Abstract
The role of host response-related factors in the fast progression of liver disease in individuals co-infected with HIV and HCV viruses remains poorly understood. This study compared patterns of cytokines, caspase-1 activation, endotoxin exposure in plasma as well as interferon signaling in peripheral blood mononuclear cells from HIV/HCV co-infected (HIV+/HCV+), HCV mono-infected (HIV−/HCV+), HIV mono-infected (HIV+/HCV−) female patients and HIV- and HCV-uninfected women (HIV−/HCV−) who had enrolled in the Women's Interagency HIV Study (WIHS). HIV+/HCV+ women had higher plasma levels of pro-inflammatory cytokines as well as caspase-1 compared with other groups. Both HIV+/HCV+ and HIV+/HCV− women had significantly higher sCD14 levels compared with other groups. Peripheral blood mononuclear cells from HCV mono-infected patients had reduced levels of phosphorylation of STAT1 compared with other groups as well as lower basal levels of expression of the IFN-stimulated genes, OAS1, ISG15, and USP18 (UBP43). Basal expression of USP18, a functional antagonist of ISG15, as well as USP18/ISG15 ratios were increased in the HIV+/HCV+ group compared with HIV−/HCV+ and HIV+/HCV− groups. A more pronounced systemic inflammatory profile as well as increased expression ratios of USP18 to ISG15 may contribute to the more rapid progression of liver disease in HIV+/HCV+ individuals.
Introduction
Since the introduction of anti-retroviral drugs and a subsequent reduction in AIDS-related mortality, HCV infection has become one of the major causes of disease and death among HIV-infected patients (Mohsen and others 2003; Hadigan and Kottilil 2011). In fact, approximately half of the non-AIDS-related deaths among HIV-infected patients have been reported to be liver related (Bica an others 2001). Although the course of HCV-related liver disease progression from infection, to liver cirrhosis and, eventually, hepatocellular carcinoma may last for approximately 20–30 years (Di Bisceigle 2000), it is significantly accelerated in individuals co-infected with the HIV virus (Soto and others 1997; Sulkowski and others 2007; French and others 2009). In fact, HIV/HCV co-infected individuals have a more rapid progression of liver fibrosis to cirrhosis compared with individuals infected with HCV alone (Soto and others 1997; Sulkowski and others 2007; French and others 2009). Moreover, HIV/HCV co-infected individuals usually have higher blood HCV RNA loads and decreased rates of sustained virological responses to anti-HCV therapy when compared with HCV mono-infected individuals (Torriani and others 2003; Soriano and others 2004).
The relationships between the immune responses to HIV and HCV and the causes for the more rapid progression of liver disease among co-infected individuals are not well understood. Studies employing DNA arrays in peripheral blood mononuclear cells (PBMC) from HIV/HCV co-infected individuals have reported gene expression profiles in which type I interferon (IFN)-stimulated genes (ISGs) are upregulated, particularly among those individuals who failed anti-HCV therapy (Lempicki and others 2006; Sarasin-Filipowicz and others 2008; Kottilil and others 2009). These studies have suggested that despite a chronically upregulated type I IFN-induced gene signature, some responses to type I IFN may be suppressed in these individuals, accounting for a decreased ability to respond to IFN-treatment and/or control HCV viral replication.
Type I IFN stimulation usually induces the expression of hundreds of ISGs, which are responsible for their antiviral, antiproliferative, and immunomodulatory properties (de Veer and others 2001). Two important antiviral ISGs are 2′-5′-oligoadenylate synthetase-1 (OAS1), an RNase activator and inhibitor of viral replication, and interferon-induced 17 kDa protein (ISG15), an ubiquitin-like protein that conjugates with a variety of cellular targets and which has also been associated with anti-viral activity (Ritchie and others 2004). The conjugation of ISG15 with its targets (ISGylation) is reversed by the action of another protease, ubiquitin specific peptidase 18 (USP18 or UBP43) (Malakhov and others 2002). An important regulatory role for USP18 in innate immune responses to viral infection has been suggested based on evidence showing that Usp18−/− mice present enhanced interferon-mediated resistance to the cytopathic effect of several viruses (Ritchie and others 2004). Moreover, it appears that USP18 is able to downregulate interferon signaling independently of its ISG15 de-conjugating activity by interfering with the JAK-STAT pathway at the level of the IFN receptor (Malakhova and others 2006). However, there are reports that ISG15 itself may play an important role in promotion of the HCV replication cycle (Broering and others 2010; Chen and others 2010). Indeed, high ISG15 expression has been associated with unfavorable HCV genotypes, high HCV viral loads, and suboptimal responses to type I IFN treatment (Chen and others 2005; Asselah and others 2008). Investigating the roles of ISG15 and USP18 in HIV/HCV co-infection may provide potential explanations for the observed suboptimal IFN responses and control of HCV in this group of patients.
Alterations in intestinal permeability, increased levels of endotoxemia, and markers of endotoxin-induced macrophage activation, such as soluble CD14 (sCD14) and lipid-binding protein, have been well documented in patients infected with HIV (Lien and others 1998; de Oca Arjona and others 2011; Papasavvas and others 2011; Sandler and others 2011; French and others 2013), suggesting that increased intestinal permeability and its sequelae (eg, endotoxemia, macrophage activation, and production of TNFα) may also play a potential role in exacerbating HCV disease.
Recently, a role for pyroptosis, a highly inflammatory type of programmed cell death, has been reported to be responsible for CD4 T-cell depletion in HIV-1 infection (Doitsh and others 2014). In contrast to apoptosis, pyroptosis is triggered by caspase-1, rather than caspase-3 activation, and results in the release of the cell cytoplasmic contents, including pro-inflammatory cytokines. Cycles of infection, cell death, and cytokine release may then contribute to a chronic inflammatory state that promotes tissue injury and disease progression (Lamkanfi and Dixit 2010). It remains to be determined how co-infection with HCV affects this cycle in HIV-1-infected individuals.
Identification of the key host response factors involved in HIV/HCV synergy is necessary in order to understand the basis for the accelerated liver disease in co-infected individuals as well as to identify potential therapeutic targets/interventions to help prevent or slow down liver damage. The aim of this retrospective study was to evaluate potential pathways and compare differences in the host immune response-related factors among HIV/HCV co-infected (HIV+/HCV+), HCV mono-infected (HIV−/HCV+), HIV mono-infected (HIV+/HCV−), and HIV- and HCV-uninfected (HIV−/HCV−) women. In this study, we investigated the potential role of (a) chronic inflammation and pryroptosis by analyzing systemic cytokine and caspase-1 levels; (b) gut permeability and endotoxin exposure by analyzing a marker of endotoxin-mediated macrophage activation (sCD14); and (c) type I interferon signaling pathways by analyzing STAT-1 phosphorylation and ISG expression in peripheral blood mononuclear cells obtained from participants who had enrolled in the Women's Interagency HIV Study (WIHS) (Barkan and others 1998; Bacon and others 2005).
Materials and Methods
Study design
The Women's Interagency HIV Study (WIHS) is a multicenter prospective cohort study that was established in 1994 to investigate the natural history of progression of HIV infection among women. A total of 3,766 women (2,791 HIV seropositive and 975 HIV seronegative) were enrolled in either 1994–1995 (n=2,623) or 2001–2002 (n=1,143) from 6 United States cities [New York (Bronx and Brooklyn), Chicago, Los Angeles, San Francisco, and Washington, DC]. Every 6 months, participants complete a comprehensive physical examination, provide blood specimens for CD4 cell count and HIV-RNA determination, and complete an interviewer-administered questionnaire, which provides data on demographics, disease characteristics, and specific anti-retroviral use. At each semi-annual study visit, participants are shown photo-medication cards and are asked the names of specific anti-retroviral medications used since their previous study visit. The WIHS uses a standard definition of highly active anti-retroviral therapy adapted from the Department of Health and Human Services/Kaiser Panel guidelines (US Department of Health and Human Services 2008). Other specific details of the methodology have been previously described (Barkan and others 1998; Bacon and others 2005). The work described in this article was approved by the University of Louisville Institutional Review Board (IRB# 11.0737). Informed consent was obtained from all participants, and human experimentation guidelines followed those of the U.S. Department of Health and Human Services.
Study groups
Five HIV+/HCV+, 8 HIV−/HCV+, 13 HIV+/HCV−, and 6 HIV−/HCV− women documented by laboratory results were included in this study. The HCV-infected women were HCV treatment naïve and had not received previous interferon and/or ribavirin therapy. All subjects were African American and study groups were matched for age. Groups were also matched for similar CD4+ T-cell counts (for the HIV-positive groups), similar HIV-viral loads (for the HIV-positive groups), and similar HCV-viral loads (for the HCV-positive groups). Frozen plasma and PBMC specimens collected from these women during their visits to WIHS centers were obtained from the WIHS bio-repository. Table 1 shows a summary of the characteristics of the patient groups.
Table 1.
Group Demographics and Laboratory Test Results
| Variable | HIV+/HCV+ | HIV−/HCV+ | HIV+/HCV− | HIV−/HCV− | P value |
|---|---|---|---|---|---|
| Age (years) | 51.1 (9.4) | 45.4 (6.3) | 49.9 (8.2) | 45.8 (13.2) | 0.123 |
| CD4+ T count (cells/μL) | 473 (525) | 1043 (201) | 502 (536) | 999 (445) | 0.008 |
| HIV RNA load (no. of copies/mL) | 20 (2029) | — | 20 (0) | — | 0.065 |
| HCV RNA load (log10) (no. of copies/mL) | 6.21 (1.41) | 6.19 (1.27) | — | — | 0.472 |
| AST (U/L) | 25 (71.5) | 43.5 (56.0) | 19.5 (8.5) | 17.5 (6.5) | 0.131 |
| ALT (U/L) | 18 (25.5) | 29.5 (41.3) | 15.5 (13.3) | 12.5 (10.8) | 0.215 |
Numbers represent the median and interquartile range (IQR) values for all variables.
Measurement of cytokine and caspase-1 levels in plasma samples
Plasma samples were thawed, centrifuged at 10,000 g for 5 min and the supernatants were used to measure the concentrations of 10 different cytokines and chemokines (IL-1β, IL-1ra, IL-6, CXCL8 [IL-8], IL-10, IL-12p40, IL-17, IFN-γ, TNFα, and CXCL10 [IP-10]) using Milliplex MAP Multiplex kits (EMD Millipore, Billerica, MA). Plasma IL-18 levels were measured using an Instant ELISA kit (eBioscience, San Diego, CA). The levels of immunoreactive caspase-1 in plasma were also measured by ELISA (R&D Systems, Minneapolis, MN).
Measurement of endotoxin and soluble CD14 levels
Endotoxin levels in the plasma samples were measured using a kinetic QCL-Limulus Amoebocyte Lysate assay (Lonza, Walkersville, MD). The plasma levels of sCD14, which are a reflection of previous exposure to endotoxin (Landmann and others 1996), were measured using a commercial 2-site ELISA kit (Aviscera Bioscience, Santa Clara, CA).
Thawing and culture of PBMC
PBMC specimens were maintained in liquid N2 until they were assayed. After thawing, the cells (∼6–10×106 cells/vial) were diluted 1:10 in complete medium (RPMI 1640 supplemented with 10% FCS, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids) containing 0.25 U/mL Benzonase (Sigma Aldrich, St. Louis, MO) to prevent cell aggregation. The cells were incubated overnight at 37°C/5% CO2 atmosphere at a cell density of 1×106 cells/mL in complete medium. Aliquots of the cells were then either left untreated or stimulated with IFN-α and subsequently used for measurement of STAT1 phosphorylation or RNA extraction for gene expression studies.
STAT1 phosphorylation
PBMC aliquots in complete medium were transferred to a 96-well plate (1×105 cells/well) and either left untreated or stimulated with IFN-α2 (1,000 U/mL, PBL Interferon Source, Piscataway, NJ) for 30 min at 37°C/5% CO2. Subsequently, cells were lysed and total and phosphorylated-STAT1 were measured using a Human Phospho-STAT1 (Y701) cell-based ELISA (R&D Systems). Results were normalized by dividing the phospho-STAT1 fluorescence (630 nm) by the total STAT1 fluorescence (450 nm) in each well. For each sample, the phospho-STAT1 response in the presence of IFN-α2 was compared with that of the untreated cells and expressed as a percentage (% of basal). No significant differences in the basal levels of STAT1 phosphorylation were detected among the 4 study groups.
Stimulation of PBMC for gene expression studies
Aliquots of the PBMCs were cultured in 24-well plates at a cell density of 1×106/mL in complete medium and left alone or stimulated with IFN-α2 (1,000 U/mL) for 6 h at 37°C/5% CO2. The cells were subsequently harvested by centrifugation and immediately lysed using a miRVANA lysis reagent (Ambion, Austin, TX). Total RNA was isolated using a miRVANA kit according to the manufacturer's protocol, and purity and quantity were assessed spectrophotometrically using a Nanodrop 2000 apparatus (Thermo Scientific, Wilmington, DE).
Real-time PCR analysis
A reverse-transcription step was performed with total RNA aliquots using a High-capacity RNA-to-cDNA kit according to the manufacturer's protocol (Applied Biosystems, San Diego, CA). Expression levels of transcripts specific for TNFα, OAS1, ISG-15, and USP18 in unstimulated PBMCs were measured by a real-time quantitative polymerase chain reaction (qPCR). After the reverse-transcription step, cDNA aliquots were amplified in triplicates in an ABI prism 7500 sequence detection system (Applied Biosystems, Foster City, CA) using SYBR-green and transcript-specific primers (Applied Biosystems). The expression levels of the different gene transcripts were normalized to those of 18S rRNA in all the experiments (ΔCt). The relative expression in comparison to the average of the control (HIV−/HCV−) group was calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001). The sequences of the primers used were TNF (forward: 5′-CCCCCAGAGGGAAGAGTTC-3′, reverse: 5′-CAGCTT GAGG GTTTGCTACA-3′); OAS1 (forward: 5′-GCAGAAA GAGGGCGAGTTCT-3′, reverse: 5′-CCTGGGCTGTGTTG AAATGT-3′); ISG15 (forward: 5′-CCCACAGCCATGG GCT-3′, reverse: 5′-CGATCTTCTGGGTGATCTGC-3′); USP18 (forward: 5′-AGTCCCGACGTGGAACTCAG-3′, reverse: 5′-TCAGGACAGCACGACTTCAC-3′); 18S rRNA: (forward: 5′-CTCAACACGGGAAACCTCAC-3′, reverse: 5′-CGCTCC ACCAACTAAGAACG-3′).
Statistical methods
Results of the cytokine concentrations, STAT-1 phosphorylation, and gene expression studies were analyzed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Data distribution was analyzed using the D'Agostino and Pearson omnibus normality test. The Kruskall–Wallis one-way analysis of variance was performed to test for differences across the 4 study groups. The Mann–Whitney test was used for comparisons involving 2 different groups. Results were considered statistically significant at P values<0.05.
Results
Description of patient population
The demographics and laboratory values of the study groups are shown in Table 1. All the women in the 4 groups were middle age (there were no statistically significant age differences among the groups [P=0.123]). Although there were no significant differences in CD4+ T-cell counts between the 2 HIV-infected groups (HIV+/HCV+ and HIV+/HCV−), these groups had lower CD4+ T-cell counts compared with the non-HIV-infected groups (HIV−/HCV+ and HIV−/HCV−) [P=0.008]. There were no significant differences in HIV RNA loads, which were mostly undetectable in the HIV+/HCV+ and HIV+/HCV− groups [P=0.065]. HCV RNA loads were substantially elevated in both HCV-infected groups (HIV+/HCV+ and HIV−/HCV+) but there were no significant differences between them [P=0.472]. Despite a tendency for higher levels in the HIV+/HCV+ and HIV−/HCV+ groups, there were no statistically significant differences in the serum levels of the liver enzymes, AST (P=0.131) and ALT (P=0.215) among the 4 groups.
Chronic inflammation and pyroptosis: systemic cytokine and caspase-1 levels
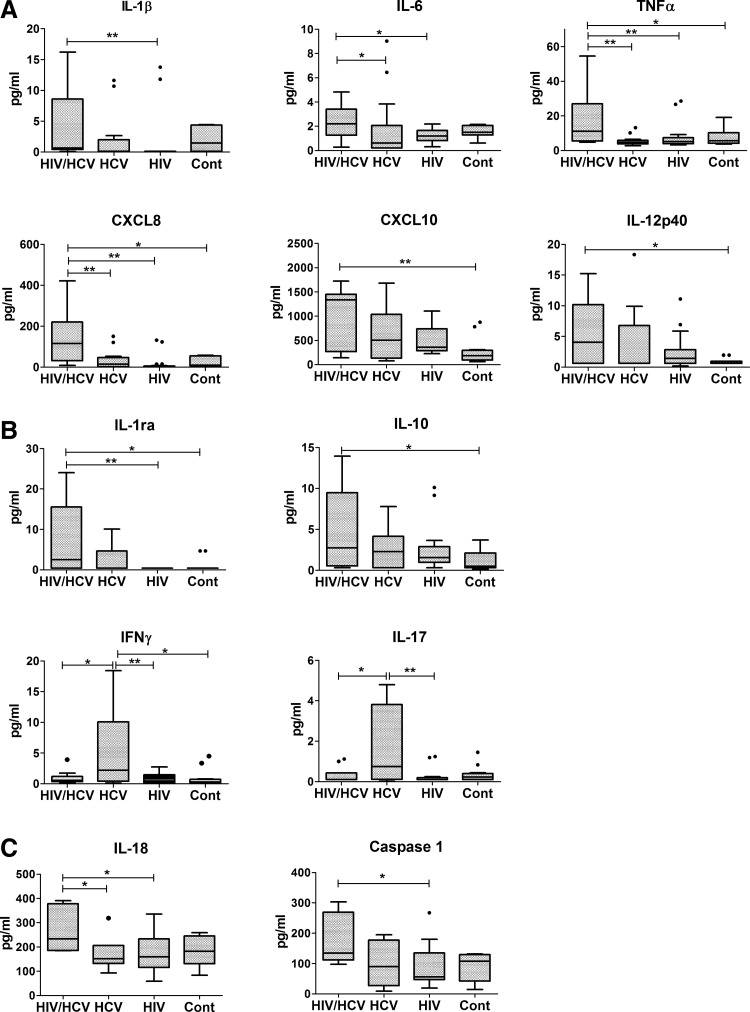
In order to investigate the potential involvement of chronic inflammation, we measured the systemic levels of several cytokines and chemokines. Figure 1A shows the results for the pro-inflammatory cytokines. As seen in this panel, the co-infected (HIV+/HCV+) group had a plasma cytokine pattern with consistently higher levels of all of the measured cytokines, including IL-1β, IL-6, TNFα, CXCL8, and CXCL10, when compared with the other groups, indicating a more pronounced systemic pro-inflammatory profile. No significant differences were observed among the other 3 groups. Results for the anti-inflammatory (IL-1ra and IL-10) and adaptive immunity cytokines (IFN-γ and IL-17) are shown in Fig. 1B. As can be seen in this panel, the HIV+/HCV+ group also tended to have higher levels of anti-inflammatory cytokines compared with the other groups. Statistically significant differences were observed between the HIV+/HCV+ group and HIV+/HCV− and control HIV−/HCV− groups for IL-1ra but only with the control HCV−/HIV− group in the case of IL-10. In contrast to the more prominent pro-inflammatory profile of the HIV+/HCV+ group, levels of the adaptive-immunity cytokines were found to be higher in the HIV−/HCV+ group compared with other groups. Finally, due to the heightened pro-inflammatory profile seen in the co-infected group and based on a recent report identifying pyroptosis as a driver of CD4+ T-cell depletion in HIV infection (Doitsh and others 2014), we investigated the plasma levels of caspase-1 and IL-18, a cytokine that, similar to IL-1β, is cleaved by caspase-1. As shown in Fig. 1C, higher median levels of both IL-18 and caspase-1 were found in the co-infected (HIV+/HCV+) group. Statistically significant differences were found for both mono-infected groups in the case of IL-18 and with the HIV+/HCV− group in the case of caspase-1. Taken together, these results suggest that HIV/HCV co-infection may result in a heightened chronic inflammatory profile with increased caspase-1 levels.
FIG. 1.
Plasma cytokine and caspase-1 profiles in HIV+/HCV+, HIV−/HCV+, HIV+/HCV−, and HIV−/HCV− groups. Plasma samples were assayed for the indicated pro-inflammatory (A) and anti-inflammatory and adaptive immunity cytokines/chemokines (B) using a bead-based multiplex assay. IL-18 and caspase-1 levels (C) were determined by ELISA as indicated in the “Materials and Methods” section. Box plots depict the 25%–75% interquartile range, and the horizontal bar depicts the median. *P<0.05; **P<0.01.
Gut permeability and endotoxin exposure: soluble CD14 levels
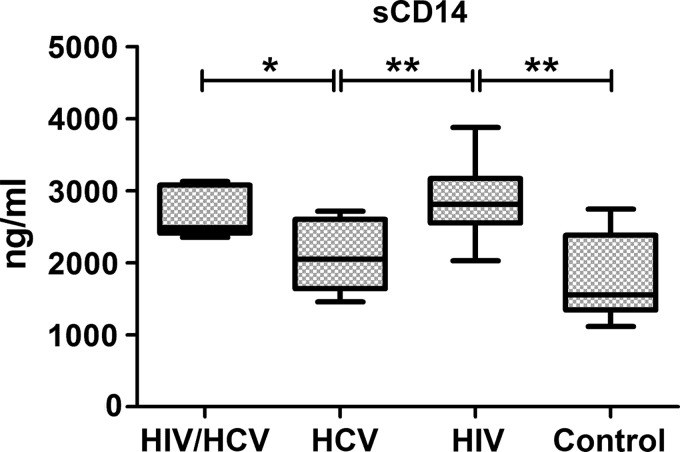
Measurement of endotoxin levels in the plasma samples using a limulus amoebocyte lysate-based assay failed to show any statistically significant differences among the groups (results not shown). However, since endotoxin levels can be affected by a variety of endogenous (ie, endotoxin-binding substances, platelet content) or exogenous factors (ie, fat content in the diet, smoking, and alcohol consumption) (Erridge and others 2007; Romaschin and others 2012; Balagopal and others 2012), the levels of sCD14, a more stable marker of previous endotoxin exposure (Landmann and others 1996), were investigated. An analysis of plasma sCD14 levels showed statistically significant differences among the groups (P=0.004). The 2 HIV-infected groups (HIV+/HCV+ and HIV+/HCV−) had significantly higher sCD14 levels, compared with the HIV−/HCV+ and HIV−/HCV− groups (Fig. 2), but there were no statistically significant differences between the 2 HIV-infected groups. These results suggest that HIV-infected patients, irrespective of HCV infection, may have increased gut permeability and exposure to endotoxin.
FIG. 2.
Soluble CD14 levels in plasma samples from HIV+/HCV+, HIV−/HCV+, HIV+/HCV−, and HIV−/HCV− groups. sCD14 levels were measured using a 2-site commercial ELISA kit as described in the “Materials and Methods” section. Box plots depict the 25%–75% interquartile range, and the horizontal bar depicts the median. *P<0.05; **P<0.01.
IFN signaling: type I IFN-induced STAT-1 phosphorylation
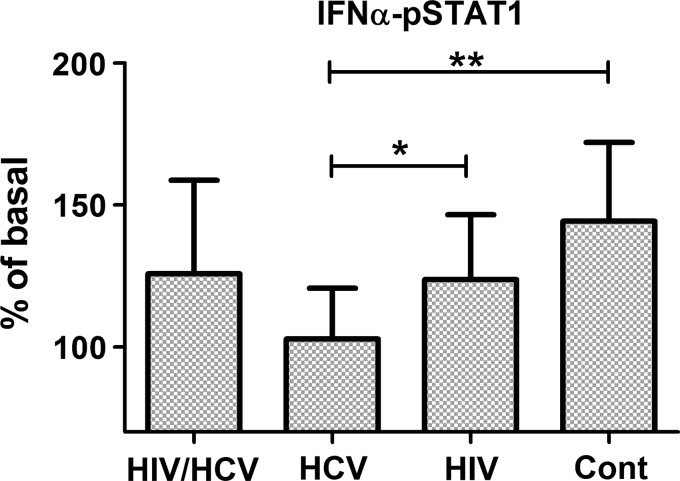
To investigate whether HIV or HCV infection modulates the ability of PBMCs to respond to type-I interferon, PBMCs were exposed to IFN-α2 and the levels of phospho-STAT1 were measured as an indicator of interferon responsiveness. As seen in Fig. 3, the mean levels of STAT-1 phosphorylation (expressed as% of the levels in the absence of IFN-α) were only significantly reduced in the HIV−/HCV+ group in comparison with the control uninfected group (P=0.006). In the case of the 2 HIV-infected groups (HIV+/HCV+ and HIV+/HCV−), there were only moderately reduced STAT1 phosphorylation levels that were not statistically different from the control group. These results suggest that type I IFN receptor-mediated signaling is particularly compromised in HCV-infected patients.
FIG. 3.
Type I IFN-induced STAT-1 phosphorylation in PBMCs from HIV+/HCV+, HIV−/HCV+, HIV+/HCV−, and HIV−/HCV− groups. PBMC samples were thawed, allowed to rest overnight in culture, and then left untreated or exposed to IFN-α (1,000 U/mL) for 30 min at 37°C. STAT-1 phosphorylation was measured using a cell-based ELISA as described in the “Materials and Methods” section. Results are expressed as % of basal, comparing phosphorylation in the presence of IFN-α2 with the baseline levels in untreated PBMCs. Bar graphs represent the averages and SD *P<0.05; **P<0.01.
IFN signaling: ISG expression levels
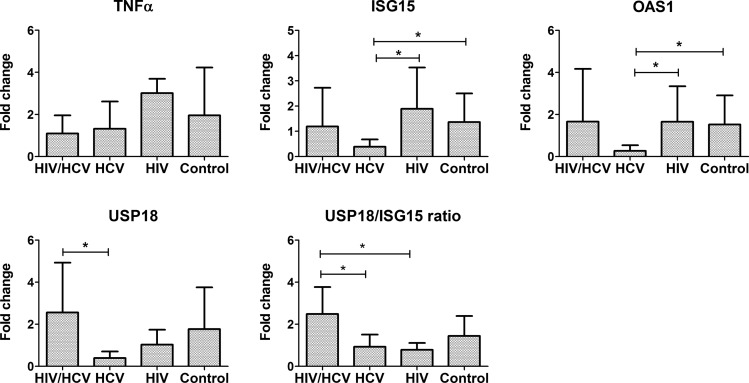
The levels of expression of several ISGs (OAS1, ISG15, and USP18) as well as of TNFα in unstimulated PBMC were investigated, and results are shown in Fig. 4. There were no statistically significant differences in the basal (unstimulated) expression levels of TNFα among the 4 study groups (P=0.201). The basal expression of OAS-1, ISG15, and USP18 followed a similar pattern to that observed for STAT-1 phosphorylation, with the lowest levels found in the HIV−/HCV+ group. Differences in the expression of OAS1 among the 4 groups were statistically significant (P=0.048) and approached significance in the case of ISG15 (P=0.085). Interestingly, the expression of USP18 was significantly increased in the HIV+/HCV+ group in comparison to the HIV−/HCV+ group (P=0.015). When USP18/ISG15 ratios were calculated, the HIV+/HCV+ group had significantly higher ratios compared with the HIV−/HCV+ (P=0.033) and the HIV+/HCV− groups (P=0.048). The expression levels of OAS1 and ISG15 after stimulation of the PBMC in vitro with type I IFN were also investigated. However, under the experimental conditions used, all 4 groups showed induction of the 2 genes, with no significant differences among the groups (results not shown). These results are consistent with observed defects in IFN-signaling in HCV-infected patients and suggest that HIV/HCV co-infection may restrict the inhibitory effect on IFN-signaling but be associated with a dysregulation in the expression of USP18/ISG15.
FIG. 4.
Expression of transcripts for TNFα, OAS-1, ISG15, and USP18 in unstimulated PBMCs from HIV+/HCV+, HIV−/HCV+, HIV+/HCV−, and HIV−/HCV− groups. PBMC samples were thawed, allowed to rest overnight in culture, and then lysed; total RNA was isolated. Expression of the different genes was measured by quantitative PCR, and relative expression over the average of the control (HIV−/HCV−) group was calculated based on the −ΔΔCt method, as described in the “Materials and Methods” section. Bar graphs represent the average and SD *P<0.05.
Discussion
The purpose of this study was to investigate potential differences in host response-related factors between HIV and HCV co-infected and HIV or HCV mono-infected women. We found that HIV+/HCV+ co-infected women had a more prominent plasma pro-inflammatory cytokine profile and higher levels of plasma caspase-1 when compared with HCV+/HIV−, HIV+/HCV−, and HCV−/HIV− women. Reduced IFN-mediated signaling, as evidenced by reduced levels of phosphorylation of STAT1 on exposure to type I IFN, was particularly evident in the HIV−/HCV+, but not the co-infected group. In agreement, the basal expression levels of the IFN-inducible genes, OAS1, ISG15, and USP18 were also reduced in the HCV-monoinfected (HIV−/HCV+) group. In contrast, the co-infected HIV+/HCV+ group showed higher expression levels of USP18 compared with the HIV−/HCV+ group and significantly increased USP18 to ISG15 ratios compared with the other groups.
In the case of plasma cytokines, the HIV+/HCV+ group showed consistently higher median levels of pro-inflammatory cytokines, including IL-1β, IL-6, TNFα, CXCL8, and CXCL10. Due to the recent report linking pyroptosis, a caspase-1-dependent and highly pro-inflammatory type of programmed cell death with CD4+ T-cell depletion in HIV-1 infection (Doitsh and others 2014), the pro-inflammatory profile of the co-infected patients prompted us to investigate the plasma levels of IL-18 and caspase-1. Our results showed indeed higher levels of both caspase-1 and IL-18 in the HIV+/HCV+ group, raising the possibility that enhanced pyroptosis in this group may be associated with a chronic inflammatory state. A potential contributing factor for the higher levels of pro-inflammatory cytokines could also be increased intestinal permeability, which has been reported to affect HIV-infected individuals (Mehandru and others 2004; Kotler 2005; French and others 2013). In our study, both HIV-infected groups had significantly higher plasma levels of sCD14, a marker of macrophage exposure/activation by endotoxin, compared with the HIV−/HCV+ and HIV−/HCV− groups. While these findings are suggestive of elevated exposure to endotoxin, by themselves, they fail to explain the marked systemic pro-inflammatory profile seen in HIV+/HCV+ women, as HIV+/HCV− women had lower pro-inflammatory plasma cytokines despite having comparable levels of sCD14. A potential explanation is that HCV infection may lead to alteration of the responsiveness of liver macrophages or Kupffer cells to endotoxin, resulting in the higher levels of pro-inflammatory cytokines in co-infected patients.
It is possible that pyroptosis and endotoxin stimulation may potentiate each other. It is known that Toll-like receptor stimulation often acts in concert with caspase-1-activating Nod-like receptors, increasing the susceptibility of cells to undergo caspase-1 activation in response to cytosolic recognition of host- or pathogen-derived danger signals (Bergsbaken and others 2009). These mechanisms may not only aggravate the HIV infection but also the higher levels of pro-inflammatory cytokines may play an important role in promoting or accelerating liver injury (Lien and others 1998; Kotler 2005; de Oca Arjona and others 2011; Sandler and others 2011; French and others 2013). TNFα, for example, has been reported to be an important factor in promoting hepatocyte cell damage in response to a variety of toxic insults (Yin and others 1999). A very different profile was observed with the 2 adaptive immunity-related cytokines, IFN-γ and IL-17, in which the HIV−/HCV+ group showed the highest median levels compared with the other groups. This may be related to an ongoing anti-viral immune response in these women. On the other hand, the lower levels seen in the HIV+/HCV+ group may be, in part, the result of HIV-related immunosuppressive mechanisms on T-cell immunity. These defects as well as suboptimal anti-viral T-cell responses would allow for a failure to control HCV infection, resulting in accelerated liver damage.
Our results showing reduced phosphorylation of STAT1 suggested a defective IFN-mediated signaling, particularly in the HCV-infected compared with the other groups. Indeed, HCV has been reported to downregulate IFN-mediated signaling (Gale and Foy 2005). Inhibition of IFN-signaling pathways would interfere with both innate and adaptive immunity mechanisms that control viral replication, resulting in inadequate anti-viral immunity. The reasons behind the low levels of STAT1 phosphorylation in the HIV−/HCV+ but not in the HIV+/HCV+ group remain to be investigated. In this regard, there is evidence of increased expression of IFN-induced genes in the latter group (Lempicki and others 2006; Sarasin-Filipowicz and others 2008; Kottilil and others 2009), suggesting that co-inection may result in comparatively more IFN activity.
Concurrent with the lower levels of STAT1 phosphorylation in the HIV−/HCV+ group found in this study, the basal expression levels of the IFN-induced genes, OAS1, ISG15, and USP18, followed a similar pattern, thus reinforcing the idea of interference with IFN signaling mechanisms. Interestingly, the HIV+/HCV+ group had the highest levels of expression of USP18 and USP18 to ISG15 ratios. USP18 is a protease that de-conjugates ISG15 from its cellular targets and thus antagonizes ISG15 function, including its anti-viral activity (Malakhov and others 2002). Thus, increased USP18 levels and particularly its ratio to that of ISG15 may result in further reduced antiviral activity in HIV+/HCV+ patients, contributing to the failure to control HCV infection during anti-HCV treatment. Failure to respond to interferon treatment has been correlated with higher liver levels of USP18 among chronic hepatitis C patients (Chen and others 2011), thus highlighting its important role in regulating anti-viral responses.
In addition to type I IFN, the expression of USP18 is also induced by LPS via interferon regulatory factor 3 (IRF-3) (Malakhova and others 2002). While it is possible that increased exposure to endotoxin may contribute to the higher USP18 expression seen in the HIV+/HCV+ co-infected patients, the fact that HIV-monoinfected patients did not have the same USP18/ISG15 profile despite also having elevated sCD14 levels suggests that endotoxin is not the only factor responsible.
In summary, results of our study provide evidence for a pronounced systemic inflammatory profile in HIV/HCV-co-infected patients. The responsible mechanisms are not yet clear, but inflammation associated with HIV-induced pyroptotic CD4+ T-cell death as well as with increased endotoxin exposure may play a role. In addition, dysregulation of USP18/ISG15 expression may contribute to the failure to control HCV infection and the more rapid progression of liver disease in HIV+/HCV+ individuals. The relatively small sample size of our study is an important limitation and urges caution when making generalizations. Additional studies with larger sample sizes are needed to validate our results.
Acknowledgments
This work was partially supported by NIH U01AI-034994-17REV. Data in this article were collected by the Women's Interagency HIV Study (WIHS); Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Asselah T, Bieche I, Narguet S, Sabbagh A, Laurendeau I, Ripault MP, Boyer N, Martinot-Peignoux M, Valla D, Vidaud M, Marcellin P. 2008. Liver gene expression signature to predict response to pegylated interferon plus ribavirin combination therapy in patients with chronic hepatitis C. Gut 57:516–524 [DOI] [PubMed] [Google Scholar]
- Bacon M, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA. 2005. The women's interagency HIV study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 12:1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal A, Gama L, Franco V, Russell JN, Quinn J, Higgins Y, Smeaton LM, Clements JE, Thomas DL, Gupta A. 2012. Detection of microbial translocation in HIV and SIV infection using Limulus amebocyte lysate assay is masked by serum and plasma. PLos One 7:e41258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J. 1998. The women's interagency HIV study. Epidemiology 9:117–125 [PubMed] [Google Scholar]
- Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. 2001. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 32:492–497 [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. 2009. Pyroptosis: host cell death and inflammation. Nat Rev Immunol 7:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering R, Zhang X, Kottilil S, Trippler M, Jiang M, Lu M, Gerken G, Schlaak JF. 2010. The interferon stimulated gene 15 functions as a proviral factor for the hepatitis C virus and as a regulator of the IFN response. Gut 59:1111–1119 [DOI] [PubMed] [Google Scholar]
- Chen J, Sun J, Meng L, Heathcote J, Edwards AM. 2010. ISG15, a ubiquitin-like interferon-stimulated gene, promotes hepatitis C virus production in vitro: implications for chronic infection and response to treatment. J Gen Virol 91:382–388 [DOI] [PubMed] [Google Scholar]
- Chen L, Borozan I, Field J, Sun J, Tannis LL, Coltescu C, Heathcote J, Edwards AM, McGilvray ID. 2005. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C infection. Gastroenterology 128:1437–1444 [DOI] [PubMed] [Google Scholar]
- Chen L, Li S, McGilvray I. 2011. The ISG15/USP18 ubiquitin-like pathway (ISGylation system) in hepatitis C virus infection and resistance to interferon therapy. Int J Biochem Cell Biol 43:1427–1431 [DOI] [PubMed] [Google Scholar]
- de Oca Arjona MM, Marquez M, Soto MJ, Rodriguez-Ramos C, Terron A, Vergara A, Arizcorreta A, Fernandez-Gutierrez C, Giron-González JA. 2011. Bacterial translocation in HIV-infected patients with HCV cirrhosis: implications in hemodynamic alterations and mortality. J AIDS 56:42–47 [DOI] [PubMed] [Google Scholar]
- de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams BR. 2001. Functional classification of interferon-stimulated genes identified using microarrays. J Leuk Biol 69:912–920 [PubMed] [Google Scholar]
- Di Bisceigle AM. 2000. Natural history of hepatitis C: its impact on clinical management. Hepatology 31:1014–1018 [DOI] [PubMed] [Google Scholar]
- Doitsh G, Galloway NLK, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Muñoz-Arias I, Greene WC. 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge C, Attina T, Spickett CM, Webb DJ. 2007. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 86:1286–1292 [DOI] [PubMed] [Google Scholar]
- French AL, Evans CT, Agniel DM, Cohen MH, Peters M, Landay AL, Desai SN. 2013. Microbial translocation and liver disease progression in HIV/hepatitis C co-infected women. J Infect Dis 208:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AL, Gawel SH, Hershow RC, Benning L, Hessol NA, Levine AM, Anastos K, Augenbraun M, Cohen MH. 2009. Trends in mortality and causes of death for women with HIV in the US: a ten year study. J AIDS 51:399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M, Foy EM. 2005. Evasion of intracellular host defence by hepatitis C virus. Nature 436:939–945 [DOI] [PubMed] [Google Scholar]
- Hadigan C, Kottilil S. 2011. Hepatitis C virus infection and co-infection with Human Immunodeficiency virus. JAMA 306:294–301 [DOI] [PubMed] [Google Scholar]
- Kotler DP. 2005. HIV infection and the gastrointestinal tract. AIDS 19:107–117 [DOI] [PubMed] [Google Scholar]
- Kottilil S, Yan MY, Reitano KN, Zhang X, Lempicki R, Roby G, Daucher M, Yang J, Cortez KJ, Ghany M, Polis MA, Fauci AS. 2009. Human immunodeficiency virus and hepatitis C infections induce distinct immunologic imprints in peripheral mononuclear cells. Hepatology 50:34–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. 2010. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe 8:44–54 [DOI] [PubMed] [Google Scholar]
- Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. 1996. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun 64:1762–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempicki RA, Polis MA, Yang J, McLaughlin M, Koratich C, Huang DW, Fullmer B, Wu L, Rehm CA, Masur H, Lane HC, Silverman KE, Fauci AS, Kotilil S. 2006. Gene expression profiles in hepatitis C virus (HCV) and HIV coinfection: class prediction analyses before treatment predict the outcome of anti-HCV therapy among HIV-coinfected persons. J Infect Dis 193:1172–1177 [DOI] [PubMed] [Google Scholar]
- Lien E, Aukrust P, Sundan A, Müller F, Froland SS, Espevik T. 1998. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: Correlation to disease progression and clinical events. Blood 92:2084–2092 [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. 2002. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem 277:9976–9981 [DOI] [PubMed] [Google Scholar]
- Malakhova O, Malakhov M, Hetherington C, Zhang DE. 2002. Lipopolysaccharide activates the expression of ISG15-specific protease UBP43 via interferon regulatory factor 3. J Biol Chem 277:14703–14711 [DOI] [PubMed] [Google Scholar]
- Malakhova OA, Kim KI, Juo J-K, Zou W, Suresh Kumar KG, Fuchs SY, Shuai K, Zhang DE. 2006. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J 25:2358–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsen AH, Easterbrook PJ, Taylor C, Portmann B, Kulasegaram R, Murad S, Wiselka M, Norris S. 2003. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut 52:1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasavvas E, Azzoni L, Foulkes A, Violari A, Cotton MF, Pistilli M, Reynolds G, Yin X, Glencross DK, Stevens WS, McIntyre JA, Montaner LJ. 2011. Increased microbial translocation in ≤180 days old perinatally human immunodeficiency virus-positive infants as compared with human immunodeficiency virus-exposed uninfected infants of similar age. Pediatr Infect Dis J 30:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie KJ, Hahn CS, Kim KI, Yan M, Rosario D, Li L, de la Torre JC, Zhang DE. 2004. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nature Med 10:1374–1378 [DOI] [PubMed] [Google Scholar]
- Romaschin AD, Klein DJ, Marshall JC. 2012. Bench to bedside review: Clinical experience with the endotoxin activity assay. Crit Care 16:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC; INSIGHT SMART Study Group. 2011. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 203:780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin-Filipowicz M, Oakeley EJ, Duong FH, Christen V, Terracciano L, Filipowicz W, Heim MH. 2008. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci U S A 105:7034–7039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano V, Perez-Olmeda M, Rios P, Núñez M, García-Samaniego J, González-Lahoz J. 2004. Hepatitis C virus (HCV) relapses after anti-HCV therapy are more frequent in HIV-infected patients. AIDS Res Hum Retrovir 20:353–363 [DOI] [PubMed] [Google Scholar]
- Soto B, Sánchez-Quijano A, Rodrigo L, del Olmo JA, García-Bengoechea M, Hernández-Quero J, Rey C, Abad MA, Rodríguez M, Sales Gilabert M, González F, Mirón P, Caruz A, Relimpio F, Torronteras R, Leal M, Lissen E. 1997. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol 26:1–5 [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, Moore RD, Afdhal NH, Thomas DL. 2007. Rapid fibrosis among HIV/Hepatitis C virus co-infected adults. AIDS J 21:2209–2216 [DOI] [PubMed] [Google Scholar]
- Torriani FJ, Ribeiro RM, Gilbert TL, Schrenk UM, Clauson M, Pacheco DM, Perelson AS. 2003. Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) dynamics during HCV treatment in HCV/HIV coinfection. J Infect Dis 188:1498–1507 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services and Henry J. Kaiser Family Foundation Panel on Clinical Practices for the Treatment of HIV Infection [DHHS]. 2008. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Available at http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf
- Yin M, Wheeler MD, Kono H, Bradford BU, Galluci RM, Luster MI, Thurman RG. 1999. Essential role of tumor necrosis factor α in alcohol-induced liver injury in mice. Gastroenterology 117:942–952 [DOI] [PubMed] [Google Scholar]