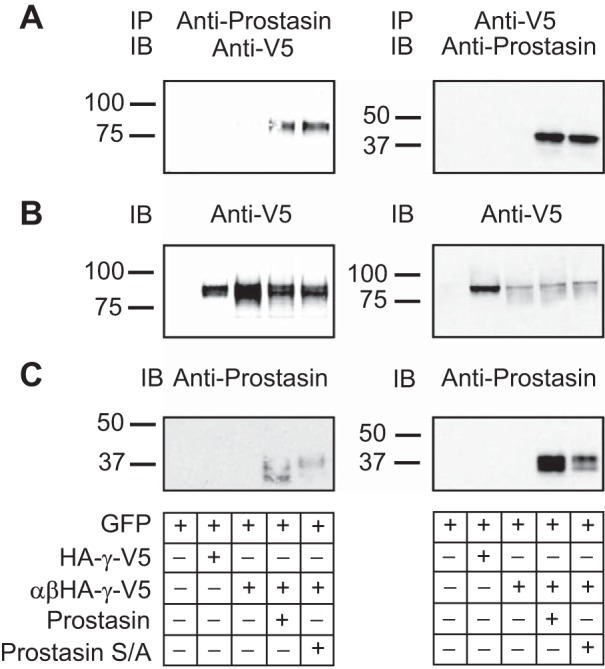
Both wild-type and catalytically inactive prostasin coimmunoprecipitate with ENaC. Coimmunoprecipitation experiments were performed with extracts from HEK-293H cells expressing GFP, HA-γ-V5, αβHA-γ-V5, αβHA-γ-V5 and wild type prostasin, or αβHA-γ-V5 and mutant S/A prostasin as described in experimental procedures. Immunoprecipitates were subjected to SDS-PAGE and IB with the indicated antibodies. A: IP assays for the γ-subunit and prostasin. B and C: representative blots using anti-V5 and prostasin antibodies, respectively. Numbers on the left of the images represent the mobility of Bio-Rad Precision Plus protein standards (in kDa). Data are representative of four independent experiments.
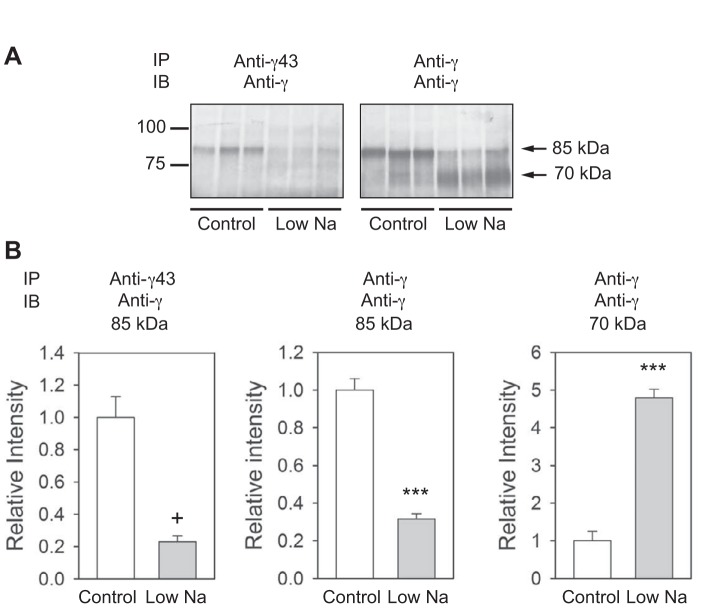
The mineralocorticoid aldosterone regulates Na+ reabsorption and K+ secretion in the distal nephron (for reviews, see Refs. 35 and 43). Reduced Na+ intake in experimental animals prompts aldosterone release, which increases ENaC subunit abundance and promotes proteolytic processing of the α- and γ-subunits (15, 31). Our previous studies (4, 10) have suggested that proteases augment ENaC activity by releasing inhibitory fragments from the α- and γ-subunits and that proteolytic cleavage per se is not sufficient to activate ENaCs. Channel activation requires cleavage at multiple sites with release of imbedded inhibitory tracts. To determine whether reduced Na+ intake results in the release of the tract 143–186 from the γ-subunit, we used anti-γ43 or anti-γ subunit antibodies to immunoprecipitate ENaCs from membranes prepared from the kidneys of rats fed a normal or low-Na+ diet. Membrane extracts were incubated with either anti-γ43 or anti-γ subunit COOH-terminal antibodies, and immunoprecipitated ENaC was recovered and subject to SDS-PAGE and immunoblot analysis with anti-γ COOH-terminal antibody (Fig. 5). With both antibodies, we observed an ∼85-kDa polypeptide, presumably representing the full-length rat γ-subunit, under control conditions, as previously described (15). When animals were maintained on a low-Na+ diet, we observed less of the full-length γ-subunit with both antibodies. While a 70-kDa polypeptide was recovered with anti-γ antibody from animals on a low-Na+ diet (15), this polypeptide was largely absent when the anti-γ43 antibody was used for immunoprecipitation. These results suggest that dietary salt restriction promotes γ-subunit cleavage as well as dissociation of the γ-subunit inhibitory tract from the COOH-terminal γ-subunit fragment.
Fig. 5.
Dietary salt restriction promotes the release of the inhibitory tract from the γ-subunit COOH-terminal fragment. A: extracts of membranes prepared from the kidneys of rats fed a normal or low-Na+ diet were incubated with either the anti-γ43 or anti-γ antibody, as described in experimental procedures. Proteins were recovered with protein G conjugated to agarose, separated by SDS-PAGE, electrophoretically transferred to nitrocellulose membranes, and immunoblotted with the anti-γ antibody. Numbers on the left of the image represent the mobility of Bio-Rad Precision Plus protein standards (in kDa). Numbers on the right indicate the predicted molecular mass of the bands. B: quantification of band densities. Values are means ± SE from three animals kept with a low-Na+ diet and paired controls. Statistically significant differences in protein abundance between the experimental and control groups are indicated as +P < 0.005 and **P < 0.001 by an unpaired Student's t-test.
DISCUSSION
The final amount of Na+ and K+ eliminated in the urine is determined in the distal segments of the nephron, including the late distal convoluted tubule, connecting tubule, and collecting duct. Dietary salt restriction stimulates aldosterone secretion from the adrenal gland, which promotes Na+ absorption as well as K+ secretion in the distal nephron. Both aldosterone infusion and dietary salt restriction substantially augment ENaC activity in principal cells (15, 18). ENaC-mediated whole cell conductance in collecting duct principal cells is at least 50 times greater in rats maintained on a low-Na+ diet than on a normal Na+ diet (20), although dietary salt restriction only produces a two- to fourfold increase in ENaC surface expression (19). This suggests that the change in the number of channels at the plasma membrane that occurs in response to dietary salt restriction cannot account, per se, for the observed increase in ENaC function. Taken together, these studies suggest that multiple mechanisms account for the observed effects of dietary salt restriction on whole cell ENaC activity.
Compelling evidence indicates that the release of imbedded inhibitory tracts from the α- and γ-subunits by proteases increases ENaC Po (4, 9, 25, 41). Both aldosterone infusion and dietary salt restriction promote maturation and cleavage of ENaC α- and γ-subunits (15). It has been recently reported that a 70-kDa COOH-terminal γ-subunit cleavage fragment was seen in kidney homogenates of rats that received aldosterone, whereas a 75-kDa fragment was noted in rats treated with both aldosterone and the serine protease inhibitor camostat (47). These observations suggest that a camostat-sensitive serine protease promotes the release of the inhibitory tract from the COOH-terminal γ-subunit cleavage fragment. Svenningsen and colleagues, using a monoclonal antibody raised against the human γ-subunit inhibitory tract, found that this region is released from channels exposed to plasmin (45), a protease that cleaves the γ-subunit after Lys194 (33, 45). To determine whether the γ-subunit inhibitory tract is released from the channel complex in animals maintained on a Na+-restricted diet, we generated an antibody against residues 131–187 in the γ-subunit (anti-γ43), which includes the inhibitory tract spanning the furin and putative prostasin cleavage sites. We found that dietary salt restriction results in loss of the inhibitory tract from the COOH-terminal γ-subunit cleavage fragment (Fig. 5), indicating that, under these conditions, the γ-subunit is cleaved twice and the tract between the RKRR143 and RKRK186 sites is released from the γ-subunit. We propose that the release of the γ-subunit inhibitory tract contributes to the increased ENaC activity registered in rats maintained on a low-Na+ diet.
Prostasin is one of many proteases that may be involved in ENaC processing and activation in vivo. This protease is associated with the plasma membrane through a GPI anchor and can be secreted in response to signals that activate phosphatidylinositol-specific phospholipase C (12). Prostasin is released into renal tubules, and its urinary concentration increases in response to aldosterone infusion in rats (29). A correlation between aldosterone levels and urinary prostasin excretion has been reported in humans (27). To gain further insights regarding the mechanism(s) by which prostasin activates ENaC, we conducted biochemical and functional experiments with wild-type prostasin and a catalytically inactive mutant (S238A). We (4) have previously reported that mutant (S238A) prostasin cleaved the γ-subunit and increased ENaC activity similarly to wild-type prostasin. Using anti-γ43 antibody, we found that both wild-type prostasin and catalytically inactive mutant prostasin promoted the release of the intervening tract between the putative furin and γRKRK186 cleavage sites. Whereas aprotinin prevented ENaC activation by mutant S238A prostasin, the mutant did not bind to aprotinin (Fig. 3). Taken together, our results suggest that mutant S238A prostasin facilitates the release of the imbedded inhibitory tract from the γ-subunit and channel activation by an endogenous aprotinin-sensitive protease expressed in Xenopus oocytes.
How does wild-type prostasin activate ENaC? Our coimmunoprecitation experiments suggest that both ENaC and prostasin (wild type or mutant S238A) are present in a multiprotein complex. Prostasin-dependent cleavage of ENaC has been suggested to occur at the plasma membrane, as deletion of the consensus motif for the GPI anchor attachment at the prostasin COOH-terminus prevents ENaC activation (3, 49). We speculate that the association of prostasin with ENaC facilitates γ-subunit cleavage by proteases that are recruited to this protein complex by prostasin as well as by catalytically inactive prostasin (Fig. 6). We hypothesize that wild-type prostasin itself may cleave the γ-subunit, as suggested by previous studies (4, 47). Prostasin has been shown to both interact with other proteases as well as facilitate zymogen conversion. For example, prostasin and matriptase form a reciprocal zymogen activation complex that is required for epithelial development and homeostasis (17). The activation of the prostasin-matriptase complex can occur independent of prostasin catalytic activity and prostasin zymogen conversion (17), suggesting that prostasin may serve as a nonproteolytic cofactor for the activation of heterologous trypsin-like serine proteases. It is also possible that the binding of prostasin to ENaC exposes regions of the channel that are otherwise inaccessible, facilitating γ-subunit cleavage by other proteases.
Fig. 6.
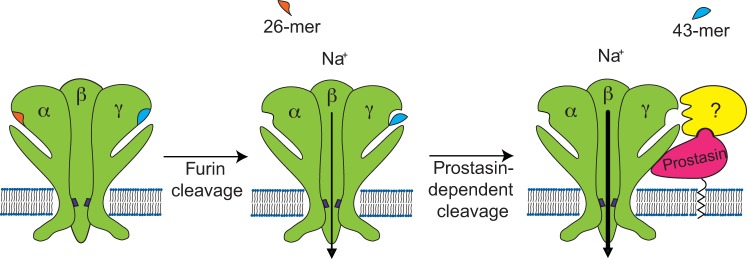
Hypothetical mechanism of activation of ENaC by serine proteases. ENaC α- and γ-subunits contain embedded inhibitory tracts in their extracellular loops. ENaC subunits are assembled in a trimeric structure in the biosynthetic pathway. During maturation in the trans-Golgi network, the pro-protein convertase furin cleaves the α-subunit twice, releasing a 26-mer inhibitory tract, and the γ-subunit at a single site preceding the γ inhibitory tract. Channels lacking proteolytic cleavage by furin have a very low open probability (Po) in the presence of external Na+. Channels that have been processed by furin and therefore lack the α-subunit inhibitory tract have an intermediate Po of ∼0.3–0.4. Prostasin is associated with the plasma membrane through a glycosylphosphatidylinositol anchor and interacts with ENaC. We propose that prostasin can cleave ENaC as well as recruit other serine proteases to the ENaC-prostasin complex, which eventually can cleave the γ-subunit, releasing the inhibitory peptide. The release of the inhibitory peptide from the γ-subunit promotes a further increase in Po. Channels lacking the inhibitory tract in both the α- and γ-subunits have a Po that approaches 1.
In summary, the results of our study revealed that a catalytically inactive mutant prostasin activates ENaC in Xenopus oocytes via facilitating the release of an imbedded inhibitory tract from the γ-subunit by an endogenous aprotinin-sensitive protease. As proteases often function within a catalytic cascade, our results suggest that prostasin may, in part, work “upstream” of other protease(s) that directly cleave ENaC by acting as a scaffold for protease binding. Furthermore, our results indicate that dietary salt restriction promotes both cleavage and release of an imbedded inhibitory tract from the γ-subunit, which likely accounts, in part, for the increased Na+ reabsorption observed in animals on a low-Na+ diet.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-065161, R01-DK-59659, R01-DK-084060, and P30-DK-079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.C., G.M.M., L.G.P., G.F., R.P.H., and T.R.K. conception and design of research; M.D.C., G.M.M., L.G.P., G.F., A.C.R., and R.P.H. performed experiments; M.D.C., G.M.M., A.C.R., R.P.H., and T.R.K. analyzed data; M.D.C., G.M.M., A.C.R., R.P.H., and T.R.K. interpreted results of experiments; M.D.C. prepared figures; M.D.C. and T.R.K. drafted manuscript; M.D.C., L.G.P., G.F., R.P.H., and T.R.K. edited and revised manuscript; M.D.C., G.M.M., L.G.P., G.F., A.C.R., R.P.H., and T.R.K. approved final version of manuscript.
REFERENCES
- 1.Adachi M, Kitamura K, Miyoshi T, Narikiyo T, Iwashita K, Shiraishi N, Nonoguchi H, Tomita K. Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J Am Soc Nephrol 12: 1114–1121, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Adebamiro A, Cheng Y, Johnson JP, Bridges RJ. Endogenous protease activation of ENaC: effect of serine protease inhibition on ENaC single channel properties. J Gen Physiol 126: 339–352, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasen D, Vuagniaux G, Fowler-Jaeger N, Hummler E, Rossier BC. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J Am Soc Nephrol 17: 968–976, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol 286: C190–C194, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol Cell Physiol 267: C1682–C1690, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Carattino MD, Hill WG, Kleyman TR. Arachidonic acid regulates surface expression of epithelial sodium channels. J Biol Chem 278: 36202–36213, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem 283: 25290–25295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its α subunit. J Biol Chem 281: 18901–18907, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Carter P, Wells JA. Dissecting the catalytic triad of a serine protease. Nature 332: 564–568, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Chen LM, Skinner ML, Kauffman SW, Chao J, Chao L, Thaler CD, Chai KX. Prostasin is a glycosylphosphatidylinositol-anchored active serine protease. J Biol Chem 276: 21434–21442, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem 277: 8338–8345, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Eladari D, Chambrey R, Picard N, Hadchouel J. Electroneutral absorption of NaCl by the aldosterone-sensitive distal nephron: implication for normal electrolytes homeostasis and blood pressure regulation. Cell Mol Life Sci 71: 2879–2895, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Frateschi S, Keppner A, Malsure S, Iwaszkiewicz J, Sergi C, Merillat AM, Fowler-Jaeger N, Randrianarison N, Planes C, Hummler E. Mutations of the serine protease CAP1/Prss8 lead to reduced embryonic viability, skin defects, and decreased ENaC activity. Am J Pathol 181: 605–615, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Friis S, Uzzun Sales K, Godiksen S, Peters DE, Lin CY, Vogel LK, Bugge TH. A matriptase-prostasin reciprocal zymogen activation complex with unique features: prostasin as a non-enzymatic co-factor for matriptase activation. J Biol Chem 288: 19028–19039, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frindt G, Ergonul Z, Palmer LG. Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol 131: 617–627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frindt G, Palmer LG. Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol 297: F1249–F1255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frindt G, Sackin H, Palmer LG. Whole-cell currents in rat cortical collecting tubule: low-Na diet increases amiloride-sensitive conductance. Am J Physiol Renal Fluid Electrolyte Physiol 258: F562–F567, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Corniere N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kashlan OB, Adelman JL, Okumura S, Blobner BM, Zuzek Z, Hughey RP, Kleyman TR, Grabe M. Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel α subunit reveals a mechanism of channel activation by proteases. J Biol Chem 286: 649–660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashlan OB, Kleyman TR. Epithelial Na+ channel regulation by cytoplasmic and extracellular factors. Exp Cell Res 318: 1011–1019, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleyman TR, Satlin LM, Hallows KR. Opening lines of communication in the distal nephron. J Clin Invest 123: 4139–4141, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koda A, Wakida N, Toriyama K, Yamamoto K, Iijima H, Tomita K, Kitamura K. Urinary prostasin in humans: relationships among prostasin, aldosterone and epithelial sodium channel activity. Hypertens Res 32: 276–281, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Narikiyo T, Kitamura K, Adachi M, Miyoshi T, Iwashita K, Shiraishi N, Nonoguchi H, Chen LM, Chai KX, Chao J, Tomita K. Regulation of prostasin by aldosterone in the kidney. J Clin Invest 109: 401–408, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orce GG, Castillo GA, Margolius HS. Inhibition of short-circuit current in toad urinary bladder by inhibitors of glandular kallikrein. Am J Physiol Renal Fluid Electrolyte Physiol 239: F459–F465, 1980 [DOI] [PubMed] [Google Scholar]
- 31.Pacha J, Frindt G, Antonian L, Silver RB, Palmer LG. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 25–42, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passero CJ, Mueller GM, Myerburg MM, Carattino MD, Hughey RP, Kleyman TR. TMPRSS4-dependent activation of the epithelial sodium channel requires cleavage of the γ-subunit distal to the furin cleavage site. Am J Physiol Renal Physiol 302: F1–F8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem 283: 36586–36591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel AB, Chao J, Palmer LG. Tissue kallikrein activation of the epithelial Na channel. Am J Physiol Renal Physiol 303: F540–F550, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol; 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picard N, Eladari D, El Moghrabi S, Planes C, Bourgeois S, Houillier P, Wang Q, Burnier M, Deschenes G, Knepper MA, Meneton P, Chambrey R. Defective ENaC processing and function in tissue kallikrein-deficient mice. J Biol Chem 283: 4602–4611, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Planes C, Leyvraz C, Uchida T, Angelova MA, Vuagniaux G, Hummler E, Matthay M, Clerici C, Rossier B. In vitro and in vivo regulation of transepithelial lung alveolar sodium transport by serine proteases. Am J Physiol Lung Cell Mol Physiol 288: L1099–L1109, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol 71: 361–379, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 290: F1488–F1496, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Shi S, Carattino MD, Hughey RP, Kleyman TR. ENaC regulation by proteases and shear stress. Curr Mol Pharmacol 6: 28–34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem 269: 24379–24383, 1994 [PubMed] [Google Scholar]
- 43.Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem 285: 30363–30369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spraggon G, Hornsby M, Shipway A, Tully DC, Bursulaya B, Danahay H, Harris JL, Lesley SA. Active site conformational changes of prostasin provide a new mechanism of protease regulation by divalent cations. Protein Sci 18: 1081–1094, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skott O. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol 20: 299–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tolino LA, Okumura S, Kashlan OB, Carattino MD. Insights into the mechanism of pore opening of acid-sensing ion channel 1a. J Biol Chem 286: 16297–16307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchimura K, Kakizoe Y, Onoue T, Hayata M, Morinaga J, Yamazoe R, Ueda M, Mizumoto T, Adachi M, Miyoshi T, Shiraishi N, Sakai Y, Tomita K, Kitamura K. In vivo contribution of serine proteases to the proteolytic activation of γENaC in aldosterone-infused rats. Am J Physiol Renal Physiol 303: F939–F943, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Vallet V, Pfister C, Loffing J, Rossier BC. Cell-surface expression of the channel activating protease xCAP-1 is required for activation of ENaC in the Xenopus oocyte. J Am Soc Nephrol 13: 588–594, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol 11: 828–834, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Wen D, Cornelius RJ, Yuan Y, Sansom SC. Regulation of BK-α expression in the distal nephron by aldosterone and urine pH. Am J Physiol Renal Physiol 305: F463–F476, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]