Abstract
Interferons (IFNs) are generally considered antiviral cytokines, yet the newly discovered IFN-λ4 is linked with the failure to clear hepatitis C virus (HCV) infection either spontaneously or in response to treatment. IFN-λ4 can be generated only by individuals who carry the IFNL4-ΔG allele (rs368234815), which is the strongest known host factor for predicting clearance of HCV. The ancestral IFNL4-ΔG allele is the major variant in Africans while the minor variant in Asians, suggesting very strong negative genetic selection for this allele—most likely driven by an infectious agent other than HCV. IFN-λ4 most closely resembles IFN-λ3, but these proteins share only 29% amino-acid identity, and, in contrast to IFN-λ3, IFN-λ4 is only weakly secreted. Nevertheless, IFN-λ4 signals through the IFN-λ receptor complex and induces expression of IFN-stimulated genes via the Janus kinase-signal transducer and activator of transcription signaling pathway. Although the IFNL4-ΔG variant is strongly associated with the failure to clear HCV infection, HCV-infected patients who carry this allele have lower baseline HCV RNA levels in the absence of treatment. Resolving the paradoxical functions of IFN-λ4, which appears to induce antiviral activity yet impair effective clearance of HCV, may yield critical new insights into the immunologic response to HCV infection and IFN biology.
Introduction
Interferons (IFNs) are characterized, in large part, by their ability to induce antiviral activity in target cells that express the matching cognate receptor complexes. In fact, the very name of this cytokine family refers to their ability to “interfere” with viral replication within host cells. The most recently discovered protein to be classified as an IFN is IFN-λ4 (gene symbol: IFNL4). This name was assigned by the Human Genome Organization Gene (HUGO) Nomenclature Committee in conjunction with the Nomenclature Committee of the International Cytokine and Interferon Society on the basis of the sequence similarity between IFN-λ4 and other IFN-λ proteins, as well as evidence that IFN-λ4 can induce antiviral responses through activation of the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway and expression of interferon-stimulated genes (ISGs). We discovered the IFNL4 gene and the IFN-λ4 protein through genetic and genomic studies of hepatitis C virus (HCV) infection that demonstrated very strong associations between IFNL4-ΔG, the genetic variant that creates IFN-λ4, and the failure to clear HCV infection either spontaneously or in response to treatment with pegylated IFN-α and ribavirin (Prokunina-Olsson and others 2013). It appears, therefore, that IFN-λ4 impairs HCV clearance by an as-yet-undefined mechanism. Here, we summarize the current knowledge of IFN-λ4 and discuss questions that should be addressed to resolve its paradoxical biologic activities.
IFN-λ1, IFN-λ2, and IFN-λ3
The first 3 members of the IFN-λ family were discovered simultaneously through computational predictions based on genomic sequence. In 2003, 2 independent research groups, using different nomenclatures, reported the discovery of these proteins (Kotenko and others 2003; Sheppard and others 2003). In late 2012, in conjunction with granting an official name and gene symbol for IFNL4, the HUGO Nomenclature Committee changed the official symbols for these genes from IL29, IL28A, and IL28B to IFNL1, IFNL2, and IFNL3, respectively. Those changes are consistent with the widespread recognition that these cytokines function primarily as IFNs, not as interleukins. The proteins encoded by these 3 genes, IFN-λ1, IFN-λ2 and IFN-λ3, are highly similar to each other. The amino-acid identity between IFN-λ2 and IFN-λ3 is ∼96%, and these genes are also almost identical in their noncoding sequences, including upstream and downstream flanking regions (Kotenko and others 2003; Sheppard and others 2003; Fox and others 2009). The identity between IFN-λ1 and IFN-λ2/-λ3 is ∼81% (Kotenko and others 2003; Sheppard and others 2003). IFN-λ1, IFN-λ2, and IFN-λ3 are classified as type-III IFNs, because they signal through a receptor complex that is distinct from the receptors used by type I and type II IFNs (de Weerd and Nguyen 2012).
Viral infection can induce expression of the type III IFNs in a variety of cell types (Kotenko and others 2003; Sheppard and others 2003). These cytokines signal through a heterodimeric receptor complex consisting of IFN-λR1, the ligand-binding chain, and IL-10R2, the accessory chain (Kotenko and others 2003; Sheppard and others 2003; Donnelly and Kotenko 2010). IFNs induce antiviral activity in cells that express the corresponding cell surface receptors, and IFN-λR1 expression is largely restricted to cells of epithelial origin (Sommereyns and others 2008; Mordstein and others 2010). These cell types include epidermal, bronchial, and gastrointestinal epithelial cells, as well as hepatocytes. In contrast, most types of leukocytes do not express IFN-λ receptors, and, therefore, are not responsive to IFN-λ (Diegelmann and others 2010; Dickensheets and others 2013). Consequently, type III IFNs have a more limited functional range than the type I IFNs (IFN-α/β), whose receptors are widely expressed on most somatic cell types. Thus, type III IFNs may have evolved specifically to render protection to the epithelium.
The binding of a type III IFN to IFN-λR1 leads to a rapid conformational change that facilitates the recruitment of IL-10R2 to the complex (Gad and others 2009; Miknis and others 2010). After a ternary complex consisting of a type III IFN, IFN-λR1, and IL-10R2 is assembled, the receptor-associated tyrosine kinases, JAK1 and TYK2, are activated to mediate tyrosine phosphorylation of the intracellular domain of the IFN-λR1 chain. This results in the formation of phosphotyrosine-containing peptide motifs that provide docking sites for STAT proteins. For type III IFNs, signaling results in the formation of IFN-stimulated gene factor 3 (ISGF3) transcription factor complexes, consisting of STAT1, STAT2, and IRF-9. Once assembled, ISGF3 translocates from the cytosol to the nucleus, where it binds to interferon-stimulated response elements (ISRE) in the promoters of numerous ISGs. The proteins encoded by these ISGs mediate a variety of antiviral and anti-proliferative activities. The genes induced by type III IFNs appear to be essentially the same as those induced by IFN-α (Doyle and others 2006; Marcello and others 2006), and the downstream biological activities induced by either group appear to be very similar.
In addition to their ability to activate the JAK/STAT signaling pathway, the IFN-lambda subtypes (ie, IFN-λ1, 2 and 3) can also induce the activation of other signaling pathways, including p38 mitogen-activated protein (MAP) kinase and c-Jun N-terminal kinase (JNK), in certain cell types (Zhou and others 2007). In addition, it was reported that signaling by IFN-λ1 through the canonical IFN-λR1/IL-10R2 receptor complex results in the activation of p90 ribosomal protein S6 kinase (RSK1) and its downstream effector, eukaryotic initiation factor 4B (eIF4B), in several tumor cell lines, including a human colon carcinoma (HT-29) and a human retinal epithelial cell line (ARPE-19) (Kroczynska and others 2011).
Although type I and type III IFNs signal through distinct receptors, the primary intracellular signaling pathway activated by type I and type III IFNs is common to both. However, there are a number of important differences in the antiviral activities induced by type I versus type III IFNs. First, as mentioned earlier, type I IFN receptors are broadly expressed in virtually all somatic cells, including most types of leukocytes. In contrast, type III IFN receptors are predominantly expressed by nonhematologic cell types, especially cells of epithelial origin such as bronchial epithelium, gastrointestinal epithelium, and keratinocytes. Second, viral infection studies in several mouse models have shown that type III IFNs play a more prominent role than type I IFNs in mediating antiviral protection against certain types of viruses which preferentially infect gastrointestinal and/or respiratory epithelium. For example, Pott and coworkers (2011) found that IFN-lambda receptor gene knockout mice (Ifnlr1−/−) are markedly impaired in their ability to control an infectious challenge with live rotavirus; whereas mice which lack type I IFN receptors (ie, Ifnar1 knockouts) respond similarly to wild-type mice. Furthermore, treatment with type-III IFN (IFN-λ) but not type-I IFN (IFN-α) protected wild-type mice against rotavirus infection. These findings indicate that type III IFN, but not type I IFN, plays a more dominant role in host defense against certain types of viral infections. There is also increasing evidence, based on infectious challenge studies in mice, that type III IFN functions along with type I IFN to mediate protection against infection by respiratory viruses such as influenza A virus and respiratory syncytial virus which preferentially infect airway epithelial cells (Jewell and others 2010; Mordstein and others 2010). Early clinical trials of recombinant IFN-λ1 for the treatment of chronic hepatitis C indicate that its antiviral effects are similar to recombinant IFN-α, yet it induces fewer adverse effects due to the more limited range of cells which express IFN-λR1 (Muir and others 2010).
Discovery of IFN-λ4
The discovery of IFN-λ4 was facilitated by genome-wide association studies (GWAS) of HCV infection. In 2009, GWAS results reported by 3 independent groups identified a number of single nucleotide polymorphism (SNP) markers located upstream of IFNL3 (formerly IL28B) in chromosomal region 19q13.13 as associated with response to treatment with pegylated IFN-α and ribavirin among patients with chronic hepatitis C (Ge and others 2009; Suppiah and others 2009; Tanaka and others 2009). These SNPs were in strong linkage disequilibrium (LD) with each other, that is, genotypes based on these SNPs were highly correlated and, as a result, provided very similar genetic associations. Subsequent reports confirmed these findings and showed that these SNP markers were also associated with the likelihood of spontaneous HCV clearance (Thomas and others 2009; Rauch and others 2010). Specifically, a genotype for the rs12979860 SNP (located ∼3 kb upstream of IFNL3) was reported to be the host factor that was most strongly associated with a response to pegylated IFN-α and ribavirin treatment for HCV-infected patients (Thompson and others 2010). Individuals who carried the rs12979860-T allele (ie, C/T and T/T genotypes) were less likely to respond to treatment than those with 2 copies of the rs12979860-C allele. Furthermore, the rs12979860-T allele was found to be more common in Africans than in Europeans or Asians (Thomas and others 2009), which explained, in part, previous observations that African Americans were less likely to respond to treatment for hepatitis C. On the basis of these findings, a genetic test for rs12979860 was introduced to help predict the likelihood of treatment response in HCV infection and, in 2010, the FDA recommended that the “IL28B” genotype be routinely assessed in clinical trials of new agents for the treatment of chronic hepatitis C (Pacanowski and others 2012).
In addition to the association with improved viral clearance, other, seemingly inconsistent phenotypes were also linked to the rs12979860 genotype. Notably, the rs12979860-T allele, which was associated with a poorer treatment response, was also associated with lower pretreatment HCV RNA levels (Ge and others 2009), which was a well-established predictor of treatment success. In addition, rs12979860-T, as well as the strongly linked rs8099917-G allele, was shown to be associated with higher intrahepatic expression of ISGs before treatment (Honda and others 2010; Urban and others 2010). Thus, the range of phenotypes associated with the rs12979860-T allele was paradoxical, because it encompassed decreased treatment-induced viral clearance in the face of higher ISG expression levels and lower HCV RNA levels before treatment.
Some of the SNPs near IFNL3 that are in strong LD with rs12979860 are potentially functional, and a number of hypotheses have been proposed to explain how genetic variation within or near IFNL3 might impair HCV clearance [reviewed in Horner and Gale (2013)]. Two earlier studies of IFNL3 mRNA expression in lymphocytes suggested that expression levels were greater in individuals with a genotype which was favorable for treatment response (Suppiah and others 2009; Tanaka and others 2009), while another study did not observe a similar effect (Ge and others 2009). Studies of IFNL3 expression in liver have also yielded inconsistent results. Fukuhara and others (2010) reported that intrahepatic expression of “IL28” (ie, IFNL2 and IFNL3 combined) mRNA was significantly lower in individuals who carried the unfavorable (for HCV clearance) rs8099917-G allele; however, other studies found no association between intrahepatic IFNL3 expression and genotypes for either rs8099917 (Honda and others 2010) or rs12979860 (Urban and others 2010), the SNPs reported by GWAS. A nonsynonymous coding variant in IFNL3 (rs8103142, Arg70Lys) that is in strong LD with rs12979860 does not appear to alter the potency or function of IFN-λ3 (Urban and others 2010). Recently, McFarland and others (2014) suggested that the rs4803217 polymorphism, which lies in the 3′ untranslated region of IFNL3 and is in strong LD with rs12979860, is a functional variant which affects the stability of IFNL3 transcripts by influencing adenosine-uridine-rich element-mediated decay of IFNL3 mRNA and the binding of HCV-induced microRNAs during infection. In an earlier study of patients of European ancestry who were treated with pegylated IFN-α and ribavirin, associations with treatment response were comparable for the rs4803217 and rs12979860 genotypes (de Castellarnau and others 2012), which would be expected based on the high LD between these polymorphisms. A previous analysis of spontaneous clearance of HCV in an Egyptian population reported that the rs4803217 genotype provided a somewhat weaker overall association than a genotype for the rs12979860 GWAS marker itself (Pedergnana and others 2012). Thus, attempts to identify a functional genetic variant within IFNL3 or a functional mechanism linked to IFNL3 have failed to yield consistent results. More broadly, functional studies aimed specifically at IFN-λ3 beg the question of how variation in a single member of the closely related type III IFN group could account for the apparently contradictory phenotypes that have been observed.
The IFNL4 gene was discovered through sequencing of RNA samples derived from primary human hepatocytes (PHH) that had been treated with polyinosinic:polycytidylic acid (poly I:C, a synthetic double-stranded RNA) to mimic HCV infection. Early in 2013, we reported the discovery of a previously unrecognized transiently induced region upstream of IFNL3 that proved to contain a novel gene among several other transcripts (Fig. 1a) (Prokunina-Olsson and others 2013). Furthermore, we showed that IFNL4 is controlled by a heritable (germline) dinucleotide frameshift variant, denoted IFNL4-ΔG/TT (rs368234815, originally designated as ss469415590), which is located in exon 1 of IFNL4 (Fig. 1b). The IFNL4-ΔG allele creates the open reading frame for the full-length IFN-λ4 protein, whereas the alternative allele at this locus (IFNL4-TT) does not create IFN-λ4 (Prokunina-Olsson and others 2013). Thus, IFNL4-ΔG is a functional variant that controls the generation of IFN-λ4.
FIG. 1.
Location of the interferon (IFN)-λ gene family and genome-wide association study markers rs12979860 and rs8099917 on chromosome 19 (a); exonic structure of IFNL4 with the location of the IFNL4 rs12979860 (“IL28B”) and IFNL4 rs368234815 (IFNL4–ΔG) polymorphisms (b).
The rs12979860 variant, which is still commonly referred to as “IL28B,” is actually located within intron 1 of IFNL4 (Fig. 1b) (Prokunina-Olsson and others 2013), and, therefore, is more properly called IFNL4 rs12979860. LD between IFNL4-ΔG, which creates IFN-λ4, and the (unfavorable) IFNL4 rs12979860-T allele is very high for Asians (r2=1.0) and Europeans (r2>0.9) (Prokunina-Olsson and others 2013), which means that in these racial groups IFNL4-ΔG and IFNL4 rs12979860-T almost always are inherited together. For such highly linked variants, it can be difficult or impossible to determine which variant is more strongly associated with outcome and, therefore, more likely to be causal. However, we found that, among Africans, LD between IFNL4-ΔG and IFNL4 rs12979860-T is weaker than in other populations (r2∼0.7). This enabled us to demonstrate that HCV RNA decline after 28 days of treatment with pegylated IFN-α and ribavirin was more strongly associated with a genotype for IFNL4-ΔG than IFNL4 rs12979860 among African American patients who had enrolled in the Virahep study (Fig. 2) (Prokunina-Olsson and others 2013). Among the same patients, IFNL4-ΔG was also more strongly associated with other measures of treatment response, including sustained virologic response (SVR), although those differences did not reach statistical significance. We found consistent results for genotype associations with spontaneous HCV clearance in African Americans (Prokunina-Olsson and others 2013; Aka and others 2014). Among patients of European ancestry, we found that, due to strong LD, IFNL4-ΔG and IFNL4 rs12979860 genotypes yielded similar or identical results (Prokunina-Olsson and others 2013); however, since then, others have reported that response to treatment with pegylated IFN-α and ribavirin is more strongly associated with a genotype for IFNL4-ΔG than IFNL4 rs12979860 among Europeans (Bibert and others 2013; Franco and others 2014).
FIG. 2.
Median decrease in hepatitis C virus (HCV) RNA (log10 IU/mL) in African American participants in Virahep-C study during the first 28 days of treatment with pegylated IFN-α and ribavirin. P=0.015 for a comparison of mean differences in HCV RNA levels at day 28 for each of the 3 genotype groups for ss469415590 (ie, rs368234815, IFNL4–ΔG) with the respective groups for IFNL4 rs12979860 (“IL28B”). From Prokunina-Olsson and others (2013) [By permission, Nature Publishing].
A number of host factors have been shown to predict response to treatment for chronic hepatitis C. Previously, Thompson and his colleagues showed that among such factors, IFNL4 rs12979860 genotype was the strongest factor for response to treatment with pegylated IFN-α and ribavirin (Thompson and others 2010). By extrapolation, the new data suggest that IFNL4-ΔG genotype may be the single strongest host factor for predicting such a treatment response.
IFN-λ4 Sequence and Function
IFN-λ4 most closely resembles IFN-λ3, but these proteins share only ∼30% amino-acid identity. IFN-λ4 is most similar to IFN-λ1, 2, and 3 within the first and last (A and F) helices, the sequences that correspond to the region where type III IFNs interact with the IFN-λR1 chain, their primary receptor. IFN-λ4 is very distinct from IFN-λ1, 2, and 3 in the D helix, which is the predicted binding region for the IL-10R2 chain, the second chain of the IFN-λ receptor complex (Prokunina-Olsson and others 2013). Despite this sequence dissimilarity, based on structural modeling studies, Hamming and others (2013) concluded that the overall structure of IFN-λ4 is similar to that of IFN-λ1, 2, and 3, and independent work from 2 groups now indicates that IFN-λ4 can signal through the IFN-λ receptor complex.
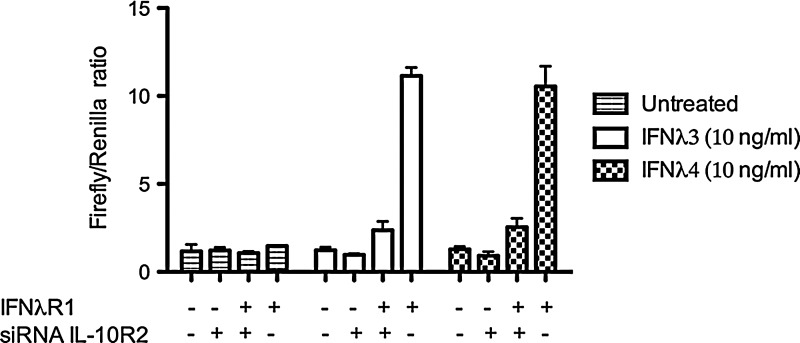
Hamming and others (2013) have generated biologically active recombinant IFN-λ4 in an Escherichia coli expression system. Using HEK293 cells, which express the IL-10R2 chain well but IFN-λR1 poorly (Meager and others 2005), these investigators demonstrated that ectopic expression of the IFN-λR1 chain in HEK293 cells reconstitutes responsiveness to IFN-λ4 (Hamming and others 2013) (Fig. 3). They also showed that knockdown of endogenous IL-10R2 expression using a silencing RNA (siRNA) markedly diminished responsiveness to IFN-λ4 in these cells (Hamming and others 2013). These experiments showed that IFN-λ4 signals through the IFN-λR1/IL-10R2 receptor complex and that both receptors are required for signaling. The authors also suggested additional protein residues as being responsible for binding of the IL10R2 receptors, with all of these residues being nonconserved between IFN-λ3 and IFN-λ4. New, independent work from the Prokunina-Olsson laboratory shows similar results. In HepG2-ISRE-Luc cells that were transiently transfected with an IFNL4 expression construct, either silencing of IFN-λR1 by siRNA or blocking of IL-10R2 with an anti-IL-10R2 antibody inhibited IFN-λ4 signaling (Muchmore and others 2013).
FIG. 3.
Recombinant IFN-λ4 signals through the IFN-λ receptor complex. HEK293 cells were, as indicated, transfected with IFN-λR1 and/or treated with silencing RNA against IL-10R2. The cells were also transfected with luciferase reporter constructs for Renilla, which is constitutively expressed, and Firefly, which is IFN inducible. Adapted from Hamming and others (2013) [By permission, Nature Publishing].
IFN-λ4 can induce expression of ISGs via the JAK-STAT signaling pathway and exert antiviral effects. In transfection experiments, we demonstrated that IFN-λ4 induces STAT1 and STAT2 phosphorylation, activates an ISRE-Luc reporter gene, induces expression of multiple ISGs, and generates an antiviral response against HCV (Prokunina-Olsson and others 2013). Using their recombinant IFN-λ4 protein, Hamming and others confirmed that IFN-λ4 induces expression of ISGs. Furthermore, despite the structural differences between IFN-λ4 and IFN-λ3 noted earlier, these investigators also showed that the levels of ISG induction and anti-viral activity are similar for IFN-λ4 and IFN-λ3 (Hamming and others 2013). The antiviral potency of IFN-λ4 was found to be quite similar to that of IFN-λ3 when tested on 2 different hepatic cell lines, Huh7 and HepG2. IFN-λ4 and IFN-λ3 also exhibited comparable potency when assayed for their ability to induce antiviral activity against 2 different human coronaviruses, HCoV-229E and MERS-CoV, in cultures of primary human bronchial epithelial cells. The antiviral activity of IFN-λ3 and IFN-λ4 correlated with equivalent induction levels of several ISGs, including IFIT1, MX1, and OASL. The similar antiviral activity observed for IFN-λ3 and IFN-λ4 is also noteworthy, because it has been shown that IFN-λ3 is more potent than IFN-λ1 or IFN-λ2 (Dellgren and others 2009). These in vitro results appear to be consistent with the observational studies among individuals with chronic hepatitis C (noted above) in which the unfavorable “IL28B” host genotypes were associated with higher expression of ISGs and lower HCV RNA levels.
Several recent studies have explored hepatic expression of IFNL4. Due to the location of the defining IFNL4-ΔG/TT polymorphism in the first exon of IFNL4 and the splicing composition of the IFNL4 region, 10 transcripts are expressed after induction, but only 1 of these generates the full-length IFN-λ4 protein (Prokunina-Olsson and others 2013). The transcript that includes IFNL4-ΔG and produces full-length IFN-λ4 protein (designated JN806234 or NM_001276254) is very similar to transcript JN806227, which produces a prematurely terminated protein due to the presence of the IFNL4-TT allele and the resulting frameshift (Prokunina-Olsson and others 2013). For this reason, it is impossible to exclusively measure mRNA expression of the JN806234 transcript that produces full-length IFNL4. However, IFNL4 expression can be examined via an assay that measures both JN806234 (ie, the IFNL4-ΔG transcript that generates full-length protein) and JN806230, a transcript which is similar to JN806234, but carries the IFNL4-TT allele and, therefore, generates a nonfunctional protein. Alternatively, it is possible to use “allele-specific” assays that differentiate all transcripts which include the IFNL4-ΔG allele from all transcripts that include the IFNL4-TT allele (Prokunina-Olsson and others 2013).
Using the former approach, Amanzada and others (2013) analyzed mRNA expression in the IFNL4 region from liver biopsy specimens of patients with chronic hepatitis C, chronic hepatitis B, or nonviral liver diseases, as well as patients without liver disease. These investigators found IFNL4 region transcripts to be present in ∼50% of the liver specimens from patients with HCV infection, but none of the specimens from patients with other liver diseases or normal liver. This finding suggests that induction of IFNL4 mRNA in the liver might be specific to HCV infection. In the liver biopsies from patients with HCV infection in which IFNL4 region mRNA (ie, JN806234 and JN806227) could be detected, the expression level was positively correlated with liver HCV RNA levels, but not with IFNL4-ΔG genotype. The authors interpreted this finding to suggest that the level of hepatic HCV RNA impacts the level of IFNL4 transcription (irrespective of IFNL4-ΔG genotype). Consistent with previous studies based on GWAS SNPs, Amanzada and others detected higher ISG expression in carriers of the IFNL4–ΔG allele compared with those without this allele (ie, those with the IFNL4–TT/TT genotype). Among IFNL4–ΔG carriers, the expression of IFNL4 was positively correlated with ISG expression (Amanzada and others 2013).
Using allele-specific expression assays, Konishi and others (2013) analyzed IFNL4 region mRNA expression levels in liver tissue from transplant recipients with chronic hepatitis C who were treated with pegylated IFN-α and ribavirin. Overall, they reported IFNL4 region mRNA expression in 78 of 80 (98%) recipients. Among the recipients who carried IFNL4-ΔG, hepatic expression of IFNL4-ΔG mRNA was significantly lower in patients who achieved SVR compared with those who did not; whereas among the recipients with the IFNL4-TT/TT genotype, treatment response did not differ by IFNL4-TT mRNA levels. These investigators also found that hepatic expression of 2 ISGs (ISG15 and USP18) was higher in the IFNL4-ΔG carriers and that, among IFNL4-ΔG carriers, ISG levels were positively correlated with IFNL4-ΔG expression levels. Consistent with Konishi and others, Honda and others (2014) found that hepatic expression of IFNL4 and ISGs was positively correlated in patients with chronic hepatitis C. These investigators also reported that ISG expression in the liver and blood was positively correlated, but only among the patients with the rs8099917-T/T genotype (ie, individuals who cannot generate IFN-λ4).
Individuals who carry IFNL4–ΔG allele may express low levels of IFN-λ4 protein that induces low, but persistent ISG expression in the liver, which makes these cells refractory to stimulation by IFN-α. As noted earlier, these individuals have higher basal levels of ISG expression despite being less likely to respond efficiently to pegylated IFN-α and ribavirin therapy. Some recent studies have examined the potential cross-regulatory effects of IFN-λ on IFN-α responsiveness. Makowska and others (2011) found that pretreating mice with IFN-λ2 induced a state of refractoriness to a secondary challenge with IFN-α in vivo, and Francois-Newton and others (2011) showed that pretreating PHH with type I or type III IFN in vitro suppressed subsequent responses to IFN-α but not to IFN-λ1. These studies, which indicate that pretreatment with IFN-λ can inhibit responsiveness to a secondary challenge with IFN-α, may be relevant to the biological activities of IFN-λ4. Another recent study suggests that the mechanism of action for IFN-λ4 could involve the regulation of IFN-λR1 expression. Measuring the expression of IFN-λR1 in liver biopsies from HCV-infected patients and from noninfected controls, Duong and others (In press) found IFN-λR1 expression to be much higher in the biopsies from individuals with chronic hepatitis C. Among the HCV-infected patients, IFN-λR1 expression was significantly higher in those who carried the IFNL4 rs12979860-T allele, which is in very strong LD with the IFNL4-ΔG allele, and in patients who had failed to respond to treatment with pegylated-IFN-α/ribavirin compared with responders. In PHH samples, IFN-λR1 expression could be induced with IFN-α, and the expression of IFN-λR1was significantly stronger in samples that carried IFNL4 rs12979860-T. These results suggest the possibility that IFN-λ4 impairs HCV clearance via a mechanism involving up-regulation of IFN-λR1.
Recently, Sheahan and others applied laser capture microdissection to cultures of PHHs in order to isolate and transcriptionally profile HCV-infected cells, as well as adjacent cells that were uninfected. The observed transcriptional response was dominated by an innate antiviral immune signature that was greater and more diverse in infected cells than noninfected cells. Cells from donors who carried IFNL4-ΔG were infected at higher frequencies, and the antiviral program in infected cells from such donors was less robust than in infected cells from donors with the IFNL4-TT/TT genotype. These results suggest that IFN-λ4 may impair the antiviral program required for effective HCV clearance (Sheahan and others 2014).
The poor correlation between ISG expression in the liver and blood among patients who can generate IFN-λ4 could be linked to observations that the secretion of IFN-λ4 appears to be weak. In general, IFNs are secreted proteins; however, we found IFN-λ4 to be primarily intracellular and hypothesized that this could be due to a weak signal peptide (Prokunina-Olsson and others 2013). Hamming and others confirmed that secretion of IFN-λ4 is impaired, but concluded that this is not due to a weak signal peptide. In experiments in which the signal peptides for IFN-λ3 and IFN-λ4 were exchanged, the secretion of a chimeric protein consisting of IFN-λ3 with the IFN-λ4 signal peptide remained strong and the secretion of an IFN-λ4/IFN-λ3 signal peptide chimera remained weak. Moreover, Hamming and others (2013) showed that N-linked glycosylation is required for IFN-λ4 to be efficiently secreted. In contrast, IFN-λ3 is not glycosylated, and it does not require glycosylation for secretion. Therefore, the impaired secretion of IFN-λ4 might be due to inefficient post-translational glycosylation. It is also possible that intracellular accumulation of nonglycosylated IFN-λ4 could be cytotoxic and result in cell death. If IFN-λ4 is not efficiently secreted, this protein could conceivably become extracellular within the liver as a result of cell lysis. Notably, this appears to be the case for at least one of the type I IFNs, IFN-κ (Buontempo and others 2006).
Negative Selection for the IFNL4–ΔG Allele
Full-length protein-coding IFNL4 transcripts were initially predicted only in humans and other primates (Prokunina-Olsson and others 2013); however, an additional genomic analysis has identified IFNL4 in a number of nonprimate mammals, although not in mice and rats (Tang and others 2013). Except for humans, all species with available IFNL4 genomic sequence have been found to be monomorphic for the IFNL4-ΔG allele that supports the IFN-λ4 open reading frame (Tang and others 2013).
The IFNL4-ΔG allele underwent very strong negative genetic selection with replacement of the ancestral IFNL4–ΔG allele by the derived IFNL4-TT variant in non-African populations. Consistent with earlier reports based on IFNL4 rs12979860 (Thomas and others 2009), the allele frequency for IFNL4-ΔG varies markedly by racial ancestry. In HapMap populations, ∼95% of Africans, ∼54% of Europeans, and ∼13% of Asians carry at least 1 copy of this variant (Prokunina-Olsson and others 2013). These allele frequencies are consistent with a report (that preceded the discovery of IFNL4) demonstrating that the chromosomal region harboring IFNL4 was the most highly selected human IFN region (Manry and others 2011). It seems unlikely that this selection was driven by HCV. Infection with HCV is now relatively common worldwide (Shepard and others 2005); however, risk factors that account for the bulk of HCV transmission (blood transfusions, contaminated medical injections, and injection drug use) arose primarily in the twentieth century. Furthermore, HCV usually causes a slowly progressive chronic infection that is unlikely to markedly impair reproduction.
With regard to other infectious agents, neither an analysis based on the IFNL4 rs12979860 SNP (Martin and others 2010) nor published GWAS results (Kamatani and others 2009) provide any evidence that IFNL4–ΔG might impair spontaneous clearance of hepatitis B virus, perhaps consistent with the recent observation (noted above) that IFNL4 is not expressed in HBV-infected liver (Amanzada and others 2013). Griffiths and others found that individuals with the CT or TT genotypes for IFNL4 rs12979860 (ie, likely IFNL4–ΔG carriers) had more frequent episodes of severe herpes labialis (Griffiths and others 2013), a condition resulting from reactivation of herpes simplex virus type 1. To date, GWAS for other infectious diseases have not reported associations with variants in the IFN-λ region.
Future Directions
The discovery of IFN-λ4 appears to explain the strong genetic associations observed between GWAS markers within the IFN-λ gene cluster and HCV clearance, while simultaneously raising a number of questions concerning the functional mechanism and full clinical implications of this novel member of the IFN-λ family. Functional similarities and differences with IFN-λ3, its chromosomal neighbor, and closest relative in terms of amino-acid sequence may provide some clues regarding this mechanism. Both IFN-λ4 and IFN-λ3 signal through IFN-λ receptor complex, activate the JAK-STAT pathway to induce ISGs, and exhibit strong antiviral activity in vitro. On the other hand, IFN-λ4 differs from IFN-λ3 in its IL-10R2-binding region sequence, its weaker secretion, and, on the basis of the IFNL4–ΔG genotype associations, its impairment of viral clearance in HCV-infected individuals.
As indicated earlier, there is now strong evidence that IFN-λ4 signals through the IFN-λR1/IL-10R2 receptor complex despite relatively low sequence similarity with IFN-λ3 in the region which binds to the IL-10R2 chain. There is also evidence that IFN-λ4, unlike most IFNs, is a poorly secreted protein. It is possible that IFN-λ4 impairs HCV clearance by impeding receptor binding of the other members of the IFN-λ family either due to the observed differences in structure or secretion. Future studies are likely to expand our understanding of the secretion and signaling efficiency of IFN-λ4.
A recent report suggests that, among patients with chronic hepatitis C who carry the IFNL4-ΔG allele, lower expression of IFNL4 mRNA was associated with a higher rate of SVR in response to pegylated IFN-α and ribavirin therapy (Konishi and others 2013). If that is the case, regulatory genetic variants or clinical factors that might modulate IFNL4 expression could impact HCV clearance in individuals who carry IFNL4-ΔG, and the measurement of IFN-λ4 in peripheral blood might be a clinically useful biomarker. However, the weak secretion of IFN-λ4 may make it challenging to develop assays to detect IFN-λ4 in blood.
Treatment for chronic hepatitis C is improving rapidly due to the development of direct acting antiviral agents (DAAs) that target various aspects of the HCV life cycle. DAA regimens promise to markedly increase SVR rates and eliminate the adverse effects associated with IFN-α treatment. In a phase 2b trial of 362 patients, SVR in response to the combination of faldaprevir (a protease inhibitor) and deleobuvir (a polymerase inhibitor) was lower in the patients with the CT or TT genotypes for IFNL4 rs12979860 (Zeuzem and others 2013). In a trial of patients treated with sofosbuvir (a polymerase inhibitor) along with ribavirin, the IFNL4-ΔG allele was associated with slower early viral decay (Meissner and others 2014). These results suggest that the IFNL4-ΔG genotype may be relevant for IFN-α-free DAA regimens; however, with the treatment of sufficient duration, it appears that the genetic effect of IFNL4-ΔG can be overcome and almost all patients may respond to treatment (Lawitz and others 2014). However, DAA regimens promise to be very expensive (Lancet 2014), and treatment costs might be reduced if the duration of therapy could be personalized on the basis of the IFNL4-ΔG genotype and other factors.
The strong selection pressure against the IFNL4-ΔG allele in human populations could have resulted from an infectious agent that is now extinct. It is also possible, however, that IFN-λ4 plays an important role in contemporary infectious diseases other than HCV. Associations with other infections might be detected through studies based on the IFNL4-ΔG genotype or through GWAS. It also seems plausible that the IFNL4-ΔG genotype could be associated with an impaired response to IFN-based treatment for other clinical conditions, similar to the associations that have been observed for the treatment of chronic hepatitis C. IFN-α is used in some regimens for the treatment of chronic hepatitis B; however, data on associations between genotype for GWAS markers linked to IFNL4-ΔG and response to IFN-α-based treatment are mixed (Jilg and Chung 2013). A study of the treatment of multiple sclerosis with IFN-β found no association with the IFNL4 rs12979860 genotype (Malhotra and others 2011).
Finally, it is notable that the detection of IFN-λ4 involved the complementary use of 2 different genomic approaches, GWAS and RNA-seq. This discovery raises the question of whether genomic approaches might lead to the discovery of other important immunological genes.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author Disclosure Statement
The authors are inventors of patent applications filed by the National Cancer Institute for the IFNL4-ΔG (rs368234815) genotype-based test and for the IFN-λ4 protein.
References
- Aka PV, Kuniholm MH, Pfeiffer RM, Wang AS, Tang W, Chen S, Astemborski J, Plankey M, Villacres MC, Peters MG, Desai S, Seaberg EC, Edlin BR, Strickler HD, Thomas DL, Prokunina-Olsson L, Sharp GB, O'Brien TR. 2014. Association of the IFNL4-ΔG allele with impaired spontaneous clearance of hepatitis C virus. J Infect Dis 209(3):350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzada A, Kopp W, Spengler U, Ramadori G, Mihm S. 2013. Interferon-λ4 (IFNL4) transcript expression in human liver tissue samples. PLoS One 8(12):e84026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibert S, Roger T, Calandra T, Bochud M, Cerny A, Semmo N, Duong FHT, Gerlach T, Malinverni R, Moradpour D, Negro F, Müllhaupt B, Bochud P-Y; the Swiss Hepatitis C Cohort Study. 2013. IL28B expression depends on a novel TT/-G polymorphism which improves HCV clearance prediction. J Exp Med 210(6):1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buontempo PJ, Jubin RG, Buontempo CA, Wagner NE, Reyes GR, Baroudy BM. 2006. Antiviral activity of transiently expressed IFN-kappa is cell-associated. J Interferon Cytokine Res 26(1):40–52 [DOI] [PubMed] [Google Scholar]
- de Castellarnau M, Aparicio E, Parera M, Franco S, Tural C, Clotet B, Martínez MA. 2012. Deciphering the interleukin 28B variants that better predict response to pegylated interferon-α and ribavirin therapy in HCV/HIV-1 coinfected patients. PLoS One 7(2):e31016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerd NA, Nguyen T. 2012. The interferons and their receptors—distribution and regulation. Immunol Cell Biol 90(5):483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellgren C, Gad HH, Hamming OJ, Melchjorsen J, Hartmann R. 2009. Human interferon-lambda3 is a potent member of the type III interferon family. Genes Immun 10(2):125–131 [DOI] [PubMed] [Google Scholar]
- Dickensheets H, Sheikh F, Park O, Gao B, Donnelly RP. 2013. Interferon-lambda (IFN-lambda) induces signal transduction and gene expression in human hepatocytes, but not in lymphocytes or monocytes. J Leukoc Biol 93(3):377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann J, Beigel F, Zitzmann K, Kaul A, Göke B, Auernhammer CJ, Bartenschlager R, Diepolder HM, Brand S. 2010. Comparative analysis of the lambda-interferons IL-28A and IL-29 regarding their transcriptome and their antiviral properties against hepatitis C virus. PLoS One 5(12):e15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly RP, Kotenko SV. 2010. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res 30(8):555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-Pour F, Sivakumar P, Chan C, Birks C, Foster D, Clegg CH, Wietzke-Braun P, Mihm S, Klucher KM. 2006. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 44(4):896–906 [DOI] [PubMed] [Google Scholar]
- Duong FHT, Trincucci G, Boldanova T, Calabrese D, Campana B, Krol I, Durand SC, Heydmann L, Zeisel MB, Baumert TF, Heim MH. Hepatic IFN-λ receptor 1 expression is induced in chronic hepatitis C and correlates with IFN-λ3 minor alleles and with non-response to IFN-α therapies. J Exp Med(In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BA, Sheppard PO, O'Hara PJ. 2009. The role of genomic data in the discovery, annotation and evolutionary interpretation of the interferon-lambda family. PLoS One 4(3):e4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco S, Aparicio E, Parera M, Clotet B, Tural C, Martinez MA. 2014. IFNL4 ss469415590 variant is a better predictor than rs12979860 of pegylated interferon-alpha/ribavirin therapy failure in hepatitis C virus/HIV-1 coinfected patients. AIDS 28(1):133–136 [DOI] [PubMed] [Google Scholar]
- Francois-Newton V, Magno de Freitas Almeida G, Payelle-Brogard B, Monneron D, Pichard-Garcia L, Piehler J, Pellegrini S, Uze G. 2011. USP18-based negative feedback control is induced by type I and type III interferons and specifically inactivates interferon alpha response. PLoS One 6(7):e22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara T, Taketomi A, Motomura T, Okano S, Ninomiya A, Abe T, Uchiyama H, Soejima Y, Shirabe K, Matsuura Y, Maehara Y. 2010. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology 139(5):1577–1585, 1585.e1571–e1573. [DOI] [PubMed] [Google Scholar]
- Gad HH, Dellgren C, Hamming OJ, Vends S, Paludan SR, Hartmann R. 2009. Interferon-λ is functionally an interferon but structurally related to the interleukin-10 family. J Biol Chem 284(31):20869–20875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. 2009. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461(7262):399–401 [DOI] [PubMed] [Google Scholar]
- Griffiths SJ, Koegl M, Boutell C, Zenner HL, Crump CM, Pica F, Gonzalez O, Friedel CC, Barry G, Martin K, Craigon MH, Chen R, Kaza LN, Fossum E, Fazakerley JK, Efstathiou S, Volpi A, Zimmer R, Ghazal P, Haas J. 2013. A systematic analysis of host factors reveals a Med23-interferon-λ regulatory axis against herpes simplex virus type 1 replication. PLoS Pathog 9(8):e1003514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming OJ, Terczyńska-Dyla E, Vieyres G, Dijkman R, Jørgensen SE, Akhtar H, Siupka P, Pietschmann T, Thiel V, Hartmann R. 2013. Interferon lambda 4 signals via the IFNλ receptor to regulate antiviral activity against HCV and coronaviruses. EMBO J 32(23):3055–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Sakai A, Yamashita T, Nakamoto Y, Mizukoshi E, Sakai Y, Yamashita T, Nakamura M, Shirasaki T, Horimoto K, Tanaka Y, Tokunaga K, Mizokami M, Kaneko S. 2010. Hepatic ISG expression is associated with genetic variation in IL28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology 139(2):499–509 [DOI] [PubMed] [Google Scholar]
- Honda M, Shirasaki T, Shimakami T, Sakai A, Horii R, Arai K, Yamashita T, Sakai Y, Yamashita T, Okada H, Murai K, Nakamura M, Mizukoshi E, Kaneko S. 2014. Hepatic interferon-stimulated genes are differentially regulated in the liver of chronic hepatitis C patients with different interleukin-28B genotypes. Hepatology 59(3):828–838 [DOI] [PubMed] [Google Scholar]
- Horner SM, Gale M., Jr.2013. Regulation of hepatic innate immunity by hepatitis C virus. Nat Med 19(7):879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell NA, Cline T, Mertz SE, Smirnov SV, Flaño E, Schindler C, Grieves JL, Durbin RK, Kotenko SV, Durbin JE. 2010. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. Journal of Virology 84(21):11515–11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilg N, Chung RT. 2013. One more piece in the interleukin 28B gene puzzle? The case of hepatitis B. Hepatology 57(3):870–872 [DOI] [PubMed] [Google Scholar]
- Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, Kubo M, Tsunoda T, Kamatani N, Kumada H, Puseenam A, Sura T, Daigo Y, Chayama K, Chantratita W, Nakamura Y, Matsuda K. 2009. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet 41(5):591–595 [DOI] [PubMed] [Google Scholar]
- Konishi H, Motomura T, Matsumoto Y, Harimoto N, Ikegami T, Yoshizumi T, Soejima Y, Shirabe K, Fukuhara T, Maehara Y. 2013. Interferon-lambda4 genetic polymorphism is associated with the therapy response for hepatitis C virus recurrence after a living donor liver transplant. J Viral Hepat [Epub ahead of print]; DOI: 10.1111/jvh.12154 [DOI] [PubMed] [Google Scholar]
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 4(1):69–77 [DOI] [PubMed] [Google Scholar]
- Kroczynska B, Joshi S, Eklund EA, Verma A, Kotenko SV, Fish EN, Platanias LC. 2011. Regulatory effects of ribosomal S6 kinase 1 (RSK1) in IFNlambda signaling. J Biol Chem 286(2):1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet. 2014. Only just the beginning of the end of hepatitis C. Lancet 383(9914):281. [DOI] [PubMed] [Google Scholar]
- Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. 2014. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet 383(9916):515–523 [DOI] [PubMed] [Google Scholar]
- Makowska Z, Duong FHT, Trincucci G, Tough DF, Heim MH. 2011. Interferon-β and interferon-λ signaling is not affected by interferon-induced refractoriness to interferon-α in vivo. Hepatology 53(4):1171–1180 [DOI] [PubMed] [Google Scholar]
- Malhotra S, Morcillo-Suarez C, Brassat D, Goertsches R, Lechner-Scott J, Urcelay E, Fernandez O, Drulovic J, Garcia-Merino A, Martinelli Boneschi F, Chan A, Vandenbroeck K, Navarro A, Bustamante MF, Rio J, Akkad DA, Giacalone G, Sanchez AJ, Leyva L, Alvarez-Lafuente R, Zettl UK, Oksenberg J, Montalban X, Comabella M. 2011. IL28B polymorphisms are not associated with the response to interferon-beta in multiple sclerosis. J Neuroimmunol 239(1–2):101–104 [DOI] [PubMed] [Google Scholar]
- Manry J, Laval G, Patin E, Fornarino S, Itan Y, Fumagalli M, Sironi M, Tichit M, Bouchier C, Casanova JL, Barreiro LB, Quintana-Murci L. 2011. Evolutionary genetic dissection of human interferons. J Exp Med 208(13):2747–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, Rice CM. 2006. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 131(6):1887–1898 [DOI] [PubMed] [Google Scholar]
- Martin , Maureen P, Qi Y, James Goedert J, Shehnaz Hussain K, Gregory Kirk D, Hoots KW, Buchbinder S, Carrington M, Thio CL. 2010. IL28B polymorphism does not determine outcomes of hepatitis B Virus or HIV infection. J Infect Dis 202(11):1749–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland AP, Horner SM, Jarret A, Joslyn RC, Bindewald E, Shapiro BA, Delker DA, Hagedorn CH, Carrington M, Gale M, Jr., Savan R. 2014. The favorable IFNL3 genotype escapes mRNA decay mediated by AU-rich elements and hepatitis C virus-induced microRNAs. Nat Immunol 15(1):72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. 2005. Biological activity of interleukins-28 and −29: comparison with type I interferons. Cytokine 31(2):109–118 [DOI] [PubMed] [Google Scholar]
- Meissner EG, Bon D, Prokunina-Olsson L, Tang W, Masur H, O'Brien TR, Herrmann E, Kottilil S, Osinusi A. 2014. IFNL4-DeltaG genotype is associated with slower viral clearance in hepatitis C, genotype-1 patients treated with sofosbuvir and ribavirin. J Infect Dis [Epub ahead of print]. DOI: 10.1093/infdis/jit.827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miknis ZJ, Magracheva E, Li W, Zdanov A, Kotenko SV, Wlodawer A. 2010. Crystal structure of human interferon-lambda1 in complex with its high-affinity receptor interferon-lambdaR1. J Mol Biol 404(4):650–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Gunther S, Drosten C, Michiels T, Staeheli P. 2010. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol 84(11):5670–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore B, Tang W, Porter-Gill P, Kohaar I, Liu L, Brand N, Park H, Dickensheets H, Sheikh F, Rehermann B, Donnelly RP, O'Brien TR, Prokunina-Olsson L. 2013. Identification and characterization of interferon-λ4 (IFN-λ4), a novel class-2 cytokine which impairs clearance of hepatitis C virus (Abstract 182). Cytokine 63(3):286 [Google Scholar]
- Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, Flamm S, Gordon SC, Marotta P, Vierling JM, Lopez-Talavera JC, Byrnes-Blake K, Fontana D, Freeman J, Gray T, Hausman D, Hunder NN, Lawitz E. 2010. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology 52(3):822–832 [DOI] [PubMed] [Google Scholar]
- Pacanowski M, Amur S, Zineh I. 2012. New genetic discoveries and treatment for hepatitis C. JAMA 307(18):1921–1922 [DOI] [PubMed] [Google Scholar]
- Pedergnana V, Abdel-Hamid M, Guergnon J, Mohsen A, Le Fouler L, Theodorou I, Mohamed MK, Fontanet A, Plancoulaine S, Abel L. 2012. Analysis of IL28B variants in an egyptian population defines the 20 kilobases minimal region involved in spontaneous clearance of hepatitis C virus. PLoS One 7(6):e38578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott J, Mahlakõiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW. 2011. IFN-λ determines the intestinal epithelial antiviral host defense. Proc Nat Acad Sci USA 108(19):7944–7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I, Chen S, Brand N, Tarway M, Liu L, Sheikh F, Astemborski J, Bonkovsky HL, Edlin BR, Howell CD, Morgan TR, Thomas DL, Rehermann B, Donnelly RP, O'Brien TR. 2013. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 45(2):164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, Bochud M, Battegay M, Bernasconi E, Borovicka J, Colombo S, Cerny A, Dufour J-F, Furrer H, Günthard HF, Heim M, Hirschel B, Malinverni R, Moradpour D, Müllhaupt B, Witteck A, Beckmann JS, Berg T, Bergmann S, Negro F, Telenti A, Bochud P-Y. 2010. Genetic variation in IL28B Is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology 138(4):1338–1345 [DOI] [PubMed] [Google Scholar]
- Sheahan T, Imanaka N, Marukian S, Dorner M, Liu P, Ploss A, Rice CM. 2014. Interferon lambda alleles predict innate antiviral immune responses and hepatitis C virus permissiveness. Cell Host Microbe 15(2):190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. 2005. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 5(9):558–567 [DOI] [PubMed] [Google Scholar]
- Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4(1):63–68 [DOI] [PubMed] [Google Scholar]
- Sommereyns C, Paul S, Staeheli P, Michiels T. 2008. IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog 4(3):e1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, Riordan S, Sheridan D, Smedile A, Fragomeli V, Muller T, Bahlo M, Stewart GJ, Booth DR, George J. 2009. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 41(10):1100–1104 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, Ito Y, Mita E, Tanaka E, Mochida S, Murawaki Y, Honda M, Sakai A, Hiasa Y, Nishiguchi S, Koike A, Sakaida I, Imamura M, Ito K, Yano K, Masaki N, Sugauchi F, Izumi N, Tokunaga K, Mizokami M. 2009. Genome-wide association of IL28B with response to pegylated interferon-[alpha] and ribavirin therapy for chronic hepatitis C. Nat Genet 41(10):1105–1109 [DOI] [PubMed] [Google Scholar]
- Tang W, Dennis M, Prokunina-Olsson L. 2013. Comparative analysis of biological activity of 11 mammalian paralogs of the novel human interferon IFNλ-4 (IFNL4) associated with viral clearance. Presented at the 63rd Annual Meeting of The American Society of Human Genetics, October2013, Boston, MA, [Google Scholar]
- Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M. 2009. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461(7265):798–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, Urban T, Afdhal NH, Jacobson IM, Esteban R, Poordad F, Lawitz EJ, McCone J, Shiffman ML, Galler GW, Lee WM, Reindollar R, King JW, Kwo PY, Ghalib RH, Freilich B, Nyberg LM, Zeuzem S, Poynard T, Vock DM, Pieper KS, Patel K, Tillmann HL, Noviello S, Koury K, Pedicone LD, Brass CA, Albrecht JK, Goldstein DB, McHutchison JG. 2010. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in hepatitis C virus-1 patients. Gastroenterology 139(1):120–129 [DOI] [PubMed] [Google Scholar]
- Urban TJ, Thompson AJ, Bradrick SS, Fellay J, Schuppan D, Cronin KD, Hong L, McKenzie A, Patel K, Shianna KV, McHutchison JG, Goldstein DB, Afdhal N. 2010. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology 52(6):1888–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S, Soriano V, Asselah T, Bronowicki J-P, Lohse AW, Mullhaupt B, Schuchmann M, Bourliere M, Buti M, Roberts SK, Gane EJ, Stern JO, Vinisko R, Kukolj G, Gallivan J-P, Bocher W-O, Mensa FJ. 2013. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med 369(7):630–639 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. 2007. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol 81(14):7749–7758 [DOI] [PMC free article] [PubMed] [Google Scholar]