Abstract
In this study, we provide the first comprehensive annotation of canine interferon-λ (CaIFN-λ, type III IFN). Phylogenetic analysis based on genomic sequences indicated that CaIFN-λ is located in the same branch with Swine IFN-λ1 (SwIFN-λ), Bat IFN-λ1 (BaIFN-λ), and human IFN-λ1 (HuIFN-λ1). CaIFN-λ was cloned, expressed in Escherichia coli, and purified to further investigate the biological activity in vitro. The recombinant CaIFN-λ (rCaIFN-λ) displayed potent antiviral activity on both homologous and heterologous animal cells in terms of inhibiting the replication of the New Jersey serotype of vesicular stomatitis virus (VSV), canine parvovirus, and influenza virus A/WSN/33 (H1N1), respectively. In addition, we also found that rCaIFN-λ exhibits a significant antiproliferative response against A72 canine tumor cells and MDCK cells in a dose-dependent manner. Furthermore, CaIFN-λ activated the JAK-STAT signaling pathway. To evaluate the expression of CaIFN-λ induced by virus and the expression of IFN-stimulated genes (ISGs) induced by rCaIFN-λ in the MDCK cells, we measured the relative mRNA level of CaIFN-λ and ISGs (ISG15, Mx1, and 2′5′-OAS) by quantitative real-time PCR and found that the mRNA level of CaIFN-λ and the ISGs significantly increased after treating the MDCK cells with viruses and rCaIFN-λ protein, respectively. Finally, to evaluate the binding activity of rCaIFN-λ to its receptor, we expressed the extracellular domain of the canine IFN-λ receptor 1 (CaIFN-λR1-EC) and determined the binding activity via ELISA. Our results demonstrated that rCaIFN-λ bound tightly to recombinant CaIFN-λR1-EC (rCaIFN-λR1-EC).
Introduction
Interferons (IFNs) were first discovered for their ability to interfere with influenza virus replication and are now recognized as the first line of defense against viral infections and have important roles in increasing the lyticpotential of natural killer (NK) cells and immune surveillance for malignant cells (Lindenmann 1982; Biron 1998; Chill and others 2003; Dupuis and others 2003; Ank and others 2006; Tezuka and others 2012; Li and others 2013). IFNs play a critical role in modulating the innate and adaptive immune systems, and there are 3 types of IFNs (types I, II, and III) distinguished based on their receptor complex (Smith and others 2005). Type I IFNs include α, β, ω, κ, ɛ, τ, ζ and δ subtypes, which were well identified and characterized. Some of subtypes, including IFN-α and IFN-β, play an important role in innate and adaptive immune defenses against viral replication and in immune cell maturation (Biron 1998; Le Bon and others 2003), but some subtypes of type I IFN, including IFN-δ and IFN-τ, are not virally inducible and produced constitutively by embryonic trophectoderm and involved in maternal recognition during pregnancy and allow the pregnancy to become established (Roberts and others 1999; Demmers and others 2001). The IFN-δ has been identified in some hoofed animals, including porcine, sheep, and horse, while IFN-τ was only found in ruminants (Lefevre and others 1998; Cochet and others 2009). The IFN-ω shows a high activity against many virus infections and are found in human, pig, cat, and rabbit (Hauptmann and Swetly 1985; Charlier and others 1993; Yang and others 2007; Zhao and others 2009). The type I IFNs have been exploited for the systemic treatment of human HIV, hepatitis B and C infection (Levin and others 1982; Manion and others 2012), but beyond that, type I IFNs play an important role in livestock farming, and IFN-α is well studied in pigs and used as a powerful adjuvant for a recombinant protein vaccine against foot-and-mouth disease virus in swine (Cheng and others 2006, 2007a, 2007b). As to type II and type III IFNs, both have one member each, IFN-γ and IFN-λ, respectively.
Type III IFNs were first identified and referred to as IFN-λ1, IFN-λ2, and IFN-λ3 (also known as IL-29, IL-28a, and IL-28b) in 2002 by 2 independent groups (Kotenko and others 2002; Sheppard and others 2003). Both groups also reported the novel receptor, IFN-λR1 (also known asIL-28RA), through which (upon interaction with IL-10R2) type III IFNs mediate their biological activities. Type I and type III IFNs represent the first line of defense against viral infection in mammals, and both of them share many biological activities. For example, type III IFNs are directly induced in response to viral infection and have similar mechanisms of activating the JAK-STATs and MAPK signaling pathways, and antiproliferative and antitumor strategies, though they are not highly homologous (only 15%–20% amino acid identity) to type I IFN (Ank and others 2006, 2008; Zitzmann and others 2006; Iversen and Paludan 2010; Kotenko 2011; Zhou and others 2011a). In contrast to type I IFNs, the type III IFN's receptor complex includes IFN-λR1 and IL-10R2, while the type IIFN's receptor complex is comprised of IFNAR1 and IFNAR2 (Sheppard and others 2003). Furthermore, the expression distribution of IFN-λR1 is different from IFNAR1 and IFNAR2, and the absence of IFN-λR1 in the central nervous system and bone marrow endows type III IFN treatments with less side effects in humans (Witte and others 2009). As type III IFNs display antiviral activities and the distribution of their receptor complex is distinct from the type I IFN receptor, IFN-λ has the potential for in vivo antiviral activity with fewer side effects than IFN-α (Friborg and others 2013). Several studies show that IFN-λ share the potential for treatment of chronic Hepatitis C infection (Pagliaccetti and Robek 2010; Thomas and others 2012; Friborg and others 2013). To date type III IFNs have been identified in humans, mice, chicken, cattle, bats, and canines (Kotenko and others 2002; Sheppard and others 2003; Lasfar and others 2006; Karpala and others 2008; Diaz-San Segundo and others 2011; Zhou and others 2011b). Numerous type III IFNs have also been identified in the publicly available whole-genome sequence data from a variety of other species (www.ncbi.nlm.nih.gov).
Recently, several canine IFNs have been identified: IFN-α, IFN-β, IFN-γ, IFN-ɛ, and IFN-κ (belonging to types I and II) (Himmler and others 1987; Devos and others 1992; Zucker and others 1992; Iwata and others 1996). One canine IFN-λ has been identified (Yang and others 2013) while its function remains unclear.
Here, phylogenetic analyses of type III IFNs indicated that canine IFN-λ is IFN-λ1. Canine IFN-α7 and canine IFN-λ were cloned and expressed in Escherichia coli. Recombinant canine IFN-λ (rCaIFN-λ) displayed both antiviral activity and antiproliferation activity. In addition, rCaIFN-λ induced increased expression of IFN-stimulated genes (ISGs) than the control. Additionally, rCaIFN-λ activated the JAK-STAT pathway. These results indicate that CaIFN-λ behaves like its homologs in other species and has the potential to be developed as a novel protein pharmaceutical.
Materials and Methods
Virus stocks and cells
The New Jersey serotype of Vesicular stomatitis virus (VSV) and the canine parvovirus were propagated in the Madin-Darby bovine kidney (MDBK) cell line (ATCC CCL-22). The influenza viruses A/WSN/33(H1N1) (H1N1-WSN), A/PR8/34(H1N1) (H1N1-PR8), and A/California/04/2009(H1N1) (H1N1-CA04) were propagated in 10-day-old SPF chicken embryonated eggs. The MDBK, MDCK (ATCC CCL-34), A72 canine tumor, and human amnion WISH (ATCC CCL-25) cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM; GIBICO) supplemented with 10% heat-inactivated fetal bovine serum (FBS; GIBICO).
Phylogenetic analyses of canine IFN-λ
To intensively study canine IFN-λ, 23 IFN sequences (Table 1) from 10 species acquired from NCBI (www.ncbi.nlm.nih.gov/) were used to perform an alignment using ClustalW (EBI, www.ebi.ac.uk/clustalw) (Thompson and others 2002). A phylogenetic tree of the newly discovered canine IFN-λ sequences and other publicly available representative IFN sequences was constructed using the MAGA program (version 5.0) and neighbor-joining analysis with the following parameters: method=NJ, substitution model=Poisson correction method, and 1000 bootstrap replicates (Tamura and others 2011).
Table 1.
Query Sequences Used for the Phylogenetic Analyses
| Species | Gene | Accession No. |
|---|---|---|
| Canine | IFN-α5 | NM_001006647.1 |
| Canine | IFN-α6 | NM_001007128.1 |
| Canine | IFN-α7 | NM_001006654.1 |
| Canine | IFN-α8 | NM_001007130.1 |
| Canine | IFN-β1 | NM_001135787.1 |
| Canine | IFN-ɛ | KC527684.1 |
| Canine | IFN-κ | KC754971.1 |
| Canine | IFN-γ | NM_001003174.1 |
| Canine | IFN-λ | KC754970.1 |
| Chicken | IFN-λ3 | NM_001128496.1 |
| Human | IFN-λ1 | NM_172140.1 |
| Human | IFN-λ2 | NM_172138.1 |
| Human | IFN-λ3 | NM_172139.2 |
| Machin | IFN-λ4 | KC525948.1 |
| Murine | IFN-λ2 | NM_001024673.2 |
| Murine | IFN-λ3 | NM_177396.1 |
| Swine | IFN-λ1 | NM_001142837.1 |
| Swine | IFN-λ3 | NM_001166490.1 |
| Baboon | IFN-λ4 | KC525947.1 |
| Chimpanzee | IFN-λ4 | NM_001276259.1 |
| Bat | IFN-λ1 | HQ201956.1 |
| Bat | IFN-λ2 | HQ201955.1 |
| Silurana | IFN-λ3 | NM_001171766.1 |
Construction of recombinant expression plasmids
The construction of recombinant expression plasmids for CaIFNs and the extracellular domain of CaIFN-λR1 (CaIFN-λR1-EC) was performed as previously described (Yang and others 2013) based on the gene sequences of rCaIFN-λ (ATCC: KC754970.1), CaIFN-α7 (ATCC:AB125936.1), and CaIFN-λR1 (ATCC: XM_850017.3). The CaIFN-λR1-EC sequence was compared and selected according to the known extracellular domain of HuIFN-λR1. Briefly, PCR products encoding the mature CaIFNs and CaIFN-λR1-EC protein were cloned into the pET21a vector (Novagen) to generate the recombinant plasmids pET21a/CaIFNs and pET21a/CaIFN-λR1-EC; sequences were verified by sequencing. In addition, a termination codon was added ahead of the 6-His sequence in pET21a/CaIFN-λR1-EC to generate CaIFN-λR1-EC protein without a 6-His tag, while CaIFNs had a 6-His tag.
Expression and purification of rCaIFN-λ, CaIFN-α7, and CaIFN-λR1-EC
Protein expression and purification was performed as previously described (Yang and others 2013). Briefly, the recombinant plasmids were transformed into E. coli strain BL21 (DE3). Cells were grown in LB medium, and recombinant protein expression was induced with IPTG. The cells were collected by centrifugation, and the precipitation was disrupted by ultrasonication. The CaIFNs and CaIFN-λR1-EC purifications were performed using nickel-chelating Sepharose (GE Healthcare) and cation-exchange columns (GE Life), respectively. Then, all of the purified proteins were fractionated by gel filtration on a Superdex-200 column (GE Healthcare) according to the supplier's instructions. The purity of the proteins was analyzed by 12% SDS-PAGE, and their concentrations were assessed using a BCA protein assay kit (CW Bio) according to the supplier's instructions.
ELISA for the binding of rCaIFN-λ to CaIFN-λR1-EC
To analyze the binding activity of rCaIFN-λ to CaIFN-λR1-EC, ELISAs were performed as previously described (Xue and others 2010). Briefly, 1 μg rCaIFN-λ was added to wells that had been coated with rCaIFN-λR1-EC protein (500 ng/well) overnight at 4°C and then incubated at 37°C for 1 h, and washed with PBST (0.2% Tween) 6 times. The wells were then incubated with rabbit anti-His-tag antibody at 37°C for 1 h and washed 4 times. HRP-labeled secondary antibody (1:10,000) was added for 1 h at 37°C and washed 4 times. The color was developed using a mixture of TMB (tetramethylbenzidine) and H2O2, and the reaction was stopped using 50 μL 1 M H2SO4. Absorbance values were measured in an ELISA plate reader at 450 nm. The rCaIFN-α7 protein and BSA were used as control. All experiments were performed in triplicate.
Antiviral activities assay of rCaIFN-λ in vitro
The antiviral activities of rCaIFN-λ and rCaIFN-α7 were determined via the cytopathic effect (CPE) inhibition method based on the VSV/MDCK, VSV/MDBK, VSV/WISH, CPV/MDCK, and WSN/MDCK systems according to previously described protocols (Armstrong 1971; Taira and others 2005). Briefly, cells were cultured in 96-well plates until they reached monolayer status at 37°C in humid air with 5% CO2. Then, the cells were washed and stimulated with 100 μL of 4 fold serial dilutions of rCaIFN-λ for 12 h, and the cells were then challenged with 100 TCID50 viruses per well and cultured until the CPE of virus-infected cells without rCaIFN-λ treatment appeared. Cultures were stained with crystal violet. The rCaIFNs titers (U/mg) are expressed as the reciprocal of the dilutions that led to 50% virus-induced cell lysis by the Reed–Muench method.
Antiproliferation activity assay of rCaIFN-λ in canine cell lines
The antiproliferation activity of rCaIFN-λ and rCaIFN-α7 in the MDCK and A72 cell lines was measured as previously described (Loveland and others 1992; Tsang and others 2007). Briefly, MDCK and A72 cells were cultured in 96-well plates with DMEM containing 10% bovine serum at 37°C in humid air with 5% CO2 until the cells reached monolayer status. A dilution series of rCaIFN-λ was then added to the cells, which were incubated for 72 h. Then, 20 μL (5 mg/mL) MTT solution was added to each well and mixed gently. The cells were further incubated for 4 h, and DMSO was added to each well to elute the dye after the media was removed by aspiration. The color intensity at 490 nm was measured using an ELISA plate reader. The data were reported as the percentage of growth, which was calculated as (OD of sample/OD of mock)×100%.
JAK-STAT pathway activation by rCaIFN-λ
The ability of rCaIFN-λ and rCaIFN-α7 to activate the JAK-STAT signal pathway was measured by the IFN-stimulated response element (ISRE)-luciferase assay, as previously described with some modifications (Schindler and Darnell 1995; Zhao and others 2012). Briefly, the MDCK cells were cultured in 24-well plates with DMEM containing 10% bovine serum at 37°C in humid air with 5% CO2 until the cells reached monolayer status. Then, the cells were transfected with 250 ng of ISRE-luc plasmids containing 50 ng of the reference Renilla luciferase reporter vector pRL-TK (Promega) using Lipofectamine 2000 (Invitrogen). Following incubation at 37°C for 24 h, 10U rCaIFN-λ (the antiviral units of rCaIFN were determined according to the anti-VSV activity at 12 h post-IFN treatment) was added to each well and gently mixed prior to incubation for 6 h. Then, the cells were lysed in Reporter Lysis Buffer (Promega), and luciferase activity was measured. All tests were performed in triplicate. The results are reported as the relative luciferase activity presented as the ratio of the firefly luciferase activity to the renilla luciferase activity.
Real-time quantitative PCR assay of virus-induced rCaIFNs and rCaIFNs-induced ISGs in MDCK cells
A real-time quantitative PCR assay was used to quantify the expression levels of CaIFN-λ and CaIFN-α7 induced by virus at different time points. Briefly, MDCK cells were infected with VSV, H1N1-WSN strain, H1N1-PR8 strain, or H1N1-CA04 strain at 0.01 m.o.i. in 12-well plates, and the cell cultures were collected at 2, 4, 8, and 12 h after infection for RNA isolation. To evaluate the mRNA levels of chosen ISGs at different time points after rCaIFNs treatment, MDCK cells were treated with 100 U/mL rCaIFN-λ and rCaIFN-α7 for the indicated durations. The antiviral units of rCaIFN-λ and rCaIFN-α7 were determined according to the anti-VSV activity at 12 h post-IFN treatment. Cell samples were collected at 2, 4, 8, 12, and 24 h post rCaIFNs treatment for RNA extraction. Untreated MDCK cells were collected at 0 h as a calibrator to evaluate the mRNA levels of rCaIFNs and the chosen ISGs. β-actin was used as a housekeeping gene for sample normalization.
Total RNA was extracted from the MDCK cells with anRNA isolation kit (Qiagen) following the manufacturer's instructions. qRT-PCR was performed using an ABI-Prism 65H0 sequence detection system and SYBR green PCR master mix (Newpep), as previously described (Qu and others 2013). Samples were analyzed in triplicate. Levels of mRNA were calculated using the 2–ΔΔCt method, which expresses mRNA in treated cells relative to mock-infected cells after normalizing to β-actin. The primers for qRT-PCR are listed in Table 2.
Table 2.
Primer Sequence Used in This Study
| Gene | Primer name | Sequence 5′-3′ |
|---|---|---|
| OAS | OAS-forward | TTCACATCATCTCCACTT |
| OAS-reverse | ACCCTTGACAACTTTAGA | |
| ISG15 | ISG15-forward | TCTGTGCCCCTGGAGGACTTGA |
| ISG15-reverse | TGCTGCTTCAGCTCTGATGCCA | |
| Mx1 | Mx1-forward | GAATCCTGTACCCAATCATGTG |
| Mx1-reverse | TACCTTCTCCTCATATTGGCT | |
| β-actin | β-actin-forward | CGAGACATTCAACACCCCAAC |
| β-actin-reverse | AGCCAGGTCCAGACGCAAG | |
| CaIFN-λ | CaIFN-λ-forward | GTTCCAGTCTCTGTCACC |
| CaIFN-λ-reverse | CCAGTTCTTCCAGGAGAG | |
| CaIFN-α7 | CaIFN-α7-forward | GAGATGGTCCGAGCAGAA |
| CaIFN-α7-reverse | TCATTCCTTCCTCCTGATTCT |
Results
Phylogenetic analysis shows that CaIFN-λ is related to IFN-λ1
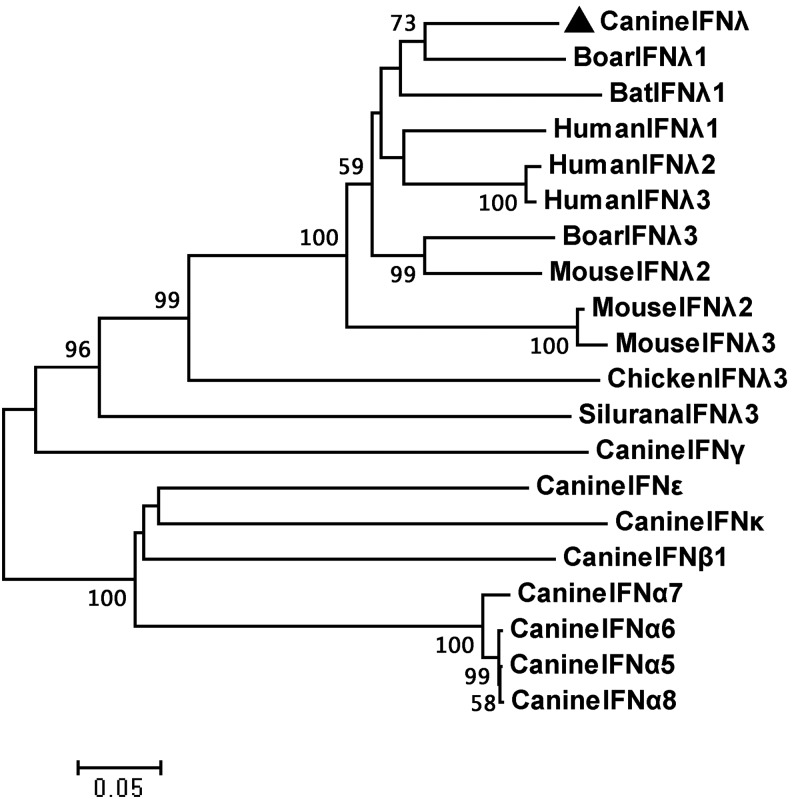
To gain insight into the canine IFN-λ, BLASTn was performed using the IFN-λ sequences from different species in GenBank, and a phylogenetic tree was constructed using the MAGA program and neighbor-joining analysis method based on the DNA sequences. The nucleic acid sequence analysis revealed that the newly found CaIFN-λ had high similarity to swine IFN-λ1 (SwIFN-λ1), human IFN-λ1 (HuIFN-λ1), and bat IFN-λ1 (BaIFN-λ1), the sequence identity values were 82.7%, 80.5%, and 75.9% respectively. The amino acid sequence analysis results showed that CaIFN-λ also has high identity to SwIFN-λ1, HuIFN-λ1, and BaIFN-λ1, the values were 77.4%, 73.2%, and 70% respectively (Table 3). The CaIFN-λ, SwIFN-λ1, BaIFN-λ1, and HuIFN-λ1 genes formed a phylogenetic cluster, and the CaIFN-λ gene displayed the highest similarity to SwIFN-λ1 in the model tree (Fig. 1).
Table 3.
Homology (%) of Nucleotides (Top Line) and Amino Acids (Bottom Line) Among Each Canine Interferon Subtype and Other Reported IFNE-λs
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | *** | 5.6 | 8 | 9.3 | 9.3 | 17.2 | 9.3 | 3.3 | 3.3 | 3.8 | 4.8 | 11.5 | 8.1 | 94.4 | 5.2 | 12.4 | 14.4 | 99.4 | 10.2 | 9.8 | 9.4 | 10 | 7 | BaboIFN-λ4 |
| 2 | 21.8 | *** | 72.9 | 3 | 3 | 18.2 | 3 | 2.3 | 4.8 | 6.4 | 6.6 | 75.9 | 46.9 | 6.9 | 76.7 | 74.8 | 74.7 | 6.5 | 68.4 | 66.7 | 11.1 | 75.5 | 72.7 | BatIFN-λ1 |
| 3 | 25.1 | 66 | *** | 6.9 | 4.1 | 18 | 6.9 | 3.9 | 2.8 | 7.2 | 3.6 | 77.8 | 52.6 | 8.5 | 71.3 | 80.1 | 79.4 | 8.1 | 72.5 | 70.3 | 7.8 | 74 | 80.1 | BatIFN-λ2 |
| 4 | 7.8 | 9.1 | 6.4 | *** | 99.6 | 96.3 | 99.6 | 43.1 | 35.6 | 3.6 | 13.8 | 5.7 | 12.3 | 9.1 | 3 | 3 | 3.2 | 9.6 | 3 | 3 | 2.8 | 5.3 | 5.1 | CaIFN-α5 |
| 5 | 7.8 | 9.1 | 6.4 | 99.5 | *** | 96.3 | 99.6 | 44.6 | 35.5 | 3.6 | 13.8 | 5.9 | 5.9 | 9.1 | 3 | 3 | 3.2 | 9.6 | 3 | 3 | 2.8 | 5.3 | 5.1 | CaIFN-α6 |
| 6 | 7.8 | 9.1 | 3.2 | 93 | 93.6 | *** | 96.3 | 23.4 | 20.9 | 20.2 | 21 | 17.8 | 18.2 | 16.3 | 18.2 | 18.9 | 19.3 | 17.6 | 18.4 | 16.8 | 19.6 | 18.6 | 19.9 | CaIFN-α7 |
| 7 | 7.8 | 9.1 | 6.4 | 98.9 | 99.5 | 93 | *** | 44.4 | 35.5 | 3.6 | 13.8 | 8.7 | 5.9 | 9.1 | 3 | 3 | 3.2 | 9.6 | 3 | 3 | 2.8 | 5.3 | 5.1 | CaIFN-α8 |
| 8 | 6.7 | 5.9 | 5.4 | 26.9 | 26.9 | 22 | 26.3 | *** | 40.6 | 3.6 | 14.6 | 2.1 | 4.1 | 3.7 | 3.2 | 2.1 | 3.9 | 3.3 | 2.9 | 2.9 | 5 | 2 | 3.2 | CaIFN-β1 |
| 9 | 1.7 | 5.9 | 5.3 | 27.8 | 27.8 | 28.3 | 27.3 | 32.3 | *** | 3 | 11.3 | 4.3 | 7.5 | 2.6 | 2.7 | 2.7 | 2.7 | 3.5 | 2.8 | 6.9 | 5.6 | 2.5 | 2.8 | CaIFN-ζ |
| 10 | 2.4 | 11.4 | 3 | 10.2 | 10.2 | 10.8 | 10.2 | 7.2 | 4.2 | *** | 4.2 | 4.2 | 5.8 | 3 | 6.4 | 4.6 | 4.6 | 3.8 | 9.2 | 9 | 2.4 | 6.2 | 8.4 | CaIFN-γ |
| 11 | 4.5 | 4.2 | 3.6 | 24.6 | 24.6 | 25.1 | 24.6 | 26.9 | 31.6 | 12.7 | *** | 2.3 | 3.9 | 5.4 | 2.3 | 6.5 | 6.4 | 4.8 | 6.4 | 4.3 | 4.6 | 3.6 | 6 | CaIFN-κ |
| 12 | 24 | 70 | 69.5 | 4.8 | 4.8 | 4.8 | 4.8 | 5.9 | 5.9 | 10.2 | 4.2 | *** | 48.5 | 8.3 | 80.6 | 77.8 | 76.4 | 11.9 | 70.9 | 69.1 | 8.5 | 82.7 | 77.1 | CaIFN-λ |
| 13 | 17.3 | 31.7 | 33.3 | 4.8 | 4.8 | 4.8 | 5.4 | 7.5 | 5.9 | 4.8 | 5.9 | 35.5 | *** | 7.8 | 45.3 | 49 | 49.4 | 8 | 15.2 | 11.2 | 8.1 | 49.9 | 50.4 | ChIFN-λ3 |
| 14 | 91.6 | 22.9 | 26.8 | 7.3 | 7.3 | 6.7 | 7.3 | 6.1 | 1.7 | 6.6 | 5 | 25.7 | 21.8 | *** | 10.6 | 10.9 | 10.9 | 94.8 | 10.9 | 10 | 3.1 | 7.8 | 7.6 | ChimpIFN-λ4 |
| 15 | 24.6 | 67.5 | 63.6 | 5.3 | 5.3 | 8 | 5.3 | 5.4 | 4.3 | 6.6 | 7 | 73.2 | 29.6 | 25.7 | *** | 75.8 | 77.3 | 10 | 67.2 | 64.9 | 5.6 | 79 | 73.5 | HuIFN-λ1 |
| 16 | 22.3 | 63.4 | 68.7 | 9.1 | 9.1 | 13.4 | 9.1 | 1.6 | 8 | 8.4 | 2.5 | 64.7 | 31.7 | 22.3 | 69.5 | *** | 98.1 | 12.8 | 75.6 | 73.2 | 11.5 | 73.4 | 77.6 | HuIFN-λ2 |
| 17 | 22.3 | 64.9 | 69.2 | 9.6 | 9.6 | 13.4 | 9.6 | 8.1 | 14.4 | 9 | 3.1 | 67.9 | 32.8 | 22.3 | 71.9 | 95.9 | *** | 14.8 | 75.9 | 73.5 | 11.5 | 74 | 78.9 | HuIFN-λ3 |
| 18 | 98.9 | 22.3 | 25.1 | 7.3 | 7.3 | 6.7 | 7.3 | 6.7 | 7.3 | 2.4 | 5 | 24 | 17.3 | 92.7 | 24 | 23.5 | 23.5 | *** | 10.4 | 9.8 | 9.4 | 8.7 | 7.2 | MaIFN-λ4 |
| 19 | 21.8 | 56 | 57.5 | 6.4 | 6.4 | 9.1 | 15 | 3.2 | 11.2 | 10.8 | 4.7 | 57.4 | 32.3 | 22.3 | 56.5 | 64.2 | 64.8 | 21.8 | *** | 97.1 | 7.2 | 68.4 | 76.3 | MuIFN-λ2 |
| 20 | 20.1 | 53.4 | 53.9 | 10.2 | 10.2 | 10.2 | 10.2 | 2.2 | 10.7 | 9 | 2.6 | 53.7 | 30.6 | 20.1 | 53.4 | 61.1 | 61.7 | 20.1 | 93.3 | *** | 7.2 | 66.8 | 74.4 | MuIFN-λ3 |
| 21 | 18.4 | 20.1 | 23.5 | 8.9 | 8.9 | 4.5 | 8.9 | 6.1 | 8.4 | 6.6 | 2.8 | 23.5 | 23.5 | 20.1 | 19 | 23.5 | 24.6 | 17.9 | 21.8 | 22.3 | *** | 7.2 | 5.2 | SiIFN-λ3 |
| 22 | 20.7 | 61.8 | 66.5 | 7.5 | 7.5 | 7 | 7.5 | 4.8 | 6.4 | 7.2 | 3.1 | 77.4 | 30.6 | 21.8 | 72.8 | 61.3 | 64.4 | 20.7 | 52.9 | 50.8 | 21.2 | *** | 74.1 | SwIFN-λ1 |
| 23 | 24 | 60.7 | 70.3 | 7 | 7 | 7 | 7 | 4.3 | 9.1 | 7.2 | 5.1 | 65.8 | 30.6 | 25.7 | 60 | 68.7 | 70.3 | 24 | 61.7 | 58.5 | 20.7 | 60.2 | *** | SwIFN-λ3 |
Same sequence.
FIG. 1.
Phylogenetic tree analysis of several IFNs in canine and other species. The newly identified CaIFN-λ was labeled with black triangle. The phylogenetic tree was generated with the MAGA program (version 5.0) using the neighbor-joining method.
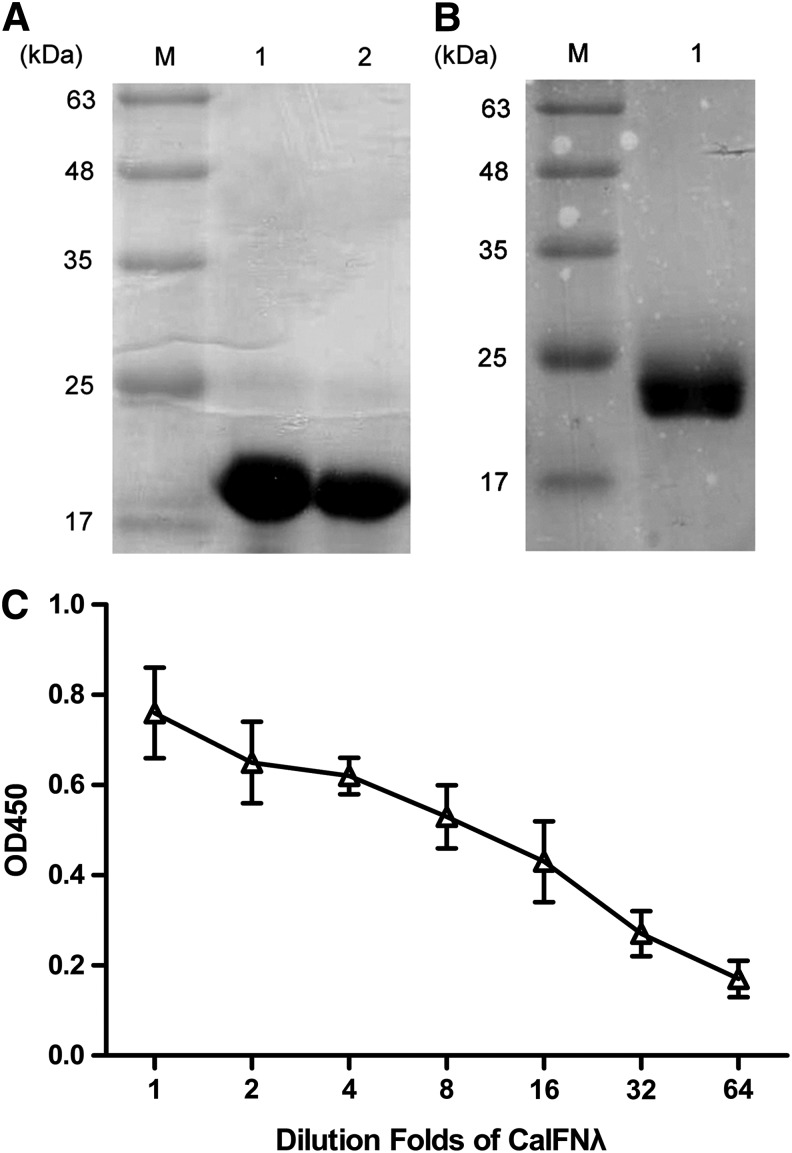
Expression and purification of CaIFNs and CaIFN-λR1-EC in E. coli and rCaIFN-λ binds to CaIFN-λR1-EC in vitro
The expression and purification of rCaIFNs and CaIFN-λR1-EC were performed as described previously (Yang and others 2013). The purity of the rCaIFNs and CaIFN-λR1-EC protein was ∼98% as judged by SDS-PAGE assessment (Fig. 2A, B). The rCaIFN-α7, rCaIFN-λ, and CaIFN-λR1-EC proteins also migrated at their expected sizes: ∼20, 20, and 24 kDa, respectively.
FIG. 2.
SDS-PAGE analysis of purified rCaIFNs expressed in Escherichia coli. BL21(DE3) and the binding activity of rCaIFN-λ to rCaIFN-λR1-EC in vitro according to ELISA assay. Lane M, molecular weight markers in kDa; lane 1 (A), lane 2 (A), lane 1 (B) represent purified rCaIFN-λ, rCaIFN-α7, and rCaIFN-λR1-EC respectively. The rCaIFN-λ was added in the wells coated with rCaIFN-λR1-EC, then the rabbit anti-His-tag antibody was added to detect the rCaIFN-λ (6 his-tag) that binding to rCaIFN-λR1-EC (C). Data are mean (n=3)±SEM.
ELISAs were used to evaluate the binding activity of rCaIFN-λ to rCaIFN-λR1-EC. The extra cellular domain of the CaIFN-λR1 protein was expressed in E. coli strain BL21 (DE3), and then excess purified CaIFN-λR1-EC was used to coat the wells of a plate for ELISAs. We found that the signal intensity was directly proportional to the concentration of rCaIFN-λ protein added to the wells, indicating that rCaIFN-λ bound to CaIFN-λR1-EC (Fig. 2C).
rCaIFN-λ has a wide range of antiviral activity
The purified rCaIFN-λ protein produced in E. coli was tested via the CPE inhibition assay using VSV/MDCK, VSV/MDBK, VSV/WISH, CPV/MDCK, and H1N1-WSN strain/MDCK systems. The rCaIFN-λ protein demonstrated unequal biological activities in different systems, such as higher antiviral activity in the VSV/MDCK and VSV/MDBK systems than other systems. The antiviral activity of rCaIFN-λ was more effective than rCaIFN-α7 on the CPV/MDCK and H1N1-WSN strain/MDCK systems but less active than rCaIFN-α7 on the VSV/MDCK and VSV/MDBK systems (Table 4).
Table 4.
Antiviral Activities of rCaIFN-λ and rCaIFN-α on Different Systems
| Antiviral activity (×106U/mg) on the cells | |||||
|---|---|---|---|---|---|
| CaIFN | VSV/MDCK | VSV/MDBK | VSV/WISH | CPV/MDCK | WSN/MDCK |
| IFN-λ | 0.55 | 0.056 | <0.00006 | 0.035 | 0.0012 |
| IFN-α | 198.10 | 10.1 | <0.00006 | 0.012 | 0.0006 |
Data are shown as the mean of 3 independent experiments. Data are mean (n=3)±SEM.
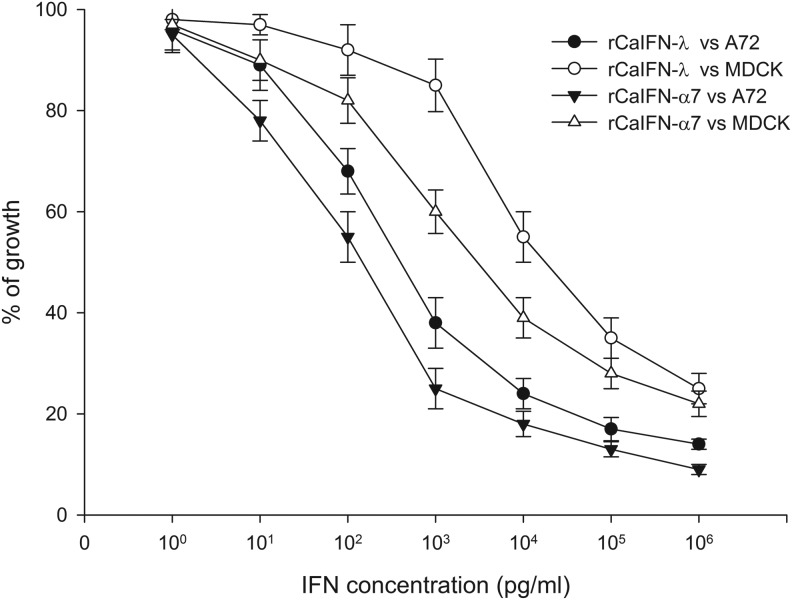
Assessment of antiproliferation activity on MDCK and A72 cell lines in vitro
The proliferation inhibition activities of rCaIFN-λ and rCaIFN-α7 were tested on the MDCK and A72 cell lines. As shown in Fig. 3, cell growth was inhibited by 50% when ∼600 pg/mL rCaIFN-λ or 220 pg/mL rCaIFN-α7 was added to A72 malignant tumor cells. For MDCK cells, ∼20,000 pg/mL rCaIFN-λ and 4500 pg/mL rCaIFN-α7 resulted in 50% cell growth inhibition, respectively. Both rCaIFN-λ and rCaIFN-α7 displayed significant proliferation inhibition activities in a dose-dependent manner, but the antiproliferation activity of rCaIFN-λ was lower than that of rCaIFN-α7 on both MDCK and A72 cell lines.
FIG. 3.
Comparison of the antiproliferation activity in 2 canine cell lines between rCaIFN-λ and rCaIFN-α7 produced in E. coli. BL21(DE3). Data are mean (n=3)±SEM.
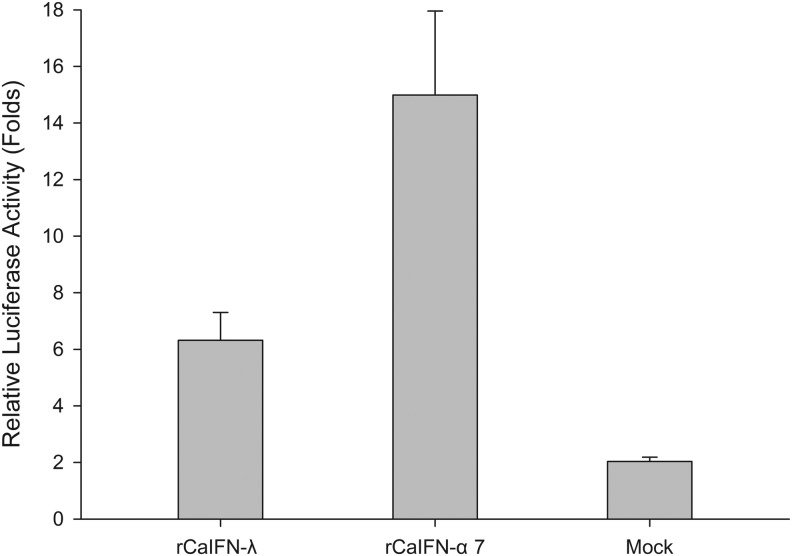
rCaIFN-λ activates the JAK-STAT signaling pathway
To determine whether rCaIFN-λ could activate the JAK-STAT pathway, rCaIFN-λ was incubated with MDCK cells transfected with ISRE-luc plasmids. Luciferase fluorescence was then determined using a luminometer. Comparing to the untreated group, the expression of luciferase were significantly induced in the rCaIFN-λ-treated and rCaIFN-α7-treated groups (Fig. 4), indicating that both rCaIFN-λ and rCaIFN-α7 activate the JAK-STAT signaling pathway in vitro. The potency of rCaIFN-α7 activation was ∼2 fold greater than that of rCaIFN-λ.
FIG. 4.
Luciferase reporter assay. Relative luciferase activity is presented as the ratio of the reporter plasmid and the reference Renilla luciferase reporter plasmid. Data are mean (n=3)±SEM.
CaIFN-λ expression can be induced in MDCK cells by several viruses
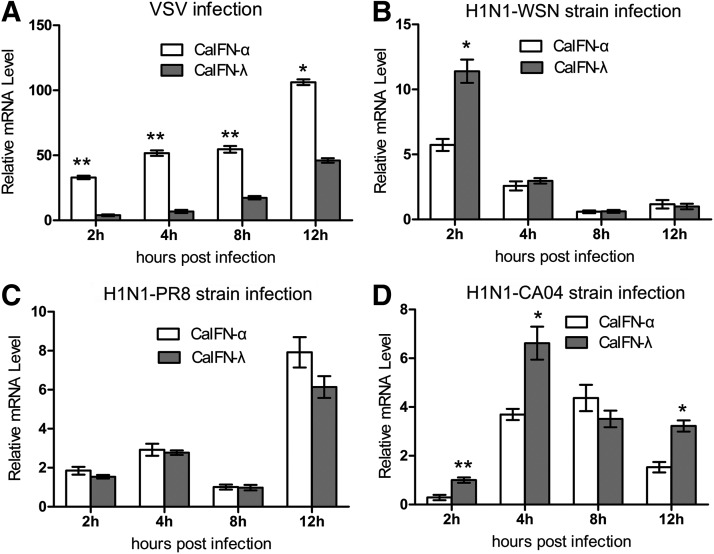
IFNs are produced in response to viral infection and induce an antiviral state in virtually all cell types. To determine whether CaIFN-λ is induced by viral infection in MDCK cells, real-time qPCR was used to measure CaIFN-λ mRNA expression. The relative mRNA production of CaIFN-λ and CaIFN-α7 were compared, and the results demonstrated that the expression of both CaIFN-λ and CaIFN-α7 was induced by VSV, H1N1-WSN strain, H1N1-PR8 strain, and H1N1-CA04 strain (Fig. 5).
FIG. 5.
Virus-induced expression of CaIFN-λ and CaIFN-α7. MDCK cells were infected with VSV (A), H1N1-WSN strain (B), H1N1-PR8 strain (H1N1) (C), and H1N1-CA04 strain (D) at 0.01 m.o.i. in 12-well plate. At the indicated time postinfection, cells were harvested for RNA isolation, and virus-induced expression of CaIFN-λ and CaIFN-α7 was assayed by real-time q-PCR. Data are mean (n=3)±SEM. A value of P<0.05 was considered statistically significant. *P<0.05 and **P<0.01. The asterisks were masked on the top of higher columns.
In VSV-infected cells, CaIFN-λ and CaIFN-α7 were induced to a higher level with rapid kinetics compared with that in influenza virus-infected cells. Both the CaIFN-λ and CaIFN-α7 expression induced by VSV reached a peak at 12 h p.i. In addition, the expression of CaIFN-λ was less than that of CaIFN-α7 (Fig. 5A). In all 3 influenza virus-infected groups, the expression of CaIFN-λ and CaIFN-α7 behaved differently from that of VSV-infected cells. The results of the influenza virus-infected groups indicated that expression of CaIFN-λ and CaIFN-α7 reached peak at different time points after infection by H1N1-WSN strain, H1N1-PR8 strain, and H1N1-CA04 strain, that is, 2, 12, and 4–8 h p.i., respectively (Fig. 5B, D). Furthermore, the expression of CaIFN-λ in the H1N1-WSN strain-infected and H1N1-CA04 strain-infected groups was higher than the expression of CaIFN-α7, while their expression levels were comparable in the H1N1-PR8 strain-infected group (Fig. 5C). The different expression behaviors of CaIFN-λ and CaIFN-α7 in the influenza virus-infected groups may be related to the different pathogenicity of these 3 influenza viruses.
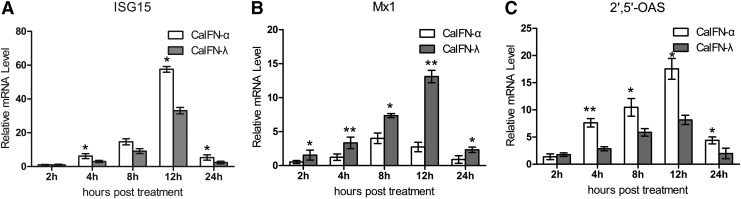
rCaIFN-λ induces the expression of ISGs in MDCK cells
To elucidate the effect of CaIFN-λ on the expression of ISGs, we used real-time qPCR to measure the mRNA expression of ISG15, Mx, and OAS in MDCK cells following a 12 h treatment with recombinant CaIFNs. ISG15 is an ubiquitin-like protein modifier, which is reversibly conjugated to different viral and cellular proteins, mediating considerable antiviral responses (Yanguez and others 2013). 2′,5′-OAS is also an important antiviral protein responsible for the IFN antiviral effect. We found that the ISG15 and 2′,5′-OAS mRNA levels stably increased during both rCaIFN-λ and rCaIFN-α7 treatments (Fig. 6A), reaching a peak at 12 h p.i. and then sharply declining at 24 h p.i. Comparing the rCaIFN-λ and rCaIFN-α7-treated groups, we found that the ISG15 and 2′,5′-OAS mRNA levels induced by rCaIFN-α7 were greater than those induced by rCaIFN-λ.
FIG. 6.
The expression of ISGS induced by rCaIFN-λ and rCaIFN-α7. After MDCK cells were incubated with rCaIFN-λ and rCaIFN-α7 in the same concentration (100 U/mL, there were 100 antiviral activity units of IFN in 1 mL DMEM, and the antiviral activities of rCaIFN-λ and rCaIFN-α7 were determined by the cytopathic effect inhibition assay using VSV on MDCK cells), the cells were harvested at the indicated times post-treatment for RNA extraction and cDNA preparation. The transcriptional levels of ISG15 (A), Mx1 (B), and 2′,5′-OAS (C) were assayed by real-time PCR. Data are mean (n=3)±SEM.
Mx1 protein was selected as an indicator induced by CaIFNs. Mx proteins are IFN-induced members of the dynamin superfamily of large GTPases, and both type I (α/β) and type III (λ) IFNs can induce the expression of Mx proteins. These proteins can inhibit a wide range of viruses by blocking an early stage of the replication cycle (Haller and others 2007). Some studies show that Mx1 protein can also inhibit the replication of influenza virus in mice (Staeheli and others 1986; Arnheiter and others 1996). Here, we found that the Mx mRNA level stably increased during treatment from 2 to12 h p.i. in both groups, followed by a sharp decline at 24 h p.i. (Fig. 6B). In contrast to the expression behavior of ISG15 and 2′5′-OAS, the Mx1 mRNA level induced by rCaIFN-λ was higher than that induced by rCaIFN-α7. As Mx1 protein can inhibit the replication of influenza virus, these results indicate that CaIFN-λ may inhibit H1N1-WSN strain replication better than CaIFN-α7 in MDCK cells.
Discussion
IFNs are defined by their ability to induce resistance to viral infection. Although all types of IFNs stimulate innate and adaptive immune mechanisms that contribute to the clearance of viral infections, only type I and type III IFNs are directly produced in response to viral infections. Type III IFNs, which include IFN-λ1, 2, and 3, were first discovered in 2002/2003 and mediate antiviral responses similar to Type I IFNs via a distinct receptor complex. IFN-λ1 is more effective than the other 2 family members. In canines, 3 type-III IFN family genes were predicated using a combination of protein sequence and genomics-based methods (Fox and others 2009), one of the dog IFN-λ genes is located on chromosome 24 and has a single predicted exon; the other 2 are multi-exon genes that are neighbors of unknown distance and orientation on chromosome 1. However, only one IFN-λ genes located on chromosome 24 is obtained in MDCK cells using RT-PCR method in our study and it has been confirmed that the single-exon gene produces a protein (Yang and others 2013), and the other 2 multi-exon genes might not transcript in MDCK cells or might be pseudogenes. However, the exact function of the canine typeIII IFN is still unclear. In this study, we provide the first detailed report of the functional characteristics of the newly found CaIFN-λ protein.
Our nucleic acid and amino acids sequence analysis revealed that the newly found CaIFN-λ has high homology to swine IFN-λ1 (SwIFN-λ1), human IFN-λ1(HuIFN-λ1), and bat IFN-λ1(BaIFN-λ1), indicating that CaIFN-λ is CaIFN-λ1. In addition, a previous study confirmed that the CaIFN-λ gene is located on chromosome 24 of the canine genome (Yang and others 2013), which is consistent with the predicted results that there are 3 IFN-λs in canines and that CaIFN-λ1 is located on chromosome 24 (Fox and others 2009). Thus, the newly found CaIFN-λ is homologous to IFN-λ1.
The binding of IFNs to their receptors is the first step to initiate the signal transduction to achieve antiviral action. Here, we determined whether rCaIFN-λ interacted with rCaIFN-λR1-EC protein using ELISAs. The results showed that rCaIFN-λ bound to recombinant CaIFN-λR1-EC protein with high affinity.
To explore the antiviral potency of CaIFN-λ, we systematically analyzed its antiviral activities in different cells and against different viruses. The results showed that there are some differences in antiviral activity against various viruses between rCaIFN-λ and rCaIFN-α7. For instance, rCaIFN-α7 was more active against VSV, but rCaIFN-λ was more active against CPV and influenza virus (H1N1-WSN strain). These results suggest different functions for these IFNs in vivo, which may be related to the different distributions of their receptors.
Type I IFNs are considered to execute their antiproliferative function via cell-cycle arrest and/or by inducing apoptosis. To explore whether CaIFN-λ inhibited proliferation, the MDCK and A72 (tumor cell) lines were selected. CaIFN-λ exhibited an antiproliferation function in a dose-dependent manner, and the inhibitory effects of CaIFN-λ on tumor cells were better than on MDCK cells, indicating that CaIFN-λ has the potential of to be an antitumor drug.
Previous studies demonstrate that IFN-λ is preferentially induced by intranasal influenza A virus infection and is the predominant IFN induced by influenza A virus infection in vivo (Jewell and others 2010). In the experiments described herein, we examined the mRNA level of CaIFN-λ in influenza virus- and VSV-infected cells using real-time qPCR. We found that the expression of CaIFN-λ is higher than that of CaIFN-α7 in both the H1N1-WSN strain- and H1N1-CA04 strain-infected groups, while the expression of CaIFN-λ and CaIFN-α7 were comparable in the H1N1-PR8 strain-infected group. Perhaps the best interpretation of these results is that IFN-λ plays a more important role than CaIFN-α7 in anti-influenza A virus infection.
HuIFN-λs signals through an IFN-λR1 and IL-10R2 receptor complex to induce a subset of type I-associated genes (Kotenko and others 2003; Dumoutier and others 2004), which explains some of their type I IFN-like properties. To explore whether CaIFN-λ had type I IFN-like properties in MDCK cells, the expression levels of 3 ISGs were measured. Like IFN-λs in other species, CaIFN-λ induced the expression of ISG15, Mx1, and 2′,5′-OAS in MDCK cells. However, CaIFN-λ clearly contrasted with type I IFNs. We found that the ISG15 and 2′,5′-OAS mRNA levels induced by rCaIFN-α7 were greater than those induced by rCaIFN-λ, but the Mx1 levels displayed the opposite response. Previous results show that Mx1 protein can inhibit the replication of influenza virus in mice (Staeheli and others 1986; Arnheiter and others 1996); so the higher expression of Mx1 induced by rCaIFN-λ might endow IFN-λ with better antiviral activity.
Overall, these results indicate that CaIFN-λ is an important mediator of the antiviral and antitumor response. These data also suggest a potential therapeutic role for type III IFNs.
Acknowledgments
This study was funded by grants from the National Key Technologies Research and Development Program of China (2013ZX10004-610), Ministry of Science and Technology of China Program 973 (Grant No. 2012CB955501 and 2012CB518903), Ministry of Science and Technology of China Program 863 (Grant No. 2011AA10A215), the National Natural Science Foundation of China (NSFC) (Grant No. 31100644 and 81101253), and the Key Research Program of the Chinese Academy of Sciences (Grant No. KSZD-EW-Z-005). Wenjun Liu is a principal investigator of the National Natural Science Foundation of China Innovative Research Group (Grant No. 81021003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Author Disclosure Statement
No competing financial interests exist.
References
- Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. 2008. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol 180(4):2474–2485 [DOI] [PubMed] [Google Scholar]
- Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. 2006. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol 80(9):4501–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JA. 1971. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol 21(4):723–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheiter H, Frese M, Kambadur R, Meier E, Haller O. 1996. Mx transgenic mice—animal models of health. Curr Top Microbiol Immunol 206:119–147 [DOI] [PubMed] [Google Scholar]
- Biron CA. 1998. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol 10(5):383–390 [DOI] [PubMed] [Google Scholar]
- Charlier M, L'Haridon R, Boisnard M, Martal J, Gaye P. 1993. Cloning and structural analysis of four genes encoding interferon-omega in rabbit. J Interferon Res 13(5):313–322 [DOI] [PubMed] [Google Scholar]
- Cheng G, Chen W, Li Z, Yan W, Zhao X, Xie J, Liu M, Zhang H, Zhong Y, Zheng Z. 2006. Characterization of the porcine alpha interferon multigene family. Gene 382:28–38 [DOI] [PubMed] [Google Scholar]
- Cheng G, Zhao X, Chen W, Yan W, Liu M, Chen J, Zheng Z. 2007a. Detection of differential expression of porcine IFN-alpha subtypes by reverse transcription polymerase chain reaction. J Interferon Cytokine Res 27(7):579–587 [DOI] [PubMed] [Google Scholar]
- Cheng G, Zhao X, Yan W, Wang W, Zuo X, Huang K, Liu Y, Chen J, Wang J, Cong W, Liu M, Gao H, Chen J, Lu Y, Zheng Z. 2007b. Alpha interferon is a powerful adjuvant for a recombinant protein vaccine against foot-and-mouth disease virus in swine, and an effective stimulus of in vivo immune response. Vaccine 25(28):5199–5208 [DOI] [PubMed] [Google Scholar]
- Chill JH, Quadt SR, Levy R, Schreiber G, Anglister J. 2003. The human type I interferon receptor: NMR structure reveals the molecular basis of ligand binding. Structure 11(7):791–802 [DOI] [PubMed] [Google Scholar]
- Cochet M, Vaiman D, Lefevre F. 2009. Novel interferon delta genes in mammals: cloning of one gene from the sheep, two genes expressed by the horse conceptus and discovery of related sequences in several taxa by genomic database screening. Gene 433(1–2):88–99 [DOI] [PubMed] [Google Scholar]
- Demmers KJ, Derecka K, Flint A. 2001. Trophoblast interferon and pregnancy. Reproduction 121(1):41–49 [DOI] [PubMed] [Google Scholar]
- Devos K, Duerinck F, Van Audenhove K, Fiers W. 1992. Cloning and expression of the canine interferon-gamma gene. J Interferon Res 12(2):95–102 [DOI] [PubMed] [Google Scholar]
- Diaz-San Segundo F, Weiss M, Perez-Martin E, Koster MJ, Zhu J, Grubman MJ, de los Santos T. 2011. Antiviral activity of bovine type III interferon against foot-and-mouth disease virus. Virology 413(2):283–292 [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. 2004. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J Biol Chem 279(31):32269–32274 [DOI] [PubMed] [Google Scholar]
- Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, Al Ghonaium A, Tufenkeji H, Frayha H, Al-Gazlan S, Al-Rayes H, Schreiber RD, Gresser I, Casanova JL. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet 33(3):388–391 [DOI] [PubMed] [Google Scholar]
- Fox BA, Sheppard PO, O'Hara PJ. 2009. The role of genomic data in the discovery, annotation and evolutionary interpretation of the interferon-lambda family. PLoS One 4(3):e4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friborg J, Levine S, Chen C, Sheaffer AK, Chaniewski S, Voss S, Lemm JA, McPhee F. 2013. Combinations of lambda interferon with direct-acting antiviral agents are highly efficient in suppressing hepatitis C virus replication. Antimicrob Agents Chemother 57(3):1312–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Stertz S, Kochs G. 2007. The Mx GTPase family of interferon-induced antiviral proteins. Microbes Infect 9(14–15):1636–1643 [DOI] [PubMed] [Google Scholar]
- Hauptmann R, Swetly P. 1985. A novel class of human type I interferons. Nucleic Acids Res 13(13):4739–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A, Hauptmann R, Adolf GR, Swetly P. 1987. Structure and expression in Escherichia coli of canine interferon-α genes. J interferon Res 7(2):173–183 [DOI] [PubMed] [Google Scholar]
- Iversen MB, Paludan SR. 2010. Mechanisms of type III interferon expression. J Interferon Cytokine Res 30(8):573–578 [DOI] [PubMed] [Google Scholar]
- Iwata A, Saito T, Mizukoshi-iwata N, Fujino M, Katsumata A, Hamada K, Sokawa Y, Ueda S. 1996. Cloning and expression of the canine interferon-β gene. J Interferon Cytokine Res 16(10):765–770 [DOI] [PubMed] [Google Scholar]
- Jewell NA, Cline T, Mertz SE, Smirnov SV, Flano E, Schindler C, Grieves JL, Durbin RK, Kotenko SV, Durbin JE. 2010. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J Virol 84(21):11515–11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpala AJ, Morris KR, Broadway MM, McWaters PG, O'Neil TE, Goossens KE, Lowenthal JW, Bean AG. 2008. Molecular cloning, expression, and characterization of chicken IFN-lambda. J Interferon Cytokine Res 28(6):341–350 [DOI] [PubMed] [Google Scholar]
- Kotenko SV. 2011. IFN-lambdas. Curr Opin Immunol 23(5):583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. 2002. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 4(1):69–77 [DOI] [PubMed] [Google Scholar]
- Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, Reuhl K, Dickensheets H, Sheikh F, Donnelly RP, Raveche E, Kotenko SV. 2006. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res 66(8):4468–4477 [DOI] [PubMed] [Google Scholar]
- Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol 4(10):1009–1015 [DOI] [PubMed] [Google Scholar]
- Lefevre F, Guillomot M, D'Andrea S, Battegay S, La Bonnardiere C. 1998. Interferon-delta: the first member of a novel type I interferon family. Biochimie 80(8–9):779–788 [DOI] [PubMed] [Google Scholar]
- Levin S, Hahn T, Rosenberg H, Bino T. 1982. Treatment of life-threatening viral infections with interferon alpha: pharmacokinetic studies in a clinical trial. Isr J Med Sci 18(4):439–446 [PubMed] [Google Scholar]
- Li Q, Kawamura K, Tada Y, Shimada H, Hiroshima K, Tagawa M. 2013. Novel type III interferons produce anti-tumor effects through multiple functions. Front Biosci (Landmark Ed) 18:909–918 [DOI] [PubMed] [Google Scholar]
- Lindenmann J. 1982. From interference to interferon: a brief historical introduction. Philos Trans R Soc Lond B Biol Sci 299(1094):3–6 [DOI] [PubMed] [Google Scholar]
- Loveland BE, Johns TG, Mackay IR, Vaillant F, Wang ZX, Hertzog PJ. 1992. Validation of the MTT dye assay for enumeration of cells in proliferative and antiproliferative assays. Biochem Int 27(3):501–510 [PubMed] [Google Scholar]
- Manion M, Rodriguez B, Medvik K, Hardy G, Harding CV, Schooley RT, Pollard R, Asmuth D, Murphy R, Barker E, Brady KE, Landay A, Funderburg N, Sieg SF, Lederman MM. 2012. Interferon-alpha administration enhances CD8+ T cell activation in HIV infection. PLoS One 7(1):e30306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccetti NE, Robek MD. 2010. Interferon-lambda in HCV Infection and Therapy. Viruses 2(8):1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Yang L, Meng S, Xu L, Bi Y, Jia X, Li J, Sun L, Liu W. 2013. The differential antiviral activities of chicken interferon alpha (ChIFN-alpha) and ChIFN-beta are related to distinct interferon-stimulated gene expression. PLoS One 8(3):e59307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RM, Ealy AD, Alexenko AP, Han CS, Ezashi T. 1999. Trophoblast interferons. Placenta 20(4):259–264 [DOI] [PubMed] [Google Scholar]
- Schindler C, Darnell JE., Jr.1995. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 64:621–651 [DOI] [PubMed] [Google Scholar]
- Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4(1):63–68 [DOI] [PubMed] [Google Scholar]
- Smith PL, Lombardi G, Foster GR. 2005. Type I interferons and the innate immune response—more than just antiviral cytokines. Mol Immunol 42(8):869–877 [DOI] [PubMed] [Google Scholar]
- Staeheli P, Haller O, Boll W, Lindenmann J, Weissmann C. 1986. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell 44(1):147–158 [DOI] [PubMed] [Google Scholar]
- Taira O, Watanugi I, Hagiwara Y, Takahashi M, Arai S, Sato H, Maehara N. 2005. Cloning and expression of canine interferon-alpha genes in Escherichia coli. J Vet Med Sci 67(10):1059–1062 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka Y, Endo S, Matsui A, Sato A, Saito K, Semba K, Takahashi M, Murakami T. 2012. Potential anti-tumor effect of IFN-lambda2 (IL-28A) against human lung cancer cells. Lung Cancer 78(3):185–192 [DOI] [PubMed] [Google Scholar]
- Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, Rotman Y, Liang TJ. 2012. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology 142(4):978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG. 2002. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter 2:Unit 2 3. [DOI] [PubMed] [Google Scholar]
- Tsang SL, Leung PC, Leung KK, Yau WL, Hardy MP, Mak NK, Leung KN, Fung MC. 2007. Characterization of murine interferon-alpha 12 (MuIFN-alpha12): biological activities and gene expression. Cytokine 37(2):138–149 [DOI] [PubMed] [Google Scholar]
- Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, Sabat R, Wolk K. 2009. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun 10(8):702–714 [DOI] [PubMed] [Google Scholar]
- Xue Q, Yang L, Liu X, Liu W. 2010. Molecular characterization of feline type I interferon receptor 2. J Interferon Cytokine Res 30(2):81–88 [DOI] [PubMed] [Google Scholar]
- Yang L, Xu L, Li Y, Li J, Bi Y, Liu W. 2013. Molecular and functional characterization of canine interferon-epsilon. J Interferon Cytokine Res 33(12):760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LM, Xue QH, Sun L, Zhu YP, Liu WJ. 2007. Cloning and characterization of a novel feline IFN-omega. J Interferon Cytokine Res 27(2):119–127 [DOI] [PubMed] [Google Scholar]
- Yanguez E, Garcia-Culebras A, Frau A, Llompart C, Knobeloch KP, Gutierrez-Erlandsson S, Garcia-Sastre A, Esteban M, Nieto A, Guerra S. 2013. ISG15 regulates peritoneal macrophages functionality against viral infection. PLoS Pathog 9(10):e1003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Cheng G, Jiao Y, Yan W, Liu M, Zheng Z. 2012. Cloning and characterization of porcine interferon-delta-related genes identified by genomic database screening. J Interferon Cytokine Res 32(8):378–385 [DOI] [PubMed] [Google Scholar]
- Zhao X, Cheng G, Yan W, Liu M, He Y, Zheng Z. 2009. Characterization and virus-induced expression profiles of the porcine interferon-omega multigene family. J Interferon Cytokine Res 29(10):687–693 [DOI] [PubMed] [Google Scholar]
- Zhou P, Cowled C, Marsh GA, Shi Z, Wang LF, Baker ML. 2011a. Type III IFN receptor expression and functional characterisation in the pteropid bat, Pteropus alecto. PLoS One 6(9):e25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Cowled C, Todd S, Crameri G, Virtue ER, Marsh GA, Klein R, Shi Z, Wang LF, Baker ML. 2011b. Type III IFNs in pteropid bats: differential expression patterns provide evidence for distinct roles in antiviral immunity. J Immunol 186(5):3138–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann K, Brand S, Baehs S, Goke B, Meinecke J, Spottl G, Meyer H, Auernhammer CJ. 2006. Novel interferon-lambdas induce antiproliferative effects in neuroendocrine tumor cells. Biochem Biophys Res Commun 344(4):1334–1341 [DOI] [PubMed] [Google Scholar]
- Zucker K, Lu P, Esquenazi V, Miller J. 1992. Cloning of the cDNA for canine interferon-gamma. J Interferon Res 12(3):191–194 [DOI] [PubMed] [Google Scholar]