Abstract
Significance: Hyaluronic acid (HA, or hyaluronan) is a ubiquitous naturally occurring polysaccharide that plays a role in virtually all tissues in vertebrate organisms. HA-based hydrogels have wound-healing properties, support cell delivery, and can deliver drugs locally.
Recent Advances: A few HA hydrogels can be customized for composition, physical form, and biomechanical properties. No clinically approved HA hydrogel allows for in vivo crosslinking on administration, has a tunable gelation time to meet wound-healing needs, or enables drug delivery. Recently, a thiolated carboxymethyl HA (CMHA-S) was developed to produce crosslinked hydrogels, sponges, and thin films. CMHA-S can be crosslinked with a thiol-reactive crosslinker or by oxidative disulfide bond formation to form hydrogels. By controlled crosslinking, the shape and form of this material can be manipulated. These hydrogels can be subsequently lyophilized to form sponges or air-dried to form thin films. CMHA-S films, liquids, and gels have been shown to be effective in vivo for treating various injuries and wounds in the eye in veterinary use, and are in clinical development for human use.
Critical Issues: Better clinical therapies are needed to treat ophthalmic injuries. Corneal wounds can be treated using this HA-based crosslinked hydrogel. CMHA-S biomaterials can help heal ocular surface defects, can be formed into a film to deliver drugs for local ocular drug delivery, and could deliver autologous limbal stem cells to treat extreme ocular surface damage associated with limbal stem cell deficiencies.
Future Directions: This CMHA-S hydrogel increases the options that could be available for improved ocular wound care, healing, and regenerative medicine.

Barbara Wirostko, MD
Scope and Significance
Better clinical therapies are needed to treat ophthalmic surface injuries. Hyaluronic acid (HA, or hyaluronan) is a ubiquitous naturally occurring polysaccharide that plays a role in virtually all tissues in vertebrate organisms. HA-based hydrogels have wound-healing properties, support cell delivery, and can deliver drugs locally. Indeed, HA-derived gels product can help heal ocular surface defects, can be formed into a film to deliver drugs for local ocular drug delivery, and have the potential to deliver autologous limbal stem cells to treat extreme ocular surface damage associated with limbal stem cell deficiencies. A few clinical HA products have been optimized hydrogels for composition, physical form, and biomechanical properties.
Translational Relevance
A thiolated carboxymethyl HA (CMHA-S) was used to produce crosslinked hydrogels. CMHA-S can be crosslinked with a thiol-reactive crosslinker or by oxidative disulfide bond formation to form hydrogels. These hydrogels can be subsequently lyophilized to form sponges or air-dried to form thin films. No clinically approved HA hydrogel allows for in vivo crosslinking during administration and tunable gelation to meet wound-healing needs. Crosslinked CMHA-S films, liquids, and gels have been shown to be more effective than native HA in vivo for treating various ocular injuries and wounds in the eye in veterinary use, and are in clinical development for human use.
Clinical Relevance
Ocular surface wound healing is a highly regulated process that requires proliferation and migration of epithelial cells on the ocular surface. When the process is altered by surgical intervention or by changes in conditions of ocular surface disease due to trauma, systemic disease, this can result in delayed corneal epithelial wound healing. Currently, the standard of care includes temporizing measures that have various inherent issues with application and administration. A biomaterial that could be topically applied to the ocular surface and deliver compounds to activate corneal epithelial cells and protect the ocular surface to promote corneal wound healing would be particularly valuable.
Background
The highly regulated process of corneal and ocular surface wound healing requires the proliferation and migration of epithelial cells and interactions between epithelial cells, inflammatory cells, and stromal fibroblasts. As noted earlier, when the process is altered, delayed corneal epithelial wound healing can result in corneal defects that will not resolve or “close.” This impaired corneal wound healing can lead to persistent corneal epithelial erosions and/or deeper stromal defects that result in corneal scarring, ulceration and infections, opacification, corneal neovascularization, and, ultimately, visual compromise.1,2 Currently, the standard of care includes lubricants, ointments, bandage lenses, amniotic membrane grafts, autologous serum eye drops, and, as a last measure, corneal transplants and tarsorraphies. Although many of these work as temporizing measures, they have various inherent issues with application and administration.1 A commercial biomaterial that could be applied topically to the ocular surface and could (1) deliver therapeutic molecules to activate corneal epithelial cells and/or stromal keratocytes to proliferate and/or migrate, as well as (2) protect the ocular surface, would be useful to promote corneal wound healing in the clinic.
Biomaterials can facilitate the wound-healing process, thus reducing the healing time and leading to a healthier, more native-like repaired tissue. HA is a ubiquitous naturally occurring polysaccharide that plays a role in virtually all tissues in vertebrate organisms. HA-based biomaterials have become particularly attractive, because HA is prevalent throughout the body, has anti-inflammatory properties, is non-immunogenic, and has been shown to play a role in the wound-healing process, including on the ocular surface.3,4 During tissue injury, high-molecular-weight HA in the extracellular matrix is degraded to lower-molecular-weight HA. Moreover, different molecular sizes of HA have different effects on various cell types involved in wound healing, such as monocytes and fibroblasts, thus affecting rates of healing, inflammation, and scar formation, although the data are conflicting.5 Commercially available HA is derived from animal (usually avian) or fermentation sources, and can be obtained in various molecular sizes, that is, from 80 to 2,000 kDa, thus controlling the molecular weight. When exogenous HA is administered as a solution in vivo, it is quickly degraded by the body.6,7 To combat such rapid degradation, various chemical modifications of HA have been made to generate crosslinkable HA derivatives that can be manufactured into materials with varied shapes, forms, and biophysical and biochemical properties.8,9 These properties can be manipulated and tailored to design products for specific clinical applications that can meet the needs for extracellular matrix reconstruction, wound healing, and sustained local delivery of drugs and cells to fight infections, scarring, and enhance healing.8–16 For example, to make the CMHA-S hydrogels for ocular indications, the molecular size range of the CMHA-S macromonomer is tightly controlled and product specific. Once the hydrogel is formed, the molecular size of the resulting polymeric network is essentially infinite. The practical aspects of the development of HA-based medical products, and a survey of chemically modified and native HA products approved for clinical use have previously been summarized.17
CMHA-S is one type of chemically modified HA that has been utilized in a variety of applications, including wound repair and adhesion prevention.18,19 CMHA-S is produced by first carboxymethylating HA, followed by introduction of crosslinkable thiol residues.19,20 CMHA-S can then be crosslinked with either a thiol-reactive crosslinker, such as poly(ethylene glycol) diacrylate, or by oxidative disulfide bond formation to form hydrogels (Fig. 1). A key advantage of both chemically modifying and crosslinking HA is that the resulting derivatives and gels degrade more slowly than native HA, retain their shape, and, thus, remain in the body for weeks to months. The rate of enzymatic degradation can be controlled by altering the extent of modification and the degree of crosslinking to optimize the residence time in a particular location in the body. The hydrogels can also be lyophilized to form sponges or air-dried to form thin films.21,22 CMHA-S films and gels have already been shown effective in vivo for the treatment of injured vocal folds, scar-free healing after endoscopic sinus surgery, preventing stenosis after tracheal injury, preventing postsurgical adhesions, and treating skin and corneal wounds in various animal models.13,14,23–27 Further, this material releases encapsulated growth factors slowly over timescales of weeks to months both in vitro and in vivo.28,29 For example, wound healing was accelerated in various disease and injury models when growth factors were continuously released from topical hydrogels as films and gels into full-thickness wounds.23,28,30
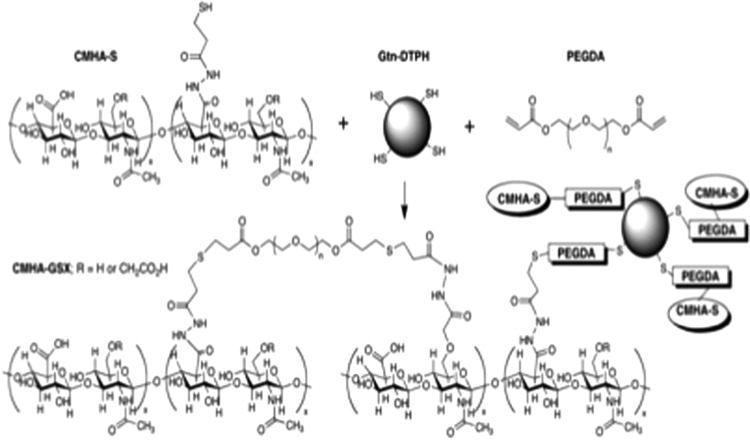
Figure 1.
An example of crosslinking thiol-modified carboxymethyl hyaluronic acid (CMHA-with thiol-modified gelatin) (Gtn-DTPH) using the bifunctional crosslinker poly(ethylene glycol) diacrylate (PEGDA) to form a thioether crosslinked semi-synthetic matrix (CMHA-GSX).
Several companies share the development rights for this unique CHMA-S polymer and are actively developing it for wound-healing indications as well as for local therapeutic cell and drug delivery. BioTime, Inc. (Alameda, CA) has established an FDA Device Master File for manufacture of certain CMHA-S materials, and has products in development for topical wound healing (Premvia™), cell delivery (Renevia™), and adhesion prevention (ReGlyde™) in humans. Jade Therapeutics, Inc. (Salt Lake City, UT), holds a sublicense for ophthalmic uses and indications from BioTime and is in preclinical development stage for products intended for use in ophthalmic wound and corneal repair as well as ocular drug delivery in humans. Finally, SentrX Animal Care, Inc. (Salt Lake City, UT) currently has global distribution of veterinary products for the healing of dermal and corneal wounds, for dry eye, and for preventing postsurgical adhesions in horses, dogs, cats, and exotic pets.
Discussion
A significant medical need exists for a better means to treat corneal and ocular wounds due to various traumas and diseases, as well as for an improved platform to deliver drugs and cells that can be used to heal these ocular injuries locally.1,31 The current clinical standard of care for dry eye in the EU and Asia is, in fact, the use of topical HA lubricants. However, uncrosslinked native HA products are poorly retained and rapidly degraded, thus requiring frequent daily administration.32 Crosslinked CMHA-S hydrogels not only offer intrinsic ocular wound healing with anti-inflammatory and anti-adhesive capabilities that are well recognized with HA but also have the capability to degrade more slowly. Thus, crosslinked CMHA-S would act as a local lubricant as well as a highly biocompatible vehicle to deliver drugs in a sustained manner with less frequent ocular administration.13,33,34 Such drugs may include growth factors or small molecules to stimulate epithelial cell proliferation or migration, or to simultaneously treat or prevent infections. Furthermore, chronic ocular wounds that are associated with limbal stem cell deficiencies, secondary to trauma, autoimmune disorders, and infections are the most difficult to heal.1 Such eyes very often have complications of corneal haze, corneal neovascularization, stromal scarring, and severe photophobia and pain.2 These hydrogels can serve as a matrix to deliver autologous stem cells.35 Therefore, crosslinked CMHA-S could be utilized to treat corneal and ocular wounds by combining the intrinsic scar-free wound-healing properties of CMHA-S hydrogels with the delivery of drugs and/or therapeutic multipotent stem or progenitor cells.
Translational experience to date
Jade Therapeutics has recently reconfirmed the ocular safety and tolerability of crosslinked CMHA-S in vivo, confirming the results found by Yang et al. and SentrX.13 In a 5-day pilot study, CMHA-S gel versus Ringers Lactate was applied topically four times a day to a corneal debridement model (New Zealand White rabbits). The CMHA-S gel demonstrated excellent ocular biocompatibility and normal histology, and was capable of delivering recombinant human growth hormone (rHGH) topically.30 In cell culture and in animal models of delayed wound healing, the data show that delivery of rHGH locally from these CMHA-S films have the potential to treat these more recalcitrant cases.30,36 The rationale for the choice of rHGH was based on the existing data for the role of rHGH in wound healing systemically. Moreover, recent cellular mechanistic data and in vivo data have confirmed the ability of rHGH to stimulate and morphologically activate corneal epithelial stem cells to migrate.36,37 A pilot study has shown excellent safety and tolerability for this crosslinked CMHA-S when delivered subconjunctivally.38
In terms of drug delivery, the ability to maintain therapeutic agents, particularly proteins, in a bioactive state during formulation and controlled release is an important capability of this HA polymer technology; this is particularly important for achieving multiday release of expensive large molecules.39 The ability to deliver large molecules in a sustained manner over weeks to months from crosslinked CMHA-S hydrogels is supported by previous research both in vivo and in vitro. For example, wound healing was accelerated in a diabetic mouse model when basic fibroblast growth factor was continuously released from the CMHA-S hydrogels into full-thickness wounds.23,40 Other proteins and small molecules that have been delivered both in vitro and in vivo from these CMHA-S gels and films include the following growth factors:28,41–45
• Basic fibroblast growth factor
• Insulin-like growth factor-1
• Vascular endothelial growth factor
• Angiopoietin-1
• Keratinocyte growth factor
• Platelet-derived growth factor
• Transforming growth factor-β1
• Bone morphogenetic protein
• Sunitinib
• Mitomycin C
Clinical experience to date
Wound healing is a complicated and very highly regulated pathway on the ocular surface that varies in its process longitudinally, temporally, and spatially. To have effective wound healing, the process requires a delicate interplay of cells, destructive and constructive enzymes, growth factors, inflammatory chemokines and cytokines, and extracellular matrices that continue to change and be modified based on the origin of insult and the duration of pathology. In ophthalmology, an acute corneal defect could be treated with a topical HA alone, but if the limbal stem cells are deficient due to traumatic burn and chemical injuries, systemic autoimmune processes, or diabetes, the epithelial defect could be present for weeks to months. Such chronic defects will likely need the application of growth factors in combination with a topical HA with perhaps even physical corneal epithelial debridement, as noted earlier. If the defect becomes infected, the management can change again and require frequent hourly topical antibiotics, thus necessitating the need for an alternative way of dosing these antimicrobials through a sustained release HA formulation such as the films in preclinical development. It is these types of clinical scenarios and wound-healing challenges to which current research and development efforts for human and veterinary products are directed.
As previously mentioned, the degree of crosslinking of the CMHA-S hydrogels can be varied to adjust the rheological properties, effective pore size, and rates of diffusion of molecules of different sizes and chemical properties. Moreover, both the biodegradation rate of the hydrogel and the release rate of molecules from the hydrogel can be fine tuned. CMHA-S can be fabricated as gels, emulsions, thin films, sponges, powders, or delivered as a liquid and allowed to crosslink in situ (Fig. 2). Specifically for ocular applications, the gel can be formulated into an eye drop or a film that would allow a “bandage” type approach to wound care as well as local drug delivery. The veterinary eye drop formulation has been shown to improve corneal wound healing in rabbit models and is being sold globally for management of opththalmic injuries in dogs, cats, and horses.13 In addition, we have shown that crosslinked CMHA-S can be fabricated as a dried thin film that rehydrates to a transparent thin hydrogel (Fig. 3). These films have been capable of successfully releasing proteins over weeks to months in a linear near zero-order release fashion in vitro with confirmed efficacy in vivo; proteins have also previously been released from similar, thicker hydrogels.23,40 Moreover, we have successfully delivered antibiotics to the ocular surface via these films in vivo over days for corneal ulcers (Fig. 4).46 Current research is directed at optimizing in vitro release for various doses of therapeutic proteins and small molecules such as antibiotics intended to treat corneal wounds from drug-loaded, sterile films.
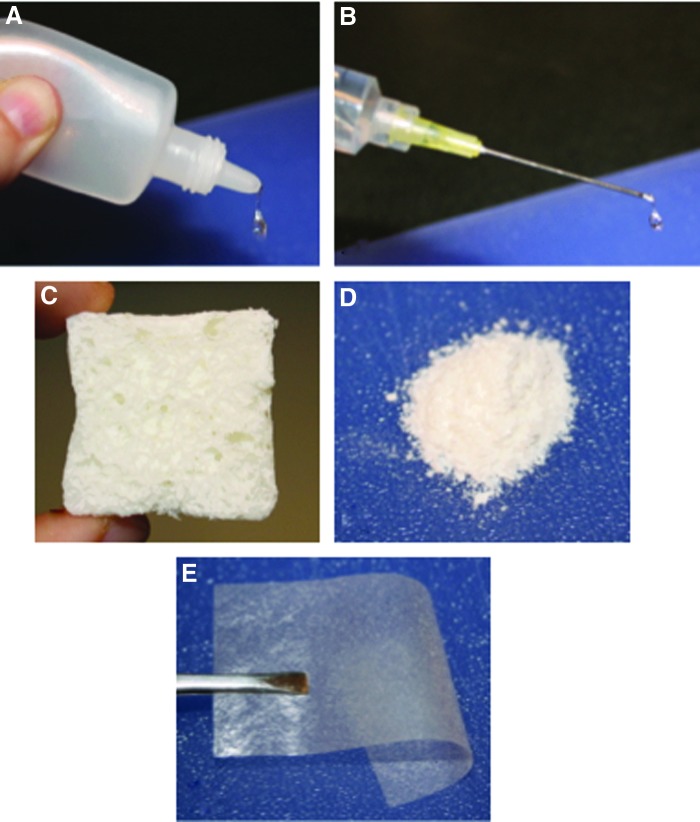
Figure 2.
Various formulations of crosslinked CMHA-S. (A) Gel drops designed for delivery through an eye dropper. (B) Liquid polymer solution delivered via syringe and designed to crosslink in situ. (C) Hydrogel lyophilized to form a sponge. (D) Hydrogel dried and ground to form a powder. (E) Hydrogel dried to form a thin, flexible film. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 3.

Rehydrated 8 mm CMHA-S film (left) and corresponding 6 mm dried film (right). Note that although the dried film is opaque, the rehydrated film is transparent. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 4.
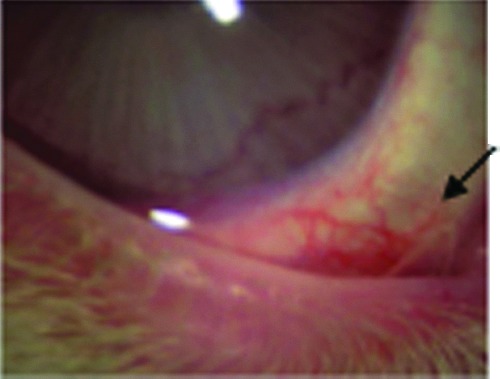
Transparent CMHA-S film in the lower inferior cul de sac in a rabbit (arrow). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Although the use of crosslinked CMHA-S materials for ophthalmic applications has yet to enter the clinical stage in humans, clinical studies in dogs have been undertaken to support their use as a tear replacement for keratoconjunctivis sicca (KCS) and for treating corneal ulcers.33,34,47 For KCS, the studies demonstrated that the CMHA-S gel drops, administered twice to thrice daily, reduced ocular irritation, hyperemia, and discharge, and increased ocular comfort as assessed by pet owners in as little as 2 weeks. Further, the CMHA-S gel was superior in reducing these symptoms compared with a solution of non-crosslinked HA at a similar concentration, and owners were more satisfied with the outcomes when using CMHA-S gel. This is important, as animals with tear film deficiencies can be difficult to medicate given their ocular discomfort; however, owners found that the improvement in ocular comfort provided by CMHA-S gel eased the problems usually seen in topical medication. In the case of corneal ulcers, CMHA-S gel was used in dogs when the ulcers had not healed in an average of 25 days by a variety of other treatments. After twice daily treatment with the CMHA-S gel, the corneal ulcers healed in an average of 13 days, and in as little as 7 days in some cases. Similar results have also been found when treating ulcers in cats (Fig. 5). It should be noted that the gel alone was not enough to aid in healing ulcers with a devitalized, non-adherent epithelium where defective epithelial basement membrane was the reason for lack of epithelial healing.48 In these spontaneous superficial chronic corneal erosions, physical methods to disrupt the basement membrane, such as with diamond burr debridement, are required.49 It would appear that use of CMHA-S can be valuable as a supplement to such ophthalmic treatments but not instead of them.
Figure 5.
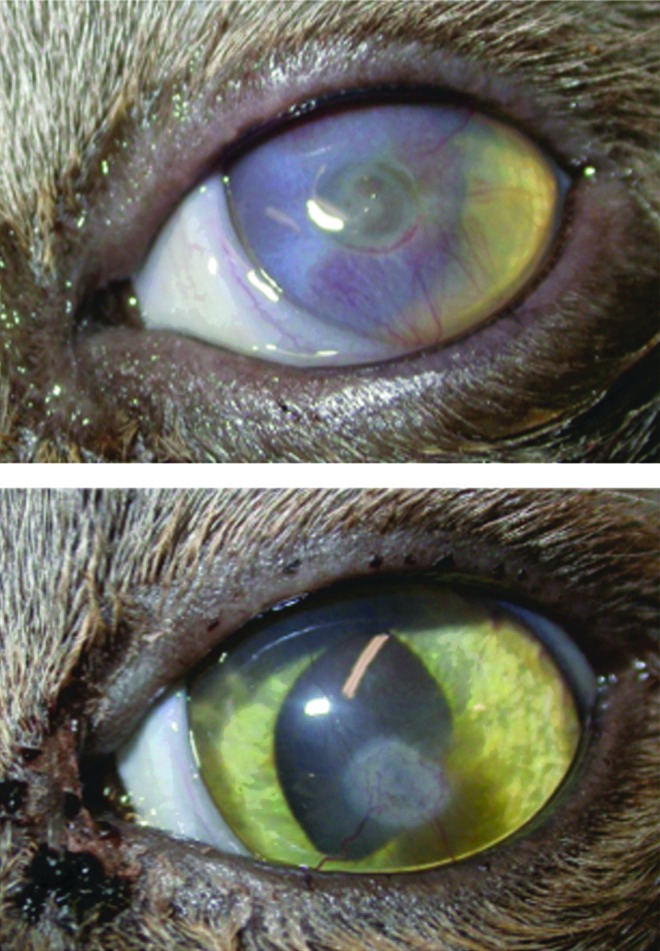
Top: Corneal ulcer in a cat that had not healed in 35 days. Bottom: The corneal ulcer healed after treating for 10 days with the CMHA-S gel. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Innovation
As a local sustained delivery polymer, crosslinked CMHA-S offers an advantage over daily frequently administered topical and locally injected HAs clinically for ocular wound healing. It increases the options that could be available for improved ocular wound care, healing, and regenerative medicine. This tunable sustained approach for lubrication and delivery of drugs for ocular wound healing has the following advantages:
• A CMHA-S hydrogel film can be placed on the ocular surface under the lids with local retention for days to weeks.
• The CMHA-S can be delivered initially as a liquid CMHA-S solution that crosslinks to a gel in situ within minutes through a small gauge needle.
• The CMHA-S hydrogel can be delivered as a gel subconjunctivally with excellent tolerability.38
• The CMHA-S hydrogel improves corneal epithelial surface healing as a topical gel compared with non-crosslinked HA solutions.13,47
Summary
Wound healing is a complicated and very highly regulated pathway in all parts of the body that varies in its process longitudinally, temporally, and spatially. To have effective wound healing, the process requires a delicate interplay of cells, destructive and constructive enzymes, growth factors, inflammatory chemokines and cytokines, and extracellular matrices that continue to change and be modified. The development of a safe and well-tolerated HA-based hydrogel that can not only serve as a matrix for healing but also offer intrinsic well-recognized traits to support wound healing and be used to deliver therapeutic cells and drugs to promote healing would be clinically important. However, challenges exist not only in understanding the full extent of the intrinsic capabilities of the biopolymer but also in understanding the role and interplay of the biopolymer hydrogel and the therapy within specific tissues over time in this very active process. Together, these variables can impact the timing of the intervention as well as the timing and need for cell and/ or drug therapy.
Ultimately, we anticipate that these developments and advances in new materials for use in ocular wound healing will be able to meet the clinical unmet needs of patients. The ideal products would be those that can address the need, be easy to administer, and be efficacious. An inherent challenge is that so often the underlying pathology and inciting event is very different among patients, yet the end result—a wound—is the same. To develop and market clinically useful products for human and veterinary use, we should learn and understand the heterogeneity of the diseases in order to better target and select groups of patients to define and meet their unmet clinical needs. Very often, the better we understand the underlying pathology, the better we can develop the right tool to enhance and augment the natural healing and regenerative response. Without this fundamental knowledge, even the best products can fail in their clinic due to the incorrect patient population and/or the incorrect study design. Products, by their nature, need to be elegant in their simplicity with a direct and targeted approach to wound repair and tissue regeneration. That is, we should embrace the biological complexity, engineer versatile solutions, and deliver simple and easy-to-use products.50 We are optimistic that crosslinked CMHA-S hydrogels will become affordable, reimbursable, effective, and easy-to-use products for treating ophthalmic wounds in human patients as they already are in veterinary patients.
Take-Home Message.
This crosslinked CMHA-S sustained delivery technology as an ocular surface wound-healing therapeutic has potential to offer clinical advantages such as
• Improved local drug delivery, resulting in less systemic exposure with higher targeted local tissue drug concentrations
• Improved patient compliance to therapy, especially in cases of chronic and/ or very frequent administered therapies
• Reduced burden of illness given better outcomes
• Improved visual function and quality of life
Abbreviations and Acronyms
- CMHA-GSX
thiolated carboxymethyl hyaluronic acid-thioether crosslinked semi-synthetic matrix
- CMHA-S
thiolated carboxymethyl hyaluronic acid
- FDA
Food and Drug Administration
- Gtn-DTPH
thiol-modified gelatin
- HA
hyaluronic acid or hyaluronan
- KCS
keratoconjunctivis sicca
- PEGDA
poly(ethylene glycol) diacrylate
- rHGH
recombinant human growth hormone
Acknowledgments and Funding Sources
The authors thank Drs. T. Zarembinski and J. Bahr-Davidson for providing helpful comments. Partial financial support was from National Science Foundation SBIR No. 1315150 and the Department of Defense SBIR contract No. W81XWH-14-C-0025.
Author Disclosure and Ghostwriting
Barbara Wirostko, MD, is the cofounder and Chief Scientific Officer of Jade Therapeutics. Glenn D. Prestwich, PhD, is the inventor of the CMHA-S technology and a consultant to BioTime, SentrX, and Jade Therapeutics. Brenda K. Mann, PhD, is the Vice President of R&D for SentrX and a consultant to Jade Therapeutics. David L. Williams, MA, VetMD, PhD, FRCVS, is a consultant and advisor to SentrX. The authors were responsible for all writing, content preparation, and review of this article. There was no ghostwriting.
About the Authors
Barbara Wirostko, MD, is cofounder and Chief Scientific Officer of Jade Therapeutics, Salt Lake City, UT. Dr. Wirostko maintains an academic position as a Clinical Adjunct Associate Professor in Ophthalmology and as Adjunct Associate Professor in the Department of Bioengineering at the University of Utah. She completed her glaucoma fellowship at Cornell University, NY and received her Ophthalmology training as well as her MD at Columbia University, College of Physicians and Surgeons in NY. She is an active member and fellow of the American Academy of Ophthalmology, ARVO, Women in Ophthalmology, and local state societies. Brenda K. Mann, PhD, is Vice President for Research & Development at SentrX Animal Care. She is a Research Associate Professor in the Department of Bioengineering at the University of Utah. She was a founding faculty member of the Keck Graduate Institute of Applied Life Sciences, and now serves on its Advisory Council. Dr. Mann is also a member of the External Advisory Board for the Department of Bioengineering at the University of Louisville, and has served as Director of the Salt Lake Valley Science and Engineering Fair since 2006. Glenn D. Prestwich, PhD, is Presidential Professor of Medicinal Chemistry and launched and directs the Entrepreneurial Faculty Program at the University of Utah. He cofounded eight life science start-ups, including Echelon Biosciences, Sentrx Animal Care, Glycosan BioSystems, and GlycoMira. During his 37 years as a faculty member, he has published more than 600 technical papers and book chapters and 30 issued patents, and has trained more than 125 postgraduate scientists. David L. Williams, MA, VetMD, PhD, FRCVS, qualified from the Department of Veterinary Medicine at the University of Cambridge in 1988. He worked at the Animal Health Trust (AHT) in Newmarket before undertaking a PhD at the Royal Veterinary College (RVC) in London. He is associate lecturer at Cambridge, running the ophthalmology clinic. Dr. Williams teaches pathology in St John's College, where he is a fellow and director of studies in veterinary medicine. He also lectures in animal welfare and ethics.
References
- 1.Jeng BH. Treating the nonhealing epithelial defect—an overview of standard and investigational therapies for persistent corneal epithelial defects. Cataract Refract Surg Today Europe 2011;25–28 [Google Scholar]
- 2.Sacchetti M, Lambiase A, Cortes M, et al. Clinical and cytological findings in limbal stem cell deficiency. Graefes Arch Clin Exp Ophthalmol 2005;243:870–876 [DOI] [PubMed] [Google Scholar]
- 3.Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. J Int Med 1997;242:27–33 [DOI] [PubMed] [Google Scholar]
- 4.Chen WYJ, Abatangelo G. Functions of hyaluronan in wound repair. Wound Rep Regen 1999;7:79–89 [DOI] [PubMed] [Google Scholar]
- 5.Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One 2011;6:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurent TC, Fraser JR. Hyaluronan. FASEB J 1992;6:2397–2404 [PubMed] [Google Scholar]
- 7.Lindqvist U, Tolmachev V, Kairemo K, Astrom G, Jonsson E, Lundqvist H. Elimination of stabilized hyaluronan from the knee joint in healthy men. Clin Pharmacokinet 2002;41:603–613 [DOI] [PubMed] [Google Scholar]
- 8.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 2011;23:H41–H56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prestwich GD, Kuo J-W. Chemically-modified HA for therapy and regenerative medicine. Curr Pharm Biotechnol 2008;9:242–245 [DOI] [PubMed] [Google Scholar]
- 10.Allison D, Grande-Allen K. Hyaluronan: a powerful tissue engineering tool. Biomaterials 2006;12:2131–2140 [DOI] [PubMed] [Google Scholar]
- 11.Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release 2011;155:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderhooft JL, Acoutlabi M, Magda JJ, Prestwich GD. Rheological properties of crosslinked hyaluronan-gelatin hydrogels for tissue engineering. Macromol Biosci 2009;9:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G, Espandar L, Mamalis N, Prestwich GD. A crosslinked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Vet Ophthalmol 2010;13:144–150 [DOI] [PubMed] [Google Scholar]
- 14.Yang G, Prestwich GD, Mann BK. Thiolated carboxymethyl hyaluronic acid-based biomaterials enhance wound healing in rats, dogs, and horses. ISRN Vet Sci 2011;Article ID 851593:7 pages [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawyer T, McIntosh K, Clavijo C, Potekhina L, Mann BK. Formulation changes affect material properties and cell behavior in HA-based hydrogels. Int J Cell Biol 2012;Article ID 737421:9 pages [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khetan S, Guvendiren M, Legant W, Cohen D, Chen C, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater 2013;12:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo JW. Practical Aspects of Hyaluronan Based Medical Products. Boca Raton, FL: Taylor & Francis, 2006 [Google Scholar]
- 18.Prestwich GD. Clinical biomaterials for scar-free healing and localized delivery of cells and growth factors. Adv Wound Care 2010;1:394–399 [Google Scholar]
- 19.Shu XZ, Liu Y, Prestwich GD. Modified macromolecules and methods of making and using thereof. U.S. Patent 7,981,871 (July19, 2011) [Google Scholar]
- 20.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 2002;3:1304–1311 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Shu XZ, Prestwich GD. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials 2005;26:4737–4746 [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Ahmad S, Shu XZ, Sanders RK, Kopesec SA, Prestwich GD. Accelerated repair of cortical bone defects using a synthetic extracellular matrix to deliver human demineralized bone matrix. J Orthop Res 2006;24:1454–1462 [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Cai S, Shu XZ, Shelby J, Prestwich GD. Release of basic fibroblast growth factor from a cross-linked glycosaminoglycan hydrogel promotes wound healing. Wound Repair Regen 2007;15:245–251 [DOI] [PubMed] [Google Scholar]
- 24.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng 2006;12:2171–2180 [DOI] [PubMed] [Google Scholar]
- 25.Proctor M, Proctor K, Shu XZ, McGill LD, Prestwich GD, Orlandi RR. Composition of hyaluronan affects wound healing in the rabbit maxillary sinus. Am J Rhinol 2006;20:206–211 [PubMed] [Google Scholar]
- 26.Sondrup C, Liu Y, Shu XZ, Prestwich GD, Smith ME. Cross-linked hyaluronan-coated stents in the prevention of airway stenosis. Otolaryngol Head Neck Surg 2006;135:28–35 [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Skardal A, Shu XZ, Prestwich GD. Prevention of peritendinous adhesions using a hyaluronan-derived hydrogel film following partial-thickness flexor tendon injury. J Orthop Res 2008;26:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pike D, Caib S, Pomraninga K, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials 2006;27:5242–5251 [DOI] [PubMed] [Google Scholar]
- 29.Overman JJ, Clarkson AN, Wanner IB, et al. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A 2012;109:E2230–E2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafii MJ, Wirostko B, Zarembinski T, et al. HyStem, a bio-absorbable protein delivery polymer: safety, and efficacy in a corneal debridement model. Poster presentation No. 5048. ARVOMay2013, Seattle, WA [Google Scholar]
- 31.Weichel ED, Colyer MH, Ludlow SE, Bower KS, Eiseman AS. Combat ocular trauma visual outcomes during Operations Iraqi and Enduring Freedom. Ophthalmology 2008;115:2235–2245 [DOI] [PubMed] [Google Scholar]
- 32.Shimmura S, Ono M, Shinozaki K, et al. Sodium hyaluronate eyedrops in the treatment of dry eyes. Br J Ophthalmol 1995;79:1007–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams D, Mann B. A Crosslinked HA-based hydrogel ameliorates dry eye symptoms in dogs. Int J Biomater 2013;Article ID 460437:8 pages [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams DL, Mann BK. Efficacy of a crosslinked hyaluronic acid-based hydrogel as a tear film supplement: a masked controlled study. PLoS One 2014;9(6):99766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espandar L, Bunnell B, Wang GY, Gregory P, McBride C, Moshirfar M. Adipose-derived stem cells on hyaluronic acid-derived scaffold: a new horizon in bioengineered cornea. Arch Ophthalmol 2012;130:202–208 [DOI] [PubMed] [Google Scholar]
- 36.Ding J, Wirostko B, Sullivan D. Human growth hormone promotes corneal epithelial cell migration in vitro. Cornea 2014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demling RH. The role of anabolic hormones for wound healing in catabolic states. J Burns Wounds 2005;4:46–62 [PMC free article] [PubMed] [Google Scholar]
- 38.Zarembinski T, Doty NJ, Erickson IE, Srinivas R, Wirostko BM, Tew WP. Thiolated hyaluronan-based hydrogels crosslinked using oxidized glutathione: an injectable matrix designed for ophthalmic applications. Acta Biomater 2014;10:94–103 [DOI] [PubMed] [Google Scholar]
- 39.Piscal DS, Kosloski MP, Balu-lyer SV. Delivery of therapeutic proteins. J Pharm Sci 2010;99:2557–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005;26:6054–6067 [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Li H, Shu XZ, Gray SD, Prestwich GD. crosslinked hyaluronan hydrogels containing mitomycin C reduce postoperative abdominal adhesions. Fertil Steril 2005;83(Suppl 1):1275–1283 [DOI] [PubMed] [Google Scholar]
- 42.Hosack LW, Firpo MA, Scott JA, Prestwich GD, Peattie RA. Microvascular maturity elicited in tissue treated with cytokine-loaded hyaluronan-based hydrogels. Biomaterials 2008;29:2336–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis by in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials 2010;31:4630–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peattie RA, Pike DB, Yu B, et al. Effect of gelatin on heparin regulation of cytokine release from hyaluronan-based hydrogels. Drug Deliv 2008;15:363–371 [DOI] [PubMed] [Google Scholar]
- 45.Sanders WG, Hogrebe PC, Grainger DW, Cheung AK, Terry CM. A biodegradable perivascular wrap for controlled, local and directed drug delivery. J Control Release 2012;161:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rafii MJ, Wirostko B, Gum G, Godfrey K, Lee HK. Safety, tolerability, of ocular sustained-release (SR) moxifloxacin (MX) hydrogel films in New Zealand White (NZW) rabbits for corneal ulcers. Poster presentation No. 4704. ARVOMay2014, Orlando, FL [Google Scholar]
- 47.Williams D. Speeding the healing of corneal ulcers. Vet Pract 2014;2:28–29 [Google Scholar]
- 48.Bentley E, Abrams GA, Covitz D, et al. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (SCCED) in dogs. Invest Ophthalmol Vis Sci 2001;42:2262–2269 [PubMed] [Google Scholar]
- 49.Gosling AA, Labelle AL, Breaux CB. Management of spontaneous chronic corneal epithelial defects (SCCEDs) in dogs with diamond burr debridement and placement of a bandage contact lens. Vet Ophthalmol 2013;16:83–88 [DOI] [PubMed] [Google Scholar]
- 50.Prestwich GD. Engineering a clinically-useful matrix for cell therapy. Organogenesis 2008;4:42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]