Abstract
Significance: Nutrition is one of the most basic of medical issues and is often ignored as a problem in the management of our chronic wound patients. Unfortunately, malnutrition is widespread in our geriatric patients even in nursing homes in developed countries. Attention to basic nutrition and providing appropriate supplements may assist in the healing of our chronic wounds.
Recent Advances: Recent research has revealed the epidemiology of malnutrition in developed countries, the similarities to malnutrition in developing countries, and some of the physiologic and sociologic causes for this problem. More information is now available on the biochemical effects of nutrient deficiency and supplementation with macronutrients and micronutrients. In some cases, administration of isolated nutrients beyond recommended amounts for healthy individuals may have a pharmacologic effect to help wounds heal.
Critical Issues: Much of the knowledge of the nutritional support of chronic wounds is based on information that has been obtained from trauma management. Due to the demographic differences of the patients and differences in the physiology of acute and chronic wounds, it is not logical to assume that all aspects of nutritional support are identical in these patient groups. Before providing specific nutritional supplements, appropriate assessments of patient general nutritional status and the reasons for malnutrition must be obtained or specific nutrient supplementation will not be utilized.
Future Directions: Future research must concentrate on the biochemical and physiologic differences of the acute and chronic wounds and the interaction with specific supplements, such as antioxidants, vitamin A, and vitamin D.

Joseph Andrew Molnar, MD, PhD, FACS
Scope and Significance
This review discusses the current knowledge of nutrition as related to the patient with chronic wounds. We describe the demographic and physiologic differences between acute and chronic wound patients and define the problem of malnutrition in developed countries. A basic understanding of the need for macronutrients and micronutrients and the value of specific supplementation is provided.
Translational Relevance
Much of the current knowledge of nutrition in relation to wound healing is inferred from the management of acute wounds and may not be relevant to the patient with chronic wounds. This review describes the current state of available research on the topic and demonstrates the limitations of this information to guide future research. Additional studies that evaluate the supplementation with specific nutrients in patients with chronic wounds will translate into improved patient care.
Clinical Relevance
This review provides the clinician with knowledge of basic nutrition, the epidemiology of malnutrition in the chronic wound patient, and how to screen these patients for malnutrition. The reader will recognize that even an obese patient may have specific nutrient deficiencies. The literature is reviewed regarding the value of macronutrient and micronutrient supplementation. Recommendations for supplementation are provided when appropriate to assist in the care of patients.
Background
The problem of nutrition and chronic wounds
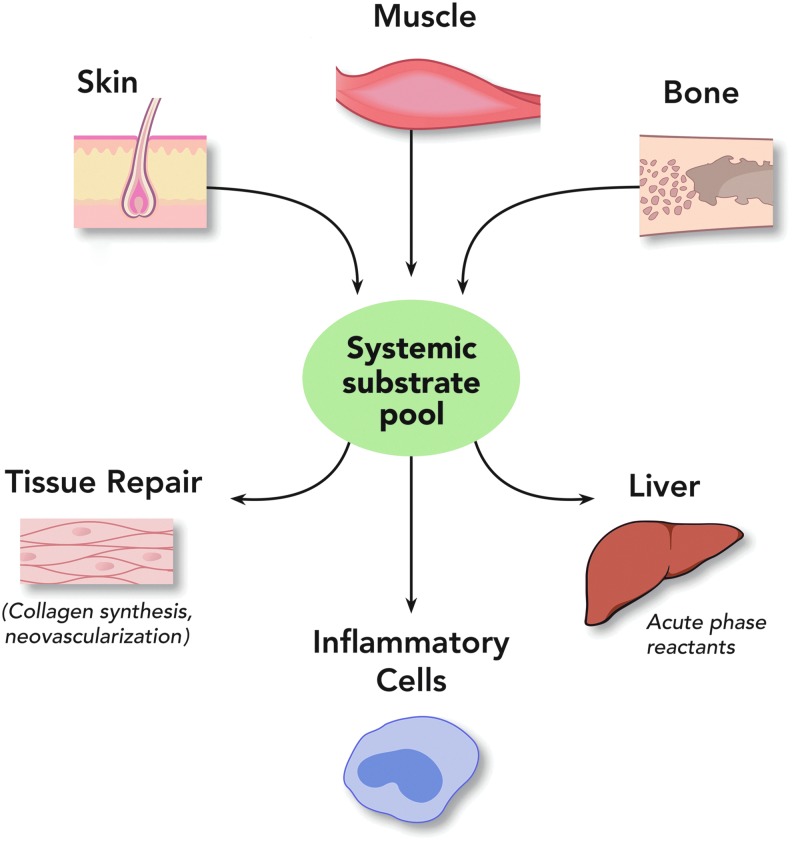
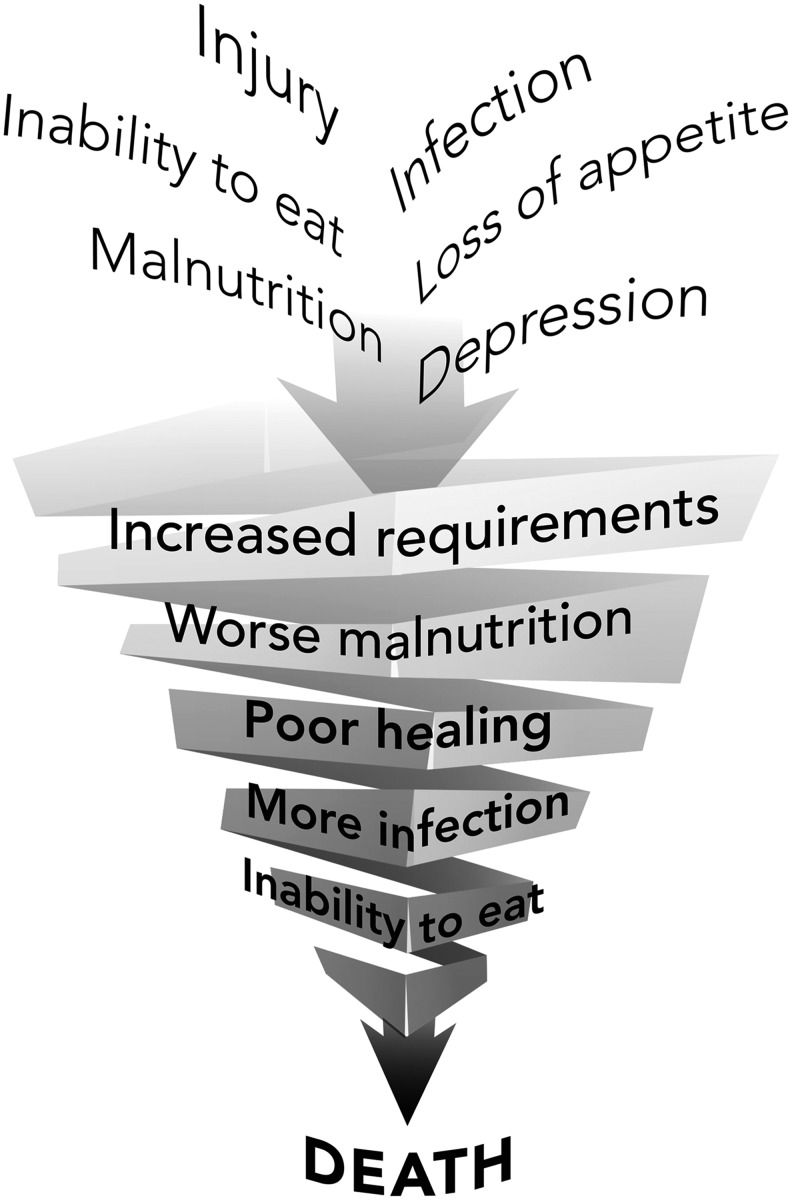
In the 20th century, extensive research on the metabolic response to injury increased our interest in the relationship of nutrition and wound healing. In the 1930s, Cuthbertson defined the concept of peripheral to visceral redistribution of metabolic substrates as part of the adaptive mechanism to heal wounds in our critically ill trauma patients (Fig. 1).1 With this concept the carcass of the body provides substrate, especially amino acids, for the healing wound in an injured patient who is unable to eat. As the metabolic reserves dwindled, these patients suffered from general protein calorie under nutrition. Until the introduction of total parenteral nutrition by Dudrick et al., little could be done for critically ill patients due to limited ability to provide enteral nutrition.2 Total parenteral nutrition stimulated the interest of surgeons in nutrition and wound healing. Initially, it was assumed that more was better and soon discovered the consequences of overnutrition of the critically ill with driving hypermetabolism with increased caloric intake, fatty livers, and elevated CO2 production and respiratory compromise in these patients.3,4 Continued research has refined the appropriate guidelines for nutritional management of the critically ill to optimize nutrition without the consequences of excess.5,6 The majority of this research concentrates on the patient with acute wounds due to trauma.
Figure 1.
In periods of metabolic stress, the carcass is broken down to provide substrate, especially amino acids, to support essential organ function such as in the liver and for the production of acute-phase reactions. This general process is also applicable to periods of undernutrition. Paradoxically, in the case of a skin wound, substrate may be obtained from the skin to help heal this wound. With permission from WFUSM Plastic Surgery Collection. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
To determine the nutritional needs of the patient with chronic wounds, it is important to realize that the typical patient is not like the trauma patient. Trauma patients are usually young and chronic wound patients are usually old. Trauma patients are typically healthy prior to the injury but chronic wound patients often have coexisting disease such as diabetes and other endocrine disorders—hypertension, peripheral vascular disease, renal disease, and hepatic disorders to mention a few. While there are some commonalities due to the similar basic phases of the healing wound, the acute wound is not exactly the same as the chronic wound. As a result, metabolic management approaches to acute wound care and trauma management may not apply universally to the chronic wound patient.
In many respects, studying the chronic wound patient has been more difficult than studying the trauma patient. Trauma patients are in a controlled environment in the hospital or intensive care unit, making them easier to study than the ambulatory patient. Animal models for acute wounds are much easier to develop than chronic wound models. As a result, much less information is available on nutrition and the chronic wound patient. This review will attempt to provide the reason that this is important, summarize the limited information on nutrition and chronic wounds, and provide recommendations to help in the management of these patients.
Nutrition concepts
To make sense of the nutritional needs of the wound patient, it is important to understand some basic concepts and terminology. All nutrient intake can be broken down into macronutrients, micronutrients, and water. Macronutrients are defined by ASPEN Guidelines and Standards as “nutrients present in the body and required in the greatest amount (e.g., carbohydrates, proteins, lipids).”7 Amino acids are the building blocks of protein throughout the body. Some individual amino acids (arginine, glutamine, and methionine) have been supplemented in addition to the protein in the diet as adjunct pharmacologic nutrients for wound healing.
Carbohydrates may be used to provide carbon skeletons for amino acid synthesis but only for those amino acids that are nutritionally dispensable (nonessential). Indispensible (essential) amino acids must be provided in the diet as a component of protein fed or as a keto-acid for all indispensible amino acids except lysine, threonine, and histidine.8 Fatty acids and cholesterol are nutrients that can be catabolized via beta oxidation to form cellular energy (ATP) and have important cellular functionality such as insulating membranes for nerve axons and are also necessary to form the lipid bilayer essential for organelle and cell membranes.
Micronutrients are defined as “nutrients present and required in the body in minute quantities (e.g., vitamins, trace elements).”7 Micronutrients include the vitamins and certain minerals. These minerals are often referred to as trace elements. Certain minerals, such as calcium, magnesium, and phosphorus (macrominerals), which are present in large quantities in the bone and other tissues, are not considered trace elements. One of the most common functions of micronutrients, and some macrominerals, is to serve as necessary cofactors for enzymatic reactions.
Essential (or indispensable) nutrients are those that cannot be synthesized in the body, such as vitamin C and minerals. Conditionally essential (or conditionally indispensable) nutrients are those that are necessary in the diet under defined metabolic circumstances where the needs of the body cannot be met by endogenous synthesis mechanisms. Providing optimal nutrition must be understood as a dynamic process as patient needs change based on the amount of exercise they do and comorbidities. For example, an adequately nourished patient may become acutely malnourished over a short period of time due to the increased metabolic needs of an injury or sepsis. Under these circumstances, a wound that was healing well may be diminished until nutritional deficits can be met. This phenomenon can explain the observed problem of slowed healing of our outpatients with repeated bouts of urosepsis or respiratory infections.
Epidemiology of malnutrition
One might suspect that in the wealthy countries of the world with easy access to food, malnutrition in the outpatient ambulatory setting is a thing of the past. There are a number of issues that must be accounted for to explain the problems of malnutrition in the developed world. At the beginning of the 20th century, the average life expectancy for an individual in the United States was only 49.2 years of age. However, at the end of the same century, this average life expectancy had risen to 79 years of age. At the end of the 20th century, ∼12.8% of the U.S. population was over 65 years of age.9 It is estimated that by 2050, 22% of the global population will consist of individuals over the age of 60 years.10 In a nation whose citizens are continually living longer, it is important to consider the nutrition needs and implications of deficiencies in this growing population.
The term malnutrition encompasses a variety of nutritional concerns, such as undernutrition resulting from decreased or inadequate food intake, overnutrition caused by excessive food consumption, and specific nutrient deficiencies.11 In regards to malnutrition in the elderly, the term usually refers to the overall undernutrition of an individual. However, this condition often results in either protein-calorie malnutrition (PCM) or specific vitamin and mineral deficiencies; in some cases both may occur.12 In 2006, ∼5–10% of older individuals living independently were considered malnourished. These statistics are seen to increase to 30–60% of institutionalized and 35–65% of hospitalized elderly. The highest proportion of malnourishment is found within the nursing home populations, with up to 85% of these patients exhibiting poor nutritional status.13 Past data from the National Health and Nutrition Examination Survey (NHANES) indicates that roughly 16% of elderly individuals living independently in the United States consume <1,000 kcal/day, increasing the probability of undernutrition and chronic deficiencies.14 Collins et al. reported that up to 30% of the Australian elderly community is at high risk of being malnourished; additionally, a 1998 regional survey reported that the home-nursed elderly group exhibited a moderate-to-high risk of developing malnutrition using the Australian Nutrition Screening Initiative tool (Fig. 2).15
Figure 2.
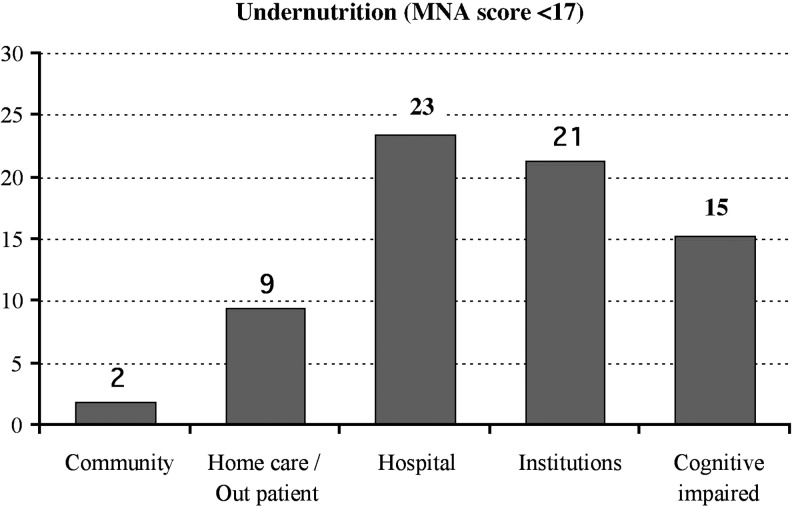
The MNA tool is widely used. In this figure, ∼35,000 elderly subjects were assessed in different housing settings. A score of <17 using the MNA is categorized as undernourished elderly. Hospital, cognitively impaired, and institutions (nursing homes, assisted care, and long-term care) have the greatest percentage of undernourished elderly; however, home health care and outpatient patients are at risk for undernutrition. MNA, Mini Nutritional Assessment. Adapted from Guigoz45 with permission from The Journal of Nutrition, Health, and Aging.
Three separate studies conducted in the United States on three different racial groups provide a comparison of the individuals who are undernourished, at risk of malnutrition, or well-nourished in each of these populations. The groups studied were elderly Hispanic, non-Hispanic whites, and inner-city African Americans; all of whom were independent and residing within the community setting. The highest rates of undernutrition were noted in the African American elderly (1.6%), followed by the Hispanic elderly (1.0%). In a selection of 420 non-Hispanic white elderly, none of these individuals were undernourished. Approximately 38.6% of the African American elderly were considered at risk of malnutrition. In comparison, only 27.0% of Hispanic elderly and 15.0% of non-Hispanic white elderly were determined to be at risk for malnutrition.16 This information indicates that minority populations are at a greater risk of developing malnutrition.
Physiological and sociological causes of malnutrition
Many age-associated implications put the elderly at an increased risk of developing nutritional deficiencies. These include medical, psychological, physiological, social, and economic difficulties commonly identified in individuals of increased age.12
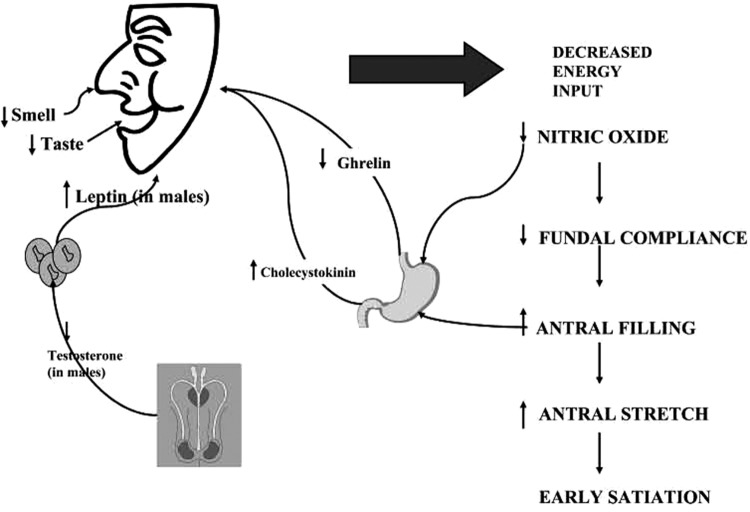
One of the leading causes of malnutrition in the elderly population is decreased food intake associated with a loss of appetite (Fig. 3). This depressed appetite is related to several commonalities of this age group, such as low physical activity or complete immobilization, general pain, social isolation, comorbidities (depression, dementia, stroke, obesity, etc.), deterioration in the senses of taste and smell, vision changes, oral dysfunctions (dry mouth, sores, dysphagia, tooth loss, and issues with mastication), limited self-feeding, and polypharmacy. In fact, the regular use of multiple medications has been determined to contribute largely to geriatric hospitalization. Commonly noted nutrition-related drug reactions are loss of appetite, decreased taste acuity, altered taste, xerostomia, nausea and vomiting, and mental dysfunction.12 Approximately 250 drugs, and counting, have been reported to affect the senses of taste and smell, directly affecting consumption.13 The known side-effects of constipation, nausea, and anorexia would suggest the cautious use of narcotics to manage pain in senior citizens with wounds. At the same time, not treating the pain of the wound will also lead to anorexia. In some cases, appropriate topical management of the wound may ease the pain without the side-effects of the medication.
Figure 3.
With age, appetite is decreased through a variety of mechanisms making it difficult for some geriatric patients to meet metabolic needs without supplements. This is especially true when demands are increased by the presence of wounds. Adapted from Wilson and Morley111 with permission from The American Physiological Society.
Retirement from a previous career and the physical inability to work affect the financial status of many elderly individuals. Those with limited financial flexibility are predisposed to poor nutritional intake due to the inability to obtain nutrient-dense, healthy foods.17 It has been identified that as many as 40% of elderly individuals have an income of less than $6,000 per year, spending approximately $25–35 per week on food alone. In times of financial distress, many individuals will prioritize household bills and medication costs before healthful foods.11 In correlation, the degree to which an individual perceives that he/she is dependent on others negatively affects overall intake. Nutritional intake is reduced the more an individual feels that he/she is dependent on another.17
The death of a spouse or friend is not uncommon in the elderly population and contributes significantly to nutritional intake. The surviving spouse is often subjected to social isolation, loneliness, depression, financial worry, and consequently malnutrition.11 Depression is considered a major factor responsible for decreased intake and weight loss in both independent and hospitalized elderly.9 According to surveys of elderly persons living independently, those individuals who eat alone tend to eat much less, further expediting the development of deficiencies.13
Many older adults may have been diagnosed with a number of different disease states, such as diabetes, hypertension, or dyslipidemia. Due to these previously established conditions, the patient may have been placed on a therapeutic diet as treatment. However, therapeutic diets are considered a causative agent of weight loss in this older population. These diets may be disregarded or modified substantially in hospital or institutional settings.9
PCM in the elderly
Typically, caloric needs decrease with age due to decreased activity, decreased muscle mass, and slowing of metabolic rate. However, protein, fluid, vitamin, and mineral recommendations may remain relatively stable. It becomes difficult to decrease caloric intake to avoid obesity and still meet the needs of protein and micronutrients. Thus, a nutrient-dense diet is recommended to concentrate the most effective levels of nutrients within a limited caloric threshold.18 A required micronutrient supplement will be necessary.
PCM results from overall underconsumption of both protein and energy. In general, PCM is characterized by weight loss and a decrease in lean body mass (LBM).11 Unintended weight loss in the elderly is an important clinical indicator of malnutrition. It has been reported that weight loss of more than 4% of total body weight in 1 year is predictive of forthcoming mortality in older adults.19 Therefore, it is important to identify PCM early to reduce the likelihood of further complications.
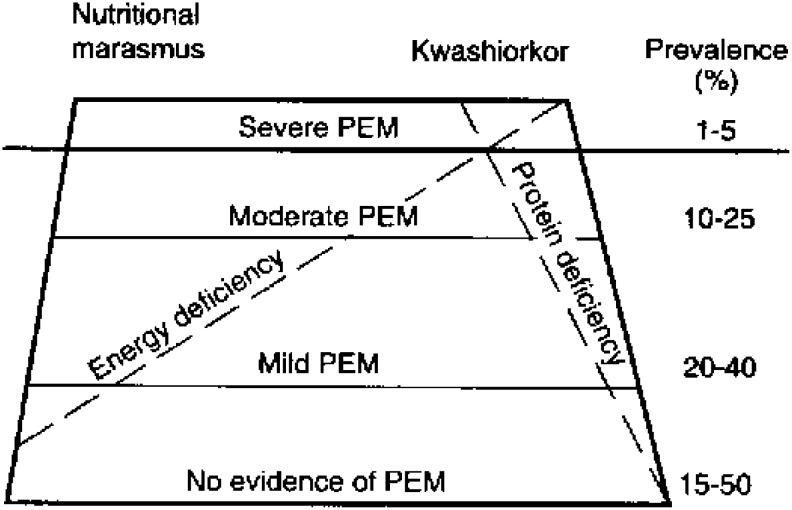
Three specific varieties of PCM may occur in the elderly. Sometimes they are described using the historical terms of marasmus, kwashiorkor, or marasmic kwashiorkor (Fig. 4).11 While originally used to describe malnutrition in children in developing countries, conceptually these states are also seen in elderly individuals living independently in the community or nursing home. Marasmus is characterized clinically by a decrease in both fat and muscle mass, inadequate caloric intake, body wasting, and normal serum albumin levels due to normal visceral organ function.11 Kwashiorkor is clinically described as an inadequate consumption of protein, a well-nourished appearance, edema, and decreased serum albumin levels. This individual may appear obese but be dangerously malnourished, as visceral organ function may be compromised. The term sarcopenic obesity has been used to describe this condition.20 This type of malnutrition is most commonly seen in hospitalized patients experiencing illness, inflammatory stress, or infection who receives excessive calories but inadequate protein.19 While marasmus may take many months to develop, a kwashiorkor state can develop in a matter of weeks when an already nutritionally compromised patient develops an infection. It is important to note that in most cases the elderly develop characteristics of both conditions.11
Figure 4.
In this graphical depiction of PEM, conditions where energy and protein deficiencies coexist define nutritional marasmus, which is frequently seen in the undernourished elderly patient. Protein deficiency with limited energy deficiency defines kwashiorkor and some recent estimates suggest that up to 50% of residents in nursing homes do not receive enough protein in their diet. PEM, protein energy malnutrition. Adapted from Latham112 with permission from the Food and Agricultural Organizations of the United Nations.
The presence of a wound may increase metabolic demands due to the metabolic response to injury. In addition, large quantities of protein can be lost in wound exudates each day.21 It is not uncommon to see large pressure ulcers or large leg wounds that represent 5–10% body surface area.21,22 The presence of a chronic wound can increase protein requirements by 250% and calorie requirements by 50% in order to maintain an appropriate LBM.23 All of these protein losses should be replaced in order to prevent further PCM and complications to wound healing. Losses in LBM occur when inadequate protein is available, causing body protein to be broken down to provide amino acids to the wound site for healing. If glucose (energy or calories) is insufficient, then additional protein will be broken down so that amino acids can be used for glucose synthesis in the liver via the alanine shunt pathway.24 This breakdown of body proteins is the contributing factor to losses of LBM.
When considering the effects of PCM on wound healing, it is important to recognize the implications that losses in LBM have on wound healing, particularly chronic wounds. In an individual who has lost up to 20% LBM, wound healing will take biological precedence over restoration of LBM. Only at a critical point for survival, a loss of 30% LBM, will the body postpone wound healing to address LBM. However, any loss of LBM associated with PCM may negatively affect wound healing. Losses of 10% LBM are associated with impaired immunity and risk of infection, losses of 20% LBM decrease the rate of wound closure and cause the skin to thin, losses of 30% LBM halt healing and predispose the patient to new wound formation, and losses of 40% LBM often result in death (Fig. 5).21,25
Figure 5.
In the geriatric or chronically ill patient, there is a complex interaction between anorexia, infection, wounds, and metabolic needs. In a patient with marginal nutrition and a wound, infection (even if remote from the wound such as urinary tract infection) may increase metabolic demands and limit wound healing if the metabolic losses are not replaced. Such infections also commonly lead to more anorexia. The result is a vicious spiral potentially leading to the death of the patient if the cycle is not broken with appropriate interventions. With permission from WFUSM Plastic Surgery Collection.
These negative consequences of protein loss are exemplified in all four stages of wound healing (hemostasis, inflammation, proliferation, and remodeling). Phagocytes, monocytes, lymphocytes, leukocytes, and macrophages require protein for their formation and are necessary for an immune response. An insufficient immune response will delay the progression of the wound from the inflammation stage to the proliferation phase. PCM reduces fibroblast activity in both the proliferation phase and remodeling phase, preventing angiogenesis and new collagen formation.18 As collagen is the predominant protein of the healing wound, this may have profound consequences.
Occurrence of micronutrient deficiency in the elderly
By underconsuming key nutrients for a prolonged period of time, elderly patients are placed at risk for malnutrition caused by micronutrient deficiency.11 The most common micronutrient deficiencies among the elderly include vitamin D, zinc, and vitamin B12 (cobalamin), which may have a significant impact on wound healing. However, keep in mind that all water-soluble vitamins and many minerals are required as cofactors for metabolic reactions in the body. Although the amount of each micronutrient required is small, sustained underconsumption combined with metabolic stress can create conditions where reactions are less efficient and body repair cannot be optimized.
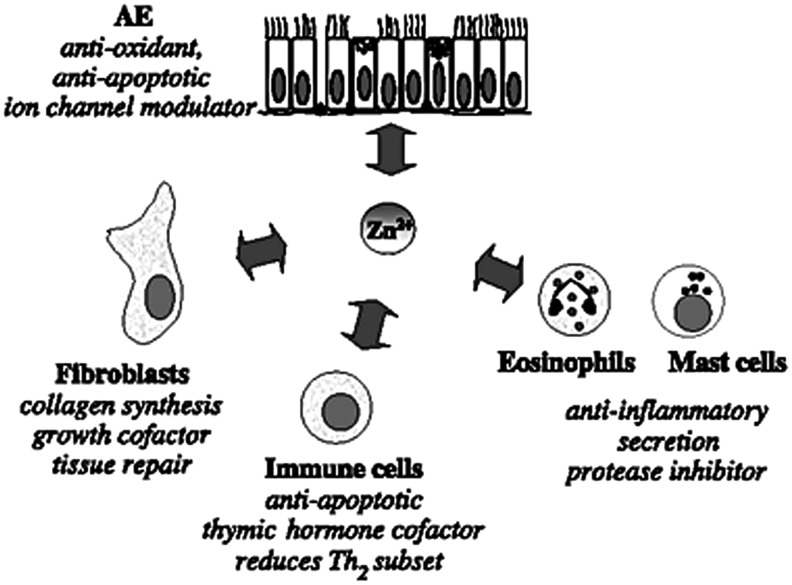
A decline in consumption of dietary zinc with the process of aging has been noted in both developed and developing nations.26 Therefore, the elderly are included among the individuals most likely to be affected by a zinc deficiency. Due to the involvement of zinc in critical functions within the immune system, deficiency can lead to depressed immunity and increased susceptibility to infection.25,26 Research (in vivo) has shown that decreased levels of zinc can reduce the cytotoxicity of natural killer cell, impair phagocytosis in macrophages and neutrophils, and reduce the number of granulocytes. Zinc deficiency also impairs functions of specific immunity; both B-cells and T-cells are affected in the presence of low zinc levels.27 As a result of a zinc deficiency, both B-cell precursors and mature B-cells are reduced in number. This is understood to be the result of premature apoptosis of these specific cells. Further, T-cells undergo a reduction in functionality, increase in autoreactivity and alloreactivity, and decrease in number. The reduced concentration of B-cells and T-cells reduces the capability of the immune system to produce adequate antibodies relative to antigens present.26 In combination, the effects of zinc deficiency predispose the elderly to a suppression of the immune system and a decreased immune response with injury (Fig. 6).28
Figure 6.
Although this figure depicts the interaction of zinc with AE cells, it demonstrates the role that zinc has in collagen synthesis and tissue repair, as an component of the antioxidant system, as well as antiapoptotic and anti-inflammatory actions. Zinc deficiency will negatively influence wound healing while oversupplementation appears to have no or potentially negative consequences. Adapted from Zalewski et al.113 with permission from Elsevier. AE, airway epithelial.
Deficiency (<20 ng/mL) and insufficiency (<30 ng/mL) of vitamin D in the serum has been noted in the elderly population and recent literature indicates that ∼20% of the worldwide population over the age of 50 have a reduced capability to maintain sufficient levels in the serum even with aggressive supplementation.29 In addition, more individuals with a higher body mass index (BMI) have been reported with vitamin D deficiency when compared with individuals with lower BMI. Data related to vitamin D deficiency and wound healing are limited. However, recent research efforts have identified the presence of vitamin D receptors in a number of different tissue types where their presence was previously unknown. Zhang et al. noted that cathelicidin, an antimicrobial peptide that is induced by vitamin D, promotes wound healing.30 The conclusion of these reviewers is that vitamin D and its receptor signaling “regulates not only structural integrity but also transport functions of different epithelial barriers.” Burkievcz et al. and Kalava et al. noted a higher incidence of reduced levels of vitamin D in venous and pressure ulcer patients, respectively.31,32 It should be noted that the researchers were unsure whether the reduced level impacted the healing of these wounds or whether it was a comorbidity associated with the patient populations. Research directed toward the impact of vitamin D on wound healing is increasing and new developments related to this nutrient should be reported in the forthcoming literature.
Ascorbic acid is well known for its biological role as an antioxidant and as a cofactor in the hydroxylation of proline and lysine to hydroxyproline and hydroxylysine needed for the synthesis of collagen. If levels of ascorbic acid were deficient, then one would suspect a decreased synthesis of collagen due to decreased hydroxylation of lysine and proline. Since the classic observations by British naval physicians, scurvy, and its effect on the healing wound, deficiency has been avoided by simple dietary intake of citrus fruits. One would think that with food availability in modern society, vitamin C deficiency would be a thing of the past. Unfortunately, multiple recent publications have demonstrated scurvy and vitamin C deficiency in developed countries.33–35 Nancy Strange presented a case study at SAWC, 2012, where data from the Indiana University School of Medicine, IU Health, Indianapolis, found that 14 of 15 individuals with delayed wound healing had ascorbic acid levels below normal ranges.36 One case was present in the state of Florida, known for its large production of citrus fruit.35
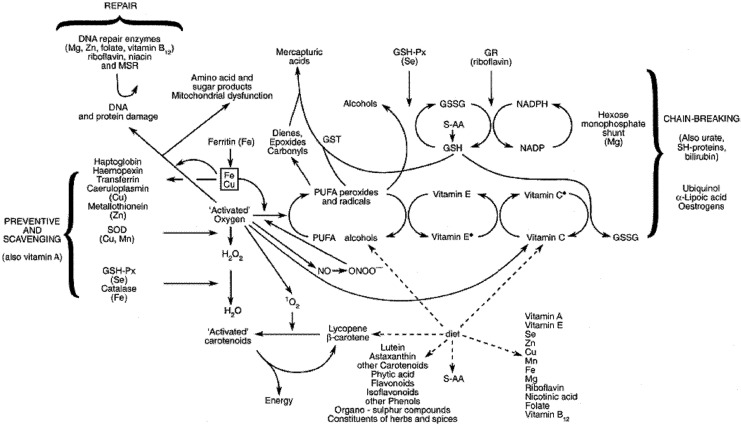
Oxidative stress is a measure related to the balance of pro-oxidants and antioxidants in the body. In a recent research publication, Wonisch et al. reported that oxidative stress (more pro-oxidants) increased with age and BMI in a population of 2,190 male subjects undergoing a health examination.37 Recently, Bryan et al. published a review article that explore the role of reactive oxygen species (ROS) in constructive inflammation and wound healing.38 Leukocytes, utilizing ROS, clean compromised tissue and eliminate pathogenic organisms; however, a long-term imbalance of pro-oxidants may compromise the wound healing rate and efficiency of new tissue deposition. Numerous micronutrients are involved in maintaining the antioxidant capacity of the individual, including vitamins A, E, K, and C; β-carotene and other carotenoids; and the minerals selenium and zinc. Several research trials have evaluated the efficacy of micronutrient supplementation as related to wound healing to be discussed later. Unfortunately, many experiments used “cocktails” of antioxidants, micronutrients, protein, and individual amino acids so the independent contribution of each nutrient is often difficult to elucidate (Fig. 7).
Figure 7.
The antioxidant defense system is complex with coupled oxidation/reduction reactions and free-radical-quenching activities. Dietary input of selected vitamins and minerals, unsaturated fatty acids, and various phytochemicals and flavonoids allow for regeneration of central antioxidants maintaining the balance between pro- and antioxidants. Adapted from Strain,114 with permission from Cambridge University Press.
Finally, achlorhydria, or decreased gastric acid secretions, has an increased prevalence in the elderly.12 Achlorhydria is caused by gastric atrophy frequently associated with aging.39 Due to the reduced state of acid secretion, bacteria are able to grow uninhibited in the small intestine, often referred to as “blind loop syndrome.”12,39 In turn, this imbalance can reduce the solubility and bioavailability of critical nutrients, such as calcium, iron, folate, vitamin B6, and vitamin B12.12 Limited gastric secretions results in the inability to free vitamin B12 from food or transport proteins; this mode of malabsorption is the most common cause of this nutrient deficiency in the elderly.39 It is often difficult to recognize vitamin B12 deficiency because clinical manifestations are not always pronounced and serum levels are not indicative. However, elevation of plasma levels of total homocysteine and methylmalonic acid is typically present in concurrence with a vitamin B12 deficiency; therefore, these values are used to more accurately diagnose a deficiency.40 Vitamin B12 acts as a methyl transfer cofactor converting homocysteine to the indispensible (essential) amino acid methionine.8
Nutritional assessment of patients
In past research, nutrition screening of older individuals has been difficult.41 One of the most published tools for identifying malnutrition in the elderly populations of the United States is the Mini Nutritional Assessment (MNA).42 Since publication, it has been considered one of the most valid tools for identification of malnutrition in the elderly population and cited in more than 400 scientific research publications. While it was designed for use in geriatric individuals, it has been shown to be useful across varying demographics.43,44
First, the MNA is a brief evaluation used to screen specifically for elderly individuals at risk for malnutrition and has been validated by three studies since 1991. This tool includes a series of responses to questions from four assessment categories: anthropometric, general, dietary, and subjective. Within these categories, patients are examined for weight changes, lifestyle characteristics, degree of mobility, medication use, food and fluid intake, ability to self-feed, and their opinion of their own general health and nutrition status.16 From the responses, each individual is given a score between 0 and 30; this correlates with their suspected nutrition level. Scores above 24 indicate no concern for malnutrition in that individual, scores between 17 and 23.5 identify a risk for malnutrition, and individuals scoring<17 are likely to be protein-calorie malnourished.16 Cumulative research (21 studies) using the MNA has shown that ∼2% of elderly living independently in the community have an MNA score <17. Further research (25 studies) indicates that 9% of elderly receiving care at home or outpatient services have an MNA score <17. However, the highest percentage (35 studies) of elderly individuals scoring <17 on the MNA are representative of the hospital setting.45
While the MNA evaluation only requires 15 min to complete, a shorter form has been validated for nutrition screening in lower risk elderly populations. The Mini Nutritional Assessment–Short Form (MNA-SF) is split into two steps. The first step involves a survey of six questions and requires <5 min to complete. As with the full MNA, the responses to the questionnaire are affiliated with a certain score. The maximum score for the MNA-SF is 14; any score ≤11 indicates a nutritional risk. Only if a nutritional risk is determined will the patient proceed to the second step of the evaluation, or the full-MNA questionnaire. This form of nutrition screening is the most beneficial in healthy or community-dwelling elderly.45
In a study performed by Lamghamp-Henken et al., the full-MNA evaluation and MNA-SF were shown to provide advantages in determining malnutrition in elderly individuals with pressure ulcers (stages I–IV) in comparison to serum protein levels alone.46 Thus, nutrition screening using the MNA could be used to identify developing malnourishment in elderly individuals with chronic skin wounds before complications to wound healing may occur.
Using the MNA, elderly individuals at risk for malnutrition can be identified for early nutrition intervention. However, no tool is without limitations.47 All data gathered from the MNA are based upon personal responses to this questionnaire; therefore, some bias may exist. Additionally, patients with cognitive impairment or an inability to speak will require assistance from a friend or family member in order to complete the survey.16
In addition to the MNA assessment tool, several other tests or techniques may be used to assess nutrition status of the patient. Skin fold measurements from various locations on the body (triceps, subscapula, suprailiac, abdomen, and thigh) can be used to assess fat, fat-free mass, and muscle size. This test should be performed by someone with experience to reduce variability.8 BMI and information regarding sudden unexpected weight loss can provide valuable information about body composition and reserve nutritional capacity of the patient. Recent advances in instrumentation have made bioelectrical impedance measurements, or use of conductance to calculate LBM, more affordable and reliable.
Recently, Jensen et al. published international consensus guidelines for adult starvation and disease-related malnutrition that may provide a good starting point for assessment of the nutrition status of patients undergoing treatment for wound care.48 Serum albumin is routinely assessed as a marker of visceral protein status and has been reported as declining 0.8 g/L per decade for individuals over the age of 60. Volpato et al. noted in a retrospective study of 1,117 deaths that serum albumin and high-density lipoprotein cholesterol (HDL-C) can be used to distinguish groups that are at risk for mortality.49 The identified three subgroups with different prognoses: high risk (low albumin, <3.8 g/L), intermediate risk [high albumin (>3.8 g/L) and low HDL-C (<47 mg/dL)], and low risk [high albumin and high HDL-C (>47 mg/dL)].
Numerous studies have demonstrated an increased risk for developing pressure ulcers using variables, such as BMI, recent weight loss, lowered serum albumin, reduced food intake, and protein intake/kg of body mass.50 In many of the reported cases, data are confounded due to the fact that serum albumin and prealbumin are acute-phase responders and may not be accurate indicators of the nutrition status if the patient is metabolically stressed. Since serum levels of albumin are an objective measurement that may already be available from laboratory tests such as a complete metabolic panel, they should not be ignored. When used in conjunction with other nutritional assessment tools and clinical history, they can provide useful information on the trends of the nutritional status of the patient. Whether the origin of the decreased value is from an inflammatory stress such as infection or due to nutritional deficits, normalization of the value is a positive sign of improved metabolic state (Fig. 5). Amir et al. in a recent review (2012) found that albumin levels were one of the most significant independent predictors of poor wound healing outcomes in chronic wounds.51
Many clinicians are not aware of the potential nutritional information in the complete blood count. Presence of a microcytic hypochromic anemia may represent iron deficiency, as well as anemia of chronic disease associated with PCM. Megaloblastic anemia may represent folate or B12 deficiency.8 Total lymphocyte count may also be a useful indicator of nutritional status.52
Chronic Wounds and Managing Nutrition Intervention
Not all wounds are the same. For example, the key to healing a vascular insufficiency wound is not the same as a pressure ulcer or a stasis ulcer even though they share some common pathways of chronic inflammation. Nonetheless, principles learned from one type of wound population may be helpful in the management of other wounds. This is particularly true of nutrition, the common denominator for all wound patients. As more information is available of nutritional interventions in pressure ulcers and stasis ulcer, it is prudent to review this in more detail.
Preventing the development of pressure ulcers is the most cost-effective methodology and involves reducing risk through management of intrinsic and extrinsic variables. Intrinsic factors include limited mobility and poor nutrition, the strongest predictors of pressure ulcer formation.53 van Anholt et al. indicated that the risk for an elderly patient to develop a pressure ulcer is two to three times higher when the patient is malnourished or underweight; however, this does not indicate that a nonmalnourished patient is protected from developing a pressure ulcer.54 Lamghamp-Henken et al. used an MNA tool for patients presenting with pressure ulcers and found that only 3 out of 23 subjects were considered well-nourished, whereas 20 were considered at risk for malnutrition or malnourished.46 Hengstermann et al., using an MNA instrument, found that the nutrition status of patients with pressure ulcers was significantly reduced in comparison to nonpressure ulcer patients and that the fraction of malnourished patients was twice as much in the pressure ulcer group than the age weighted control group.55 Haydock and Hill reported that wound healing was impaired in both mild and moderate/severe malnourished patients and that the “direction in which the patient was moving metabolically at the time of wounding” was more important than the degree of weight loss.56,57
Macronutrient supplementation
Total energy needs
Adequate energy needs to be provided to the patient, either through enteral or parenteral delivery methods, to provide for positive energy and nitrogen balance, if possible. All macro- and micronutrients need to be provided in quantities appropriate to support the functioning of catabolic and anabolic cycles necessary to support life. The European Pressure Ulcer Advisory Panel (EPUAP) recommended a minimum of 30–35 kcal/kg of body weight for patients with pressure ulcers. We have previously discussed malnutrition (protein/energy) as a significant risk factor for the development of chronic wounds. Distribution of macronutrient calories should be similar to dietary distribution in traditional diet regimes (45–60% carbohydrate, 25–30% fat, and 15–20% protein); however, additional protein may be needed to support the increased amino acid supply necessary for collagen synthesis and wound healing. For patients who are underweight or losing weight, the National Pressure Ulcer Advisory Panel (NPUAP) recommends increasing the caloric intake to 35–40 kcal/kg of body weight.58 Thompson and Fuhrman recommend careful monitoring of repletion and suggest the use of indirect calorimetry to modify the diet to meet the patients' metabolic needs.57 If the total energy needs of the patient are not met, then supplementation with additional micronutrients, protein, amino acids, or other nutritional components will most likely be unsuccessful.
Protein
Protein needs are increased in patients under metabolic stress or recovering from surgical procedures, range from 1.0 to 2.0 g/kg of body weight depending on the diagnosis.52 Chernoff reported that the elderly have an increased need for exogenous protein of at least 1.0 g/kg of body weight for normal maintenance and that “inadequate protein intake contributes to a decrease in the reserve capacity, increased skin fragility, decreased immune function, poorer healing, and longer recuperation after illness.”59 The Agency for Healthcare Research and Quality (AHRQ) recommend 30–35 kcal/kg of body weight per day, protein between 1.25 and 1.5 g/kg of body weight, and fluid intake of 1 mL/(kcal·day−1). Their recommendations are based on NPUAP/EPUAP findings in 2009.60 Legendre et al. noted that the prevalence of protein deficiency was higher in outpatients presenting with venous leg ulcers (27%) than in an age matched control group (2%) as defined by a serum albumin level below 3.5 g/L.61 Further, anthropometric measurement (BMI) was not sufficient to detect the protein deficiency and the authors suggested assessment of serum albumin, prealbumin, or C-reactive protein to assess protein status. Patients with an identified protein deficiency were associated with poor healing and the occurrence of wound complications.
Iizaka et al. estimated the protein intake required to achieve nitrogen balance in hospitalized adults with pressure ulcers.62 They noted that the average protein requirement for patients with pressure ulcers was 0.95 g/(kg·day−1), but that wound severity and condition of the patient may result in a range of 0.75–1.30 g/(kg·day−1). The authors hypothesized that patients may lose more protein due to hypercatabolism, rather than direct loss from wound exudate and the severity of the pressure ulcer. The authors estimated protein catabolism by measuring the urinary 3-methylhistidine-to-creatinine ratio.
Choo et al. recently published a review of research related to nutritional intervention(s) and the treatment of pressure ulcers.63 The authors reviewed six nutrition intervention research studies conducted since 2001, with five of the six studies using enriched protein supplements as part of the intervention. As with many of these studies, enriched proteins were included in the supplement with additional micronutrients and/or calories. Only one study of the review looked at protein supplementation alone (collagen protein) and pressure ulcer healing was significantly improved over the control treatment. The other four trials that used additional protein supplementation along with micronutrient supplementation demonstrated increased healing when compared with the control groups.
Care needs to be taken to ensure that adequate calories are provided to the patient to spare amino acids from being used to support caloric rather than protein synthesis needs. While inadequate protein consumption is characterized by edema in peripheral vessels due to decreased oncotic pressure, oversupplementation may not promote additional protein synthesis and can result in dehydration due to increased oncotic pressure.52
Arginine and glutamine in wound healing
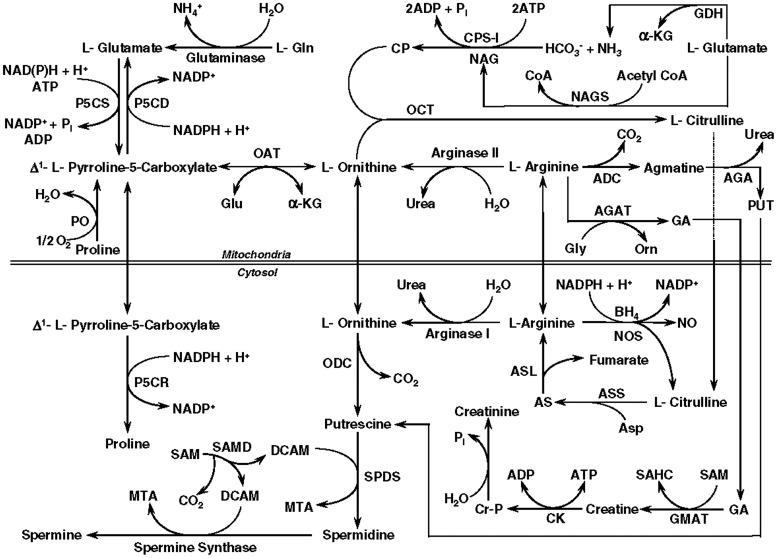
Arginine is considered a conditionally indispensable (conditionally essential) amino acid, needed in the diet only under conditions of metabolic stress. Under normal circumstances, it is synthesized in the kidney and liver from gut-derived citrulline (Fig. 8).64 Due to the ability of the body to manufacture arginine, it is not normally an essential part of our diets. However, in situations, such as sepsis, trauma, and wounds, the needs of the body may not be met by this endogenous mechanism.
Figure 8.
Arginine is central in metabolic pathways related to proline, a collagen precursor; glutamine, a nitrogen shuttle molecule and NO production, involved in neovascularization. As a result, it may become an essential amino acid in periods of metabolic stress. ADC, arginine decarboxylase; AGA, agmatinase; AGAT, arginine:glycine amidinotransferase; α-KG, α-ketoglutarate; AS, argininosuccinate; ASL, argininosuccinate lyase; Asp, aspartate; ASS, argininosuccinate synthase; BH4, (6R)-5,6,7,8-tetrahydro-L-biopterin; CK, creatine kinase; CP, carbamoylphosphate; CPS-I, carbamoylphosphate synthetase-I (ammonia); Cr-P, creatine-phosphate; DCAM, decarboxylated S-adenosylmethionine; GA, guanidinoacetate; GDH, glutamate dehydrogenase; Gln, glutamine; Glu, glutamate; GMAT, guanidinoacetate N-methyltransferase; MTA, methylthioadenosine; NAG, N-acetylglutamate; NAGS, N-acetylglutamate synthase; NO, nitric oxide; NOS, nitric oxide synthase; OAT, ornithine aminotransferase; OCT, ornithine carbamoyltransferase; ODC, ornithine decarboxylase; P5CD, pyrroline-5-carboxylate dehydrogenase; P5CR, pyrroline-5-carboxylate reductase; P5CS, pyrroline-5-carboxylate synthase; PO, proline oxidase; PUT, putrescine; SAHC, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SAMD, S-adenosylmethionine decarboxylase; SPDS, spermidine synthase. Adapted from Fang et al.115 with permission from Elsevier.
There are a number of theoretical possibilities of how arginine may affect wound healing. Proline is necessary for the synthesis of collagen and arginine is a precursor via the pathway of Arginine→Ornithine→glutaminesemialdehyde→Proline. It has been suggested that when the production of collagen is high, arginine may be an essential precursor of this amino acid.65,66 Arginine is also a precursor for nitric oxide, an essential compound for proper wound healing.67–69 Arginine, as well as ornithine, stimulates T-cells and the production of growth hormone, which may also aid the healing wound.64–66
In the 1970s Seifter et al. observed that growing animals required more arginine than mature animals and demonstrated that injured rats healed better with arginine supplementation based on measurements of wound-breaking strength.70,71 Subsequently, this was confirmed by others and ultimately applied to human studies. In a study of healthy human subjects and a polytetraflouroethylene tubing model of healing, collagen deposition was shown to be improved in volunteers given supplements of 30 g a day of arginine.72 In a study more relevant to the chronic wound patient, similar results were found in patients, 67–82 years of age, receiving such supplementation.73 Forty-three nonmalnourished subjects with stage III or IV pressure ulcers were included in a multicountry, randomized, controlled, double-blind, parallel group trial by van Anholt et al.54 They provided patients with a high protein arginine and micronutrient-rich supplement and found significantly improved healing. Yatabe et al. found that pressure ulcer patient's serum arginine was decreased and supplementation with an arginine solution improved wound healing.74 Leigh et al. determined the dose response of arginine supplementation with pressure ulcers, with no improvement of healing rate with 9 versus 4.5 g/day of supplementation.75 Others have demonstrated improved healing in diabetic foot ulcers with supplementation of the diet with arginine.76 Similar results were found with stasis ulcers.77 In a randomized controlled trial, Debats et al. did not find improved healing with skin graft donor sites in patients who received intravenous supplementation with arginine.78 However, this study may be misleading due to the fact the arginine was given intravenously and trying to measure differences in skin graft donor sites might be difficult since such wounds normally heal quite well. Stechmiller and Schols review some of the recent studies in more detail.79,80
Glutamine is the most abundant free amino acid in the human body; however, similar to arginine, it has been found to be conditionally indispensable (conditionally essential) in periods of metabolic stress. It has a unique role in that it is involved in shuttling nitrogen in the body. Glutamine is essential for the integrity of the gut mucosa, but is also used as an energy source via breakdown to glutamate and α-ketoglutarate entering the Krebs cycle. The nitrogen may then be used for the production of other nitrogen-containing molecules. Recently, this has been noted in neutrophils that may preferentially use glutamine as an energy source at a rate faster than glucose. In these cells of rapid turnover, nitrogen may be used in the production of needed amino acids, and purines and pyrimidines for DNA and RNA synthesis.81 Its metabolic functions include provision of NADPH and increasing insulin sensitivity.81 Glutamine also appears to have a role in leukocyte apoptosis, superoxide production, antigen processing, and phagocytosis.82,83 The suggested anti-inflammatory and immune activity may include activation of nuclear factor–α β and cytokine release.84 Glutamine is a precursor for glutathione, an important antioxidant necessary for stabilization of cell membranes, transporting amino acids and drugs across membranes, and a cofactor for enzymatic reactions.82 Due to this role in nitrogen metabolism, it is essential that glutamine be present in the body in adequate quantities.
The need for glutamine supplementation remains controversial. There is evidence in situations of trauma, burns, and sepsis that glutamine supplementation improves gut function, decreases septic complications, and improves insulin sensitivity, suggesting the presence of the amino acid in insufficient quantities.85,86 Many have assumed that the same situation is true for patients with chronic wounds but this is not clear. Most of the literature in this regard is confused with combination supplementation with arginine or other micronutrients.87–90 Indeed, patients with chronic wounds generally do not have an inflammatory and metabolic state similar to the patient with sepsis or trauma. Sepsis and trauma patients are hypermetabolic but patients with chronic malnutrition tend to be hypometabolic. However, in states of chronic malnutrition, the metabolic needs for glutamine may be increased in some situations due to atrophy of the gut mucosa and needs to improve the metabolism of the gut to allow other nutrients to be adequately absorbed. In addition, malnutrition in chronic wound patients may lead to a decrease in muscle mass and a relative deficiency of total body glutamine. With this information and the relative safety of glutamine supplementation it is reasonable to supplement select patients with evidence of malnutrition and healing wounds. However, like arginine supplementation, it should never be considered a substitute for correction of PCM but a supplement to improvement to overall protein intake. Without adequate overall protein intake, supplementation with glutamine or arginine is of no value.
Fat
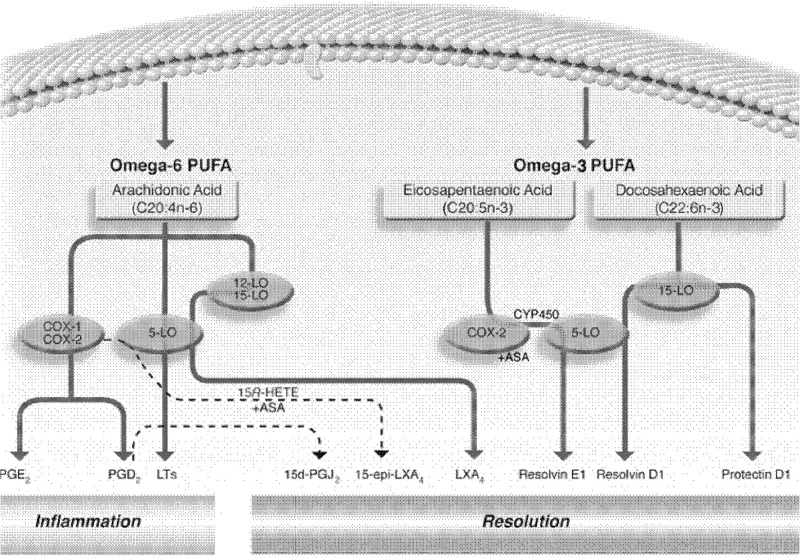
Fat must be an integral part of the diet and humans must consume essential fatty acids, linoleic acid, and α-linolenic acid in their diet for normal metabolism. Turek published a review in 2007 on fats and their influence on wound healing.91 Turek notes that besides their use as an energy source in the body and components of the lipid bilayer, fatty acids are the precursors for physiological substances, such as prostaglandins, thromboxanes, and leukotrienes. These compounds originate from omega-3 and 6 fatty acids and have different physiological effects in the human. Omega-3 fatty acids (docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA]) have been reported as being antithrombotic, vasodilators, and are anti-inflammatory whereas omega-6 fatty acids (ARA) have been reported as being platelet aggregating, inflammatory, and vasoconstrictive.
Turk et al. reported that in mice subjected to colonic wounding, DHA-fed animals started to gain weight between days 3 and 6 post-trauma whereas EPA- and corn-oil-fed animals did not.92 The authors suggested that “DHA may facilitate events that expedite the later stages of wound healing, perhaps by resolving inflammation.” This agrees with the data of Turek who suggested that in order to facilitate an adequate inflammatory response in early wound healing, omega-6 to omega-3 fatty acid ratio should remain above 10:1 until wound healing reaches the proliferative and remodeling stages.91
Theilla et al. reported that an enteral or parenteral formula enriched in fish oil and antioxidants (increased levels of vitamin C, vitamin E, vitamin A, copper, manganese, and zinc) when compared to isonitrogenous/isocaloric formulas resulted in decreased progression of pressure ulcers and a decrease in the blood level of C-reactive protein.94 The authors suggested that the results may be that “omega-3 PUFAs may have attenuated the inflammatory response in a manner that minimizes tissue injury while avoiding suppression of those components of the inflammation necessary for subsequent wound healing” (Fig. 9).93
Figure 9.
Polyunsaturated fatty acids in the diet are commonplace with overconsumption of omega-6 and limited consumption of omega-3 fatty acids in most Western diets. As this figure depicts, omega-6 fatty acids act as precursors for many proinflammatory/prothrombotic compounds (cyclooxygenases, prostaglandins, and leukotrienes) whereas omega-3 fatty acids are less thrombotic and inflammatory. Ratios of omega-6 to omega-3 fatty acids in the range of 10:1 have been suggested during proliferative and remodeling stages of wound healing while an optimum ratio after wound healing to promote cardiovascular health would be <3:1. Adapted from González-Périz and Clària116 with permission from Joan Clària.
In a similar study, Theilla et al. found that an enteral formula enriched with EPA, γ-linolenic acid, and vitamins A, C, and E resulted in a significantly lower incidence of new pressure ulcers in patients with acute lung injury.94
Research on specific fatty acid supplementation, the ratio of omega-6 to omega-3 fatty acids in the diet, and when to supplement in relation to the stage of wound healing progression needs to be further researched. Data from many of these research trials are again confounded due to the “cocktail of nutrients” approach taken.
Supplementation of micronutrients
Zinc
Over 200 zinc-containing enzymes and metalloenzymes are present in the body that are involved in wound healing, participate in antioxidant function (superoxide dismutase), cell replication, nucleic acid metabolism, tissue repair, and growth.95 Despite this, some suggest that zinc supplementation is only recommended when a zinc deficiency is determined (serum and wound assay) as excess zinc supplementation can interfere with other cation absorption, specifically iron and copper.96 Dorner et al. recommend zinc supplementation up to 40 mg elemental zinc/day (176 mg zinc sulfate) for up to 10 days to enhance wound healing if a zinc deficiency is indicated.58 Other recommendations are often higher being 220 mg of zinc sulfate (50 mg elemental zinc) twice a day for 2 weeks.97 Many of the research trials that evaluate supplementation of zinc to assist in wound healing have zinc as a component of a multinutrient supplement. A number of these supplements contain arginine, glutamine, ascorbic acid, protein, and other micronutrients, in addition to zinc, and have had variable impacts on wound healing.15,54,87,98,99 Research trials using these multinutrient component supplements make it difficult to determine the individual nutrient contribution to wound healing. A review on the impact of zinc in wound healing, in both enteral and topical administration routes, has been written by Lansdown et al., in which topical zinc application to surgical wounds seems to consistently augment wound healing.100
Ascorbic acid
Patients consuming suboptimal doses of vitamin C will not immediately exhibit decreased collagen synthesis and delayed wound healing immediately.101 Results from NHANES III report that of 15,769 patients in the sample pool, 14% of the men and 10% of the women exhibited severe vitamin C deficiency (<11 μM) and 20% of men and 17% of women had a marginal vitamin C deficiency (11–23 μM).102
Tanaka and Molnar recommend that although the data on vitamin C supplementation on chronic wound healing are inconclusive, the influence of ascorbic acid on collagen formation, antioxidant capacity, and immunomodulation suggests that individuals with small wounds or pressure ulcers consider supplementation with 500 to 1,000 mg of ascorbic acid daily in divided doses for optimal utilization.103 For patients with more severe wounds, such as burns over a large area, doses can be increased to 1–2 g/day. A recent review of research trials by Ellinger and Stehel looking at the efficacy of vitamin supplementation on wound healing concluded that “clear evidence for pressure ulcer prevention by oral supplementation of single antioxidant vitamins or ONS (Oral Nutrient Supplement) containing protein/amino acids, trace elements and vitamins not available.”104 Further, after evaluating numerous randomized control treatments, the authors noted that “convincing evidence exists only for a protein and energy rich ONS providing daily at least 500 mg of vitamin C, 17 mg of zinc and arginine in pressure ulcer therapy.” A general review of the literature on vitamin C and its impact on morbidity and mortality was recently published by Lykkesfeldt and Poulson.105
Vitamin A
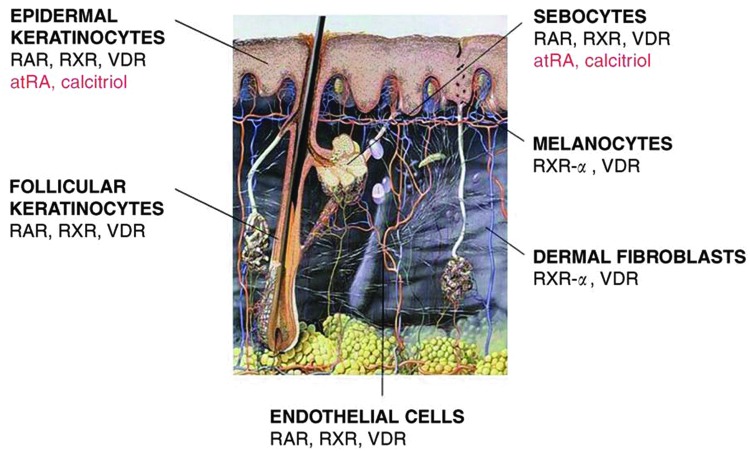
Vitamin A is an essential fat-soluble vitamin that has been used topically in dermatology for many years for the management of such conditions as photodamage, psoriasis, and to aid healing after such treatments as dermabrasion. It has been found to stimulate epithelial growth, fibroblasts, and ground substance as well as having an anti-inflammatory effect in open wounds. The literature supports a positive effect of supplemental vitamin A in acute wounds and healing of fractures, burns, bowel, and radiation-induced injury.8,106,107 Indeed, vitamin A seems to function as a hormone altering the activity of epithelial cells, melanocytes, fibroblasts, and endothelial cells through a family of retinoic acid receptors (Fig. 10).108
Figure 10.
Vitamin A effectively works as a hormone-stimulating retinoid receptors that alter the function of keratinocytes, endothelial cells, fibroblasts, melanocytes, and sebocytes. atRA, all-trans retinoic acid; RAR, retinoic acid receptor; RXR, retinoid X receptors; RXR-α, retinoid X receptor type; VDR, vitamin D receptor. Adapted from Reichrath108 with permission from Thieme. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The effect of vitamin A on patients with chronic wounds is less clear except in the case of the patient being treated with corticosteroids for inflammatory diseases such as rheumatoid arthritis. For these individuals, vitamin A has a unique role in nutritional management. Since the early studies of Ehrlich et al., investigators have noted that vitamin A supplementation can counteract the negative effects of glucocorticoids on wound healing.109 Wicke et al. demonstrated in a rat model that administration of methylprednisone significantly reduced hydroxyproline synthesis by ∼50% on day 17 of treatment (methylprednisone) but supplementation of steroid-fed animals with either all trans-retinoic or 9-cis retinoic acids increased hydroxyproline content to near normal levels.110 There was also a positive effect on collagen production on animals not receiving steroids.
The mechanism for this effect has been controversial, as steroids have an effect on all stages of wound healing, there are many possibilities. Ehrlich et al. suggested that the mechanisms may have been through stabilization of the lysozomes.109 Wicke et al. found that vitamin A reversed the effect of steroids to decrease the levels of transforming growth factor beta (TGFβ) and insulin-like growth factor-1 (IGF1) in the wound.110 Whether this effect is desirable in chronic wounds is remain to be seen as TGFβ is one of the proposed mediators of chronic inflammation in the chronic wound. Clearly additional work will be required to determine the value of vitamin A in chronic wounds.
Stechmiller suggests oral administration to patients at 10,000–15,000 IU/day vitamin A to enhance wound healing in patients receiving corticosteroids.96 In our clinic, we recommend 20,000–25,000 IU. Treatment must be provided as a short course of 10–14 days to be safe and used with caution in individuals who may have deficiency of the carrier protein, retinol binding protein, due to liver malfunction or severe PCM.
Summary
Modern understanding of nutrition and wound healing is largely derived from the research dealing with nutrition in trauma and sepsis. The information obtained from these young, previously healthy, hypermetabolic patients may not completely translate to the chronic wound patient who is typically elderly with coexisting disease, chronically malnourished, and hypometabolic. Patients with chronic wounds should undergo a nutritional assessment as part of their general wound management evaluation. This is necessary due to the modern epidemic of malnutrition even in industrialized countries and the fact that even obese patients may suffer from protein and micronutrient malnutrition. Protein and caloric deficits must be replaced and micronutrients should be supplemented in patients with malnutrition. Zinc and vitamin C are recommended supplements in patients with wounds due to the central role in collagen metabolism and inflammation. Vitamin A has a unique role in the management of the patient treated with corticosteroids. Arginine and glutamine supplementation may be helpful but only if protein and calories are present in adequate quantities in the diet.
Abbreviations and Acronyms
- ADC
arginine decarboxylase
- AGA
agmatinase
- AGAT
arginine:glycine amidinotransferase
- AHRQ
Agency for Healthcare Research and Quality
- α-KG
a-ketoglutarate
- ARA
arachidonic acid
- AS
argininosuccinate
- ASL
argininosuccinate lyase
- Asp
aspartate
- ASPEN
American Society of Parenteral and Enteral Nutrition
- ASS
argininosuccinate synthase
- ATP
adenosine triphosphate
- atRA
all-trans retinoic acid
- BH4
(6R)-5,6,7,8-tetrahydro-L-biopterin
- BMI
body mass index
- CK
creatine kinase
- CP
carbamoylphosphate
- CPS-I
carbamoylphosphate synthetase-I (ammonia)
- Cr-P
creatine-phosphate
- DCAM
decarboxylated S-adenosylmethionine
- DHA
docosahexaenoic acid
- DNA
deoxyribonucleic acid
- EPA
eicosapentaenoic acid
- EPUAP
European Pressure Ulcer Advisory Pane
- GA
guanidinoacetate
- GDH
glutamate dehydrogenase
- Gln
glutamine
- Glu
glutamate
- GMAT
guanidinoacetate N-methyltransferase
- HDL
high-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- IGF1
insulin-like growth factor-1
- LBM
lean body mass
- MNA
Mini Nutritional Assessment
- MNA-SF
Mini Nutritional Assessment–Short Form
- MTA
methylthioadenosine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NAG
N-acetylglutamate
- NAGS
N-acetylglutamate synthase
- NHANES III
National Health and Nutrition Examination Survey III
- NO
nitric oxide
- NOS
nitric oxide synthase
- NPUAP
National Pressure Ulcer Advisory Panel
- OAT
ornithine aminotransferase
- OCT
ornithine carbamoyltransferase
- ODC
ornithine decarboxylase
- ONS
oral nutrient supplement
- P5CD
pyrroline-5-carboxylate dehydrogenase
- P5CR
pyrroline-5-carboxylate reductase
- P5CS
pyrroline-5-carboxylate synthase
- PCM
protein-calorie malnutrition
- PO
proline oxidase
- PUFAs
polyunsaturated fatty acids
- PUT
putrescine
- RAR
retinoic acid receptor
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- RXR
retinoid X receptors
- RXR-α
retinoid X receptor type
- SAHC
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- SAMD
S-adenosylmethionine decarboxylase
- SPDS
spermidine synthase
- TGFβ
transforming growth factor beta
- VDR
vitamin D receptor
Acknowledgments and Funding Sources
The authors wish to acknowledge the patient assistance of Betsy Wilson in the preparation of this manuscript. No outside funding sources were used in the preparation of this manuscript.
Author Disclosures and Ghostwriting
Joseph Andrew Molnar, MD, PhD, FACS: Medical Advisory Board, Healogics, Inc., Speaker's Bureau, Integra Life Sciences, Inc. M. Jane Underdown, MS1: No competing financial interests exist. W. Andrew Clark, PhD, RD: Chief Technology Officer, RTD Neutraceuticals LLC. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Joseph A. Molnar, MD, PhD, FACS, is Professor of Plastic and Reconstructive Surgery and Regenerative Medicine at Wake Forest University School of Medicine. He is the Medical Director of the Wound Care and Hyperbaric Center and the Associate Director of the Burn Unit. He remains actively involved in acute reconstructive surgery and hand surgery as well as the management of chronic wounds and burns. His research interests include nutrition, wound healing, and regenerative medicine with an emphasis on skin substitutes and negative pressure wound therapy. M. Jane Underdown, MS1, is a graduate of East Tennessee State University with a BS in Allied Health with a concentration in Nutrition and Foods. She has research experience in the development of a topical antioxidant gel for burn patients and is currently attending Quillen College of Medicine. W. Andrew Clark, PhD, RD, is a Professor of Clinical Nutrition and Associate Dean of Research and Clinical Practice in the College of Clinical and Rehabilitative Health Sciences at East Tennessee State University. Dr. Clark has extensive experience with nutrition research in both academic and industrial settings. His research interests are related to absorption of fat-soluble nutrients and the impact of oxidative stress in wound healing, cardiac patients, and infertile women.
References
- 1.Cuthbertson DP. Observations on the disturbance of metabolism produced by injury to the limbs. Q J Med 1932;1:233–246 [Google Scholar]
- 2.Dudrick SJ, Willmore DW, Vars HM, Rhoads JE. Can intravenous feeding as the sole means of nutrition support growth in the child and restore weight loss in an adult? An affirmative answer. Ann Surg 1969;169:974–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ireton-Jones C, Liepa GU. Carbohydrates and wound healing. In: Molnar JA, ed. Nutrition and Wound Healing. Boca Raton, FL: CRC Press, 2007:1–14 [Google Scholar]
- 4.Burke JS, Wolfe RR, Mullany CJ, Matthews DW, Bier DM. Glucose requirements following burn injury. Ann Surg 1979;190:274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molnar JA, Burke JF. Nutritional aspects of surgical physiology. In: Burke JF, ed. Surgical Physiology. Philadelphia, PA: W.B. Saunders Company, 1983:249–269 [Google Scholar]
- 6.Watters CA, Tredget EE, Cooper C. Nutrition and wound healing in burns, trauma, and sepsis. In: Milnar JA, ed. Nutrition and Wound Healing. Boca Raton, FL: CRC Press, 2007:219–260 [Google Scholar]
- 7.Teitelbaum D, Guenter P, Howell WH, Kochevar ME, Roth J, Seidner DL. Definition of terms, style, and conventions used in A.S.P.E.N. guidelines and standards. Nutr Clin Pract 2005;20:281–285 [DOI] [PubMed] [Google Scholar]
- 8.Gropper SS, Smith JL, Groff JL. Advanced Human Nutrition and Metabolism, 5th ed. Belmont, CA: Wadsworth, 2009 [Google Scholar]
- 9.Morley JE. Nutrition in the older person. In: Shils ME, ed. Modern Nutrition in Health and Diseases. 10th ed., Philadelphia: Lippincott Williams & Wilkins, 2006:1531–1538 [Google Scholar]
- 10.Miller MD, Thomas JM, Cameron ID, et al. BMI: a simple, rapid and clinically meaningful index of under-nutrition in the oldest old? Br J Nutr 2009;101:1300–1305 [DOI] [PubMed] [Google Scholar]
- 11.Chen CCH, Schilling LS, Lyder CH. A concept analysis of malnutrition in the elderly. J Adv Nurs 2001;36:131–142 [DOI] [PubMed] [Google Scholar]
- 12.Pirlich M, Lochs H. Nutrition in the elderly. Best Pract Res Clin Gastroenterol 2001;15:869–884 [DOI] [PubMed] [Google Scholar]
- 13.Brownie S. Why are elderly individuals at risk of nutritional deficiency? Int J Nurs Pract 2006;12:110–118 [DOI] [PubMed] [Google Scholar]
- 14.Endoy MP. Anorexia among older adults. Am J Nurse Pract 2005;9:31–38 [Google Scholar]
- 15.Collins CE, Kershaw J, Brockington S. Effect of nutritional supplements on wound healing in home-nursed elderly: a randomized trial. Nutrition 2005;21:147. [DOI] [PubMed] [Google Scholar]
- 16.Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition The Mini Nutritional Assessment. Clin Geriatr Med 2002;18:737–757 [DOI] [PubMed] [Google Scholar]
- 17.Woo J. Nutrition in the elderly. J Hong Kong Geriatr Soc 2000;3:15–18 [Google Scholar]
- 18.Harris CL, Fraser C. Malnutrition in the institutionalized elderly: the effects of wound healing. Ostomy Wound Manage 2004;50:54–63 [PubMed] [Google Scholar]
- 19.Wells JL, Dumbrell AC. Nutrition and aging: assessment and treatment of compromised nutritional status in frail elderly patients. Clin Interv Aging 2006;1:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen GL, Hsiao PY, Wheeler D. Adult nutrition assessment tutorial. JPEN J Parenter Enteral Nutr 2012;36:267–274 [DOI] [PubMed] [Google Scholar]
- 21.Russell L. The importance of patients' nutritional status in wound healing. Br J Nurs 2001;10(Suppl 6):S42, S44-9 [DOI] [PubMed] [Google Scholar]
- 22.Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46:381–386 [DOI] [PubMed] [Google Scholar]
- 23.Breslow RA, Hallfrisch J, Guy C, Crawley B, Goldberg AP. The importance of dietary protein in healing pressure ulcers. J Am Geriatr Soc 199;209:63–72 [DOI] [PubMed] [Google Scholar]
- 24.Demling RH. Nutrition, anabolism, and the wound healing process: an overview. J Plast Surg 2009;9:65–94 [PMC free article] [PubMed] [Google Scholar]
- 25.Evans C. Malnutrition in the elderly: a multifactorial failure to thrive. Perm J 2005;9:38–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad AS, Beck FWJ, Bao B, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 2007;85:837–844 [DOI] [PubMed] [Google Scholar]
- 27.Helge K, Rink L. Zinc-altered immune function. J Nutr 2003;133:1452S–1456S [DOI] [PubMed] [Google Scholar]
- 28.Prasad AS. Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol 2012;26:66–69 [DOI] [PubMed] [Google Scholar]
- 29.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;84:1080S. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wu S, Sun J. Vitamin D, vitamin D receptor and tissue barriers. Tissue Barriers 2013;1:e23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkievcz CJC, Skare TL, Malafaia OM, Nassif PAN, Ribas CSG, Santos LRP. Vitamin D deficiency in patients with chronic venous ulcers. Rev Col Bras Cir 2012;39:60–63 [PubMed] [Google Scholar]
- 32.Kalava UR, Cha SS, Takahashi PY. Association between vitamin D and pressure ulcers in older ambulatory adults: results of a matched case-control study. Clin Interv Aging 2011;6:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olmedo JM, Yiannias JA, Windgassen EB, Gornet MK. Scurvy: a disease almost forgotten. Int J Dermatol 2006;45:909–913 [DOI] [PubMed] [Google Scholar]
- 34.Popovich D, McAlhany A, Adewumi AO, Barnes MM. Scurvy: forgotten but definitely not gone. J Pediatr Health Care 2009;23:405–415 [DOI] [PubMed] [Google Scholar]
- 35.Raynaud-Simon A, Cohen-Bittan J, Gouronnec A, Pautas E, Senet P, Verny M. Scurvy in hospitalized elderly patients. J Nutr Health Aging 2010;14:407–410 [DOI] [PubMed] [Google Scholar]
- 36.Strange N. Diagnostic criteria to identify vitamin C deficiency in patients with wounds. The Symposium on Advanced Wound Care (SAWC). Baltimore, MD, 2012, pp. 12–14 [Google Scholar]
- 37.Wonisch W, Falk A, Sundi I, Winklhofer-Roob BM, Lindschinger M. Oxidative stress increases continuously with BMI and age with unfavorable profiles in males. Aging Male 2012;15:159–165 [DOI] [PubMed] [Google Scholar]
- 38.Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S, Hunt JA. Reactive oxygen species (ROS)–A family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cells Mater 2012;24:249–265 [DOI] [PubMed] [Google Scholar]
- 39.Andrès E, Loukili NH, Noel E, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004;171:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke R, Evans JG, Schneede J, et al. Vitamin B12 and folate deficiency in later life. Age Ageing 2004;33:34–41 [DOI] [PubMed] [Google Scholar]
- 41.Soini H, Routasalo P, Lagström . Characteristics of the Mini-Nutritional Assessment in elderly home-care patients. Eur J Clin Nutr 2004;58:64–70 [DOI] [PubMed] [Google Scholar]
- 42.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr 2008;27:5–15 [DOI] [PubMed] [Google Scholar]
- 43.Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc 2010;58:1734–1738 [DOI] [PubMed] [Google Scholar]
- 44.Yatabe MS, Taguchi F, Ishida I, et al. Mini Nutritional Assesssment as a useful method of predicting the development of pressure ulcers in elderly impatients. J Am Geriat Soc 2013;61:1698–1704 [DOI] [PubMed] [Google Scholar]
- 45.Guigoz Y. The mini nutritional assessment (MNA) review of the literature–what does it tell us? J Nutr Health Aging 2006;10:466–487 [PubMed] [Google Scholar]
- 46.Lamghamp-Henken B, Hudgens J, Stechmiller JK, Herrlinger-Garcia KA. Mini nutritional assessment and screening scores are associated with nutritional indicators in elderly people with pressure ulcers. J Am Diet Assoc 2005;105:1590–1596 [DOI] [PubMed] [Google Scholar]
- 47.Vellas B, Villars H, Abellan G, et al. Overview of the MNA–its history and challenges. J Nutr Health Aging 2006;10:456–465 [PubMed] [Google Scholar]
- 48.Jensen GL, Mirtallo J, Compher C, et al. Adult starvation and disease-related malnutrition. A proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. J Parenter Enteral Nutr 2010;34:156–159 [DOI] [PubMed] [Google Scholar]
- 49.Volpato S, Leveille SC, Corti M, Harris TB. The value of serum albumin and high-density lipoprotein cholesterol in defining mortality risk in older persons with low serum cholesterol. J Am Ger Soc 2001;49:1142–1147 [DOI] [PubMed] [Google Scholar]
- 50.Horn SD, Bender SA, Bergstrom N, et al. Description of the national pressure ulcer long-term care study. J Amer Geriat Soc 2002;50:1816–1825 [DOI] [PubMed] [Google Scholar]
- 51.Amir O, Liu A, Chang ALS. Stratification of highest-risk patients with chronic skin ulcers in a Stanford retrospective cohort includes diabetes, need for systemic antibiotics, and albumin levels. Ulcers 2012;72:1–7 [Google Scholar]
- 52.Shils M, Shike M, Ross AC, Caballero B, Cousins RJ. (Eds). Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams and Wilkins, 2006 [Google Scholar]
- 53.Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infectious Dis 2002;35:1390–1396 [DOI] [PubMed] [Google Scholar]
- 54.van Anholt RD, Sobotka L, Meijer EP, et al. Specific nutritional support accelerates pressure ulcer healing and reduces wound care intensity in non-malnourished patients. Nutrition 2010;26:867–872 [DOI] [PubMed] [Google Scholar]
- 55.Hengstermann S, Fischer A, Steinhagen-Thiessen E, Schulz R. Nutrition status and pressure ulcer: What we need for nutrition screening. JPEN J Parenter Enteral Nutr 2007;31:288–294 [DOI] [PubMed] [Google Scholar]
- 56.Haydock DA, Hill GL. Improved wound healing response in surgical patients receiving intravenous nutrition. Br J Surgery 1987;74:320–323 [DOI] [PubMed] [Google Scholar]
- 57.Thompson C, Fuhrman MP. Nutrients and wound healing: still searching for the magic bullet. Nutr Clin Pract 2005;20:331–347 [DOI] [PubMed] [Google Scholar]
- 58.Dorner B, Posthauer ME, Thomas D. 2009. The role of nutrition in pressure ulcer prevention and treatment: National Pressure Ulcer Advisory Panel White Paper. www.npuap.org (accessed July13, 2013) [DOI] [PubMed]
- 59.Chernoff R. Protein and older adults. J Am Coll Nutr 2004;23:627S–630S [DOI] [PubMed] [Google Scholar]
- 60.National Guidelines Clearing House. Pressure Ulcer Prevention Protocol, www.guideline.gov/content.aspx?id=36059 (accessed July13, 2013)
- 61.Legendre C, Debure C, Meaume S, Lok C, Golmard JL, Senet P. Impact of protein deficiency on venous ulcer healing. J Vasc Surg 2008;48:688–693 [DOI] [PubMed] [Google Scholar]
- 62.Iizaka S, Matsuo J, Konya C, Sekine R, Sugama J, Sanada H. Estimation of protein requirements according to nitrogen balance for older hospitalized adults with pressure ulcers according to wound severity in Japan. J Am Geriar Soc 2012;60:2027–2034 [DOI] [PubMed] [Google Scholar]
- 63.Choo TS, Hayter M, Watson R. The effectiveness of nutritional intervention(s) and the treatment of pressure ulcers–A systematic literature review. Int J Nurs Prac 2013;19(Suppl 1):19–27 [DOI] [PubMed] [Google Scholar]
- 64.Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009;37:153–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barbul A. Proline precursors to sustain mammalian collagen synthesis. J Nutr 2008;138:2021S–2024S [DOI] [PubMed] [Google Scholar]
- 66.Wu G, Bazer FW, Burghardt RC, et al. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 2011;40:1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Debats IBJG, Wolfs TGAM, Gotoh T, Cleutjens JPM, Peutz-Kootstra CJ, van der Hulst RRWJ. Role of arginine in superficial wound healing in man. Nitric Oxide 2009;21:175–183 [DOI] [PubMed] [Google Scholar]
- 68.Zunić G, Supić G, Magić Z, Drasković B, Vasiljevska M. Increased nitric oxide formation followed by increased arginase activity induces relative lack of arginine at the wound site and alters whole nutritional status in rats almost within the early healing period. Nitric Oxide 2009;20:253–258 [DOI] [PubMed] [Google Scholar]
- 69.Zhang XJ, Chinkes DL, Wolfe RR. The anabolic effect of arginine on proteins in skin wound and muscle is independent of nitric oxide production. Clin Nutr 2008;27:649–656 [DOI] [PubMed] [Google Scholar]
- 70.Seifter E, Rettura G, Barbul A, Levenson SM. Arginine: an essential amino acid for the injured rat. Surgery 1978;84:224–230 [PubMed] [Google Scholar]
- 71.Kavalukas SL, Barbul A. Nutrition and wound healing: an update. Plast Reconstr Surg 2011;127(Suppl 1):38S–43S [DOI] [PubMed] [Google Scholar]
- 72.Barbul A, Lazarou S, Efron DT, Wasserkrug HI, Yoshimura NN, Efron G. Arginine enhances wound healing in humans. Surgery 1990;108:331–337 [PubMed] [Google Scholar]
- 73.Kirk SJ, Hurson M, Regan MC, Holt DR, Wasserkrug HI, Barbul A. Arginine stimulates wound healing and immune function in aged humans. Surgery 1993;114:155–160 [PubMed] [Google Scholar]
- 74.Yatabe J, Saito F, Ishida I, et al. Lower plasma arginine in enteral tube-fed patients with pressure ulcer and improved pressure ulcer healing after arginine supplementation by Arginaid Water. J Nutr Health Aging 2011;15:282–286 [DOI] [PubMed] [Google Scholar]
- 75.Leigh B, Desneves K, Rafferty J, et al. The effect of different doses of an arginine-containing supplement on the healing of pressure ulcers. J Wound Care 2012;21:150–156 [DOI] [PubMed] [Google Scholar]
- 76.Sipahi S, Gungor O, Gunduz M, Cilci M, Demirci MC, Tamer A. The effect of oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine and glutamine on wound healing: a retrospective analysis of diabetic haemodialysis patients. BMC Nephrol 2013;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McMahon L, Tamary H, Askin M, et al. A randomized phase II trial of arginine butyrate with standard local therapy in refractory sickle cell leg ulcers. Br J Haematol 2010;151:516–524 [DOI] [PubMed] [Google Scholar]
- 78.Debats IB, Booi DI, Wehrens KM, et al. Oral arginine supplementation and the effect on skin graft donor sites: a randomized clinical pilot study. J Burn Care Res 2009;30:417–426 [DOI] [PubMed] [Google Scholar]
- 79.Schols JMGA, Heyman H, Meijer EP. Nutritional support in the treatment and prevention of pressure ulcers: an overview of studies with an arginine enriched oral nutritional supplement. J Tissue Viability 2009;18:72e–79e [DOI] [PubMed] [Google Scholar]
- 80.Stechmiller JK, Childress B, Cowan L. Arginine supplementation and wound healing. Nutr Clin Pract 2005;20:52–61 [DOI] [PubMed] [Google Scholar]
- 81.Soeters PB, Grecu I. Have we enough glutamine and how does it work? A clinician's view. Ann Nutr Metab 2012;60:17–26 [DOI] [PubMed] [Google Scholar]
- 82.Newsholme P. Why is L-glutamine metabolism important to cells of the immune systemin health, postinjury, surgery or infection? J Nutr 2001;131:2515S–2522S [DOI] [PubMed] [Google Scholar]
- 83.Ardawi MS. Glutamine and glucose metabolism in human peripheral lymphocytes. Metabolism 1988;37:99–103 [DOI] [PubMed] [Google Scholar]
- 84.Wischmeyer PE. Glutamine: mode of action in critical illness. Crit Care Med 2007;35:S541–S544 [DOI] [PubMed] [Google Scholar]
- 85.Peng X, Yan H, You Z, Wang P, Wang S. Clinical and protein metabolic efficacy of glutamine granules-supplemented enteral nutrition in severely burned patients. Burns 2005;31:342–346 [DOI] [PubMed] [Google Scholar]
- 86.Pattanshetti V, Powar R, Godhi A, Metgud S. Enteral glutamine supplementation reducing infectious morbidity in burns patients: a randomised controlled trial. Indian J Surg 2009;71:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blass SC, Goost H, Tolba RH, et al. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: a PRCT. Clin Nutr 2012;31:469–475 [DOI] [PubMed] [Google Scholar]
- 88.Coëffier M, Déchelotte P. Combined infusion of glutamine and arginine: does it make sense? Curr Opin Clin Nutr Metab Care 2010;13:70–74 [DOI] [PubMed] [Google Scholar]
- 89.Wong A, Chew-Childs A, Ong L, Wang CC, Zhang SH, Young S. The use of specialized amino acid mixture in pressure ulcer healing rates–a randomized controlled trial (abstract). In the European Society of Clinical Nutrition and Metabolism, Barcelona Spain, 2012 [Google Scholar]
- 90.Benati G, Gasparoni R, Coppola D. Supplementing with arginine, glutamine, and beta hydroxylmethylbutyrate (beta HMB) can improve pressure ulcer healing, reduce pain and frequency of dressing changes, improving cost (abstract). In the European Society for Clinical Nutrition and Metabolism, Barcolena Spain, 2012 [Google Scholar]
- 91.Turek JJ. Fat and wound healing. In: Molnar JA, ed. Nutrition and Wound Healing. Boca Raton, FL: CRC Press, 2007:27–47 [Google Scholar]
- 92.Turk HF, Monk JM, Fan Y, Callaway ES, Weeks B, Chapkin RS. Inhibitory effects of omega-3 fatty acids on injury-induced epidermal growth factor receptor transactivation contribute to delayed wound healing. Am J Physiol Cell Physiol 2013;304:C905–C917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Theilla M, Schwartz B, Cohen J, Shapiro H, Anbar R, Singer P. Impact of a nutritional formula enriched is fish oil and micronutrients on pressure ulcers in critical care patients. Am J Crit Care 2012;21:e102–e109 [DOI] [PubMed] [Google Scholar]
- 94.Theilla M, Singer P, Cohen J, DeKeyser F. A diet enriched in eicosapentanoic acid, gamma-linolenic acid and antioxidants in the prevention of new pressure ulcer formation in critically ill patients with acute lung injury; A randomized, prospective, controlled study. Clin Nutr 2007;26:752–757 [DOI] [PubMed] [Google Scholar]
- 95.Stipanuk MH, Caudill MA. Biochemical, Physiological, and Molecular Aspects of Human Nutrition, 3rd ed. St. Louis, MO: Elsevier Saunders, 2013 [Google Scholar]
- 96.Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Prac 2010;25:61–68 [DOI] [PubMed] [Google Scholar]
- 97.Posthauer ME. Do patients with pressure ulcers benefit from oral zinc supplementation? Adv Skin Wound Care 2005;18:471–472 [DOI] [PubMed] [Google Scholar]
- 98.Alves CC, Torrinhas RS, Giorgi R, et al. Short-term specialized enteral diet fails to attenuate malnutrition impairment of experimental open wound acute healing. Nutrition 2010;26:873–879 [DOI] [PubMed] [Google Scholar]
- 99.Cereda E, Gini A, Pedrolli C, Vanotti A. Disease-specific, versus standard, nutritional support for the treatment of pressure ulcers in institutionalized older adults: a randomized controlled trial. J Am Geriatr Soc 2009;57:1395–1402 [DOI] [PubMed] [Google Scholar]
- 100.Lansdown ABG, Mirastschijski U, Stubbs N, Scanlon E, Agren MS. Zinc in wound healing: theoritical, experimental, and clinical aspects. Wound Repair Reg 2007;12:2–16 [DOI] [PubMed] [Google Scholar]
- 101.Crandon JH, Lund CC, Dill DB. Experimental human scurvy. N Engl J Med 1940;223:353–369 [Google Scholar]
- 102.Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health 2004;94:870–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tanaka H, Molnar JA. Vitamin C and Wound Healing. In: Molnar JA, ed. Nutrition and Wound Healing. Boca Raton, FL: CRC Press, 2007:121–148 [Google Scholar]
- 104.Ellinger S, Stehel P. Efficacy of vitamin supplementation in situations with wound healing disorders: results from clinical intervention studies. Curr Opin Clin Nutr Metab Care 2009;12:588–595 [DOI] [PubMed] [Google Scholar]
- 105.Lykkesfeldt J, Poulson HE. Is vitamin C supplementation beneficial? Lessons learned from randomized controlled trials. Br J Nutr 2010;103:1251–1259 [DOI] [PubMed] [Google Scholar]
- 106.Gottschlich M. Fat-Soluable Vitamins and Wound Healing. In: In: Molnar JA, ed. Nutrition and Wound Healing. Boca Raton, FL: CRC Press, 2007:149–171 [Google Scholar]
- 107.Burgess C. Topical vitamins. J Drugs Dermatol 2008;7(7 Suppl):s2–s6 [PubMed] [Google Scholar]
- 108.Reichrath J, Lehmann B, Carlberg C, Varani J, Zouboulis CC. Vitamins as Hormones. Horm Metab Res 2007;39:71–84 [DOI] [PubMed] [Google Scholar]
- 109.Ehrlich HP, Hunt TK. Effects of cortisone and vitamin A on wound healing. Ann Surg 1968;167:324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wicke C, Halliday B, Allen D, et al. Effects of steroids and retinoids on wound healing. Arch Surg 2000;135:1265–1270 [DOI] [PubMed] [Google Scholar]
- 111.Wilson MM, Morley JE. Invited review: aging and energy balance. J Appl Physiol (1985) 2003;95:1728–1736 [DOI] [PubMed] [Google Scholar]
- 112.Latham MC. Human Nutrition in the Developing World. Food and Nutrition Series–No. 29, FAO, Food and Agricultural Organization of the United Nations, ISSN 1014–3181, 1997 [Google Scholar]
- 113.Zalewski PD, Truong-Tran AQ, Grosser D, Jayaram L, Murgia C, Ruffin RE. Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets. A review. Pharmacol Ther 2005;105:127–149 [DOI] [PubMed] [Google Scholar]
- 114.Strain JJ. Disturbances of micronutrient and antioxidant status in diabetes. Proc Nutr Soc 1991;50:591–604 [DOI] [PubMed] [Google Scholar]
- 115.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition 2002;18:872–879. Review [DOI] [PubMed] [Google Scholar]
- 116.González-Périz A, Clària J. Resolution of adipose tissue inflammation. ScientificWorldJournal 2010;10:832–856. Review [DOI] [PMC free article] [PubMed] [Google Scholar]