Abstract
The purpose of this study was to determine if heat inhibits α2-adrenergic vasocontraction, similarly to α1-adrenergic contraction, in isolated human skeletal muscle feed arteries (SMFA) and elucidate the role of the temperature-sensitive vanilloid-type transient receptor potential (TRPV) ion channels in this response. Isolated SMFA from 37 subjects were studied using wire myography. α1 [Phenylephrine (PE)]- and α2 [dexmedetomidine (DEX)]-contractions were induced at 37 and 39°C with and without TRPV family and TRPV4-specific inhibition [ruthenium red (RR) and RN-1734, respectively]. Endothelial function [acetylcholine (ACh)] and smooth muscle function [sodium nitroprusside (SNP) and potassium chloride (KCl)] were also assessed under these conditions. Heat and TRPV inhibition was further examined in endothelium-denuded arteries. Contraction data are reported as a percentage of maximal contraction elicited by 100 mM KCl (LTmax). DEX elicited a small and variable contractile response, one-fifth the magnitude of PE, which was not as clearly attenuated when heated from 37 to 39°C (12 ± 4 to 6 ± 2% LTmax; P = 0.18) as were PE-induced contractions (59 ± 5 to 24 ± 4% LTmax; P < 0.05). Both forms of TRPV inhibition restored PE-induced contraction at 39°C (P < 0.05) implicating these channels, particularly the TRPV4 channels, in the heat-induced attenuation of α1-adrenergic vasocontraction. TRPV inhibition significantly blunted ACh relaxation while denudation prevented heat-induced sympatholysis without having an additive effect when combined with TRPV inhibition. In conclusion, physiological increases in temperature elicit a sympatholysis-like inhibition of α1-adrenergic vasocontraction in human SMFA that appears to be mediated by endothelial TRPV4 ion channels.
Keywords: heat, feed arteries, alpha adrenergic, sympatholysis, TRPV ion channels
during muscle contraction, the normal vasoconstriction response to stimulation of α1- and α2-adrenoceptors is attenuated in a process termed functional sympatholysis (4, 25). Inspired by the theory that exercise-induced heat generation plays a role in mediating this local inhibition of α-adrenergic vasoconstriction (4), our group recently tested the effect of heat on α1-adrenergic responsiveness in isolated human skeletal muscle feed arteries (SMFA) (14, 15). In 2011, Ives et al. (15) reported that simply heating these isolated feed arteries from 37 to 39°C (approximate temperature of muscle at rest and exercise, respectively; Ref. 28) attenuated α1-induced adrenergic vasocontraction by ∼40%. It was later determined that this sympatholytic effect of heat could be prevented by inhibiting endothelial nitric oxide synthase (eNOS) (14), implicating nitric oxide (NO) as a key player in heat-induced sympatholysis. While these studies support the concept of heat-induced sympatholysis, they do not explain how these denervated arteries sense the increase in temperature to initiate the relaxing response.
Within the endothelial and smooth muscle cells of the vasculature exists a family of temperature-sensitive ion channels, known as the vanilloid-type transient receptor potential (TRPV) ion channels, which seems poised to serve as the link between heat exposure and sympatholysis. In fact, while investigating the function of type IV TRPV (TRPV4) ion channels Watanabe et al. (36) reported that heating cultured cells, including mouse aorta endothelial cells, to temperatures similar to those employed by Ives et al. (14, 15) resulted in an influx of calcium that was prevented by treating cells with the TRPV antagonist ruthenium red (RR) or by the genetic inhibition of the TRPV4 channels specifically. Considerable additional evidence indicates that the activation of the TRPV ion channels by other stimuli, like shear stress and endogenous ligands, can modulate vascular responsiveness and enhance endothelial-dependent dilation (2, 7, 8, 19, 21, 24, 35, 39). Therefore, given their known impact on vascular function and sensitivity to physiological temperatures (2, 6, 33), it seems plausible that the TRPV ion channels, especially TRPV4 ion channels, play a role in sensing and mediating the sympatholytic effect of heat in human SMFA (14, 15). However, this potential mechanism has yet to be elucidated.
Therefore, the purpose of this study was to better characterize α-adrenergic function in human SMFA and explore the possibility that the TRPV ion channels, particularly the TRPV4 channel, act as the link between elevated temperature and blunted α-adrenergic vasocontraction (14, 15). As the function of the α2-adrenoceptors, which has yet to be documented in these arteries, is believed to differ from their α1-counterparts in distribution and sensitivity to sympatholysis (18, 23), we first sought to compare the effects of α1- and α2-agonists on human SMFA and determine whether α2-induced vasocontraction is also inhibited in the heat. We then tested two hypotheses regarding the role of TRPV ion channels in the restoration of vascular function mediated by α1- and α2-adrenoceptors in the heat. Specifically, based on the observation of Watanabe et al. (36) that TRPV inhibition prevented the response of cultured endothelial cells to heat, we first hypothesized that the inhibition of these ion channels and/or endothelial denudation would result in the restoration of α-adrenergic vasocontraction at 39°C to levels observed at 37°C. Second, we tested the hypothesis that this restorative effect of TRPV inhibition would be associated with altered endothelial function and not altered smooth muscle function.
METHODS
Subjects and General Procedures
Thirty-seven diverse subjects (22 male, 15 female, 22–71 yr) undergoing routine melanoma-diagnosis-related surgery donated SMFA that were removed during the normal course of the surgery. Vessels from subjects who had undergone chemotherapy were not included in this study as this was a contraindication for the surgery. Other medical conditions and medications used by the subjects were noted, but no exclusion criteria were based on these data (Table 1). All protocols used in this study were approved by the Institutional Review Board of the University of Utah and Salt Lake City Veterans Affairs Medical Center, and informed written consent was obtained from all subjects before surgery. All protocols were carried out in accordance with the Declaration of Helsinki.
Table 1.
Subject characteristics
| Means ± SE | Normal Range | |
|---|---|---|
| Vital characteristics | ||
| Age | 53 ± 2 | — |
| Male/female (n) | 22/15 | — |
| Height, cm | 175 ± 2 | — |
| Weight, kg | 88 ± 3 | — |
| Body mass index, kg/m2 | 28 ± 1 | — |
| Systolic blood pressure, mmHg | 128 ± 3 | — |
| Diastolic blood pressure, mmHg | 78 ± 2 | — |
| Mean arterial blood pressure, mmHg | 94 ± 2 | — |
| Blood and plasma | ||
| Glucose, mg/dl | 96 ± 3 | 65–110 |
| Blood urea nitrogen, mg/dl | 16 ± 1 | 6–21 |
| Creatinine, mg/dl | 1.0 ± 0.1 | 0.52–0.99 |
| Lactate dehydrogenase, U/l | 317 ± 28 | 313–618 |
| Hemoglobin, g/dl | 14 ± 0.3 | 12–16 |
| White blood cells, ×103/μl | 7 ± 0.3 | 3.6–10.6 |
| Red blood cells, ×106/μl | 4.7 ± 0.1 | 4.0–5.2 |
| Platelets, ×103/μl | 244 ± 12 | 150–400 |
| Hematocrit, % | 43 ± 1 | 36–46 |
| Cardiovascular medication (users/n) | ||
| Diuretic | 3/37 | — |
| Ca2+ channel blocker | 3/37 | — |
| β-Blocker | 2/37 | — |
| Angiotensin blocker | 1/37 | — |
| Angiotensin-converting enzyme inhibitor | 5/37 | — |
| Statin | 10/37 | — |
| Vessel location (n/total) | ||
| Axillary | 27/37 | — |
| Inguinal | 10/37 | — |
Vessel Harvest and Preparation
SMFAs from the axillary and inguinal regions were obtained during melanoma-related node dissection surgeries at the Huntsman Cancer Hospital and the Salt Lake City Veterans Affairs Hospital. Patients were anaesthetized using a standard protocol including propofol, fentanyl, benzodiazepines, and succinylcholine. After lymph node dissection, feed arteries entering muscles in the axillary (e.g., serratus anterior, or latissimus dorsi) or inguinal (e.g., quadriceps femoris or hip adductors) regions were identified and classified as SMFA based on entry into a muscle bed, structure, blood color, and pulsatile bleed pattern. The vessels were ligated, excised, and then immediately placed in cold (∼4°C), normal physiological saline solution (NPSS), which consisted of 125 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 18 mM NaHCO3, 0.026 mM Na2EDTA, and 11.2 mM glucose. Note that the pH of the NPSS in the bath was maintained at 7.4 ± 0.5 at all temperatures throughout the entirety of the experiment. Vessels were then brought to the laboratory for experimentation within 15 min of harvesting.
Once at the laboratory, feed arteries were dissected (to remove all perivascular adipose and other tissue) under a stereomicroscope and cut into six to eight ring segments (∼2 mm in length) while immersed in cold NPSS. Rings were then mounted in wire myography chambers (700 MO; DMT Systems, Aarhus, Denmark) in cold NPSS that was continuously aerated with carbogen gas (95% O2-5% CO2) throughout the experiment. The diameter and length of the mounted rings were assessed using a calibrated micrometer eyepiece. Myography chambers were then gradually warmed to a baseline temperature of 37°C during a 30-min equilibration period before beginning the protocol. During the protocol, NPSS was exchanged every 15 min except during the concentration-response curves described below.
Vessel Function Protocols
Before the administration of TRPV inhibitors all artery segments underwent a length-tension protocol (15) at 37°C to assure that the arteries were at the length (Lmax) that would elicit the greatest contractile response to a single exposure to 100 mM KCl dissolved in NPSS (LTmax). Specifically, LTmax was reached when increasing the length of the arteries increased the response to 100 mM KCl by <10% (15). Following the length tension protocol, half of the rings were continuously incubated with a TRPV antagonist to assess the role of the TRPV ion channels in modulating the responsiveness of the arteries. Given that multiple TRPV ion channels may potentially be involved in mediating the sympatholytic effect of heat, RR (30 μM) (29), a nonspecific TRPV antagonist (6, 29), was applied to the arteries to inhibit the activity of the family of TRPV channels. As the TRPV4 ion channel has been reported to be activated by similar levels of heat, as utilized in this study, previously in arterial preparations (36), the selective TRPV4 antagonist RN-1734 (20 μM) (3, 34) was applied to a different set of arteries (n = 7) to explore the role of this specific ion channel in the sympatholytic effect of heat.
After allowing the treated rings to incubate in RR or RN-1734 for 15–20 min, α-adrenergic responsiveness of all rings (TRPV inhibited and control rings) was assessed at 37°C by generating concentration-response curves to the α1-agonist phenylephrine (PE; 10−9-10−3 logM) and the α2-agonist dexmedetomidine (DEX; 10−10-10−3 logM). Due to the variability of individual vessel ring caliber, vasocontractile responses for each ring were normalized to the maximum response to 100 mM KCl during the length tension protocol (i.e., LTmax) (15). As described previously (14), the endothelium-dependent vasorelaxation was assessed with concentration-response curves to acetylcholine (ACh; 10−7-10−3 logM) following preconstriction with PE to ∼70% of the maximum PE response. Note that the order of the PE and DEX concentration-response curves was alternated in a balanced manner to account for any ordering effect.
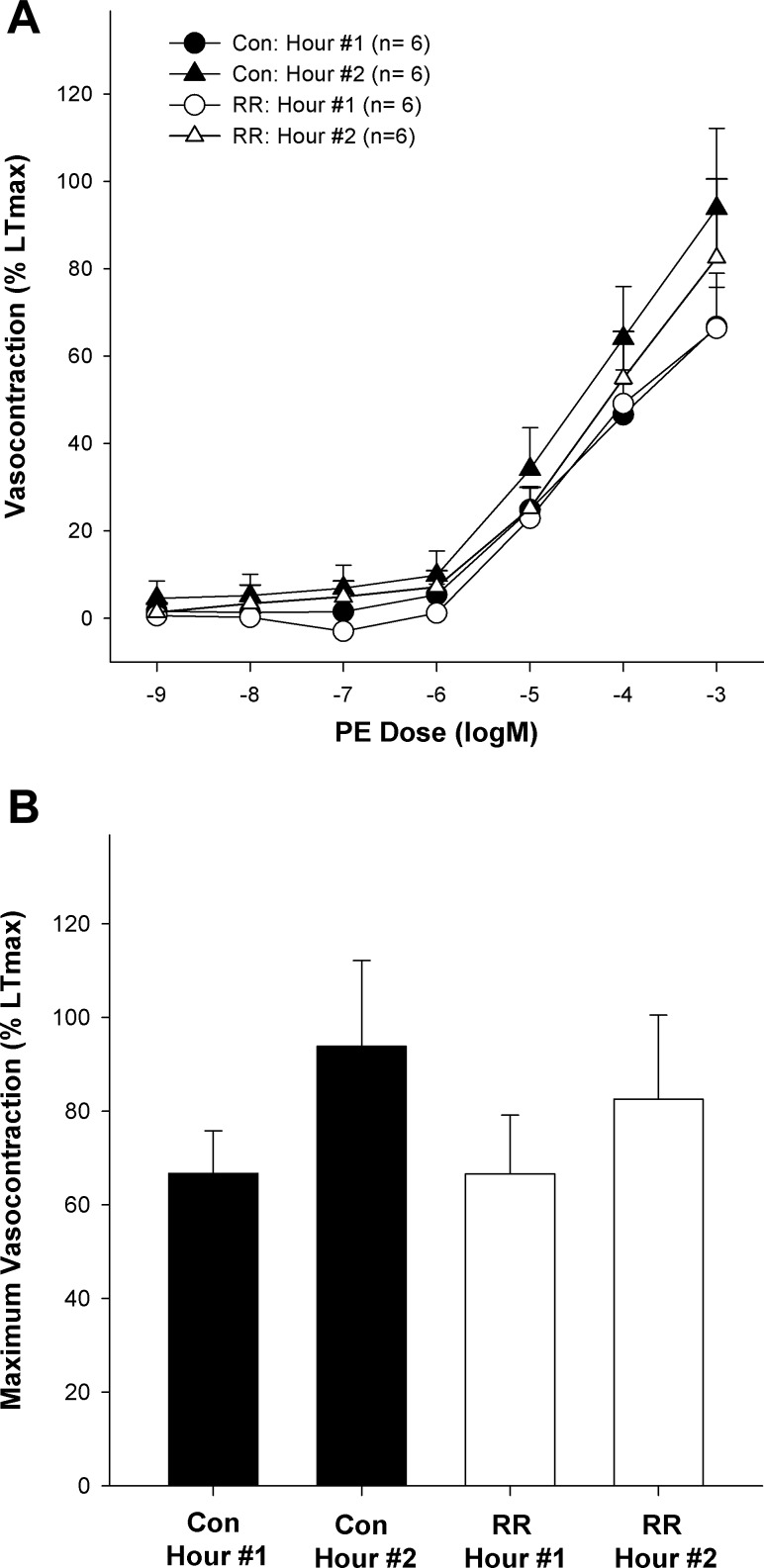
The chambers were then heated from 37 to 39°C over a period of 10 min. After a 20- to 30-min equilibration period, the concentration-response curves for PE, DEX, and ACh were performed once again as described above. As prior exposure to heat has been reported to alter adrenergic responsiveness (27) we, like others (15, 20), always performed the highest temperature phase last. Given the potential for a time effect in this design, we performed a time control experiment in a set of six arteries by generating concentration-response curves to PE (10−9-10−3 logM), with the temperature held constant at 37°C at hour 1 and hour 2 of the experiment in the presence and absence of RR.
As previous research has suggested that the sympatholytic effect of heat may be mediated by the endothelium (14), we also sought to determine whether the effect of the TRPV ion channels on vascular function was endothelium dependent by performing the control and RR experiments in endothelium denuded arteries (n = 6). Arteries were denuded by passing 2 ml of air through the lumen of the artery before it was dissected and mounted on the wire myograph (22). Once mounted, denudation was verified by the vessel rings exhibiting <10% relaxation to ACh (10−3) at 37°C.
Another set of arteries was used to assess the effect of heat and TRPV inhibition on smooth muscle function. Following the length tension protocol described earlier, rings were rinsed with NPSS and allowed to recover to baseline tension. To test receptor-independent smooth muscle vasocontraction with and without TRPV inhibition, KCl was added to the NPSS buffer at 37 and 39°C to progressively raise the concentration of KCl in the bath from 10 to 100 μM, with and without RR. Endothelium-independent relaxation was assessed with concentration-response curves to the NO donor sodium nitroprusside (SNP; 10−9-10−4 logM) in all conditions (37 and 39°C with and without RR) following preconstriction with PE. As pilot studies suggested that prior exposure to SNP may alter vascular responsiveness, each ring used in the SNP protocol was exposed to only one of the four conditions (37 or 39°C, with or without RR).
Statistical Analyses
All myography tension data were acquired at 4 Hz with an analog-to-digital data acquisition system (Biopac Systems, Goleta, CA). As described above, unless otherwise stated, contraction data are represented as a percentage of the maximum contraction elicited by 100 mM KCl during the length tension protocol (i.e., %LTmax) for each arterial ring, and relaxation data are represented as the magnitude of relaxation from preconstriction tension divided by the magnitude of preconstriction tension for each arterial ring (i.e., %relaxation). As the arterial rings do not always reach peak relaxation or tension at the highest drug concentration, maximum relaxation and contraction data, regardless of concentration, are presented in addition to the concentration-response curve data. Sensitivity of the arteries to PE, DEX, KCl, and SNP was assessed by calculating the logEC50 with a sigmoidal parameter {(a + b − a)/[1 + 10(x−c)]} as described previously (15).
Statistical analyses were performed using SPSS statistical software (SPSS version 17; SPSS, Chicago, IL), while graphs and figures were created using Sigma Plot version 11 (Systat Software, San Jose, CA). Note that values presented in text and figures are means ± SE, unless otherwise stated. Repeated-measures ANOVA followed by Tukey's post hoc test was used to determine if α1- and α2-stimulation resulted in significant contractions above baseline levels. A paired t-test was used to examine differences between maximal α1- and α2-induced vasocontraction. Repeated-measures ANOVA followed by Tukey's post hoc test was used to examine the effect of temperature and TRPV inhibition and denudation on arterial responsiveness for each concentration-response curve, maximum response, and logEC50. The level of significance for all tests was set a priori at α = 0.05.
RESULTS
Vessel Characteristics
Twenty-seven SMFA from the axillary region and ten SMFA from the inguinal region were harvested for this study. The average internal diameter of the feed arteries was 515 ± 40 μm. The maximum response to the length tension protocol (LTmax) was 1,076 ± 135 mg at Lmax, with a starting tension of 1,254 ± 82 mg.
Vessel Function
α1- and α2-Adrenergic responsiveness.
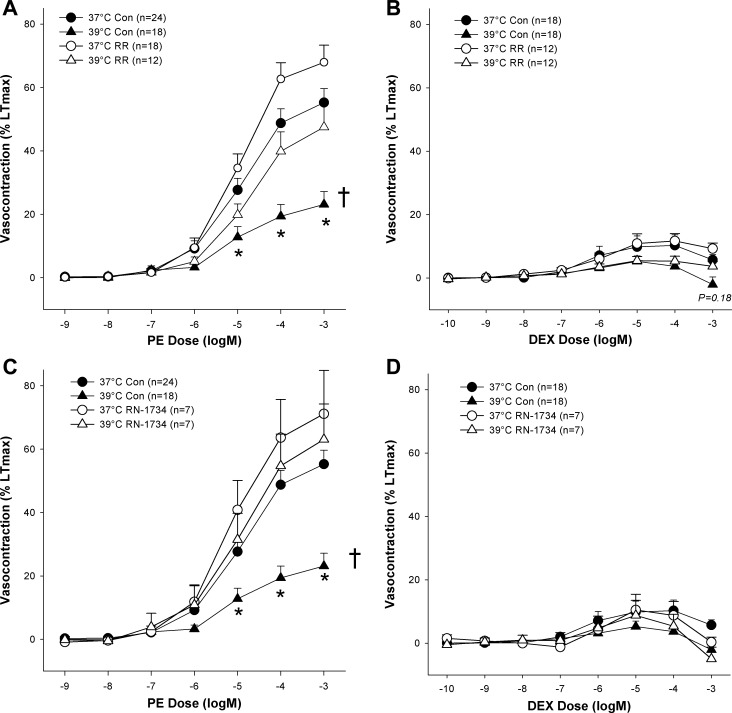
At 37°C, exposure to the α1-agonist PE and the α2 agonist DEX resulted in significant contractions above baseline tension (P < 0.05), with higher concentrations of DEX tending to elicit relaxation from the tension generated in response to lower concentrations of DEX (Fig. 1). Interestingly, responsiveness to DEX proved to be more varied from artery to artery with many arteries exhibiting little-to-no response to DEX while several others exhibited strong responses to DEX (∼45% LTmax). This variance was not accounted for by age nor gender, although females did tend to have a greater response to DEX than males (17 ± 6 and 8 ± 3, respectively, P = 0.18). Nevertheless, Fig. 1 illustrates that on average the magnitude of maximal α2-induced contractions was far less than the magnitude of the maximal α1-induced contractions (59 ± 5 and 12 ± 4% LTmax, respectively; P < 0.001).
Fig. 1.
Effect of temperature with and without vanilloid-type transient receptor potential (TRPV) family and TRPV4-specific inhibition [ruthenium red (RR) and RN-1734, respectively] on α1 [phenylephrine (PE)]- and α2 [dexmedetomidine (DEX)]-induced adrenergic vasocontraction. A: effect of temperature and RR on PE concentration-response curve. B: effect of temperature and RR on DEX concentration-response curve. C: effect of temperature and RN-1734 on PE concentration-response curve. D: effect of temperature and RN-1734 on DEX concentration-response curve. *Response to concentration significantly different than same concentration in the 37°C control condition (P < 0.05). †Concentration-response curve significantly different than 37°C control curve (P < 0.05).
Effect of TRPV inhibition and heat on α-adrenergic function.
Treating arteries with RR or RN-1734 had no significant effect on baseline tension at 37°C (P > 0.05). Heating human SMFA from 37 to 39°C resulted in a small, yet significant, increase in baseline tension (35 ± 15 mg; P < 0.05). While statistically significant, it should be noted that this change in basal tension only represents an increase equivalent to 3% LTmax. Interestingly, inhibition of the TRPV ion channels prevented the heat-related increase in baseline tension such that no significant change in basal tone was observed when heated in the presence of RR (−8 ± 6 mg; P > 0.05).
As illustrated in Fig. 1, α1-adrenergic responsiveness, as assessed by concentration-response curves with PE, was significantly attenuated at 39°C (24 ± 4% LTmax) compared with the 37°C control condition (P < 0.05). α2-Adrenergic contraction with DEX also exhibited a similar tendency for decreased vasocontraction when comparing 37 to 39°C (12 ± 4, 6 ± 2% LTmax), but due to the already recognized variability in the responses, this heat-induced sympatholysis did not reach statistical significance (P = 0.18). As hypothesized, treating arteries with RR restored α1-adrenergic contraction at 39°C to levels observed under the 37°C control condition, such that there was no significant difference between the 37°C control response curve and the 39°C RR curve (P > 0.05) or maximum contraction elicited by the drugs (PE: 48 ± 7% LTmax, and DEX: 14 ± 3% LTmax respectively; P > 0.05). Similarly, no evidence of heat-induced sympatholysis of α-adrenergic vasocontraction at 39°C was observed in arteries treated with RN-1734 (65 ± 11% LTmax; Fig. 1, C and D).
To determine if the restorative effect of RR and RN-1734 on the heat-induced attenuation of α-adrenergic vasocontraction was due to the blocking of the sympatholytic effect of heat or if it was acting through some other mechanism, we also examined the effect of the drugs on adrenergic responsiveness of the arterial rings at 37°C (Fig. 1). Initially, due to a tendency that failed to reach statistical significance, it appeared that PE- and DEX-induced vasocontraction may be potentiated by TRPV inhibition at 37°C, but, despite raising the n to increase statistical power, no significant effect of RR on PE- or DEX-induced vasocontraction at 37°C was observed (P > 0.30). It should also be noted that neither temperature nor treatment with RR or RN-1734 elicited significant differences between any other pairings besides those including the 39°C control condition and did not significantly alter the sensitivity of the arteries (i.e., logEC50), which averaged −4.9 ± 0.1 logM for PE and −7.3 ± 0.2 logM for DEX.
Effect of heat and TRPV inhibition on endothelial function.
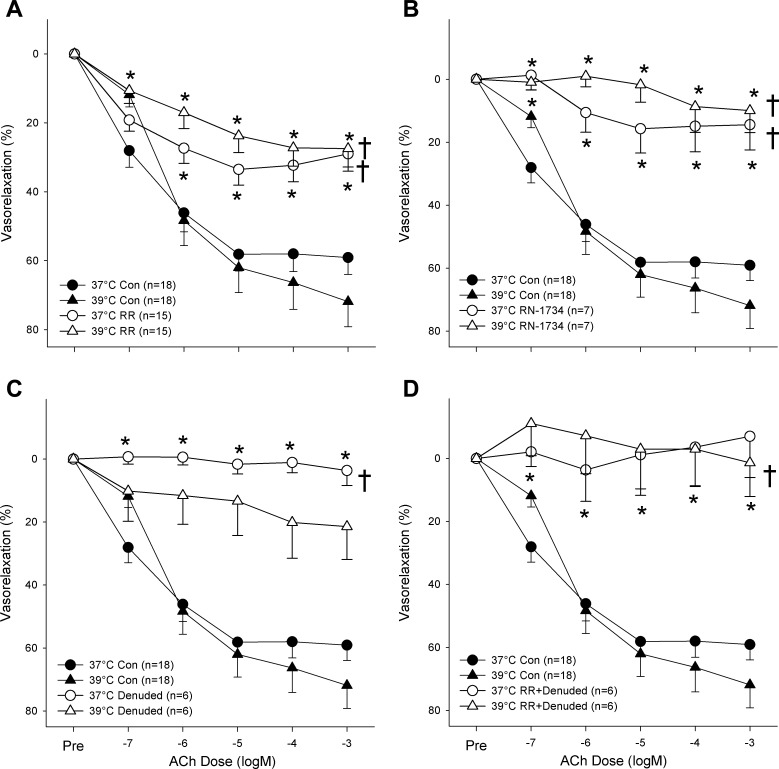
As illustrated in Fig. 2A, heating from 37 to 39°C tended to augment but ultimately had no significant effect on endothelial-dependent relaxation induced by exposure to ACh (maximum relaxation: 65 ± 5 and 75 ± 8%, respectively; P > 0.05) while the application of RR significantly and strongly attenuated ACh-induced vasorelaxation compared with control condition at both 37 and 39°C (P < 0.05). It is worth noting that relaxation in the presence of RR was similarly blunted at 37 and 39°C such that no significant difference between the two conditions was observed (maximal relaxation: 38 ± 4 and 32 ± 5%; P > 0.05). As illustrated in Fig. 2B, inhibition of the TRPV4 channels with RN-1734 yielded a similar significant attenuation of ACh-induced dilation at 37°C (20 ± 8% relaxation) and 39°C (17 ± 7% relaxation). As TRPV inhibition appeared to affect endothelial function, ACh-induced dilation with RR was tested in endothelial-denuded arteries (Fig. 2, C and D). There was no significant difference in ACh-induced dilation between endothelial-denuded arteries with or without RR.
Fig. 2.
Effect of heating with and without TRPV family and TRPV4-specific inhibition (RR and RN-1734, respectively) on endothelium-dependent, acetylcholine-induced (ACh) vasorelaxation. A: effect of heat and RR on ACh concentration-response curve. B: effect of heat and RN-1734 on ACh concentration-response curve. C: effect of heat, RR, and endothelial denudation on ACh concentration-response curve. D: effect of heat, RR, and endothelial denudation on ACh concentration-response curve. *Response to concentration significantly different than the same concentration in the 37°C control condition (P < 0.05). †Concentration-response curve significantly different than 37°C control curve (P < 0.05).
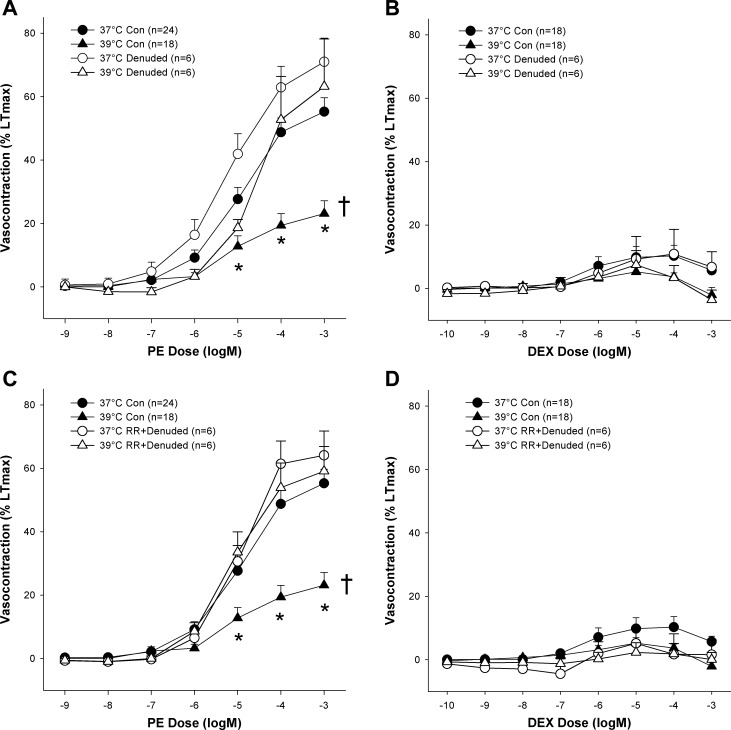
Like TRPV inhibition, endothelial denudation prevented the sympatholytic effect of heat on α1-adrenergic contraction (Fig. 3A; P > 0.05). Furthermore, no additive effect of TRPV inhibition was revealed in endothelium-denuded arteries suggesting that the effect of the TRPV ion channels in this response is largely endothelially mediated. Endothelial denudation with or without RR had no statistically discernible effect on α2-adrenergic contraction at 37 or 39°C (Fig. 3, B and D). Additionally, it should be noted that neither temperature nor treatment with RR or RN-1734 elicited significant differences between any other pairings besides those including the control condition.
Fig. 3.
Effect of endothelial denudation and TRPV family ion channel inhibition (RR) on heat-induced sympatholysis of α1 (PE) and α2 [dexmedetomidine (DEX)] adrenergic vasocontraction. A: effect of temperature and endothelial denudation on the PE concentration-response curve. B: effect of temperature and endothelial denudation on the DEX concentration-response curve. C: effect of temperature and nonspecific TRPV inhibition (RR) with endothelial denudation on the PE concentration-response curve. D: effect of temperature and nonspecific TRPV inhibition (RR) with endothelial denudation on the DEX concentration-response curve. *Response to concentration significantly different than same concentration in the 37°C control condition (P < 0.05). †Concentration-response curve significantly different than 37°C control curve (P < 0.05).
Effect of heat and TRPV inhibition on smooth muscle function.
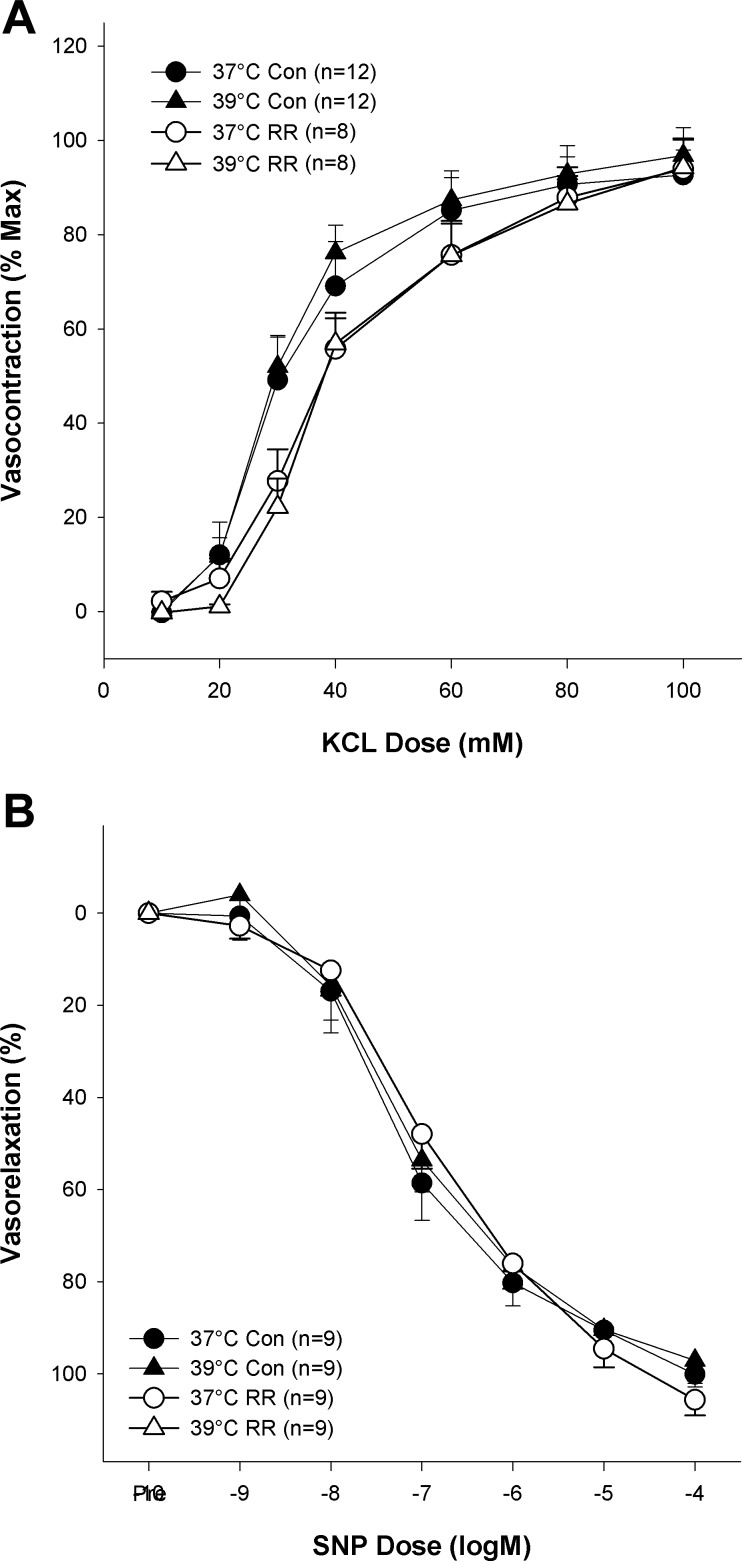
Figure 4 illustrates that receptor-independent vasocontraction (KCl) was unaffected by heating from 37 to 39°C (maximum contraction: 94 ± 5 and 97 ± 6% LTmax respectively; P > 0.05). Additionally, the inhibition of the TRPV channels with RR did not significantly alter KCl-induced contraction at 37°C (maximum contraction: 95 ± 4% LTmax; P > 0.05) or 39°C (maximum contraction: 95 ± 6% LTmax; P > 0.05). Neither heat nor TRPV inhibition significantly altered the sensitivity of the arteries to KCl (EC50 = 34 ± 3 mM). Likewise, neither temperature nor RR significantly affected endothelium-independent relaxation as assessed by cumulative exposures to SNP (P > 0.05). Finally, as illustrated in Fig. 5, time had no significant effect on PE-induced vasocontraction (P > 0.05).
Fig. 4.
Effect of heating and TRPV ion channel inhibition (RR) on smooth muscle function. A: effect of heat and RR on endothelium-independent vasocontraction [potassium chloride (KCl)] concentration-response curve. B: effect of heating and RR on endothelial independent vasorelaxation [sodium nitroprusside (SNP)] concentration-response curve.
Fig. 5.
Effect of time on α1 (PE)-induced arterial responsiveness with and without TRPV family inhibition (RR). A: effect of time on concentration-response curve to PE with and without RR. B: effect of time on maximum response to PE with and without RR.
DISCUSSION
In this study we sought to better characterize α-adrenergic function in human SMFA and determine if the temperature-sensitive TRPV ion channels act as the mechanistic link between elevated temperature and attenuated α-adrenergic responsiveness in these arteries. This study resulted in three novel observations. First, we described the relative roles of the α1- and α2-adrenoceptors in human SMFA and documented that α2-mediated vasocontraction is not as clearly inhibited by heat as α1-mediated vasocontraction. Second, we observed that inhibiting the TRPV ion channels, particularly the TRPV4 channel, results in the restoration of α1-adrenergic vasocontraction at 39°C, thereby implicating this channel in the sympatholytic effect of heat. Third, we determined that heat-induced sympatholysis, and the role of the TRPV ion channels in this phenomenon, occur in an endothelium-dependent manner.
Characterization α1- and α2-Vasocontraction in Human SMFA
Others have reported that the contribution of α2-mediated constriction is greater in distal resistance arterioles than in proximal conduit arteries (38). With the feed artery lying somewhere between the proximal conduit and distal resistance arteries (16, 17, 30), the role of the α2-receptors in human SMFA is unknown. Therefore, we performed concentration-response curves with both α1- and α2-agonists (PE and DEX, respectively) to determine what contribution the α2-receptors make at the level of the feed artery. Indeed, the stimulation of α2-adrenoceptors resulted in significant vasocontraction above baseline; however, the magnitude of responsiveness varied dramatically from artery to artery with some exhibiting little-to-no response to DEX and others exhibiting robust responses on the order of ∼45% LTmax. Additionally, among many subjects higher concentrations of DEX (>10−4 M) tended to result in relaxation and not constriction, as observed at the lower concentrations. The reason for this large variability is unclear but may possibly be related to the heterogeneity among subjects participating in this study who varied considerably in terms of sex, age, and health history (31). Despite the variability of responses, on average the magnitude of the maximum α2-induced contraction was relatively small, amounting to approximately only 20% of the magnitude of maximum α1-induced vasocontraction (Fig. 1, A and B). It is possible that the in vitro isolation itself may have diminished the responsiveness of the α2-adrenoreceptors as others have reported that in vitro isolation of arteries decreases α2-adrenergic contraction by ∼60% (13). However, it is important to note that even if our in vitro response was 40% of what would be observed in vivo, the corrected maximal α2-adrenergic contraction would still amount to only 45% of its α1-counterpart. Therefore, our data indicate that the α2-adrenoceptor plays less of a role than the α1-adrenoceptor in regulating the diameter and tone of human SMFA.
Much controversy exists as to the extent to which each α-receptor subtype is inhibited under sympatholytic conditions. Several studies have reported that α1-adrenoceptors are insensitive or less sensitive to sympatholysis than their α2-counterparts (1, 5, 32, 38) while others have reported that both α-subtypes are equally sensitive to sympatholysis (26). As illustrated in Fig. 1, both α1- and α2-induced vasocontraction tended to be inhibited when heated to 39°C, but only α1-induced contraction was significantly inhibited by the heat. At first glance it seems that the α2-receptors are not sensitive to heat-induced sympatholysis as they were not significantly inhibited at 39°C; however, upon further examination the tendency for heat-induced sympatholysis is evident in the α2-induced contraction (Fig. 1, B and D). Thus it seems possible that the lack of a significant attenuation of α2-induced vasocontraction may be more related to the relatively small and varied vasocontraction associated with α2-induced contractions rather than a lack of sensitivity to heat. Further research is needed to determine if these adrenoceptors do, in fact, differ in terms of sensitivity to heat. Additionally, the varied nature of the DEX response may have also obscured the effect of other conditions like TRPV inhibition and denudation on α2-induced vasocontraction, which revealed no clear trends.
Role of TRPV Channels in Heat-induced Sympatholysis of Adrenergic Vasocontraction
Given their known temperature sensitivity (2, 6, 11, 21, 36) and interaction with vascular function ranging from coronary to cutaneous circulation (7, 10, 21, 37, 40), we hypothesized that the TRPV ion channels would serve as the link between elevated temperature and attenuated α-adrenergic contraction in isolated human SMFA. Indeed, as illustrated in Fig. 1A, the inhibition of the TRPV ion channel family with RR restored α1-adrenergic vasocontraction at 39°C to levels observed at 37°C implying that the TRPV ion channels play a role in mediating heat-induced sympatholysis. Such inhibition had no significant effect on α2-adrenergic vasocontraction (Fig. 1B). Having observed that nonspecific TRPV inhibition restored α1-adrenergic contraction in the heat, we sought to determine if a single member of the TRPV family could be implicated in this response. Given their sensitivity to temperatures ranging from 25 to 39+°C (2) and their previously described relationship with endothelium-dependent dilation (21, 41), we explored the possibility that the TRPV4 ion channel mediates the sympatholytic effect of heat. As illustrated in Fig. 1C, similar to arteries treated with nonspecific TRPV inhibition, arteries treated with the TRPV4-specific inhibitor RN-1734 exhibited normal levels of vasocontraction in the face of the heat stimulus. Thus these data indicate that the TRPV4 ion channel is specifically involved in mediating the sympatholytic effect of heat, which is in agreement with previous research that has documented that heating cultured rat aortic endothelial cells elicits an intracellular calcium flux that was prevented by inhibiting the TRPV4 channels (36). Thus it appears that the TRPV4 ion channel senses, either directly or indirectly (2, 36), the increase in temperature and subsequently initiates the sympatholytic effect of heat. Interestingly, although TRPV inhibition at 37°C tended to augment adrenergic contraction in some cases, this did not do occur consistently or significantly suggesting that the TRPV channels have only a minor influence on α1- and α2-induced vasocontraction under seemingly normothermic conditions.
Potential Mechanisms of Involvement of TRPV Channels in Heat-Induced Sympatholysis
As we have previously determined that heat-induced sympatholysis is the result of increased NO production (14), it seems likely that the activation of the TRPV4 ion channels with heat attenuates adrenergic vasocontraction by precipitating an increase in endothelial NO production. The likelihood of this potential explanation is supported by several lines of evidence. Using isolated endothelial cells and arteries from rats, Kohler et al. (21) found that applying moderate warmth (∼37°C) increased the activity of the TRPV4 ion channels and reported that such an increase in activity did in fact augment NO-dependent vasodilation. Furthermore, the putative relationship between TRPV activation and NO production is supported by reports that the activation of TRPV1 ion channels increases eNOS activity and NO production in isolated skeletal muscle arterioles and endothelial cells (7, 19).
Although we did not measure NO production in the current study, we did probe the role of the endothelium in this phenomenon by assessing endothelial-dependent vasorelaxation with ACh and examining the effect of heat on endothelium-denuded arteries. As illustrated in Fig. 2, when the TRPV ion channels were inhibited, with RR or RN-1734, ACh-induced relaxation was potently attenuated thereby supporting the relationship between the TRPV channels and the endothelium that has been reported previously (21). If the TRPV channels in the endothelium initiated that sympatholytic effect of heat, it stands to reason that one would find augmented ACh-induced vasorelaxation in the heated condition. Interestingly, although such a tendency existed, we did not observe a significant potentiation of ACh-induced relaxation, possibly due to the heterogeneity among subjects. Nevertheless, more concrete evidence that the TRPV ion channels mediate the sympatholytic effect of heat in an endothelium-dependent manner comes from the current experiments that indicated that the endothelium is vital for the sympatholytic effect of heat. Specifically, that TRPV inhibition in conjunction with endothelial denudation had no additive effect on the response to PE in denuded arteries (Fig. 3). Considering that denudation prevented the sympatholytic effect of heat, and that TRPV inhibition attenuated endothelium-dependent relaxation while preventing the sympatholytic effect of heat, it seems likely that the TRPV4 ion channels mediate the response in an endothelium-dependent manner and not by way of altered smooth muscle function, which was not significantly affected by heat and TRPV inhibition (Fig. 4).
It is worth noting that, like eNOS inhibition (14), denudation had no significant effect on α1- or α2-vasocontraction at 37°C indicating that endothelial activity does not significantly suppress adrenergic contraction under normothermic conditions. Additionally, it is important to note that the arteries in this preparation were studied in isolation, thus precluding a role of the central nervous system in the sympatholytic effect of heat. Nevertheless, it should be noted that even with careful dissection it is likely impossible to completely remove all nerve fibers from the SMFA and therefore there remains the possibility that TRPV ion channels on sensory and/or sympathetic nerve endings embedded in the artery wall mediate the vascular response in a manner similar to the axon reflex that mediates cutaneous hyperemia in response to local heating (12, 37).
Experimental Considerations
As prior exposure to heat has been reported to alter adrenergic responsiveness (27), we, like others (15, 20), performed the highest temperature phase last. Recognizing that with this design the observed decreases in adrenergic responsiveness may be due to time and not temperature, we performed a time control experiment with PE in SMFA. As illustrated in Fig. 5, and in agreement with prior work (15), adrenergic vasocontraction was preserved over the duration of the experiment indicating that time was likely not a factor in the decreased adrenergic responsiveness we observed with heating. Due to the limited supply of human SMFA we limited the time control experiment to PE-induced contractions reasoning that if time had an effect on adrenergic contraction it would likely affect PE-induced and DEX-induced contraction similarly.
Based on previous research (36), we hypothesized that the TRPV4 ion channel would mediate the sympatholytic response to heat, but given the potential overlap in temperature sensitivity by other members of the TRPV family we could not rule out the possibility that other TRPV ion channels were involved in mediating the response. Therefore, we used the chemical RR, a nonspecific TRPV ion channel inhibitor, to screen for the role of the TRPV family in the sympatholytic effect of heat. Once the data using RR pointed to a role for TRPV ion channels, a more specific inhibitor, RN-1734, was utilized to pinpoint which TRPV channel was involved. With regard to RR it is important to consider that it is not completely specific to the TRPV ion channels and may affect other channels like the cold-sensitive TRPM8 channels, which are not likely to be active under the warm conditions of our experiment, and ryanodine receptors (8). Thus the possibility exists that these other channels may account, in part, for some of the results of this experiment. Nevertheless, the response to stimuli, like heat, by tissues treated with RR and by tissues in which the TRPV channels have been genetically nullified, document good agreement (9, 24, 36). Additionally, it should be noted that in the current study there was good agreement between the effects of RR and RN-1734 and there was no significant impact of RR on baseline tension, like in the work of Kohler et al. (21). Thus it appears that RR had the majority of its effects on the heat-sensitive TRPV ion channels and not on other putative targets of the drug.
As documented in Table 1, the subjects participating in this study were heterogeneous in terms of age, sex, and health history and therefore do not reflect a specific subset of the population but rather represent humans as a whole. Furthermore, although the SMFA were harvested during sentinel node biopsies, performed to determine if melanoma has spread from the skin, this was of little significance as, in the majority of these cases, the cancer was determined not to have had systemic consequences. Thus the conclusions of this study are likely applicable to the population as whole.
Conclusions
In this study we sought to describe the functional roles of α1- and α2-adrenoceptors in SMFA and explore the possibility that the TRPV ion channels, particularly the TRPV4 channel, act as the link between elevated temperature and blunted α-adrenergic vasocontraction. We conclude that the contribution of the α2-receptors in human SMFA is minimal, more varied, and not as clearly inhibited by heat compared with the α1-receptors. As hypothesized, the inhibition of TRPV ion channels, particularly the TRPV4 channels, restored α1-adrenergic vasocontraction at 39°C to levels observed at 37°C, thereby implicating the TRPV ion channels as a component of the heat-induced sympatholysis acting in an endothelial-dependent manner. Thus it appears that physiological increases in temperature, typical of the muscle bed during moderate intensity exercise, likely activate the endothelial TRPV4 ion channels to modulate vascular function, ultimately yielding a sympatholysis-like attenuation of α-adrenergic vasocontraction.
GRANTS
We are grateful for financial support provided by National Heart, Lung, and Blood Institute Grant PO1-HL-091830 and Veterans Affiars Merit Grant E6910R.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.G., S.J.I., and R.S.R. conception and design of research; J.R.G., S.J.I., S.-Y.P., R.H.A., J.R.H., M.T.M., G.S.T., and J.D.T. performed experiments; J.R.G., S.J.I., and R.S.R. analyzed data; J.R.G., S.J.I., S.-Y.P., C.W., and R.S.R. interpreted results of experiments; J.R.G. prepared figures; J.R.G. and R.S.R. drafted manuscript; J.R.G., S.J.I., S.-Y.P., R.H.A., J.R.H., M.T.M., G.S.T., C.W., J.D.T., and R.S.R. edited and revised manuscript; J.R.G., S.J.I., S.-Y.P., R.H.A., J.R.H., M.T.M., G.S.T., C.W., J.D.T., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for gracious participation and the surgical staff for generous assistance.
REFERENCES
- 1.Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha-1-adrenoceptor and alpha-2-adrenoceptor constriction to metabolic inhibition during rat skeletal-muscle contraction. Circ Res 69: 174–184, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol (Oxf) 203: 99–116, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol 302: H634–H642, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev 29: 159–163, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol 90: 172–178, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Ching LC, Kou YR, Shyue SK, Su KH, Wei J, Cheng LC, Yu YB, Pan CC, Lee TS. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1. Cardiovasc Res 91: 492–501, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci 119: 19–36, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297: H1096–H1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gifford JR, Heal C, Bridges J, Goldthorpe S, Mack GW. Changes in dermal interstitial ATP levels during local heating of human skin. J Physiol 590: 6403–6411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guler AD, Lee HS, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22: 6408–6414, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henriksen O. Sympathetic reflex control of blood flow in human peripheral tissues. Acta Physiol Scand, Suppl 143: 33–39, 1991 [PubMed] [Google Scholar]
- 13.Ikeoka K, Faber JE. ANG II reverses selective inhibition of α2-adrenoceptor sensitivity after in vitro isolation of arterioles. Am J Physiol Heart Circ Physiol 265: H1988–H1995, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Ives SJ, Andtbacka RH, Kwon SH, Shiu YT, Ruan T, Noyes RD, Zhang QJ, Symons JD, Richardson RS. Heat and α1-adrenergic responsiveness in human skeletal muscle feed arteries: the role of nitric oxide. J Appl Physiol 113: 1690–1698, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ives SJ, Andtbacka RHI, Noyes RD, McDaniel J, Amann M, Witman MA, Symons JD, Wray DW, Richardson RS. Human skeletal muscle feed arteries studied in vitro: the effect of temperature on alpha(1)-adrenergic responsiveness. Exp Physiol 96: 907–918, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ives SJ, Andtbacka RH, Noyes RD, Morgan RG, Gifford JR, Park SY, Symons JD, Richardson RS. alpha(1)-Adrenergic responsiveness in human skeletal muscle feed arteries: the impact of reducing extracellular pH. Exp Physiol 98: 256–267, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ives SJ, Andtbacka RHI, Park SY, Donato AJ, Gifford JR, Noyes RD, Lesniewski LA, Richardson RS. Human skeletal muscle feed arteries: evidence of regulatory potential. Acta Physiol (Oxf) 206: 135–141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jendzjowsky NG, Delorey DS. Short-term exercise training augments α2-adrenoreceptor-mediated sympathetic vasoconstriction in resting and contracting skeletal muscle. J Physiol 591: 5221–5233, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, Papp Z, Edes I, Porszasz R, Toth A. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol 73: 1405–1412, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Kluess HA, Buckwalter JB, Hamann JJ, Clifford PS. Elevated temperature decreases sensitivity of P2X purifiergic receptors in skeletal muscle arteries. J Appl Physiol 99: 995–998, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26: 1495–1502, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H(2)O(2) is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res 108: 566–573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGillivray-Anderson KM, Faber JE. Effect of acidosis on contraction of microvascular smooth-muscle by alpha-1-adrenoceptors and alpha-2-adrenoceptors–implications for neural and metabolic-regulation. Circ Res 66: 165–173, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962 [DOI] [PubMed] [Google Scholar]
- 26.Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol 547: 971–976, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan AJ, Gisolfi CV. Responses of rat mesenteric-arteries to norepinephrine during exposure to heat-stress and acidosis. J Appl Physiol 78: 38–45, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol 21: 1757–1762, 1966 [DOI] [PubMed] [Google Scholar]
- 29.Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation–a novel mechanism involved in myogenic constriction. Circ Res 95: 1027–1034, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand 168: 511–518, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Tejera N, Balfagon G, Marin J, Ferrer M. Gender differences in the endothelial regulation of alpha2-adrenoceptor-mediated contraction in the rat aorta. Clin Sci (Lond) 97: 19–25, 1999 [PubMed] [Google Scholar]
- 32.Thomas GD, Hansen J, Victor RG. Inhibition of α2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol 266: H920–H929, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Vincent F, Duncton MA. TRPV4 agonists and antagonists. Curr Top Med Chem 11: 2216–2226, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Wang YX, Wang J, Wang C, Liu J, Shi LP, Xu M, Wang C. Functional expression of transient receptor potential vanilloid-related channels in chronically hypoxic human pulmonary arterial smooth muscle cells. J Membr Biol 223: 151–159, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Wong BJ, Fieger SM. Transient receptor potential vanilloid type 1 channels contribute to reflex cutaneous vasodilation in humans. J Appl Physiol 112: 2037–2042, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of alpha-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol 555: 545–563, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Zhang DX, Gutterman DD. Transient receptor potential channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol 57: 133–139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53: 532–538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]