Abstract
Human interleukin-29 (IL-29), a helical cytokine with interferon-like activities, is currently being developed as a clinical biotherapeutic to treat chronic hepatitis C infection and some cancers. As such, the World Health Organization (WHO) has recognized a need for biological standardization of IL-29 and the establishment of an internationally available reference reagent of IL-29. In order to accomplish this, an international collaborative study that evaluates WHO candidate reference reagents of IL-29 was instigated by the National Institute for Biological Standards and Control (NIBSC) in 2010 and was carried out in the succeeding year. Two preparations of human sequence recombinant IL-29, one expressed in murine NS0 cells and the other in Escherichia coli, were formulated and lyophilized at NIBSC before evaluation in the collaborative study for their suitability to serve as a reference reagent. The preparations were tested by 6 laboratories from 4 countries using in vitro bioassays and also evaluated for thermal stability within the NIBSC laboratory. On the basis of the results of the collaborative study, both preparations, 07/212 (NS0-derived) and 10/176 (E. coli-derived) were judged sufficiently active and stable to serve as a reference reagent. However, since IL-29 produced in E. coli is in development for clinical applications, it was recommended that the preparation coded 10/176 be established as the WHO international reference reagent for human IL-29. This recommendation was accepted, and the IL-29 preparation coded 10/176 was formally established by the WHO ECBS at its meeting in October 2012 as the WHO international reference reagent for IL-29 with an assigned unitage of 5,000 reference units per ampoule.
Introduction
Interleukin-29 (IL-29) is the prototypic member of a small family of 3 closely related cytokines, IL-28A, IL-28B, and IL-29, which share common functional and structural features with a class of numerous cytokines that act through class II cytokine receptor family receptors (Kotenko and others 2003; Sheppard and others 2003; Langer and others 2004; Li and others 2009; Donnelly and Kotenko 2010). This class includes type I interferons (IFN), type II IFN or IFN-γ, and the IL-10 family. IL-29 is distantly related to both IL-10 and type I IFN-α families, has antiviral activity, and is alternatively designated as IFN-λ1, which is a type III IFN (IL-28A=IFN-λ2 and IL-28B=IFN-λ3). The gene encoding IL-29 has 5 exons and is located on the long arm of human chromosome 19 in close proximity to the IL-28A and IL-28B genes. The IL-29 gene encodes a mature, secreted IL-29 protein of 181 amino acids, which includes 1 potential N-glycosylation site and whose 3D structure is that of a monomeric α-helical protein, topologically similar to IL-10 and other members of the IL-10 family of cytokines (Miknis and others 2010). Similar to type I IFNs, IL-29 is induced by viral infections in many cells types, including dendritic cells, monocytes/macrophages, and various tumor-derived cell lines (Li and others 2009; Donnelly and Kotenko 2010). It has been shown to inhibit the replication of several viruses, including hepatitis C virus (HCV) in vitro (Li and others 2009; Pagliaccetti and Robek 2010).
IL-29, as well as IL-28 (A and B), interacts with a heterodimeric class II cytokine receptor that consists of the affinity converter IL-10Rβ chain and an orphan class II receptor chain designated IL-28Rα or, alternatively, IFN-λR1 (Kotenko and others 2003; Sheppard and others 2003). Although IL-28/-29 receptors are distinct from those used by type I IFNs, it appears that IL-29 (and IL-28A and B) triggers identical JAK-STAT signaling pathways in susceptible cells and induces interferon-stimulated response elements (ISREs). In common with type I IFN, IL-29 upregulates several known IFN responsive genes, including MxA, 2-5A synthetase, and class I MHC antigen. Thus, the activities of IL-29 demonstrated to date are the same as those documented for type I IFNs, namely antiviral (both in vitro and in vivo), immunostimulatory, and antiproliferative activity, although more selective and appearing to be weaker than type I IFN in the cell-based systems used (Meager and others 2005; Li and others 2009; Donnelly and Kotenko 2010). The selective activity results from the variable expression pattern of IL-28Rα among cell types. While the IL-10Rβ chain is ubiquitous, IL-28Rα is expressed at the highest levels in epithelial cells, melanocytes, and hepatocytes and at the lowest levels in primary central nervous system cells. Although blood immune cells, for example, monocytes and lymphocytes, express IL-28Rα, they exhibit impaired response to IL-28/-29 due to the secretion of a short spliced variant of IL-28Rα that inhibits induction of JAK-STAT activation (Meager and others 2005; Li and others 2009; Witte and others 2009; Donnelly and Kotenko 2010; Dickensheets and others 2013).
The characterization of IL-29 as an “antiviral” cytokine has paved the way for its development as a therapeutic clinical product, especially for patients with chronic HCV infection. Although pegylated IFN-α2 and ribavirin in combination is the current “standard of care” therapy for these patients, it is associated with undesirable hematological and neurological side effects. In comparison, since functional receptors for IL-29 are expressed at low levels in T cells and NK cells and not at all in hematopoietic precursor cells, IL-29 appears to have a few side effects (Donnelly and Kotenko 2010; Pagliaccetti and Robek 2010; Dickensheets and others 2013). In addition, genetic studies have implicated the IL-28/29 cytokine family in both the natural and therapy-induced resolution of HCV infection (Pagliaccetti and Robek 2010; Thursz and others 2011; Dolganiuc and others 2012; Hayes and others 2012). Several new inhibitors, for example, protease-, polymerase-, and cyclophilin inhibitors are currently in phase 3 trials with the aim of delivering effective, safe, and shorter duration of therapy when compared with the current standard of care (Aronsohn and others 2013).
The potential of pegylated IL-29 as an alternative biotherapeutic agent to pegylated IFN-α2 for the treatment of patients with chronic HCV infection was demonstrated in a phase Ib trial of pegylated IL-29 (IFN-λ1) with or without ribavirin (Muir and others 2010; Ramos 2010) and in a recently completed “EMERGE” phase IIb trial according to which treatment naïve patients with HCV genotypes 1 or 4 were treated with pegylated IL-29 (IFN-λ1) or pegylated IFN-α2a. Pegylated IL-29 appeared to have fewer of the undesirable side effects of IFN-α2 and to have a good safety profile in chronic HCV-infected patients (Muir and others 2010). In the “EMERGE” study, similar sustained virological response rates for the 2 regimens (IFN-α2a or IL-29) and similar viral breakthrough and post-treatment response rates were evident. However, IL-29-treated patients experienced less hematological toxicity and, as in phase Ib trial, fewer side effects (musculoskeletal and flu-like symptoms) were noted (Muir and others, Presented at American Association for the Study of Liver diseases, November 2012). Furthermore, combinations of lL-29 with direct-acting antiviral agents are highly efficient in suppressing HCV replication (Friborg and others 2013). In addition, studies have shown that IL-29 also has independent antitumor activity, and co-operates with type I IFN to elicit more efficient direct antitumor activities (Li and others 2009; Donnelly and Kotenko 2010; Fujie and others 2011), suggesting its usefulness for therapy of certain cancer types. As a consequence, other IL-29-based prototypes, for example, IL-29 linked to a stabilized dimer of Fab, are being investigated (Liu and others 2013).
Potency measurements of IL-29 products used for clinical treatments of HCV infection and other possible indications, for example, malignancies, require standardized bioassays. Since an international reference standard for IL-29 would facilitate measurement of the potency and stability of therapeutic preparations of IL-29 and, in addition, measurement of IL-29 levels for research purposes, the need for an international reference reagent for use in standardizing bioassays that measure the potency of IL-29 was recognized and endorsed by the World Health Organization Expert Committee on Biological Standardization (WHO ECBS) at its meeting in October 2010. Consequently, the National Institute for Biological Standards and Control (NIBSC) co-ordinated an international collaborative study to undertake this objective. Two candidate preparations of IL-29, both of which were lyophilized, were sent to participants for potency determinations in their in vitro bioassays with specific aims:
(i) To assess the relative activity of the 2 ampouled IL-29 preparations in different bioassays for assessing the influence of individual bioassay formats on the estimates of potency.
(ii) To compare the activities of the ampouled preparations with “in-house” standards of IL-29, where available.
(iii) To compare the activities of the ampouled preparations with type I human IFN standards, for example, IFN-α2, where available.
Materials and Methods
Materials used for the study: preparation of ampouled lyophilized IL-29
Two preparations of recombinant human (rh)IL-29 were kindly donated to the WHO (see Acknowledgments section). One preparation was expressed in murine NS0 myeloma cells, while the other preparation was expressed in Escherichia coli. Trial fills were conducted, and the biological activity of the lyophilized preparations was compared with the bulk material in a reporter gene assay based on induced secretion of soluble alkaline phosphatase from human HEK cells harboring the interferon-stimulated response element (ISRE) promoter linked to alkaline phosphatase gene (LaFleur and others 2001; Meager and others 2005). A formulation containing both human serum albumin (HSA) and bovine casein, previously successfully used for lyophilization of IFN-β (Meager and Gaines Das 2005), was found to best preserve the activity of IL-29 after lyophilization and was, therefore, chosen for trial and definitive fills. Since the trial lyophilizations performed appropriately in the bioassay, the preparations were filled into ampoules and final lyophilization was carried out at NIBSC as per the procedures used for International Biological Standards (WHO Expert Committee on Biological Standardization 2006).
Formulation solutions were prepared using nonpyrogenic, sterile 6-salt phosphate-buffered saline (NaCl: 140 mM; KCl: 2 mM; Na2HPO4·12H2O: 8 mM; KH2PO4: 1.5 mM; CaCl2·2H2O: 0.9 mM; MgCl2·6H2O: 0.5 mM: pH 7.4) and de-pyrogenated glassware. After addition of the appropriate quantities of HSA and bovine casein and re-adjustment of pH (to pH 7.4 with sodium hydroxide), solutions were filtered using sterile nonpyrogenic 0.2 μm cellulose acetate filters (Schleicher & Schuell Microscience GmbH) and stored at 4°C. The appropriate amount of the bulk rhIL-29 preparation was added to the formulation solution (∼4,000 mL) on the day of the definitive fills and dispensed into ampoules in 1.0 mL aliquots to provide final rhIL-29 concentrations of 1.0 μg/mL* (NS0-derived rhIL-29, 07/212) and 0.5 μg/mL* (E. coli-derived rhIL-29, 10/176), respectively. Final compositions are shown in Table 1. For the study, the 2 preparations were coded as described in Table 1.
Table 1.
Materials Used in Study
| Ampoule contents | |||||||
|---|---|---|---|---|---|---|---|
| Ampoule code | Study code | No. of ampoules in stock | IL-29 (predicted mass, μg) | Expression system | Excipientsa | Reconstitution volume | Custodian and storage |
| 07/212 | A | ∼3,800 | 1 | Murine NS0 | 0.3% Bovine casein, 1% HSA, 6-salt PBS, pH 7.4 | 1 mL distilled water | NIBSC Potters Bar, HERTS, United Kingdom Stored at −20°C |
| 10/176 | B | ∼3,900 | 0.5 | Escherichia coli | |||
Bovine casein was certified to be sourced from countries where BSE is not known to exist.
BSE, bovine spongiform encephalopathy; HSA, human serum albumin; IL-29, interleukin-29; PBS, phosphate-buffered saline.
For each fill, a percentage of ampoules were weighed. The mean fill weights are shown in Table 2. Each solution was lyophilized, and the ampoules were sealed under dry nitrogen by heat fusion of the glass and stored at −20°C in the dark. Residual moisture of each preparation, measured by the coulometric Karl–Fischer method (Mitsubishi CA100), is shown in Table 2. Headspace oxygen content was determined by frequency-modulated spectroscopy using the Lighthouse FMS-760 Instrument (Lighthouse Instruments; LLC). Testing for microbial contamination using total viable count method did not show any evidence of microbial contamination. A low level of endotoxin was detected in the preparations; the levels were deemed to be insignificant to the performance of assays, particularly as it is diluted out in assay procedures and the cell lines used for assays are generally insensitive due to a lack of endotoxin receptors.
Table 2.
Mean Fill Weights and Residual Moisture Content of Candidate Preparation
| Ampoule code | Study code | Mean fill weight (g) | CV fill weight % | Mean residual moisture % | CV residual moisture % | Mean headspace oxygen % | CV headspace oxygen % |
|---|---|---|---|---|---|---|---|
| 07/212 | A | 1.0045 (139) | 0.156 | 0.389 (12) | 16.38 | 0.46 (12) | 31.32 |
| 10/176 | B | 1.0017 (228) | 0.190 | 0.419 (12) | 21.35 | 0.77 (12) | 14.82 |
The numbers in parentheses indicate the number of determinations. Residual moisture of each preparation was measured by the coulometric Karl–Fischer method (Mitsubishi CA100). Headspace oxygen content was determined by frequency-modulated spectroscopy (Lighthouse FMS-760).
Participants
Coded ampoules were dispatched in December 2011 to 9 laboratories in 6 countries. The participants comprised 3 control laboratories, 1 contract research laboratory, and 5 academic scientists. Six participants submitted data (2 participants withdrew due to inability to assay materials and 1 due to not being able to receive materials). Participants are referred to by a code number allocated at random (Appendix A).
Assay methods and study design
A summary of the bioassay methods used by the individual laboratories in the study is given in Table 3. Antiviral assays that were based on the IL-29-induced reduction of cytopathic effect in cultured human cell lines challenged with encephalomyocarditis virus and measurements of vital stain absorption at the end of assays were used by laboratories 1, 2, and 5. Laboratory 3 used vesicular stomatitis virus (VSV) for viral challenge in their antiviral assay (Kotenko and others 2003). Two laboratories, 5 and 6, used reporter gene assay methods in which IL-29-induced enzyme activity was measured after a defined time interval. For example, laboratory 5 measured alkaline phosphatase activity in the supernatants of transfected HEK 293 cells harboring the secreted alkaline phosphatase cDNA linked to the ISRE promoter after a 48 h stimulation with IL-29 (LaFleur and others 2001; Meager and others 2005). In contrast, laboratory 6 measured luminescence generated after IL-29 stimulation of firefly luciferase activity in transfected HuH7 cells harboring an IFN-regulated firefly luciferase construct (Lallemand and others 2008). Two laboratories measured “early” IL-29 STAT-1 activation using different methods. Laboratory 4 determined the quantity of phospho-tyrosine STAT-1 in cell lysates by an ELISA method after IL-29 stimulation, while laboratory 3 used an electrophoretic mobility shift assay (EMSA) to assess STAT-1 activation (Kotenko and others 2003).
Table 3.
Individual Laboratory Codes and Assays Used by Study Participants
| Laboratory code | Cells/cell line | Assay type | Assay duration (h) | Read-out | Reference |
|---|---|---|---|---|---|
| 1 | Human HEP2G liver carcinoma | Antiviral/cytopathic effect reduction/EMCV challenge | 48 | Colorimetric/crystal violet stain | — |
| 2 | Human A549 adenocarcinoma | Antiviral/cytopathic effect reduction/EMCV challenge | 48 | Colorimetric/crystal violet stain | — |
| 3A | Human retinal pigmented epithelial ARPE-19 | Antiviral/cytopathic effect reduction/VSV challenge | 48 | Colorimetric/crystal violet stain | Kotenko and others 2003 |
| 3B | Chinese hamster ovary cells transfected with IFN-λR1 | EMSA/STAT-1 induction | 0.33 (20 min) | SDS-PAGE/STAT-1 protein band shift | Kotenko and others 2003 |
| 4 | Human HEP2G liver carcinoma | Induction of phospho-tyrosine STAT-1 | 0.5 (30 min) | Measurement of phosphoY-STAT-1 by ELISA | — |
| 5A | Human NCTC2544 keratinocyte | Antiviral/cytopathic effect reduction/EMCV challenge | 48 | Colorimetric/amido blue black stain | — |
| 5B | Human HEK transfected with ISRE-SEAP | Reporter gene (Alkaline phosphatase) | 48–72 | Supernatant SEAP activity by ELISA | LaFleur and others 2001, Meager and Gaines Das 2005 |
| 6 | Human HuH7 liver carcinoma transfected with IFN-regulated Firefly luciferase | Reporter gene (Luciferase) | 16 | Luminescence (Firefly luciferase) | Lallemand and others 2008 |
EMCV, encephalomyocarditis virus; EMSA, electrophoretic mobility shift assay; HEK, human embryonic kidney; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; SEAP, soluble extracellular alkaline phosphatase; VSV, vesicular stomatitis virus.
Participants were asked to assay all samples concurrently on a minimum of 2 separate occasions using their own routine bioassay methods. Participants were requested to perform at least 4 dilutions of each preparation using freshly reconstituted ampoules for each assay. Where available, they were asked to include their own in-house reference material in their assay. Participating laboratories were sent 3 ampoules each of study samples coded A and B as detailed in Table 1. Participants were requested to return their raw assay data and also their own calculations of potency of the study samples relative to A or to other in-house reference materials they included in their assays.
Statistical analysis
Where possible, the potencies of the study samples A and B were compared by calculating the potency of B relative to A by analysis of the raw assay data at NIBSC. The assays were analyzed using a weighted logistic parallel line model, using the full dose-response curve, using the European Directorate for Quality of Medicines and Healthcare (EDQM) assay analysis software, Combistats (http://combistats.edqm.eu/). In some instances, the assays were analyzed using a simple parallel-line model based on a linear portion of the dose-response curve (Finney 1978). Assay validity was assessed by the usual analysis of variance tests for linearity and parallelism and by visual inspection of the plotted dose-response curve.
Potencies within laboratories were combined using geometric means, and intra-laboratory variability was expressed as geometric coefficients of variation (%GCV) (Kirkwood 1979). Overall potencies were calculated as geometric means of the individual laboratory means, and inter-laboratory variability was expressed as %GCVs between laboratory means.
Stability studies
Accelerated degradation studies
Samples of the candidate standards 07/212 and 10/176 (study samples A and B) were stored at elevated temperatures (+4°C, 20°C, 37°C, 45°C, and 56°C) for 42 and 20 months, respectively, and assayed at NIBSC using a reporter gene assay (LaFleur and others 2001; Meager and others 2005). Samples were tested concurrently with those stored at the recommended storage temperature of −20°C, and with baseline samples stored at −70°C. For each material, at least 3 assays were performed with each temperature replicated across 4 plates within each assay. The assays were analyzed as described for the main collaborative study, and the potencies of the samples stored at different temperatures were expressed relative to the appropriate −70°C baseline samples. Not all samples could be fitted on all plates, so a balanced layout was used that resulted in 2 independent estimates of each temperature relative to the −70°C baseline sample for each assay. These were combined as unweighted geometric means.
Stability after reconstitution
Samples were reconstituted and stored at temperatures of +4°C and +20°C for periods of 1, 7, and 8 days. They were then assayed concurrently with a freshly reconstituted sample. The assays were analyzed as described for the main collaborative study, and the potencies of the stored samples were expressed relative to the freshly reconstituted sample. Two independent reporter gene assays (LaFleur and others 2001; Meager and others 2005) were performed, with each replicated over 3 plates. Not all combinations of reconstitution time and temperature could be included on each plate. A balanced layout was used, with a freshly reconstituted sample on each plate, resulting in 2 estimates of potency relative to a freshly reconstituted sample for each time/temperature combination for each assay. These were combined as unweighted geometric means.
Stability on freeze-thaw
Samples of 10/176 were reconstituted and subjected to approximately 4 freeze-thaw cycles. They were then assayed concurrently with a freshly reconstituted sample. The assays were analyzed as described for the main collaborative study, and the potencies of the frozen-thawed samples were expressed relative to the freshly reconstituted sample. Two independent reporter gene assays were performed, with each sample replicated over 4 plates within each assay.
Results
Data received
Six out of the 9 laboratories that were sent the coded samples for the collaborative study contributed data which were derived using different assay methods (Table 3).
Laboratory 1 returned raw data from 4 assays, which were analyzed using the full sigmoid dose response. Laboratory 2 returned data from 4 assays, along with their own calculation of titers for the samples. Where possible, the raw data were analyzed using the full sigmoid dose response. However, the dynamic range of the assay was covered in a limited number of dilution steps, and 1 assay could not be analyzed. Potencies of B relative to A were also calculated based on the titers calculated by the laboratory. These results are referred to as laboratory 2B, with 2A being the NIBSC calculations from the raw data.
Laboratory 3 returned only photographic representations of their assays' results. In the IL-29-stimulated STAT-1 phosphorylation, EMSAs (3B), gel-shift autoradiographs of assays performed on 3 separate occasions were submitted. For antiviral assays with VSV as challenge virus (3A), color photographs of the stained cells in 96-well microtiter plates (2 assays, 2 plates each) at the assay termination point were submitted. Since no numerical assay readings were submitted, NIBSC was unable to perform a statistical analysis, for either assay, from these photographs. However, Laboratory 3 supplied their own in-house analyses, for which account was taken in general terms in the final assessment of assay data from all laboratories.
Laboratory 4 returned data from a single assay, using an in-house control as a standard curve. Potencies for triplicates of samples A and B were calculated relative to the standard curve, and the potency of B relative to A was calculated as the ratio of these.
Laboratory 5 returned data from 3 antiviral assays (laboratory 5A) and 3 reporter gene assays (laboratory 5B), each with multiple plates. They were analyzed as linear parallel line assays, after taking a log transformation of the assay read-out.
Laboratory 6 provided data from 2 assays of A against the WHO International Standard for IFN-α2b (95/566), and 2 assays for sample B against the same standard. Samples A and B were not assayed concurrently. There was evidence of nonparallelism of 95/566 against both A and B, with the samples having different maximum responses. There were also big differences in the overall response level across different plates. Approximate potencies of B relative to A were calculated as ratios of the potencies of B and A against 95/566, which were calculated using a linear parallel line model based on a restricted linear part of the dose-response curves. The responses of 95/566 appeared to differ between assays, with higher responses in the assays of sample B compared with those of sample A. It is possible that apparent differences in potencies of A and B may be due to differences in the response of 95/566 across assays, rather than genuine differences between samples.
Dose response of samples A and B in different assays
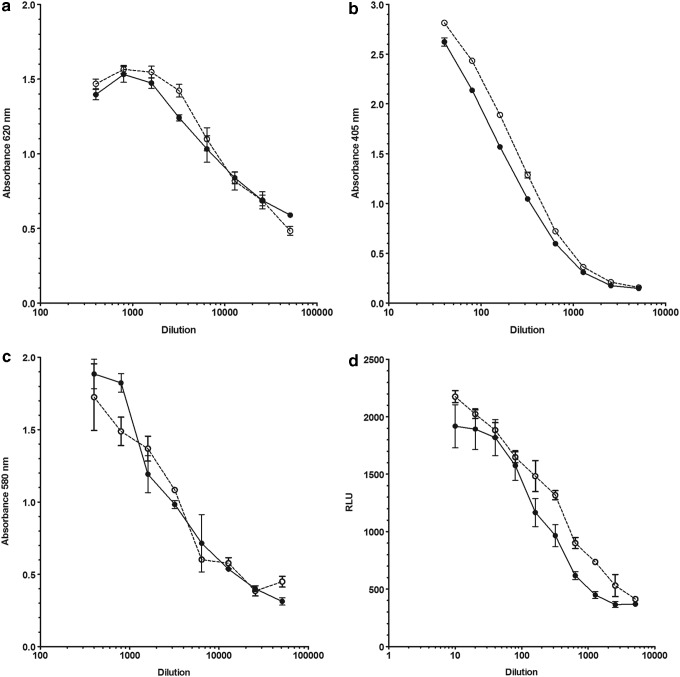
Both samples A and B exhibited dose responses in all assays that would allow either of them to serve as a reference material. Representative dose-response curves of A and B obtained in antiviral and reporter gene assays are shown in Figure 1. With regard to results derived from assays in which local IL-29 reference preparations or type I IFN (IFN-α or IFN-β) or type II IFN (IFN-γ) were included for comparative purposes, the numbers of assays were few and could provide only limited data and conclusions. Where analyzable, assays in which a “local” IL-29 reference preparation was included, for example, Laboratories 2 and 5, the dose-response curves were parallel with those of A and B. In contrast, assays in which type I or II IFNs (laboratories 5 and 6) were titrated with A and B yielded results that indicated nonparallelism of their dose-response curves to those of A or B. In addition, type I IFN (IFN-α2b or IFN-β) gave higher maximal responses.
FIG. 1.
Illustrative examples of dose-response curves obtained with interleukin-29 (IL-29) preparations coded A (closed circles) and B (open circles) using different IL-29 assays. Human NCTC2544 keratinocyte cell line-based antiviral assay (a), human HEK ISRE SEAP-based reporter gene assay (b), human HEP2G carcinoma cell line-based antiviral assay (c), reporter gene assay using transfected human HuH7 cells harboring interferon (IFN)-regulated firefly luciferase construct (d).
Potencies of sample B relative to A
The laboratory geometric mean potencies of sample B relative to sample A are shown in Table 4, along with the between-assay variability measured by the %GCV. Potencies are expressed as a percentage of that of A. The agreement between assays within laboratories is good, with %GCVs ranging between 3% and 13%, where it was possible to calculate them. This indicates that for the majority of laboratories, the samples A and B behave reproducibly within the different assay systems.
Table 4.
Geometric Mean Potencies of Sample B Relative to Sample A (as % of A)
| Laboratory | B as % of A | Inter-assay %GCV |
|---|---|---|
| 1 | 113 | 13.4 |
| 2A | 63 | 3.1 (sample A only) |
| 2B | 58 | 7.9 (sample A) |
| 5.9 (sample B) | ||
| 4 | 410 | N/A-1a |
| 5A | 164 | 10.1 |
| 5B | 134 | 8.1 |
| 6 | 23 | N/A-2b |
N/A-1, single assay.
N/A-2, A and B not assayed concurrently and approximate potencies only.
GCV, geometric coefficients of variation.
The laboratory mean potencies of sample B relative to sample A are more variable between laboratories. The results from laboratory 2 (2A and 2B) are alternative calculations from the same assay data, and so are in close agreement. The anti-viral and reporter gene assays from laboratory 5 are in reasonable agreement with each other, and with the assays of laboratory 1. Laboratory 4 obtains a much higher potency of B relative to A (410%) than the other laboratories, but this is based on a single assay. While it has not been possible to analyze the EMSA photographs submitted by laboratory 3, these clearly show that B has a much higher potency than A. For instance, from the participant's analysis, 1/100 dilution of B was equivalent to 10 ng/mL of an in-house IL-29 reference preparation; whereas this corresponded to a 1/10 dilution of A, that is, B was 10-fold more potent than A in their EMSA. In addition, the antiviral assays submitted by laboratory 3 indicated a greater potency of B, ∼5-fold more potent than A from their in-house analysis. In contrast, laboratory 6 obtained a lower potency for B relative to A (23%); however, as noted earlier, this is an approximate value derived from separate assays of A and B against 95/566, the international standard (IS) for IFN-α2b. These assays exhibited considerable variability in response between plates/assays, with the response for 95/566 being much higher in the assays of sample B than in the assays of sample A. The apparent low potency of B relative to A may, therefore, be partly due to the behavior of 95/566 in the assays, rather than a genuine difference between samples A and B.
Stability studies
Accelerated degradation studies
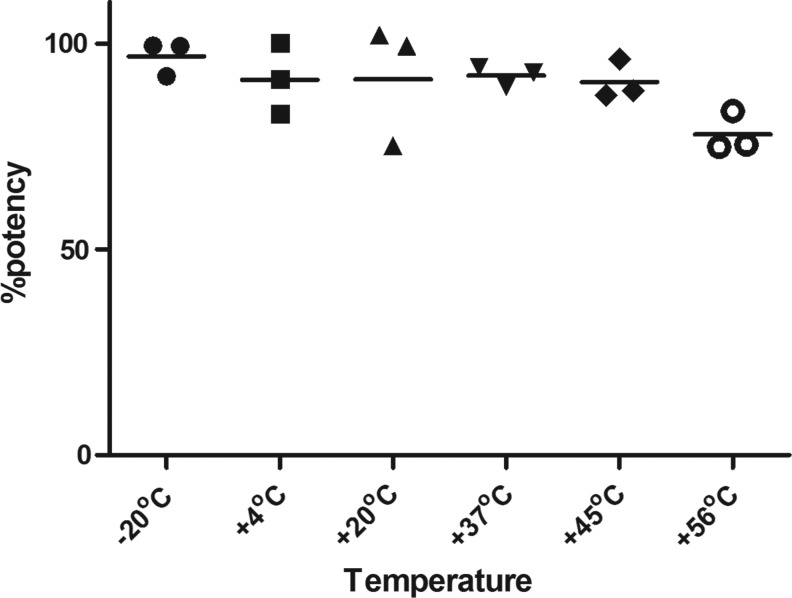
For this, reporter gene assays in which samples were stored at different temperatures were analyzed and the potencies were expressed relative to the samples stored at −70°C. Both samples A and B exhibited high thermal stability in lyophilized form; however, only results for sample B (candidate standard coded 10/176) are presented here. The results for assay 1 were considerably more variable than for assays 2 and 3, and did not show the expected trend of increased degradation with increased temperature. In particular, the estimate for the +20°C sample is low at 75.1% (of the −70°C sample) compared with the estimate of 96.2% for the +45°C sample. The results for assays 2 and 3 were more consistent, and they indicate little degradation at lower temperatures, with an increasing trend with higher temperatures and estimates for the +56°C sample of around 75% of the −70°C sample. The geometric mean potencies are shown in Figure 2.
FIG. 2.
Geometric mean potencies (expressed as a percentage) of samples of 10/176 stored at elevated temperatures relative to −70°C sample of 10/176.
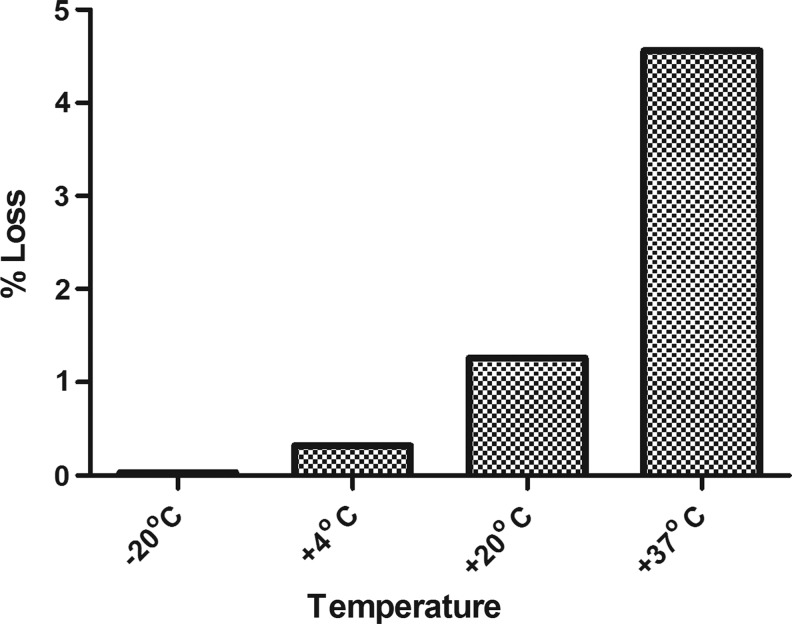
The usual Arrhenius model (Kirkwood 1977) was applied to the combined data from assays 2 and 3, to obtain predictions of % loss per year at the different temperatures. The data were a good fit to the model, and the results are shown in Figure 3. The results indicate that 10/176 is stable, with a predicted loss of below 0.1% per year when stored at −20°C. It also appears to be sufficiently stable for transportation at ambient temperatures, with a predicted % loss per month of 0.4% at +37°C.
FIG. 3.
Predicted loss in potency (%) of 10/176 per year at different temperatures.
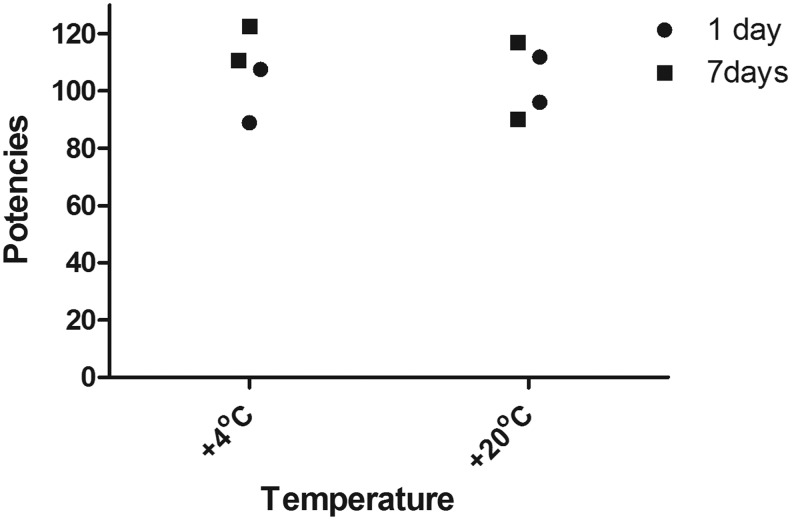
Further studies showed that the potencies of the reconstituted ampoules of sample B (10/176) are not diminished after storage at temperatures of +4°C and +20°C for approximately 7 days as shown in Figure 4 (expressed as a percentage of the freshly reconstituted ampoule). Data indicate that 10/176 is very stable after reconstitution (Fig. 4). Although there is no evidence of any loss in potency after 8 days of storage at +20°C, it is recommended that 10/176 is used immediately after reconstitution. In addition, we found no pattern of increased loss of bioactivity in the reporter gene assay used after subjecting the reconstituted contents of the ampoule to 4 freeze-thaw cycles (data not shown).
FIG. 4.
The figure shows the geometric mean potencies of stored (1, 7 days) samples of 10/176 expressed as a percentage relative to freshly reconstituted samples of 10/176.
Discussion
From the results of this study, both A and B candidate standards for IL-29 were sufficiently highly active in all of the assays used by participants and could be considered for adoption as a WHO international reference reagent for IL-29. However, there was high variation in the potency estimates for A and B and in the ratio of estimates, for example, B/A (Table 4). Such variations in potencies, although not unusual in bioassays of different types, may be attributed to structural differences between the IL-29 proteins in A and B. Sample A contains IL-29 derived from the murine NS0 myeloma cell line and, as IL-29 has 1 potential N-linked and 6 O-linked glycosylation sites, would be secreted as a glycoprotein in contrast to the nonglycosylated sample B expressed in E. coli (Kotenko and others 2003). In addition, IL-29 in A has a carboxy-histidine tag to aid purification, which is lacking in the preparation in B. Such differences, while not qualitatively affecting the activities seen in assays, could impact binding affinity/avidity to IL-29 receptors expressed on the surface of assay cells and, thus, affect responses quantitatively.
It is notable that the phospho-YSTAT-1 induction assay of laboratory 4 showed the highest difference in potency. The phosphorylation of tyrosine on STAT-1 is an “early” event that occurs within minutes of receptor firing. The greater potency of B as opposed to A suggests that the E. coli-derived IL-29 triggers the phosphorylation more intensely than the NS0-derived IL-29, an explanation also supported by laboratory 3's similar observation in their EMSA data (data not shown), where phosphorylation of STAT-1 was measured within 20 min after the addition of IL-29 to the cells. Data from laboratory 3 as per the participant's analysis showed an order of magnitude difference between the 2 preparations, A and B. Since both STAT-1 phosphorylation assays were of relatively short duration, they may exhibit “unusual” responsiveness; this is based on the limited data available from these assays. Nevertheless, wide differences in potencies of A and B were also observed with other assay types; for example, antiviral and reporter gene assays where the assay duration was much longer than the phosphorylation assays. The antiviral assays of laboratories 1, 3, and 5 indicated that B was more potent than A, whereas those of laboratory 2 gave the opposite result. While giving a similar higher potency of B to A as found in the antiviral assays of laboratory 5, the results of reporter gene assays of laboratory 5 were in complete contrast to those of the reporter gene assays of laboratory 6, where A was apparently ∼4-fold more potent than B (Table 4). However, the results of laboratory 6 appear to have been influenced by plate-to-plate variation. It appears, therefore, that differences in the potency of A and B are likely reflected by differences in the protein structures along with differences in the types of assays used in the study, though further confirmatory studies are indicated.
A comparison of the IL-29 preparations with the IS of IFN-α2b, 95/566 which was included in this study because of the shared antiviral effects of both IFN-α and IL-29, showed that the 2 IFN types behaved differently in these assays.
Despite the finding of wide potency estimates for samples A and B, both of them were clearly demonstrated to be active among the (disparate) range of bioassays employed by participants. Both exhibited dose responses in all assays that would enable either of them to serve as a reference material. Both exhibited high stability and were suitable to serve as a reference material. Based on the product in clinical trials, reconstitution and freeze-thaw studies were conducted only on samples of the E. coli-derived candidate preparation (coded 10/176). These studies showed that 10/176 is stable at the storage temperature of −20°C, and its activity appears stable after reconstitution and freeze-thaw cycles. Therefore, on the basis that E. coli-derived, nonglycosylated IL-29 is currently the only product developed for clinical use, it was proposed that candidate standard B (code 10/176) be accepted as the WHO reference reagent for IL-29 and assigned a value of 5,000 units/ampoule. This was agreed by the participants, and a report of the study was submitted to the WHO ECBS for consideration at their annual meeting in October 2012. The WHO ECBS formally accepted the proposal and established 10/176 as the first WHO international reference reagent for IL-29 in October 2012 (ECBS report WHO/BS/2012.2197).
Appendix A: Participants in the Study
The following participants contributed data to the study. In this article, each laboratory has been identified by a number from 1 to 6 that is not related to this order of listing.
Dr. Kathryn Zoon and Joseph Bekisz, National Institutes of Health (NIH), National Institute for Allergy and Infectious Diseases (NIAID), Cytokine Biol Sec, 50 South Dr, Building 50, Room 5511, Bethesda, MD 20892.
Dr. Ray Donnelly, Division of Therapeutic Proteins, Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA), Building 29A, Room 3B15, 29, LincolnDrive, Bethesda, MD 20892.
Professor Guido Antonelli and Caroline Scagnolari, Full Professor of Virology, Laboratory of Virology, Department of Molecular Medicine, Sapienza University, Viale di Porta Tiburtina 28, 00185 Rome, Italy.
Dr. Sergei Kotenko, Department of Biochemistry and Molecular Biology, University Hospital Cancer Center, University of Medicine and Dentistry of New Jersey (UMDNJ), 185 South Orange Av, Newark, New Jersey, 07103.
Professor Michael Tovey, INSERM Director of Research, Laboratory of Biotechnology and Applied Pharmacology, Ecole Normale Supérieure de Cachan, Cachan, France.
Ms. Paula Dilger, Mr. Christopher Bird, and Dr. Anthony Meager, Cytokine and Growth Factor Section, Biotherapeutics Group, NIBSC, Blanche Lane, South Mimms, Potters Bar, Herts, EN6 3QG, United Kingdom.
Acknowledgments
The authors are very grateful to the manufacturers (Shenandoah and R&D Systems) for the supply of candidate materials and to the participating laboratories (Appendix A) for performing the laboratory tests. They are also grateful to Paul Matejtschuk and Kiran Malik for assistance with pilot fills of IL-29 preparations and to staff of the Standards Processing Division, NIBSC, for lyophilizing and dispatching the candidate materials of the study.
Author Disclosure Statement
All the authors from NIBSC have no conflicts of interest to declare.
The mass content of bulk rhIL-29 preparations was determined by the manufacturers. Since the protein content of the ampoules cannot be verified by direct measurement of absolute mass, the content of rhIL-29 per ampoule is assumed to be that calculated from the dilution of the bulk rhIL-29 preparation of the protein mass content assigned by the manufacturer, and the volume of formulated solution delivered to the ampoule. This value is given as “predicted μg.”
References
- Aronsohn A, Muir AJ, Swan T, Jensen D. 2013. Is the HCV pipeline heading in the right direction? Gastroenterology 144:482–485 [DOI] [PubMed] [Google Scholar]
- Dickensheets H, Sheikh F, Park O, Gao B, Donnelly RP. 2013. Interferon-lambda (IFN-λ) induces signal transduction and gene expression in human hepatocytes, but not in lymphocytes or monocytes. J Leukoc Biol 93:377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolganiuc A, Kodys K, Marshall C, Saha B, Zhang S, Bala S, Szabo G. 2012. Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells. PLoS One 7:e44915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly RP, Kotenko SV. 2010. Interferon lambda: a new addition to an old family. J Interferon Cytokine Res 30:555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ. 1978. Statistical methods in biological assay, 3rd ed. London: Charles Griffin [Google Scholar]
- Friborg J, Levine S, Chen C, Sheaffer AK, Chaniewski S, Voss S, Lemm JA, McPhee F. 2013. Combinations of lambda interferon with direct-acting antiviral agents are highly efficient in suppressing hepatitis C virus replication. Antimicrob Agents Chemother 57:1312–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujie H, Tanaka T, Tagawa M, Kaijun N, Watanabe M, Suzuki T, Nakayama K, Numasaki M. 2011. Antitumor activity of type III interferon alone or in combination with type I interferon against human non-small cell lung cancer. Cancer Sci 102:1977–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CN, Imamura M, Aikata H, Chayama K. 2012. Genetics of IL28B and HCV—response to infection and treatment. Nat Rev Gastroenterol Hepatol 9:406–417 [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. 1977. Predicting the stability of biological standards and products. Biometrics 33:736–742 [PubMed] [Google Scholar]
- Kirkwood TBL. 1979. Geometric means and measures of dispersion. Biometrics 35:908–909 [Google Scholar]
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. 2003. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 4:69–77 [DOI] [PubMed] [Google Scholar]
- LaFleur DW, Nardelli B, Tsarev T, Mather D, Feng T, Semenuk M, Taylor K, Buergin M, Chinchilla D, Roschke V, Chen G, Ruben SM, Coleman TA, Moore PA. 2001. Interferon-kappa, a novel type I interferon expressed in human keratinocytes. J Biol Chem 276:39765–39771 [DOI] [PubMed] [Google Scholar]
- Lallemand C, Meritet JF, Erickson R, Grossberg SE, Roullet E, Lyon-Caen O, Lebon P, Tovey MG. 2008. Quantification of neutralizing antibodies to human type I interferons using division-arrested frozen cells carrying an interferon-regulated reporter-gene. J Interferon Cytokine Res 28:393–404 [DOI] [PubMed] [Google Scholar]
- Langer JA, Cutrone EC, Kotenko S. 2004. The class II cytokine receptor (CRF2) family: overview and patterns of receptor-ligand interactions. Cytokine Growth Factor Rev 15:33–48 [DOI] [PubMed] [Google Scholar]
- Li M, Liu X, Zhou Y, Su SB. 2009. Interferon-λs: the modulators of antivirus, antitumor, and immune responses. J Leucoc Biol 86:22–32 [DOI] [PubMed] [Google Scholar]
- Liu D, Chang CH, Rossi EA, Cardillo TM, Goldenberg DM.2013. Interferon-λ1 linked to a stabilized dimer of Fab potently enhances both antitumor and antiviral activities in targeted cells. PLoS One 8:e63940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meager A, Gaines Das R. 2005. Biological standardization of human interferon beta: establishment of a replacement World Health Organization international biological standard for human glycosylated interferon beta. J Immunol Methods 306:1–15 [DOI] [PubMed] [Google Scholar]
- Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. 2005. Biological activity of interleukins-28 and -29: comparison with type I IFNs. Cytokine 31:109–118 [DOI] [PubMed] [Google Scholar]
- Miknis Z, Magracheva E, Li W, Zhanov A, Kotenko SV, Wlodawer A. 2010. Crystal structure of the complex of human interferon-λ1 with its high affinity receptor–λR1. J Mol Biol 404:650–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, Flamm S, Gordon SC, Marotta P, Vierling JM, Lopez-Talavera JC, Byrnes-Blake K, Fontana D, Freeman J, Gray T, Hausman D, Hunder NN, Lawitz E. 2010. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology 52:822–832 [DOI] [PubMed] [Google Scholar]
- Pagliaccetti NE, Robek MD. 2010. Interferon-λ in HCV infection and therapy. Viruses 2:1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos EL. 2010. Preclinical and clinical development of pegylated interferon-lambda 1 in chronic hepatitis C. J Interferon Cytokine Res 30:591–595 [DOI] [PubMed] [Google Scholar]
- Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnel S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4:63–68 [DOI] [PubMed] [Google Scholar]
- Thursz M, Yee L, Khakoo S. 2011. Understanding the host genetics of chronic hepatitis B and C. Semin Liver Dis 31:115–127 [DOI] [PubMed] [Google Scholar]
- WHO Expert Committee on Biological Standardization, 2006. Fifty-fifth Report. Recommendations for the preparation, characterization and establishment of international and other biological reference standards: WHO Technical Report Series, 932, p. 73 [Google Scholar]
- Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, Sabat R, Wolk K. 2009. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun 10:702–714 [DOI] [PubMed] [Google Scholar]