Abstract
Recent studies suggest the ability of the central nervous system to detect changes in osmolality is mediated by products of the genes encoding the transient receptor potential vanilloid-1 (TRPV1) or vanilloid-4 (TRPV4) channel. The purpose of the present study was to determine whether deletion of TRPV1 and/or TRPV4 channels altered thirst responses to cellular dehydration in mice. Injection of 0.5 or 1.0 M NaCl produced dose-dependent increases in cumulative water intakes of wild-type (WT), TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice. However, there were no differences in cumulative water intakes between WT versus any other strain despite similar increases in plasma electrolytes and osmolality. Similar results were observed after injection of hypertonic mannitol. This was a consistent finding regardless of the injection route (intraperitoneal vs. subcutaneous) or timed access to water (delayed vs. immediate). There were also no differences in cumulative intakes across strains after injection of 0.15 M NaCl or during a time-controlled period (no injection). Chronic hypernatremia produced by sole access to 2% NaCl for 48 h also produced similar increases in water intake across strains. In a final set of experiments, subcutaneous injection of 0.5 M NaCl produced similar increases in the number of Fos-positive nuclei within the organum vasculosum of the lamina terminalis and median preoptic nucleus across strains but significantly smaller number in the subfornical organ of WT versus TRPV1−/−V4−/− mice. Collectively, these findings suggest that TRPV1 and/or TRPV4 channels are not the primary mechanism by which the central nervous system responds to cellular dehydration during hypernatremia or hyperosmolality to increase thirst.
Keywords: hypernatremia, water intake, organum vasculosum of the lamina terminalis, subfornical organ, cellular dehydration
the central nervous system plays a pivotal role in body fluid homeostasis through its ability to sense changes in osmotic pressure and subsequently alter fluid intake, secretion of antidiuretic hormone, natriuresis, and sympathetic nerve activity (5, 19, 30, 42, 45, 50). Physiologically, changes in extracellular osmotic pressure are typically the result of changes in the extracellular concentration of Na+ and/or Cl− to produce cellular dehydration. The most influential set of osmosensitive neurons is located within the forebrain lamina terminalis (5). This region of the brain contains several structures including two circumventricular organs: the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO). A number of laboratories have demonstrated that acute or chronic hypernatremia increases Fos immunoreactivity in the OVLT and SFO (21, 36, 40, 46). Second, in vivo and in vitro electrophysiological studies have reported that acute hypernatremia or hyperosmolality increases the discharge of neurons in both structures (11, 12, 14, 17, 28, 35, 38). Third, in vitro electrophysiological studies indicate that SFO and OVLT neurons are intrinsically osmosensitive (2, 11, 12). Finally, lesion of the lamina terminalis largely attenuates or abolishes thirst, antidiuretic hormone secretion, and sympathetic and/or pressor responses to hyperosmolality (3, 7, 20, 27, 31, 41, 51). While the relative contribution of the OVLT versus SFO to these osmoregulatory responses may vary across species, these studies highlight the importance of these structures in osmoregulatory processes.
Recent studies performed in OVLT neurons suggest the underlying osmosensory process in these neurons is mediated by members of the transient receptor potential vanilloid (TRPV) family (5, 11, 12). TRPV channels contain six transmembrane spanning segments and a pore-loop domain that are sensitive to temperature, chemical, and mechanical/osmotic stimuli (34, 37). Ciura and Bourque (11, 12) have postulated that the intrinsic osmosensitivity of OVLT neurons is mediated by a capsaicin-insensitive, NH2-terminal variant of the TRPV1 gene. Although it is unclear whether TRPV1 channels are expressed in the OVLT (10, 26), a series of elegant in vitro studies showed that excitatory responses of OVLT neurons to hyperosmolality were blocked by the nonselective TRPV blocker ruthenium red or were absent in TRPV1−/− mice (11). Consistent with a role for TRPV1, OVLT neurons showed an increase in conductance in response to hyperosmolality or cellular shrinking via pipette suction (11, 12). These responses were abolished by a selective TRPV1 antagonist SB366791 or were absent in TRPV1−/− but not TRPV4−/−, mice (12). On the other hand, TRPV4 mRNA and immunoreactivity have been reported in the forebrain lamina terminalis (23). Moreover, TRPV4−/− versus wild-type (WT) mice have lower numbers of Fos-positive neurons in the ventral lamina terminalis after an acute sodium load (23).
Despite these observations, the functional impact of a deleted TRPV1 or TRPV4 gene remains controversial. As noted above, TRPV4−/− mice have a slightly attenuated thirst response to an acute sodium load (23). Ciura and Bourque (11) reported that TRPV1−/− mice drank slightly less water than WT mice after an intraperitoneal injection of hypertonic NaCl. In marked contrast, our laboratory reported that TRPV1−/− and WT mice ingested similar amounts of water in response to a range of acute and chronic hypernatremic challenges (46). In addition, acute and chronic sodium loading produced a similar increase in the number of Fos-positive neurons in the lamina terminalis of WT versus TRPV1−/− mice (46). Given the controversial data regarding the functional role of TRPV1 versus TRPV4 channels, the purpose of the present study was to perform a comprehensive analysis of osmoregulatory thirst in TRPV1−/−, TRPV4−/−, and double knockout TRPV1−/−V4−/− mice. Cumulative water intake was measured in response to both acute and chronic sodium loads. We employed several different paradigms including varied injection routes (intraperitoneal vs. subcutaneous) and timed access to water (delayed vs. immediate) to permit direct comparisons to previous studies (11, 23, 46). Parallel experiments also investigated whether deletion of TRPV1 and/or TRPV4 genes disrupted Fos activation in neurons of the OVLT, SFO, and median preoptic nucleus.
MATERIALS AND METHODS
All of the experimental procedures conform to the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State College of Medicine.
Animals.
Double knockout mice for the TRPV1 and TRPV4 (TRPV1−/−V4−/−) channels were obtained from Dr. Wolfgang Liedtke (Duke University). TRPV4−/− mice were made previously by deletion of exon 12, which encodes the pore-loop and adjacent transmembrane domains (23). These mice were crossed with TRPV1−/− mice (Jackson Laboratory, Bar Harbor, ME) that lack the exon encoding the fifth and sixth transmembrane and the pore-loop domains (9). Within our laboratory, the TRPV1−/−V4−/− mice were crossed with C57BL/6 mice (Charles River Laboratories) to obtain TRPV1−/− and TRPV4−/− strains. Animals were housed individually in a temperature-controlled room (22–24°C) and maintained on a 12:12-h light-dark cycle (lights on at 6 AM) at least 1 wk before experiments began. The room was solely dedicated for these experiments. Standard laboratory chow (Harlan Teklad Global Diet no. 2018) and deionized water were provided ad libitum except where noted. Experiments were conducted between 8 AM and 1 PM. The total number of animals used in the present study is as follows: WT (C57BL/6, n = 30), TRPV1−/− (n = 26), TRPV4−/− (n = 24), and TRPV1−/−V4−/− (n = 23).
Thirst studies.
To determine the contribution of TRPV1 and TRPV4 channels to thirst stimulated by cellular dehydration, we studied male mice (initial age: 12–15 wk) of four different strains: WT or C57BL/6 (Charles River Laboratories or Jackson Laboratories), TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/−. At least 1 wk before experiments began, mice were housed singly and acclimated to the testing procedures daily. Briefly, food was removed from all cages at least 1 h before and throughout studies to eliminate any possibility that the recent ingestion of food impacted subsequent water intake. Each mouse was weighed and then handled identically to an injection. Finally, graduate 10-ml drinking tubes (± 0.05 ml) were placed and remained on cages throughout the entire study. Two different groups of mice were studied in two separate experimental series (see below).
Experimental series 1.
The first series of experiments was a randomized, within-subjects design, and each treatment was separated by >3 days. WT (n = 13), TRPV1−/− (n = 13), TRPV4−/− (n = 12), and TRPV1−/−V4−/− (n = 10) mice received an acute injection of 0.15, 0.5, or 1.0 M NaCl (10 μl/g body wt). To distinguish between thirst stimulated by hypernatremia versus hyperosmolality, mice also received an injection of 0.7 M mannitol dissolved in 0.15 M NaCl (10 μl/g body wt). Injections were performed intraperitoneally and subcutaneously using 30-gauge needles; however, the same route of injection was used only once per week. A final time-control experiment was performed in which the testing procedures were identical except mice did not receive an injection. Access to water in all tests was delayed for 30 min. Then, cumulative water intakes (± 0.05 ml) were measured at 30, 60, 90, and 120 min. In addition, water bottles were weighed (nearest 0.05 g) before and after the drinking tests to confirm the observed intakes. If there was a difference between the observed water intake and weight of the water bottles, the test was repeated on another day. However, this was rarely encountered (<5 times). Experimental series 1 lasted ∼5–6 wk.
Experimental series 2.
The second series of experiments was a randomized, within-subjects design, and each treatment was separated by >3 days. WT (n = 12), TRPV1−/− (n = 9), TRPV4−/− (n = 8), and TRPV1−/−V4−/− (n = 9) mice received an acute injection of 0.15, 0.5, or 1.0 M NaCl (0.3 ml sc) or 0.7 M mannitol dissolved in 0.15 M NaCl (0.3 ml sc) using 30-gauge needles. In contrast to experimental series 1, these mice were given immediate access to water as previously described (46). In addition, to investigate whether TRPV1 and/or TRPV4 channels contributed to thirst stimulated by chronic hypernatremia, the same mice were given access to food and a 2% NaCl solution for 48 h. Then, food was removed and the 2% NaCl was replaced with water. To examine whether deletion of TRPV1 and/or TRPV4 genes did not result in a general disruption of thirst independent of osmotic or hypernatremic stimuli, mice received an injection of the β-adrenergic agonist dl-isoproterenol hydrochloride (0.04 μg, 0.25 ml sc) or were made hypovolemic by two successive injections of the loop diuretic furosemide (0.5% Lasix, 0.25 ml sc) separated by 2 h. Again, mice had immediate access to water in all experiments except after injection of furosemide. In this experiment, water bottles were returned at 2 h after the second injection. Cumulative water intakes (± 0.05 ml) were measured at 15, 30, 60, and 120 min. Experimental series 2 lasted ∼4 wk.
Blood sampling and analysis of plasma electrolytes and osmolality.
A final set of experiments using a between-group design was conducted to determine whether the acute NaCl injections produced similar changes in plasma electrolytes and osmolality across mouse strains. Each treatment used both naïve mice and mice from experimental series 1 at least 2 wk after all behavioral studies were completed. The procedures were identical to those described in experimental series 1. Mice received an injection of 0.15, 0.5, or 1.0 M NaCl (10 μl/g body wt sc) but had no access to water. At 25 min, mice were anesthetized with 2–3% isoflurane (in 100% O2) and instrumented with a catheter in the left carotid artery. The entire procedure lasted <5 min. A blood sample (70 μl) was collected immediately through the carotid catheter and analyzed for electrolytes using an I-STAT1 and 6+ cartridges (Abbott; East Windsor, NJ). Additional blood (300 μl) was collected into a microcentrifuge tube containing heparin (3 units) and centrifuged (10,000 g, 1 min). Osmolality was determined in triplicate using a freezing-point depression osmometer (model 3320; Advanced Instruments). Plasma protein was measured by protein refractometry (Refractometer Veterinary ATC, VWR International).
Fos immunocytochemical studies.
To determine whether lack of the TRPV1 and/or TRPV4 genes disrupted neuronal activation in the lamina terminalis, Fos immunoreactivity was quantified in the OVLT, SFO, and median preoptic nucleus (MnPO) after an acute sodium load. At least 2 wk after completion of all thirst experiments described in experimental series 2, these same mice received an injection of 0.15 or 0.5 M NaCl (0.3 ml sc) and were returned to home cages without access to food or water for 90 min. Then, animals were anesthetized with isoflurane (3% in 100% O2). Mice were then perfused transcardially with isotonic saline (1 ml) followed by 4% paraformaldehyde (4 ml, 4°C). Brains were postfixed overnight in 4% paraformaldehyde (4°C), transferred to 30% sucrose for 1–2 days, and sectioned at 40 μm using a vibratome. Sections were collected into two serially adjacent sets and stored in 10 mM phophate-buffered saline at 4°C for 2–3 days or cryoprotectant (52) at −20°C. Fos immunoreactivity was visualized as described previously in our laboratory (44, 46) using a rabbit Fos polyclonal anti-Fos antibody (1:10,000, PC-38; EMD Biosciences), ABC Vectastain Kit (Vector Laboratories, Burlingame, CA), and Ni-enhanced DAB reaction. The number of Fos-positive nuclei was quantified from a representative section(s) of the OVLT, dorsal MnPO, ventral MnPO, and two sections in the SFO. Counts from the two levels of SFO or dorsal and ventral MnPO were averaged or combined, respectively.
Genotyping.
Genomic DNA was isolated from tail snips (0.5 cm) using DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). The primers used for TRPV1 genotyping were as specified by The Jackson Laboratory (oIMR0297, 5′-ACGAGACTAGTGAGACGTG-3′; oIMR1561, 5′-CCTGCTCAACATGCTCATTG-3′; oIMR1562, 5′-TCCTCATGCACTTCAGGAAA-3′) at a concentration of 0.2 μM (oIMR0297 and oIMR1561) or 0.4 mM (oIMR1562). The PCR reaction mixture contained 20 mM Tris (pH 8.0), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM each dNTP, 2% DMSO, 1 unit Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA), and ∼100 ng of genomic DNA. PCR cycling conditions were 94°C (3 min) followed by 35 amplification cycles (94°C, 30 s; 55°C, 1 min; 72°C, 1 min) and 72°C (10 min). The WT and null alleles generated either a 984-bp or a 600-bp product, respectively. The primers for the TRPV4 PCR (F, 5′-CATGAAATCTGACCTCTTGTCCCC-3′ and R, 5′-TTGTGTACTGTCTGCACACCAGGC-3′) were used at a concentration of 0.4 μM. PCR reaction mixture contained 1× Herculase buffer (Stratagene, La Jolla, CA), 0.2 mM each dNTP, 2% DMSO, 1.25 units Herculase Enhanced DNA polymerase, 0.6 units Platinum Taq DNA Polymerase (Invitrogen), and ∼100 ng of genomic DNA. PCR cycling conditions were 94°C (3 min) followed by 35 amplification cycles (94°C, 30 s; 68°C, 1.5 min; 72°C, 2 min) and 72°C for 10 min. The wild-type and null alleles generated either a 2.1- or a 1.1-kb product, respectively. The PCR reaction product (15 μl) was ran on a 1.5% agarose gel containing 0.5 μg/ml ethidium bromide for 1 h at 70 V, and the PCR products were visualized with a ultraviolet transilluminator.
Statistical analysis.
Values are expressed as means ± SE. Cumulative water intakes were analyzed in two ways: 1) two-way ANOVA (strain and time) between WT and a single strain to assess whether a strain differed from WT, and 2) a two-way ANOVA (strain and time) across all four strains. Both statistical analyses yielded the same results. The reported P values reflect the latter method. Post hoc tests were made using a layered Bonferroni with correction (Systat 10.2, Systat Software). All other data (Fos-positive nuclei, electrolytes) were analyzed by a two-way ANOVA with independent t-tests with a layered Bonferroni analysis when significant F values were obtained.
RESULTS
Genotyping of WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice.
The PCR product generated from tail snips of WT contained the expected 984-bp and 2100-bp fragments corresponding to the TRPV1 and TRPV4 alleles, respectively (Fig. 1). TRPV1−/− mice displayed the shorter fragment or null allele at 600 bp for TRPV1 gene. TRPV4−/− mice had the predicted null allele at 1100 bp for the TRPV4 gene. Double-knockout or TRPV1−/−V4−/− displayed both null alleles for the TRPV1 and TRPV4 gene.
Fig. 1.
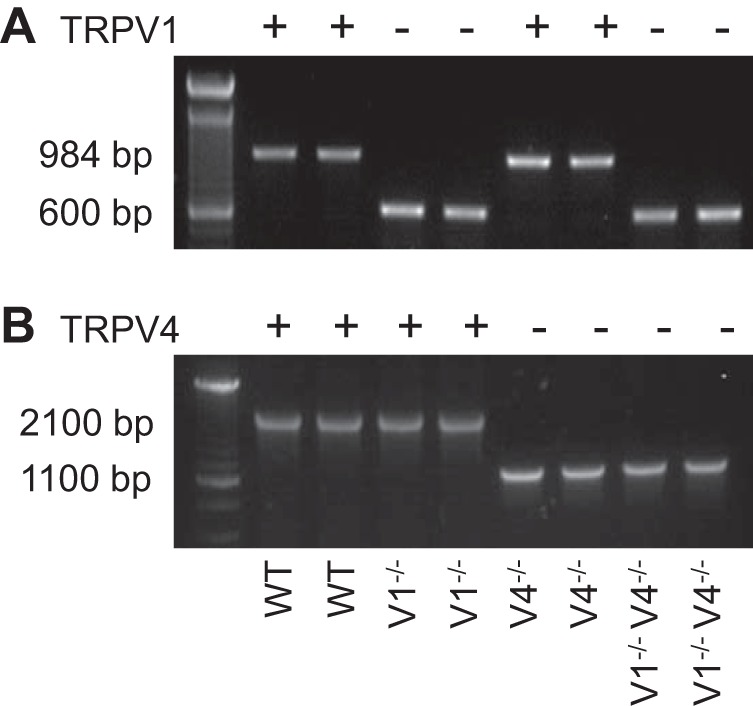
Representative example of PCR products from wild-type (WT), TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice for transient receptor potential vanilloid-1 (TRPV1) allele (A) and vanilloid-4 (TRPV4) allele (B). The WT allele for the TRPV1 and TRPV4 genes were 984 and 2100 bp, respectively. The truncated allele for the TRPV1 and TRPV4 genes were 600 and 1100 bp, respectively. The genotype of every mouse in the current study was confirmed via PCR.
Effect of TRPV1 and/or TRPV4 gene deletion on osmotically stimulated thirst.
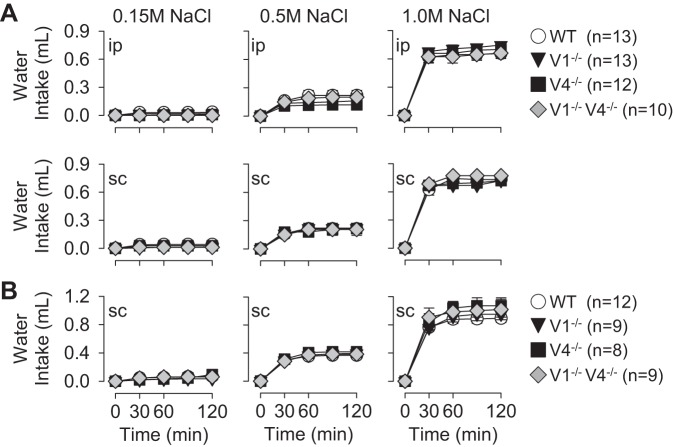
A major goal of the present study was to determine whether deletion of the TRPV1 and/or TRPV4 gene disrupted thirst stimulated by cellular dehydration and produced by injection of hypertonic NaCl. Acute injection of 0.5 and 1.0 M NaCl produced dose-dependent increases in water intake across all strains including WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice (Fig. 2). However, there were no differences in water intake across strains at any time. This observation was consistent regardless of the route of injection (intraperitoneal versus subcutaneous, Fig. 2A, experimental series 1) or whether mice had delayed versus immediate access to water (Fig. 2, A vs. B, experimental series 1 vs. 2). In addition, there were no differences in water intake across strains after a control injection of 0.15 M NaCl. Time-control studies (experimental series 2), in which water intake was measured but no injection was performed, indicate there were no differences in water intake across strains at 120 min (WT: 0.03 ± 0.02 ml, TRPV1−/−: 0.01 ± 0.01 ml, TRPV4−/−: 0.02 ± 0.02 ml, TRPV1−/−V4−/−: 0.04 ± 0.03 ml, P > 0.9 from overall ANOVA). In addition, there were no differences in body weight across groups or cumulative water intakes when normalized to 20 g body weight (data not shown).
Fig. 2.
Cumulative water intakes as a function of time of WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice after injection of 0.15, 0.5, and 1.0 M NaCl. A, experimental series 1: mice were injected intraperitoneally or subcutaneously (10 μl/g body wt) and given access to water 30 min later. B, experimental series 2: mice were injected subcutaneously (0.3 ml) and given immediate access to water. Injection of 0.5 and 1.0 M NaCl produced dose-dependent increases in water intake. However, there were no differences across WT versus TRPV1−/−, TRPV4−/−, or TRPV1−/−V4−/− mice in any treatment paradigm.
Analysis of plasma electrolytes and plasma osmolality after injection of NaCl indicate there were no differences across WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice (Fig. 3). Injection of 0.5 or 1.0 M NaCl produced dose-dependent increases in plasma [Na+], [Cl−], and osmolality in all strains. Again, there were no differences across strains. As expected, plasma protein concentration decreased significantly after injection of 0.5 or 1.0 M NaCl (data not shown).
Fig. 3.
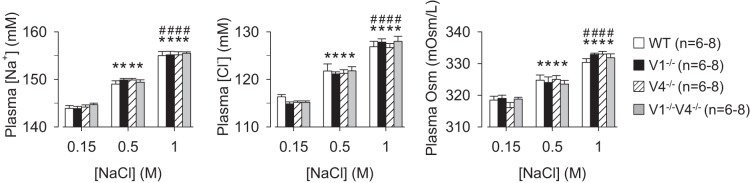
Plasma [Na+], [Cl+], and osmolality, of WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice at 30 min after injection of 0.15, 0.5, or 1.0 M NaCl (10 μl/g body wt sc). Injection of 0.5 or 1.0 M NaCl produced dose-dependent increases in plasma [Na+], [Cl+], and osmolality in all groups; however, there were no differences in any variable across groups. *P < 0.01 vs. 0.15 M within same strain; #P < 0.01, 0.5 vs. 1.0 M within same strain.
Since the majority of studies investigating the intrinsic osmosensitivity of hypothalamic neurons have increased bath osmolality using mannitol rather than NaCl (11, 12), an additional set of acute experiments was conducted using hyperosmolality via addition of mannitol (0.7 M dissolved in 0.15 M NaCl) as a stimulus for thirst. Injection of 0.7 M mannitol (or 1.0 osmol/l) significantly increased water intake across all strains. However, there were no differences in cumulative water intakes across strains regardless of the route of injection (intraperitoneal or subcutaneously, Fig. 4A, experimental series 1) or whether animals had delayed or immediate access to water (Fig. 4, A vs. B, experimental series 1 vs. 2).
Fig. 4.
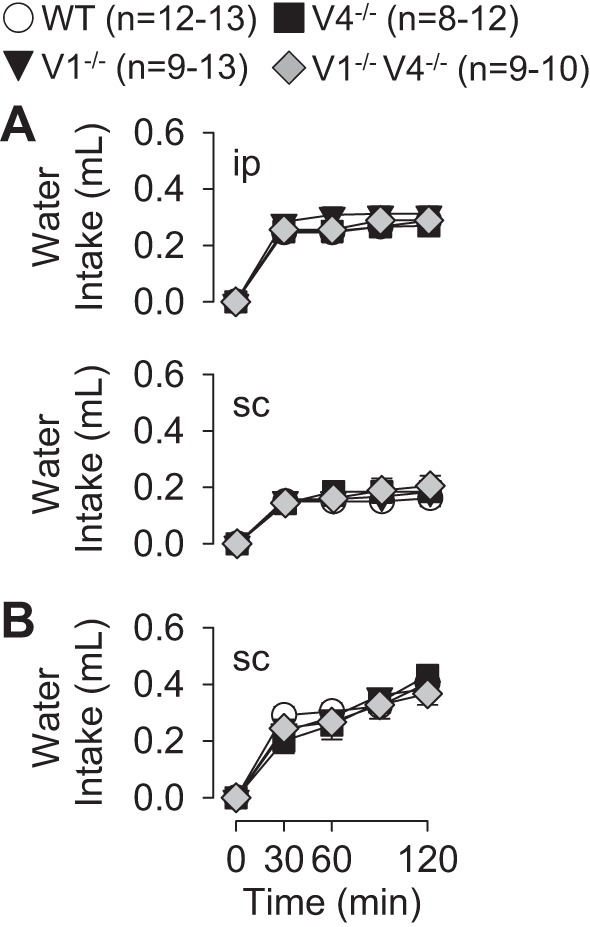
Cumulative water intakes as a function of time of WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice after injection of 0.7 M mannitol. A, experimental series 1: mice were injected intraperitoneally or subcutaneously (10 μl/g body wt) and given access to water 30 min later. B, experimental series 2: mice were injected subcutaneously (0.3 ml) and given immediate access to water. There were no differences across strains within each paradigm.
When mice were given access to 2% NaCl, all strains ingested similar amounts over 48 h (WT: 6.5 ± 0.6 ml/day, TRPV1−/−: 7.2 ± 0.3 ml/day, TRPV4−/−: 5.9 ± 0.4 ml/day, TRPV1−/−V4−/−: 5.8 ± 0.5 ml/day). When water bottles were returned to the cages, all groups drank similar amounts of water (Fig. 5).
Fig. 5.
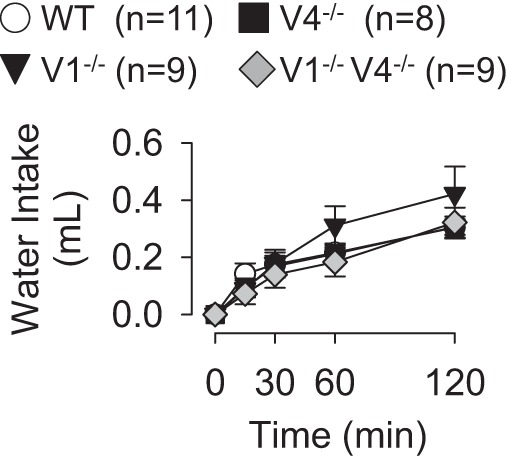
Cumulative water intakes as a function of time of WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice after sole access to 2% NaCl for 48 h (experimental series 2). There were no differences in absolute water intakes between WT versus TRPV1−/−, TRPV4−/−, or TRPV1−/−V4−/− mice.
It is noteworthy that similar statistical results were obtained for all experiments when cumulative water intakes were normalized to 20 g body weight (data not shown).
Effect of TRPV1 and/or TRPV4 gene deletion on thirst stimulated by extracellular fluid volume deficits.
To determine whether deletion of the TRPV1 and/or TRPV4 resulted in a general disruption of thirst, cumulative water intakes were measured after injection of two agents commonly used to induce extracellular fluid volume deficits without changes in plasma electrolytes or osmolality. Injection of the β-adrenergic agonist isoproterenol increased water intake in all strains of mice, but cumulative water intakes were not different across strains (120 min: WT, 0.18 ± 0.03 ml; TRPV1−/−: 0.24 ± 0.03 ml; TRPV4−/−: 0.20 ± 0.04 ml; TRPV1−/−V4−/−: 0.16 ± 0.02 ml; P > 0.3 from overall ANOVA). Injection of furosemide also increased water intake in all mice (120 min: WT, 0.78 ± 0.07 ml; TRPV1−/−: 0.66 ± 0.05 ml; TRPV4−/−: 0.79 ± 0.07 ml; TRPV1−/−V4−/−: 0.57 ± 0.09 ml), but there were no statistical differences between groups (P > 0.1 from overall ANOVA). Similar statistical results were obtained for both isoproterenol and furosemide tests when cumulative water intakes were normalized to 20 g body weight (data not shown).
Effect of acute hypernatremia on Fos activation in the forebrain lamina terminalis.
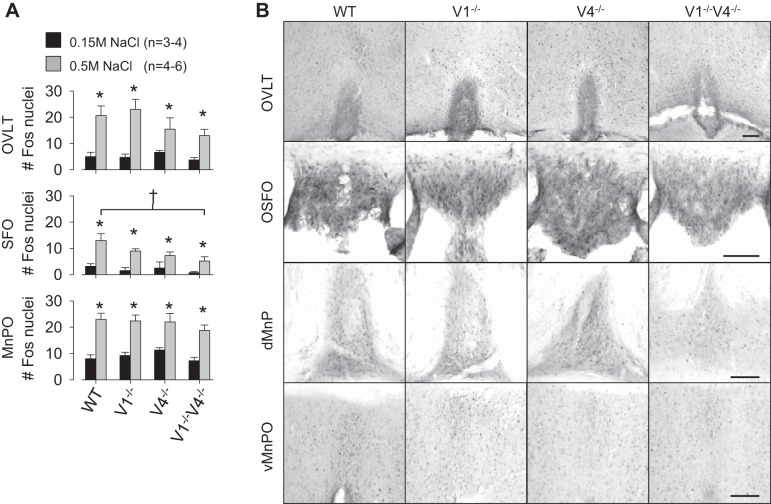
A final set of experiments was performed to determine whether deletion of the TRPV1 and/or TRPV4 gene attenuated Fos activation in the forebrain lamina terminalis in response to an acute sodium load. Few Fos-positive cells were observed in the OVLT, MnPO, or SFO of any strain after injection of 0.15 M NaCl (Fig. 6A). Injection of 0.5 M NaCl significantly increased the number of Fos-positive cells in all areas of all strains (Fig. 6). However, there were no differences in the number of Fos-positive nuclei in the OVLT between WT versus TRPV1−/− (P > 0.8), TRPV4−/− (P > 0.4), or TRPV1−/−V4−/− (P > 0.3) mice treated with 0.5 M NaCl. Similarly, there were no differences in the MnPO between WT versus TRPV1−/− (P > 0.8), TRPV4−/− (P > 0.8), or TRPV1−/−V4−/− (P > 0.5) mice treated with 0.5 M NaCl. For SFO, there were no differences in the number of Fos-positive nuclei between WT and TRPV1−/− (P > 0.2) or TRPV4−/− (P > 0.02) mice treated with 0.5 M NaCl. However, the number of Fos-positive nuclei in the SFO was significantly less in TRPV1−/−V4−/− versus WT mice after injection of 0.5 M NaCl (Fig. 6). Plasma electrolytes and osmolality at 90 min were significantly elevated after the injection of 0.5 M NaCl (data not shown).
Fig. 6.
A: means ± SE of Fos-positive nuclei in the lamina terminalis (OVLT), subfornical organ (SFO), and median preoptic nucleus (MnPO) of WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice after injection of 0.15 or 0.5 M NaCl (0.3 ml sc). Injection of 0.5 M NaCl increase the number of Fos-positive in all three brain regions of all strains (*P < 0.05). The only difference across strains was a smaller number of Fos-positive nuclei in the SFO of TRPV1−/−V4−/− versus WT mice (†P < 0.05). B: examples of Fos-positive nuclei in the OVLT, SFO, dorsal MnPO (dMnPO), and ventral MnPO (vMnPO) in WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice after injection of 0.5 M NaCl (0.3 ml sc). Black bar represents 100 μm in each area.
DISCUSSION
The role of TRPV1 and/or TRPV4 channels in osmoregulatory thirst remains controversial. The purpose of the present study was to determine the extent by which deletion of TRPV1 and/or TRPV4 genes alters thirst stimulated by cellular dehydration and produced by acute injection of hyperosmotic NaCl or mannitol employing a number of different protocols used previously (11, 46). The present findings provide several new key observations: 1) acute hypernatremia produced dose-dependent increases in water intake between WT versus TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice, but cumulative water intakes did not differ across strains at any time regardless of the experimental protocol; 2) acute injection of 0.5 or 1.0 M NaCl produced similar increases in plasma [Na+], [Cl−], and osmolality across strains; 3) cumulative water intakes did not differ between strains after acute injection of hypertonic mannitol; 4) access to 2% NaCl for 48 h produced similar increases in water intake of WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice; and 5) the number of Fos-positive cells was similar within the OVLT and MnPO of different strains but significantly lower in the SFO between WT and TRPV1−/−V4−/− mice. Collectively, these findings suggest that TRPV1 and/or TRPV4 channels may not be the primary mechanism by which the central nervous system detects changes in plasma hypernatremia or hyperosmolality.
A number of studies have indicated that the primary set of osmosensory neurons is located in the OVLT and SFO of the forebrain lamina terminalis (5, 45, 50). Recent and elegant electrophysiology studies performed in vitro have suggested that the osmosensitivity of isolated OVLT neurons is a mechanical process mediated by the TRPV1, but not TRPV4, gene products (11, 12). However, the present study demonstrates that cellular dehydration produced by acute injection of hyperosmotic NaCl or mannitol produced similar increases in water intake in WT versus TRPV1−/− mice. This was a consistent observation regardless of the route of injection (intraperitoneal vs. subcutaneous) or whether mice had immediate versus delayed access to water. These findings confirm a previous report from our laboratory (46). The lack of a difference in cumulative water intakes cannot be explained by the ability of TRPV1−/− mice to excrete the NaCl load more efficiently as we (46) and others (11) have reported that acute NaCl injections produce similar increases in plasma [Na+] or osmolality between WT and TRPV1−/− mice. Indeed, the present study confirms this observation as plasma electrolytes and osmolality did not differ between WT and TRPV1−/− mice (or TRPV4−/− and TRPV1−/−V4−/−) after injection of 0.5 or 1.0 M NaCl. If osmoregulatory function was compromised in TRPV1−/− mice, it may be expected that an acute NaCl load should produce a greater increase in plasma electrolytes or osmolality due to a reduced capacity to concentrate urine and effectively excrete the NaCl load. Furthermore, compared with WT mice, TRPV1−/− mice did not have an elevated basal intake over the testing period, which could have masked a response to acute NaCl loads as reported previously (46). In addition, these mice drank normally in response to nonosmotic challenges such as isoproterenol or furosemide thereby suggesting that deletion of TRPV1 and TRPV4 channels did not alter thirst responses in general. Altogether, these findings suggest that TRPV1 channels do not play a pivotal role in thirst stimulated by cellular dehydration.
What explains the discrepancy between the previous study of Ciura and colleagues (11) versus the findings of the present study and Taylor and colleagues (46)? It is not clear but it is unlikely to be due to differences in experimental methods since the present study incorporated a variety of experimental protocols including those of previous studies (11, 46). The only apparent methodological difference is that Ciura and colleagues removed food at the time of injection, whereas the present study removed food 1 h before injections to limit the impact of recent food ingestion on subsequent water intake. While Ciura and colleagues (11) reported an attenuated water intake of TRPV1−/− versus WT mice after an acute NaCl load, the reported difference was small (∼20–25%). In other words, in the study by Ciura and colleagues (11), deletion of the TRPV1 gene slightly attenuated the water intake stimulated by acute hypernatremia. Yet, lesions of the lamina terminalis or anteroventral region of the third ventricle in mice have been reported to significantly reduce water intake to acute hypernatremia by >75% (20, 27). Collectively, these behavioral data would suggest that deletion of the TRPV1 gene produces slight or no differences in thirst stimulated by acute increases in plasma sodium concentration or osmolality.
TRPV4 channels have also been implicated in central and peripheral osmoregulatory processes (22, 23, 29). TRPV4 mRNA and immunoreactivity has been reported in the ventral lamina terminalis (23). Furthermore, Liedtke and colleagues (23) reported a smaller number of Fos-positive nuclei within the OVLT and a smaller water intake after an acute NaCl load in TRPV4−/− versus WT mice. In contrast, in vitro electrophysiological data suggests TRPV4 channels are not involved in the intrinsic osmosensitivity of isolated OVLT neurons (12). In the current study, TRPV4−/− mice ingested similar amounts of water as WT mice over a range of hypernatremic and hyperosmotic stimuli. TRPV4−/− and WT mice also displayed similar number of Fos-positive neurons after acute NaCl load. While these findings contradict the original report of Liedtke and colleagues (23), it does corroborate the in vitro electrophysiological data (12). Again, the reason for the discrepancy remains unclear. The prior study of Liedtke and colleagues (23) administered a single injection of 0.5 M NaCl (0.4 ml/10 g body wt ip) that stimulated a robust increase in water intake, osmolality, and Fos immunoreactivity. The magnitude of these changes exceeded those of the present study after injection of 0.5 or 1.0 M NaCl and likely attributed to the much larger NaCl load. Although TRPV4−/− mice drank significantly less than WT, the difference was again <15% (23). In the current study, thirst responses were tested over a range of hypernatremic and hyperosmotic stimuli using a number of different experimental protocols. Collectively, these findings suggest TRPV4 channels do not play a prominent role in thirst stimulated by hypernatremia or hyperosmolality.
Since previous reports indicated a role for both TRPV1 and TRPV4 channels in osmoregulatory thirst (11, 23), we measured cumulative water intakes in TRPV1−/−V4−/− mice (double knockout) after a number of different stimuli. Surprisingly, these mice ingested similar amounts of water versus WT mice in response to acute hypernatremia or hyperosmolality despite similar increases in plasma electrolytes and osmolality in response to 0.5 or 1.0 M NaCl. The number of Fos-positive nuclei was also not different between WT and TRPV1−/−V4−/− in the OVLT or MnPO after injection of 0.5 M NaCl; however, there was a statistically smaller number of Fos-positive cells within the SFO of TRPV1−/−V4−/− versus WT mice. This is consistent with the presence of osmosensitive neurons in the SFO (1, 2). Collectively, the combined deletion of TRPV1 and TRPV4 channels did not further disrupt thirst stimulated by hypernatremia or hyperosmolality.
In the present study, we used Fos immunocytochemistry as an index of neuronal activation within the lamina terminalis. The approach has been used by a number of investigators to identify neurons responsive to hypernatremia and other stimuli (21, 36, 40, 46). Although a common interpretation of Fos immunoreactivity is a synaptic and/or membrane depolarization, it is possible that neurons in the OVLT, SFO, and MnPO were responsive to the acute hypernatremia but might have displayed a significantly blunted discharge response in TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− versus WT mice. Hypernatremia also stimulates the secretion of antidiuretic hormone from the posterior pituitary (5, 42), and TRPV channels on magnocellular neurons within the supraoptic nucleus have been postulated to participate in this response (5, 39). We did not quantify the number of Fos-positive neurons within the supraoptic (or hypothalamic paraventricular nuclei), but Fos activation was present in these regions across all strains (unpublished observations). Future experiments will be necessary to provide a comprehensive analysis of antidiuretic hormone (and oxytocin) secretion across a number of paradigms in these mice.
There are several explanations for the lack of apparent differences in thirst or Fos immunoreactivity between WT versus TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice. First, TRPV1 and/or TRPV4 channels do not play a pivotal role osmosensation and thirst responses stimulated by cellular dehydration. Alternatively, another channel either plays a primary role or contributes only after deletion of the TRPV1 and TRPV4 gene at development to cause an adaptive mechanism within osmoregulatory circuits. For example, TRPV2 channels respond to changes in osmolality (24) and have been reported in the OVLT and SFO (33). In addition, extracellular hypertonicity produces cellular shrinking and regulatory volume increase accomplished by the influx of inorganic and organic osmolytes (4, 8) and secondary movement of water. In magnocellular neurons, one such organic osmolyte postulated to participate in hypertonicity-evoked changes in electrical activity is taurine (5, 13). Whether such mechanisms contribute to osmosensory transduction in OVLT neurons has not been tested, but a recent study by Ciura and colleagues (12) suggests the osmosensitivity of OVLT neurons is strictly a mechanical process. As recently reviewed (50), a second possibility is extracellular acidification. In vascular smooth muscle cells or renal mesangial cells, hyperosmolality can increase extracellular pH (32, 43) and increase activity or expression of the Na+/H+ exchanger. This raises the possibility that Na+/H+ exchanger or acid-sensing ion channels could participate in osmosensory transduction. Presently, none of these possibilities has been directly tested. Finally, a series of recent reports suggest the central nervous system detects changes in [Na+] through the voltage-dependent Nax channel (15, 16). While these studies report NaX knockout versus WT mice have a lower Na+ intake or preference during dehydration or lateral ventricle infusion of hypertonic NaCl, cumulative water intakes were not different between strains during these same experimental manipulations. Moreover, the threshold for activation of the NaX channel was reported 157 mM Na+. So far, the available data would not support a role for Nax channel as a central mediator of thirst responses to changes in osmolality or [Na+], but its function warrants further investigation.
A potential confounding variable in the present study is that the majority of experimental manipulations such as the injection of hypertonic NaCl or mannitol may produce visceral pain to subsequently alter thirst responses or central Fos immunoreactivity. Therefore, it is theoretically possible that TRPV1−/− and/or TRPV4−/− mice have an attenuated thirst response but this is masked by removal of the painful stimulus. While this might explain the lack of apparent differences between strains, it does not explain the discordance between the findings of Ciura and Bourque (11) versus the present study or Taylor and colleagues (46). In fact, such experimental manipulations have been consistently used in both rats and mice to investigate the central control of thirst and vasopressin secretion (6, 18, 20, 23, 46). It is also possible that nociceptive activity or the lack thereof may impact Fos immunoreactivity in circumventricular organs. However, we are unaware of a role for such structures in pain responses or central processing of nociceptive inputs. Finally, it is noteworthy that the electrophysiological studies performed to date have focused on the underlying osmosensitivity of OVLT neurons (11, 12). The exact contribution of OVLT neurons to osmoregulatory thirst may be species dependent. For example, classical studies performed in dogs reported that selective lesion of the OVLT greatly diminished thirst and vasopressin secretion stimulated by an intravenous infusion of hypertonic NaCl but not in response to hemorrhage (47, 48). Lesion of the SFO in dogs does not affect these thirst or vasopressin responses to hypertonic NaCl (49). Studies performed in sheep indicate that lesion of the OVLT only does not greatly disrupt osmoregulatory thirst (31). Instead, osmoregulatory thirst was attenuated in animals with combined lesion of the OVLT and SFO, OVLT, or MnPO, or the entire lamina terminalis (31). In rodents, lesions of the AV3V region largely attenuate or eliminate osmotically induced drinking (7, 20, 27), whereas lesion of the SFO produce small deficits in rodents (18, 25). To date, there are no data regarding the contribution of OVLT neurons as selective lesions have not been produced and evaluated. Collectively, these data highlight the potential species differences and multiple forebrain hypothalamic sites that may mediate osmoregulatory responses. Whether the osmosensory processes differs across these structures or between neurons participating in different osmoregulatory responses (thirst, vasopressin secretion, sympathetic nerve activity, natriuresis) remains to be determined.
Perspectives and Significance
In summary, deletion of the TRPV1 and/or TRPV4 gene did not disrupt thirst stimulated by acute hypernatremia or hyperosmolality. In addition, acute hypernatremia produced similar increases in the number of Fos-positive neurons within the lamina terminalis of WT, TRPV1−/−, TRPV4−/−, and TRPV1−/−V4−/− mice. Collectively, these findings suggest that products encoded by the TRPV1 and/or TRPV4 genes do not greatly contribute to osmoregulatory thirst. Moreover, these findings highlight that the molecular identity of putative signaling mechanisms by which neurons sense changes in osmotic pressure or cell volume to underlie osmosensory processes remains unknown.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute R01 HL-113270 (to S. D. Stocker), an American Heart Association Established Investigator Grant (to S. D. Stocker), and the Pennsylvania Department of Health using Tobacco CURE Funds (to S. D. Stocker). The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.K., J.C., K.N.B., and S.D.S. conception and design of research; B.K., J.C., J.L., S.S.S., and S.D.S. performed experiments; B.K., J.C., J.L., S.S.S., and S.D.S. analyzed data; B.K., J.C., J.L., S.S.S., K.N.B., and S.D.S. interpreted results of experiments; B.K. and S.D.S. drafted manuscript; B.K., J.C., J.L., S.S.S., K.N.B., and S.D.S. edited and revised manuscript; B.K., J.C., J.L., S.S.S., K.N.B., and S.D.S. approved final version of manuscript; S.D.S. prepared figures.
ACKNOWLEDGMENTS
The authors thank Shane Miller for technical assistance, and Dr. Wolfgang Liedtke for generously providing the TRPV1−/−V4−/− mice. We also thank Dr. John J. McCarthy for assistance in genotyping.
REFERENCES
- 1.Anderson JW, Smith PM, Ferguson AV. Subfornical organ neurons projecting to paraventricular nucleus: whole-cell properties. Brain Res 921: 78–85, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Anderson JW, Washburn DL, Ferguson AV. Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience 100: 539–547, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Antunes VR, Yao ST, Pickering AE, Murphy D, Paton JF. A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J Physiol 576: 569–583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arieff AI, Guisado R. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int 10: 104–116, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9: 519–531, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Buggy J, Johnson AK. Anteroventral third ventricle periventricular ablation: temporary adipsia and persisting thirst deficits. Neurosci Lett 5: 177–182, 1977 [DOI] [PubMed] [Google Scholar]
- 7.Buggy J, Johnson AK. Preoptic-hypothalamic periventricular lesions: thirst deficits and hypernatremia. Am J Physiol Regul Integr Comp Physiol 233: R44–R52, 1977 [DOI] [PubMed] [Google Scholar]
- 8.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31: 5067–5077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26: 9069–9075, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciura S, Liedtke W, Bourque CW. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: a mechanical process involving TRPV1 but not TRPV4. J Neurosci 31: 14669–14676, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol 507: 463–471, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutman MB, Ciriello J, Mogenson GJ. Effects of plasma angiotensin II and hypernatremia on subfornical organ neurons. Am J Physiol Regul Integr Comp Physiol 254: R746–R754, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Hiyama TY, Watanabe E, Okado H, Noda M. The subfornical organ is the primary locus of sodium-level sensing by Na(x) sodium channels for the control of salt-intake behavior. J Neurosci 24: 9276–9281, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiyama TY, Watanabe E, Ono K, Inenaga K, Tamkun MM, Yoshida S, Noda M. Na(x) channel involved in CNS sodium-level sensing. Nat Neurosci 5: 511–512, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Honda K, Negoro H, Dyball RE, Higuchi T, Takano S. The osmoreceptor complex in the rat: evidence for interactions between the supraoptic and other diencephalic nuclei. J Physiol 431: 225–241, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosutt JA, Rowland N, Stricker EM. Impaired drinking responses of rats with lesions on the subfornical organ. J Comp Physiol Psychol 95: 104–113, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Johnson AK. The sensory psychobiology of thirst and salt appetite. Med Sci Sports Exerc 39: 1388–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Johnson RF, Beltz TG, Thunhorst RL, Johnson AK. Investigations on the physiological controls of water and saline intake in C57BL/6 mice. Am J Physiol Regul Integr Comp Physiol 285: R394–R403, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. J Neurosci 15: 2609–2627, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner SG, Markworth S, Poole K, Smith ES, Lapatsina L, Frahm S, May M, Pischke S, Suzuki M, Ibanez-Tallon I, Luft FC, Jordan J, Lewin GR. The molecular and cellular identity of peripheral osmoreceptors. Neuron 69: 332–344, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Liedtke W, Friedman JM. Abnormal osmotic regulation in TRPV4−/− mice. Proc Natl Acad Sci USA 100: 13698–13703, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedtke WB. TRPV channels' function in osmo- and mechanotransduction. In: TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, edited by Liedtke WB, Heller S. Boca Raton, FL: CRC, 2007, Chapt. 22 [PubMed] [Google Scholar]
- 25.Lind RW, Thunhorst RL, Johnson AK. The subfornical organ and the integration of multiple factors in thirst. Physiol Behav 32: 69–74, 1984 [DOI] [PubMed] [Google Scholar]
- 26.Mannari T, Morita S, Furube E, Tominaga M, Miyata S. Astrocytic TRPV1 ion channels detect blood-borne signals in the sensory circumventricular organs of adult mouse brains. Glia 61: 957–971, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 107: 263–270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAllen RM, Pennington GL, McKinley MJ. Osmoresponsive units in sheep median preoptic nucleus. Am J Physiol Regul Integr Comp Physiol 259: R593–R600, 1990 [DOI] [PubMed] [Google Scholar]
- 29.McHugh J, Keller NR, Appalsamy M, Thomas SA, Raj SR, Diedrich A, Biaggioni I, Jordan J, Robertson D. Portal osmopressor mechanism linked to transient receptor potential vanilloid 4 and blood pressure control. Hypertension 55: 1438–1443, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol 16: 340–347, 2004 [DOI] [PubMed] [Google Scholar]
- 31.McKinley MJ, Mathai ML, Pennington G, Rundgren M, Vivas L. Effect of individual or combined ablation of the nuclear groups of the lamina terminalis on water drinking in sheep. Am J Physiol Regul Integr Comp Physiol 276: R673–R683, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Miyata Y, Muto S, Yanagiba S, Asano Y. Extracellular Cl− modulates shrinkage-induced activation of Na+/H+ exchanger in rat mesangial cells. Am J Physiol Cell Physiol 278: C1218–C1229, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Nedungadi TP, Dutta M, Bathina CS, Caterina MJ, Cunningham JT. Expression and distribution of TRPV2 in rat brain. Exp Neurol 237: 223–237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilius B. TRP channels in disease. Biochim Biophys Acta 1772: 805–812, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Nissen R, Bourque CW, Renaud LP. Membrane properties of organum vasculosum lamina terminalis neurons recorded in vitro. Am J Physiol Regul Integr Comp Physiol 264: R811–R815, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience 60: 255–262, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol 428: 183–207, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Sayer RJ, Hubbard JI, Sirett NE. Rat organum vasculosum laminae terminalis in vitro: responses to transmitters. Am J Physiol Regul Integr Comp Physiol 247: R374–R379, 1984 [DOI] [PubMed] [Google Scholar]
- 39.Sharif Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 9: 93–98, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Shi P, Martinez MA, Calderon AS, Chen Q, Cunningham JT, Toney GM. Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus. J Physiol 586: 5231–5245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sladek CD, Johnson AK. Effect of anteroventral third ventricle lesions on vasopressin release by organ-cultured hypothalamo-neurohypophyseal explants. Neuroendocrinology 37: 78–84, 1983 [DOI] [PubMed] [Google Scholar]
- 42.Sladek CD, Johnson AK. Integration of thermal and osmotic regulation of water homeostasis: the role of TRPV channels. Am J Physiol Regul Integr Comp Physiol 305: R669–R678, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soleimani M, Bookstein C, McAteer JA, Hattabaugh YJ, Bizal GL, Musch MW, Villereal M, Rao MC, Howard RL, Chang EB. Effect of high osmolality on Na+/H+ exchange in renal proximal tubule cells. J Biol Chem 269: 15613–15618, 1994 [PubMed] [Google Scholar]
- 44.Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol 563: 249–263, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stocker SD, Monahan KD, Browning KN. Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Curr Hypertens Rep 15: 538–546, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor AC, McCarthy JJ, Stocker SD. Mice lacking the transient receptor vanilloid potential 1 channel display normal thirst responses and central Fos activation to hypernatremia. Am J Physiol Regul Integr Comp Physiol 294: R1285–R1293, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Thrasher TN, Keil LC. Regulation of drinking and vasopressin secretion: role of organum vasculosum laminae terminalis. Am J Physiol Regul Integr Comp Physiol 253: R108–R120, 1987 [DOI] [PubMed] [Google Scholar]
- 48.Thrasher TN, Keil LC, Ramsay DJ. Lesions of the organum vasculosum of the lamina terminalis (OVLT) attenuate osmotically-induced drinking and vasopressin secretion in the dog. Endocrinology 110: 1837–1839, 1982 [DOI] [PubMed] [Google Scholar]
- 49.Thrasher TN, Simpson JB, Ramsay DJ. Lesions of the subfornical organ block angiotensin-induced drinking in the dog. Neuroendocrinology 35: 68–72, 1982 [DOI] [PubMed] [Google Scholar]
- 50.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol 588: 3375–3384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veerasingham SJ, Leenen FH. Excitotoxic lesions of the ventral anteroventral third ventricle and pressor responses to central sodium, ouabain and angiotensin II. Brain Res 749: 157–160, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Watson RE, Jr., Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7: 155–159, 1986 [DOI] [PubMed] [Google Scholar]