Abstract
We tested the hypothesis that older muscle has greater metabolic economy (ME) in vivo than young, in a manner dependent, in part, on contraction intensity. Twenty young (Y; 24 ± 1 yr, 10 women), 18 older healthy (O; 73 ± 2, 9 women) and 9 older individuals with mild-to-moderate mobility impairment (OI; 74 ± 1, 7 women) received stimulated twitches (2 Hz, 3 min) and performed nonfatiguing voluntary (20, 50, and 100% maximal; 12 s each) isometric dorsiflexion contractions. Torque-time integrals (TTI; Nm·s) were calculated and expressed relative to maximal fat-free muscle cross-sectional area (cm2), and torque variability during voluntary contractions was calculated as the coefficient of variation. Total ATP cost of contraction (mM) was determined from flux through the creatine kinase reaction, nonoxidative glycolysis and oxidative phosphorylation, and used to calculate ME (Nm·s·cm−2·mM ATP−1). While twitch torque relaxation was slower in O and OI compared with Y (P ≤ 0.001), twitch TTI, ATP cost, and economy were similar across groups (P ≥ 0.15), indicating comparable intrinsic muscle economy during electrically induced isometric contractions in vivo. During voluntary contractions, normalized TTI and total ATP cost did not differ significantly across groups (P ≥ 0.20). However, ME was lower in OI than Y or O at 20% and 50% MVC (P ≤ 0.02), and torque variability was greater in OI than Y or O at 20% MVC (P ≤ 0.05). These results refute the hypothesis of greater muscle ME in old age, and provide support for lower ME in impaired older adults as a potential mechanism or consequence of age-related reductions in functional mobility.
Keywords: bioenergetics, mitochondria, creatine kinase, glycolysis, oxidative phosphorylation
while many of the changes in neuromuscular properties that occur with advanced age, such as declines in strength and contractile velocity (5, 64), can be detrimental to physical function, some age-related physiological changes may act in a compensatory manner and, thereby, help maintain function to some degree. For instance, it has been suggested that a greater proportion of type I muscle fibers (35, 55, 56), slowed contractile properties (62, 71), and slower motor unit discharge rates, particularly during maximal contractions (8, 12, 37, 38, 68), may place older muscle at an economic advantage (41, 49). While age-related differences in muscle metabolic economy (ME; mass-normalized torque produced per unit ATP consumed) have been shown in rat muscle (13, 32), a systematic analysis of muscle ME in aging humans has only recently begun (41, 54). Such a focus on muscle ME is critical to informing studies of age-related changes in the energy cost of whole body activities, such as walking, which generally involve measures of oxygen consumption, rather than ATP consumption, per se (33, 58).
Economy of muscle contraction is a multifactorial issue, which could be influenced by metabolic processes, mechanical properties, neural input, and coordinated activation of agonists, synergists, and antagonists. Therefore, a complete understanding of economy of muscle contractions requires a collective effort using a combination of approaches. A recent study by Layec et al. (54) indicated lower ME in older compared with young adults during dynamic plantar flexion exercise. However, given that the age-related alterations in muscle energetics vary among muscles (51, 65), this finding does not rule out different adaptations in other locomotory muscles.
Here, we employ isometric contractions of the tibialis anterior, a single-joint muscle responsible for the majority of dorsiflexor force production, to evaluate muscle ME in young and older adults. Such an approach controls for many of the mechanical and coordination parameters that influence muscle ME during dynamic contractions. We also include a measure of economy during stimulated contractions, which isolates the properties of the muscle itself, thus eliminating variability in neural input as a confounder. The information gained through this controlled approach, using simultaneous metabolic and torque measures, helps identify 1) the conditions under which age-related differences in muscle ME are evident, and 2) the potential sources of these differences.
The ME of muscle contraction in vivo is established, in part, by muscle fiber composition, as type II fibers have been shown to be less efficient than type I fibers in both humans (26, 30) and animals (11, 19), including during isometric contractions. This difference in efficiency is due, in part, to a lower cross-bridge cycling duty ratio (30) and lower cost of calcium handling (73) in type I fibers. Further, according to Henneman's size principle (31), an increasing proportion of type II muscle fibers are recruited as voluntary contraction intensity increases. As such, it can be hypothesized that in mixed muscle, ME will decrease with increasing contraction intensity, as a greater proportion of less efficient, type II fibers are recruited to contribute to force production. Although conflicting evidence exists (3, 63, 70), an inverse relationship between contraction intensity and ME has been observed in young adults, along with an association between average ME and type II fiber area (34).
With advanced age, there can be a modest shift in fiber type toward a greater proportion of type I fibers (23, 35, 55, 56, 77), suggesting that older muscle may be inherently more economical than young on the basis of fiber-type composition. It can be further hypothesized that a lower proportion of type II fibers in older muscle (23, 35, 55, 56, 77) should protect against a decline in ME with increasing contraction intensity, as relatively fewer type II fibers would contribute to force production at greater intensities in older adults. However, this hypothesis has not been tested in aged humans. Furthermore, very little attention has focused on muscle metabolic function in older individuals with physical impairments. Information regarding muscle ME may be useful for understanding the mechanisms of impaired physical function in older populations, as muscle physiological properties have been suggested to play a role in the development of frailty in aging (75).
The purpose of this study was to quantify the effects of old age and physical impairment on ME of the ankle dorsiflexor muscles during isometric contractions. Electrically stimulated twitch contractions were used to determine the inherent muscular component of ME, while voluntary isometric contractions at 20%, 50%, and 100% maximal voluntary contraction (MVC) were used to distinguish neural and muscular contributions to ME.
Consistent with the reported shift toward a slower muscle phenotype (35, 55, 56, 77), we hypothesized that twitch economy would be greater in older individuals compared with young, regardless of functional status. Assuming that a voluntary contraction at 20% MVC would recruit primarily economical, slow-twitch muscle fibers, regardless of age, we hypothesized that ME would not differ across groups at this intensity. It was anticipated that the impact of age-related differences in fiber type (35, 55, 56) on ME would become apparent at 50% MVC, as essentially all motor units in the tibialis anterior muscle are recruited at this intensity (16). Therefore, we hypothesized that ME would be greater in older compared with young muscle at 50% MVC. At 100% MVC, we expected that slower motor unit discharge rates (8, 12, 37, 38, 68) would add to the economic advantage of older individuals, resulting in an even greater age-related difference in ME during maximal contractions.
METHODS
Twenty young (24 ± 1 yr, 10 women), 18 older healthy (73 ± 2 yr, 9 women), and 9 older individuals with signs of mobility impairments (74 ± 1 yr, 7 women) took part in the study. To minimize potential effects of exercise habits and disease, and focus on age, all participants were relatively sedentary (<60 min of structured activity per week), free of cardiovascular and neuromuscular disorders, and not taking any medications known to affect neuromuscular or metabolic function. Physician's consent for participation was obtained for all older individuals prior to study. All participants completed two visits, one to the Muscle Physiology Laboratory at the University of Massachusetts, where preliminary measures of participant characteristics and muscle properties were obtained and familiarization with study procedures was accomplished. The second visit was to the Magnetic Resonance Research Center at the Yale University School of Medicine, where metabolic measures and magnetic resonance images of the leg were obtained. The study procedures were reviewed and approved by the Institutional Review Board at the University of Massachusetts and by the Human Investigations Committee at the Yale University School of Medicine.
Preliminary Testing
Physical function.
All older individuals completed the short physical performance battery (SPPB) (25), prior to participation. The SPPB is a test of physical function that involves balance tasks, chair rises, and a 6-m walk, and is predictive of future mortality (25). Participants are scored on a scale of 0–12, with a score of 12 indicating no mobility impairments, and a score of 7–11 indicating early mobility impairments and increased risk of loss of mobility or mortality (25). The older healthy group comprised individuals who scored a 12 on the SPPB, while the older impaired group comprised those who scored less than 12 (range 9–11). As a score of 12 introduces a ceiling effect (such that both a sedentary but unimpaired person and a highly trained person would each score a 12), the functional difference between an individual who scores 11 and one who scores 12 can vary. In a 6-year follow-up study, all-cause mortality rate was 1.3 per 100 people for individuals who scored a 12, and 2.0 per 100 people for individuals who scored an 11 (25).
Strength.
With participants lying supine, the foot was secured into a custom-built apparatus, with the ankle fixed at 30° of plantar flexion. This device is designed to measure isometric dorsiflexion torque, and has been described elsewhere (49, 52, 74). Because of the difficulty in isolating leg preference based only on the dorsiflexor muscles, leg dominance was not determined. The right leg was used for all except three participants, who had previously had ankle (n = 1) or knee (n = 2) surgery on the right leg. The signal from the force transducer was sampled at 500 Hz. Participants performed three MVCs, each lasting 3–4 s and separated by 2 min of rest. Additional MVC trials were conducted if the two highest values differed by more than 10%. Peak torque obtained during these trials was recorded as the MVC. After determining maximal stimulus intensity based on the compound muscle action potential (M-wave, see below), participants were asked to produce one additional MVC, during which a 50-Hz supramaximal stimulus was applied to the peroneal nerve for 500 ms to determine the central activation ratio, a measure of the completeness of voluntary activation (42). All participants achieved at least 90% complete activation.
Contractile properties.
To quantify muscle contractile properties for use as an indirect measure of fiber type composition, a stimulating electrode (1 cm in diameter, 1-cm interelectrode distance; Grass Technologies, West Warwick, RI) was secured over the peroneal nerve. A 1-cm diameter gold disk recording electrode (Grass Technologies) was placed over the belly of the tibialis anterior muscle with a reference electrode over the tibial tendon at the ankle. A ground electrode was placed between the stimulating and recording electrodes. A single stimulus (200 μs in duration) was applied to the nerve at 115% of the intensity required to elicit a maximal electrical (M-wave) response from the muscle. The corresponding twitch response was processed using a custom-written MatLab (Mathworks, Natick, MA) program to determine peak torque (Nm), which was also expressed relative to muscle CSA (N·m/cm−2) and maximal isometric strength (% MVC). The half-relaxation time for twitch torque (T1/2, ms) was also determined.
Physical activity.
To ensure that participants were relatively sedentary and to compare habitual physical activity levels between groups, activity was quantified with a uniaxial accelerometer (Actigraph GT1M, Pensacola, FL), which each participant wore over a 10-day period during all waking hours. Data were sampled at 20 Hz and stored in 60-s epochs. Participants also completed a physical activity log to corroborate the accelerometer data. Using a custom-written MatLab program, total physical activity counts for each participant were averaged across a minimum of 7 days, including at least one weekend day and excluding testing days.
Muscle Size Measure
To calculate maximal fat-free muscle cross-sectional area (mCSA) for use in the calculation of ME, magnetic resonance images of the leg were obtained using a 1.5-Tesla magnet (Siemens Sonata, Munich, Germany). Participants were positioned supine, and a phased array coil was placed over the leg and positioned in the isocenter of the magnet. Serial T1-weighted axial images were obtained from the tibial plateau to the lateral malleolus, using the following parameters: 90° flip angle, 11-ms echo time, 4-mm slice thickness with no gap, 400-ms repetition time, a 210 × 210-mm field of view, and a 512 × 512 reconstruction matrix. In most cases, these images were obtained during the metabolic testing visit to the Yale Magnetic Resonance Research Center. Where scheduling conflicts prohibited collection of the images at Yale, participants agreed to an additional visit to the Amherst Community Imaging Center at Cooley Dickinson Hospital, where images were collected on a 1.5-Tesla system (Siemens Espree, Munich, Germany), using the same parameters described above.
Custom-written MatLab software was used to outline the dorsiflexor muscle group in each slice and separate pixels into muscle, fat, and connective tissue on the basis of signal intensity (44). Total area was determined for each tissue type, and the five consecutive slices with the largest fat-free mCSA were re-analyzed to ensure accuracy in the area calculations. The average of the three adjacent slices with the largest mCSA was used to define maximal mCSA (cm2). This method of obtaining the mCSA provides an accurate reflection of muscle volume during isometric contractions of the ankle dorsiflexor muscles in young and older adults (29). To adjust for expected age-related differences in muscle mass (and, therefore, torque-producing capacity), the torque-time integral (TTI; see below) obtained for the metabolic calculations was expressed relative to mCSA (Nm·s·cm−2).
Muscle Metabolic and Torque Measures
Each participant was positioned supine on the patient bed of a 4-T magnet (Bruker Biospin, Rheinstetten, Germany), with the foot strapped into the custom-built force apparatus and a probe assembly containing a 7-cm circular proton (1H) and a 3 × 4 cm phosphorous (31P) coil secured over the belly of the tibialis anterior muscle. The muscle was then positioned in the isocenter of the magnet. The FASTMAP procedure (72) was used for local shimming on the muscle water peak, which resulted in a mean width at half height of 10.3 ± 1.4 Hz for the phosphocreatine (PCr) peak, indicating excellent magnetic field homogeneity in the muscle region of interest.
After optimizing the magnet for metabolic measures, the MVC was quantified as described above, and two brief (3–4 s) MVCs, separated by 2-min rest, were performed 2 min prior to the metabolic measures, to “warm up” the muscle and standardize metabolic conditions across participants.
2-Hz stimulated contractions.
As described above for determination of contractile properties, electrical stimulation was applied to the peroneal nerve at 115% of the intensity required to elicit a maximal M-wave. This stimulation was provided at 2 Hz for 3 min. A sample torque recording from this stimulated protocol is shown in Fig. 1. Spectra were acquired for 1 min (plus 8 dummy pulses) prior to stimulation, throughout the 3 min of stimulation and for 10 min following the stimulation. The pulse parameters included a 100-μs hard pulse with a nominal 60° flip angle, 8-kHz spectral width, 2,048 data points and 2-s repetition time (TR). The TTI (Nm/s) was calculated for each twitch contraction, expressed relative to mCSA (cm2), and averaged across the 3-min protocol. Because of participant discomfort (n = 1) or technical difficulties (n = 2), the 2-Hz protocol was obtained from six of the nine older impaired individuals.
Fig. 1.
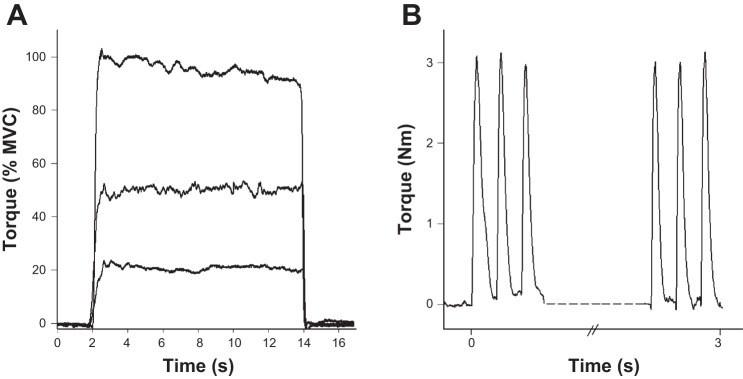
Sample torque recordings from a young female participant showing the voluntary (A) and stimulated (B) contraction protocols. A: voluntary contractions of the ankle dorsiflexors were performed for 12 s each, at 20%, 50%, and 100% maximum voluntary contraction (MVC). B: peroneal nerve was stimulated at 2 Hz to induce twitch contractions of the ankle dorsiflexors for 3 min. Shown here are the first three and final three twitch responses from a sample recording.
Voluntary contractions.
Following the 2-Hz protocol and while keeping the participant in the same position within the magnet, the stimulating electrodes were removed from the leg to improve the phosphorus signal-to-noise ratio. Following 5 min of rest, participants performed a 3- to 4-s MVC to ensure the muscle was not fatigued. Next, the participant completed a series of 12 dorsiflexor contractions (6 contractions at 20% MVC, 3 at 50% MVC, and 3 at 100% MVC, in random order), each lasting 12 s. Participants were provided with real-time feedback of their torque via a light-emitting diode box upon which actual and target torque were displayed. Sample torque traces from one trial at each contraction intensity are shown in Fig. 1. To avoid fatigue, participants were provided 3-min rest following the 20% and 50% MVC contractions, and 10-min rest following the 100% MVC contractions. Spectra were acquired for 1 min (plus 8 dummy pulses) prior to (resting baseline), throughout, and for 3 (20% and 50% MVC conditions) or 10 (100% MVC) min following each 12-s contraction. The pulse parameters were the same as those described above for stimulated measures.
For each of the 12-s contractions, the TTI (Nm/s) was calculated using MatLab and expressed relative to mCSA (cm2). This normalized TTI was then averaged across trials at each contraction intensity, for each participant. Variability in torque production was also quantified as the coefficient of variation (%) in relative torque (% MVC) and averaged across trials for each intensity.
Spectral Processing and Metabolic Calculations
Using a custom-written MatLab program, all free-induction decays were zero-filled and Fourier-transformed prior to line-fitting. A 10-Hz line broadening was applied, and spectra were manually phased. After frequency correction to set the PCr peak to 0 Hz, spectra were aligned temporally and averaged within each contraction intensity (six contractions at 20% MVC, 3 at 50% MVC, 3 at 100% MVC). Using NUTS software (Acorn NMR, Livermore, CA), the broad peak in the spectra due to phosphorous in bone was removed with a polynomial fit to obtain a flat baseline. To improve the signal-to-noise ratio, spectra then were time-averaged as follows: for the 2-Hz stimulated contractions: 1-min averaging at rest, 10-s averaging during stimulation, and 30-s averaging during recovery; for voluntary contractions: 1-min averaging at rest, 4-s averaging during each contraction, and for the first 16 s of recovery. Eight-second averaging was used for the remainder of the recovery in the 20% MVC and 50% MVC conditions and for the first 5 min of recovery for 100% MVC. Thirty-second averaging then was used for minutes 5–10 in the 100% MVC condition. The area under the peaks corresponding to PCr, Pi, and phosphomonoesters were quantified by fitting each peak with a Lorenzian-shaped curve. Where splitting of the Pi peak was evident (n = 4 participants at 100% MVC), two curves were used to fit Pi, and the weighted average of the two peaks was used (49).
Concentrations of Pi and PCr were calculated using the assumption that [Pi] + [PCr] = 42.5 mM in resting muscle (60). Peak areas were corrected for partial saturation effects using correction factors calculated from 24 partially saturated (TR = 2 s) and 24 fully relaxed (TR = 30 s) spectra in a subset of participants (8 young, 8 older). Intracellular pH was calculated on the basis of the chemical shift between Pi and PCr (61).
ATP cost of contraction.
Because under our experimental conditions there is no net change in [ATP], ATP production is equivalent to ATP consumption. The rates of ATP production through the creatine kinase (CK) reaction (ATPCK), nonoxidative glycolysis (ATPGLY), and oxidative phosphorylation (ATPOX) were calculated at each intensity (48, 49, 74).
The rate of net PCr breakdown by the CK reaction was established by the rate of decline in PCr determined using linear regression of the four data points obtained for each 12-s contraction (at 0, 4, 8 and 12 s), as follows:
Oxidative ATP synthesis was calculated on the basis of the assumptions that resting [ATP] = 8.2 mM (28), and the phosphorylation potential ([Pi][ADP]/[ATP]) is regulated with a Km of 0.11 (83), as follows:
where Vmax (mM ATP/s), the theoretical maximal muscle oxidative capacity, was calculated from the 100% MVC condition as the product of the rate constant of PCr recovery (kPCr) following the 12-s maximal contraction and resting [PCr] (60). [ADP] was calculated on the basis of the assumption that free creatine and Pi are equal in muscle (83), as follows:
where [Pi] and [PCr] were averaged across the 12-s contraction, and kCK is the equilibrium constant of the CK reaction corrected for pH, as previously described by Golding et al. (21) and assuming [Mg2+] = 1 mM.
ATPGLY was calculated from changes in pH and PCr during each 12-s contraction (rates were calculated using linear regression for each). Proton consumption by the CK reaction, buffering, and oxidative proton production also were taken into account, as follows:
where θ is the proton stoichiometry for the CK reaction coupled with ATP hydrolysis and m accounts for the protons generated by oxidative phosphorylation (39), each averaged across the 12-s contraction. Under our conditions (i.e., no net acidosis at any contraction intensity), proton efflux was assumed to be negligible (40). Overall buffering (β) was computed as the sum of inherent buffering, and buffering from bicarbonate and inorganic phosphate (39).
The total rate of ATP production (ATPTOT, mM/s) was calculated as the sum of fluxes through each of the three metabolic pathways. The total amount of ATP produced by each pathway (mM) at each intensity, was determined by multiplying the corresponding rate by the total contraction duration (i.e., 12 s). To evaluate the relative contributions of ATP production from oxidative pathways, the oxidative ratio was calculated as ATPOX/(ATPCK + ATPGLY + ATPOX).
Our methods for calculating the cost of a single muscle twitch from 3 min of 2-Hz stimulation have been described previously (74). Briefly, the initial rate of change in PCr was used to calculate twitch cost, as follows:
where dPCr/dtt=0 is the initial rate of change in PCr, derived from an exponential fit of the time course of the decline in PCr over the 3-min protocol (17).
Muscle Metabolic Economy
For voluntary contractions ME was calculated for each intensity as the normalized TTI produced per unit ATP (Nm·s·cm−2·mM ATP−1). Estimation of twitch economy was accomplished for each individual using the average twitch TTI for the 3 min of stimulation and ATPTOT calculated from the cost of a single twitch (Nm·s·cm−2·mM ATP−1).
Statistical Analyses
One-way ANOVA was used to compare group (age, height, body mass, and physical activity counts) and muscle (MVC, mCSA, contractile properties, twitch economy, and oxidative capacity) characteristics. Two-factor (group, contraction intensity), repeated-measures ANOVAs with Greenhouse-Geisser corrections were used to compare muscle metabolic variables, pathway-specific ATP production, total ATP cost, TTI, variability in torque, and ME between groups and across contraction intensities. Where significant effects were found, post hoc pairwise comparisons were performed. Linear regression analyses were used to explore relationships between twitch economy and ME values at different contraction intensities. Data are presented as means ± SE, and statistical significance (two-sided) was set at P = 0.05. We appreciate that the smaller number of participants in the older impaired group could impact the significance of the observed differences. Therefore, where comparisons involving the older impaired group approached significance, effect sizes were computed using Cohen's d, to determine the magnitude of the effect independent of sample size (9).
RESULTS
Study group characteristics are presented in Table 1. The older healthy and impaired groups were similar in age (P = 0.89). Height (P = 0.46) and body mass (P = 0.25) did not differ across groups, and daily physical activity did not differ between young and older healthy individuals (P = 0.72). However, physical activity counts were lower in the older impaired group compared with either young (P = 0.02) or healthy older individuals (P < 0.001).
Table 1.
Group characteristics
| Young (n = 20) | Older Healthy (n = 18) | Older Impaired (n = 9) | |
|---|---|---|---|
| Age, yr | 23.5 ± 0.53 | 73.2 ± 1.3* | 74.0 ± 1.4* |
| Height, m | 1.6 ± 0.05 | 1.7 ± 0.02 | 1.6 ± 0.03 |
| Body mass, kg | 66.8 ± 2.6 | 74.5 ± 2.4 | 75.8 ± 3.5 |
| Physical activity, counts·day−1·1000−1 | 212.2 ± 17.6 | 234.1 ± 24.0 | 108.5 ± 13.2*‡ |
Data are expressed as means ± SE for each group.
Significantly different from young (P ≤ 0.02).
Significantly different from older healthy (P ≤ 0.02).
Baseline Muscle Characteristics
Group data for baseline muscle characteristics are presented in Table 2. MVC torque (P = 0.58) and the central activation ratio (P = 0.22) were not different across groups. Fat-free mCSA was smaller in the older impaired group, compared with young (P = 0.02) or older healthy (P = 0.002) but was similar between young and older healthy groups (P = 0.53). Neither kPCr nor Vmax differed among groups (P = 0.29, P = 0.54, respectively), indicating similar in vivo muscle oxidative capacities in the study groups.
Table 2.
Muscle characteristics
| Young | Older Healthy | Older Impaired | |
|---|---|---|---|
| MVC, Nm | 38.9 ± 2.0 | 34.0 ± 2.0 | 32.2 ± 3.7 |
| Central activation ratio | 0.97 ± 0.02 | 0.98 ± 0.01 | 0.92 ± 0.07 |
| Peak twitch torque, Nm·cm−2 | 0.49 ± 0.02 | 0.46 ± 0.02 | 0.46 ± 0.02 |
| Peak twitch torque, % MVC | 11.2 ± 0.4 | 12.0 ± 0.8 | 11.8 ± 0.5 |
| T1/2 twitch torque relaxation, ms | 95.6 ± 3.5 | 130.3 ± 5.3* | 144.4 ± 7.4* |
| mCSA, cm2 | 9.7 ± 0.53 | 10.0 ± 0.33 | 7.7 ± 0.54*‡ |
| Muscle oxidative capacity | |||
| kPCr, s−1 | 0.025 ± 0.001 | 0.026 ± 0.001 | 0.029 ± 0.004 |
| Vmax, mM ATP/s | 0.99 ± 0.03 | 0.97 ± 0.05 | 1.08 ± 0.16 |
Data are expressed as means ± SE for each group. MVC, maximal voluntary contraction; mCSA, muscle fat- free cross-sectional area; kPCr, rate constant for PCr recovery following 12-s maximal contraction; Vmax, maximal muscle oxidative capacity.
Significantly different from young (P ≤ 0.02).
Significantly different from older healthy (P ≤ 0.02).
Torque Production and Metabolic Changes During Voluntary Contractions
As designed, the TTI increased with increasing contraction intensity (P < 0.001, Fig. 2A). There was no significant difference in normalized TTI across groups (P = 0.20) and no group × intensity interaction (P = 0.28), indicating similar torque production, relative to mCSA, among all groups at each contraction intensity (Fig. 2A).
Fig. 2.
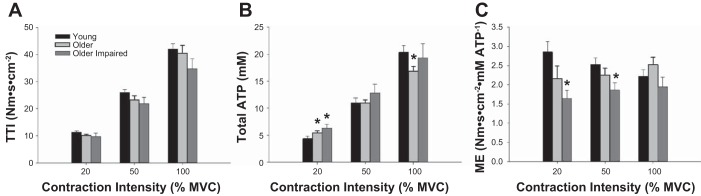
Torque-time integral (TTI), ATP cost, and metabolic economy. Normalized TTI (A), total ATP production (B), and metabolic economy (C) during voluntary contractions at 20%, 50%, and 100% MVC. A: as designed, the TTI, normalized to fat-free muscle size, increased with increasing contraction intensity (P < 0.001), with no differences across groups (P = 0.20). B: total ATP cost of contraction was not different across groups (P = 0.39), increased with increasing contraction intensity (P < 0.001), and showed a group × intensity interaction (P = 0.04). C: overall, metabolic economy (ME) was similar across contraction intensities (P = 0.21) and was lower in older impaired individuals than young or older healthy (P ≤ 0.04). A group × intensity interaction was also observed for ME (P = 0.04). Data are expressed as means ± SE for each group. *Significantly different from young (P ≤ 0.05).
The CV of torque production differed by contraction intensity (P < 0.001), such that variability was greater at 20% (P < 0.001) and 100% (P = 0.001) than at 50% MVC, with no significant difference between 20% and 100% MVC (P = 0.90; Fig. 3). There was a main effect of group, as overall torque variability was greater in older impaired compared with young (P = 0.001) and tended to be greater in the impaired than the healthy older group (P = 0.07), but was not different between young and healthy older (P = 0.37). There was also a group × intensity interaction (P = 0.04), as there was less torque variability in the young group than older healthy (P = 0.01) or impaired groups (P = 0.001) at 20% MVC, with no significant group differences at 50% (P ≥ 0.61) or 100% MVC (P ≥ 0.19). The torque CV was similar between the first and last contractions at 20% MVC in young (P = 0.40), older healthy (P = 0.11) and older impaired groups (P = 0.21), indicating that the observed difference was not due to a differential learning effect across groups.
Fig. 3.
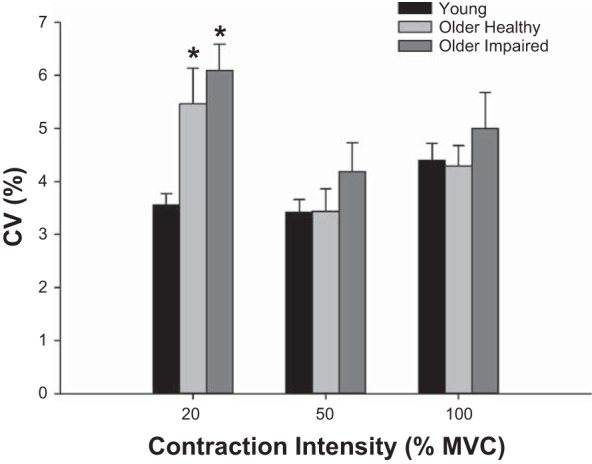
Torque variability during voluntary contractions. Coefficient of variation (CV) in torque production in each group during 12-s voluntary isometric contractions at 20%, 50%, and 100% MVC. Overall, the CV was greater at 20% and 100% MVC than at 50% (P ≤ 0.01) and was greater in older impaired compared with young (P = 0.001). There was also a group × intensity interaction (P = 0.04). Data are expressed as means ± SE for each group. *Significantly different from young (P ≤ 0.05).
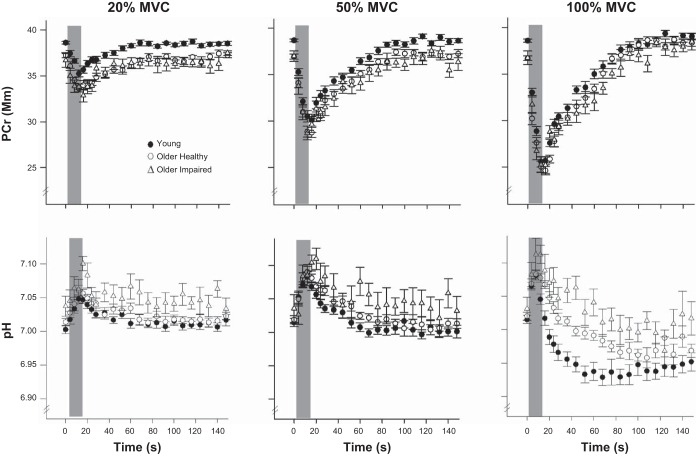
Selected metabolic variables are presented in Table 3. Resting [PCr] was greater in young than in older healthy (P = 0.001) or older impaired (P < 0.001) groups, but was not different at baseline across contraction intensities (P = 0.51). Resting pH did not differ across groups (P = 0.16) or contraction intensity (P = 0.20). Mean changes in [PCr] and pH during the contractions are shown for each group in Fig. 4. Phosphocreatine at the end of contraction (PCrend, % rest) decreased with increasing contraction intensity (P < 0.001), but these changes were similar across groups (P = 0.77). Likewise, the minimum pH reached following contraction decreased with increasing contraction intensity (P < 0.001), with no significant differences across groups (P = 0.65). There were no significant age × intensity interactions for [PCr]rest (P = 0.53), pHrest (P = 0.22), PCrend (P = 0.84), or pHend (P = 0.30).
Table 3.
Muscle metabolic variables: voluntary contractions
| 20% MVC | 50% MVC | 100% MVC | |
|---|---|---|---|
| [PCr]rest, mM | |||
| Young | 38.6 ± 0.14 | 38.7 ± 0.14 | 38.6 ± 0.18 |
| Older Healthy* | 37.3 ± 0.29 | 36.8 ± 0.26 | 36.6 ± 0.26 |
| Older Impaired* | 36.7 ± 0.74 | 36.9 ± 0.56 | 36.7 ± 0.73 |
| pHrest | |||
| Young | 7.00 ± 0.01 | 7.01 ± 0.01 | 7.01 ± 0.01 |
| Older Healthy | 7.01 ± 0.01 | 7.02 ± 0.01 | 7.02 ± 0.01 |
| Older Impaired | 7.03 ± 0.01 | 7.04 ± 0.02 | 7.03 ± 0.02 |
| PCrend (% rest)† | |||
| Young | 90.8 ± 0.8 | 77.7 ± 1.6 | 65.4 ± 1.4 |
| Older Healthy | 89.5 ± 0.9 | 77.9 ± 1.2 | 66.2 ± 1.1 |
| Older Impaired | 92.4 ± 1.2 | 77.1 ± 2.5 | 67.3 ± 3.7 |
| pHmin† | |||
| Young | 6.99 ± 0.01 | 6.98 ± 0.01 | 6.91 ± 0.01 |
| Older Healthy | 7.00 ± 0.01 | 6.98 ± 0.01 | 6.93 ± 0.01 |
| Older Impaired | 7.00 ± 0.02 | 6.99 ± 0.02 | 6.93 ± 0.02 |
Data are expressed as means ± SE for each group. [PCr]rest, resting PCr concentration; pHrest, resting muscle pH; PCrend, PCr concentration at the end of contraction, relative to rest; pHmin, minimum muscle pH following contraction.
Significantly different from young (P ≤ 0.001).
Significantly different across intensities (P ≤ 0.001).
Fig. 4.
Metabolite changes during voluntary contractions. Changes in PCr (top) and pH (bottom) during 12-s voluntary contractions and first 2 min of recovery for 20%, 50%, and 100% MVC conditions in young, older healthy, and older impaired groups. Shaded area indicates contraction period. Data are expressed as means ± SE for each group.
ATP Production and Cost of Voluntary Contractions
Total ATP cost.
As expected, ATPTOT increased with increasing intensity (P < 0.001; Fig. 2B) but was not different across groups (P = 0.39). A group × intensity interaction (P = 0.001) was observed, such that ATPTOT was greater in older healthy (P = 0.05) and older impaired (P = 0.02) groups, compared with young at 20% MVC. ATPTOT was greater in young compared with older healthy individuals at 100% MVC (P = 0.02). No other significant group differences were observed for ATPTOT (P ≥ 0.16).
ATP production by pathway.
Contributions from each of the three metabolic pathways (ATPCK, ATPGLY, ATPOX) to the total ATP produced during the 12-s voluntary contractions are shown in Fig. 5. The ATP produced by each pathway increased with increasing contraction intensity (P < 0.001). Overall, ATPCK (Fig. 5A) was not different across groups (P = 0.45); however, there was a group × intensity interaction (P = 0.04). Post hoc analysis revealed that at 100% MVC, ATPCK was greater in young than in older healthy individuals (P = 0.02), with no significant group differences at 20% (P ≥ 0.19) or 50% MVC (P ≥ 0.60). ATPGLY (Fig. 5B) was different across groups (P = 0.01), and showed a group × intensity interaction (P < 0.001). Specifically, ATPGLY was greater in young than older healthy (P = 0.002) or older impaired (P = 0.05) individuals at 100% MVC, with no other significant group differences (P ≥ 0.31). There was a main effect of group (P = 0.05) for ATPOX (Fig. 5C), as it was lower in young individuals than in older healthy (P = 0.009) or older impaired (P = 0.03), with no significant difference between older impaired and older healthy groups (P = 0.21). There was no group × intensity interaction (P = 0.42) for ATPOX.
Fig. 5.
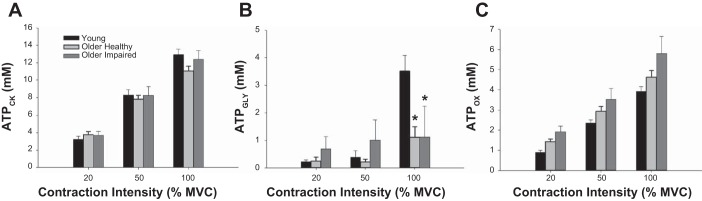
Metabolic pathways and ATP production. Total ATP (mM) produced by the creatine kinase reaction (ATPCK; A), nonoxidative glycolysis (ATPGLY; B) and oxidative phosphorylation (ATPOX, C) during 12-s voluntary isometric contractions at 20%, 50%, and 100% MVC. A: ATPCK was similar across groups (P = 0.45), increased with increasing contraction intensity (P < 0.001), and showed a group × intensity interaction (P = 0.04). B: ATPGLY increased with increasing contraction intensity (P = 0.03), but was overall greater in young than older healthy or older impaired groups (P ≤ 0.05) and had a group × intensity interaction (P < 0.001). C: ATPOX increased with increasing contraction intensity (P ≤ 0.001), and, overall, was lower in young than older healthy or older impaired individuals (P ≤ 0.03). Data are expressed as means ± SE for each group. *Significantly different from young (P ≤ 0.02).
Oxidative ratio.
The proportion of ATP produced oxidatively did not vary across contraction intensities (P = 0.08) but was different across groups (Fig. 6). This ratio was greater in the older impaired than the young group (P < 0.001). There was also a group × intensity interaction (P < 0.001), such that the oxidative ratio was lower in young individuals than either older group at 50% (P ≤ 0.001) and 100% MVC (P ≤ 0.001), but not at 20% MVC (P ≥ 0.35). There were no significant differences between older healthy and older impaired groups at any intensity (P ≥ 0.27).
Fig. 6.
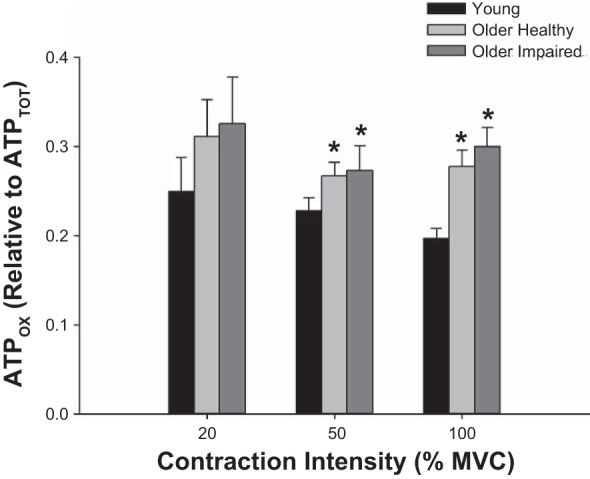
Oxidative ratio. Proportion of ATP produced by oxidative phosphorylation relative to total ATP in each group during 12-s voluntary isometric contractions is shown at 20%, 50%, and 100% MVC. Overall, the oxidative ratio was not different across intensities (P = 0.08). A group × intensity interaction was observed (P < 0.001). *Significantly different from young (P ≤ 0.001).
Metabolic Economy During Voluntary Contractions
Metabolic economy for each group at each contraction intensity is shown in Fig. 2C. Economy differed among groups (P = 0.05), but not across contraction intensities (P = 0.21). Specifically, ME was lower overall in the older impaired group than either the young (P = 0.009) or older healthy groups (P = 0.04), with no significant difference between the latter two groups (P = 0.21). There was also a group × intensity interaction (P = 0.04), as ME was greater in young than in older impaired individuals at 20% (P = 0.02) and 50% MVC (P = 0.02) and tended to be greater in the older healthy than older impaired group at 20% (P = 0.07) and 50% MVC (P = 0.07). The computed effect sizes indicated that, although these differences in ME between older healthy and older impaired subjects did not reach statistical significance, they are physiologically significant at 20% MVC (d = 0.70) and 50% MVC (d = 0.72). There were no significant group differences at 100% MVC (P ≥ 0.10). Linear regression analyses with all participants combined (n = 38) showed that, while ME at 50% and 100% MVC were strongly associated with each other (r = 0.61; P < 0.001), ME at 20% was not associated with ME at 50% (r = 0.22; P = 0.14) or 100% MVC (r = 0.09; P = 0.54), suggesting that factors regulating ME were different at 20% compared with 50% and 100% MVC.
Torque, ATP Production, and Economy During Stimulated Twitch Contractions
Normalized peak twitch torque at baseline was not different across groups (P = 0.87), nor was baseline twitch TTI (P = 0.15; Table 2). Resting [PCr] (P = 0.11) and pH (P = 0.94) prior to stimulated contractions were not different across groups (Table 4). The pHmin (P = 0.39) and [PCr]end (P = 0.37) following the 3-min stimulation protocol were also comparable across groups (Table 4), indicating an overall similar metabolic response to the stimulation. Average TTI (P = 0.15) and ATP cost (P = 0.49) per twitch did not differ across groups (Table 4). As a result, twitch economy was not different across groups (P = 0.86; Table 4), indicating similar inherent muscle economy.
Table 4.
Muscle metabolic variables: stimulated contractions
| Young | Older Healthy | Older Impaired | |
|---|---|---|---|
| [PCr]rest, mM | 37.8 ± 0.4 | 37.2 ± 0.4 | 36.1 ± 0.7 |
| pHrest | 7.02 ± 0.01 | 7.03 ± 0.01 | 7.04 ± 0.01 |
| PCrend, % rest | 67.9 ± 1.6 | 64.4 ± 2.3 | 68.8 ± 2.9 |
| pHmin | 6.92 ± 0.01 | 6.94 ± 0.01 | 6.94 ± 0.01 |
| Twitch TTI, Nm·s·cm−2 | 0.033 ± 0.002 | 0.035 ± 0.004 | 0.042 ± 0.005 |
| Twitch cost, mM ATP/twitch | 0.17 ± 0.02 | 0.19 ± 0.02 | 0.20 ± 0.04 |
| Twitch economy, Nm·s·cm−2·mM ATP−1 | 0.24 ± 0.04 | 0.22 ± 0.04 | 0.26 ± 0.05 |
Data are means ± SE for each group. TTI, torque-time integral.
DISCUSSION
The purpose of this study was to investigate potential differences due to age and mobility status in muscle ME during stimulated and voluntary isometric contractions of varying intensity. We hypothesized that the economy of a single twitch would be greater in both older groups compared with young, consistent with a slower muscle phenotype in aging. During voluntary contractions, we hypothesized that muscle ME would be similar across groups at 20% MVC, but greater in older than young at 50% and 100% MVC due to the increasing influence of differing fiber type recruitment and motor unit discharge rates. Contrary to our hypothesis, twitch economy was not different across groups of young, older healthy, and older impaired individuals. Likewise, there was no significant difference in ME during voluntary contractions between young and older healthy individuals, despite slower contractile properties in the older group. Notably, ME was, in fact, lower in older impaired individuals than in young or older healthy adults, particularly at submaximal contraction intensities, despite similar muscle oxidative capacity and normalized torque production across groups. This observed difference in ME during voluntary, but not stimulated contractions, suggests that factors other than intrinsic muscle properties are involved in the determination of in vivo economy in humans, as discussed below.
The ankle dorsiflexor muscles play an important role during walking, as they are active for ∼75% of the gait cycle (6). Likewise, adequate dorsiflexor function is needed to prevent falls in older adults (24, 59), suggesting a strong functional relevance for this muscle group during gait. However, we cannot rule out the possibility that age-related differences in ME may vary by muscle group, as has been demonstrated with other bioenergetics variables, such as muscle oxidative capacity (51).
Metabolic Economy During Voluntary Contractions
Influence of contraction intensity.
Although data from electrically stimulated contractions consistently indicate higher ATP cost as stimulation frequency increases (2, 17, 20), the impact of voluntary contraction intensity on ME is less clear. Some reports suggest a positive relationship between voluntary contraction intensity and ME (70), while other reports suggest a negative relationship (34), and still others report no change in economy across intensities (3, 63). In the current investigation, muscle ME did not differ across intensities during short-duration contractions of the ankle dorsiflexor muscles. One potential explanation for differences across studies is the muscle that was investigated. It is possible that intensity-dependent changes in ME may be more apparent in a mixed muscle, where differences in recruitment of fiber type would be more profound than in the TA, which is ∼76–84% type I fibers (35).
Economy in healthy older adults.
Our previous work clearly showed greater reliance on oxidative ATP production in older compared with young muscle during fatiguing isometric contractions (43, 48, 49), a result that was replicated here (Figs. 5 and 6). Metabolic economy was not reported in these earlier investigations, and ATP cost was not adjusted for muscle mass. In addition, the contraction protocols in these previous studies involved sustained (48) or intermittent (49) maximal contractions, both designed to induce muscle fatigue. A retrospective analysis of a subset of the data from Lanza et al. (49) indicated greater ME in older compared with young adults (41), which provided an important impetus for the current study. A strength of our new study design was the use of brief muscle contractions, allowing us to examine muscle ME independent of fatigue. To our knowledge, this study is the first to prospectively document age-related differences in ME during ankle dorsiflexion, and to extend this finding to the case of older adults with mild-to-moderate mobility impairments.
Although a relationship between age and ME has been shown in premenopausal women for the ankle plantar flexor muscles (33), we did not observe a significant difference in ME between our young and older healthy groups in the current study. Our result is also contradictory to a recent report demonstrating lower economy in older muscle compared with young during dynamic contractions of the ankle plantar flexor muscles (54). During a 5-min submaximal contraction protocol, Layec et al. (54) report higher ATP cost, relative to Watts produced, in older healthy individuals compared with young. In the current investigation, we accounted for fat-free muscle mass and used an isometric paradigm to control for the complexity of interacting factors that contribute to dynamic control, such as coordinated neural input, muscle endurance, and muscle activation and relaxation mechanics. Under such isometric conditions, we did not observe a difference in economy between young and older healthy groups. Therefore, it seems likely that in older healthy individuals, the neural and mechanical factors involved in dynamic movements may be an important source of inefficiency, consistent with previous reports (34, 54). In addition, the greater overall energy cost of dynamic compared with isometric contractions (69) may also contribute to these differences between studies, although this hypothesis awaits further testing.
Aging and mobility impairment.
Although ME was similar between young and older healthy individuals, our older impaired group had lower ME compared with young and older healthy individuals at the submaximal contraction intensities. Such a difference across groups suggests that factors other than age alone are contributing to this reduced ME. It is likely that the observed greater variability in torque of the older impaired group (discussed in more detail below) contributes to the observed difference in ME. This becomes a particularly attractive hypothesis given the absence of differences in muscle oxidative capacity (kPCr, Vmax) in the older impaired compared with young and older healthy groups (Table 2). However, lower ME was also observed in the older impaired group at 50% MVC, where variability in torque was similar across groups. Such a finding suggests that additional factors may be involved.
The muscles of older individuals with mobility impairments often exhibit significant atrophy (36, 67, 81) and lower motor unit discharge rates (66, 80). These changes likely combine with lower habitual physical activity (Table 1), contributing to poor functional capacity. Further, animal models suggest that efficiency of Ca2+ transport in the sarcoplasmic reticulum (Ca2+ transported per unit ATP) is reduced in muscles of older rats compared with young rats (18). Such changes in the neuromuscular system of older individuals with physical impairment could place their muscles at an economic disadvantage, despite an apparently greater proportion of type I muscle fibers and adequate voluntary muscle activation, as indicated by the similar normalized TTI values reported here for all groups.
Although partial denervation of older muscle has been documented in animal models (14, 84), such changes have not to our knowledge been directly documented in humans. If present in our older subjects, denervated muscle fibers would be included in the mCSA, but would not be metabolically or mechanically active during contraction. As such, the presence of denervated fibers could reduce mass-normalized torque and decrease ME. In the current investigation, however, we did not observe any significant differences in mass-normalized torque across groups (Fig. 2). Thus, it seems unlikely that such alterations in innervation contributed to the differences in ME observed in the current investigation.
ATP Production
We have used the classic construct of bioenergetics as being accomplished during contractions by glycolysis and oxidative phosphorylation, with temporal and spatial buffering by the creatine kinase reaction (4, 82). We note that this model does not include potential contributions from the net breakdown of ADP to AMP, which also plays a role in the maintenance of energy homeostasis (27).
Consistent with previous reports (48–50), we have shown here that ATPGLY was greater in young than either older group at 100% MVC (Fig. 5). Notably, a previous study showed that this age-related difference in glycolytic flux during maximal contractions is not due to a limitation in glycolytic ATP production in older muscle (49). The difference in absolute ATPGLY observed here between young and old did not result in an age-related difference in ME at 100% MVC.
The relative contribution from ATPOX (oxidative ratio; Fig. 6) was greater in older individuals, compared with young at 100% MVC, despite similar kPCr and Vmax across all groups. This result is consistent with previous studies of muscle bioenergetics in aging (7, 43, 48, 49). The current study also extends information regarding submaximal contraction intensities (7, 43), where we found a greater oxidative ratio in both older groups compared with young at 50% MVC, but not at 20% MVC. This greater relative ATPOX in the older groups is also consistent with our observed main effect of group for the absolute ATPOX (Fig. 5). Together, these results indicate that both absolute and relative ATPOX are greater in older individuals than young at moderate-to-high contraction intensities. However, such an oxidative phenotype does not seem to confer any economic advantage in the unfatigued muscle of older healthy adults.
Torque Variability
Presumably, the variability of torque production at low contraction intensities (Fig. 3) introduced inefficiency to the neuromuscular response of the older groups. The finding that the CV for torque did not change in any group over the course of the six contractions at 20% MVC suggests that a learning effect was not the source of this difference between young and old. The exact mechanism by which this occurs, and the impact of this effect on mobility in older adults, await future clarification. However, Saugen and Vollestad (70) propose that motor unit recruitment and rate coding patterns have a profound impact on the intensity-related increase in economy they observed in healthy young adults. They suggested that the force oscillations typically observed at low motor unit firing rates reduce the economy of the contraction compared with greater intensity, fully fused contractions (70). Further, it has been shown that variability in motor unit firing rates increases with age (78), particularly at low contraction intensities (15), likely due to the larger motor units of older muscle (22, 46) that make precise force production at low contraction intensities difficult (76).
Our findings of greater torque variability in older individuals at 20% MVC may, therefore, explain a portion of the greater ATP cost and lower ME observed in the older impaired group at this intensity, despite their similar relative TTI (Fig. 2). Notably, torque variability was not different across groups at 50% MVC, despite lower ME in the older impaired group at this intensity. This result suggests that variable mechanisms contribute to age-related differences in ME across intensities, an observation that is further supported by the lack of associations between ME at 20% MVC and that at 50% or 100% MVC (r ≤ 0.22).
Inherent Muscle Economy
The inclusion of a stimulated paradigm here allowed us to investigate inherent economy of the muscle, independent of potential age-related differences in neural drive, such as increased co-contraction (45, 57). Consistent with a recent retrospective analysis of data from the quadriceps muscles (10), we found no difference in inherent muscle economy across age groups (Table 2). While we did not have a direct measure of fiber type composition, the lower resting [PCr] (Table 3), (47, 79) and slower T1/2 of force relaxation in the older groups (Table 2) were consistent with a shift toward a slower fiber type composition in these individuals (53). Despite this apparent difference in fiber type, twitch economy was not different across groups, suggesting that 1) factors other than fiber type are predominantly contributing to inherent muscle ME in vivo in humans; 2) any economic advantage due to type I fiber composition (11, 26, 30, 34) was offset in the older adults by increased energy needs of the sodium-potassium, sarcoplasmic reticulum Ca2+ (as discussed above) or myosin ATPases; 3) force relaxation rate in vivo does not adequately reflect fiber type composition in the context of aging, particularly as we did not observe a difference in peak twitch torque; or 4) the relatively small age-related shift in fiber type composition, from ∼76% to ∼84% type I, that has been documented in the human tibialis anterior muscle (35) may not be sufficient to produce measurable differences in whole muscle twitch economy. The solution to this apparent paradox of a slower, but not more economical, phenotype in older muscle is not clear at this time. Overall, the lack of difference in inherent muscle economy by age provides strong evidence that the lower ME during voluntary contractions in our older mobility-impaired group was due to differences arising proximal to the site of stimulation.
We note that our twitch cost (mM ATP per twitch; Table 2) results are in contrast to our previous study, which showed lower ATP cost of twitch contractions in older compared with young men (74). The twitch cost for young men in the current study was comparable to that of Tevald et al. (74) (means ± SE = 0.18 ± 0.02 mM ATP per twitch in both cases), whereas twitch cost for the older men in the current study was greater than in the previous cohort (0.19 ± 0.02 vs. 0.13 ± 0.01, respectively). While the source of this difference in studies is not clear, it is possible that differences in physical activity are a contributing factor. The older men in the study of Tevald et al. (74) had lower physical activity counts than the young participants, while there were no differences in physical activity between the young and older healthy groups in the current investigation.
Single Muscle Metabolic Economy and Whole Body Energy Costs in Aging
As the field moves forward to elucidate the potential role of muscle ME in age-related changes in the energy cost of whole body exercise such as walking, it is imperative to keep in mind the distinctions between single-muscle economy (mass—normalized torque or power per unit ATP consumed—from all sources) and whole body energy costs (generally evaluated using a measure of oxygen consumption). These approaches are both necessary but focus on different sets of factors and likely involve different mechanisms and solutions. As the complexity of the paradigm increases, so too do the number of potential influences on the outcome variables. Such is the challenge of truly integrative physiological research. By starting with a well-controlled model established by previous studies of older adults, the present study contributes to the emerging literature by providing new and unique evidence of lesser ME in mobility-impaired older adults during submaximal, voluntary contraction intensities, and suggests that attention to the control of muscular work will be fruitful in future studies.
Conclusions
We have shown that inherent dorsiflexor muscle twitch economy in vivo is similar in young, older healthy and older mobility-impaired adults, despite slower contractile properties suggestive of more economical, type I muscle in the older groups. During nonfatiguing, voluntary contractions, our results indicate that muscle ME does not differ in healthy older adults compared with young. However, when extended to mobility-limited older adults, a reduction in muscle ME is evident, particularly at submaximal contraction intensities. Our analyses suggest that greater variability in torque production contributes to the reduced ME at submaximal intensities, although additional factors may also play a role.
Perspectives and Significance
This work adds important new information to our understanding of the physiological mechanisms associated with functional declines in older individuals. As most activities of daily living involve submaximal contractions, our observation of lower ME in mobility-impaired older adults has implications for daily energy cost and the experience of symptomatic fatigue in this population. Although it has been suggested that muscle fatigue may be related to symptomatic fatigue in frail older individuals (1), evidence for such a connection is lacking. It is possible that muscle ME may provide a physiological link between muscle and symptomatic fatigue, although further investigation is necessary. Future work should extend these findings to other muscle groups, more complex contraction protocols, and conditions of muscle fatigue.
GRANTS
This study was funded by National Institutes of Health Grants R01-AG21094 and K02-AG023582.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.D.C., R.G.L., and J.A.K. conception and design of research; A.D.C., A.T., R.G.L., and J.P.D. performed experiments; A.D.C., A.T., R.G.L., J.P.D., and J.A.K. analyzed data; A.D.C., A.T., R.G.L., and J.A.K. interpreted results of experiments; A.D.C. prepared figures; A.D.C. drafted manuscript; A.D.C., A.T., R.G.L., and J.A.K. edited and revised manuscript; A.D.C., A.T., R.G.L., J.P.D., and J.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the participants for their time and enthusiasm, Prof. John Buonaccorsi for guidance about statistical analyses, Bryce Jones, and Dr. Linda Chung for logistical help, Dr. Douglas Befroy for technical assistance, and Logan Maynard for data analysis work.
REFERENCES
- 1.Avlund K. Fatigue in older adults: an early indicator of the aging process? Aging Clin Exp Res 22: 100–115, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol 465: 203–222, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boska M. ATP production rates as a function of force level in the human gastrocnemius/soleus using 31P MRS. Magn Reson Med 32: 1–10, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Brooks GA, Fahey TD, White TP. Exercise Physiology: Human Bioenergetics and Its Applications. Mountain View, CA: Mayfield, 1996 [Google Scholar]
- 5.Callahan DM, Foulis SA, Kent-Braun JA. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve 39: 692–702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol 95: 3426–3437, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chilibeck PD, Paterson DH, McCreary CR, Marsh GD, Cunningham DA, Thompson RT. The effects of age on kinetics of oxygen uptake and phosphocreatine in humans during exercise. Exp Physiol 83: 107–117, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Christie A, Kamen G. Short-term training adaptations in maximal motor unit firing rates and afterhyperpolarization duration. Muscle Nerve 41: 651–660, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988 [Google Scholar]
- 10.Conley KE, Jubrias SA, Cress ME, Esselman P. Exercise efficiency is reduced by mitochondrial uncoupling in the elderly. Exp Physiol 98: 768–777, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol 79: 147–166, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton BH, Jakobi JM, Allman BL, Rice CL. Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol (Oxf) 200: 45–55, 2010 [DOI] [PubMed] [Google Scholar]
- 13.De Haan A, de Ruiter CJ, Lind A, Sargeant AJ. Age-related changes in force and efficiency in rat skeletal muscle. Acta Physiol Scand 147: 347–355, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol 45: 389–393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol 13: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Erim Z, De Luca CJ, Mineo K, Aoki T. Rank-ordered regulation of motor units. Muscle Nerve 19: 563–573, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Foley JM, Meyer RA. Energy cost of twitch and tetanic contractions of rat muscle estimated in situ by gated 31P NMR. NMR Biomed 6: 32–38, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Gafni A, Yuh KC. A comparative study of the Ca2+-Mg2+ dependent ATPase from skeletal muscles of young, adult and old rats. Mech Ageing Dev 49: 105–117, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol (1985) 89: 695–703, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Giannesini B, Izquierdo M, Le Fur Y, Cozzone PJ, Bendahan D. In vivo reduction in ATP cost of contraction is not related to fatigue level in stimulated rat gastrocnemius muscle. J Physiol 536: 905–915, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golding EM, Teague WE, Jr., Dobson GP. Adjustment of K′ to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol 198: 1775–1782, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Gordon T, Hegedus J, Tam SL. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res 26: 174–185, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Gouzi F, Maury J, Molinari N, Pomies P, Mercier J, Prefaut C, Hayot M. Reference values for vastus lateralis fiber size and type in healthy subjects over 40 years old: a systematic review and metaanalysis. J Appl Physiol (1985) 115: 346–354, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Grabiner MD, Owings TM, Pavol MJ. Lower extremity strength plays only a small role in determining the maximum recoverable lean angle in older adults. J Gerontol A Biol Sci Med Sci 60: 1447–1450, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49: M85–M94, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Han YS, Geiger PC, Cody MJ, Macken RL, Sieck GC. ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol (1985) 94: 2188–2196, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33: 109–120, 1974 [PubMed] [Google Scholar]
- 29.Hasson CJ, Kent-Braun JA, Caldwell GE. Contractile and non-contractile tissue volume and distribution in ankle muscles of young and older adults. J Biomech 44: 2299–2306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J 79: 945–961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957 [DOI] [PubMed] [Google Scholar]
- 32.Hepple RT, Hagen JL, Krause DJ, Baker DJ. Skeletal muscle aging in F344BN F1-hybrid rats. II. Improved contractile economy in senescence helps compensate for reduced ATP-generating capacity. J Gerontol A Biol Sci Med Sci 59: 1111–1119, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Hunter GR, Bickel CS, Del Corral P, Byrne NM, Hills AP, Larson-Meyer DE, Bamman MM, Newcomer BR. Age, muscle fatigue, and walking endurance in pre-menopausal women. Eur J Appl Physiol 111: 715–723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve 24: 654–661, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Jakobsson F, Borg K, Edstrom L. Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropathol (Berl) 80: 459–468, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52: 80–85, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59: 1334–1338, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol (1985) 79: 1908–1913, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q 10: 43–63, 1994 [PubMed] [Google Scholar]
- 40.Kemp GJ, Taylor DJ, Styles P, Radda GK. The production, buffering and efflux of protons in human skeletal muscle during exercise and recovery. NMR Biomed 6: 73–83, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev 37: 3–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 19: 861–869, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol (1985) 93: 1813–1823, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol (1985) 88: 662–668, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol (1985) 91: 1341–1349, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Kung TA, Cederna PS, van der Meulen JH, Urbanchek MG, Kuzon WM, Jr., Faulkner JA. Motor unit changes seen with skeletal muscle sarcopenia in oldest old rats. J Gerontol A Biol Sci Med Sci 69: 657–665, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kushmerick MJ, Moerland TS, Wiseman RW. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc Natl Acad Sci USA 89: 7521–7525, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol (1985) 99: 1736–1744, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol 583: 1093–1105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 577: 353–367, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab 37: 88–99, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. In vivo oxidative capacity varies with muscle and training status in young adults. J Appl Physiol (1985) 107: 873–879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol Cell Physiol 272: C638–C649, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Layec G, Trinity JD, Hart CR, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK, Richardson RS. In vivo evidence of an age-related increase in ATP cost of contraction in the plantar flexor muscles. Clin Sci (Lond) 126: 581–592, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Lexell J. Human aging, muscle mass, and fiber type composition. J. Gerontol. A Biol. Sci. Med. Sci. 50 Spec No: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988 [DOI] [PubMed] [Google Scholar]
- 57.Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve 25: 858–863, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Malatesta D, Simar D, Dauvilliers Y, Candau R, Borrani F, Prefaut C, Caillaud C. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol (1985) 95: 2248–2256, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Melzer I, Benjuya N, Kaplanski J, Alexander N. Association between ankle muscle strength and limit of stability in older adults. Age Ageing 38: 119–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer RA. Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol Cell Physiol 257: C1149–C1157, 1989 [DOI] [PubMed] [Google Scholar]
- 61.Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem 248: 7276–7278, 1973 [PubMed] [Google Scholar]
- 62.Narici MV, Bordini M, Cerretelli P. Effect of aging on human adductor pollicis muscle function. J Appl Physiol (1985) 71: 1277–1281, 1991 [DOI] [PubMed] [Google Scholar]
- 63.Newcomer BR, Boska MD. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve 20: 336–346, 1997 [DOI] [PubMed] [Google Scholar]
- 64.Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol (1985) 98: 211–220, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Picard M, Ritchie D, Thomas MM, Wright KJ, Hepple RT. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Aging Cell 10: 1047–1055, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve 22: 1094–1103, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Roubenoff R, Parise H, Payette HA, Abad LW, D'Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med 115: 429–435, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Rubinstein S, Kamen G. Decreases in motor unit firing rate during sustained maximal-effort contractions in young and older adults. J Electromyogr Kinesiol 15: 536–543, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Ryschon TW, Fowler MD, Wysong RE, Anthony A, Balaban RS. Efficiency of human skeletal muscle in vivo: comparison of isometric, concentric, and eccentric muscle action. J Appl Physiol (1985) 83: 867–874, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Saugen E, Vollestad NK. Nonlinear relationship between heat production and force during voluntary contractions in humans. J Appl Physiol (1985) 79: 2043–2049, 1995 [DOI] [PubMed] [Google Scholar]
- 71.Scaglioni G, Narici MV, Maffiuletti NA, Pensini M, Martin A. Effect of ageing on the electrical and mechanical properties of human soleus motor units activated by the H reflex and M wave. J Physiol 548: 649–661, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen J, Rycyna RE, Rothman DL. Improvements on an in vivo automatic shimming method [FASTERMAP]. Magn Reson Med 38: 834–839, 1997 [DOI] [PubMed] [Google Scholar]
- 73.Szentesi P, Zaremba R, van Mechelen W, Stienen GJ. ATP utilization for calcium uptake and force production in different types of human skeletal muscle fibres. J Physiol 531: 393–403, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tevald MA, Foulis SA, Lanza IR, Kent-Braun JA. Lower energy cost of skeletal muscle contractions in older humans. Am J Physiol Regul Integr Comp Physiol 298: R729–R739, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theou O, Jones GR, Jakobi JM, Mitnitski A, Vandervoort AA. A comparison of the relationship of 14 performance-based measures with frailty in older women. Appl Physiol Nutr Metab 36: 928–938, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol (1985) 92: 1004–1012, 2002 [DOI] [PubMed] [Google Scholar]
- 77.Trappe SW, Costill DL, Fink WJ, Pearson DR. Skeletal muscle characteristics among distance runners: a 20-yr follow-up study. J Appl Physiol (1985) 78: 823–829, 1995 [DOI] [PubMed] [Google Scholar]
- 78.Vaillancourt DE, Larsson L, Newell KM. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging 24: 25–35, 2003 [DOI] [PubMed] [Google Scholar]
- 79.Vandenborne K, Walter G, Ploutz-Snyder L, Staron R, Fry A, De Meirleir K, Dudley GA, Leigh JS. Energy-rich phosphates in slow and fast human skeletal muscle. Am J Physiol Cell Physiol 268: C869–C876, 1995 [DOI] [PubMed] [Google Scholar]
- 80.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve 25: 17–25, 2002 [DOI] [PubMed] [Google Scholar]
- 81.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60: 324–333, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40: 1271–1296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walter G, Vandenborne K, Elliott M, Leigh JS. In vivo ATP synthesis rates in single human muscles during high intensity exercise. J Physiol 519: 901–910, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang ZM, Zheng Z, Messi ML, Delbono O. Extension and magnitude of denervation in skeletal muscle from ageing mice. J Physiol 565: 757–764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]