Abstract
Mitochondrial dysfunction in animal models of heart failure is associated with downregulation of the peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α pathway. To test whether PGC-1α is an appropriate therapeutic target for increasing mitochondrial biogenesis and improving function in heart failure, we used a transgenic (TG) mouse model of moderate overexpression of PGC-1α (∼3-fold) in the heart. TG mice had small increases in citrate synthase activity and mitochondria size in the heart without alterations in myocardial energetics or cardiac function at baseline. In vivo dobutamine stress increased fractional shortening in wild-type mice, but this increase was attenuated in TG mice, whereas ex vivo isolated perfused TG hearts demonstrated normal functional and energetic response to high workload challenge. When subjected to pressure overload by transverse aortic constriction (TAC), TG mice displayed a significantly greater acute mortality for both male and female mice; however, long-term survival up to 8 wk was similar between the two groups. TG mice also showed a greater decrease in fractional shortening and a greater increase in left ventricular chamber dimension in response to TAC. Mitochondrial gene expression and citrate synthase activity were mildly increased in TG mice compared with wild-type mice, and this difference was also maintained after TAC. Our data suggest that a moderate level of PGC-1α overexpression in the heart compromises acute survival and does not improve cardiac function during chronic pressure overload in mice.
Keywords: peroxisome proliferator-activated receptor-γ coactivator 1α, heart failure, cardiac hypertrophy, mitochondrial biogenesis, energetics
increasing evidence implicates mitochondrial dysfunction in the development of a plethora of cardiac pathologies, including heart failure (16). Impaired myocardial energetics associated with quantitative and qualitative changes in mitochondria have been demonstrated in human end-stage heart failure (19). We have previously shown that defective mitochondrial biogenesis occurred early during the development of cardiac dysfunction, before evident clinical symptoms, suggesting that mitochondrial biogenesis plays an important role in the development and progression of heart failure (10).
Peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α has been identified as an inducible upstream regulator of mitochondrial biogenesis that determines mitochondrial mass and function. Decreased mitochondrial biogenesis in heart failure has been associated with decreased PGC-1α gene expression as well as downregulation of its downstream target genes (8). Furthermore, deletion of PGC-1α in mice accelerates pressure overload-induced cardiac hypertrophy and failure (2), raising the intriguing possibility that PGC-1α can be targeted to increase mitochondrial biogenesis and improve cardiac energetics and function in heart failure.
Several attempts have been made over the last several years to enhance mitochondrial biogenesis in the heart by overexpression of PGC-1α, but the outcomes were not as promising as anticipated. Lehman et al. (14) showed that cardiac overexpression of PGC-1α results in a progressive and uncontrolled increase in mitochondrial number that disrupts the cardiomyocyte sarcomeric structure, leading to contractile dysfunction. Similarly, using an inducible model of PGC-1α overexpression, Russell et al. (23) found myofilament derangement and reversible cardiomyopathy in mice. In those studies, PGC-1α expression was driven by the α-myosin heavy chain promoter. The authors proposed that an excessive induction of PGC-1α could be detrimental. More recently, Lynn et al. (15) used a mouse model of mild PGC-1α overexpression (4-fold) and reported that a transient upregulation of PGC-1α worsened the cardiac response to ischemia-reperfusion injury. However, the effect of moderate and sustained upregulation of PGC-1α in pathological cardiac hypertrophy and heart failure has not been tested.
In the present study, we examined whether a mild upregulation of PGC-1α could maintain mitochondrial biogenesis and preserve cardiac function during chronic cardiac stress. We found that cardiac mitochondrial morphology and function were maintained in a transgenic (TG) mouse model overexpressing PGC-1α by 3-fold in the heart. Although the expression of genes involved in mitochondrial biogenesis in TG mice after chronic pressure overload was maintained at a level similar to that in wild-type (WT) sham-operated mice, cardiac function and long-term survival of the mice were not improved.
MATERIALS AND METHODS
Animal experiments.
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of the University of Washington. MCK-PGC-1α mice (where MCK is muscle-form creatine kinase) were kindly provided by Dr. Zoltan Arany and backcrossed to the FVB background for at least six generations. Mice were kept on a 12:12-h light-dark cycle at 22°C with water and food ad libitum.
Isolated perfused mouse heart preparation and 31P NMR spectroscopy.
Ex vivo cardiac function and myocardial energetics were assessed in isolated hearts perfused in the Langendorff mode in conjunction with 31P NMR spectroscopy (12). In brief, excised hearts were initially perfused at 80 mmHg with modified Krebs Henseleit (KH) buffer consisting of 118 mmol/l NaCl, 25 mmol/l NaHCO3, 5.3 mmol/l KCl, 2.0 mmol/l CaCl2, 1.2 mmol/l MgSO4, and 0.5 mmol/l EDTA equilibrated with 95% O2 and 5% CO2 (pH 7.4) and enriched with 10 mmol/l glucose and 0.5 mmol/l pyruvate. After stabilization, the glucose- and pyruvate-containing KH buffer was switched to a mixed-substrate KH buffer consisting of 5.5 mmol/l glucose, 1.2 mmol/l lactate, 0.4 mmol/l long-chained fatty acids (bound to 1.2% BSA), and 50 μU/ml insulin. Temperature was maintained at 37.5°C throughout the protocol. After 10 min of equilibration, baseline function was monitored for 10 min at a fixed end-diastolic pressure of 8–10 mmHg via a water-filled balloon inserted into the left ventricle (LV). After baseline, the Ca2+ concentration in the mixed-substrate KH buffer was changed from 2 to 4 mM to induce an acute increase in cardiac function (i.e., high workload challenge).
During baseline and high workload periods, dynamic changes in cardiac content of phosphocreatine (PCr), ATP, and Pi were monitored using 31P NMR spectroscopy simultaneously with continuous recording of LV function via a data-acquisition system (PowerLab, AD Instruments, Colorado Springs, CO). Spectra were obtained by averaging 120 free induction decays over a time period of 5 min at a pulse angle of 60°, acquisition time of 0.4 s, and recycle time of 2.14 s. Frequency-domain NMR spectra were obtained by Fourier transformation of the free induction decays and analyzed using 20-Hz exponential multiplication and zero and first-order phase corrections. The average of γ- and β-ATP peak areas obtained at baseline was set to 100% and used as the reference value for all peaks in the 31P NMR spectra.
Transverse aortic constriction surgery.
Eight- and sixteen-week-old mice underwent transverse aortic constriction (TAC) or sham surgery as previously described (7, 25). Briefly, mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (85 mg/Kg). The aorta was exposed via a left thoracotomy, and a constriction was created using a 7-0 ligature around the vessel and tied against a 28-gauge blunt needle. Sham surgeries were performed as described above without performing the constriction of the aorta. All animals received a subcutaneous injection of Meloxicam (5 mg/kg) for pain relief before the surgery and every 24 h for the next 3 days.
Assessment of cardiac function in vivo.
Murine transthoracic echocardiography was conducted in mice using a VEVO 770 High-Resolution Imaging System (VisualSonics, Toronto, ON, Canada) equipped with a 707B scanhead, centered at 30 MHz (15- to 45-MHz imaging band), with a 20-mm lateral field of view, a 12.7-mm focal length, and resolution of 55 μm. Echocardiography measurements were performed under isoflurane (1%) anesthesia. Cardiac function was measured within 1 wk before surgical procedures (week 0) and at 2, 4, and 8 wk after surgery. Acute increase of cardiac performance was induced by a single intraperitoneal injection of dobutamine (3 mg/kg body wt) in a separate cohort of mice. Echocardiograms were recorded at baseline (preinjection) and 10 min after the injection.
RNA isolation and real-time PCR.
Total RNA was isolated from frozen LV tissue using the RNeasy kit (Qiagen), and cDNA was synthesized using Omniscript (Qiagen) reverse synthase and random hexamers according to manufacturer's instructions. Real-time PCR was performed using SYBR green (Bio-Rad). The primer sequences are shown in Table 1. The real-time PCR results for mRNA levels of each gene were normalized to 18S rRNA levels. Results are presented as mean fold changes relative to the respective WT sham-operated mice.
Table 1.
Primer sequences for real-time PCR
| Gene | Forward | Reverse |
|---|---|---|
| 18S rRNA | 5′-GTAACCCGTTGAACCCCATT-3′ | 5′-CCATCCAATCGGTAGTAGCG-3′ |
| Peroxisome proliferator-activated receptor-γ coactivator-1α | 5′-AGCCGTGACCACTGACAACGAG-3′ | 5′-GCTGCATGGTTCTGAGTGCTAAG-3′ |
| Sarco(endo)plasmic reticulum Ca2+-ATPase 2 | 5′-GGAGATGCACCTGGAAGACT-3′ | 5′-CCACACAGCCGACGAAA-3′ |
| Brain natriuretic peptide | 5′-GCCAGTCTCCAGAGCAATTCA-3′ | 5′-GGGCCATTTCCTCCGACTT-3′ |
| Optic atrophy 1 | 5′-TGGCTCCCGACACAAAGGAAACTA-3′ | 5′-ATCCGTCTTGGATGCACAGGATGA-3′ |
| Mitofusin 1 | 5′-ACAGATGTCACCACAGAGCTGGAT-3′ | 5′-TTGGAGAGCCGCTCATTCACCTTA-3′ |
| Adenine nucleotide translocator 1 | 5′-ACTTCGCCTTCAAAGACAAGTACA-3′ | 5′-GCGCCAGAACTGCTTATGG-3′ |
| Estrogen-related receptor A | 5′-TGCTGCTGCTTAATCCGATCTCCT-3′ | 5′-TGGAGCCTGCTTGGAGTTATTGCT-3′ |
| Medium-chain acyl-CoA dehydrogenase | 5′-TGGCGATGAAGGTTGAACTCGCTA-3′ | 5′-GCTGATTGGCAATGTCTCCAGCAA-3′ |
| Cytochrome c, somatic | 5′-TGGAAGAACAGCCAGTGCTCTTGA-3′ | 5′-CCCGGCAATTCCAGGGCTTTATTT-3′ |
| Transcription factor A, mitochondrial | 5′-GTCCATAGGCACCGTATTGC-3′ | 5′-CCCATGCTGGAAAAACACTT-3′ |
| 7S DNA | 5′-GGCCTACTTTCATCAACATAGCCGTC-3′ | 5′-GGACTAATGATTCTTCACCGTAGGTGCG-3′ |
| Cytochrome oxidase II | 5′-ACGAAATCAACAACCCCGTA-3′ | 5′-GGCAGAACGACTCGGTTATC-3′ |
| ATP synthase F0 subunit 6 | 5′-ATGGCATTAGCAGTCCGGCTTACA-3′ | 5′-TGTAATGGTAGCTGTTGGTGGGCT-3′ |
Mitochondrial isolation and polarography assays.
Mitochondria were isolated as previously described (3). The O2 consumption of isolated mitochondria was measured by a Clark electrode as previously described (13). Pyruvate and malate were used as the electron donors to complex I in the presence of ADP. Succinate was next added to measure complex I- and II-supported respiration, and rotenone was added next to inhibit complex I and measure complex II-supported respiration. Antimycin A was added last to measure the residual O2 consumption not accounted by the electron transfer chain, and it was subtracted from the previous measurements.
Biochemical assays.
Enzyme activities were measured in isolated mitochondria solubilized in CelLytic buffer (Sigma). Citrate synthase activity and succinate dehydrogenase were measured as previously described (11, 22, 27).
Detection of H2O2 production.
The rate of H2O2 production in intact mitochondria was determined using the oxidation of the fluorogenic indicator Amplex red in the presence of horseradish peroxidase (18). In a typical experiment, 30 μg of mitochondria in a 96-well plate were incubated at 30°C, and H2O2 production was initiated by the addition of the substrates indicated in the respective figure.
Electron microscopy.
The mitochondrial ultrastructure was studied in freshly collected LV tissues by electron microscopy. Samples were dissected in 1- to 2-mm3 sections, immediately fixed with 2% glutaraldehyde, postfixed with 1% osmium tetroxide, and embedded in epon resin. For each sample, five to six randomly chosen fields at a magnification of ×10,000 were used for the quantification of mitochondria number. The size of each individual mitochondrion was measured in the same micrographs using ImageJ software.
Statistical analysis.
Comparisons among groups were performed by one-way ANOVA followed by Tukey's post hoc comparisons. Data from the isolated perfused heart experiments were analyzed by repeated-measures ANOVA followed by Bonferroni's post hoc analysis. Kaplan-Meier survival curves were compared using the log-rank test (Mantel-Cox test). The hazard ratio for mortality was determined using Mantel-Haenszel analysis and presented as the hazard ratio with 95% confidence intervals. All analyses were performed using GraphPad Prism 4.0. All data are expressed as means ± SE. Statistical significance was tested at the P ≤ 0.05 level.
RESULTS
PGC-1α overexpression in the heart increased mitochondrial size.
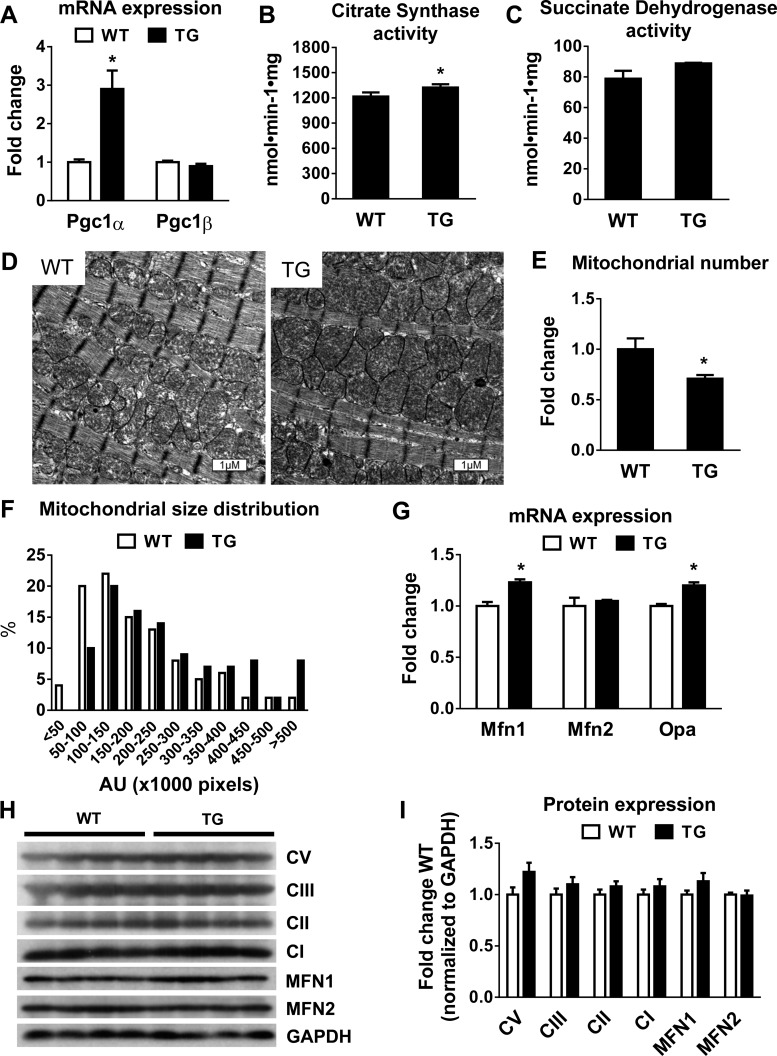
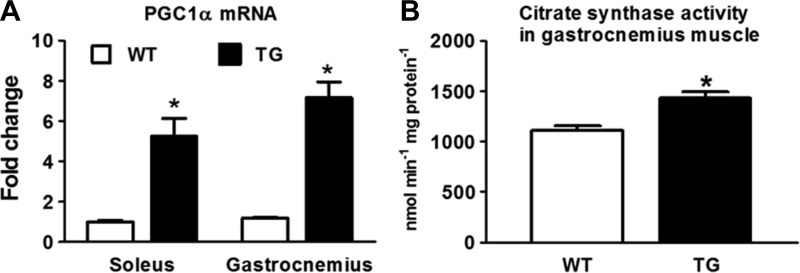
To test the effects of PGC-1α-mediated mitochondrial biogenesis as a potential therapeutic approach for mitochondrial dysfunction and heart failure, we studied a TG mouse line that overexpressed PGC-1α driven by the MCK promoter. The expression of PGC-1α mRNA in the heart was increased by threefold in TG mice compared with WT mice (Fig. 1A), and this was accompanied by ∼10% increases in citrate synthase and succinate dehydrogenase activity (Fig. 1, B and C). No change was observed in PGC-1β mRNA levels. The moderate increase of PGC-1α mRNA in this model provided an opportunity to normalize mitochondrial biogenesis in heart failure models without causing cardiac toxicity or cardiomyopathy, as described in previous reports of PGC-1α overexpression using the α-myosin heavy chain promoter. In TG hearts, there was a small increase in mitochondrial size, whereas mitochondrial number was lower, resulting in no changes in the overall mitochondrial density (Fig. 1, D–F). TG hearts showed a moderate increase in the expression of the fusion genes optic atrophy 1 and mitofusin (Mfn)1 (Fig. 1G), consistent with the role of PGC-1α in the regulation of mitochondrial dynamics (17, 28). However, no significant changes were observed at the protein level in the expression of representative proteins of the electron transport chain or in MFN1 and MFN2 proteins (Fig. 1, H and I). Moreover, we found that PGC-1α expression was increased by approximately fivefold in the soleus muscle and by approximately sevenfold in the gastrocnemius muscle of TG mice (Fig. 2A). Consequently, citrate synthase activity was increased significantly in the gastrocnemius muscle of TG mice (Fig. 2B).
Fig. 1.
Mitochondrial mass, morphology, and gene expression in the mouse heart with peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α overexpression. A–C: mRNA levels of PGC-1α and PGC-1β (n = 4; A), citrate synthase (CS) activity (n = 6; B), and succinate dehydrogenase activity (n = 4–6; C) in wild-type (WT) and PGC-1α-overexpressing transgenic (TG) mouse hearts. D: representative electron microscopy images illustrating mitochondrial morphological characteristics in WT and TG hearts. E and F: mitochondria number (E) and size distribution (F) in WT and TG hearts. AU, arbitrary units. G: mRNA levels of mitochondrial fusion proteins optic atrophy 1 (Opa1), mitofusin (Mfn)1, and Mfn2 in WT and TG hearts (n = 4). H: Western blot from WT and TG heart extracts. Representative proteins are shown from complex V (CV; ATP synthase, H+ transporting, mitochondrial F1 complex, α1-subunit), complex III (CIII; ubiquinol-cytochrome c reductase core protein II), complex II (CII; succinate dehydrogenase complex subunit B), complex I [CI; NADH dehydrogenase (ubiquinone) 1β subcomplex subunit 8], MFN1, and MFN2. I: quantitation of protein expression normalized to GAPDH and expressed as fold changes from WT mice (n = 4). *P < 0.05 vs. WT mice.
Fig. 2.
PGC-1α expression and CS activity in skeletal muscle. A and B: mRNA levels of PGC-1α in soleus and gastrocnemius muscles (A) and CS activity in gastrocnemius muscles from 3-mo-old WT mice (n = 4) and PGC-1α TG mice (n = 4; B). *P < 0.05 vs. WT mice.
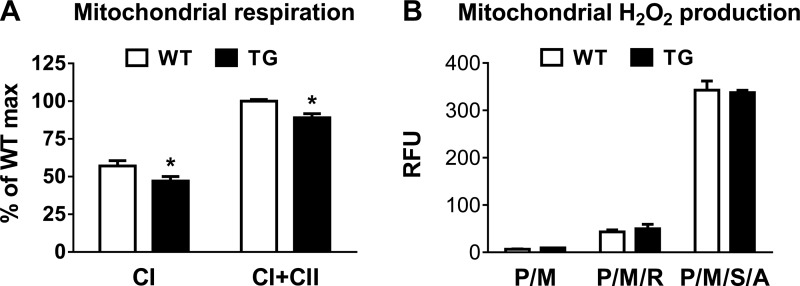
To determine whether the overexpression of PGC-1α altered mitochondrial function, we measured O2 consumption in isolated cardiac mitochondria using a Clark electrode. State 3 respiration supported by complex I substrates (pyruvate/malate/ADP) or complex I + II substrates (pyruvate/malate/succinate/ADP) was decreased by 10–15% in TG cardiac mitochondria (Fig. 3A). To test whether the impairment was also associated with changes in ROS production, we measured H2O2 production in isolated cardiac mitochondria using Amplex red but found no significant changes between WT and TG groups in any of the conditions we tested (Fig. 3B).
Fig. 3.
Mitochondrial function in mouse hearts with PGC-1α overexpression. A: state 3 respiration rates in isolated mitochondria supported by CI substrates [pyruvate/malate (P/M)] or CI + CII substrates (pyruvate/malate/succinate; n = 4). B: H2O2 production in isolated mitochondria incubated with P/M in the presence or absence of rotenone (R), succinate (S), or antimycin A (A; n = 3). RFU, relative fluorescence units. *P < 0.05 vs. WT mice.
PGC-1α overexpression offered no advantage in myocardial energetics and contractile performance in the normal heart.
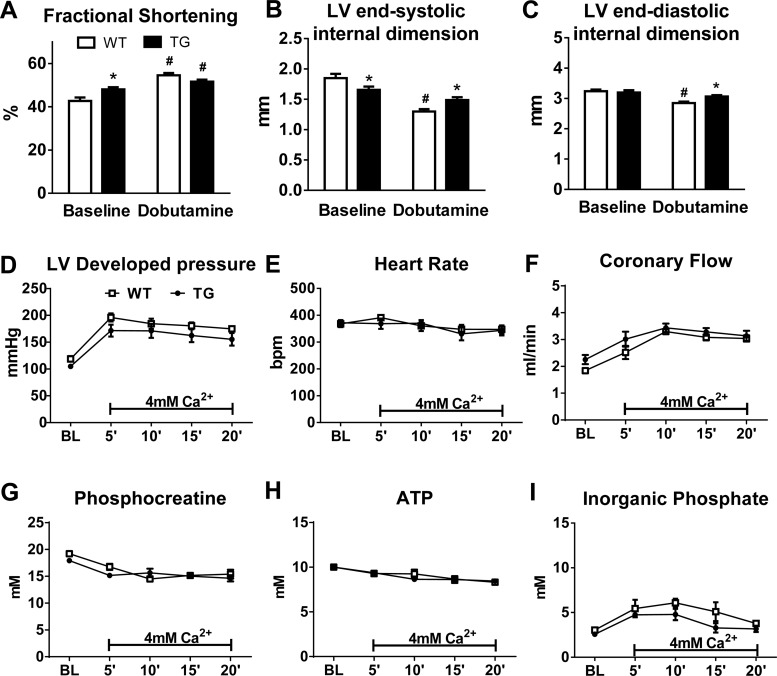
To test whether the decreased mitochondrial respiration affected cardiac function, we assessed in vivo cardiac function by echocardiography in mice at baseline and in response to dobutamine challenge. Cardiac function, as measured by LV fractional shortening, was slightly higher at baseline in TG mice. However, the increase in fractional shortening after dobutamine challenge was attenuated in TG mice (∼4%) compared with WT mice (∼22%; Fig. 4, A–C).
Fig. 4.
Cardiac function in PGC-1α TG hearts. A–C: fractional shortening (A), left ventricular (LV) end-systolic dimension (B), and LV end-diastolic dimension (C) at baseline (BL) and 10 min after dobutamine injection (intraperitoneal) in 5- to 6-mo-old WT (n = 8) and TG (n = 9) mice. *P < 0.05, WT vs. TG mice with the same treatment; #P < 0.05, BL vs. dobutamine in the same genotype. D–F: LV developed pressure (D), heart rate [in beats/min (bpm); E], and coronary flow (F) measured in isolated perfused hearts at BL and during a high workload challenge (4 mM Ca2+ in the perfusate) in WT (n = 5) and TG (n = 6) mice. G–I: concentrations of phosphocreatine (G), ATP (H), and Pi (I) as assessed by 31P NMR spectroscopy in isolated perfused hearts subjected to a high workload challenge from 3- to 4-mo-old WT (n = 3) and TG (n = 3) mice.
To determine whether increasing PGC-1α expression affected myocardial ATP production, we measured high-energy phosphate content in isolated perfused hearts using 31P NMR spectroscopy both at baseline and during a high workload challenge. During the high workload challenge, the Ca2+ concentration in the perfusate was doubled to stimulate increased force of contraction. At baseline, LV developed pressure was slightly reduced in TG hearts at similar heart rates, although this did not reach statistical significance (P = 0.073; Fig. 4, D and E). Coronary flow was not statistically different in TG hearts at baseline (Fig. 4F). Myocardial energetic status was essentially unchanged in TG hearts, as PCr, ATP, or Pi content were similar to those in WT hearts (Fig. 4, G–I). When stimulated for higher contractile performance, both WT and TG hearts demonstrated similar increases of LV developed pressure with no changes noted in heart rate (Fig. 4, D and E). Coronary flow was significantly increased to a similar degree in both WT and TG hearts (Fig. 4F). No significant differences were observed in PCr, ATP, or Pi during the high workload challenge (Fig. 4, G–I). These data demonstrate that a moderate overexpression of PGC-1α does not offer any functional or energetic advantage to hearts at normal or high workload conditions.
Acute mortality was increased in PGC-1α TG mice subjected to pressure overload.
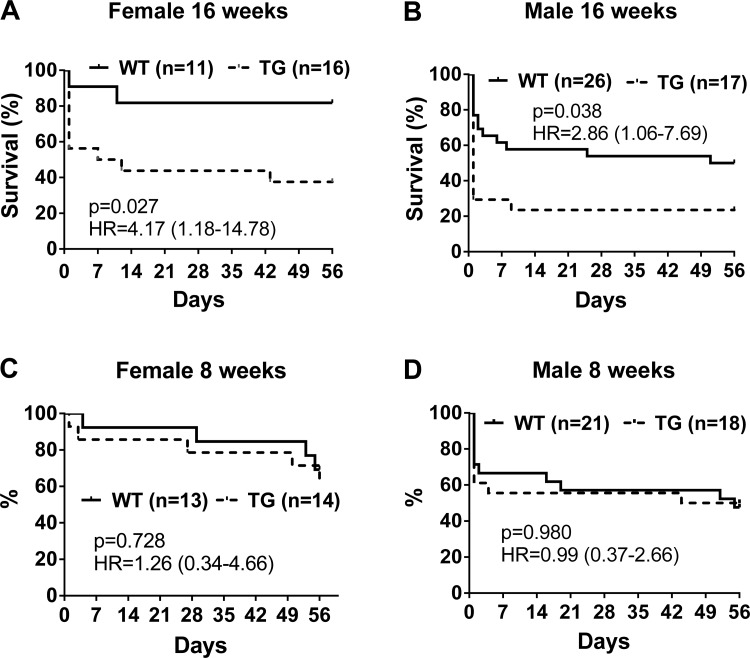
To determine whether overexpression of PGC-1α protected the heart from pressure overload-induced hypertrophy and failure, we subjected 16-wk-old TG and WT mice to sham or TAC surgery. Interestingly, we observed a significantly higher acute mortality in both male and female TG mice after TAC (Fig. 5, A and B), whereas there was minimal acute mortality after sham surgery for either genotype (data not shown). Approximately 70% of male TG mice and 45% of female TG mice died within 2 h of TAC surgery compared with 30% of male WT mice and 10% of female WT mice during the same period. We subsequently performed surgery in a cohort of younger mice (8 wk old). The acute mortality of 8-wk-old WT mice subjected to TAC surgery was similar to 16-wk-old mice. The acute mortality in TG mice subjected to TAC surgery, however, was markedly lower in 8-wk-old mice compared with 16-wk-old mice (Fig. 5, C and D), raising the possibility that long-term overexpression of PGC-1α compromised the acute responses to stress. Despite the differences in acute mortality, there was no difference in the chronic survival of WT and TG mice in the 8 wk after TAC surgery for both male and female mice.
Fig. 5.
Kaplan-Meier survival curve during the 8 wk after transverse aortic constriction (TAC). Female (A and C) and male (B and D) PGC-1α TG and WT mice were subjected to TAC or sham surgery at 16 wk (A and B) or 8 wk (C and D) of age. Statistical significance as well as hazard ratios (HR) with 95% confidence intervals are shown in each graph.
Cardiac function was not improved in TG mice despite the normalization of mitochondrial biogenesis in pressure overload-induced cardiac hypertrophy.
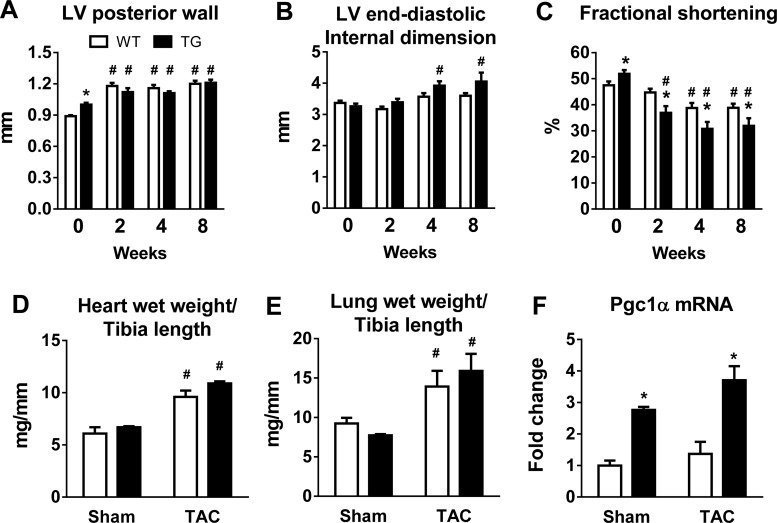
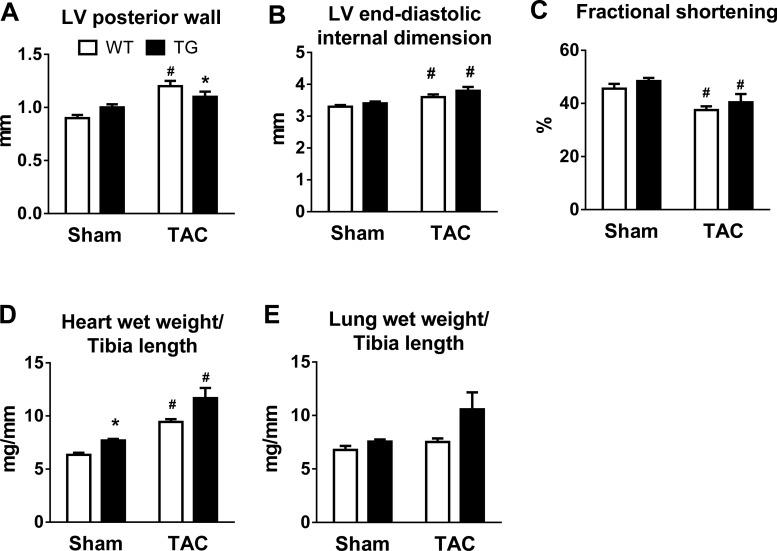
TAC induced a significant and similar increase in LV posterior wall thickness in WT and TG mice at 2 wk, which persisted throughout the 8-wk period (Fig. 6A). LV chamber dimension was similar before surgery but increased progressively after TAC in TG mice but not in WT mice (Fig. 6B). LV fractional shortening also showed a greater decline in TG mice after TAC compared with WT mice (Fig. 6C). Furthermore, both lung and heart weights were increased 8 wk after TAC, consistent with the echocardiographic results of cardiac hypertrophy and LV dysfunction (Fig. 6, D and E). PGC-1α expression remained elevated in TG mice subjected to TAC compared with WT mice subjected to TAC (Fig. 6F). In mice subjected to TAC at 8 wk of age, a mild LV dysfunction was observed, and there was no difference between the two genotypes (Fig. 7).
Fig. 6.
Cardiac responses to chronic pressure overload in WT and TG mice subjected to TAC at 16 wk of age. A–C: LV posterior wall thickness (A), LV end-diastolic internal dimension (B), and LV fractional shortening (C) in WT and TG male mice before and at 2, 4, and 8 wk after TAC surgery. *P < 0.05 vs. WT mice; #P < 0.05 vs. week 0. D–F: heart wet weights (D) and lung wet weights (E) normalized to tibia length as well as PGC-1α mRNA levels (F) 8 wk after sham surgery (n = 4–7) or TAC (n = 4–13). *P < 0.05 vs. WT mice with the same treatment; #P < 0.05 vs. sham surgery in the same genotype.
Fig. 7.
Cardiac responses to chronic pressure overload in WT and TG mice subjected to TAC at 8 wk of age. A–C: LV posterior wall thickness (A), LV end-diastolic internal dimension (B), and LV fractional shortening (C) in WT and TG male mice 8 wk after TAC (n = 9–10) or sham (n = 7–8) surgery. D and E: heart wet weights (D) and lung wet weights (E) normalized to tibia length 8 wk after sham (n = 6) or TAC (n = 6–7) surgery. *P < 0.05 vs. WT sham-operated mice; #P < 0.05 vs. sham surgery in the same genotype.
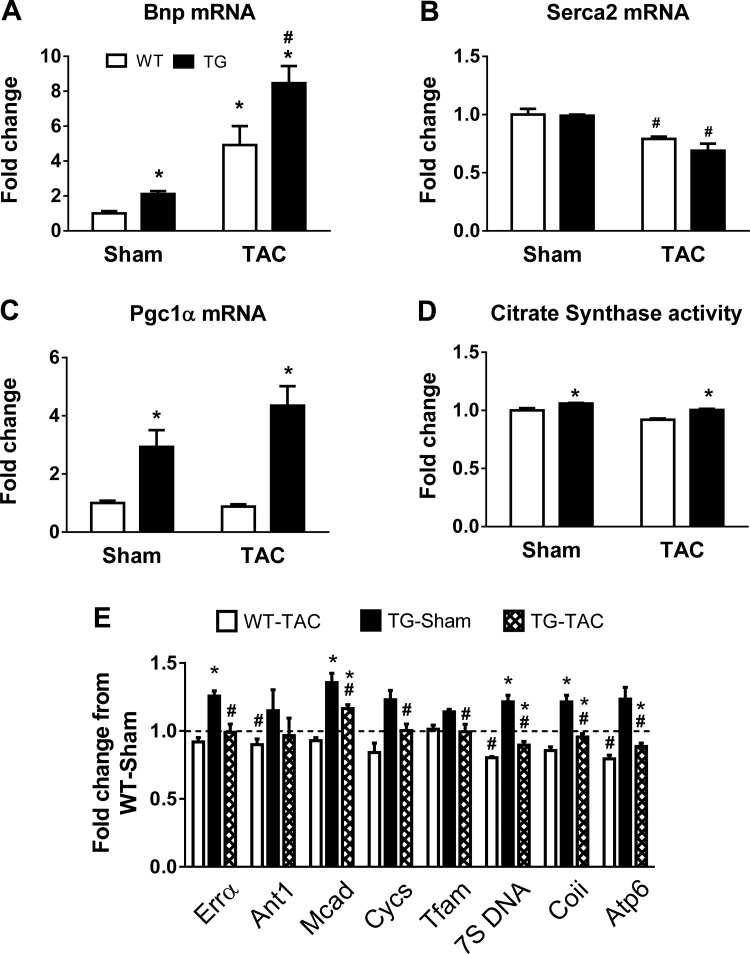
To assess whether PGC-1α overexpression sustained mitochondrial biogenesis during the development of pathological hypertrophy, we measured the expression of genes related to pathological cardiac hypertrophy as well as PGC-1α target genes involved in mitochondrial biogenesis and oxidative metabolism. The expression of brain natriuretic peptide was significantly increased in both genotypes 8 wk after TAC, and the increase was greater in TG mice (Fig. 8A). Sarco(endo)plasmic reticular Ca2+-ATPase 2a mRNA was significantly decreased in both WT and TG mice 8 wk after TAC (Fig. 8B). The expression of PGC-1α (Fig. 8C) and mitochondrial genes previously reported to be under the control of PGC-1α was moderately decreased in WT mice subjected to TAC (Fig. 8E). In TG sham-operated mice, the expression of these genes was 20–30% higher, and after TAC it was normalized to WT sham-operated levels. The activity of citrate synthase, a mitochondrial matrix enzyme often used as a marker of mitochondrial mass, was slightly higher in TG sham-operated mice and remained higher in TG mice subjected to TAC compared with WT mice subjected to TAC (Fig. 8D). Taken together, PGC-1α overexpression maintained the expression of mitochondria-related gene expression as well as mitochondrial mass in mouse hearts with pressure overload-induced hypertrophy. However, these effects did not result in improvements of cardiac function or survival in response to chronic pressure overload.
Fig. 8.
Cardiac gene expression in WT and TG mice subjected to TAC at 8 wk of age. A–C: mRNA levels of brain natriuretic peptide (Bnp; A), cardiac sarco(endo)plasmic reticulum Ca2+-ATPase 2 (Serca2; B), and PGC-1α (C). D and E: CS activity (D) and mRNA levels of PGC-1α downstream targets (E) reflecting mitochondrial biogenesis in sham-operated and TAC hearts of WT and TG mice 8 wk after surgery (n = 4 mice/group). The dotted line indicates the WT sham-operated group. *P < 0.05 vs. WT mice with the same treatment; #P < 0.05 vs. sham surgery in the same genotype.
DISCUSSION
In the present study, we demonstrated that a threefold increase in PGC-1α expression led to very modest changes in mitochondrial volume density, morphology, and function in the normal mouse heart. Moreover, PGC-1α overexpression did not affect myocardial energetics or cardiac function under unstressed conditions. However, long-term overexpression of PGC-1α rendered mice more vulnerable to acute cardiac stress and failed to protect against cardiac dysfunction caused by chronic pressure overload despite sustained expression of PGC-1α and its target genes for mitochondrial biogenesis.
Heart failure is characterized by decreases in mitochondrial biogenesis and ATP production, and these changes have been associated with the downregulation of PGC-1α in the heart (8, 17). In addition, genetic ablation of the PGC-1α gene exacerbates cardiac dysfunction in mouse models of heart failure (2), suggesting that mitochondrial biogenesis mediated by PGC-1α is important for the adaptation to chronic stress. However, previous efforts to overexpress PGC-1α using the α-myosin heavy chain promoter led to robust proliferation of mitochondria in cardiac myocytes that displaced myofilament proteins, resulting in cardiomyopathy (23). In the present study, we were able to induce a moderate increase of PGC-1α expression in the heart, which did not affect mitochondrial volume, mass, or function in normal mouse hearts. Furthermore, PGC-1α overexpression preserved mitochondrial mass in pressure-overloaded hearts, as evidenced by citrate synthase activity; however, it failed to prevent the development of cardiac dysfunction. Similarly, a recent study (15) reported that transient induction of PGC-1α in mice by about fourfold did not significantly change the mitochondrial DNA copy number but decreased the contractile recovery after ischemia-reperfusion injury. The authors in that study concluded that the detrimental effect was due to the induction of adenine nucleotide translocator 1 gene expression by PGC-1α. In the present study, we did not detect any significant change in adenine nucleotide translocator 1 gene expression in TG mice. These differences could be due to the mode of PGC-1α overexpression in the mouse models. Lynn et al. (15) induced PGC-1α expression for only 3 days in the adult heart, whereas in our study long-term PGC-1α overexpression was initiated early in life by the MCK promoter. Nevertheless, in both cases, modest PGC-1α overexpression in the heart had a detrimental effect rather than a protective effect. Thus, PGC-1α does not appear to be an appropriate target for cardioprotection during acute or chronic stress.
PGC-1α was originally identified as a cold inducible regulator of mitochondrial biogenesis in brown adipose tissue (BAT). Subsequent studies (14, 21, 26) have also demonstrated that PGC-1α was able to drive mitochondrial biogenesis in multiple tissues such as BAT, white adipose tissue, the liver, skeletal muscle, and the heart. The heart is a highly oxidative organ with mitochondria occupying ∼30% of the cell volume in the cardiac myocyte, making it the cell type with the highest mitochondrial content. The functional impact of further upregulation of mitochondrial biogenesis in the heart has been less clear. We show here that a moderate upregulation of PGC-1α in cardiac myocytes does not increase the content of mitochondria or alter myocardial energetics in the heart. Unlike in other cell/tissue types [e.g., BAT (21)], increasing PGC-1α expression in cardiac myocytes does not enhance oxidative metabolism or contractile performance in vivo. In a previous study (23), high-level overexpression of PGC-1α led to aberrant mitochondrial biogenesis and the development of cardiomyopathy. Recent findings have shown that PGC-1α/β deletion is detrimental during development but not in adult hearts (17). Deletion of both PGC-1α/β leads to lethal cardiomyopathy, whereas deletion of both isoforms in adulthood appears to be well tolerated, at least in nonstressed conditions. This suggests that mitochondrial biogenesis in the adult heart may be controlled by an additional mechanism that is activated after maturation. It may also explain why modest PGC-1α overexpression did not have a major effect in cardiac mitochondrial volume or mass in our study.
Interestingly, despite the unimpressive effects on mitochondrial biogenesis, long-term and moderate upregulation of PGC-1α compromises rather than enhances the cardiac response to stress. It is unclear whether these effects are due to alterations of mitochondrial function as mitochondrial respiration or ROS production are largely unchanged by PGC-1α overexpression. Recent studies have revealed other important biological functions of PGC-1α in addition to its role in mitochondrial biogenesis, particularly in regard to angiogenesis. For example, PGC-1α has been shown to be responsible for exercise-induced angiogenesis in skeletal muscle (4) as well as regulating angiogenesis in skeletal muscle after an ischemic insult (1). Furthermore, mice with cardiac deletion of PGC-1α develop a profound peripartum cardiomyopathy, which can be rescued by proangiogenic therapy (20). In the present study, we found similar coronary flow rates in MCK-PGC-1α hearts compared with WT hearts. There were no changes in the expression of angiogenic markers in the heart, such as VEGF or CD31 (data not shown). It does not, however, rule out the possibility that increased vascularity in peripheral tissues can affect cardiac response to stress in vivo. Further investigation is warranted.
In the present study, we found that the survival rate was higher in female mice than in male mice for both phenotypes in response to pressure overload. This is consistent with the reported sex differences in the cardiac responses to stress. Male mice have been shown to be more susceptible to the development of pressure overload-induced cardiac hypertrophy and cardiac ischemia-reperfusion injury (5, 6, 24). Furthermore, the prevalence of cardiovascular disease and heart failure in premenopausal women is lower compared with men, and this has been attributed primarily to hormonal differences (9). It is also possible that the degree of constriction resulted from the ligation against a 28-gauge needle is more severe for the male mice, which are ∼20% larger than age-matched female mice. Nevertheless, within the same sex, TG mice consistently presented a higher mortality, suggesting that the detrimental effects of PGC-1α overexpression are not sex specific.
In summary, the present study assessed whether a moderate level of PGC-1α overexpression could restore mitochondrial biogenesis and improve cardiac function during chronic pressure overload conditions. The results demonstrate that promotion of PGC-1α expression does not improve cardiac energetics and function and that long-term overexpression, even at a moderate level, unfavorably affects the cardiac response to stress. These findings illustrate the complexity of PGC-1α-driven transcriptional circuits in the heart and suggest that caution should be exercised when targeting PGC-1α as a therapy.
GRANTS
The work was supported in part by National Institutes of Health Grants HL-067970, HL-110349, and RR-029021 and by the American Heart Association (a Scientist Development Grant to S. C. Kolwicz, Jr., and a postdoctoral fellowship award to C. F. Lee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.K., L.G.M., S.C.K., and R.T. conception and design of research; G.K., L.G.M., S.C.K., and C.F.L. performed experiments; G.K., L.G.M., S.C.K., and C.F.L. analyzed data; G.K., L.G.M., S.C.K., and R.T. interpreted results of experiments; G.K., L.G.M., S.C.K., and C.F.L. prepared figures; G.K. drafted manuscript; G.K., L.G.M., S.C.K., C.F.L., and R.T. edited and revised manuscript; G.K., L.G.M., S.C.K., C.F.L., and R.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Zoltan Arany for providing the MCK-PGC-1α mice and for helpful discussions.
REFERENCES
- 1.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451: 1008–1012, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-γ coactivator 1α. Proc Natl Acad Sci USA 103: 10086–10091, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehm EA, Jones BE, Radda GK, Veech RL, Clarke K. Increased uncoupling proteins and decreased efficiency in palmitate-perfused hyperthyroid rat heart. Am J Physiol Heart Circ Physiol 280: H977–H983, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1α mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci USA 106: 21401–21406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross HR, Lu L, Steenbergen C, Philipson KD, Murphy E. Overexpression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion injury in male, but not female, transgenic mice. Circ Res 83: 1215–1223, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Cross HR, Murphy E, Koch WJ, Steenbergen C. Male and female mice overexpressing the β2-adrenergic receptor exhibit differences in ischemia/reperfusion injury: role of nitric oxide. Cardiovasc Res 53: 662–671, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Menendez L, Karamanlidis G, Kolwicz S, Tian R. Substrain specific response to cardiac pressure overload in C57BL/6 mice. Am J Physiol Heart Circ Physiol 305: H397–H402, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 551: 491–501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 127: e6-e245, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karamanlidis G, Bautista-Hernandez V, Fynn-Thompson F, Del Nido P, Tian R. Impaired mitochondrial biogenesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. Circ Heart Fail 4: 707–713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerner J, Turkaly PJ, Minkler PE, Hoppel CL. Aging skeletal muscle mitochondria in the rat: decreased uncoupling protein-3 content. Am J Physiol Endocrinol Metab 281: E1054–E1062, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Kolwicz SC, Jr., Tian R. Assessment of cardiac function and energetics in isolated mouse hearts using 31P NMR spectroscopy. J Vis Exp 2010: 2069, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3: 965–976, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynn EG, Stevens MV, Wong RP, Carabenciov D, Jacobson J, Murphy E, Sack MN. Transient upregulation of PGC-1α diminishes cardiac ischemia tolerance via upregulation of ANT1. J Mol Cell Cardiol 49: 693–698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marin-Garcia J, Goldenthal MJ. Mitochondrial centrality in heart failure. Heart Fail Rev 13: 137–150, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM, Kelly DP. A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res 114: 626–636, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghaddas S, Hoppel CL, Lesnefsky EJ. Aging defect at the QO site of complex III augments oxyradical production in rat heart interfibrillar mitochondria. Arch Biochem Biophys 414: 59–66, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Nakae I, Mitsunami K, Omura T, Yabe T, Tsutamoto T, Matsuo S, Takahashi M, Morikawa S, Inubushi T, Nakamura Y, Kinoshita M, Horie M. Proton magnetic resonance spectroscopy can detect creatine depletion associated with the progression of heart failure in cardiomyopathy. J Am Coll Cardiol 42: 1587–1593, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del Monte F, Hilfiker-Kleiner D, Karumanchi SA, Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485: 333–338, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 56: 2062–2069, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator-1α promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 94: 525–533, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E. Estrogen receptor-β mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol 288: H469–H476, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 16: 349–360, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Yen HC, Oberley TD, Gairola CG, Szweda LI, St Clair DK. Manganese superoxide dismutase protects mitochondrial complex I against adriamycin-induced cardiomyopathy in transgenic mice. Arch Biochem Biophys 362: 59–66, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, Collia D, Chen Z, Wozniak DF, Leone TC, Kelly DP. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab 12: 633–642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]