Abstract
Food intake depends on a tightly controlled interplay of appetite hormones and the enteric (ENS) and central nervous system. Corticosterone (CORT) levels, which are mainly studied with regard to stress, are also increased during fasting. However, the role of CORT in the ENS remains elusive. Therefore, we investigated whether CORT modulates activity of enteric neurons and whether its intracellular regulator, 11β-hydroxysteroid dehydrogenase (HSD) type 1, is present in the myenteric plexus, using immunohistochemistry and RT-qPCR. Effects of CORT on neuronal activity and expression of neuronal markers in the myenteric plexus were assessed via Ca2+ imaging and RT-qPCR, respectively, whereas modulations in mixing behavior were measured by video imaging. 11β-HSD-1 was present in enteric neurons along the gastrointestinal tract, and its expression increased after fasting (control: 0.58 ± 0.09 vs. fasted: 1.5 ± 0.23; P < 0.05). CORT incubation significantly reduced neuronal Ca2+ transients in tissues stimulated by electrical pulses (control: 1.31 ± 0.01 vs. CORT: 1.27 ± 0.01, P < 0.01) and in cultured neurons (control: 1.85 ± 0.03 vs. CORT: 1.76 ± 0.03, P < 0.05). CORT decreased small intestinal mixing (P < 0.05). Incubation of muscle myenteric plexus preparations with CORT induced an increase in cannabinoid receptor 1 (CB1, P < 0.05) and synaptobrevin (P < 0.05) but not in 11β-HSD-1 mRNA expression. In addition, fasting induced significant elevations in synaptobrevin (P < 0.05) and CB1 (P < 0.01) mRNA expression. In conclusion, we suggest CORT to be a downstream factor in a feeding state-related pathway that modulates important proteins in the fine tuning of enteric neurotransmission and gastrointestinal motility.
Keywords: appetite, neuromodulation
fasting and feeding cycles were shown to influence the activity of neurons in the enteric nervous system (ENS), a highly specialized network embedded in the intestinal wall along the entire length of the gastrointestinal tract (32). These nerves coordinate different gastrointestinal functions, including digestion of food, propulsion of gut content, and absorption of nutrients. The ENS circuits controlling intestinal function are built up by intrinsic primary afferent neurons, interneurons, and motor neurons, which form ganglionated networks located in the submucous and myenteric nerve plexus. These neurons are activated by the presence of luminal contents and modulated by hormone levels that wax and wane depending on the feeding status (15). Satiety is induced by the rise of cholecystokinin, pancreatic polypeptide, and peptide YY, whereas hunger is associated with an increase in ghrelin (27).
In addition to these appetite hormones and their well-studied food intake-related effects in ENS and the central nervous system (CNS), corticosterone (CORT), one of the stress hormones, has also been shown to be involved in the regulation of appetite. Stress has been reported to affect food intake in a bidirectional way in humans, leading to increased eating in 70% and weight loss in 30% of individuals via a complex interplay with various appetite hormones (e.g., leptin, insulin, and neuropeptide Y) (1). These observations clearly demonstrate that stress is able to modulate hunger; however, interestingly, hunger itself has been shown to induce alterations in stress hormone (CORT) levels (25). Although it is clear that CORT levels rise during hunger to provide energy to fuel hepatic gluconeogenesis by mobilizing substrate, its role in the ENS during hunger remains unknown (9).
CORT has, upon crossing the blood-brain barrier, profound effects in the CNS as it binds to cytoplasmic or membrane-bound mineralocorticoid receptors (type I) or glucocorticoid (type II) receptors, which after translocation in the nucleus function as transcription factors (30). Derived from CNS studies, CORT has been recognized to target enzymes involved in neurotransmitter synthesis and ion channel and receptor expression (20). Plasma CORT levels depend on production in the adrenal cortex in response to the activation of the hypothalamic-pituitary-adrenal (HPA) axis by stressors. Activity of this axis is controlled by a tightly regulated feedback mechanism that is modulated by the endocannabinoid system. Endocannabinoids, via cannabinoid receptor 1 (CB1), mediate CORT-induced fast feedback inhibition of the HPA axis at the paraventricular nucleus (PVN) of the hypothalamus (14). Next to plasma CORT concentrations, intracellular CORT levels are regulated by 11β-hydroxysteroid dehydrogenase (HSD) type 1, an enzyme that is abundantly present in liver, adipose tissue, and the CNS (10). 11β-HSD-1 is a bidirectional enzyme that predominantly acts as a reductase to generate active CORT intracellularly from inert 11-dehydrocorticosterone. In the CNS, 11β-HSD-1 has been suggested to play an important role in HPA axis feedback regulation by modulating CORT levels (20). Moreover, the high expression of 11β-HSD-1 in hippocampus and cerebellum suggests a role for this enzyme, together with CORT, in behavior, cognition, neuronal development, and structure (18).
We hypothesize that CORT is an important neuromodulator of appetite-related fine tuning of ENS activity. Therefore, in this study, we investigate the role of elevated CORT levels during fasting in the ENS of mouse small intestine using Ca2+-imaging and intestinal motility studies. We start by assessing plasma CORT levels and 11β-HSD-1 expression in the ileum after fasting, and, considering the negative feedback role of endocannabinoids, we also measure the local effect of CORT on CB1 expression in the ileum. Interestingly, Burdyga et al. (7) have shown that CB1 expression in vagal afferent neurons increased after 24 h of fasting, demonstrating the involvement of endocannabinoids in the control of appetite. Our data expand the understanding of the effects fasting and feeding have on the physiology of the ENS, which can be of therapeutic importance in view of food intake-related diseases such as obesity, bulimia, and anorexia nervosa.
METHODS
Animals
Male C57BL/6N mice (8–9 wk) were housed in the animal facility of the KU Leuven and randomly assigned to three groups each subjected to different food access conditions (control, fasted, and refed). Control mice were fed regular chow ad libitum, fasted mice were fasted for 20 h, and refed mice were fasted for 20 h and subsequently refed for 3 h.
Immunostaining
After the mice were killed by cervical dislocation (a euthanasia procedure approved by the Ethical Animal Committee of the KU Leuven, Belgium), the stomach and intestines were removed, cut along the mesentery, and pinned open with mucosa facing up. Tissues were fixed in 4% paraformaldehyde (Merck, Darmstadt, Germany) containing Krebs solution for 30 min on ice. Subsequently, after being washed with PBS (1.54 mM KH2PO4, 155.17 mM NaCl, and 2.71 mM Na2HPO4, pH 7.4), the mucosal and circular muscle layers were removed by dissection. Next, longitudinal muscle myenteric plexus (LMMP) preparations were permeabilized in PBS with 0.5% Triton X-100 containing 4% donkey serum to block aspecific binding sites. Primary antibodies (Table 1) were diluted in donkey serum and incubated for 24 h at 4°C. After being washed with PBS (3 × 10 min), the cells were incubated for 2 h at 4°C with the appropriate secondary antibodies (Table 1). Immunoreactivity was visualized using a BX-41 fluorescence microscope (Olympus, Aartselaar, Belgium) with a metal halide light source and specific filter cubes (excitation/dichroic mirror/emission in nm) for blue (325–375/400/435–485), green (460–495/505/510–550), and red (570–590/595/600–660) fluorescent probes. Confocal images were recorded on a Zeiss LSM510 (Meta) confocal/multiphoton microscope (Cell Imaging Core). Six consecutive images (0.5 μm apart) were used to make maximum projections as shown in Figs. 1 and 2.
Table 1.
Primary and secondary antibodies used in immunohistochemistry experiments
| Primary | Host | Dilution | Company | Secondary | Company |
|---|---|---|---|---|---|
| HuC/D | Mouse | 1:1,000 | Molecular Probes, Invitrogen | Alexa Fluor 488 | Molecular Probes, Invitrogen |
| 11β-HSD-1 | Sheep | 1:1,000 | Gift S. Webster (11) | Alexa Fluor 594 | Molecular Probes, Invitrogen |
| nNOS | Rabbit | 1:400 | Santa Cruz Biotechnologies | AMCA | Jackson Immuno Labs |
| Calretinin | Rabbit | 1:2,000 | Chemicon International | AMCA | Jackson Immuno Labs |
| Neurofilament M | Rabbit | 1:1,000 | Millipore | Alexa Fluor 488 | Molecular Probes, Invitrogen |
11β-HSD-1, 11β-hydroxysteroid dehydrogenase type 1.
Fig. 1.
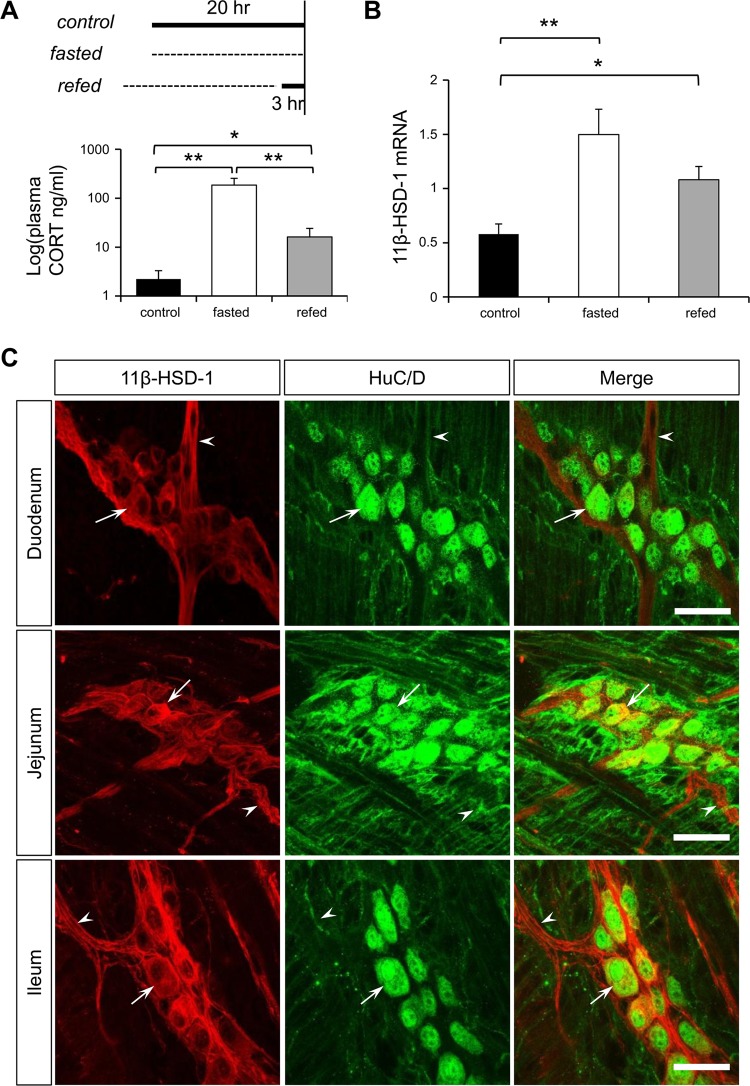
A: experimental setup of different food intake conditions [control (ad libitum), fasted (20 h), refed (for 3 h)] and the resulting CORT plasma levels obtained by an enzyme immune assay (n = 6). B: 11β-hydroxysteroid dehydrogenase (11β-HSD-1) mRNA expression in muscle myenteric plexus preparations of ileum in the control condition and after fasting and refeeding (n = 10). P < 0.05 (*) and 0.01 (**). C: immunohistochemical staining for 11β-HSD-1 (red) and HuC/D (green) in mouse myenteric plexus of duodenum, jejunum, and ileum. Arrows and arrowheads indicate a typical example of an 11β-HSD-1-positive cell body and fiber, respectively. Scale bar: 50 μm.
Fig. 2.
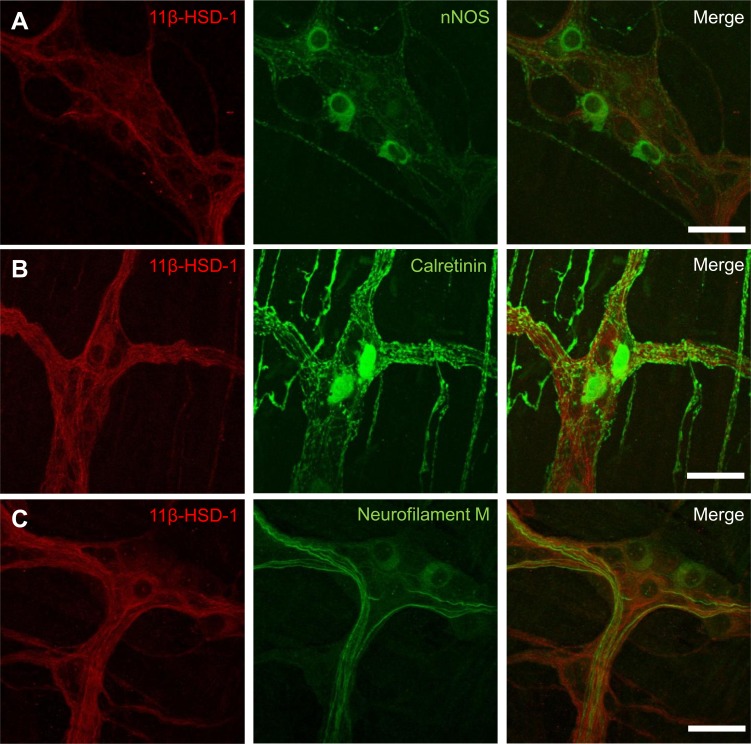
Immunohistochemical staining for 11β-HSD-1 (red) in myenteric plexus of mouse ileum in combination with nNOS (green, A), calretinin (green, B), and neurofilament M (green, C). Scale bar: 50 μm.
Plasma CORT Measurements
Animals (n = 6 animals/group) were rapidly decapitated (a euthanasia procedure approved by the Ethical Animal Committee of the KU Leuven, Belgium) at 10:00 A.M., and trunk blood was collected. Subsequently, EDTA (10%) and aprotinin (5%) were added after which the samples were spun for 10 min at 2,000 g. Supernatant plasma was rapidly frozen using liquid nitrogen and stored at −20°C until used. CORT enzyme immunoassay was performed according to the manufacturer's protocol (Cayman Chemical). Briefly, the amount of CORT in 50 μl of plasma, diluted 1:100 and 1:500, was quantified after binding competition with CORT-acetylcholinesterase (AChE) conjugate (Tracer) in a mouse monoclonal anti-rabbit IgG-coated 96-well plate. The absorbance of 5-thio-2-nitrobenzoic acid, the end product of the AChE reaction, was read at a wavelength between 405 and 420 nm, and results were calculated using a standard curve.
Quantitative Real-Time PCR
RNA samples of mouse small intestinal tissue were prepared using a QIAGEN RNeasy Mini Kit (QIAGEN, Hilden, Germany) and reverse transcribed to cDNA with Superscript II Reverse Transcriptase. The obtained cDNA served as a template for the PCR reaction, which was run on a LightCycler 480 system (Roche Diagnostics, Vilvoorde, Belgium) using LightCycler 480 SYBR Green I Master mix. Differences in 11β-HSD-1 (forward: CTCCAGAAGGTAGTGTCTCGC, reverse: CCTTGACAATAAATTGCTCCGCA), synaptobrevin (forward: GGAGTGATCTGCGCCATCAT, reverse: GGGCAGACTCCTCAGGGATT), CB1 (forward: CTGGTTCTGATCCTGGTGGT, reverse: TGTCTCAGGTCCTTGCTCCT), and mRNA expression in muscle myenteric plexus preparations were measured using specific primer sequences. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward: CCCCAATGTGTCCGTCGTG, reverse: GCCTGCTTCACCACCTTCT) was used as housekeeping gene, and for each gene a standard curve was created to obtain PCR efficiencies. Relative expression levels of all samples were calculated with the LightCycler 480 software and were expressed relative to GAPDH and corrected for interrun variability.
Tissue Incubation
A piece of ileum was pinned out in a Sylgard-lined dish, mucosa facing up. The mucosal layers were completely removed, and circular muscle strips were peeled off as much as possible. Next, tissues were washed three times with sterile Krebs solution (in mM: 120.9 NaCl, 5.9 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 14.4 NaHCO3, 11.5 glucose, and 2.5 CaCl2) and incubated with CORT (100 nM, 20 h) (Sigma-Aldrich, St. Louis, MO) or DMSO (0.01%) dissolved in medium [DMEM F-12 (1:1) enriched with 1% glutamine, 0.5% penicillin, and 0.5% streptomycin (Lonza Group, Basel, Switzerland)], gently shaken at 37°C, and continuously gassed with 5% CO2. After incubation, RNA was extracted as described before.
Primary Myenteric Mouse Cultures
Mice were killed by cervical dislocation, and the ileum was collected in sterile Krebs solution at room temperature (RT), cooled on ice for 10 min. The tissue was cut into 1-cm pieces and put over a sterile pipette after which an incision was made along the mesentery to facilitate stripping off the outer layers, containing the myenteric plexus. Collected tissues were preserved in Krebs solution on ice (4°C). Subsequently, tissues were washed in Krebs and Hanks' balanced salt solution (in mM: 126.6 NaCl, 5.36 KCl, 1.2 NaH2PO4, 14.4 NaHCO3, 0.44 KH2PO4, 10 glucose, 2.9 sucrose, and 11 HEPES). Next, the isolated LMMP preparations were digested in a collagenase (14.67 mg/ml)/protease (10 mg/ml)/albumin (5% in PBS) (GIBCO Invitrogen, Merelbeke, Belgium) solution for 8 min at 37°C. The enzymatic digestion was stopped by adding a Krebs solution with 10% fetal bovine serum (FBS) at RT. Next, the suspension was centrifuged three times for 5 min (500 g, RT) after which the supernatant was subsequently discarded. At the end of the third washing step, the pellet was resuspended in “complete” medium [DMEM F-12 (1:1) enriched with 10% FBS, 1% glutamine, 0.5% penicillin, and 0.5% streptomycin (Lonza Group)]. The cells were inoculated on round glass cover slips coated with poly-d-lysine hydrobromide (0.5 mg/ml in borate buffer) and laminin (20 μg/ml in PBS) (both from Sigma-Aldrich). The cultures were allowed to grow for 24 h incubated at 37°C, continuously gassed with 5% CO2. Next, the complete medium was replaced by a serum-free medium to which nerve growth factor (0.05%; Alomone Laboratories, Jerusalem, Israel) and neuron (0.2%)- and glia (0.2%)-specific (GIBCO Invitrogen) supplements were added (16).
Ca2+ Imaging
Preparation.
The Ca2+-imaging experiments were performed on primary myenteric mouse cultures and mouse small intestine LMMP preparations (ileum). LMMP preparations were stretched over a small inox ring and immobilized by a matched rubber O ring (35). Subsequently, mouse cultures and LMMP preparations were incubated with CORT or vehicle (DMSO).
Fluo 4 loading.
Primary myenteric mouse cultures/LMMP preparations were loaded with 5/1 μM fluo 4 acetoxymethyl (20 min) and washed in HEPES/Krebs solution (10 min, RT). Cultures were first transferred to a cover glass chamber before mounting on the microscope stage. Changes in intracellular Ca2+ concentrations were reflected in fluo 4 fluorescence intensity and recorded at 525/50 nm. Cultures/LMMP preparations were continuously perfused with HEPES/Krebs, and neurons were identified by depolarization (75 mM K+, RT) for 5 s. After a recovery period of 3 min, cultures were either acutely exposed to CORT or stimulated in an electrical field (×Hz).
Imaging setup.
Neuronal activity was monitored using an inverted Zeiss Axiovert 200 M microscope (Carl Zeiss, Oberkochen, Germany) with TILL Poly V light source (TILL Photonics, Gräfelfing, Germany), cooled CCD camera (PCO Sensicam-QE, Kelheim, Germany), and TILL-VisION (TILL Photonics) software.
Analysis.
All image analysis was performed with custom-written routines in Igor Pro (Wavemetrics, Lake Oswego, OR). Regions of interest were drawn, after which the average Ca2+ signal intensity was calculated, normalized to the initial fluo 4 values, and reported as F/F0. The Ca2+-response data (amplitude and peak duration) were compared between control and CORT groups.
Video Imaging of Mixing Behavior
Segments of ileum were suspended in an organ bath filled with Krebs solution kept at 37°C and continuously bubbled with 95% O2-5% CO2 (pH 7.4). Tetrodotoxin (TTX) (1 μM, 10 min), nifedipine (1 μM, 10 min), CORT (1 µM, 20 min), or vehicle containing Krebs solution (DMSO, 20 min) was used to investigate contractile behavior. After baseline was recorded (0 cm pH2O), the intraluminal pressure was subsequently elevated to 1, 2, and 4 cm pH2O at the oral side and filmed for 90 s (1 min interval between recordings). All movies (90 s, 10 Hz frame rate) were recorded with and sampled on a Sensoray 611 PCI Frame Grabber. Images were read into Igor Pro (Wavemetrics) and analyzed using custom-written algorithms based on Hennig et al. (19) to assess intestinal swelling and high-frequency mixing behavior (32).
Statistics
Unless stated otherwise, all data are presented as means ± SE. Distributions were tested for normality with a Kolmogorov Smirnov test. Depending on the distribution, data from different conditions were compared using parametric one-way ANOVA, nonparametric Mann Whitney U-test, or Kruskal Wallis ANOVA. Differences were considered significant if P < 0.05. Statistical analysis was performed with Statistica (StatSoft).
RESULTS
Sources of Fasting-Induced CORT Elevation
To address the effects elevated CORT levels as during fasting have on the ENS, we first investigated what possible sources of CORT were involved. The increased plasma CORT levels, as measured by a CORT enzyme immune assay, indicate the involvement of HPA axis activation during fasting (control: 6.4 ± 3.1 nM, fasted: 530 ± 200 nM, P < 0.01). This effect was partially restored after a 3-h refeeding period (refed: 47 ± 23 nM, n = 6) compared with the fasted (P < 0.01) and the control (P < 0.05) condition (Fig. 1A). Apart from an increase in circulating CORT levels, fasting also induced a significant increase in 11β-HSD-1 mRNA expression in muscle myenteric plexus preparations (control: 0.58 ± 0.09 vs. fasted: 1.5 ± 0.23, P < 0.01) that remained significantly elevated even after refeeding (refed: 1.08 ± 0.12, P < 0.05) (Fig. 1B). At present no information is available about the potential expression of 11β-HSD-1 in the ENS. Using specific antibodies against 11β-HSD-1 and the pan neuronal marker HuC/D we found that 11β-HSD-1 is expressed in myenteric neurons along the gastrointestinal tract from stomach to colon (Fig. 1C shows expression in small intestine). However, immunohistochemical stainings did not show significant differences in the number of 11β-HSD-1-positive myenteric neurons in the ileum during fasting and refeeding cycles (control: 36.91 ± 2.46% vs. fasted: 31.18 ± 3.63% vs. refed: 37.79 ± 2.64, n = 4). In addition, to identify whether 11β-HSD-1 expression was selective to specific subsets of neurons, we used three other neuronal markers (nNOS, calretinin, and neurofilament M). We found that 11β-HSD-1-positive cell bodies did not colocalize with nNOS and calretinin expression and that 11β-HSD-1-expressing fibers were mainly neurofilament M negative (Fig. 2, A–C).
CORT Reduces Ca2+ Responses in Enteric Neurons
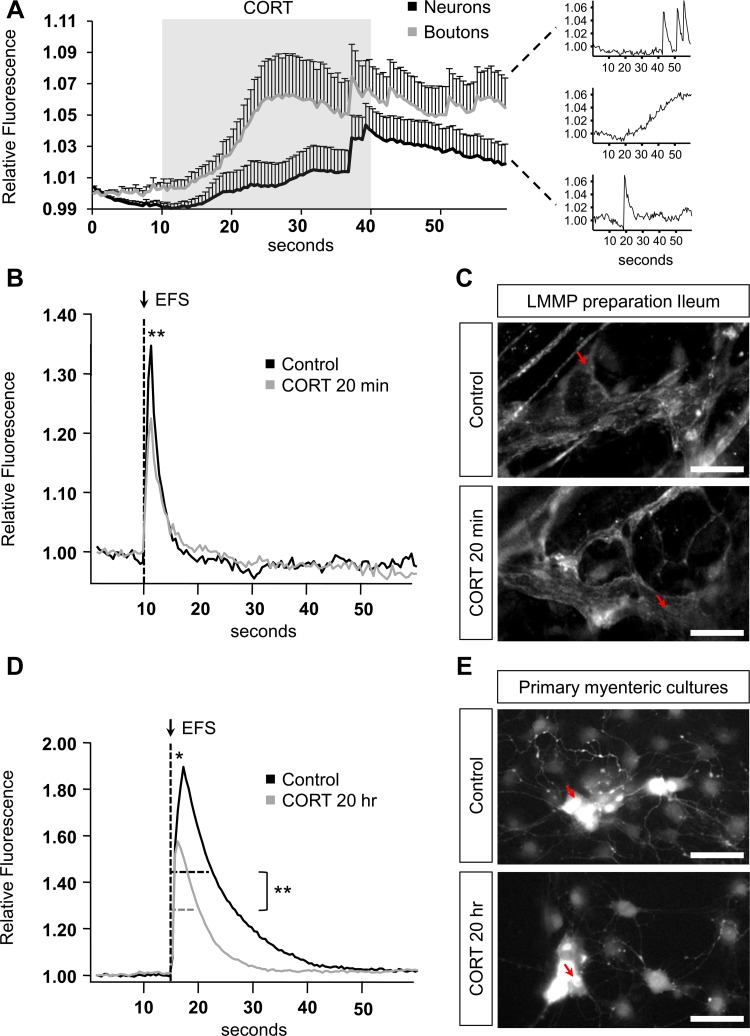
To investigate whether CORT was able to acutely affect enteric neuronal and synaptic signaling, we performed Ca2+ imaging in primary myenteric mouse cultures using a single application of CORT at a high concentration (10 µM). In both neurons and varicose release sites (boutons), CORT induced slowly rising Ca2+ responses that were quite variable in terms of amplitude and shape, as seen from large standard deviations of the average responses (Fig. 3A). These acute responses to CORT were confirmed in tissue experiments where neurons also showed CORT-induced slowly rising Ca2+ responses that were significantly higher than those induced by the vehicle (details not shown). Because these Ca2+ transients were not like any of the responses elicited by neuronal depolarization (high K+) or neurotransmitter activation, we sought to investigate whether this slow rise could reflect long-term changes in enteric neurons (6, 34). Therefore, we recorded Ca2+ signaling in LMMP preparations that were exposed to CORT (20 min), at a concentration mimicking fasting plasma CORT levels (100 nM, n = 4). Electrical stimulation in CORT-incubated tissues induced significantly lower neuronal Ca2+ amplitudes compared with vehicle-treated control tissues (control: 1.31 ± 0.01 vs. CORT: 1.27 ± 0.01, P < 0.01) (Fig. 3, B and C). Because CORT levels, as seen by others in rats, already start rising after 12 h of fasting (12), longer-term effects of CORT were studied by incubating cultured myenteric neurons with the fasting CORT concentration (100 nM) for 20 h (n = 4). Resulting Ca2+ amplitudes confirm the inhibitory effect of CORT incubation on myenteric neurons compared with time-matched control cultures (control: 1.85 ± 0.03 vs. CORT: 1.76 ± 0.03, P < 0.05) (Fig. 3, D and E). In addition to the amplitude, 20 h of CORT incubation induced a significant decrease in Ca2+-response duration (at 50% of the maximum) after electrical stimulation of the neurons (control: 9.15 ± 0.19 s vs. CORT: 8.00 ± 0.14 s, P < 0.001) (Fig. 3, D and E).
Fig. 3.
Ca2+ imaging in myenteric neurons. A: acute Ca2+ responses induced in myenteric neurons and boutons by corticosterone (CORT, 10 µM) perfusion between 10 and 40 s as indicated by the gray box. Supplemental graphs (right) show three different types of CORT-induced responses. B: electrical stimulation-induced Ca2+ responses in control and CORT-incubated longitudinal muscle myenteric plexus (LMMP) preparations. C: snapshot of the control and CORT-incubated LMMP preparation containing the corresponding neuron from graph B indicated by a red arrow. Scale bar: 100 μm. D: electrical stimulation-induced Ca2+ responses in control and CORT-incubated myenteric cultures. E: snapshot of the control and CORT-incubated myenteric cultures containing the corresponding neuron from graph D indicated by a red arrow. Scale bar: 100 μm. P < 0.05 (*) and 0.01 (**).
CORT Modulates Small Intestinal Mixing Behavior
To study whether CORT has, in addition to its inhibitory effect on neuronal Ca2+ signaling, the ability to modulate small intestinal motility, we imaged the pressure-induced contractile patterns in the mouse ileum after exposure to CORT. Contrary to guinea pig small intestine, pressure stimuli do not elicit peristaltic waves in the mouse small intestine (23, 32). Therefore, two parameters (intestinal diameter and high-frequency contractions) were assessed (Fig. 4A). To validate these parameters, we made recordings either in the presence of the Na+-channel blocker TTX, to prevent neuronal conduction, or nifedipine, an L-type Ca2+-channel blocker. TTX significantly decreased the average diameter, whereas nifedipine caused dilation (Fig. 4C). The high-frequency mixing contractions were slightly decreased by TTX and abolished by nifedipine (Fig. 4, B and D). Incubation with CORT (20 min) did not have an effect on the diameter but induced a significant decrease in high-frequency contractions (P < 0.05) (Fig. 4).
Fig. 4.
Video imaging of pressure-induced contractile patterns in the mouse ileum. A: color-coded spatiotemporal map (left) of diameter variations and contraction patterns in the mouse small intestine. A median filtered map highlights the slower changes in diameter over time (middle) that, after subtraction of the original map, generate a map (right) containing the high-frequency mixing contractions. B: spatiotemporal map of the high-frequency mixing after incubation with vehicle, tetrodotoxin (TTX), nifedipine, and CORT (1 µM) at 2 cm pH2O pressure. C: change in intestinal diameter at 1, 2, and 4 cm pH2O in control and in the presence of TTX, nifedipine, and CORT (1 µM). D: change in high-frequency mixing at 1, 2, and 4 cm pH2O in control and in the presence of TTX, nifedipine, and CORT (1 µM). P < 0.05 (*) and 0.01 (**).
Modulation of Gene Expression in CORT-Incubated Ileum
To elucidate the molecular changes underlying the CORT-induced modulations in Ca2+ buffering and intestinal motility, we investigated mRNA levels of two specific synaptic communication-related proteins (synaptobrevin and the CB1 receptor) in an in vitro setting by incubating ileal muscle myenteric plexus preparations with CORT (100 nM, 20 h). CORT incubation induced significant increases in the mRNA levels of both synaptobrevin (control: 1.87 ± 0.14 vs. CORT: 2.68 ± 0.4, P < 0.05) and CB1 (control: 0.59 ± 0.06 vs. CORT: 0.83 ± 0.11, P < 0.05) (Fig. 5, A and B). The 11β-HSD-1 mRNA expression after CORT incubation was not significantly altered, suggesting that an integrated system is required to regulate levels of the local CORT-converting enzyme (control: 0.9 ± 0.18 vs. CORT: 1.35 ± 0.28). Next, to investigate if the specific feeding state-related changes in 11β-HSD-1 mRNA expression were associated with modulations in synaptobrevin and CB1 mRNA levels, we also measured mRNA levels after fasting and refeeding in muscle myenteric plexus preparations. Here again, synaptobrevin mRNA expression (control: 2.11 ± 0.13 vs. fasted: 3.02 ± 0.32 vs. refed: 2.24 ± 0.35, P < .05) and CB1 (control: 0.56 ± 0.06 vs. fasted: 1.37 ± 0.25 vs. refed: 0.85 ± 0.16, P < 0.01) mRNA expression were increased after fasting compared with the control mice, confirming the involvement of CORT in a feeding state-related pathway affecting the enteric nervous system (Fig. 5, C and D).
Fig. 5.
A and B: ileal muscle myenteric plexus preparations incubated with control (vehicle) or CORT (100 nM) for 20 h (n = 5). mRNA expression of synaptobrevin (A) and cannabinoid receptor 1 (CB1, B). C and D: mRNA expression in muscle myenteric plexus preparations in control, fasted, and refed condition of synaptobrevin (C) and CB1 (D) (n = 10). P < 0.05 (*) and 0.01 (**).
DISCUSSION
In this study, we investigated the effect of CORT on ENS activity, expression of neuronal markers in the ENS, and intestinal motility. Resulting data show for the first time that 11β-HSD-1, the intracellular regulator of CORT levels, is expressed in the myenteric plexus along the entire gastrointestinal tract and that its intestinal mRNA expression is modulated by feeding state. In our immunohistochemical studies on whole mount preparations, we found clear evidence for CORT-converting enzyme 11β-HSD-1 expression by subsets of enteric neurons that were neither nNOS nor calretinin positive. One earlier study by Ergang et al. (13) identified the presence of 11β-HSD-1 in the mucosa of rat colon where they showed 11β-HSD-1 mRNA and protein expression that increased in response to inflammation, however, without any indication that 11β-HSD-1 would be present in enteric nerves. At present, we have not been able to identify a known subclass of neurons that expresses 11β-HSD-1. In the CNS, 11β-HSD-1 expression had been found in hippocampal, PVN, and cerebellar neurons (17, 20). Roles for 11β-HSD-1 in neural pathways were discovered by using 11β-HSD-1 null mice (18) showing that, after deletion of the 11β-HSD-1 gene, mice expressed a longer CORT peak and increased plasma CORT and ACTH concentrations, suggesting attenuated negative HPA feedback. Apart from regulating HPA control, Densmore et al. (10) demonstrated that 11β-HSD-1 was able to modulate agouti-related peptide (AGRP) expression in the arcuate nucleus of the hypothalamus to regulate appetite. AGRP mRNA in 11β-HSD-1-deficient mice on a high-fat diet was elevated in the arcuate nucleus resulting in hyperphagia, suggesting a (diet-specific) role for 11β-HSD-1 in appetite control (10). The role of 11β-HSD-1 or even the effect of CORT itself on intestinal function has never been demonstrated; however, other stress-related hormones such as corticotropin-releasing factor (CRF) and urocortins have been recognized to delay gastric emptying and increase colonic motility in healthy volunteers (33). In addition, CRF has been recognized to induce activity in guinea pig myenteric neurons via a CRF-1 receptor-dependent mechanism (4).
In the present study, we could show for the first time that CORT, at concentrations similar as observed during fasting, reduces electrical stimulation-induced Ca2+-response amplitudes in myenteric neurons in tissue and in cultures. Moreover, durations of the Ca2+ responses were also decreased by CORT incubation in myenteric neuron cultures. In the CNS, the ability of CORT to modulate neuronal activity and synaptic transmission has long been recognized. Studies by Musazzi et al. (28) in the prefrontal and frontal cortex showed that acute stress could increase the readily releasable pool of synaptic vesicles by increasing the amount of presynaptic SNARE protein complexes, of which synaptobrevin is a member. We demonstrated that CORT incubation also induces a local increase in synaptobrevin mRNA in myenteric neurons. However, apart from only a synaptic protein enhancement, we also demonstrated a CORT-induced increase in CB1 receptor expression. The increase in CB1 might be relevant regarding neuronal activity, since Boesmans et al. showed that Ca2+ buffering in myenteric neurons was inhibited by CB1 receptor activation (5). In addition, Hons et al. (22) demonstrated the inhibition of synaptic transmission in the myenteric plexus of the ileum by CB1, which was suggested to result from changes in Ca2+ influx into the presynaptic neuron. Furthermore, we were able to show that CORT decreased intestinal mixing without altering gut diameter. This CORT-induced reduction in mixing behavior is suggested to be associated with CB1 activity, since substantial evidence is available to demonstrate an inhibitory effect of cannabinoids on gastrointestinal motility (8, 29). Based on the Ca2+ and video imaging results, the elevated expression of CB1 seems to overrule any enhancing effect of CORT on synaptic transmission, which results in an overall negative effect on activity. Several mechanisms for CB1-induced inhibition of gut motility have been suggested, including the modulation of adenosine release, nonadrenergic noncholinergic excitatory transmission, and a decrease in acetylcholine release from enteric neurons in ileum and colon (2, 3, 24, 26). Local and direct CORT effects have also been shown in mouse colon, where, in contrast to our observations, CORT decreased CB1 protein expression in dorsal root ganglia innervating the colon (21). In addition, Reich et al. (31) showed significantly downregulated CB1 protein levels in the male rat hippocampus in response to chronic mild stress and upregulation in the hippocampus of female rats. These data show that the CORT-mediated modulation of CB1 expression differs not only between genders but also between central and peripheral neurons. Probably also the type of stressor is important.
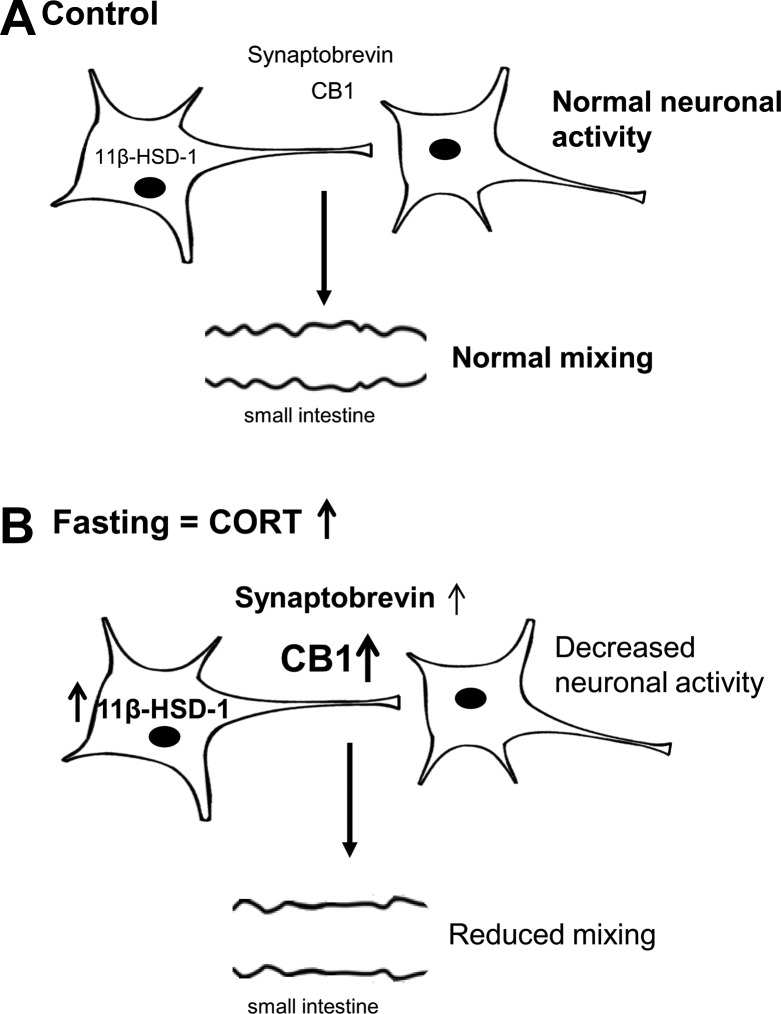
In conclusion, the present study shows a neuromodulatory role for CORT during fasting in the gastrointestinal tract leading to reduced neuronal Ca2+ responses in myenteric neurons and mixing behavior in mouse ileum. Adding further support to a role for CORT in altering enteric neuronal control, we demonstrated expression of 11β-HSD-1 in the myenteric plexus. Because fasting induced higher CORT levels, increased 11β-HSD-1 mRNA and synaptobrevin and CB1, whereas CORT application only changes the levels of the latter two, we propose a model in which modulation of synaptic communication is downstream of CORT, which in turn is a consequence of the HPA axis and local (11β-HSD-1)-mediated CORT production during fasting. We conclude that CORT is an important modulator of proteins involved in the fine tuning of enteric neurotransmission and gastrointestinal mixing movements (Fig. 6). Although more research is needed to fully unravel the mechanisms underlying the CORT-induced effects, current results confirm a role for CORT in a feeding state-related pathway affecting enteric neurons, which adds to an improved understanding of appetite physiology. This is of great importance in view of the emerging role for the ENS in appetite-regulating function and for the development of therapeutic strategies in diseases such as obesity, bulimia, and anorexia nervosa.
Fig. 6.
Schematic overview of the fasting-related CORT effects in the enteric nervous system (ENS). A: in the control condition, basal expression levels of 11β-HSD-1, synaptobrevin, and CB1 receptors are associated with normal neuronal activity and mixing patterns in the small intestine. B: during fasting, plasma CORT levels rise and induce increased expression of 11β-HSD-1, synaptobrevin, and CB1 receptors, associated with a reduction in neuronal activity and small intestinal mixing.
GRANTS
This work was funded by the Methusalem program (J. Tack) and FWO G.0510.10; G.0889.11 (P. Vanden Berghe).
DISCLOSURES
The authors disclose no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: K.L. and P.V.B. conception and design of research; K.L. performed experiments; K.L. analyzed data; K.L. and P.V.B. interpreted results of experiments; K.L. prepared figures; K.L. drafted manuscript; J.T. and P.V.B. edited and revised manuscript; J.T. and P.V.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all members of the Laboratory for Enteric NeuroScience for technical assistance and S. Webster for the 11β-HSD-1 antibody. We thank W. Boesmans for advice on the manuscript.
REFERENCES
- 1.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav 91: 449–458, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Baldassano S, Serio R, Mule F. Cannabinoid CB1 receptor activation modulates spontaneous contractile activity in mouse ileal longitudinal muscle. Eur J Pharmacol 582: 132–138, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Begg M, Dale N, Llaudet E, Molleman A, Parsons ME. Modulation of the release of endogenous adenosine by cannabinoids in the myenteric plexus-longitudinal muscle preparation of the guinea-pig ileum. Br J Pharmacol 137: 1298–1304, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisschops R, Vanden Berghe P, Sarnelli G, Janssens J, Tack J. CRF-induced calcium signaling in guinea pig small intetsine myenteric neurons involves CRF-1 receptors and activation of voltage-sensitive calcium channels. Am J Physiol Gastrointest Liver Physiol 290: G1252–G1260, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Boesmans W, Ameloot K, van den Abbeel V, Tack J, Vanden Berghe P. Cannabinoid receptor 1 signalling dampens activity and mitochondrial transport in networks of enteric neurones. Neurogastroenterol Motil 21: 958-e77, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Boesmans W, Martens MA, Weltens N, Hao MM, Tack J, Cirillo C, Vanden Berghe P. Imaging neuron-glia interactions in the enteric nervous system. Front Call Neurosci 183: 1–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons: Kinetics and role in influencing neurochemical phenotype. Am J Physiol Gastrointest Liver Physiol 299: G63–G69, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casu MA, Porcella A, Ruiu S, Saba P, Marchese G, Carai MA, Reali R, Gessa GL, Pani L. Differential distribution of functional cannabinoid CB1 receptors in the mouse gastroenteric tract. Eur J Pharmacol 459: 97–105, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Dallman MF, Warne JP, Foster MT, Pecoraro NC. Glucocorticoids and insulin both modulate caloric intake through actions on the brain. J Physiol 583.2: 431–436, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Densmore V, Morton NM, Mullins JJ, Seckl JR. 11β-hydroxysteroid dehydrogenase type 1 induction in the arcuate nucleus by high-fat feeding: a novel constraint to hyperphagia? Endocrinology 147: 4486–4495, 2006. [DOI] [PubMed] [Google Scholar]
- 11.De Sousa Peixoto RA, Turban S, Battle JH, Chapman KE, Seckl JR, Morton NM. Preadipocyte 11beta-hydroxysteroid dehydrogenase type 1 is a keto-reductase and contributes to diet-induced visceral obesity in vivo. Endocrinology 149: 1861–1868, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Djordjević J, Jasnić N, Vujović P, Djuraěvić Djordjevi I, Cvijić G. The effect of fasting on the diurnal rhythm of rat ACTH and corticosterone secretion. Arch Biol Sci 60: 541–546, 2008. [Google Scholar]
- 13.Ergang P, Vytáčková K, Švec J, Svec J, Bryndová J, Mikšík I, Pácha J. Upregulation of 11β-hydroxysteroid dehydrogenase 1 in lymphoid organs during inflammation in the rat. J Steroid Biochem Mol Biol 126: 19–25, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 151: 4811–4819, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol 72: 143–164, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gomes P, Chevalier J, Boesmans W, Roosen L, van den Abbeel V, Neunlist M, Tack J, Vanden Berghe P. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol Motil 21: 870-e62, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Sanchez EP, Romero DG, de Rodriguez AF, Warden MP, Krozowski Z, Gomez-Sanchez CE. Hexose-6-phosphate dehydrogenase and 11β-hydroxysteroid dehydrogenase-1 tissue distribution in the rat. Endocrinology 149: 525–533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris HJ, Kotelevtsev Y, Mullins JJ, Seckl JR, Holmes MC. Intracellular regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase (11beta-HSD)-1 plays a key role in regulation of the hypothalamis-pituitary-adrenal axis: analysis of 11beta-HSD-1-deficient mice. Endocrinology 142: 114–120, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol 517: 575–590, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes MC, Yau JLW, Kotelevtsev Y, Mullins JJ, Seckl JR. 11β-Hydroxysteroid dehydrogenases in the brain. Ann NY Acad Sci 1007: 357–366, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hong S, Zheng G, Wu X, Snider NT, Owyang C, Wiley JW. Corticosterone mediates reciprocal changes in CB1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology 140: 627–637, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hons IM, Storr MA, Mackie K, Lutz B, Pittman QJ, Mawe GM, Sharkey KA. Plasticity of mouse enteric synapses mediated through endocannabinoid and purinergic signaling. Neurogastroenterol Motil 24: e113–e124, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huizinga JD, Chen JH, Fang Zhu Y, Pawelka A, McGinn RJ, Bardakjan BL, Parsons SP, Kunze WA, Wu RY, Bercik P, Khoshdel A, Chen S, Yin S, Zhang Q, Yu Y, Gao Q, Li K, Hu X, Zarate N, Collins P, Pistilli M, Ma J, Zhang R, Chen D. The origin of segmentation motor activity in the intestine (Abstract). Nat Commun 5: 3326, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manara L, Croci T, Guagnini F, Rinaldi-Carmona M, Maffrand JP, Le Fur G, Mukenge S, Ferla G. Functional assessment of neuronal cannabinoid receptors in the muscular layers of human ileum and colon. Dig Liver Dis 34: 262–269, 2002. [DOI] [PubMed] [Google Scholar]
- 25.McGhee NK, Jefferson LS, Kimball SR. Elevated corticosterone associated with food deprivation upregulates expression in rat skeletal muscle of the mTORC1 repressor, REDD1. J Nutr 139: 828–834, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulè F, Amato A, Baldassano S, Serio R. Evidence for a modulatory role of cannabinoids on the excitatory NANC neurotransmission in mouse colon. Pharmacol Res 56: 132–139, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Murphy KG, Bloom SR. Gut hormones in the control of appetite. Exp Physiol 89.5: 507–516, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Musazzi L, Milanese M, Farisello P, Zapettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G, Raiteri M, Benfenati F, Bonanno G, Popoli M. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. Plos one 5: e8566, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, Mascolo N, Di Marzo, Capasso F. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 123: 227–234, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Prager EM, Johnson LR. Stress at the synapse: signal transduction mechanisms of adrenal steroids at neuronal membranes. Sci Signal 2: re5, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res 203: 264–269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roosen L, Boesmans W, Dondeyne M, Depoortere I, Tack J, Vanden Berghe P. Specific hunger- and satiety-induced tuning of guinea pig enteric nerve activity. J Physiol 590.17: 4321–4333, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stengel A, Taché Y. Neuroendocrine control of the gut during stress: corticotrophin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol 71: 219–239, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanden Berghe P, Molhoek S, Missiaen L, Tack J, Janssens J. Differential Ca2+ signaling characteristics and excitatory myenteric motor neurons in culture. Am J Physiol Gastrointest Liver Physiol 279: G1121–G1127, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Vanden Berghe P, Kenyon JL, Smith TK. Mitochondrial Ca2+ uptake regulates the excitability of myenteric neurons. J Neurosci 22: 6962–6971, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]