Abstract
DNA vaccination can generate both humoral and cellular immunity, resulting in potential prophylactic and therapeutic vaccines in variety of conditions, including hepatitis B virus (HBV) infection. Fusion of cytokine gene is one of the ways to increase the immunogenicity of DNA vaccine. Interleukin (IL)-21 has been demonstrated to play an immunomodulatory role in HBV infection. Thus, we aimed to investigate the ability of IL-21 in the regulation of middle version of HBV envelop protein (MS) DNA vaccine. Fusion plasmid encoding IL-21 linked with MS was constructed. Normal and HBV transgenic mice were immunized by plasmid. pcDNA-IL-21/S2S induced a comparable level of anti-HBs antibody and HBsAg-specific CD8+ T-cell response with pcDNA-S2S. Furthermore, the level of circulating HBsAg was decreased by induction of anti-HBs antibody and HBsAg-specific CD8+ T-cell response to both pcDNA-IL-21/S2S and pcDNA-S2S vaccination in HBV transgenic mice. Thus, immunization with DNA vaccine encoding HBV MS protein induced both T- and B-cell response by targeting the specific antigen. Furthermore, it was also revealed that MS DNA vaccination could break immune tolerance in HBV transgenic mice. But IL-21 did not strengthen immune response induced by HBV DNA immunization. Our study suggested that MS-expressing plasmid may be useful for both preventive and therapeutic methods in HBV infection. However, IL-21 does not improve the immunogenicity and efficacy of MS DNA vaccination, and thus may not be used as a therapeutic marker for chronic hepatitis B.
Introduction
An estimated 350 million people are chronically infected with hepatitis B virus (HBV), placing it among the world's most common infectious diseases (16). Chronic HBV infection often results in liver cirrhosis and hepatocellular carcinoma, leading to millions of deaths each year worldwide because of end-stage liver diseases (14). Current therapies for HBV infection include administration of nucleos(t)ide analogs or interferon (IFN)-α. These treatments are only moderately effective, and are often accompanied by severe side effects and viral resistance. Thus, there remains a need for new therapies for this serious disease.
DNA vaccination can generate both humoral and cellular immunity against the antigen encoded by plasmid vector, resulting in potential prophylactic and therapeutic vaccines in variety of conditions, such as infectious diseases, autoimmune diseases, and cancers (1,6,26,30,32). It has been demonstrated that HBV-specific DNA immunization induced anti-HBs antibody response and IFN-γ-producing CD8+ T-cells in patients and animal models (19,21,27). Furthermore, inhibition of HBV replication was also found in response to HBV Pres2/S DNA vaccination (19). However, the immunogenicity remains relatively low in large animals and nonhuman primates, despite the potentiality in small animals (28,31). Thus, it is necessary to improve the efficacy of DNA vaccination by elevation of antigen delivery and presentation, as well as by fusion of certain sequences that enhance immune response, especially cytokine genes (34). Interleukin (IL)-21 is a member of common γ-chain receptor cytokine family, which is mainly produced by activated CD4+ T-cells and NKT cells (22,29). IL-21 controls the activation, differentiation, and functions of T-cells, B-cells, and NK cells, and counteracts the inhibition effects of regulatory T-cells (20). Moreover, antigen-specific CD4+ T-cells secreting IL-21 sustained maintenance and function of specific CD8+ T-cell response, which eventually controls the chronic lymphocytic choriomeningitis virus (LCMV) infection (7,8,36). Thus, IL-21 could be a new therapeutic target for chronic viral infectious diseases.
Recent studies have also demonstrated that IL-21 contributes to the inhibition of viral replication and hepatitis B e antigen seroconversion in chronic hepatitis B (11,13,18). Thus, we hypothesized that IL-21 could regulate the HBV-specific immune response in vivo. To test this possibility, we designed effective fusion DNA vaccines containing the middle version of the HBV envelope glycoprotein (MS) gene and IL-21 gene, and evaluated the humoral and cellular immune response in both BALB/c and HBV transgenic (Tg) mice.
Materials and Methods
Plasmids constructions
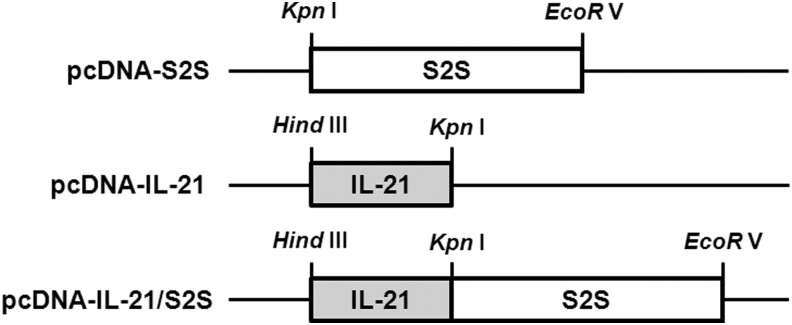
The open reading frame (ORF) for the middle version of the HBV envelope glycoprotein (MS; ayw subtype) was amplified by polymerase chain reaction (PCR) from plasmid containing HBV 1.3 genome length units (kindly provided by Dr. Michael D. Robek, Yale University) using primers 5′-CGG GTA CCA TGC AGT GGA ATT CCA CAA CC-3′ and 5′-CCG ATA TCT TAA ATG TAT ACC CAA AGA CA-3′, introducing upstream KpnI and downstream EcoRV sites for directional cloning. The MS PCR product was cleaved with KpnI and EcoRV and cloned into pcDNA3(+) plasmid after cleavage with KpnI and EcoRV, resulting the plasmid pcDNA-S2S. The ORF for mouse IL-21 was amplified by PCR from plasmid containing modified mouse IL-21 gene (kindly provided by Dr. Michael D. Robek, Yale University) using primers 5′-CCA AGC TTA TGG AGA GAA CAC TGG TCT GC-3′ and 5′-GGG GTA CCG GAG AGG TGC TGG TGA ATC AT-3′, introducing upstream HindIII and downstream KpnI sites for cloning. The IL-21 product was cleaved with HindIII and KpnI and cloned into pcDNA3(+) plasmid after cleavage with HindIII and KpnI, resulting in the plasmid pcDNA-IL-21. Otherwise, the cleaved IL-21 fragment was cloned into pcDNA-S2S plasmid after cleavage with HindIII and KpnI, resulting in the plasmid pcDNA-IL-21/S2S. A schematic representation of the plasmids is shown in Figure 1. All constructs were verified by DNA sequencing, and the plasmids for immunization were prepared using Qiagen Gigaprep kit (Qiagen, Hilden, Germany).
FIG. 1.
Schematic representation of the constructed DNA vaccine. The genes were cloned into pcDNA3(+) expression vectors and under the control of the cytomegalovirus (CMV) promoters and ATG control.
Cells and transfection
293T cells were grown in DMEM (Hyclone, Logan, UT) supplemented with 10% of heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY). The purified plasmids pcDNA-IL-21, pcDNA-S2S, and pcDNA-IL-21/S2S were transfected into 293T cells with Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA). Cells and supernatants were harvested 48 h after transfection for further experiments.
Animals and DNA immunization
Male BALB/c (H-2d) mice, 6–8 weeks of age, were purchased from Experimental Animal Center of Fourth Military Medical University (Xi'an, China). Male HBV Tg BALB/c mice were purchased from Transgenic Engineering Research Laboratory, Infectious Disease Center of PLA (Guangzhou, China). The HBV Tg mice generated high levels of viral DNA, RNA, and HBV antigens in the hepatocytes and serum 4 weeks after birth. Furthermore, they secreted infectious virus particles into the bloodstream. All animals were kept under pathogen-free condition in our animal facility, and all experiments were performed in accordance with the procedures approved by Animal Care and Use Committee of Fourth Military Medical University. Thirty normal or Tg mice were divided into six groups randomly with five mice in each group, which were immunized with pcDNA3, pcDNA-IL-21, pcDNA-S2S, pcDNA-IL-21/S2S, pcDNA-IL-21+pcDNA-S2S, or PBS respectively. The animals were injected with 100 μL of plasmid constructs (1 μg/μL of final concentration) into the quadriceps on weeks 0, 4, 8, and 12. Mice were sacrificed 16 weeks after initial injection, and serum and spleens were harvested for further studies.
Western blot
293T cells were harvested 48 h post transfection, and were lysed on ice for 15 min in lysis buffer. Supernatants were collected by centrifugation. The middle version of HBV envelope protein and IL-21 were analyzed by Western blot using anti-HepB PreS2 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and anti-mouse IL-21 antibody (eBioscience, San Diego, CA) as described previously (9,25).
Enzyme-linked immunospot assay
Splenocytes were prepared by passage the spleen through 70 μm strainers. Cells were treated with ACK lysis buffer and washed with Hanks' balanced salt solution. Splenocytes were resuspended in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS. The IFN-γ enzyme-linked immunospot assay (ELISPOT) set (BD Biosciences, San Jose, CA) was used to quantify T-cell activation. Briefly, 96-well polyvinylidene difluoride-backed plates were coated overnight with anti-mouse IFN-γ (1:200), and were blocked with DMEM supplemented with 10% FBS for 2 h. Next, 2×105 of splenocytes were seeded into each well, and were stimulated with HBV-specific peptides pools (final concentration 20 μg/mL) at 37°C overnight. The sequences of the peptides were: HBs 191–202: IPQSLDSWWTSL; HBs 353–360: VWLSVIWM; HBs 364–372: WGPSLYSIL; HBs 371–378: ILSPFLPL (3). Cells, which were stimulated with PMA/ionomycin and media, were used as positive and negative controls respectively. The plates were washed with PBS containing 0.05% Tween, and incubated with biotinylated anti-mouse IFN-γ (1:250) for 2 h at room temperature. The streptavidin-horseradish peroxidase (HRP; 1:1000) was added after washing with PBS for 1 h incubation at room temperature. The plates were again washed with PBS, and individual IFN-γ-producing cells were detected as dark spots after 20–40 min development with 3-amino-9-ethylcarbazole (AEC) chromogen-substrate. Spots were counted with an ELISPOT reader (Autoimmun Diagnostika GmbH, Straßberg, Germany) and were expressed as spot-forming cells (SFC) per 106 splenocytes. The number of specific IFN-γ-secreting cells was calculated by subtracting the negative control value from the established SFC count.
Enzyme-linked immunosorbent assay
Serum and supernatant IL-21, IFN-γ, IL-4, IL-17, HBsAg, and anti-HBs levels were determined by enzyme-linked immunosorbent assay (ELISA) following the manufacturer's protocol (IL-21: R&D Systems, Minneapolis, MN; IFN-γ, IL-4, IL-17: eBioscience, San Jose, CA; HBsAg and anti-HBs: Kehua Biotech, Shanghai, China).
Proliferation assay
A total of 2×105 of splenocytes were cultured in 96-well plates with stimulation of purified recombinant HBsAg (AbD Serotec, Oxford, United Kingdp,) with a final concentration of 10 μg/mL for 4 days. Cellular proliferation was measured with a Cell Counting Kit-8 (CCK-8; Alexis Biochemicals, San Diego, CA) as described previously (40). Briefly, 20 μL (10% of the total volume) of CCK-8 solution were added to each well for the last 4 h of culture. Wells that contained a known number of viable splenocytes were also prepared to create a calibration curve. The absorbance of the samples was measured at 450 nm using a Model 680 Microplate Reader (Bio-Rad, Hercules, CA).
Statistical analyses
Statistical significance was determined by one-way analysis of variance (ANOVA) using SPSS for Windows v13.0 (SPSS, Inc., Chicago, IL). p-Values of <0.05 were considered to indicate a significant difference.
Results
Construction and expression of DNA constructs
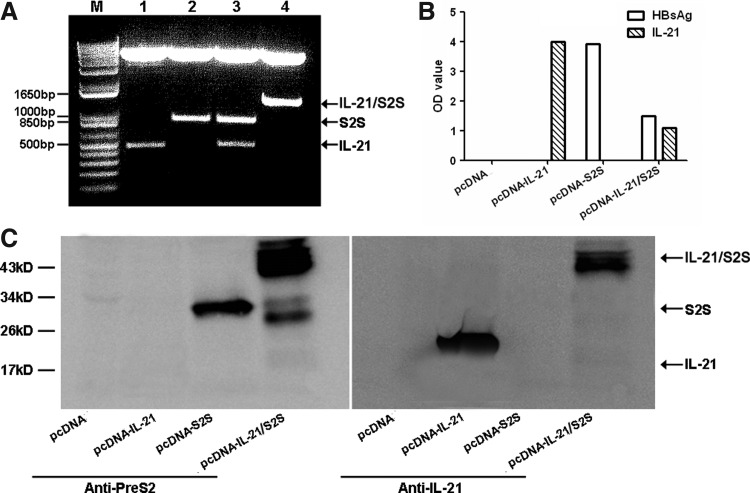
Recombinant pcDNA-IL-21, pcDNA-S2S, and pcDNA-IL-21/S2S plasmids were confirmed by restriction enzyme digestion and DNA sequencing. Digestion of the plasmids with suitable enzymes produced insertion fragments of 441 bp for IL-21 gene, 846 bp for S2S gene, and 1287 bp for IL-21-S2S gene (Fig. 2A). 293T cells were transfected with the plasmids, and supernatants as well as the cells were harvested 48 h post-transfection. ELISA and Western blot were used to detect the expression of IL-21, HBV MS protein, and fused protein IL-21-MS. As shown in Figure 2B, HBsAg was detectable in the supernatants of pcDNA-S2S and pcDNA-IL-21/S2S transfectants, while IL-21 was also measurable in pcDNA-IL-21 and pcDNA-IL-21/S2S transfectants. However, no target proteins can be detected in the supernatants of pcDNA3 transfectants. Furthermore, anti-HepB PreS2 antibody and anti-mouse IL-21 antibody was used to characterize protein expression in cell lysates. Bands consistent with the molecular weight of IL-21, MS, and fused protein were detected in transfected cells (Fig. 2C).
FIG. 2.
Construction and expression of recombinant plasmids. (A) Enzyme digestion of recombinant plasmids. M: DNA Marker; lane 1. pcDNA-IL-21 was digested by HindIII and KpnI; lane 2. pcDNA-S2S was digested by KpnI and EcoRV; lane 3. pcDNA-IL-21/S2S was digested by HindIII, KpnI and EcoRV; lane 4. pcDNA-IL-21-S2S was digested by HindIII and EcoRV. (B) The secretions of IL-21 and HBsAg were detected by enzyme-linked immunosorbent assay (ELISA) in the supernatants from 293T cells transfected with pcDNA3, pcDNA-IL-21, pcDNA-S2S, and pcDNA-IL-21/S2S. Data shown are the mean value of triplicate tests. (C) The expressions of IL-21, middle version of the HBV envelope protein (MS), and fused protein were detected by Western blot in the cell lysates from 293T cells transfected with pcDNA3, pcDNA-IL-21, pcDNA-S2S, and pcDNA-IL-21/S2S.
IL-21-fused DNA vaccination did not enhance humoral or cellular HBV-specific immune response in normal BALB/c mice
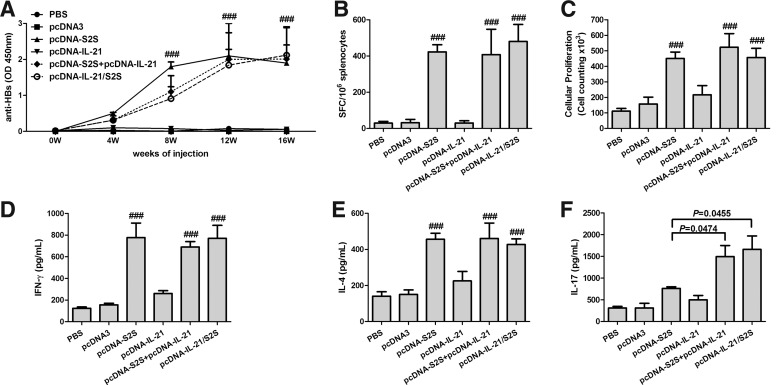
The effect of IL-21-fused immunization on HBV-specific antibody response was evaluated. Serum was collected from each normal BALB/c mouse at various times after vaccination and levels of anti-HBs were evaluated. As shown in Figure 3A, PBS, pcDNA3, and pcDNA-IL-21 did not elicit anti-HBs response after immunization. In contrast, pcDNA-S2S, pcDNA-IL-21/S2S, as well as pcDNA-S2S+pcDNA-IL-21 could elicit strong anti-HBs titers. The anti-HBs titers increased significantly 8 weeks after immunization, and reached their highest levels at 12 weeks (p<0.001 compared with PBS, pcDNA3, and pcDNA-IL-21 vaccination). However, no remarkable differences were noticed among pcDNA-S2S, pcDNA-IL-21/S2S, or pcDNA-S2S+pcDNA-IL-21 vaccination at each time point.
FIG. 3.
Specific immune response of normal BALB/c mice induced by constructed plasmids and controls. (A) Anti-HB antibody response. Groups of mice were immunized with phosphate-buffered saline (PBS), pcDNA3, pcDNA-IL-21, pcDNA-S2S, pcDNA-IL-21+pcDNA-S2S, or pcDNA-IL-21/S2S, respectively. Anti-HB antibodies were determined by ELISA at different time points after immunization. ###p<0.0001 compared with control groups. (B) Specific CD8+ T-cell responses were elicited following immunization. Sixteen weeks after first immunization, splenocytes were harvested and analyzed using an IFN-γ assay. The number of cells responding to stimulation with HBV S-peptides was represented as quantification of the number of spot-forming cells (SFC) per 106 splenocytes. ###p<0.0001 compared with PBS and pcDNA3 immunized groups. However, no significant differences were found among the three groups with MS vaccination. (C) Splenocyte proliferation in response to HBsAg. Splenocytes were stimulated with HBsAg (10 μg/mL) for 4 days, and cellular proliferation was calculated using a Cell Counting Kit-8 (CCK-8). (D), (E), and (F) Cytokine profiles of proliferating T-cells. The splenocytes were stimulated with HBsAg (10 μg/mL). The supernatants of cells were collected, and the concentrations of IFN-γ (D), IL-4 (E), and IL-17 (F) were detected by ELISA. ###p<0.0001 compared with PBS and pcDNA3 immunized groups. The data were presented as mean±standard deviation.
The effects of IL-21 fusion vaccination on HBV-specific CD8+ T-cell response were monitored by IFN-γ-producing cells by ELISPOT assay. Four peptides, which were known HBsAg-specific H-2b- and H-2d-restricted epitopes (3), were used for the stimulation. As expected, PBS-, pcDNA3-, and pcDNA-IL-21-immunized mice did not generate CD8 T-cells in response to HB peptides. Moreover, pcDNA-S2S-, pcDNA-IL-21/S2S-, and pcDNA-S2S+pcDNA-IL-21-immunized mice generated strong CD8 T-cell responses. However, there were no significant differences in SFC among the three groups with MS vaccination (p>0.05; Fig. 2B). We further investigated the lymphocyte proliferative responses to HBsAg. Cellular proliferations were notably increased in pcDNA-S2S-, pcDNA-IL-21/S2S-, and pcDNA-S2S+pcDNA-IL-21-immunized mice under HBsAg stimulation when compared with PBS-, pcDNA3-, and pcDNA-IL-21-immunized mice (p<0.001; Fig. 3C). However, no significant differences were found in cellular proliferations among pcDNA-S2S-, pcDNA-IL-21/S2S-, or pcDNA-S2S+pcDNA-IL-21-immunized mice (Fig. 3C).
To characterize the polarization of the immune responses, IFN-γ, IL-4, and IL-17 were measured as an indication of Th1, Th2, and Th17 response respectively. A total of 2×105 of splenocytes were cultured in 96-well plates with stimulation of HBsAg (10 μg/mL) for 4 days. Supernatants were harvested, and the subsets of activated Th cells were distinguished by detecting the productions of cytokines. The cytokine productions were increased in MS-immunized mice (p<0.05; Fig. 3D–F). However, IL-21 did not enhance the Th1 and Th2 response, as the levels of IFN-γ and IL-4 revealed no significant differences among pcDNA-S2S-, pcDNA-IL-21/S2S-, or pcDNA-S2S+pcDNA-IL-21-immunized mice (Fig. 3D and E). Interestingly, Th17 response were elevated by IL-21 fusion, as the release of IL-17 was increased approximately twofold in mice vaccinated with pcDNA-IL-21/S2S and pcDNA-S2S+pcDNA-IL-21 when compared with those vaccinated with pcDNA-S2S (p=0.0474 and p=0.0455 respectively; Fig. 3F).
IL-21-fused DNA vaccination downregulated HBsAg expression, but did not enhance humoral or cellular HBV-specific responses in HBV Tg mice
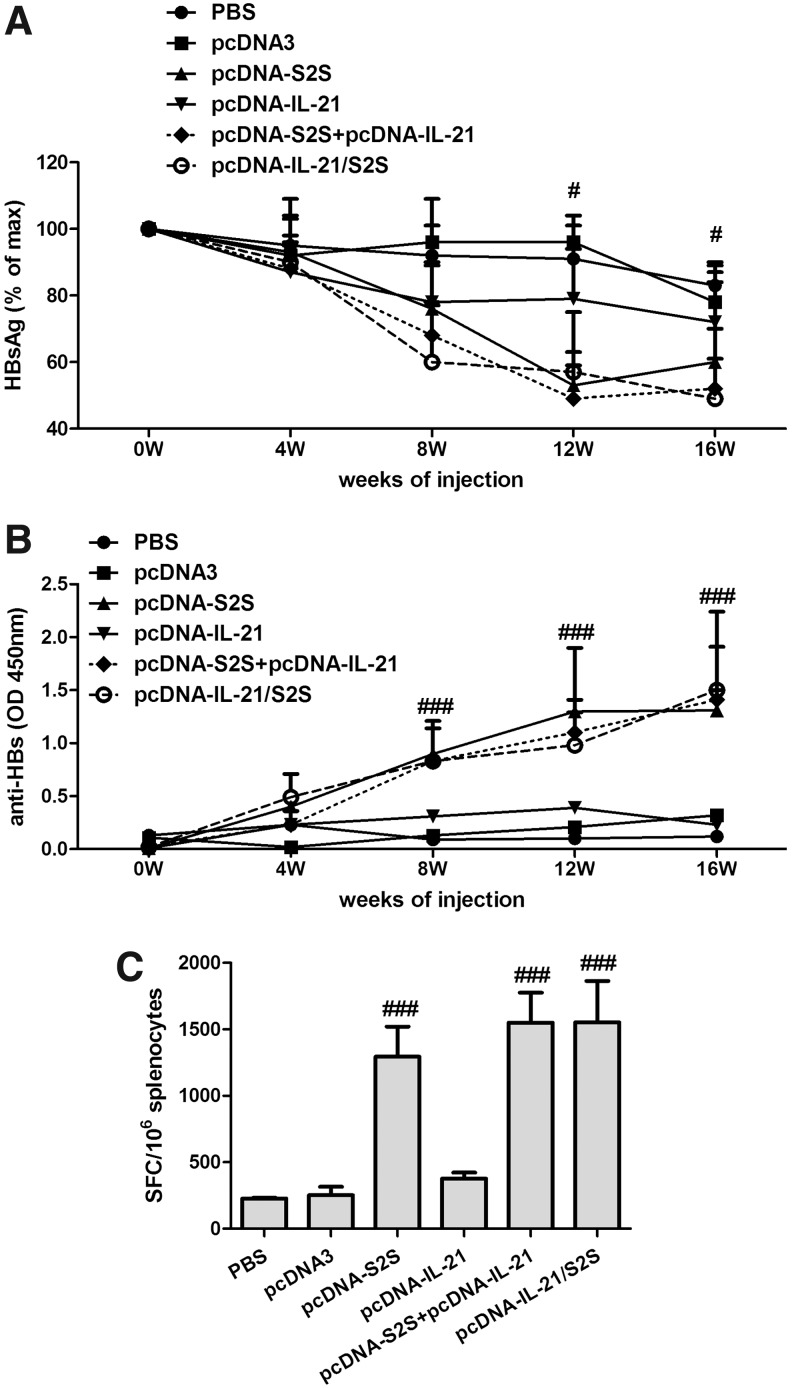
We further investigated whether the fusion DNA vaccine influenced viral clearance and HBV-specific immune responses in HBV Tg mice. Serum HBsAg decreased significantly 12 weeks after immunization in HBV Tg mice with pcDNA-S2S, pcDNA-IL-21/S2S, and pcDNA-S2S+pcDNA-IL-21 vaccination, when compared with PBS-, pcDNA3-, and pcDNA-IL-21-immunized mice (Fig. 4A). The HBsAg expressions were continuously suppressed 16 weeks post-immunization. However, the IL-21-fused DNA vaccination did not reveal efficient inhibition in circulating HBsAg than the single MS DNA immunization in HBV Tg mice.
FIG. 4.
Specific immune response of HBV transgenic BALB/c mice induced by constructed plasmids and controls. (A) Kinetics of the suppression of serum HBsAg in HBV-transgenic mice after immunization with PBS, pcDNA3, pcDNA-IL-21, pcDNA-S2S, pcDNA-IL-21+pcDNA-S2S, or pcDNA-IL-21/S2S, respectively. The serum HBsAg was quantitated by ELISA. #p<0.05 compared with PBS and pcDNA3 immunized groups. (B) Anti-HB antibody response. Groups of mice were immunized with PBS, pcDNA3, pcDNA-IL-21, pcDNA-S2S, pcDNA-IL-21+pcDNA-S2S, or pcDNA-IL-21/S2S, respectively. Anti-HB antibodies were determined by ELISA at different time points after immunization. ###p<0.0001. (C) Specific CD8+ T-cell responses were elicited following immunization. Sixteen weeks after first immunization, splenocytes were harvested and analyzed using an IFN-γ assay. The number of cells responding to stimulation with HBV S-peptides was represented as quantification of the number of spot-forming cells (SFC) per 106 splenocytes. ###p<0.0001 compared with PBS and pcDNA3 immunized groups.
We then examined the HBV-specific antibody and T-cell response in HBV Tg mice. The humoral and cellular HBV-specific immune response in HBV Tg mice represented similar trends to normal BALB/c mice. pcDNA-S2S, pcDNA-IL-21/S2S, and pcDNA-S2S+pcDNA-IL-21 vaccination induced a HBsAg-specific antibody response. However, the titers were lower than those in normal BALB/c mice, and did not reveal significant differences among the three groups (Fig. 4B). PBS-, pcDNA3-, and pcDNA-IL-21-immunized mice generated weak CD8 T-cells in response to HBs peptides in HBV Tg mice. Furthermore, pcDNA-S2S-, pcDNA-IL-21/S2S-, and pcDNA-S2S+pcDNA-IL-21-immunized mice elicited much stronger CD8 T-cell responses. However, there were no significant differences in SFC among the three groups (Fig. 4C).
Discussion
Herein, we demonstrated that MS-expressing plasmid vaccination could induce both humoral and cellular immune response in normal BALB/c mice. Moreover, the vaccination strategy could also break the immune tolerance status and eliminate HBsAg expression by induction of HBV-specific immunity in HBV Tg mice, which is consistent with many previous studies (17,21,24,42). We selected MS protein as the antigen because it contained a greater number of T-cell epitopes than the small version. Moreover, the large version (PreS1-PreS2-S) contains the HBV receptor-binding domain, which may change the tropism of the vector (3). Previous studies also revealed that a vesicular stomatitis virus-based HBV MS vaccine provided both protection for HBV infection in normal mice (3) and generated HBV-specific CD8+ T-cell and antibody responses in HBV Tg mice (4).
DNA vaccination is an attractive immunization strategy against HBV infection in both animal models and clinical trials (19,27,31,33) because it can induce a persistent T-cell and antibody response. The process of DNA immunization could mimic microbial infection when plasmids are entered artificially. Antigen-presenting cells (APCs) manipulate the expressed protein to form small peptides, which could further combine with MHC I and MHC II molecules to present on the cell surface. T-cell receptors could recognize the MHC–antigen complex, and stimulate T-cell and B-cell proliferation and differentiation (24). Thus, DNA vaccines could have both prophylaxis and therapy effects on infectious diseases. However, DNA vaccines also show limitations. Only a few injected DNA molecules were taken up by APCs, which led to a minority of the corresponding proteins being presented. Thus, the viral particles were not eliminated completely. Therefore, it is necessary to find an effective way to improve the antigen presentation or elevate the immune response to DNA vaccination. It has been demonstrated that different strategies, including fusion of IgG Fc fragment (37), heat shock protein 70 (12), IL-18 (2), cytotoxic T lymphocyte associated antigen (CTLA)-4 (42), granulocyte-macrophage colony-stimulating factor (GM-CSF) (24), and construction of epitope-based vaccine (15,35), could enhance HBV-specific CD4+ helper, CD8+ cytotoxic T-cell, and B-cell responses.
IL-21 has been proven to play an important role in HBV infection. Decreased secretion of IL-21 in younger HBV-infected patients might impede the generation of T- and B-cell responses. In contrast, acute HBV-infected adults showed increased IL-21 expression (23). Thus, IL-21 may be part of an effective hepatic immune response to HBV in an age-dependent manner. Furthermore, IL-21 may also facilitate HBeAg seroconversion in HBeAg-positive patients, which may contribute to individualization of antiviral therapy (11,18). Our previous study also found that the frequency of IL-21-secreting CD4+ T-cells was increased in patients with acute HBV infection (5). Considering the potential immunomodulatory role of IL-21, we tried to insert the IL-21 gene to the upstream of MS plasmid to make the fusion DNA vaccine. The fusion plasmid pcDNA-IL-21/S2S could be expressed and secreted in vitro, which demonstrated the successful construction of the fusion DNA vaccine. The in vivo experiments in both normal and HBV Tg BALB/c mice also revealed that the fusion IL-21/S2S vaccination as well as co-immunization of pcDNA-IL-21 and pcDNA-S2S could induce a humoral and cellular immune response. However, the titers of anti-HBs antibody and frequencies of HBV-specific CD8+ T-cells were comparable with single pcDNA-S2S immunization. The current results suggested that IL-21 may not enhance the HBV-specific immune response that is induced by MS-expressing plasmid vaccination.
We then tried to analyze why IL-21 failed to improve the immunogenicity of MS protein. The fusion plasmid could be expressed in vitro and induce strong immune response in vivo, suggesting that the injected DNA molecules were taken up by APCs, and the IL-21/S2S fusion protein was presented by APCs. The process of translation and antigen presentation did not influence IL-21 fusion. Other possible mechanisms of the immune enhancement by the fusion gene were promoting multiple T-cell proliferation and cytokine production. Thus, we measured the cellular proliferation and polarized cytokine secretions in response to HBsAg stimulation. IL-21 did not promote HBV-specific cell proliferation. Moreover, IFN-γ and IL-4 production, which presented a Th1 and Th2 response respectively, also did not remarkably increase when compared with MS-expressing plasmid immunization. Interestingly, Th17-secreting IL-17 levels were elevated in response to IL-21 fusion. This is partly because IL-21 initiated an alternative pathway to induce proinflammatory Th17 cells (10). However, Th17 cells as well as secreting IL-17 and IL22 have been demonstrated to correlate with liver inflammation but are not associated with viremia (38,39,41), which did not contribute to the antiviral immune response.
Conclusion
In summary, immunization with DNA vaccine encoding middle version of HBV envelope protein induced both a T- and B-cell response by targeting the specific antigen. Furthermore, it was also revealed that MS DNA vaccination could break immune tolerance in HBV Tg mice, since the level of circulating HBsAg was decreased. But IL-21 did not strengthen the immune response induced by HBV DNA immunization. Our study suggests that MS-expressing plasmid may be useful for both preventive and therapeutic methods in HBV infection. However, IL-21 does not improve the immunogenicity and efficacy of MS DNA vaccination, and thus may not be used as a therapeutic marker for chronic hepatitis B.
Acknowledgments
This work was supported by the grants from National Natural Science Foundation of China (No. 81072434, 31370856 and 31200650), National Key Technologies Research and Development Program of China (2012ZX10002007-001-006, 2008ZX10002-006, and 2012ZX10004-907), and a grant from Tangdu Hospital (supported Y.Z.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alarcon JB, Waine GW, and McManus DP. DNA vaccines: technology and application as anti-parasite and anti-microbial agents. Adv Parasitol 1999;42:343–410 [DOI] [PubMed] [Google Scholar]
- 2.Channarong S, Mitrevej A, Sinchaipanid N, Usuwantim K, Kulkeaw K, and Chaicumpa W. Cloning, protein expression and immunogenicity of HBs-murine IL-18 fusion DNA vaccine. Asian Pac J Allergy Immunol 2007;25:233–242 [PubMed] [Google Scholar]
- 3.Cobleigh MA, Buonocore L, Uprichard SL, Rose JK, and Robek MD. A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose. J Virol 2010;84:7513–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobleigh MA, Wei X, and Robek MD. A vesicular stomatitis virus-based therapeutic vaccine generates a functional CD8 T cell response to hepatitis B virus in transgenic mice. J Virol 2013;87:2969–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding YR, Zhang Y, Lian JQ, Li XH, Wang LX, and Huang CX. [The induced IL-21 levels in CD4(+) T cells of peripheral blood from the different clinical types of patients infected with hepatitis B virus]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2011;27:304–306, 308. [PubMed] [Google Scholar]
- 6.Donnelly JJ, Wahren B, and Liu MA. DNA vaccines: progress and challenges. J Immunol 2005;175:633–639 [DOI] [PubMed] [Google Scholar]
- 7.Elsaesser H, Sauer K, and Brooks DG. IL-21 is required to control chronic viral infection. Science 2009;324:1569–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frohlich A, Kisielow J, Schmitz I, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 2009;324:1576–1580 [DOI] [PubMed] [Google Scholar]
- 9.Hao C, Xie Y, Peng M, et al. Inhibition of connective tissue growth factor suppresses hepatic stellate cell activation in vitro and prevents liver fibrosis in vivo. Clin Exp Med 2014;14:141–150 [DOI] [PubMed] [Google Scholar]
- 10.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007;448:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Liu M, Cheng LW, et al. HBcAg-specific IL-21-producing CD4+ T cells are associated with relative viral control in patients with chronic hepatitis B. Scand J Immunol 2013;78:439–446 [DOI] [PubMed] [Google Scholar]
- 12.Li X, Yang X, Li L, Liu H, and Liu J. A truncated C-terminal fragment of Mycobacterium tuberculosis HSP70 gene enhanced potency of HBV DNA vaccine. Vaccine 2006;24:3321–3331 [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Ma S, Tang L, et al. Circulating chemokine (C-X-C Motif) receptor 5(+) CD4(+) T cells benefit hepatitis B e antigen seroconversion through IL-21 in patients with chronic hepatitis B virus infection. Hepatology 2013;58:1277–1286 [DOI] [PubMed] [Google Scholar]
- 14.Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 2012;6:531–561 [DOI] [PubMed] [Google Scholar]
- 15.Livingston BD, Newman M, Crimi C, McKinney D, Chesnut R, and Sette A. Optimization of epitope processing enhances immunogenicity of multiepitope DNA vaccines. Vaccine 2001;19:4652–4660 [DOI] [PubMed] [Google Scholar]
- 16.Lok AS, and McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661–662 [DOI] [PubMed] [Google Scholar]
- 17.Lu M, Isogawa M, Xu Y, and Hilken G. Immunization with the gene expressing woodchuck hepatitis virus nucleocapsid protein fused to cytotoxic-T-lymphocyte-associated antigen 4 leads to enhanced specific immune responses in mice and woodchucks. J Virol 2005;79:6368–6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma SW, Huang X, Li YY, et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol 2012;56:775–781 [DOI] [PubMed] [Google Scholar]
- 19.Mancini-Bourgine M, Fontaine H, Brechot C, Pol S, and Michel ML. Immunogenicity of a hepatitis B DNA vaccine administered to chronic HBV carriers. Vaccine 2006;24:4482–4489 [DOI] [PubMed] [Google Scholar]
- 20.Monteleone G, Pallone F, and Macdonald TT. Interleukin-21 (IL-21)-mediated pathways in T cell-mediated disease. Cytokine Growth Factor Rev 2009;20:185–191 [DOI] [PubMed] [Google Scholar]
- 21.Oka Y, Akbar SM, Horiike N, Joko K, and Onji M. Mechanism and therapeutic potential of DNA-based immunization against the envelope proteins of hepatitis B virus in normal and transgenic mice. Immunology 2001;103:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000;408:57–63 [DOI] [PubMed] [Google Scholar]
- 23.Publicover J, Goodsell A, Nishimura S, et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest 2011;121:1154–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qing Y, Chen M, Zhao J, et al. Construction of an HBV DNA vaccine by fusion of the GM-CSF gene to the HBV-S gene and examination of its immune effects in normal and HBV-transgenic mice. Vaccine 2010;28:4301–4307 [DOI] [PubMed] [Google Scholar]
- 25.Ren GL, Bai XF, Zhang Y, et al. Stable inhibition of hepatitis B virus expression and replication by expressed siRNA. Biochem Biophys Res Commun 2005;335:1051–1059 [DOI] [PubMed] [Google Scholar]
- 26.Robinson HL, and Pertmer TM. DNA vaccines for viral infections: basic studies and applications. Adv Virus Res 2000;55:1–74 [DOI] [PubMed] [Google Scholar]
- 27.Roy MJ, Wu MS, Barr LJ, et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 2000;19:764–778 [DOI] [PubMed] [Google Scholar]
- 28.Scheerlinck JY. Genetic adjuvants for DNA vaccines. Vaccine 2001;19:2647–2656 [DOI] [PubMed] [Google Scholar]
- 29.Spolski R, and Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol 2008;26:57–79 [DOI] [PubMed] [Google Scholar]
- 30.Stuve O, Eagar TN, Frohman EM, and Cravens PD. DNA plasmid vaccination for multiple sclerosis. Arch Neurol 2007;64:1385–1386 [DOI] [PubMed] [Google Scholar]
- 31.Tacket CO, Roy MJ, Widera G, Swain WF, Broome S, and Edelman R. Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine 1999;17:2826–2829 [DOI] [PubMed] [Google Scholar]
- 32.Tang DC, DeVit M, and Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature 1992;356:152–154 [DOI] [PubMed] [Google Scholar]
- 33.Thermet A, Rollier C, Zoulim F, Trepo C, and Cova L. Progress in DNA vaccine for prophylaxis and therapy of hepatitis B. Vaccine 2003;21:659–662 [DOI] [PubMed] [Google Scholar]
- 34.Wunderlich G, Moura IC, and del Portillo HA. Genetic immunization of BALB/c mice with a plasmid bearing the gene coding for a hybrid merozoite surface protein 1-hepatitis B virus surface protein fusion protects mice against lethal Plasmodium chabaudi chabaudi PC1 infection. Infect Immun 2000;68:5839–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W, Chu Y, Zhang R, Xu H, Wang Y, and Xiong S. Endoplasmic reticulum targeting sequence enhances HBV-specific cytotoxic T lymphocytes induced by a CTL epitope-based DNA vaccine. Virology 2005;334:255–263 [DOI] [PubMed] [Google Scholar]
- 36.Yi JS, Du M, and Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science 2009;324:1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You Z, Huang X, Hester J, Toh HC, and Chen SY. Targeting dendritic cells to enhance DNA vaccine potency. Cancer Res 2001;61:3704–3711 [PubMed] [Google Scholar]
- 38.Zhang JY, Zhang Z, Lin F, et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 2010;51:81–91 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Cobleigh MA, Lian JQ, et al. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 2011;141:1897–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Lian JQ, Huang CX, et al. Overexpression of Toll-like receptor 2/4 on monocytes modulates the activities of CD4(+)CD25(+) regulatory T cells in chronic hepatitis B virus infection. Virology 2010;397:34–42 [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Zhang Z, Luan Y, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 2014;59:1331–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou C, Peng G, Jin X, Tang J, and Chen Z. Vaccination with a fusion DNA vaccine encoding hepatitis B surface antigen fused to the extracellular domain of CTLA4 enhances HBV-specific immune responses in mice: implication of its potential use as a therapeutic vaccine. Clin Immunol 2010;137:190–198 [DOI] [PubMed] [Google Scholar]