Abstract
Biliary hyperplasia and liver fibrosis are common features in cholestatic liver disease. Melatonin is synthesized by the pineal gland as well as the liver. Melatonin inhibits biliary hyperplasia of bile duct-ligated (BDL) rats. Since melatonin synthesis (by the enzyme serotonin N-acetyltransferase, AANAT) from the pineal gland increases after dark exposure, we hypothesized that biliary hyperplasia and liver fibrosis are diminished by continuous darkness via increased melatonin synthesis from the pineal gland. Normal or BDL rats (immediately after surgery) were housed with light-dark cycles or complete dark for 1 wk before evaluation of 1) the expression of AANAT in the pineal gland and melatonin levels in pineal gland tissue supernatants and serum; 2) biliary proliferation and intrahepatic bile duct mass, liver histology, and serum chemistry; 3) secretin-stimulated ductal secretion (functional index of biliary growth); 4) collagen deposition, liver fibrosis markers in liver sections, total liver, and cholangiocytes; and 5) expression of clock genes in cholangiocytes. In BDL rats exposed to dark there was 1) enhanced AANAT expression/melatonin secretion in pineal gland and melatonin serum levels; 2) improved liver morphology, serum chemistry and decreased biliary proliferation and secretin-stimulated choleresis; and 4) decreased fibrosis and expression of fibrosis markers in liver sections, total liver and cholangiocytes and reduced biliary expression of the clock genes PER1, BMAL1, CLOCK, and Cry1. Thus prolonged dark exposure may be a beneficial noninvasive therapeutic approach for the management of biliary disorders.
Keywords: biliary epithelium, cholestasis, clock genes, melatonin, secretin
the secretory activity of the biliary epithelium is coordinately regulated by several gastrointestinal peptides/hormones including secretin that stimulates bicarbonate secretion by interaction with secretin receptors (SR, only expressed by large cholangiocytes in the liver) (1, 2, 4) by activation of the cAMP→PKA→CFTR→AE2 cascade (3–5, 34, 57). blansIn normal conditions, cholangiocytes are relatively quiescent, but during liver damage they acquire the ability to proliferate to repair the biliary epithelium and compensate for lost functional activity (2, 7, 34, 44, 45). In support of this concept, enhanced proliferation is associated with increased secretin-stimulated ductal secretion (a functional index of biliary proliferation) (3, 5, 6) evidenced by increased expression of SR→cAMP→PKA→CFTR→AE2 and secretin-stimulated bicarbonate secretion, whereas during biliary damage there is reduced secretin-induced choleresis (3–5, 34, 57). Following extrahepatic bile duct ligation (BDL, that induces the proliferation of cholangiocytes) (3), a complex network of hormones, neurotransmitters, and neuropeptides regulate the homeostasis of the biliary epithelium by autocrine and/or paracrine mechanisms (1–3, 7, 38). Biliary hyperplasia and liver fibrosis are a critical element observed during the onset of cholestasis (56). Liver fibrosis is a progress of excessive accumulation of extracellular matrix proteins that occurs during the progression of chronic liver injury including cholestasis (8, 56). Several liver cells including hepatic stellate cells, portal fibroblasts, and myofibroblasts participate in the development of fibrosis. BDL is a unique animal model of hepatic fibrosis that the primary pathological changes started from the area around the biliary epithelium (23, 50). Although the chronic obstruction of bile duct activates myofibroblasts in periductal areas, limited information exists regarding the role of cholangiocytes in the progression of biliary fibrosis (24).
Melatonin is synthetized from tryptophan by activation of the enzymes serotonin N-acetyltransferase (AANAT) and hydroxyindole-O-methyltransferase (ASMT) (62). Melatonin is released into the blood stream primarily from the pineal gland after dark exposure, and it is suppressed by light, indicating the important functions of this hormone in the regulation of circadian rhythm and several body functions (43). The hypothalamus and pituitary organs mediate many of melatonin functions including circadian rhythms, stress, and reproduction. Recently, stomach, duodenum, and the hepatobiliary system have also been identified as major sources of melatonin during the daytime (12, 44).
Abnormal melatonin synthesis and circadian rhythms are altered in patients with hepatic cirrhosis and are associated with severe liver insufficiency (58). Several studies have shown that melatonin administration has protective effects against cholestatic liver damage through its antioxidant, anti-inflammatory, and proapoptotic proprieties (19, 28, 29, 46). Recently, studies have demonstrated the antiproliferative effects of melatonin on cholangiocytes in animal models of extrahepatic cholestasis as well as cholangiocarcinoma (26, 44, 45). Several studies have also shown that melatonin circulation during darkness is a potent oncostatic signal and light exposure at night suppresses the night peak release of melatonin and seems associated with disease processes including cancer (9–11).
Interestingly, our group has shown that administration of exogenous melatonin inhibits biliary hyperplasia and secretin-stimulated ductal secretion of BDL rats (45). The proposed mechanism involves resynchronization of circadian genes such as Per1/2, Cry1/2, BMAL1, and CLOCK, which are mediators of cell mitosis. After BDL, increased/altered expression of clock genes in cholangiocytes has been described (45). During cholestasis, cholangiocytes also synthesize melatonin through activation of the rate-limiting enzyme AANAT (44). In vivo local inhibition of AANAT synthesis (by administration of AANAT Vivo-Morpholino) increases cholangiocyte proliferation, whereas in vitro overexpression has antiproliferative effects (44). These studies describe the local synthesis of melatonin by cholangiocytes and the pivotal role of this hormone in homeostasis of the biliary epithelium via an autocrine pathway. In this study, we tested the hypothesis that prolonged dark exposure after BDL increases melatonin levels released from pineal gland inhibit cholangiocyte hyperplasia and liver fibrosis by a paracrine mechanism by inducing AANAT expression and melatonin synthesis in the pineal gland.
METHODS AND MATERIALS
Materials
Reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. The mouse monoclonal antibody against proliferating cell nuclear antigen (PCNA, Sc-56) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse anti-cytokeratin-19 (CK-19) antibody was purchased from Caltag Laboratories (Burlingame, CA). Commercially available ELISA kits for measuring melatonin levels were purchased from Genway (San Diego, CA). The EIA kits (cyclic AMP EIA kit no. 581001) for the measurement of intracellular cAMP levels were purchased from Cayman Chemical (Ann Arbor, MI). The RNeasy Mini Kit for RNA purification and all primers were purchased from Qiagen (Valencia, CA).
Animal Models
Male Fischer 344 rats (175–200 g) were purchased from Charles River Laboratories (Wilmington, MA) and housed in a temperature-controlled environment (22°C). Animals were fed standard rat chow and had access to drinking water ad libitum. We used normal rats, BDL or bile duct-incannulated (BDI, for collection of bile) rats that immediately after surgery (3) were housed in a temperature-controlled environment (22°C) with 12:12-h light-dark cycles or complete dark for 1 wk. All experimental groups were used at 8:00 AM for the collection of serum, cholangiocytes, and liver samples. Before each procedure, the animals were treated with Euthasol (200–250 mg/kg body wt). Liver weight, body weight, and liver weight-to-body weight ratio (an index of the growth of liver cells including cholangiocytes) (3) were measured in all animals. All animal experiments were performed in accordance with protocols approved by Baylor Scott and White IACUC.
Freshly Isolated Cholangiocytes
Virtually pure (∼98% by histochemistry for γ-glutamyltransferase, γ-GT) (49) cholangiocytes from each animal group were obtained by immunoaffinity separation (4, 5, 31) by using a monoclonal antibody, rat IgG2a (a gift from Dr. R. Faris, Brown University, Providence, RI), against an unidentified antigen expressed by all mouse cholangiocytes (31).
Measurement of AANAT Expression in Pineal Gland and Hypothalamus Tissue and Melatonin Levels in Pineal Gland Tissue Supernatants and Serum
To validate our model, we evaluated the mRNA expression of AANAT by real-time PCR (22, 45) in total RNA (1 μg) from pineal gland and hypothalamus. Data were expressed as relative mRNA levels ± SE of AANAT-to-GAPDH ratio. To detect the mRNA expression of the selected genes, we used the RT2 Real-Time assay (SABiosciences, Frederick, MD). A ΔΔCT (delta delta of the threshold cycle) analysis was performed with normal cholangiocytes as control sample (22). We evaluated melatonin levels by ELISA kits (44) in 1) the supernatant collected from short-term (6 h) cultures of pineal gland, data expressed as pg/μg protein; and 2) serum from the four groups of animals.
Evaluation of Cholangiocyte Proliferation, IBDM, Liver Histology, and Serum Chemistry
The percentage of PCNA-positive cholangiocytes was calculated by semiquantitative immunohistochemistry (45) in paraffin-embedded liver sections (4–5 μm thick, 10 different fields analyzed from each sample obtained from 3 different animals). We determined intrahepatic bile duct mass (IBDM) in liver sections, which was measured by semiquantitative immunohistochemistry (45) as the area occupied by CK-19-positive bile ducts/total area × 100. Negative controls with the omission of the primary antibody (replaced with normal serum from the same species) were included for all samples. Sections were analyzed in a coded fashion by using an Olympus BX-51 light microscope (Tokyo, Japan) with a Videocam (Spot Insight; Diagnostic Instrument, Sterling Heights, MI). Semiquantitative analysis of the positive staining was performed with image analysis software, Image-Pro Plus (Media Cybernetics, Silver Springs, MD). About eight random fields were calculated for statistics.
We also evaluated 1) PCNA and CK-19 mRNA (by real-time PCR using 1 μg total RNA) (45) and PCNA protein expression (by immunoblots and/or FACS analysis) (45) in purified cholangiocytes from the selected groups of animals. The selected rat primers were purchased from SABiosciences (Qiagen, Valencia, CA) and designed by using sequences with the following NCBI GenBank accession numbers: PCNA, NM_022381; CK-19, NM_199498; and GAPDH, NM_017008. Immunoblots were performed in 10 μg of protein from whole cell lysate from spleen (positive control) or purified cholangiocytes. Immunoblots were normalized as ratio to β-actin, the housekeeping gene. Band intensity of immunoblots was determined by scanning video densitometry by using the phospho-imager, Storm 860 (GE Healthcare, Piscataway, NJ) and the ImageQuant TL software version 2003.02 (GE Healthcare, Little Chalfont, Buckinghamshire, UK). FACS analysis was also performed to measure PCNA protein expression by use of a C6 flow cytometer and analyzed by CFlow Software (Accuri Cytometers, Ann Arbor, MI) (45). At least 10,000 events in the light scatter (SSC/FSC) were acquired. The expression of PCNA was identified and gated on FL1-A/Count plots. The relative quantity of the selected protein (mean selected protein fluorescence intensity) was expressed as mean FL1-A (samples)/mean FL1-A (secondary antibodies only).
All paraffin-embedded liver sections (4–5 μm thick) were stained with hematoxylin and eosin and analyzed in a coded fashion by a board certified pathologist. Sections were evaluated by a BX-51 light microscope (Olympus, Tokyo, Japan). The serum levels of glutamate pyruvate transaminases (SGPT), glutamic oxaloacetic transaminase (SGOT), alkaline phosphatase (ALP), and total bilirubin were measured by a Dimension RxL Max Integrated Chemistry system (Dade Behring, Deerfield, IL) by the Chemistry Department, Baylor Scott & White Healthcare.
Measurement of Secretin-Stimulated Camp Levels and Bile Secretion
Next, we evaluated 1) basal and secretin-stimulated cAMP levels (by EIA kits) in purified cholangiocytes and bile and 2) bicarbonate secretion (functional indexes of biliary growth) (3, 34) in bile fistula rats (45). Before evaluation of cAMP levels by EIA kits, purified cholangiocytes were incubated for 1 h at 37°C (to regenerate membrane proteins damaged by proteolytic enzymes during cell purification) (31) and subsequently incubated with 0.2% BSA or secretin (100 nM) for 5 min at room temperature (4, 5).
For the evaluation of bile and bicarbonate secretion, after anesthesia rats were surgically prepared for bile collection as described (3). Briefly, when steady-state bile flow was reached (60–70 min from the intravenous infusion of Krebs-Ringer-Henseleit solution, KRH), the animals were infused with secretin (100 nM for 30 min) (34) via a jugular vein followed by intravenous infusion of KRH for 30 min. Bicarbonate levels in bile were determined by a COBAS Mira Plus automated clinical chemistry analyzer (Bohemia, NY).
Evaluation of Liver Fibrosis in Liver Sections and Expression of Fibrotic Genes in Total Liver and Purified Cholangiocytes
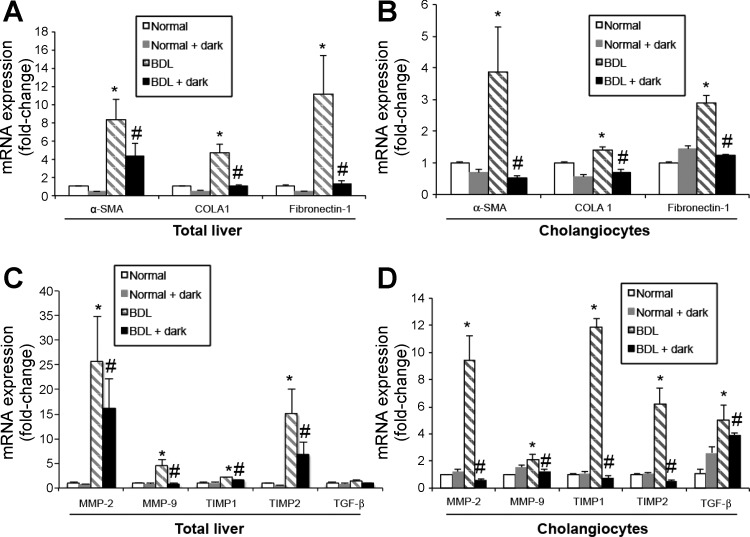
Liver fibrosis was evaluated by Sirius red staining for identifying interstitial collagen with red color in paraffin-embedded liver sections (4–5 μm thick, 10 different fields analyzed from each sample obtained from 3 different animals). Slides were scanned by a digital scanner (AperioScanscope CS System, Aperio Technologies, Oxford, UK) and processed by ImageScope. An image analysis algorithm has been used to quantify the volume fraction occupied by collagen (Sirius red-stained) fibers. The algorithm was applied on the entire sections. Typical markers of fibrosis such as α-smooth muscle actin (α-SMA) were also evaluated by immunohistochemistry in paraffin-embedded liver sections (4 μm thick). We also evaluated by real-time PCR (22) the expression of COLA1, fibronectin-1, and α-SMA, matrix metallopeptidase 2 and 9 (MMP-2, MMP-9), metallopeptidase inhibitor 1 and 2 (TIMP1 and TIMP2, tissue inhibitors of metalloproteinases), and transforming growth factor-β (TGF-β) in total liver and isolated cholangiocytes. The selected rat primers were purchased from SABiosciences (Qiagen, Valencia, CA) and designed using sequences with the following NCBI GenBank accession numbers: NM_053304 (COLA1); NM_019143 (α-SMA); NM_031004 (fibronectin-1); NM_031054 (MMP-2); NM_031055 (MMP-9); NM_053819 (TIMP1); NM_021989 (TIMP2); NM_021578 (TGF-β); CK-19, NM_199498; and NM_017008 (GAPDH).
Expression of Core Clock Genes in Isolated Cholangiocytes
The expression of the core clock genes, CLOCK, PER1, Cry1, and BAML1, was studied in isolated cholangiocytes from the selected groups of animals by real-time PCR (45). Primers used were designed against rat BAML1, Cry1, PER1, and CLOCK according to the NCBI GenBank accession numbers: NM_024362 (BMAL1), NM_198750 (Cry1), NM_001034125 (PER1); NM_021856 (CLOCK); and NM_017008 (GAPDH). Data were expressed as relative mRNA levels ± SE of the selected gene to GAPDH ratio.
Statistical Analysis
All data are expressed as means ± SE. Differences between groups were analyzed by Student's unpaired t-test when two groups were analyzed and ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test.
RESULTS
Measurement of AANAT Gene Expression in Pineal Gland and Hypothalamus Tissue and Melatonin Levels in Pineal Gland Tissue Supernatants and Serum
The transcriptional activation of the AANAT gene is the primary mechanism for the induction of melatonin biosynthesis in rodents (51). Since melatonin levels are altered by changes in light and dark cycles (35), we measured AANAT expression in the pineal gland and hypothalamus (which expresses very low levels of AANAT) and melatonin levels in pineal gland tissue supernatant and serum from the four experimental groups. There were no significant changes in AANAT expression and melatonin secretion in the pineal gland of BDL compared with normal rats (Fig. 1, A and B). As expected (27), AANAT mRNA expression and melatonin secretion increased in the pineal gland from normal and BDL + dark rats compared with their corresponding rats (Fig. 1, A and B); AANAT mRNA expression was barely detectable in the hypothalamus of normal rats and did not change significantly compared with the other groups (not shown). Similar to our previous study (45), melatonin levels were higher in the serum of BDL compared with normal rats (Table 1). Melatonin serum levels were higher in normal and BDL rats exposed to dark for 1 wk compared with the corresponding rats under light-dark cycles (Table 1).
Fig. 1.
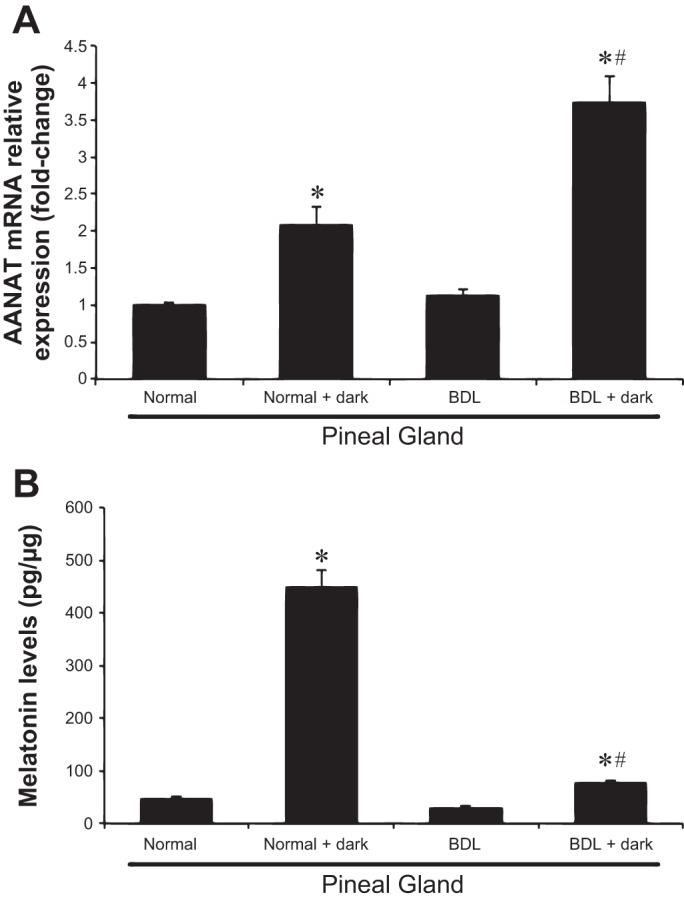
Serotonin N-acetyltransferase (AANAT) gene expression and melatonin secretion in pineal gland tissue in animals exposed to light-dark cycles or complete dark for 1 wk. AANAT mRNA expression and melatonin secretion increases in the pineal gland from normal and bile duct ligation (BDL) + dark rats compared with their corresponding rats. A: data are means ± SE of 4 experiments. *P < 0.05 compared with normal rats. #P < 0.05 BDL + dark compared with BDL. B: data (means ± SE, n = 6 experiments) are expressed as pg/μg protein. *P < 0.05 compared with normal rats. #P < 0.05 BDL + dark compared with BDL.
Table 1.
Measurement of liver, body weight and liver-to-body weight ratio; serum chemistry; % PCNA-positive cholangiocytes and IBDM; and melatonin serum levels
| Parameters | Normal + Light-Dark Cycles 1 wk | Normal + Complete Dark 1 wk | BDL + Light-Dark Cycles 1 wk | BDL + Complete Dark 1 wk |
|---|---|---|---|---|
| Liver weight, g | 8.7 ± 0.2 | 9.0 ± 0.6 | 9.8 ± 0.1* | 8.7 ± 0.5† |
| (n = 9) | (n = 7) | (n = 8) | (n = 12) | |
| Body weight, g | 217.7 ± 5.8 | 226.0 ± 5.1 | 170.2 ± 4.3* | 175.0 ± 2.7 |
| (n = 12) | (n = 7) | (n = 12) | (n = 12) | |
| Liver-to-body weight ratio, % | 4.30 ± 0.2 | 3.9 ± 0.2 | 5.6 ± 0.1* | 4.9 ± 0.3† |
| (n = 12) | (n = 7) | (n = 12) | (n = 12) | |
| Serum melatonin levels, pg/ml | 61.52 ± 8.71 | 207.0 ± 84.1 | 110.1 ± 18.4* | 145.3 ± 47.5 |
| (n = 15) | (n = 17) | (n = 15) | (n = 12) | |
| SGPT, U/l | 72.7 ± 12.1 | 156.1 ± 32.5* | 396.3 ± 95.6* | 115.7 ± 13.9† |
| (n = 9) | (n = 8) | (n = 10) | (n = 4) | |
| SGOT, U/l | 185.9 ± 24.5 | 270.0 ± 58.4‡ | 1,105.8 ± 245.4* | 249.2 ± 25.1† |
| (n = 12) | (n = 8) | (n = 11) | (n = 4) | |
| Total bilirubin, mg/dl | <0.1 | <0.1 | 11.5 ± 1.2* | 4.7 ± 1.3† |
| (n = 7) | (n = 6) | (n = 12) | (n = 4) | |
| ALP, U/l | 276.4 ± 13.7 | 244.2 ± 9.1‡ | 400.63 ± 33.0* | 357.5 ± 23n.s. |
| (n = 8) | (n = 5) | (n = 10) | (n = 4) | |
| Volume fraction occupied by collagen (Sirius red-stained) fibers | 1.8 ± 0.2 | 1.9 ± 0.2 | 6.3 ± 1.5* | 3.5 ± 0.6† |
| α-SMA+ portal MF/HSC (cells/HPF) | 1.5 ± 0.3 | 1.0 ± 0.26 | 9.7 ± 1.4 | 6.6 ± 0.7 |
P < 0.05 BDL vs. normal rats.
P < 0.05 BDL + dark vs. BDL;
not significant vs. the corresponding rats exposed to dark-light cycles.
BDL, bile duct ligation; IBDM, intrahepatic bile duct mass; SGOT, serum glutamic oxaloacetic transaminases; SGPT, serum glutamate pyruvate transaminases; ALP, alkaline phosphatase; α-SMA, α-smooth muscle actin, MF, myofibroblasts; HSC, hepatic stellate cells; HPF, high-powered field.
Evaluation of Cholangiocyte Proliferation and IBDM, Liver Histology, and Serum Chemistry
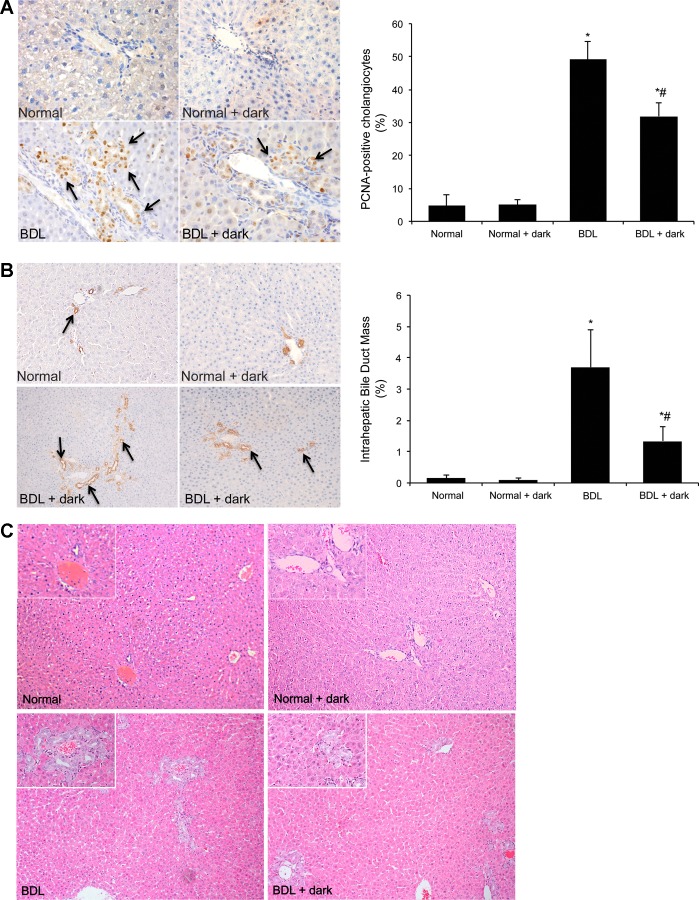
As previously shown (3), there was a decrease in the body weight of BDL compared with normal rats (Table 1). Moreover, there was no significant difference in the body weight between normal and BDL rats exposed to complete dark compared with the corresponding groups (Table 1). Consistent with reduced liver cell growth after prolonged dark exposure, there was decreased liver-to-body weight ratio in BDL rats exposed to dark compared with BDL rats exposed to light-dark cycles (controls) (Table 1). We have previously shown that in vivo administration of melatonin decreases BDL-induced biliary hyperplasia (45). Herein, we extended our previous study to determine that enhanced secretion of melatonin (from the pineal gland after prolonged exposure to dark) inhibits cholangiocyte proliferation induced by BDL (3). In agreement with previous studies (2, 3), in BDL rats there was increased percentage of PCNA-positive cholangiocytes (Fig. 2A) and IBDM (Fig. 2B) compared with normal rats (Fig. 2, A and B). Exposure of BDL rats to complete dark decreased the percentage of PCNA-positive cholangiocytes and IBDM (Fig. 2, A and B) compared with BDL controls. Prolonged dark exposure had no effect on the proliferation in normal rats. Liver architecture of normal rats exposed to 12:12-h light-dark cycles or prolonged dark was normal (score 0, no biliary proliferation or inflammation was observed) (Fig. 2C). BDL rats, which showed prominent biliary proliferation, received score 4. BDL + dark-exposed rats (that showed lower cholangiocyte proliferation compared with BDL rats) received score 3 (Fig. 2C). Serum levels of transaminases, bilirubin, and alkaline phosphatase increased in BDL compared with normal rats and significantly decreased in BDL + dark rats compared with BDL controls (Table 1); no significant difference was observed between normal rats exposed to light-dark cycles or complete dark (Table 1).
Fig. 2.
A and B: in BDL rats there was increased percentage of PCNA-positive cholangiocytes and intrahepatic bile duct mass (IBDM) compared with normal rats. Chronic exposure of BDL rats to complete dark decreases the percentage of PCNA-positive cholangiocytes and IBDM compared with BDL controls. Prolonged dark exposure had no effect on the proliferation of normal rats. Original magnification, ×40 (PCNA), ×10 (CK-19). Data are expressed as means ± SE. *P < 0.05 vs. normal rats. #P < 0.05 BDL + dark compared with BDL rats exposed to light-dark cycles. C: the architecture of normal rats exposed to 12:12-h light-dark cycles or prolonged dark was normal. BDL rats showed prominent biliary proliferation. BDL rats exposed to complete dark for 1 wk show lower cholangiocyte proliferation compared with BDL rats. Original magnification, ×10.
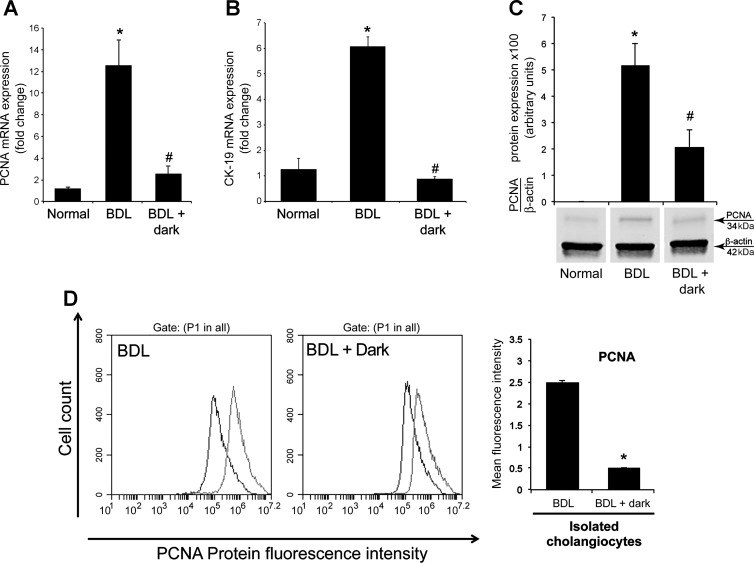
By real-time PCR, immunoblots, and FACS analysis, we demonstrated increased PCNA expression in cholangiocytes from BDL rats compared with normal rats (Fig. 3, A, C, and D). In purified cholangiocytes from BDL + dark rats, there was reduced PCNA mRNA and protein expression compared with BDL controls (Fig. 3, A, C, and D). We found a similar trend for CK-19 gene expression with a decrease in expression levels in BDL rats exposed to dark compared with BDL control rats (Fig. 3B).
Fig. 3.
Chronic exposure of BDL rats to dark for 1 wk decreases PCNA and CK-19 expression in purified cholangiocytes. PCNA (A) and CK-19 (B) expression was evaluated by real-time PCR. Data are expressed as means ± SE of 4 experiments from cumulative preparations of cholangiocytes. *P < 0.05 BDL compared with normal rats. #P < 0.05 BDL + dark compared with BDL rats. C: PCNA protein expression was evaluated by immunoblots. Data are expressed as means ± SE of 4 immunoblots reactions from cumulative preparations of cholangiocytes. *P < 0.05 BDL compared with normal rats. #P < 0.05 BDL + dark compared with BDL rats. D: PCNA protein expression evaluated by FACS analysis (n = 3). P1 represents the pool cholangiocytes population gated from the range of forward-scattered light (FSC) and side-scattered light (SSC). *P < 0.05 BDL + dark compared with BDL rats.
Measurement of cAMP Levels in Purified Cholangiocytes and Bile and Bicarbonate Secretion In Vivo
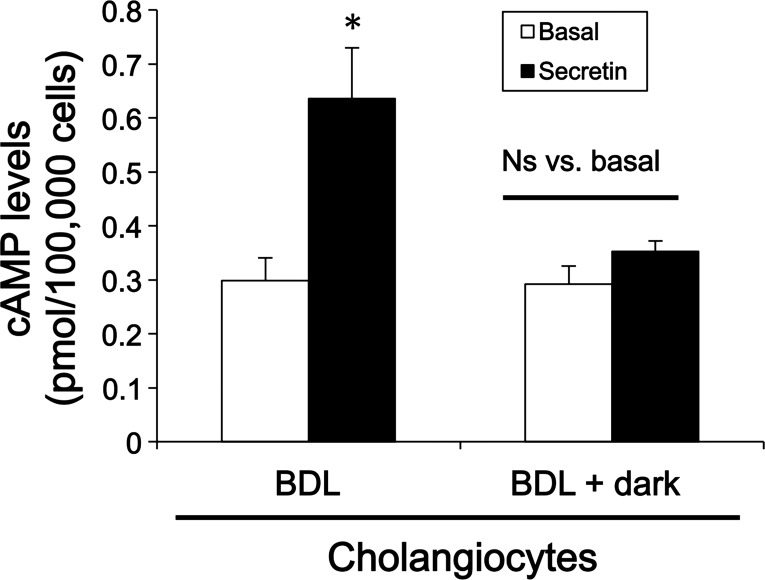
As expected (3), secretin increased cAMP and bile and bicarbonate secretion in BDL rats but did not increase cAMP levels and bile and bicarbonate secretion in BDL that were exposed to dark (Fig. 4 and Table 2).
Fig. 4.
Chronic exposure of BDL rats to dark for 1 wk decreases secretin-stimulated cAMP levels in purified cholangiocytes. Basal and secretin-stimulated cAMP levels were evaluated by ELISA. Secretin-induced cAMP levels were ablated in cholangiocytes isolated from BDL + dark rats compared with BDL rats. Data are expressed as means ± SE (n = 6). *P < 0.05 vs. the corresponding basal value of BDL rats.
Table 2.
Measurement of bile and bicarbonate secretion in BDI rats exposed to 12:12-h light-dark cycles and complete dark for 1 wk
| Bile Flow, μl·min−1·kg body wt−1 |
Bicarbonate Secretion, μEq·min−1·kg body wt−1 |
|||
|---|---|---|---|---|
| Treatments | Basal | Secretin | Basal | Secretin |
| BDI | 137.64 ± 13.35 | 188.9 ± 19.8* | 4 ± 0.3 | 11 ± 1.4* |
| BDI + Dark | 102.02 ± 9.39 | 122.46 ± 8.38† | 5.66 ± 1.4 | 7.65 ± 0.83† |
P < 0.05 vs. the corresponding basal values; †nonsignificant vs. the corresponding basal values.
BDI, bile duct incannulated.
Evaluation of Liver Fibrosis in Liver Sections and Expression of Fibrotic Genes in Total Liver and Purified Cholangiocytes
In vitro effect of melatonin on the expression of fibrotic genes.
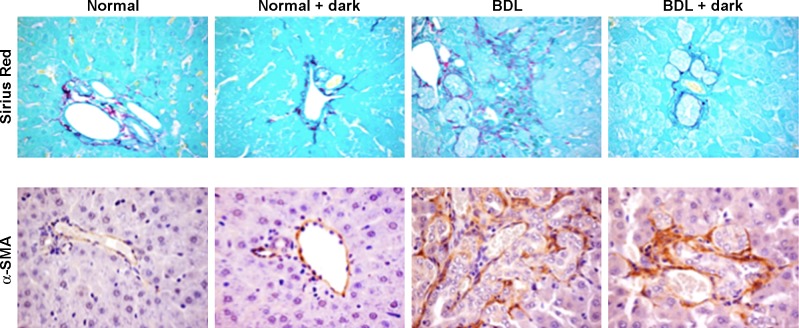
In agreement with previous studies (18, 47), in liver sections from BDL rats there was enhanced fibrosis evaluated by Sirius red staining for collagen fibers and increased expression of α-SMA compared with normal rats (Fig. 5). When BDL rats were exposed to complete dark, there was reduced fibrosis and expression of α-SMA compared with BDL rats exposed to light-dark cycles (Fig. 5). Following BDL, there was increased expression of α-SMA, COLA1, fibronectin-1, MMP-2, MMP-9, TIMP1, TIMP2, and TGF-β in total liver and cholangiocytes compared with normal rats (Fig. 6, A–D). BDL-induced increase in the expression of α-SMA, COLA1, fibronectin-1, MMP-2, MMP-9, TIMP1, TIMP2, and TGF-β was reduced by exposure of BDL rats to dark (Fig. 6, A–D).
Fig. 5.
Evaluation of fibrosis in liver sections. There was enhanced fibrosis (evaluated by Sirius red staining) and increased expression of α-smooth muscle actin (α-SMA) in BDL compared with normal rats. When BDL rats were exposed to complete dark for 1 wk, there was reduced fibrosis and expression of α-SMA compared with BDL rats exposed to light-dark cycles. Original magnification, ×40.
Fig. 6.
Prolonged exposure of BDL rats to dark decreases the expression of fibrotic markers. Following BDL, there was increased expression of α-SMA, COLA1, fibronectin-1, MMP-2, MMP-9, TIMP1, TIMP2, and TGF-β in total liver (A and C) and purified cholangiocytes (B and D) compared with normal rats. Prolonged exposure of BDL to dark decreases the expression of α-SMA, COLA1, fibronectin-1, MMP-2, MMP-9, TIMP1, TIMP2, and TGF-β in total liver samples (A and C) and purified cholangiocytes (B and D). Data are expressed as means ± SE of 4 experiments to GAPDH ratio from cumulative preparations of cholangiocytes. *P < 0.05 BDL compared with normal rats. #P < 0.05 BDL + dark compared with BDL rats.
Expression of clock genes in isolated cholangiocytes.
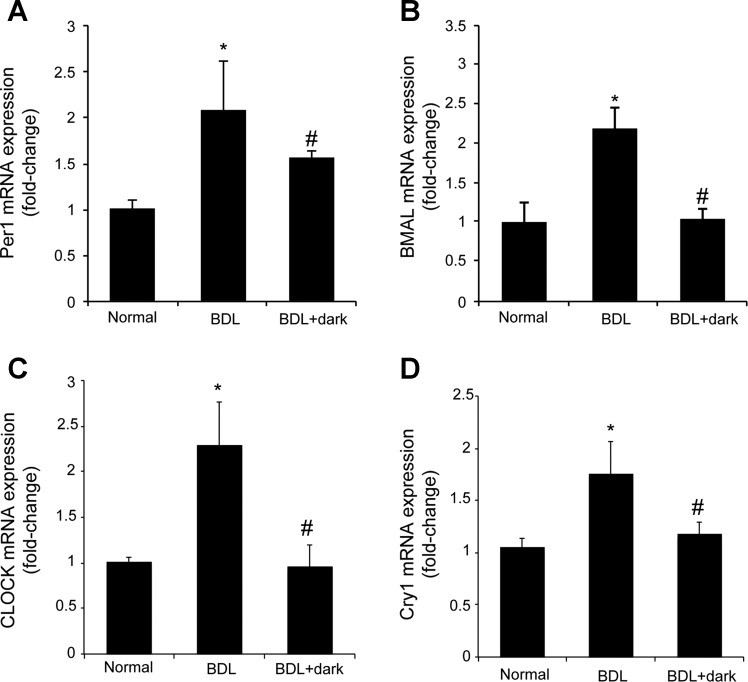
There was increased expression of the clock genes, Per1, BMAL1, CLOCK, and Cry1 in total liver samples and cholangiocytes from BDL rats compared with normal rats (Fig. 7). Exposure of BDL rats to complete dark (that increases melatonin secretion and reduces biliary hyperplasia) decreases the mRNA expression of Per1, BMAL1, CLOCK, and Cry1 compared with BDL rats exposed to light-dark cycles (Fig. 7).
Fig. 7.
There was increased expression of Per1 (A), BMAL1 (B), CLOCK (C), and Cry1 (D) in cholangiocytes from BDL rats compared with normal rats. Exposure of BDL rats to complete dark for 1 wk decreases the mRNA expression of Per1, BMAL1, CLOCK, and Cry1 compared with BDL rats exposed to 12:12-h light-dark cycles. Data were expressed as relative mRNA levels ± SE to GAPDH ratio. *P < 0.05 BDL compared with normal rats. #P < 0.05 BDL + dark compared with BDL rats.
DISCUSSION
In the present study, we evaluated the effect of prolonged dark exposure (which stimulates the release of melatonin from the pineal gland) (43, 62) on BDL-induced biliary hyperplasia and liver fibrosis. In BDL rats exposed to prolonged dark there was 1) increased AANAT mRNA expression and melatonin levels in the pineal gland and circulating melatonin levels; 2) improved liver morphology and decreased biliary proliferation and IBDM; 3) decreased secretin-stimulated ductal secretory activity; and 4) decreased fibrosis and expression of the clock genes PER1, BMAL1, CLOCK, and Cry1 in isolated cholangiocytes compared with BDL control rats. Our findings suggest that the prolonged dark exposure may be a beneficial noninvasive therapeutic approach for the management of biliary disorders through the modulation of melatonin synthesis from the pineal gland.
Melatonin synthesis in the pineal gland shows a rhythmic fashion with high levels at night and is controlled by the rate-limiting enzyme AANAT (62). We found that there was a significant elevation in serum melatonin levels when both normal and BDL rats were exposed to prolonged complete darkness. This observation is consistent with a recent study that showed a significant increase in serum melatonin in rats exposed to constant darkness for 17 days, which was similar to the nighttime elevations in animals exposed to 12:12-h light-dark cycles (60). Several other studies have demonstrated that melatonin levels increase in serum during prolonged dark exposure in rat, pig, and chicken models (14, 35, 42). Also, consistent with our previous study and those in humans with liver cirrhosis, we found that there was significant increase in serum melatonin levels in BDL compared with normal control rats (13, 45). Since there was no significant difference in AANAT expression/melatonin secretion in the hypothalamus of normal and BDL rats, the increase in melatonin serum levels observed in BDL rats is likely due to the higher secretion of melatonin by BDL cholangiocytes as part of a compensatory mechanism as we observed during biliary proliferation (44, 45). We found that, parallel with increase melatonin serum levels, there was a significant increase in AANAT gene expression in the pineal glands of the normal and BDL animals exposed to constant darkness. Similar elevations in AANAT gene expression have been observed in rats kept in constant darkness compared with those kept in standard light-dark conditions (59). Interestingly, although there was an increase in AANAT gene expression level in the pineal gland of BDL rats kept in constant darkness, there was a smaller (compared to higher levels observed in normal rats) increase in melatonin levels in the supernatants collected from the pineal glands of the BDL rats kept in constant darkness. We postulate that this reduction may represent a compensatory mechanism in the pineal gland responding to chronically elevated melatonin levels observed in BDL compared with normal control rats. Several studies have shown that daytime serum levels of melatonin are elevated in patients with liver cirrhosis, which is postulated to be due to decreased liver blood flow, lowered activity of 6β-hydroxylase, and competition with bilirubin in the intrahepatic transport system (13, 30, 52). A study has also shown that not only do patients with cirrhosis have decreased melatonin clearance, they also have decreased daily production of melatonin (33), which is consistent with our finding of decreased melatonin levels in the pineal glands of BDL rats kept in constant darkness. Further experiments are necessary to determine the contribution of the gastrointestinal tract including the biliary epithelium (12, 44) in melatonin synthesis in response to dark exposure and the possible presence of melatonin in bile.
Since melatonin has been shown to ameliorate cholestatic-induced liver damage and systemic oxidative stress (21, 55), we next demonstrated the inhibitory effect of prolonged dark exposure (a noninvasive approach) on BDL-induced increase in biliary hyperplasia, serum chemistry, and secretin-stimulated ductal secretory activity (a functional marker of biliary proliferation) (2, 3, 5, 6). In addition to the activation of biliary hyperplasia, the BDL model is also characterized by increased biliary fibrosis, which is associated with increased collagen deposition, α-SMA, and fibronectin expression in portal areas (56). It has been shown that besides hepatic stellate cells and fibroblasts, cholangiocytes are activated, express α-SMA, and synthesize matrix (61). These findings are consistent with other studies that demonstrate that melatonin ameliorates hepatic fibrosis in a number of rodent models of fibrosis (28, 54, 55). For example, melatonin has been shown to inhibit the expression and activity of MMP-2 and MMP-9 in both in vitro and in vivo models (32, 39, 41). A recent study has shown that melatonin inhibits MMP-9 by binding to its active site, which represents another possible mechanisms for the alteration in fibrosis during constant darkness (48). Melatonin has been show to diminish the secretion of TGF-β from hepatic stellate cells (25). The beneficial effects of prolonged dark exposure on biliary hyperplasia, serum chemistry, and fibrosis are likely mediated by melatonin (as confirmed by the in vitro studies) and enhanced melatonin synthesis (45). However, our study does not rule out the possibility that other factors such as follicle-stimulating hormone (37) may be released by dark exposure that could modulate biliary function (20, 36).
Since we have previously shown (45) that melatonin inhibits biliary hyperplasia in cholestatic BDL rats through downregulation of selected core clock genes, we aimed to demonstrate that reduction of biliary injury/fibrosis by complete dark exposure is associated with reduced expression of specific clock genes in the biliary epithelium. Our present study shows decreased expression of Per1, BMAL1, CLOCK, and Cry1 and supports the concept that clock genes play a key role in the modulation of liver injury (15–17). For example, the expression of fibrosis-related genes such as TGF-β, COLA1, and TIMP1 is elevated in cholestatic Per2 knockout mice, suggesting that the clock gene Per2 plays a protective role in during liver injury (16). Also, the loss of the clock gene, mPer2, has been shown to promote liver fibrosis induced by carbon tetrachloride (15). Furthermore, altered circadian rhythm of the clock gene, Cry2, has been demonstrated in fibrotic livers (17). Further studies are necessary to evaluate the circadian rhythm of Per1, BMAL1, and Cry1 expression (the clock genes decreased by dark exposure) during the autocrine/paracrine modulation of biliary disorders by melatonin.
We performed all the studies at 1 wk of BDL because we aimed to evaluate the proliferation and fibrosis in early stages of cholestasis. Thus further studies are needed to better understand the effect of continuous dark exposure on biliary injury and liver fibrosis during chronic cholestasis. The fact that epidemiological studies indicate that night shifts or longer light exposure is a risk factor for breast cancer (53) supports the clinical significance of dark therapy in human liver diseases. Supporting the clinical relevance of our studies, sleep-wake abnormalities have been observed in patients with cirrhosis (40). However, rodents are nocturnal animals, which is different from humans. Thus further studies (e.g., correlation between melatonin serum levels and liver functions in blind patients and patients under different dark-light cycles) are necessary to verify the effect of dark therapy in patients with chronic cholestatic liver diseases. Also, epidemiological studies are warranted to determine a correlation between circulating melatonin levels and liver functions in patients living in countries exposed to different cycles of dark/sunlight.
In summary, compared with our previous findings showing that increased biliary melatonin synthesis (by hepatic modulation of AANAT) inhibits biliary hyperplasia in an autocrine pathway (45), we now provide novel evidence that continuous dark exposure increases melatonin serum levels (through enhanced melatonin synthesis from the pineal gland) that, in turn, decrease biliary hyperplasia and liver fibrosis via a paracrine-regulated pathway. Modulation of melatonin levels through exposure to constant darkness may represent a novel and noninvasive approach for the environmental management of cholestatic liver diseases.
GRANTS
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White Hospital, a VA Research Career Scientist Award, a VA Merit award to G. Alpini, the NIH grants DK58411 and DK07698 to G. Alpini and S. Glaser, a CDA-2 VA Award to H. Francis (IK2 BX001760), a VA Merit Award to F. Meng, an NIH R01 award (DK082435) to S. DeMorrow, by Research Project funds from University of Rome “Sapienza,” and FIRB Accordi di Programma 2010#RBAP10Z7FS to E. Gaudio, and a VA CDA-2 Award and NIH grant DK081442 to S. Glaser.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
AUTHOR CONTRIBUTIONS
Y.H., F.M., S.D., H.F., D.R., E.G., S.G., and G.A. conception and design of research; Y.H., P.O., J.V., A.F., D.R., L.K., J.F.G., A.R., and R.M. performed experiments; Y.H., P.O., J.V., A.F., D.R., L.K., A.R., R.M., E.G., S.G., and G.A. analyzed data; Y.H., P.O., F.M., H.F., J.F.G., S.G., and G.A. interpreted results of experiments; Y.H. prepared figures; Y.H. and S.G. drafted manuscript; Y.H., P.O., F.M., S.D., J.V., H.F., A.F., D.R., L.K., J.F.G., A.R., R.M., E.G., S.G., and G.A. edited and revised manuscript; S.G. and G.A. approved final version of manuscript.
REFERENCES
- 1.Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage GD. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol 272: G1064–G1074, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Alpini G, Glaser S, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 274: G767–G775, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 81: 569–578, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 110: 1636–1643, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, LeSage G, Miller LJ, LaRusso NF. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 272: G289–G297, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Alpini G, Ulrich CD, 2nd, Phillips JO, Pham LD, Miller LJ, LaRusso NF. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 266: G922–G928, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 132: 415–431, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 115: 209–218, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blask DE, Dauchy RT, Brainard GC, Hanifin JP. Circadian stage-dependent inhibition of human breast cancer metabolism and growth by the nocturnal melatonin signal: consequences of its disruption by light at night in rats and women. Integr Cancer Ther 8: 347–353, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine 27: 179–188, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Blask DE, Dauchy RT, Sauer LA, Krause JA, Brainard GC. Light during darkness, melatonin suppression and cancer progression. Neuro Endocrinol Lett 23, Suppl 2: 52–56, 2002. [PubMed] [Google Scholar]
- 12.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 47: 2336–2348, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Celinski K, Konturek PC, Slomka M, Cichoz-Lach H, Gonciarz M, Bielanski W, Reiter RJ, Konturek SJ. Altered basal and postprandial plasma melatonin, gastrin, ghrelin, leptin and insulin in patients with liver cirrhosis and portal hypertension without and with oral administration of melatonin or tryptophan. J Pineal Res 46: 408–414, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Cevik H, Erkanli G, Ercan F, Isman CA, Yegen BC. Exposure to continuous darkness ameliorates gastric and colonic inflammation in the rat: both receptor and nonreceptor-mediated processes. J Gastroenterol Hepatol 20: 294–303, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Han Z, Yang P, Zhu L, Hua Z, Zhang J. Loss of clock gene mPer2 promotes liver fibrosis induced by carbon tetrachloride. Hepatol Res 40: 1117–1127, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Kakan X, Wang S, Dong W, Jia A, Cai C, Zhang J. Deletion of clock gene Per2 exacerbates cholestatic liver injury and fibrosis in mice. Exp Toxicol Pathol 65: 427–432, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Chen P, Kakan X, Zhang J. Altered circadian rhythm of the clock genes in fibrotic livers induced by carbon tetrachloride. FEBS Lett 584: 1597–1601, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Cong M, Liu T, Wang P, Fan X, Yang A, Bai Y, Peng Z, Wu P, Tong X, Chen J, Li H, Cong R, Tang S, Wang B, Jia J, You H. Antifibrotic effects of a recombinant adeno-associated virus carrying small interfering RNA targeting TIMP-1 in rat liver fibrosis. Am J Pathol 182: 1607–1616, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Cruz A, Padillo FJ, Torres E, Navarrete CM, Munoz-Castaneda JR, Caballero FJ, Briceno J, Marchal T, Tunez I, Montilla P, Pera C, Muntane J. Melatonin prevents experimental liver cirrhosis induced by thioacetamide in rats. J Pineal Res 39: 143–150, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Damian E, Ianas O, Badescu I, Oprescu M. Inhibitory action of pineal extract on LH and FSH. Endocrinologie 16: 257–262, 1978. [PubMed] [Google Scholar]
- 21.Esrefoglu M, Gul M, Emre MH, Polat A, Selimoglu MA. Protective effect of low dose of melatonin against cholestatic oxidative stress after common bile duct ligation in rats. World J Gastroenterol 11: 1951–1956, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis H, Glaser S, DeMorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol 295: C499–C513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgiev P, Jochum W, Heinrich S, Jang JH, Nocito A, Dahm F, Clavien PA. Characterization of time-related changes after experimental bile duct ligation. Br J Surg 95: 646–656, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Grappone C, Pinzani M, Parola M, Pellegrini G, Caligiuri A, DeFranco R, Marra F, Herbst H, Alpini G, Milani S. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol 31: 100–109, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Gu J, Zhuang L, Huang GC. Melatonin prevents H2O2-induced activation of rat hepatic stellate cells. J Pineal Res 41: 275–278, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Han Y, Demorrow S, Invernizzi P, Jing Q, Glaser S, Renzi A, Meng F, Venter J, Bernuzzi F, White M, Francis H, Lleo A, Marzioni M, Onori P, Alvaro D, Torzilli G, Gaudio E, Alpini G. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol 301: G623–G633, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardeland R. Melatonin, hormone of darkness and more: occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci 65: 2001–2018, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong RT, Xu JM, Mei Q. Melatonin ameliorates experimental hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol 15: 1452–1458, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu S, Yin S, Jiang X, Huang D, Shen G. Melatonin protects against alcoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis. Eur J Pharmacol 616: 287–292, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Iguchi H, Kato KI, Ibayashi H. Melatonin serum levels and metabolic clearance rate in patients with liver cirrhosis. J Clin Endocrinol Metab 54: 1025–1027, 1982. [DOI] [PubMed] [Google Scholar]
- 31.Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology 97: 1236–1247, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Kim SJ, Lee SR. Protective effect of melatonin against transient global cerebral ischemia-induced neuronal cell damage via inhibition of matrix metalloproteinase-9. Life Sci 94: 8–16, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Lane EA, Moss HB. Pharmacokinetics of melatonin in man: first pass hepatic metabolism. J Clin Endocrinol Metab 61: 1214–1216, 1985. [DOI] [PubMed] [Google Scholar]
- 34.LeSage G, Glaser S, Gubba S, Robertson WE, Phinizy JL, Lasater J, Rodgers RE, Alpini G. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology 111: 1633–1644, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Lewczuk B, Przybylska-Gornowicz B. The effect of continuous darkness and illumination on the function and the morphology of the pineal gland in the domestic pig. Part I: the effect on plasma melatonin level. Neuro Endocrinol Lett 21: 283–291, 2000. [PubMed] [Google Scholar]
- 36.Maldonado MD, Mora-Santos M, Naji L, Carrascosa-Salmoral MP, Naranjo MC, Calvo JR. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol Res 62: 282–270, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, Glaser S, Carpino G, Venter J, Alvaro D, Kopriva S, White M, Kossie A, Savage J, Alpini G. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol 297: G11–G26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, Taffetani S, Ueno Y, Roskams T, Phinizy JL, Venter J, Fava G, Lesage GD, Alpini G. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 128: 121–137, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Mishra A, Paul S, Swarnakar S. Downregulation of matrix metalloproteinase-9 by melatonin during prevention of alcohol-induced liver injury in mice. Biochimie 93: 854–866, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Montagnese S, De Pitta C, De Rui M, Corrias M, Turco M, Merkel C, Amodio P, Costa R, Skene DJ, Gatta A. Sleep-wake abnormalities in patients with cirrhosis. Hepatology 59: 705–712, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Paul S, Sharma AV, Mahapatra PD, Bhattacharya P, Reiter RJ, Swarnakar S. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J Pineal Res 44: 439–449, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Ralph CL, Pelham RW, MacBride SE, Reilly DP. Persistent rhythms of pineal and serum melatonin in cockerels in continuous darkness. J Endocrinol 63: 319–324, 1974. [DOI] [PubMed] [Google Scholar]
- 43.Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev 12: 151–180, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Renzi A, DeMorrow S, Onori P, Carpino G, Mancinelli R, Meng F, Venter J, White M, Franchitto A, Francis H, Han Y, Ueno Y, Dusio G, Jensen KJ, Greene JJ, Jr., Glaser S, Gaudio E, Alpini G. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology 57: 1130–1141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renzi A, Glaser S, DeMorrow S, Mancinelli R, Meng F, Franchitto A, Venter J, White M, Francis H, Han Y, Alvaro D, Gaudio E, Carpino G, Ueno Y, Onori P, Alpini G. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol 301: G634–G643, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosa DP, Bona S, Simonetto D, Zettler C, Marroni CA, Marroni NP. Melatonin protects the liver and erythrocytes against oxidative stress in cirrhotic rats. Arq Gastroenterol 47: 72–78, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg P, Sjostrom M, Soderberg C, Kinnman N, Stal P, Hultcrantz R. Attenuated liver fibrosis after bile duct ligation and defective hepatic stellate cell activation in neural cell adhesion molecule knockout mice. Liver Int 31: 630–641, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Rudra DS, Pal U, Maiti NC, Reiter RJ, Swarnakar S. Melatonin inhibits matrix metalloproteinase-9 activity by binding to its active site. J Pineal Res 54: 398–405, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Rutenburg AM, Kim H, Fischbein JW, Hanker JS, Wasserkrug HL, Seligman AM. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem 17: 517–526, 1969. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt J, Roderfeld M, Sabrane K, Zhang P, Tian Y, Mertens JC, Frei P, Stieger B, Weber A, Mullhaupt B, Roeb E, Geier A. Complement factor C5 deficiency significantly delays the progression of biliary fibrosis in bile duct-ligated mice. Biochem Biophys Res Commun 418: 445–450, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Schomerus C, Korf HW. Mechanisms regulating melatonin synthesis in the mammalian pineal organ. Ann NY Acad Sci 1057: 372–383, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med 123: 274–277, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology 16: 254–258, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Tahan G, Akin H, Aydogan F, Ramadan SS, Yapicier O, Tarcin O, Uzun H, Tahan V, Zengin K. Melatonin ameliorates liver fibrosis induced by bile-duct ligation in rats. Can J Surg 53: 313–318, 2010. [PMC free article] [PubMed] [Google Scholar]
- 55.Tahan V, Ozaras R, Canbakan B, Uzun H, Aydin S, Yildirim B, Aytekin H, Ozbay G, Mert A, Senturk H. Melatonin reduces dimethylnitrosamine-induced liver fibrosis in rats. J Pineal Res 37: 78–84, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest 74: 265–278, 1996. [PubMed] [Google Scholar]
- 57.Uriarte I, Banales JM, Saez E, Arenas F, Oude Elferink RP, Prieto J, Medina JF. Bicarbonate secretion of mouse cholangiocytes involves Na+-HCO3− cotransport in addition to Na+-independent Cl−/HCO3− exchange. Hepatology 51: 891–902, 2010. [DOI] [PubMed] [Google Scholar]
- 58.Velissaris D, Karanikolas M, Kalogeropoulos A, Solomou E, Polychronopoulos P, Thomopoulos K, Labropoulou-Karatza C. Pituitary hormone circadian rhythm alterations in cirrhosis patients with subclinical hepatic encephalopathy. World J Gastroenterol 14: 4190–4195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang GQ, Du YZ, Tong J. Daily oscillation and photoresponses of clock gene, Clock, and clock-associated gene, arylalkylamine N-acetyltransferase gene transcriptions in the rat pineal gland. Chronobiol Int 24: 9–20, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Wideman CH, Murphy HM. Constant light induces alterations in melatonin levels, food intake, feed efficiency, visceral adiposity, and circadian rhythms in rats. Nutr Neurosci 12: 233–240, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol 168: 1500–1512, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep 61: 383–410, 2009. [DOI] [PubMed] [Google Scholar]