Abstract
MicroRNA (miR)-146a and miR-146b are negative regulators of inflammatory gene expression in lung fibroblasts, epithelial cells, monocytes, and endothelial cells. The abundance of cyclooxygenase-2 (COX-2) and IL-1β is negatively regulated by the miR-146 family, suggesting miR-146a and/or miR-146b might modulate inflammatory mediator expression in airway smooth muscle thereby contributing to pathogenesis of asthma. To test this idea we compared miR-146a and miR-146b expression in human airway smooth muscle cells (hASMCs) from nonasthmatic and asthmatic subjects treated with cytomix (IL-1β, TNF-α, and IFNγ) and examined the miRNAs' effects on COX-2 and IL-1β expression. We found that cytomix treatment elevated miR-146a and miR-146b abundance. Induction with cytomix was greater than induction with individual cytokines, and asthmatic cells exhibited higher levels of miR-146a expression following cytomix treatment than nonasthmatic cells. Transfection of miR-146a or miR-146b mimics reduced COX-2 and IL-1β expression. A miR-146a inhibitor increased COX-2 and IL-1β expression, but a miR-146b inhibitor was ineffective. Repression of COX-2 and IL-1β expression by miR-146a correlated with reduced abundance of the RNA-binding protein human antigen R. These results demonstrate that miR-146a and miR-146b expression is inducible in hASMCs by proinflammatory cytokines and that miR-146a expression is greater in asthmatic cells. Both miR-146a and miR-146b can negatively regulate COX-2 and IL-1β expression at pharmacological levels, but loss-of-function studies showed that only miR-146a is an endogenous negative regulator in hASMCs. The results suggest miR-146 mimics may be an attractive candidate for further preclinical studies as an anti-inflammatory treatment of asthma.
Keywords: cyclooxygenase-2, human antigen R, interleukin-1β, miRNA-146, inflammation
asthma is characterized by inflammation, airway remodeling, and airway hyperresponsiveness. Airway smooth muscle cells function as immunomodulatory and contractile cells in the lung and contribute to the pathogenesis of asthma (59). A small subset of patients with severe asthma do not respond adequately to current therapies, exhibit corticosteroid insensitivity, and account for a large and disproportionate amount of all asthma-related health care costs (1, 10, 23). This subset of patients with poorly controlled asthma highlights the need for investigating novel anti-inflammatory drug targets.
MicroRNAs (miRNAs or miRs) are posttranscriptional regulators of gene expression that have been linked to a number of pathologies, including chronic obstructive pulmonary disease, pulmonary arterial hypertension, and idiopathic pulmonary fibrosis (14, 49, 60). MicroRNAs are differentially expressed in asthmatic human airway smooth muscle cells (hASMCs) and exhibit dynamic changes in expression in the lungs of mice during the development of allergic airway inflammation in models of asthma (11, 16, 28, 39, 45, 55). Members of the miR-146 family, consisting of miR-146a and miR-146b, are elevated in a murine model of asthma (16). miR-146a and miR-146b are negative regulators of inflammatory gene expression in numerous cell types, including monocytes (54), fibroblasts (49), endothelial (9), airway smooth muscle (34), and epithelial cells (46). Validated inflammation-related targets of the miR-146 family include cyclooxygenase-2 (COX-2) (49), human antigen R (HuR) (9), IL-1 receptor-associated kinase-1 (IRAK1) (34, 54), TNF receptor-associated factor 6 (TRAF6) (34, 54), IL-1β (41), IL-6 (34), and IL-8 (34, 46). Of these targets, only IRAK1, TRAF6, IL-6, and IL-8 have been validated in hASMCs (34). Thus, we sought to further characterize miR-146a and miR-146b expression and function in hASMCs from patients with and without asthma. We chose to investigate whether miR-146a and miR-146b regulate the expression of COX-2 and IL-1β due to their relevance to inflammation and also to determine novel cell-specific functions for the miRNAs in airway smooth muscle by investigating miR-146a regulation of HuR in these cells. The studies are stimulated by the important role of hASMCs in asthma and because expression and functional results in this cell type are limited to one study (34).
Larner-Svensson et al. and Perry and colleagues characterized miR-146 expression and function in hASMCs and human lung alveolar epithelial cells (34, 46). miR-146a expression in hASMCs was inducible by treatment with IL-1β. Treatment of human retinal pigment epithelial cells with a multicytokine cocktail consisting of IL-1β, TNF-α, and IFNγ induced significantly higher levels of miR-146a and miR-146b than treatment with IL-1β alone. A similar synergistic effect has been reported in studies of hASMCs treated with the same cytokine cocktail (22, 51). Based on these studies, we chose to investigate miR-146a and miR-146b expression and function in hASMCs that were treated with the multicytokine cocktail or “cytomix” consisting of IL-1β, TNF-α, and IFNγ that is thought to mimic the inflammatory milieu that is present in late-stage inflammation in asthmatic airways (5, 57). Before this study, only miR-146a negative regulation of IRAK1, TRAF6, IL-6, and IL-8 had been investigated in airway smooth muscle (34). We demonstrate that miR-146a and miR-146b also negatively regulate COX-2 and IL-1β in hASMCs, that miR-146a negatively regulates HuR, and that both miR-146a and miR-146b are inducible in hASMCs treated with cytomix.
METHODS AND MATERIALS
Cell culture.
Primary nonasthmatic and asthmatic hASMCs were isolated from nontransplantable donor lungs or resected lung tissue by enzymatic digestion at the University of Chicago or University of Manitoba, respectively. All tissue procurement and cell culture studies were conducted following protocols approved by the Human Research Ethics Board (University of Manitoba) and Institutional Review Boards for Protection of Human Subjects of the University of Chicago and the University of South Alabama. Cells were cultured in 5% CO2 at 37°C in DMEM supplemented with 5% FBS, 0.5 μg/l basic fibroblast growth factor, 2 μg/l epidermal growth factor, 50 units/ml penicillin, and 100 μg/ml streptomycin. Cells were growth arrested in 50:50 DMEM-Ham's F-12 supplemented with 0.25 mg/l insulin, 0.11 mg/l transferrin, and 0.1 μg/l selenium. Nonasthmatic and asthmatic hASMCs were assayed at passage 6–8. Cells were treated with cytomix (10 ng/ml IL-1β, 10 ng/ml TNF-α, 10 ng/ml IFNγ), individual cytokines (10 ng/ml), or left untreated for 20 h as previously described (22, 51). Cells were not tested for the presence of mycoplasma. Culture media and supplements were obtained from Life Technologies (Grand Island, NY), Cell Generation (Fort Collins, CO), or Sigma-Aldrich (St. Louis, MO).
RNA isolation and quantitative RT-PCR.
Total RNA was extracted using miRNeasy (Qiagen, Valencia, CA). RNA quality and concentration were assessed by spectrophotometry. miR-146a-5p and miR-146b-5p were assayed using Taqman miRNA assays (Life Technologies) according to the manufacturer's protocol with an input of 40 ng total RNA and quantified using miRNA standard curves. miRNA standards were synthesized by IDT (Coralville, IA) using sequences from miRBase (version 19) (18, 19, 29). Reverse transcription was performed using 1 μg total RNA and an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time amplification was performed using RT2 SYBR green/fluorescein quantitative RT-PCR (qPCR) Mastermix (Qiagen) and previously published primers for COX-2 (51), 18S rRNA (51), and IL-1β (48).
Mimic and inhibitor miRNA transfection.
Either miR-146a-5p or miR-146b-5p miRIDIAN duplex mimics, Caenorhabditis elegans (cel)-67 miRIDIAN negative control duplex mimics, miR-146a-5p or miR-146b-5p miRIDIAN hairpin inhibitors, or cel-67 miRIDIAN negative control inhibitors (Thermo Fisher Scientific, Waltham, MA) were transfected into hASMCs by reverse transfection at 30 nM using 0.5% (vol/vol) siPORT NeoFX (Life Technologies) as previously described (30). Mimic and inhibitor concentrations were optimized by transfecting cells with 10 or 30 nM miR-146a mimic or 30 or 90 nM 146a inhibitor (data not shown). In addition, transfection efficiency was determined by transfecting cells with 3–30 nM TEX615 siRNA (IDT). Transfection efficiency was 63% at 10 nM and 76% at 30 nM oligonucleotide.
Western blotting.
Protein samples were prepared as previously described (51). Total protein for COX-2 (15 μg), pro-IL-1β (15 μg), and HuR (10 μg) was separated by SDS-PAGE on 10% bis-Tris polyacrylamide gels in NuPAGE MOPS buffer (Life Technologies) and transferred to nitrocellulose in NuPAGE transfer buffer containing 10% methanol. Equal loading was verified using Fastblot stain (G-biosciences, St. Louis, MO). Blocking was performed using 50:50 phosphate-buffered saline/Odyssey blocking solution (LI-COR, Lincoln, NE). Anti-COX-2 (sc-1745; Santa Cruz, Dallas, TX) and anti-IL-1β (AF201NA; R&D Systems, Minneapolis, MN) antibodies were used as previously published (22, 51). Anti-HuR (sc-5261; Santa Cruz) antibody was used at a dilution of 1:500. Fluorescent secondary antibodies (IRDye 800CW; LI-COR) were detected with an Odyssey near-infrared scanner (LI-COR). Integrated intensities of protein bands were expressed relative to protein levels in control mimic or control inhibitor-treated cells as appropriate.
PGE2 assay.
Cells were treated with cytomix or left untreated for 20 h, and PGE2 abundance was determined by enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) following the manufacturer's instructions. Results were normalized to the PGE2 levels in cells transfected with control mimic and treated with cytomix.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism (version 4 for Windows; GraphPad Software, La Jolla, CA, www.graphpad.com). Results were analyzed by one-way ANOVA with Bonferroni's post hoc testing or Dunnett's post hoc testing as appropriate.
RESULTS
miR-146a and miR-146b expression in asthmatic and nonasthmatic hASMCs.
To determine the role of miR-146a and miR-146b in regulating COX-2 and IL-1β expression in hASMCs treated with cytomix, we first had to determine if miRNA expression was induced under these proinflammatory conditions. Asthmatic and nonasthmatic hASMCs were left untreated, treated with cytomix (10 ng/ml each of IL-1β, TNF-α, and IFNγ), or treated with the individual cytokines for 20 h. miR-146a and miR-146b abundance was measured using qRT-PCR. Treatment with cytomix induced expression of both both miR-146a (Fig. 1A) and miR-146b (Fig. 1B). miR-146a expression was higher in asthmatic than in nonasthmatic hASMCs treated with cytomix (Fig. 1A). Compared with cytomix, individual cytokines produced little or no upregulation of miR-146a or miR-146b. Only TNF-α increased miR-146a expression significantly in nonasthmatic hASMCs (Fig. 1A). These results demonstrated that miR-146a and miR-146b are inducible and that simultaneous stimulation with a mixture of proinflammatory cytokines is required for maximum expression. These results are consistent with previous reports of potentiation of COX-2 and IL-1β expression in hASMCs with cytomix compared with individual cytokines (22, 43, 51). miR-146a and/or miR-146b may act as negative regulators of the proinflammatory function of hASMCs under these conditions (9). To test whether changes in miR-146a or miR-146b are linked with changes in COX-2 and IL-1β expression we used miRNA mimics and inhibitors to determine effects on proinflammatory function of hASMCs.
Fig. 1.
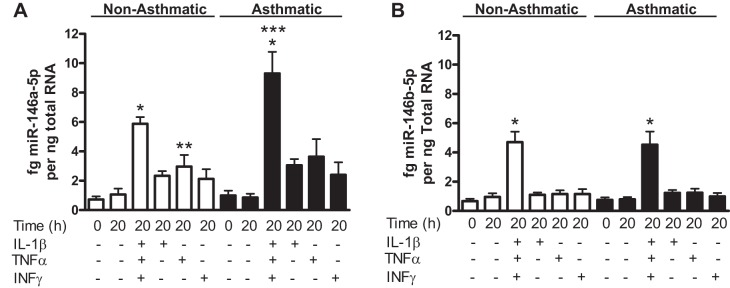
MicroRNA (miR)-146a and miR-146b abundance in cytokine-treated human airway smooth muscle cells (hASMCs). miR-146a (A) and miR-146b (B) abundance in nonasthmatic (open bars) and asthmatic (solid bars) hASMCs treated with cytomix (10 ng/ml each of IL-1β, TNF-α, and IFNγ), individual cocktail components, or left untreated for 20 h. Data (mean + SE) obtained from 5 nonasthmatic and 7 asthmatic donors; n = 10–14 cultures. *P < 0.01 vs. nonasthmatic or asthmatic 20 h untreated control; **P < 0.05 vs. nonasthmatic 20 h untreated control; ***P < 0.05 vs. nonasthmatic 20 h treated with cytomix. P values for comparisons were calculated using 1-way ANOVA with Bonferroni's test. P values for comparisons of treatments within either nonasthmatic or asthmatic groups were calculated using 1-way ANOVA with Dunnett's test.
miR-146a negatively regulates COX-2 expression in hASMCs.
COX-2 is a validated target of miR-146a in fibroblasts (49). However, miR-146b is also predicted to target COX-2 due to sequence homology with miR-146a (54), but this has not been validated experimentally. To determine if miR-146a and miR-146b can both silence COX-2 expression in hASMCs, we transfected cells with specific duplex miRNA mimics and hairpin inhibitors. Successful transfection of miRNA mimics and inhibitors was validated by qRT-PCR and transfection of fluorescently labeled oligonucleotides, which successfully transfected 76% of cells (data not shown). hASMCs were transfected with 10 and 30 nM miR-146a mimics to determine the optimal mimic concentration. Transfection with 10 nM miR-146a mimic was not sufficient to reduce COX-2 expression in hASMCs (data not shown). Transfection of 30 nM MiR-146a or 30 nM miR-146b mimics both reduced relative COX-2 mRNA (Fig. 2A) and protein (Fig. 2B) abundance in hASMCs treated with cytomix. Furthermore, miR-146a and miR-146b mimics both reduced relative PGE2 secretion (Fig. 2C). Transfection with 30 nM mimic increased miR-146a and miR-146b abundance in hASMCs by 260-fold compared with control mimic transfected cells that were treated with cytomix (data not shown). Inhibition of miR-146a by transfecting 30 nM of a hairpin inhibitor resulted in a modest but statistically significant increase in relative COX-2 protein abundance (Fig. 3). Transfecting cells with 90 nM of the miR-146a inhibitor did not have an additive effect on the enhancement of COX-2 expression (data not shown). Interestingly, inhibition of miR-146b did not increase COX-2 protein abundance (Fig. 3). These results show that both miR-146 family members can repress COX-2 expression at pharmacological levels, but only miR-146a acts as an endogenous negative regulator in hASMCs stimulated with cytomix.
Fig. 2.
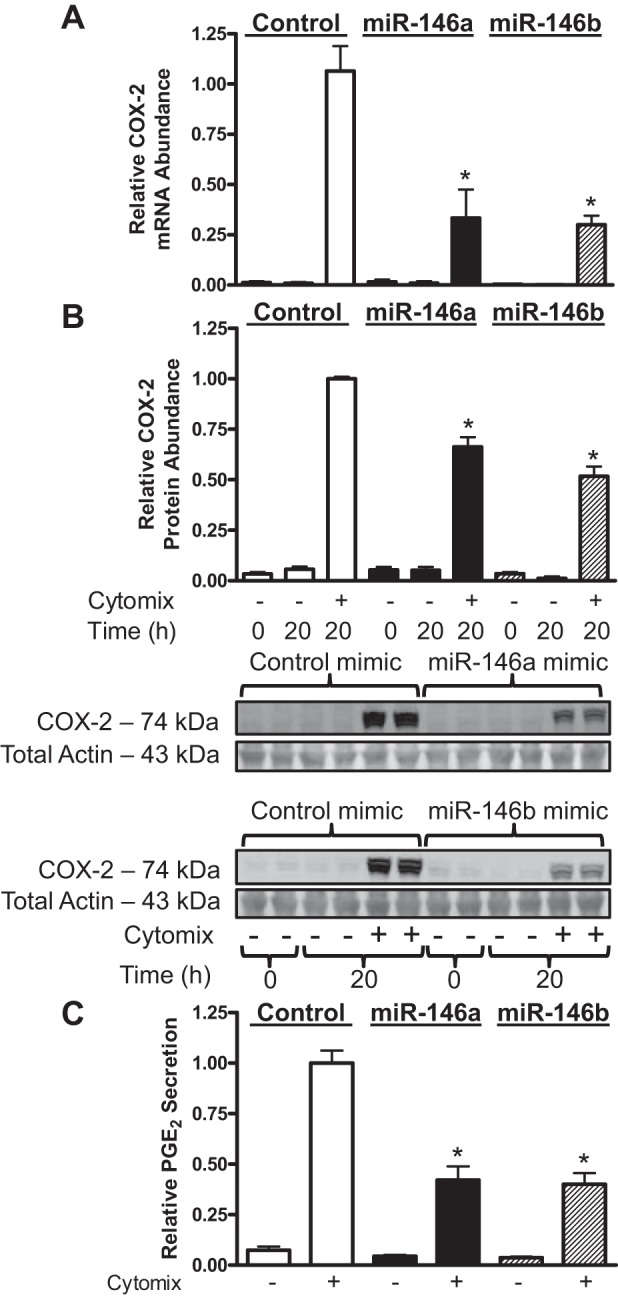
miR-146 mimics reduce cyclooxygenase-2 (COX-2) expression and PGE2 secretion in hASMCs following cytomix treatment. hASMCs were transfected with 30 nM control (open bars), miR-146a (solid bars), or miR-146b (hatched bars) mimics and then treated with cytomix (10 ng/ml each of IL-1β, TNF-α, and IFNγ) for 20 h. Relative COX-2 mRNA abundance (A), protein abundance (B), and PGE2 secretion (C) were assayed under these conditions and normalized relative to cytomix-treated control at 20 h. Data (mean + SE) obtained from 3–5 donors; n = 5–11 cultures (mRNA), n = 7–16 cultures (protein), n = 10–19 cultures (PGE2). *P < 0.01 vs. control mimic treated with cytomix, 20 h. All P values calculated using 1-way ANOVA with Dunnett's test.
Fig. 3.
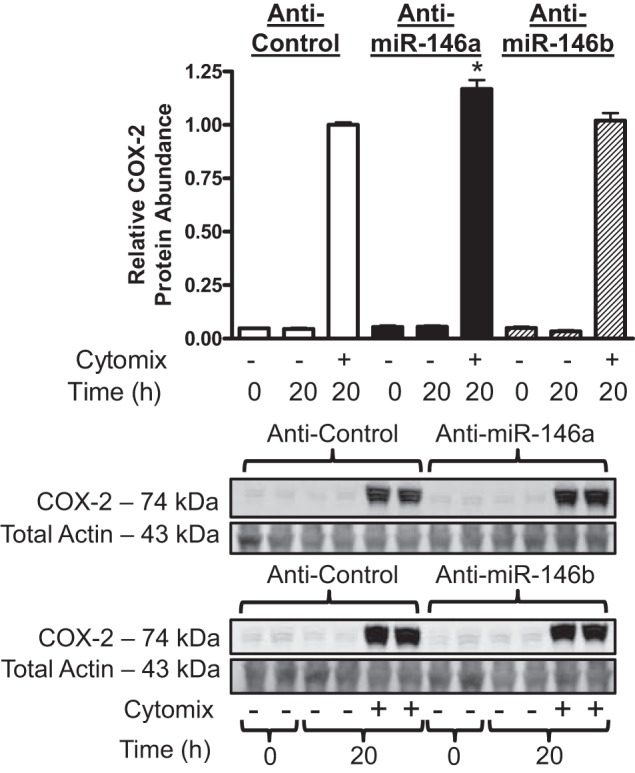
miR-146a inhibition increases COX-2 protein abundance in hASMCs following cytomix treatment. hASMCs were transfected with 30 nM control (open bars), miR-146a (solid bars), or miR-146b (hatched bars) inhibitors and then treated with cytomix (10 ng/ml each of IL-1β, TNF-α, and IFNγ) for 20 h. COX-2 protein abundance was assayed and normalized relative to cytomix-treated control at 20 h. Data (mean + SE) obtained from 4 donors; n = 7–17 cultures. *P < 0.01 vs. 20 h control inhibitor treated with cytomix. All P values calculated using 1-way ANOVA with Dunnett's test.
miR-146a negatively regulates IL-1β expression in hASMCs.
miR-146a was previously shown to reduce IL-1β expression in mouse macrophages (41). To determine if miR-146a and miR-146b negatively regulate IL-1β expression in hASMCs, we used a gain-of-function and loss-of-function approach as above. Transfection of 30 nM miR-146a or 30 nM miR-146b mimics both reduced IL-1β mRNA (Fig. 4A) and pro-IL-1β protein (Fig. 4B) abundance in hASMCs treated with cytomix. Inhibition of miR-146a but not miR-146b using 30 nM of the inhibitor modestly increased pro-IL-1β protein abundance (Fig. 5). Transfecting cells with 90 nM of the miR-146a inhibitor did not have an additive effect on the enhancement of pro-IL-1β expression (data not shown). These results indicate that both miRNAs are capable of repressing IL-1β expression at pharmacological levels, but only miR-146a acts as an endogenous negative regulator of IL-1β expression in hASMCs.
Fig. 4.
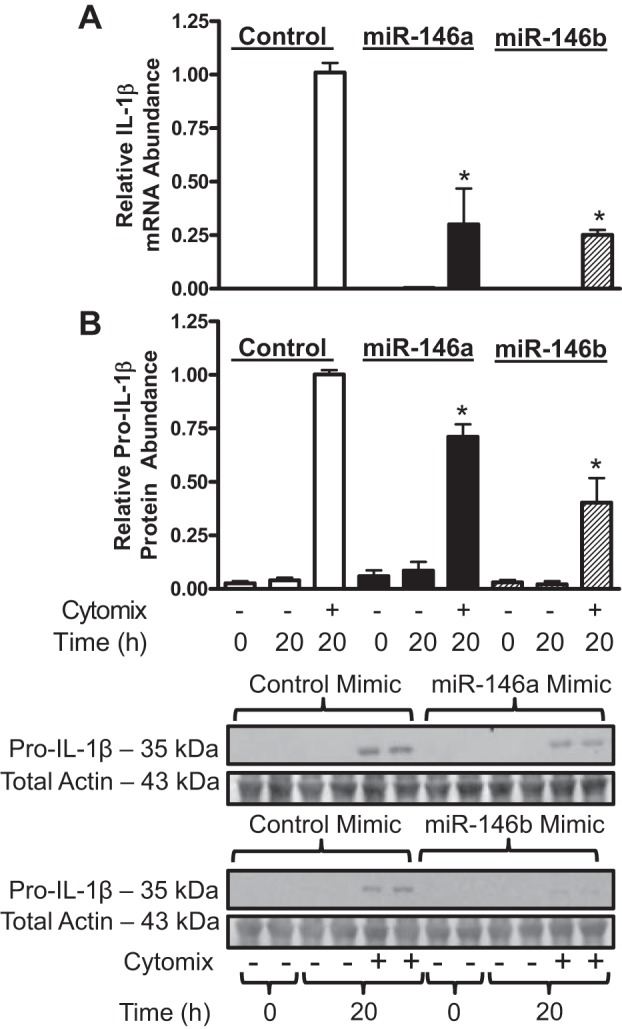
miR-146 mimics decrease IL-1β mRNA and pro-IL-1β protein abundance in hASMCs following cytomix treatment. hASMCs were transfected with 30 nM control (open bars), miR-146a (solid bars), or miR-146b (hatched bars) mimics and then treated with cytomix (10 ng/ml each of IL-1β, TNF-α, and IFNγ) for 20 h. IL-1β mRNA (A) and protein (B) abundance was assayed and normalized relative to cytomix-treated control at 20 h. Data (mean + SE) obtained from 3 to 5 donors; n = 5–11 mRNA cultures, n = 7–15 protein cultures. *P < 0.01 vs. control mimic treated with cytomix. All P values calculated using 1-way ANOVA with Dunnett's test.
Fig. 5.
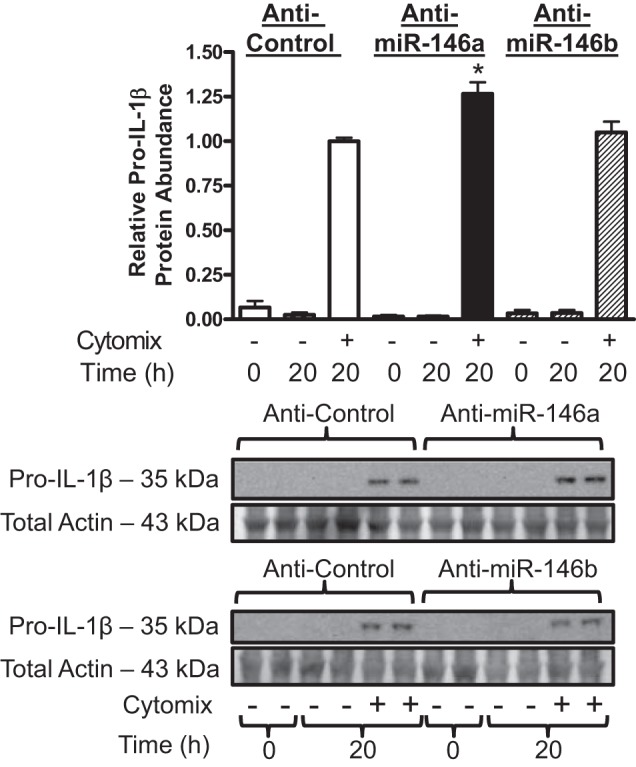
miR-146a inhibition increases pro-IL-1β protein abundance in hASMCs following cytomix treatment. hASMCs were transfected with 30 nM control (open bars), 30 nM miR-146a (solid bars), or 30 nM miR-146b (hatched bars) inhibitors and then treated with cytomix (10 ng/ml each of IL-1β, TNF-α, and IFNγ) for 20 h. IL-1β protein abundance was assayed by Western blotting and normalized relative to cytomix-treated control at 20 h. Data (mean + SE) obtained from 4 donors; n = 8–16 cultures. *P < 0.01 vs. control mimic treated with cytomix. All P values calculated using 1-way ANOVA with Dunnett's test.
miR-146a represses HuR expression in hASMCs.
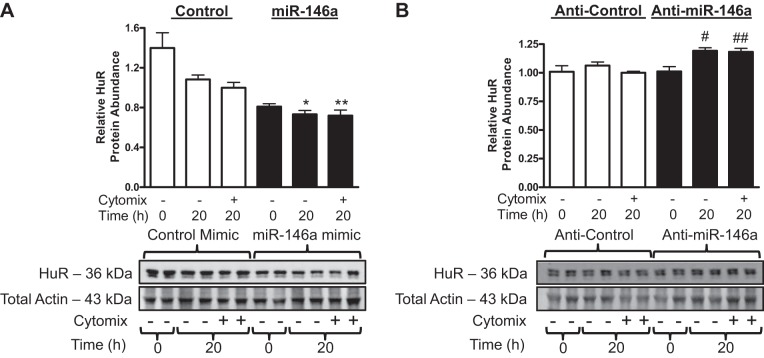
miR-146a is not predicted to directly target the 3′-untranslated region of the IL-1β transcript (3), which raises the question of what the mechanism of action is for repressing IL-1β abundance. Early studies observed that miR-146a/b repressed the expression of IRAK1 and TRAF6 (3a, 54). Later, Cheng and colleagues demonstrated that miR-146a also directly represses the expression of HuR in endothelial cells (9). HuR is downstream of IRAK1 and TRAF6 and is a direct mRNA-binding protein that stabilizes many inflammation-related mRNAs (15). Because miR-146a downregulation of cytokine synthesis was found not to be mediated by repression of IRAK1 and TRAF6 in a previous study in hASMCs (34), we chose to investigate miR-146a targeting of HuR. We used a gain-of-function and loss-of-function approach as above. Transfection of 30 nM miR-146a mimic reduced HuR protein abundance (Fig. 6A) in untreated cells and in cells that were treated with cytomix. Inhibition of miR-146a using 30 nM of the inhibitor increased HuR protein abundance (Fig. 6B) in untreated cells and in cells treated with cytomix. Transfecting the cells with 90 nM of the miR-146a inhibitor did not have an additive effect on the enhancement of HuR expression (data not shown). We did not investigate whether miR-146b negatively regulated HuR expression in hASMCs because there is no evidence that it is an endogenous negative regulator of IL-1β expression in cells treated with cytomix (see Fig. 5). Our results indicate that miR-146a can negatively regulate expression of HuR in hASMCs, which could contribute to destabilization of cytokine-stimulated mRNAs and reduced pro-IL-1β abundance.
Fig. 6.
miR-146a represses human antigen R (HuR) expression in hASMCs. hASMCs were transfected with 30 nM control (open bars), 30 nM miR-146a (solid bars) mimics (A), or inhibitors (B) and then treated with cytomix (10 ng/ml each of IL-1β, TNF-α, and IFNγ) or left untreated for 20 h. HuR protein abundance was assayed and normalized relative to control. Data (mean + SE) obtained from 4 donors; n = 8–16. *P < 0.001 vs. control mimic 20 h untreated. **P < 0.001 vs. control mimic treated with cytomix. #P < 0.05 vs. control inhibitor 20 h untreated. ##P < 0.01 vs. control inhibitor treated with cytomix. All P values calculated using 1-way ANOVA with Bonferroni's test.
DISCUSSION
Members of the miR-146 family are anti-inflammatory miRNAs that are induced in response to proinflammatory conditions (54). Here we report the novel finding that miR-146a and miR-146b expression is greatly enhanced in hASMCs treated with cytomix compared with treatment with the individual cytokines. Furthermore, we report that miR-146b is inducible in hASMCs treated with cytomix, whereas, previously, Larner-Svensson and colleagues observed no induction in hASMCs treated with IL-1β alone (34). Interestingly, we observed that miR-146a expression is greater in asthmatic hASMCs treated with cytomix, a novel finding that is supported by observations made by others of enhanced expression of cytokine-responsive genes in asthmatic hASMCs (4, 8, 27, 45). We demonstrate that miR-146a and miR-146b mimics are capable of negatively regulating the expression of COX-2 and IL-1β in hASMCs and thus are functioning as anti-inflammatory miRNAs as originally hypothesized by Taganov and colleagues (54). Furthermore, we demonstrate that, despite not being predicted to directly targeting the 3′-untranslated region of IL-1β mRNA, both miR-146a and miR-146b are capable of reducing steady-state levels of IL-1β mRNA in hASMCs. In support of this observation, we also observed that miR-146a repressed the expression of HuR in hASMCs, which may contribute to the reduction of IL-1β mRNA levels observed in this study and previous observations of miR-146a regulation of IL-6 and IL-8 by Larner-Svensson and colleagues (34). Interestingly, our loss-of-function studies indicate that only miR-146a is an endogenous negative regulator of COX-2 and IL-1β in hASMCs indicating that it is the functionally dominant miR-146 family member in hASMCs under these conditions, which is reflected by its slightly higher abundance under these conditions.
In this study, we sought to further characterize the expression and function of miR-146a and miR-146b in hASMCs. Larner-Svensson and colleagues (34) observed induction of miR-146a expression but not of miR-146b in hASMCs treated with IL-1β. We found that cytomix induced miR-146b expression as well as much higher levels of miR-146a than that obtained by treating the cells with IL-1β alone. We previously demonstrated that cytomix has a synergistic effect on protein-coding gene expression compared with the individual cytokine components and such an effect may be occurring for expression of miRNA genes as well (22, 43, 51). Cytomix activates NF-κB, ERK1/2, JNK1/2, p38 MAPK, and JAK/STAT signal transduction pathways in hASMCs (22, 51, 52). ERK1/2 and JNK1/2 regulate miR-146a and miR-146b processing, and NF-κB is a transcriptional regulator of miR-146a expression (34, 47, 54). Interestingly, p38 MAPK has not been linked to the regulation of miR-146a and miR-146b expression despite recent evidence that the pathway regulates p68 nuclear translocation and increases the miRNA processing activity of the DROSHA microprocessor complex (24). This raises the question of whether there are subtle differences in miRNA processing depending on cell type and the pathways being activated at any given time. Therefore, we investigated expression and mRNA targeting of miR146a and miR-146b in hASMCs under conditions that mimic the proinflammatory state in the airways of humans with asthma.
We observed significant increases in miR-146a expression when the cells were treated with TNF-α or cytomix. Interestingly, Larner-Svensson and colleagues observed an increase in miR-146a expression when hASMCs were treated with IL-1β (34) but our results indicated a trend toward an increase that was not statistically significant. Our results do not challenge those of Lindsay and colleagues but rather may be due to a difference in experimental design that requires corrections for multiple comparisons. These results highlight the large differences in miRNA expression that are observed when cells are treated with a multicytokine cocktail vs. individual cytokines as previously observed by Kutty and colleagues in epithelial cells (31). Furthermore, differences in expression between nonasthmatic and asthmatic cells were only evident when the cells were treated with the multicytokine cocktail that mimics the inflammatory milieu. Interestingly, no differences in basal miR-146a or miR-146b expression were observed between asthmatic and nonasthmatic cells at 0 or 20 h. These results are supported by a study by Williams and colleagues (58) who found no differences in miR-146a or miR-146b expression in samples from patients with mild asthma compared with control patients.
COX-2 was previously demonstrated to be a target of miR-146a (49), and we have confirmed this in hASMCs. Furthermore, these results indicate that miR-146a contributes to the maintenance of steady-state COX-2 expression that we previously observed in hASMCs treated with cytomix (51). Additionally, we have demonstrated that miR-146b is also capable of repressing COX-2 in hASMCs when the miRNA is present at pharmacological levels. However, miRNA inhibition experiments show that only miR-146a is an endogenous negative regulator. This negative regulatory role is modest as demonstrated by the modest increases in COX-2 protein abundance seen after inhibition of miR-146a, but a modest increase is reasonable considering that miRNAs normally function as minor regulators of individual gene expression that are capable of regulating a large number of genes (Fig. 3). miRNA-mediated reductions in COX-2 expression correlated with a reduction in PGE2 secretion. β2-Adrenoceptor heterologous desensitization has been observed in some patients with asthma, and in vitro this has been linked to cytokine-mediated induction of COX-2 and autocrine PGE2 signaling in hASMCs (2, 20, 33, 42, 50). These results suggest targeted delivery of miR-146a and miR-146b in the airways of patients with asthma that are hyporesponsive to β2-adrenoceptor agonists may help increase patient responsiveness by reducing COX-2 expression.
The miR-146a- and miR-146b-mediated reductions in PGE2 secretion observed in this study were greater in magnitude than the reduction in relative COX-2 protein abundance. This discrepancy may be due to miR-146 repression of additional enzymes involved in PGE2 synthesis. Microsomal PGE2 synthase-1 and cytosolic phospholipase A2 are induced in parallel with COX-2 to promote PGE2 biosynthesis when hASMCs are stimulated with IL-1β (43, 44). Recently, miR-146a was shown to target prostaglandin E synthase-2 in murine bone marrow-derived mesenchymal stem cells, but prostaglandin E synthase-2 mRNA is not a predicted target for miR-146a in humans (3, 40). Human miR-146a and miR-146b are predicted to target microsomal prostaglandin E synthase-1 (3). Whether miR-146a and miR-146b are repressing microsomal prostaglandin E synthase-1 expression in hASMCs is unknown and will need to be investigated in the future.
miR-146a and miR-146b are important negative regulators of the innate immune response (37). This immunoregulatory function has been attributed to repression of IRAK1 and TRAF6 (54). However, Larner-Svensson and colleagues (34) presented evidence that, although miR-146a silences IRAK1 and TRAF6 expression in hASMCs, a reduction in the transcriptional activation of IL-6 and IL-8 was not responsible for the decrease in cytokine secretion. Our results support an alternate mechanism where stability of cytokine transcripts is reduced by increased miR-146a and reduced expression of the mRNA-binding protein HuR. miR-146a represses expression of HuR protein in hASMCs (Fig. 6) as previously described in endothelial cells (9). Thus our results taken together with Larner-Svenssson et al. (34) demonstrate that members of the miR-146 family negatively regulate cytokine-responsive gene expression in hASMCs by multiple mechanisms, including direct targeting of mRNAs and repression of HuR expression. In addition, miR-146a might inhibit translation of cytokine mRNAs. This notion is supported by recent studies showing that miR-146a promotes cytosolic localization of RNA-binding motif (RBM)-4 (6, 7). RBM4 inhibits the translation of bound transcripts by a mechanism that may require colocalization of argonaute-2, and such a mechanism may be partly responsible for the effects of miR-146a in hASMCs (6, 7, 34, 35).
The ability of mimics for miR-146a or miR-146b to act as anti-inflammatory agents may be beneficial as new add-on therapy with inhaled glucocorticoids (9, 34, 46, 54). Numerous additional inflammation-related targets have been identified for the miR-146 family that support the mimics as potential anti-inflammatory agents, including C-X-C chemokine receptor type 4 (32), IRAK2 (25), Kruppel-like factor 4 (53), Rho-associated protein kinase-1 (36), and signal transducer and activator of transcription 1 (38). A number of these targets are involved in signal transduction, and, as a result, the miR-146 family has the potential to indirectly regulate the expression of a number of cytokine-responsive genes, including IL-1β (41), IL-6 (34), IL-8 (34, 46), MAPK phosphatase-1 (6), and regulated on activation, normal T cell expressed and secreted (46). Elevated miR-146a and miR-146b would likely repress Toll-like receptor (TLR)-4 and IL-1 receptor signal transduction in patients with inflammatory lung disease. TLR-4 activation on structural cells in the airways of mice is required for the development of allergic airway inflammation in response to sensitization and challenge with house dust mite extract, an allergen for which a high percentage of patients with asthma exhibit atopy (21, 56). Mattes and colleagues have demonstrated that allergic airway inflammation is attenuated in mice lacking TLR-4 following sensitization and challenge with house dust mite extract (39). Targeted delivery of miR-146a and miR-146b to the airways would mimic the effect of TLR-4 knockout that was observed in mice by antagonizing TLR-4 signaling (54).
In conclusion, miR-146a and miR-146b expression is induced in asthmatic and nonasthmatic hASMCs treated with a mixture of cytokines. miR-146a expression was higher in asthmatic hASMCs that were treated with cytomix. miR-146a but not miR-146b may be a modest endogenous negative regulator of COX-2 and IL-1β gene expression in hASMCs. Furthermore, miR-146a negatively regulates HuR expression in hASMCs. Future studies will need to investigate miR-146a and miR-146b function in mouse models of asthma to determine if the miRNAs should be targeted in asthmatic patients.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-077726 (W. T. Gerthoffer), HL-097805 (J. Solway), and HL-092588 (B. Camoretti-Mercado). A. J. Halayko is supported through the Canada Research Chairs program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.S.C. and W.T.G. conception and design of research; B.S.C. performed experiments; B.S.C. analyzed data; B.S.C. and W.T.G. interpreted results of experiments; B.S.C. prepared figures; B.S.C. drafted manuscript; B.S.C., B.C.-M., P.C.K., A.J.H., J.S., and W.T.G. edited and revised manuscript; B.S.C., B.C.-M., P.C.K., A.J.H., J.S., and W.T.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ning Cheng for technical assistance.
Some of the tissue used for this research was provided by Gift of Hope Organ & Tissue Donor Network through the generous gift of donor families with assistance at the University of Chicago by Dr. Bohao Chen.
Parts of this study were previously presented at scientific meetings and as part of a dissertation (12, 13).
REFERENCES
- 1.Adcock IM, Lane SJ. Corticosteroid-insensitive asthma: molecular mechanisms. J Endocrinol 178: 347–355, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ, Pride NB. Dose-response curves to inhaled beta-adrenoceptor agonists in normal and asthmatic subjects. Br J Clin Pharmacol 15: 677–682, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res 36: D149–D153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of miR-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene 27: 5643–5647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med 171: 1103–1108, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol 89: 958–967, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Brudecki L, Ferguson DA, McCall CE, El Gazzar M. Mitogen-activated protein kinase phosphatase 1 disrupts proinflammatory protein synthesis in endotoxin adapted monocytes. Clin Vaccine Immunol 20: 1396–1404, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brudecki L, Ferguson DA, McCall CE, El Gazzar M. MicroRNA-146a and RBM4 form a negative feed-forward loop that disrupts cytokine mRNA translation following TLR4 responses in human THP-1 monocytes. Immunol Cell Biol 91: 532–540, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan V, Burgess JK, Ratoff JC, O'Connor BJ, Greenough A, Lee TH, Hirst SJ. Extracellular matrix regulates enhanced eotaxin expression in asthmatic airway smooth muscle cells. Am J Respir Crit Care Med 174: 379–385, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med 5: 1017–1034, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, Bel E, Burney P, Chanez P, Connett G, Corrigan C, de Blic J, Fabbri L, Holgate ST, Ind P, Joos G, Kerstjens H, Leuenberger P, Lofdahl CG, McKenzie S, Magnussen H, Postma D, Saetta M, Salmeron S, Sterk P. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma European Respiratory Society. Eur Respir J 13: 1198–1208, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Collison A, Mattes J, Plank M, Foster PS. Inhibition of house dust mite-induced allergic airways disease by antagonism of microRNA-145 is comparable to glucocorticoid treatment. J Allergy Clin Immunol 128: 160–167. e4, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Comer BS, Camoretti-Mercado B, Halayko AJ, Solway J, Gerthoffer WT. MiR-146a reduces cyclooxygenase-2 expression in human airway smooth muscle cells (Abstract). Am J Respir Crit Care Med 185: A4992, 2012 [Google Scholar]
- 13.Comer BS. Cyclooxygenase-2 expression in asthmatic human airway smooth muscle cells. Univ. South Ala. 2013 [Google Scholar]
- 14.Courboulin A, Paulin R, Giguère NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Côté J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 208: 535–548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 17: 3448–3460, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garbacki N, Di Valentin E, Huynh-Thu VA, Geurts P, Irrthum A, Crahay C, Arnould T, Deroanne C, Piette J, Cataldo D, Colige A. MicroRNAs profiling in murine models of acute and chronic asthma: a relationship with mRNAs targets. PLoS One 6: e16509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerthoffer WT, Singer CA. MAPK regulation of gene expression in airway smooth muscle. Respir Physiol Neurobiol 137: 237–250, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34: D140–D144, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res 36: D154–D158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo M, Pascual RM, Wang S, Fontana MF, Valancius CA, Panettieri RA, Jr., Tilley SL, Penn RB. Cytokines regulate β-2-adrenergic receptor responsiveness in airway smooth muscle via multiple PKA- and EP2 receptor-dependent mechanisms. Biochemistry (Mosc) 44: 13771–13782, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 15: 410–416, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedges JC, Singer CA, Gerthoffer WT. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol 23: 86–94, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Hew M, Chung KF. Corticosteroid insensitivity in severe asthma: significance, mechanisms and aetiology. Intern Med J 40: 323–334, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Hong S, Noh H, Chen H, Padia R, Pan ZK, Su SB, Jing Q, Ding HF, Huang S. Signaling by p38 MAPK stimulates nuclear localization of the microprocessor component p68 for processing of selected primary microRNAs. Sci Signal 6: ra16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou J, Wang P, Lin L, Liu X, Ma F, An H, Wang Z, Cao X. MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol Baltim Md 1950 183: 2150–2158, 2009 [DOI] [PubMed] [Google Scholar]
- 27.John AE, Zhu YM, Brightling CE, Pang L, Knox AJ. Human airway smooth muscle cells from asthmatic individuals have CXCL8 hypersecretion due to increased NF-κB p65, C/EBβ, and RNA polymerase II binding to the CXCL8 promoter. J Immunol 183: 4682–4692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jude JA, Dileepan M, Subramanian S, Solway J, Panettieri RA, Jr., Walseth TF, Kannan MS. miR-140-3p regulation of TNF-α-induced CD38 expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L460–L468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 39: D152–D157, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhn AR, Schlauch K, Lao R, Halayko AJ, Gerthoffer WT, Singer CA. MicroRNA expression in human airway smooth muscle cells: Role of miR-25 in regulation of airway smooth muscle phenotype. Am J Respir Cell Mol Biol 42: 506–513, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Jaworski C, Duncan T, Cameron JE, Flemington EK, Hooks JJ, Redmond TM. Differential regulation of microRNA-146a and microRNA-146b-5p in human retinal pigment epithelial cells by interleukin-1β, tumor necrosis factor-α, and interferon-γ. Mol Vis 19: 737–750, 2013 [PMC free article] [PubMed] [Google Scholar]
- 32.Labbaye C, Spinello I, Quaranta MT, Pelosi E, Pasquini L, Petrucci E, Biffoni M, Nuzzolo ER, Billi M, Foà R, Brunetti E, Grignani F, Testa U, Peschle C. A three-step pathway comprising PLZF/miR-146a/CXCR4 controls megakaryopoiesis. Nat Cell Biol 10: 788–801, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Laporte JD, Moore PE, Panettieri RA, Moeller W, Heyder J, Shore SA. Prostanoids mediate IL-1β-induced β-adrenergic hyporesponsiveness in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 275: L491–L501, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Larner-Svensson H, Williams A, Tsitsiou E, Perry M, Jiang X, Chung K, Lindsay M. Pharmacological studies of the mechanism and function of interleukin-1β-induced miRNA-146a expression in primary human airway smooth muscle (Abstract). Respir Res 11: 68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JC, Tarn WY. RNA-binding motif protein 4 translocates to cytoplasmic granules and suppresses translation via argonaute-2 during muscle cell differentiation. J Biol Chem 284: 34658–34665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SL, Chiang A, Chang D, Ying SY. Loss of mir-146a function in hormone-refractory prostate cancer. RNA 14: 417–424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol 33: 170–177, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 142: 914–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci USA 106: 18704–18709, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matysiak M, Fortak-Michalska M, Szymanska B, Orlowski W, Jurewicz A, Selmaj K. MicroRNA-146a negatively regulates the immunoregulatory activity of bone marrow stem cells by targeting prostaglandin E2 synthase-2. J. Immunol (April 15, 2013). 10.4049/jimmunol.1202397 [DOI] [PubMed] [Google Scholar]
- 41.Opalinska JB, Bersenev A, Zhang Z, Schmaier AA, Choi J, Yao Y, D'Souza J, Tong W, Weiss MJ. MicroRNA expression in maturing murine megakaryocytes. Blood 116: e128–e138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pang L, Holland E, Knox AJ. Role of cyclo-oxygenase-2 induction in interleukin-1beta induced attenuation of cultured human airway smooth muscle cell cyclic AMP generation in response to isoprenaline. Br J Pharmacol 125: 1320–1328, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pang L, Knox AJ. Effect of interleukin-1β, tumour necrosis factor-α and interferon-γ on the induction of cyclo-oxygenase-2 in cultured human airway smooth muscle cells. Br J Pharmacol 121: 579–587, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pascual RM, Carr EM, Seeds MC, Guo M, Panettieri RA, Peters SP, Penn RB. Regulatory features of interleukin-1β-mediated prostaglandin E2 synthesis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L501–L508, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol (August 14, 2013). 10.1165/rcmb.2013-0067OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry MM, Moschos SA, Williams AE, Shepherd NJ, Larner-Svensson HM, Lindsay MA. Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells. J Immunol 180: 5689–5698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perry MM, Williams AE, Tsitsiou E, Larner-Svensson HM, Lindsay MA. Divergent intracellular pathways regulate interleukin-1β-induced miR-146a and miR-146b expression and chemokine release in human alveolar epithelial cells. FEBS Lett 583: 3349–3355, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Salinthone S, Singer CA, Gerthoffer WT. Inflammatory gene expression by human colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 287: G627–G637, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Sato T, Liu X, Nelson A, Nakanishi M, Kanaji N, Wang X, Kim M, Li Y, Sun J, Michalski J, Patil A, Basma H, Holz O, Magnussen H, Rennard SI. Reduced miR-146a increases prostaglandin E2 in chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med 182: 1020–1029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shore SA, Laporte J, Hall IP, Hardy E, Panettieri RA, Jr. Effect of IL-1 beta on responses of cultured human airway smooth muscle cells to bronchodilator agonists. Am J Respir Cell Mol Biol 16: 702–712, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Singer CA, Baker KJ, McCaffrey A, AuCoin DP, Dechert MA, Gerthoffer WT. p38 MAPK and NF-κB mediate COX-2 expression in human airway myocytes. Am J Physiol Lung Cell Mol Physiol 285: L1087–L1098, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Singer CA, Lontay B, Unruh H, Halayko AJ, Gerthoffer WT. Src mediates cytokine-stimulated gene expression in airway myocytes through ERK MAPK (Abstract). Cell Commun Signal 9: 14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun S, Zheng B, Han M, Fang X, Li H, Miao S, Su M, Han Y, Shi H, Wen J. miR-146a and Krüppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep 12: 56–62, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103: 12481–12486, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsitsiou E, Williams AE, Moschos SA, Patel K, Rossios C, Jiang X, Adams OD, Macedo P, Booton R, Gibeon D, Chung KF, Lindsay MA. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J Allergy Clin Immunol 129: 95–103, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Ulrik CS, Backer V. Markers of impaired growth of pulmonary function in children and adolescents. Am J Respir Crit Care Med 160: 40–44, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC, Jr. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis 146: 109–115, 1992 [DOI] [PubMed] [Google Scholar]
- 58.Williams AE, Larner-Svensson H, Perry MM, Campbell GA, Herrick SE, Adcock IM, Erjefalt JS, Chung KF, Lindsay MA. MicroRNA expression profiling in mild asthmatic human airways and effect of corticosteroid therapy. PLoS One 4: e5889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia YC, Redhu NS, Moir LM, Koziol-White C, Ammit AJ, Al-Alwan L, Camoretti-Mercado B, Clifford RL. Pro-inflammatory and immunomodulatory functions of airway smooth muscle: emerging concepts. Pulm Pharmacol Ther 26: 64–74, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Yang S, Cui H, Xie N, Icyuz M, Banerjee S, Antony VB, Abraham E, Thannickal VJ, Liu G. miR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J 27: 2382–2391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]