Abstract
Human induced pluripotent stem cell-derived cardiomyocyte (iPSC-CM)-based assays are emerging as a promising tool for the in vitro preclinical screening of QT interval-prolonging side effects of drugs in development. A major impediment to the widespread use of human iPSC-CM assays is the low throughput of the currently available electrophysiological tools. To test the precision and applicability of the near-infrared fluorescent voltage-sensitive dye 1-(4-sulfanatobutyl)-4-{β[2-(di-n-butylamino)-6-naphthyl]butadienyl}quinolinium betaine (di-4-ANBDQBS) for moderate-throughput electrophysiological analyses, we compared simultaneous transmembrane voltage and optical action potential (AP) recordings in human iPSC-CM loaded with di-4-ANBDQBS. Optical AP recordings tracked transmembrane voltage with high precision, generating nearly identical values for AP duration (AP durations at 10%, 50%, and 90% repolarization). Human iPSC-CMs tolerated repeated laser exposure, with stable optical AP parameters recorded over a 30-min study period. Optical AP recordings appropriately tracked changes in repolarization induced by pharmacological manipulation. Finally, di-4-ANBDQBS allowed for moderate-throughput analyses, increasing throughput >10-fold over the traditional patch-clamp technique. We conclude that the voltage-sensitive dye di-4-ANBDQBS allows for high-precision optical AP measurements that markedly increase the throughput for electrophysiological characterization of human iPSC-CMs.
Keywords: optical action potential, preclinical drug screening, QT prolongation, acquired long QT syndrome, stem cell model
recent advances in induced pluripotent stem cell (iPSC) technology now allow for the routine derivation of patient- and disease-specific human iPSC cardiomyocyte (iPSC-CM) models of cardiovascular disease. The impact of this technology has far-reaching implications, ranging from drug discovery, preclinical drug screening, mechanistic understanding of disease processes, and approaches for personalized medicine. Human iPSC-CM-based assays are emerging as a promising tool for the in vitro preclinical screening of QT interval-prolonging side effects of drugs in development. A major impediment to the widespread use of human iPSC-CM assays is the low throughput of the currently available electrophysiological tools. The patch-clamp technique allows for direct measurement of transmembrane voltage (Vm) to precisely quantify the morphology and duration of atrial and ventricular action potentials (APs). However, the patch-clamp technique is a labor-intensive technique, which involves highly skilled, experienced individuals. Recently, the utility of a genetically encoded voltage-sensitive fluorescent reporter (ArcLight) was described in human embryonic stem cell-derived CMs (6). This reporter generated robust fluorescent signals from single cells, allowing for moderate-high throughput analyses, although the temporal response was delayed relative to directly measured Vm. While voltage-sensitive dyes such as di-4-ANNEPS and di-8-ANNEPS allow for high temporal resolution signals, their inherent phototoxicity limits their use in in single cells (2). The electrochromic voltage-sensitive dye 1-(4-sulfanatobutyl)-4-{β[2-(di-n-butylamino)-6-naphthyl]butadienyl}quinolinium betaine (di-4-ANBDQBS) (1) has recently been reported to precisely track Vm in isolated, single CMs (16). Here, we report the application of the near-infrared fluorescent voltage-sensitive dye di-4-ANBDQBS for moderate-throughput electrophysiological analyses in human iPSC-CMs derived from peripheral blood mononuclear cells (PBMCs).
MATERIALS AND METHODS
Human subject research for this study was submitted to and approved by the University of Utah Institutional Review Board.
Human iPS cell generation and CM differentiation.
PBMCs were isolated using standard techniques and reprogrammed by infecting cells with lentiviruses expressing reverse tetracycline-controlled transactivator, octamer-binding transcription factor 4, Kruppel-like factor 4, sex-determining region Y-box 2, and c-Myc genes from a polycistronic cassette (pHAGE2-TetONminiCMV-hSTEMCCA) (14, 15), as recently described in Ref. 12. Infected blood cells were transferred onto mouse embryonic fibroblast (MEF) feeder cells 3 days postinfection and cultured for additional 7 days in MEF medium supplemented with 10 ng/ml human basic (b)FGF, 5 mg/ml ascorbic acid, and 2 mg/ml doxycycline. Once small colonies appeared. the medium was changed to human embryonic stem cell medium [DMEM-F-12 supplemented with Glutamax, 10 mM nonessential amino acids, 25 U/ml penicillin, 25 μg/ml streptomycin, 100 μM β-mercaptoethanol, and 20% knockout serum replacement (Invitrogen) with 10 ng/ml bFGF and 2 mg/ml doxycycline]. Colonies were selected and expanded around day 30 postinfection. After three passages on MEF feeder cells, iPSC clones were transferred to Matrigel (BD Biosciences)-coated dishes, cultured and expanded in mTeSR1 according to the manufacturer's protocol, and then gradually weaned off of doxycycline. CM differentiation was achieved by the matrix-sandwich method developed by Kamp and colleagues (18, 19).
Transmembrane potential recordings.
Spontaneously beating human iPSC-CMs (30–50 days postdifferentiation) were studied. Cells were plated at low density to facilitate single cell recordings. All experiments were performed at 36–37°C. The recording chamber was superfused with buffered physiological solution containing (in mmol/l) 126 NaCl, 4.4 KCl, 1.1 CaCl2, 1 MgCl2, 11 glucose, and 24 HEPES (pH 7.4 with NaOH). Electrodes made from borosilicate capillary tubes (8250 glass, A-M Systems) and fire polished to obtain resistances of 6–9 MΩ were filled with internal solution containing (in mmol/l) 120 KCl, 5 EGTA, 5 K2ATP, 5 MgCl2, and 10 HEPES (pH 7.2).
Vm was measured using an AxoClamp 2A amplifier (Molecular Devices) in the bridge mode with the disrupted patch technique. Vm was filtered at 10 kHz and digitized at a sampling frequency of 50 kHz with a 12-bit analog-to-digital converter (Digidata 1322A Interface, Molecular Devices). The following AP parameters were analyzed: maximum diastolic potential (MDP) and AP duration (APD). APD was calculated as the time interval between the peak maximum upstroke velocity (phase 0) and the time at 10% (APD10), 50% (APD50), and 90% of repolarization (APD90).
Dye loading.
Human iPSC-CMs were loaded with 20 μM di-4-ANBDQBS in buffer physiological solution, which was prepared daily from a stock solution of di-4-ANBDQBS dissolved in ethanol. The final concentration of ethanol in the loading solution was 0.1%. In preliminary experiments, we determined the optimum dye concentration and duration of incubation by varying the dye concentration from 5 to 25 μM for 2–10 min. Incubation of cells in 20 μM di-4-ANBDQBS for 2–5 min resulted in fluorescent signals with excellent signal-to-noise ratios and minimal cellular toxicity for the duration of the experimental conditions. Coverslips plated with human iPSC-CMs were placed in the loading solution (maintained in the dark) at 37°C and then transferred to the microscope stage, where the dye was washed out by perfusion with control buffered solution.
Optical setup for fluorescence measurements.
Coverslips plated with human iPSC-CMs were placed on the stage of an inverted microscope maintained at 36–37°C. Myocytes loaded with di-4-ANBDQBS were excited by a 200-mW red solid-state laser (660 nm) coupled to a flexible fiber optics light guide (Edmund Optics) and directed to the sample via a 692-nm dichroic mirror (Omega Optical). Backscattered fluorescence was collected through a ×25 or ×40 oil-immersion objective lens (numerical aperture: 1.3). Fluorescence was recorded by an electron multiplied (EM) charge-coupled device (CCD) camera (iXon 860, Andor Technology, Belfast, UK) connected to the video port and equipped with a 700-nm long-pass filter (Omega Optical). Movies of fluorescence were recorded with the camera using an EM gain of 300 at spatial and temporal resolutions of 64 × 64 pixels and 860 frames/s, respectively.
Exposure of our preparations to the excitation source was controlled by a system of shutters placed in the optical path of the solid-state laser and synchronized to the initiation of the EM CCD camera acquisition. All these events were recorded via a TTL output that ultimately permitted the precise time alignment of the fluorescence signals to the simultaneously acquired transmembrane potential recordings. In the present study, we used two principal illumination protocols following a procedure similar to that previously described (16). The first illumination protocol consisted of exposing the sample to brief 8-s duration episodes of laser illumination every 10 min during a total time of 30 min. During each of these episodes, fluorescence from the sample was recorded with the EM CCD camera. The second illumination protocol (“intense”) consisted of exposing the sample to long (2.5-min duration) episodes of laser illumination followed by a 3.5-min pause in the exposure to the illumination. Fluorescence of the sample was recorded while the preparation was illuminated, and the procedure was repeated three times to obtain the time course of photodynamic damage elicited by this intervention during 14.5 min.
Analysis of fluorescence recordings.
Recordings of di-4-ANBDQBS fluorescence using the EM CCD camera yielded a series of frames with resolution of 64 × 64 pixel frames at a spatial resolution of 0.21 μm/pixel. Fluorescence was averaged over the cell image, yielding an optical AP that depicted a reduction in fluorescence upon depolarization, consistent with a shift of the emission spectrum to a shorter wavelength with cellular depolarization. The di-4-ANBDQBS fluorescence signal was corrected by subtracting the intrinsic background fluorescence measured under exactly the same conditions (i.e., in the presence of laser illumination, laminin, and perfusion flow) but in the absence of myocytes. Fluorescence signals were inverted for ease of presentation and comparison with transmembrane APs. The peak value of fluorescence was calculated by averaging the fluorescence of the five frames. The optical AP amplitude was calculated as the absolute difference between the maximum and minimum fluorescent values. APD10, APD50, and APD90 were determined as the time at which repolarization of the optical AP achieved 10%, 50%, or 90% of the optical AP amplitude. Optical AP parameters were measured automatically using custom software. For each measurement, the mean value of at least four consecutive APs was calculated.
Pharmacological manipulation of repolarization.
To determine the ability of the voltage-sensitive dye to resolve electrophysiological changes in response to pharmacological manipulation, human iPSC-CMs were exposed to diltiazem or dofetilide. For these experiments, cells were field stimulated at a cycle length (CL) of 800 ms to control for baseline variations and drug-changes in spontaneous CL. After 2 min of stable pacing, control optical APs were recorded. Optical APs were recorded after cells were perfused with 1 μM diltiazem for 7 min or 50 nM dofetilide for 5 min. After a washout period of 15 min, optical AP measurements were repeated.
Measurement of conduction velocity, activation time, and repolarization time.
AP conduction velocity was measured in field-stimulated iPSC-CM preparations. To best measure conduction velocity, we selected cellular strands connecting clusters of contracting cells. The stimulating electrode was positioned as far away as possible from one of the clusters to avoid simultaneously activating the entire preparation. All measurements were done at ×25 (oil) during steady-state pacing at a CL of 700 ms. Cells were continuously bathed with normal control bathing solution containing 1 μM blebbistatin to uncouple contraction. Signals were filtered using a standard approach, which consisted of averaging the values within a four-frame sliding window along the temporal domain and a 5-pixel sliding window along the spatial domain. Conduction velocity (in cm/s) was measured from the time delay and the distance between two regions of interest located at least 170 μm apart.
Activation and repolarization times for each pixel within the CM preparation were determined as follows. The activation time was determined by calculating the time at which the first derivative of the fluorescence signal reached its minimum value. APD90 was calculated by subtracting the time at which repolarization attained 90% of the optical AP amplitude from the activation time. Activation and repolarization maps were constructed by plotting the color-coded activation and repolarization time values for each individual optical AP in the mapped region.
Statistical analysis.
Data are presented as means ± SE. In all experiments, n represents the number of independent measures. Repeated-measures Student's t-test, ANOVA, or repeated-measures ANOVA was applied to compare individual data sets. A two-tailed probability value of <0.05 was considered statistically significant.
RESULTS
Correlation between electrical and fluorescent AP recordings.
Human iPSC-CMs were plated at low density on coverslips to maximize the likelihood of encountering single isolated cells or small groups of cells encompassing 2–8 cells/cluster. Transmitted light and fluorescent images of a single iPSC-CM, together with the respective optical AP, are shown in Fig. 1. Fluorescence from a single myocyte yielded interpretable optical APs that allowed for the identification of ventricular- or atrial-like phenotypes. To confirm the fidelity of the voltage-sensitive dye to track changes in membrane potential, we simultaneously measured optical and transmembrane APs. Optical APs recorded from spontaneously beating atrial- and ventricular-like myocytes closely tracked AP recordings directly measured with the disrupted patch technique (Fig. 2). No statistical differences were observed in APD10, APD50, and APD90 measurements recorded from optical and transmembrane APs (Table 1). The tight correlation between optical and electrical signals confirm the robust temporal resolution of di-4-ANBDQBS to track the rapid changes in membrane potential that characterize APs in human iPSC-CMs.
Fig. 1.
Transmitted light image (A) and fluorescent snapshot (B) of a single human induced pluripotent stem cell-derived cardiomyocyte (iPSC-CM) loaded with 1-(4-sulfanatobutyl)-4-{β[2-(di-n-butylamino)-6-naphthyl]butadienyl}quinolinium betaine (di-4-ANBDQBS). C: optical action potentials (APs) recorded from the image in B showing a typical ventricular-like waveform. AU, arbitrary units.
Fig. 2.
Tight correlation between electrical and fluorescent AP recordings. A: simultaneous transmembrane (blue) and optical (red) APs recorded from human iPSC ventricular-like myocytes. B: simultaneous recordings from atrial-like myocytes. These images demonstrate that optical APs track transmembrane voltage (Vm) with high fidelity. Note that the timescale bars for right panels are different.
Table 1.
Comparison of optical and electrical action potentials simultaneously recorded in spontaneously beating atrial- and ventricular-like human induced pluripotent stem cell-derived cardiomyocytes
| Cycle Length, ms |
APD10, ms |
APD50, ms |
APD90, ms |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Optical | Electrical | Optical | Electrical | Optical | Electrical | Optical | Electrical | |
| Atrial | 6 | 794 ± 70 | 799 ± 68 | 49 ± 2 | 41 ± 4 | 109 ± 6 | 103 ± 8 | 171 ± 7 | 174 ± 5 |
| Ventricular | 11 | 1236 ± 118 | 1235 ± 119 | 74 ± 9 | 81 ± 5 | 214 ± 13 | 214 ± 11 | 291 ± 12 | 286 ± 10 |
Values are means ± SE; n, number of cardiomyocytes. APD10, APD50, and APD90, action potential durations at 10%, 50%, and 90% repolarization, respectively.
Stability of di-4-ANBDQBS optical AP recordings and photodynamic damage induced by an intense laser illumination protocol.
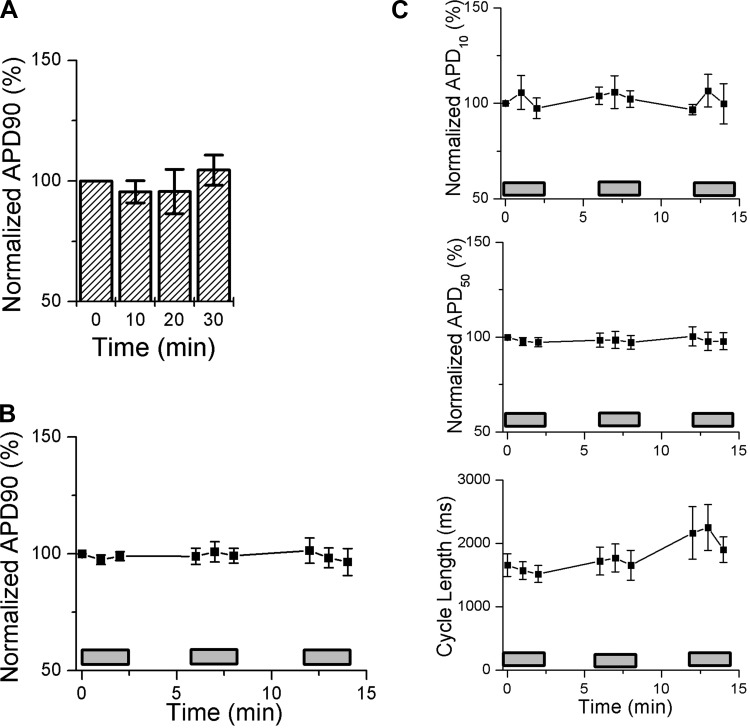
To determine the stability of optical APs over time, we recorded fluorescence signals at times of 0, 10, 20, and 30 min. APD90 did not vary over the recording period (Fig. 3A), nor did measurements of APD10, APD50, or spontaneous CL (data not shown). Next, we assessed the susceptibility to photodynamic damage using an intense laser illumination protocol that has been previously reported to perturb optical APs recordings in isolated adult ventricular myocytes loaded with either di-4-ANEPPS or di-4-ANBDQBS, respectively (13, 16). Human iPSC-CMs loaded with di-4-ANBDQBS were sequentially exposed to three cycles of 2.5 min of continuous laser illumination interspersed by brief periods of recovery. Measured optical AP parameters remained unchanged at the end of the intense illumination protocol (Fig. 3, B and C). In contrast, adult ventricular myocytes loaded with di-4-ANEPPS or di-4-ANBDQBS developed marked prolongation in APD90 during the second cycle of laser illumination (13, 16). Taken together, these data indicate that optical AP recordings in di-4-ANBDQBS-labeled human iPSC-CMs are stable over time and are relatively resistant to photodynamic damage.
Fig. 3.
Optical AP parameters measured using di-4-ANBDQBS remain stable over time and after “intense” laser exposure. A: optical APs were recorded at 10-min intervals in human iPSC-CMs loaded with di-4-ANBDQBS. AP duration (APD) at 90% repolarization (APD90) normalized to the baseline value did not change significantly over the recording period. Values are means ± SE; n = 6 independent experiments. P > 0.05 by repeated-measures ANOVA. B and C: human iPSC-CMs were exposed to an intense laser illumination protocol that has been previously reported to perturb optical APs recordings in adult ventricular myocytes loaded with either di-4-ANEPPS or di-4-ANBDQBS, respectively (13, 16). Shaded rectangles denote time periods of laser exposure. APD90 (B) and APD at 10% repolarization (APD10), APD at 50% repolarization (APD50), and cycle length (CL; C) measured from optical APs remained stable over the recording protocol. These data support the notion that di-4-ANBDQBS-labeled human iPSC-CMs generate stable optical APs that are resistant to photodynamic damage. Values are means ± SE; n = 6 independent experiments. P > 0.05 by repeated-measures ANOVA.
Characterization of repolarization time using optical AP recordings.
APD is known to vary with CL in a nonlinear fashion, and, as a result, QT intervals recorded from a surface electrocardiogram require a rate correction factor. To our knowledge, the relationship between APD and CL in human iPSC-CM has not been fully characterized. Thus, we sequentially paced ventricular-like CMs using an external field stimulator and generated repolarization restitution curves of optical APD90 versus CL (Fig. 4). As expected, the repolarization restitution curve was nonlinear and was best fit with the following power function: y = βx^α, where β = 8.5 and α = 0.44 (R2 = 0.96). This value falls in between the α-values for heart rate-corrected QT interval using the standard clinical Bazett (α = 0.5) and Fridericia (α = 0.33) correction factors.
Fig. 4.
Relationship between optical APD90 and CL for ventricular-like human iPSC-CMs. A: examples of optical APs recorded at different pacing intervals in a ventricular-like iPSC-CM. B: repolarization restitution relationship derived from optical APD90 values at variable pacing CLs. Data were fit to the following power function: y = βx^α, where β = 8.5 and α = 0.44 (R2 = 0.96). Data are means ± SE; n = 7 independent experiments.
Next, we recorded fluorescence signals in human iPSC-CMs to determine the effects of pharmacological manipulation on repolarization. To eliminate variation in baseline spontaneous CL, cells were paced at a fixed CL of 800 ms. Exposure of CMs to the L-type Ca2+ channel blocker diltiazem (1 μM) shortened APD50 and APD90 in both atrial-like (n = 16) and ventricular-like (n = 10) CMs (Fig. 5). These effects were reversible, with normalization of APD50 and APD90 after a washout period of 15 min. Next, we assessed the effects of the potent hERG channel blocker dofetilide on repolarization time. Exposure of ventricular-like CMs to 50 nM dofetilide prolonged APD90 and resulted in triangularization of the AP downstroke in ventricular-like CMs (n = 15; Fig. 5C). The effects of dofetilide were not reversible during the washout period of 15 min. Taken together, these results demonstrate the ability of di-4-ANBDQBS-based optical APs to appropriately track changes in repolarization induced by pacing and pharmacological manipulation.
Fig. 5.
Pharmacologically induced alteration in repolarization as measured in di-4-ANBDQBS-loaded human iPSC-CMs. A and B, left: representative optical APs recorded under control conditions, with perfusion with1 μM diltiazem (Diltz), and after washout (Wash) recorded from atrial-like (A) and ventricular-like (B) iPSC-CMs field stimulated at a CL of 800 ms. Right, bar graphs showing mean normalized values for optical APD10, APD50, and APD90 measurements for the treatment groups. Values are means ± SE; n = 16 paired experiments in atrial-like CMs and n = 10 for ventricular-like CMs. C, left: representative optical APs recorded under control conditions, with perfusion with 50 nM dofetilide (DOF), and after washout recorded from ventricular-like iPSC-CMs field stimulated at a CL of 800 ms. Right, bar graphs showing mean normalized values for optical APD10, APD50, and APD90 measurements for the treatment groups. Values are means ± SE; n = 15 paired experiments in ventricular-like CMs. *P < 0.05 compared with the control condition by repeated-measures ANOVA with Bonferroni correction.
Moderate-throughput electrophysiological analysis of human iPSC-CMs.
After confirmation of the precision and stability of the change in fluorescence signals using di-4-ANBDQBS, we applied this technique to a moderate-throughput electrophysiological analysis of human iPSC-CMs. We compared AP properties in CMs differentiated from two distinct iPS cell lines derived from a single patient (75 cells from cell line 1 and 134 cells from cell line 6 described in Ref. 12). CL, APD10, APD50, and APD90 were not different between CM differentiated from distinct iPS cell lines from the same individual (Fig. 6A). Given that optical AP parameters were not significantly different, we pooled together all parameters to see if any measures might allow us to distinguish subsets of CMs. The distribution of APD10 values was best fit to a Gaussian function with a single peak at 45 ms (Fig. 6B). In contrast, APD50 values were best fit to a Gaussian function with two distinct peaks at 100 and 160 ms (Fig. 6B). The distinct peaks were likely generated by two subpopulations of CMs that represent atrial- and ventricular-like morphologies. Cells with APD50 < 130 ms displayed AP morphology typical of atrial cells, including a triangular downslope, whereas APD50 >145 ms identified cells with a plateau phase typical of ventricular cells. Cells with APD50 in the 130- to 145-ms range (∼10% of cells studied) displayed intermediate AP morphologies that were more difficult to describe as purely atrial or ventricular like. Unlike APD50, the distribution of APD90 values was best fit with to a single Gaussian function. This observation suggests that APD90 measurement alone appears insufficient to discriminate between different subgroups of CMs.
Fig. 6.
Moderate-throughput analysis of human iPSC-CMs using the voltage-sensitive dye di-4-ANBDQBS. A: box plots comparing optical AP values measured from CMs differentiated from two distinct iPSC lines obtained from the same individual. There were no statistical differences in the measured parameters between the two cell lines. Small squares show means, horizontal lines show medians, large squares show SEs, error bars show SDs, and hatch marks are ±99% confidence intervals. n = 75 cells from cell line 1 (black) and 134 cells from cell line 6 (red) described in Ref. 12. B and C: histograms displaying pooled data for APD10, APD50, and APD90 measured from optical APs. Data were fit to either a single or double Gaussian function. APD10 and APD90 distributions were best fit to a single Gaussian function (red traces). The dotted gray line represents APD90 values fit to a double Gaussian function. APD50 values were well fit to a double Gaussian function (red traces) and showed two clear peaks near 100 and 160 ms. The two populations of cells may represent atrial- and ventricular-like CMs, respectively. D: superimposed AP traces for APD50 values of <130 and >155 ms, demonstrating morphologies typical for atrial-like (triangular downstroke) and ventricular-like (plateau phase) CMs. Some of the optical AP traces were filtered (using a 40-point window Savitsky-Golay algorithm) to facilitate visual presentation of the data.
Fluorescence measures of activation, repolarization, and conduction velocity.
Multielectrode arrays allow for the determination of the conductive properties and heterogeneity of repolarization in human iPSC-CM monolayers (3–5, 7–11, 17). Because CMs often do not attach uniformly to the bottom of the multielectrode array chamber, we frequently observe variability in the quality of local electrograms. To assess the quality of optical AP signals to measure the temporal-spatial properties of activation and repolarization, we focused on cellular strands connecting clusters of contracting cells. A transmitted light image and fluorescence snapshot of a representative preparation are shown in Fig. 7, A and B, together with the optical AP signal recorded from a single pixel (Fig. 7C). The high-quality optical AP signal recorded from this representative pixel demonstrated a ventricular-like morphology, characterized by a distinct plateau phase. The activation and repolarization (APD90) maps are shown in Fig. 7, D and E, respectively. The activation profile (Fig. 7D) revealed a progression of activation along the length of the CM preparation. Repolarization was relatively homogeneous across the preparation, with APD90 values ranging from 240 to 284 ms. Ventricular-like optical APs were recorded from each pixel within the preparation. Conduction velocity across the preparation was calculated by measuring the time delay and distance between two regions of interest located at either side of the preparation (Fig. 7F). The mean conduction velocity was 4.6 ± 1.2 cm/s (n = 5 independent experiments). These values are similar to the conduction velocities we recently reported in PBMC-derived iPSC-CMs measured using multielectrode array recordings (12). These proof-of-principle experiments highlight the ability to directly measure repolarization (i.e., APD90) with the voltage-sensitive dye rather than rely on estimates of repolarization based on the local field potential.
Fig. 7.
Optical mapping of a large human iPSC-CM preparation. A: transmitted light image of a human iPSC-CM preparation used for optical mapping of excitation. This stimulating electrode is located out of the field of view beyond the top left corner of the image. B: snapshot of di-4-ANBDQBS fluorescence recorded from the same preparation shown in A with a ×25 oil-immersed objective lens. The white arrow shows the direction of propagation. The white/yellow squares indicate the location of two pixels (pixels #1 and #2) from which the fluorescent signals shown in C were recorded. C: fluorescent signals (F) recorded at pixel #1 (Fpixel #1) located within the preparation and pixel #2 (Fpixel #2) from a location devoid of excitable tissue. The inset shows a single representative optical AP (top) selected from Fpixel #1 and the temporal derivative of the fluorescent signal (bottom) used to measure timing of activation (see methods). D: activation time map constructed by plotting the color-coded activation time value for each individual optical AP in the mapped region. E: APD90 map constructed by plotting the color-coded APD90 value for each individual optical AP in the mapped region. F: overlay of AP upstrokes recorded from region of interest 1 (ROI 1; red) and region of interest 2 (ROI 2; black). Signals were inverted and normalized for an easier visualization. The expanded timescale highlights the activation delay between the optical AP upstrokes recorded at the proximal and distal sites of the paced preparation. In this preparation, the conduction velocity was ∼7 cm/s.
DISCUSSION
Advances in stem cell technology now allow for the routine derivation of patient- and disease-specific human iPSC-CM models of cardiovascular disease with broad implications ranging from drug discovery, preclinical drug screening, mechanistic understanding of disease processes, and approaches in personalized medicine. However, a major impediment to realizing the full potential of stem cell-based models is the labor-intensive nature of standard electrophysiological approaches. CMs differentiated from human iPSC are heterogeneous in nature, comprising nodal-, atrial-, and ventricular-like phenotypes, based on the morphology, duration, upstroke velocity, and MDP of their respective APs (4, 5, 7, 11, 17). Currently, the patch-clamp technique is the gold-standard method that allows for distinguishing chamber-specific lineages. Recently, the utility of a genetically encoded voltage-sensitive fluorescent reporter (ArcLight) was described in human embryonic stem cell-derived CMs (6). Using cutting-edge technology, Milan and colleagues successfully generated robust voltage-dependent fluorescent signals from single cells that allowed for high throughput cellular phenotyping and physiological analyses. These authors demonstrated the power of this methodology for the serial phenotyping of differentiating cardiomyocyte populations and for drug toxicity screening. However, the temporal response of ArcLight was delayed relative to directly measured Vm (6). Voltage-sensitive dyes, such as di-4-ANNEPS and di-8-ANNEPS, allow for high temporal resolution signals, but their inherent phototoxicity limits their use in in single cell applications (2). In this context, we studied the application of the near-infrared fluorescent voltage-sensitive dye di-4-ANBDQBS for moderate-throughput electrophysiological analyses in human iPSC-CMs.
Optical APs recorded from human iPSC-CMs loaded with di-4-ANBDQBS closely tracked APs measured by the disrupted patch technique. Values of APD derived from optical APs were nearly identical to directly measured Vm. The tight correlation between optical AP and Vm signals confirm the robust temporal resolution of di-4-ANBDQBS to track rapid changes in membrane potential. Moreover, optical AP recordings in di-4-ANBDQBS-labeled human iPSC-CMs were stable over time and relatively resistant to photodynamic damage under these experimental conditions. Taken together, our data demonstrate that the voltage-sensitive dye di-4-ANBDQBS allows for high-precision optical AP measurements that markedly increase the throughput for electrophysiological characterization of human iPSC-CMs.
There are several limitations inherent in any fluorescent methodology that measures optical APs. First, there is no mechanism to measure absolute voltage, and, thus, fluorescent reporters cannot measure the maximum diastolic membrane potential, an important feature that distinguishes nodal-, atrial-, and ventricular-like iPSC-CMs. Likewise, as fluorescent units are arbitrary, one cannot measure the AP upstroke velocity, an additional parameter that discriminates between subtypes of iPSC-CMs. Despite these caveats, optical APs could be resolved from di-4-ANBDQBS-loaded single cells with sufficient signal-to-noise ratio to allow for discrimination between atrial- and ventricular-like CMs. The distribution of APD50 values revealed two distinct peaks that allowed for the qualitative discrimination between atrial-like and ventricular-like cellular phenotypes. We could not precisely distinguish between nodal- and atrial-like CMs. Nodal-like iPSC-CMs generally represent the smallest fraction (∼10%) of stem cell-derived CM subgroups (4, 5, 7, 11, 17).
Human iPSC-based CM platforms hold great promise to advance our understanding of arrhythmia mechanisms, to improve the drug discovery processes and to evaluate the safety of drugs in development. However, proper measurement of repolarization is critical for the accurate determination of risk assessment and a mechanistic understanding of arrhythmia susceptibility. Noninvasive electrophysiological assessment of human iPSC-CMs will aid in achieving the full potential of stem cell-based platforms. To that end, the voltage-sensitive dye di-4-ANBDQBS represents an alternative screening tool for electrophysiological characterization of human iPSC-CMs, by allowing for moderate-throughput high precision optical AP measurements.
GRANTS
This work was supported by the Nora Eccles Treadwell Foundation (to M. Tristani-Firouzi, K. W. Spitzer, and M. Warren), the American Heart Association (to C. J. Jou), National Heart, Lung, and Blood Institute (NHLBI) 2009 National Institutes of Health Director's Pioneer Award 7-DP1-HL-117650-05 (to I. J. Benjamin), Veterans Affairs Merit Review Award (to I. J. Benjamin), NHLBI Grant 5-R01-HL-074370-04 (to I. J. Benjamin), and NHLBI Grant R37-HL-042873-24 (to K. W. Spitzer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.L.-I., M.W., M.R., S.C., S.L., K.W.S., M.T.-F., and C.J.J. performed experiments; A.L.-I., M.W., R.L.L., M.T.-F., and C.J.J. analyzed data; A.L.-I., M.W., R.L.L., K.W.S., I.J.B., M.T.-F., and C.J.J. interpreted results of experiments; A.L.-I., M.W., and M.T.-F. prepared figures; A.L.-I., M.W., M.R., S.C., S.L., R.L.L., K.W.S., I.J.B., M.T.-F., and C.J.J. edited and revised manuscript; A.L.-I., M.W., M.R., S.C., S.L., R.L.L., K.W.S., I.J.B., M.T.-F., and C.J.J. approved final version of manuscript; M.W., M.R., S.C., R.L.L., K.W.S., I.J.B., M.T.-F., and C.J.J. conception and design of research; M.T.-F. and C.J.J. drafted manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Leslie M. Loew (University of Connecticut Health Center), who kindly provided the di-4-ANBDQBS.
REFERENCES
- 1.Fluhler E, Burnham VG, Loew LM. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry 24: 5749–5755, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Herron TJ, Lee P, Jalife J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ Res 110: 609–623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itzhaki I, Maizels L, Huber I, Gepstein A, Arbel G, Caspi O, Miller L, Belhassen B, Nof E, Glikson M, Gepstein L. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J Am Coll Cardiol 60: 990–1000, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471: 225–229, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Lahti AL, Kujala VJ, Chapman H, Koivisto AP, Pekkanen-Mattila M, Kerkela E, Hyttinen J, Kontula K, Swan H, Conklin BR, Yamanaka S, Silvennoinen O, Aalto-Setala K. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis Models Mech 5: 220–230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leyton-Mange JS, Mills RW, Macri VS, Jang MY, Butte FN, Ellinor PT, Milan DJ. Rapid Cellular phenotyping of human pluripotent stem cell-derived cardiomyocytes using a genetically encoded fluorescent voltage sensor. Stem Cell Rep 2: 163–170, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC, Wu JC. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation 127: 1677–1691, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malan D, Friedrichs S, Fleischmann BK, Sasse P. Cardiomyocytes obtained from induced pluripotent stem cells with long-QT syndrome 3 recapitulate typical disease-specific features in vitro. Circ Res 109: 841–847, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Matsa E, Rajamohan D, Dick E, Young L, Mellor I, Staniforth A, Denning C. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. Eur Heart J 32: 952–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, Seyfarth M, Sinnecker D, Schomig A, Laugwitz KL. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 363: 1397–1409, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Navarrete EG, Liang P, Lan F, Sanchez-Freire V, Simmons C, Gong T, Sharma A, Burridge PW, Patlolla B, Lee AS, Wu H, Beygui RE, Wu SM, Robbins RC, Bers DM, Wu JC. Screening drug-induced arrhythmia events using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays. Circulation 128: S3–S13, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riedel M, Jou CJ, Lai S, Lux RL, Moreno AP, Spitzer KW, Christians E, Tristani-Firouzi M, Benjamin IJ. Functional and pharmacological analysis of cardiomyocytes differentiated from human peripheral blood mononuclear-derived pluripotent stem cells. Stem Cell Rep 3: 131–141, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaffer P, Ahammer H, Muller W, Koidl B, Windisch H. Di-4-ANEPPS causes photodynamic damage to isolated cardiomyocytes. Pflügers Arch 426: 548–551, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Sommer AG, Rozelle SS, Sullivan S, Mills JA, Park SM, Smith BW, Iyer AM, French DL, Kotton DN, Gadue P, Murphy GJ, Mostoslavsky G. Generation of human induced pluripotent stem cells from peripheral blood using the STEMCCA lentiviral vector. J Vis Exp 2012: 4327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell 7: 20–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren M, Spitzer KW, Steadman BW, Rees TD, Venable P, Taylor T, Shibayama J, Yan P, Wuskell JP, Loew LM, Zaitsev AV. High-precision recording of the action potential in isolated cardiomyocytes using the near-infrared fluorescent dye di-4-ANBDQBS. Am J Physiol Heart Circ Physiol 299: H1271–H1281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471: 230–234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res 111: 1125–1136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, Xu Y, Cao H, Meng Q, Chen L, Tian T, Wang X, Li P, Hescheler J, Ji G, Ma Y. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res 21: 579–587, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]