Abstract
Formalin-inactivated respiratory syncytial virus (FI-RSV) immunization is known to cause severe pulmonary inflammatory disease after subsequent RSV infection. Ginseng has been used in humans for thousands of years due to its potential health benefits. We investigated whether ginseng would have immune modulating effects on RSV infection in mice previously immunized with FI-RSV. Oral administration of mice with ginseng increased IgG2a isotype antibody responses to FI-RSV immunization, indicating T-helper type 1 (Th1) immune responses. Ginseng-treated mice that were nonimmunized or previously immunized with FI-RSV showed improved protection against RSV challenge compared with control mice without ginseng treatment. Ginseng-mediated improved clinical outcomes after live RSV infection were evidenced by diminished weight losses, decreased interleukin-4 cytokine production but increased interferon-γ production, modulation of CD3 T-cell populations toward a Th1 response, and reduced inflammatory response. Ginseng-mediated protective host immune modulation against RSV pulmonary inflammation was observed in different strains of wild-type and mutant mice. These results indicate that ginseng can modulate host immune responses to FI-RSV immunization and RSV infection, resulting in protective effects against pulmonary inflammatory disease.
Introduction
Respiratory syncytial virus (RSV) is the leading cause of a wide spectrum of lower respiratory tract diseases, ranging from common cold-like symptoms to more serious disease, such as bronchiolitis or pneumonia (Collins and Graham 2008). RSV-associated disease is estimated to cause 64 million morbidity and 160,000 deaths throughout the world (Girard and others 2005). Inactivated viral vaccines are, in general, considered safer than live virus-based vaccines. However, children immunized with a formalin-inactivated RSV (FI-RSV) vaccine experienced much higher rates of hospitalization and 2 of them died on natural infection during the epidemic season (Fulginiti and others 1969; Kapikian and others 1969; Kim and others 1969). Some live attenuated RSV vaccines are in clinical trials (Karron and others 2005; Openshaw and Tregoning 2005). Despite several decades of extensive efforts, there is not yet a licensed vaccine against RSV.
During the past decades, considerable effort has been directed toward characterizing enhanced disease of FI-RSV immunization on infection with live RSV (Boelen and others 2000; Castilow and others 2008b). BALB/c mice that were previously immunized with FI-RSV induced higher levels of T-helper type 2 (Th2) immune responses such as IgG1 isotype antibodies and interleukin (IL)-4 cytokines (Waris and others 1997). In addition, live RSV challenge of FI-RSV immunized mice resulted in significant increases in cellularity in lungs and bronchoalveolar lavage (BAL) fluids. These cell phenotypes infiltrating the lungs include CD4 T cells, granulocytes, and eosinophils (Connors and others 1994; Waris and others 1996; Tripp and others 2001). Pulmonary histopathology of FI-RSV-immunized mice after infection with live RSV showed prominent interstitial pneumonia such as thickening of alveolar walls and infiltration of inflammatory mononuclear cells (Murawski and others 2010).
Ginseng, the root of plant Panax ginseng C.A. Meyer mainly produced in Korea, China, and America, has been used in humans for thousands of years due to its beneficial effects on improving health and is one of the most well-studied herbal medicines (Attele and others 1999). Previous studies have demonstrated that ginseng had some therapeutic and pharmacological activities, including anticancer, anti-allergy, anti-inflammatory and immunomodulatory activities (Hong and Lyu 2011; Jung and others 2012b). The major constituents of the Panax genus ginseng root include triterpenoid glycosides or saponins (also known as ginsenosides), acid polysaccharides, phenol, and polyethylene compounds (Yuan and others 2010). The ginseng roots, including Korean red ginseng extracts, contain ∼2%–3% ginsenosides (saponins), which are likely to act as adjuvants (Lu and others 2009; Yuan and others 2010). Previous studies have demonstrated that ginsenosides Rg1, Re, and acidic polysaccharide extracted from ginseng have adjuvant properties either promoting T-helper type 1 (Th1) immune responses or stimulating dendritic cells (Takei and others 2008; den Brok and others 2012; Su and others 2012). Structurally, ginsenosides comprise triterpenoidal glycosides with glucose, arabinose, xylose, or rhamnose. Ginsenosides and acidic polysaccharide components are most likely to act as adjuvants shaping the immune system and inducing Th1-type immune responses. It was also reported that pretreatment with ginseng polysaccharide suppressed acute inflammatory responses at an early phase, resulting in the enhancement of antimicrobial activities and survival protection of mice from Staphylococcus aureus-induced sepsis (Ahn and others 2006). In addition, ginseng extracts and ginsenosides were reported to exhibit anti-inflammatory effects that are associated with their properties of cytokine regulation and phagocytosis in innate immunity, as well as activation of lymphocytes (Jung and others 2012b).
RSV disease or FI-RSV vaccine-enhanced disease on live RSV infection seems to be a result of inflammatory cytokine responses. We hypothesized that ginseng treatment would diminish inflammatory disease of host cytokine responses to RSV infection or FI-RSV vaccination and RSV infection. In this study, we investigated whether ginseng has the potential preventive and therapeutic effects after RSV viral infection or immunization with FI-RSV and RSV infection in different strains and mutant mice.
Materials and Methods
Cells, virus, and reagents
The RSV A2 strain and HEp2 cells were gifts from Dr Martin Moore (Emory University) and previously described (Quan and others 2011). Korean red ginseng extract (ginseng), a concentrated form of the commercial ginseng products, was kindly provided by Korea Ginseng Corporation. Briefly, fresh roots of the Panax ginseng that had grown for 6 years were washed, steamed at 100°C for 2 to 3 h, and dried. The dried red ginseng roots were boiled in 4 to 5 volumes of water for 3 h, and the supernatants were concentrated. This preparation was designated “red ginseng extract” (∼36% water content). Polyclonal goat anti-RSV antibody and mouse anti-RSV fusion protein were purchased from Millipore. HRP-conjugated anti-goat antibody, anti-mouse antibody immunoglobulin G (IgG), IgG1, and IgG2a were purchased from Southern Biotech. Fetal bovine serum (FBS), penicillin–streptomycin, RPMI1640, and Dulbecco's modified Eagle's medium (DMEM) were purchased from GIBCO. All other chemicals were of analytical grade.
Preparation of RSV stock and FI-RSV vaccine antigens
HEp2 cells were grown in tissue culture flasks in DMEM containing 10% FBS. RSV A2 virus was added, and virus adsorption was carried out in medium without serum for 1 h at 37°C with 5% CO2. DMEM with 5% FBS was added to the flask and incubated for 2–4 days. RSV-infected cells were removed using a cell scraper, lightly sonicated (1 s on and off cycles for 20 s at the 30% amplitude), and centrifuged at 2,000 rpm for 10 min at 4°C; the supernatants were titrated by an immunoplaque assay as described next and stored at −80°C. To prepare FI-RSV vaccines, collected cell culture supernatants (2×107 PFU/mL) containing RSV after removal of cell debris (2,000 rpm, 10 min) were incubated for 3 days with formalin (1:400 v/v) at 37°C, and then purified using ultracentrifugation as previously described (Quan and others 2011). Inactivated RSV was precipitated with aluminum hydroxide (4 mg/mL) for 30 min. The working dilution of the vaccine was made in phosphate-buffered saline (PBS) immediately before use.
Treatment of mice with ginseng, FI-RSV and RSV A2 virus
Ginseng was dissolved in sterile PBS and filtered through 0.4 μm Millipore membrane. The duration of ginseng oral treatment in animal (mouse, rat, and dogs) studies has been reported to be in a wider range between 80 days and 175 days (Shibata 2001; Chan and Fu 2007; Kim and others 2013a; Park and others 2014). Thus, we followed a similar protocol of ginseng oral treatment and dose (Kim and others 2013a; Park and others 2014). For animal experiments, 6–12 week-old female BALB/c, C57BL/6 mice (Harlan Laboratories), or major histocompatibility class II (MHCII) knockout mice (B6.129S2-H2dlAb1-Ea/J; Jackson laboratory) were anesthetized by isoflurane and then ginseng was administered orally at a dose of 25 mg/kg/day for 80 or 130 days. Mice were intramuscularly immunized twice on day 8 and 36 with 1 μg of FI-RSV. To determine the effects of ginseng treatment on protection against RSV, FI-RSV immunized mice (n=10 per group) were intranasally infected with RSV A2 strain (2×106 PFU) in a volume of 100 μL. Mice were monitored daily to record weight changes. Details of this study and all animal experiments presented in this article were approved by the Georgia State University Institutional Animal Care and Use Committee (IACUC) review board and conducted under the guidelines of the Georgia State University IACUC. Georgia State University IACUC operates under the federal Animal Welfare Law (administered by the USDA) and regulations of the Department of Health and Human Services.
Antibody responses
RSV A2 virus-specific antibodies (IgG, IgG1, and IgG2a) were determined in sera by an enzyme-linked immunosorbent assay (ELISA) (Quan and others 2011). Briefly, 96-well microtiter plates were coated with 100 μL of RSV virus (3×105 PFU) per well in coating buffer at 4°C overnight. The samples were serially diluted and added onto plates after they were washed and blocked with 3% bovine serum albumin. The plates were then incubated with HRP-conjugated goat anti-mouse IgG, IgG1, and IgG2a antibodies at 37°C for 1.5 h. The substrate O-phenylenediamine (Invitrogen) in citrate-phosphate buffer (pH 5.0) containing 0.03% hydrogen peroxide was used to develop color. The optical density at 450 nm was measured using an ELISA reader.
RSV immunoplaque assay
RSV immunoplaque assay was performed as described (Quan and others 2011). Briefly, serially diluted virus stock or lung homogenates from infected mice were added to the HEp2 cell monolayer plates and incubated for 3–6 days at 37°C. After fixing with ice-cold acetone-methanol and air drying, anti-F monoclonal antibody and then HRP-conjugated anti-mouse IgG antibodies were used. Individual plaques were developed using DAB substrate (Invitrogen) and counted.
Virus titer and cytokine assays
The individual lungs were removed aseptically at day 5 postchallenge, and extracts were prepared as homogenates after challenge using frosted glass slide (Quan and others 2011). The homogenates were centrifuged at 2,000 rpm for 10 min to collect supernatants. The virus titer in the supernatant was determined by an immunoplaque assay.
Pulmonary histology of RSV-infected mice
For histological analysis of lung tissues, the lungs were then fixed via infusion through the trachea with 4% formalin, removed, immersed in 4% formalin for 24 h, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) stain. Ten sections per mouse were obtained. All images were acquired using the same camera settings. Images were acquired using a Zeiss Axiovert 100 microscope at a magnification of ×100, using an attached Canon 30D digital camera. For sections stained with PAS, the percentage of airways positive for PAS in 15 to 25 randomly selected airways was determined, as previously described (Mok and others 2007; Murawski and others 2010).
Determination of T-cell responses
At day 5 postchallenge, splenocytes and lung cells were isolated from the corresponding tissue samples. IL-4 and interferon (IFN)-γ secreting cell spots were determined on Multi-screen 96-well plates (Millipore) coated with cytokine-specific capture antibodies as described (Song and others 2010a; Kim and others 2013b). Briefly, 0.5×106 spleen cells per well or 0.1×106 lung cells per well were cultured with FI-RSV (2 μg/mL), RSV F peptide (ELQLLMQSTPPTNNR, 4 μg/mL), or RSV G peptide (WAICKRIPNKKPG, 4 μg/mL) as an antigenic stimulator. After 36 h of incubation, the spots of IL-4 or IFN-γ secreting T cells were counted using an ELISpot reader (BioSys). Cytokine ELISA was performed as previously described (Quan and others 2010; Kim and others 2013b).
Preparation of BAL and flow cytometric analysis
Five days after RSV infection, mice were sacrificed to collect BAL fluids and lung samples. BAL fluid samples were obtained by infusing 1 mL of PBS into the lungs via the trachea using a 25-gauge catheter (Exelint International Co.). Total cell numbers in lung and BAL fluid samples were counted from individual mice in each group (n=5). For flow cytometry analysis, BAL samples from 5 mice were pooled for sufficient numbers of BAL cells for quantification (percentages) of each phenotypic cell population. Cells from BAL fluids were stimulated with phorbol myristate acetate (50 ng/mL) and ionomycin (500 ng/mL) for 4 h. After staining with surface antibodies (anti-CD3, CD45, CD4, CD8α, CD11b, CD11c, and Siglec F antibodies from eBiosciences), intracellular IFN-γ cytokine staining was followed by manufacturer's manuals (BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit). The percentage of gated cells was calculated by Flow Jo software (Tree Star, Inc.).
Statistical analysis
To determine the statistical significance, a 2-tailed Student's t-test was used when comparing 2 different groups. One-way analysis of variance was used when 2 or more different groups were compared together. Data were analyzed using Prism software (GraphPad software, Inc.). A level of P<0.05 was regarded as statistically significant.
Results
Ginseng treatment increases IgG2a isotype antibody responses to FI-RSV
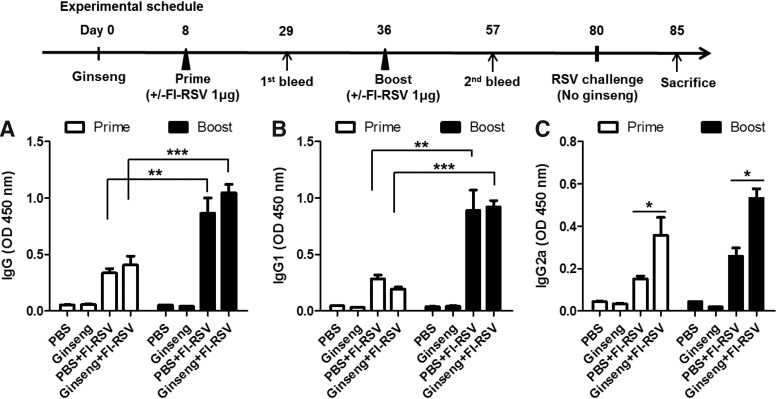
To determine whether ginseng has immune modulating effects on FI-RSV vaccination, BALB/c mice were intramuscularly immunized with FI-RSV during the 80 day-period of oral administration of ginseng 25 mg/kg body weight/day (Fig. 1). The levels of total IgG, IgG1, and IgG2a antibody responses in sera specific for RSV after prime and boost with FI-RSV were determined using RSV as an ELISA coating antigen (Fig. 1). The mock control buffer (PBS) or ginseng only (Ginseng) group did not show RSV-specific antibody responses (Fig. 1). Low levels of serum binding antibodies were induced in both groups with or without ginseng treatment after priming (Fig. 1). The levels of RSV-specific IgG were significantly increased after boost compared with those by prime immunization. The ginseng-treated FI-RSV group raised a higher level of total IgG antibody compared with the FI-RSV vaccine alone group, although there was no statistical significance (Fig. 1A). To further characterize antibody production, serum IgG subtypes IgG1 and IgG2a specific for RSV were determined after the prime and boost immunization with FI-RSV. The levels of RSV-specific IgG1 were increased after boost, which is a similar level in both groups with and without ginseng treatment (Fig. 1B). The ginseng-treated group showed an ∼2-fold increase in IgG2a antibody levels after priming or boost immunization (Fig. 1C). These results indicate that long-term oral administration of ginseng increased serum IgG2a isotype levels in mice immunized with FI-RSV, indicating an increased Th1 immune response.
FIG. 1.
Influence of ginseng on serum IgG and isotype antibodies specific for RSV. (A) Total serum IgG antibody. (B) Serum IgG1 isotype antibody. (C) Serum IgG2a isotype antibody. As shown in a diagram of experimental schedule, BALB/c mice (n=5) were orally administered ginseng at a dose of 25 mg/kg/day for 80 days. During this period, mice were intramuscularly immunized with FI-RSV. Blood samples were collected individually at 3 weeks after priming (first) and boost (second) immunizations with FI-RSV, and 100-fold diluted sera were used to determine total IgG and IgG isotypes by ELISA coated with FI-RSV. Optical densities were read at 450 nm, and values are the mean±SEM. *P<0.05; **P<0.01; ***P<0.001. PBS, PBS mock control mice; Ginseng, Ginseng mock control mice; PBS+FI-RSV, FI-RSV immunization during PBS oral treatment; Ginseng+FI-RSV, FI-RSV immunization during ginseng oral treatment. The data were presented from 2 independent experimental assays. ELISA, enzyme-linked immunosorbent assay; FI-RSV, formalin-inactivated respiratory syncytial virus; IgG, immunoglobulin G; PBS, phosphate-buffered saline.
Ginseng improves clinical outcomes in BALB/c mice after RSV infection
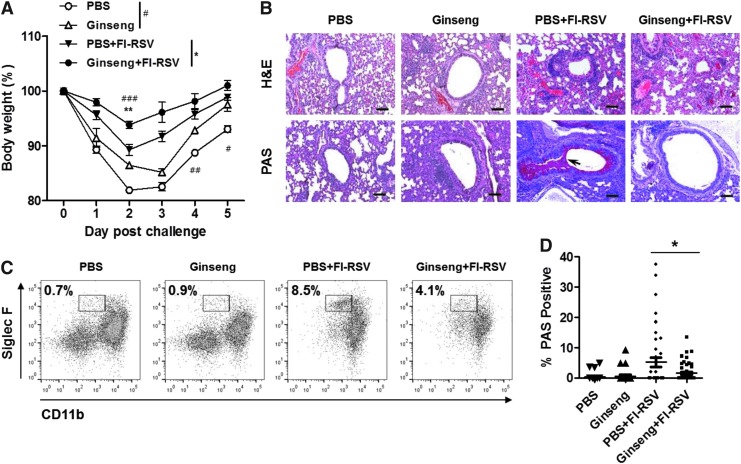
We investigated whether 80 days of oral ginseng administration could confer better protection against RSV and diminish FI-RSV vaccine-mediated lung disease against RSV challenge (Fig. 2). Ginseng pretreatment resulted in less weight loss (13%–15%) in BALB/c mice after RSV infection (2×106 PFU) compared with PBS buffer control (17%–20%) (Fig. 2). After RSV challenge of FI-RSV-immunized mice, diminished body weight loss (5%–8%) was observed in the FI-RSV-vaccinated group with ginseng treatment compared with the FI-RSV-vaccinated group without ginseng that showed 9%–13% body weight loss (Fig. 2A). To better understand the effects of ginseng on reducing RSV disease, lung histopathology was assessed after intranasal RSV challenge of the ginseng oral-administered mice that were immunized with FI-RSV. Histological H&E or PAS-stained tissue sections were prepared from lung tissues. We observed severe infiltration of abundant inflammatory cells into the bronchial lumens as well as luminal cell infiltration of the FI-RSV immunized mice after RSV infection. A trend of less inflammation was observed in ginseng-treated mice compared with untreated mice after FI-RSV immunization and then RSV infection (Fig. 2B). Mock controls showed less severe lung tissue inflammation compared with FI-RSV-immunized groups. Levels of eosinophils in BAL of the ginseng-treated and FI-RSV-vaccinated group were decreased compared with that in the FI-RSV alone group, even though ginseng could not prevent eosinophil recruitment into BAL as determined by expression of surface markers SiglecF+, CD45+, CD11b+, and CD11c− (Fig. 2C). Without FI-RSV immunization, no significant levels of eosinophils and mucus production were observed in mock control groups (PBS, Ginseng; Fig. 2C, D). Lung sections were stained with PAS to visualize mucus production (Fig. 2B). These sections were scored for the percentage of airway linings showing PAS staining (Fig. 2B, D). We observed that the ginseng-treated FI-RSV group showed less PAS-positive cells than the FI-RSV group (Fig. 2D). These results indicate that ginseng treatment improves clinical outcomes of FI-RSV vaccination-enhanced RSV inflammatory disease on RSV infection, which include the prevention of severe weight loss, less degree of infiltrates into the lung, and lowering eosinophilia as well as reducing mucus production.
FIG. 2.
Influence of ginseng on disease progression in FI-RSV immunized BALB/c mice after RSV infection. (A) Changes in body weight after challenge infection. BALB/c mice (n=5) were orally administered ginseng or PBS and intramuscularly immunized with FI–RSV or no immunization (PBS and Ginseng mock controls). After 80 days of ginseng oral treatment, FI-RSV immunized mice were intranasally infected with RSV (2×106 PFU), and monitored for changes in body weight. *P<0.05; **P<0.01 between the PBS+FI-RSV group and the Ginseng+FI-RSV group, #P<0.05; ##P<0.01; ###P<0.001 between the PBS and Ginseng groups. (B) Comparison of pulmonary histopathology after RSV infection (2×106 PFU). Lungs were prepared for tissue sections, as described in Materials and Methods, and stained with H&E to visualize infiltration of inflammatory cells or PAS to visualize mucus production (arrows). Scale bars indicate 100 μm. (C) Eosinophils. Phenotypes of CD11b+SiglecF+CD45+CD11c− eosinophils in BAL fluids. BAL cells were harvested, pooled (n=5 per BALB/c mice group), and analyzed by flow cytometry. F4/80+ macrophage and CD11c+ cells were first gated out from the total CD45+ leukocytes. Then, CD11b+SiglecF+ cells gated are presented as eosinophils. (D) Scores for bronchiolar mucus production from PAS staining. Tissue sections stained with PAS were scored as the percentage of 25 random airways positive for PAS. PBS, PBS mock control mice with RSV infection; Ginseng, Ginseng mock control mice with RSV infection; PBS+FI-RSV, FI-RSV immunization during PBS oral treatment and RSV infection; Ginseng+FI-RSV, FI-RSV immunization during ginseng oral treatment and then RSV infection. Data were collected from 2 independent experiments (n=5). Values are the mean±SEM. *P<0.05. BAL, bronchoalveolar lavage; H&E, hematoxylin and eosin; PAS, periodic acid-Schiff.
Effects of ginseng on protection against RSV in C57BL/6 mice
The BALB/c mouse strain is most commonly used for RSV pathogenesis and vaccine studies (Graham 2011). Meanwhile, C57BL/6 mice frequently provide a background for transgenic or mutant strains of mice. It is important to better understand the effects of genetic background on host immune responses and pathology to RSV infection. The nature and severity of RSV disease vary widely among infected individuals. Many factors, including virus, host, and environment, probably contribute to variable RSV disease expression. RSV disease susceptibility is considered less in C57BL/6 mice than that in BALB/c mice (Stark and others 2002; Jessen and others 2011). Therefore, testing a different strain of mice, C57BL/6 is important for further understanding the effect of ginseng on pathogenesis of RSV disease. Using a C57BL/6 mouse model, we further investigated the ginseng effects on diminishing pathology associated with RSV infection or FI-RSV vaccination and RSV infection.
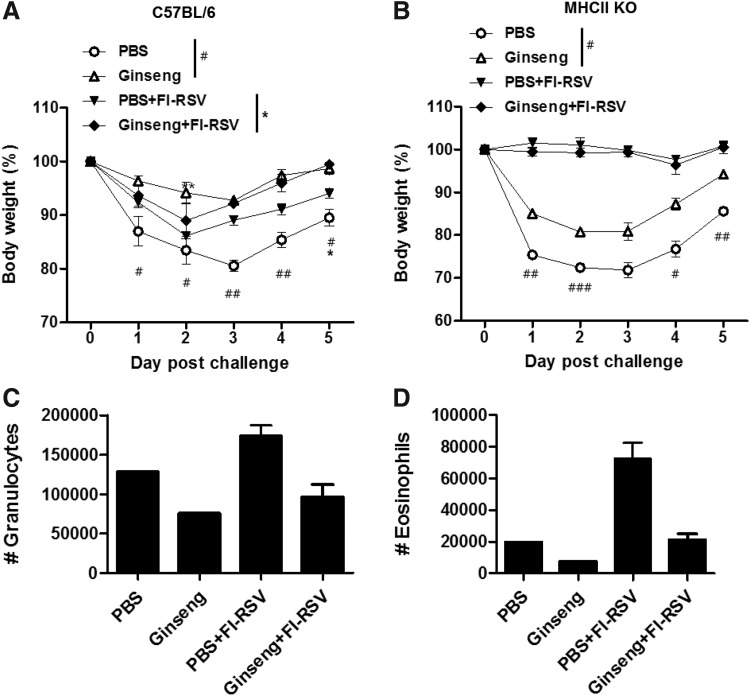
C57BL/6 mice were orally administered with ginseng 25 mg/kg body weight/day for 130 days (Figs. 3 and 4). Mice were intramuscularly immunized with FI-RSV at days 8 and 36 during ginseng treatment. After RSV challenge at day 130 when ginseng treatment was stopped, body weight changes were monitored. A pattern of less loss and faster recovery of weight loss was observed in the FI-RSV-immunized C57BL/6 group with ginseng treatment compared with the FI-RSV C57BL/6 group without ginseng (Fig. 3A). The PBS mock control C57BL/6 group showed significant weight loss on RSV infection (PBS, Fig. 3A). Interestingly, ginseng pretreatment significantly lowered weight losses due to RSV infection (Ginseng, Fig. 3A). When we determined viral loads at day 5 post RSV challenge, ∼2-fold lower lung virus titer was observed in the ginseng-treated group compared with the control group without ginseng, although there was no statistical difference (data not shown).
FIG. 3.
Influence of ginseng on RSV disease in C57BL/6 mice and MHCII KO mice. (A, B) Whole body weight changes after RSV infection in C57BL/6 mice (A) and MHCII KO mice (B). C57BL/6 mice (n=5) and MHCII KO mice (n=5) were intramuscularly immunized with FI-RSV at day 8 and 36 during ginseng or PBS oral administration for 130 days. At day 130 when ginseng treatment was stopped, mice were infected with RSV (2×106 PFU) and monitored for changes in body weight. *P<0.05 PBS+FI-RSV versus Ginseng+FI-RSV, #P<0.05; ##P<0.01; ###P<0.001 PBS versus Ginseng. PBS, PBS mock control (28 weeks old MHCII KO mice by the time of infection); Ginseng, Ginseng oral treatment mock control (28 weeks old MHCII KO mice by the time of infection). PBS+FI-RSV, FI-RSV immunization without ginseng treatment (16 months old MHCII KO mice by the time of infection); Ginseng+FI-RSV, FI-RSV immunization during ginseng oral treatment (16 months old MHCII KO mice by the time of infection). (C, D) Phenotypes of granulocytes (CD11b+F4/80−CD45+CD11c−) and eosinophils (CD11b+SiglecF+ CD45+CD11c−) in BAL fluids from C57BL/6 mice. BAL fluids from PBS and Ginseng groups were pooled (n=5). Average values of data were shown out of 2 independent experiments, and the error bars indicate SEM. MHCII, major histocompatibility class II.
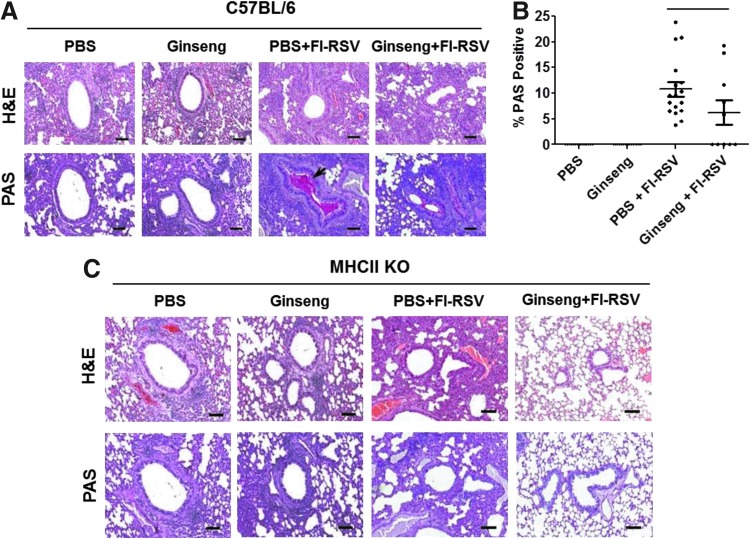
FIG. 4.
Influence of ginseng on lung histology in C57BL/6 mice and MHCII KO mice after RSV infection. (A) C57BL/6 mouse pulmonary histopathology. (B) Scores for C57BL/6 mouse bronchiolar mucus production from PAS staining. The scoring was similarly performed as in the Fig. 2D. *P<0.05 between the FI-RSV group and ginseng+FI-RSV group (t-test). (C) MHCII KO mouse pulmonary histopathology. Lungs were prepared for H&E staining to visualize infiltration of inflammatory cells or PAS staining to visualize mucus production (arrows). The treatments in groups are the same as in the Fig. 3. Scale bars indicate 100 μm.
Lung histopathology was assessed at day 5 post RSV challenge of C57BL/6 mice immunized with FI-RSV or PBS (mock) in the presence or absence of ginseng treatment (Fig. 4A). Mock control C57BL/6 mice with or without ginseng treatment did not show significant differences in H&E staining of lung tissues. Cell infiltration around bronchia was observed in both groups, but the severity was milder than that observed in FI-RSV immunized groups with or without ginseng. In addition, H&E staining showed that severe interstitial pneumonia was observed in FI-RSV immunized C57BL/6 mice with or without ginseng (Fig. 4A). The PAS staining of mucus production was moderately reduced in the ginseng treated, FI-RSV immunized C57BL/6 mouse group compared with FI-RSV only immunized C57BL/6 mice on RSV challenge (Fig. 4A, B). The levels of BAL granulocytes and eosinophils in the ginseng-treated FI-RSV C57BL/6 group after RSV challenge were significantly reduced compared with those of the FI-RSV C57BL/6 group without ginseng treatment (Fig. 3C, D). In addition, mock ginseng control C57BL/6 mice showed a decreasing trend of granulocytes and eosinophils in BAL fluid samples compared with PBS mock control mice (Fig. 3C, D). Thus, comparisons of the lungs of RSV-challenged FI-RSV-immunized mice indicate that oral administration of ginseng reduced the abnormal pathology associated with FI-RSV vaccination and RSV infection. Importantly, mock control results suggest that ginseng treatment might have a preventive potential of RSV disease as evidenced by lowering body weight losses due to RSV infection.
Effects of ginseng on protection and inflammatory disease in MHCII knockoutmice
MHC class II-knockout mice (MHCII KO) were demonstrated to be deficient in mature CD4+ T-cell-mediated immune responses (Grusby and others 1991). MHCII KO mice do not have conventional CD4 T cells that are known to contribute to the FI-RSV vaccine-induced RSV disease. Use of the MHCII KO mouse model would provide informative insights into possible inflammatory disease that is mediated by host immune components other than CD4 and MHCII molecules. Since the anti-inflammatory effects of ginseng would be moderate, protective effects of ginseng are expected to be more prominent in MHCII KO mice. The treatment of MHCII KO mice with ginseng would explain its effects on inflammatory host responses independent of CD4 and MHCII molecules.
MHCII knockout mice were orally administered with ginseng 25 mg/kg body weight/day for 130 days by following a similar ginseng treatment protocol used for C57BL/6 mice (Figs. 3 and 4). There were no significant weight changes in FI-RSV vaccinated MHCII KO mouse groups with or without ginseng. In contrast to C57BL/6 (Fig. 3A), the PBS mock control MHCII KO mice group showed severe body weight loss on RSV infection (2×106 PFU) (PBS, Fig. 3B). The ginseng mock group displayed less body weight loss and a quicker recovery pattern compared with those observed in the PBS mock control group after RSV infection (PBS vs. Ginseng, Fig. 3B).
Lung histopathology was assessed at day 5 post RSV challenge of MHCII KO mice immunized with FI-RSV or mock controls (PBS, Ginseng) in the presence or absence of ginseng treatment (Fig. 4C). Inflammatory infiltrates were meaningfully less in the MHCII KO mice immunized with FI-RSV compared with those in C57BL/6 mice. In particular, oral administration of ginseng prominently reduced interstitial pneumonia of FI-RSV vaccine-enhanced disease in MHCII KO mice on RSV challenge compared with the FI-RSV group without ginseng treatment (Fig. 4C). PBS and ginseng mock control mice displayed substantial inflammation in lungs but corresponding mock control inflammation appeared to be lower than that observed with FI-RSV mice without ginseng treatment. The levels of eosinophils in BAL samples from MHCII knockout mice were lower compared with those from C57BL/6 mice (data not shown).
Ginseng treatment modulates host immune responses toward Th1 cytokine
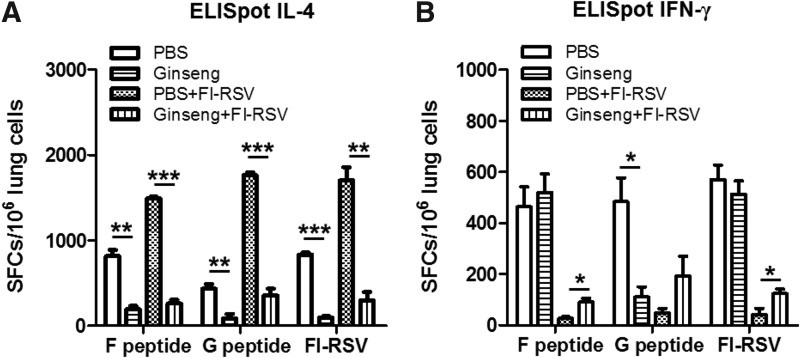
It is significant that ginseng treatment improved clinical outcomes of FI-RSV vaccine enhanced disease after RSV infection. To better understand possible mechanisms of beneficial effects by ginseng, we determined the effects of ginseng on IL-4 and IFN-γ producing lung cell responses in FI-RSV-immunized BALB/c mice at day 5 post-RSV infection (Fig. 5). Ginseng mock control mice exhibited significantly lower levels of IL-4 cytokine producing lung cells compared with those of PBS mock control mice (Fig. 5A). Ginseng-treated FI-RSV mice also showed significantly lower levels of IL-4 secreting lung cells by ∼5-fold compared with their counterparts without ginseng treatment (Fig. 5A). In addition, higher levels of IFN-γ secreting lung cells were observed in ginseng-administered and FI-RSV-vaccinated mice than those in the FI-RSV alone group (Fig. 5B). Both mock control mice displayed higher levels of IFN-γ secreting lung cells except the RSV G peptide stimulation (Fig. 5B).
FIG. 5.
Ginseng enhances IFN-γ cytokine-producing lung cells from BALB/c mice. (A) IL-4 ELISpot. (B) IFN-γ ELISpot. Cytokine (IL-4 and IFN-γ) producing lung cells isolated at day 5 postinfection (n=5 BALB/c mice per group) were determined by ELISpot after stimulation with FI-RSV (2 μg/mL), RSV F peptide (4 μg/mL) or RSV G peptide (4 μg/mL). The spots for cytokine-producing cells from the lung were counted and expressed based on 1×106 lung cells. The group labeling and treatments are the same as in the Fig. 2. Data were collected from 2 independent experiments (n=5). Values are the mean±SEM. *P<0.05; **P<0.01; ***P<0.001. IFN, interferon; IL, interleukin.
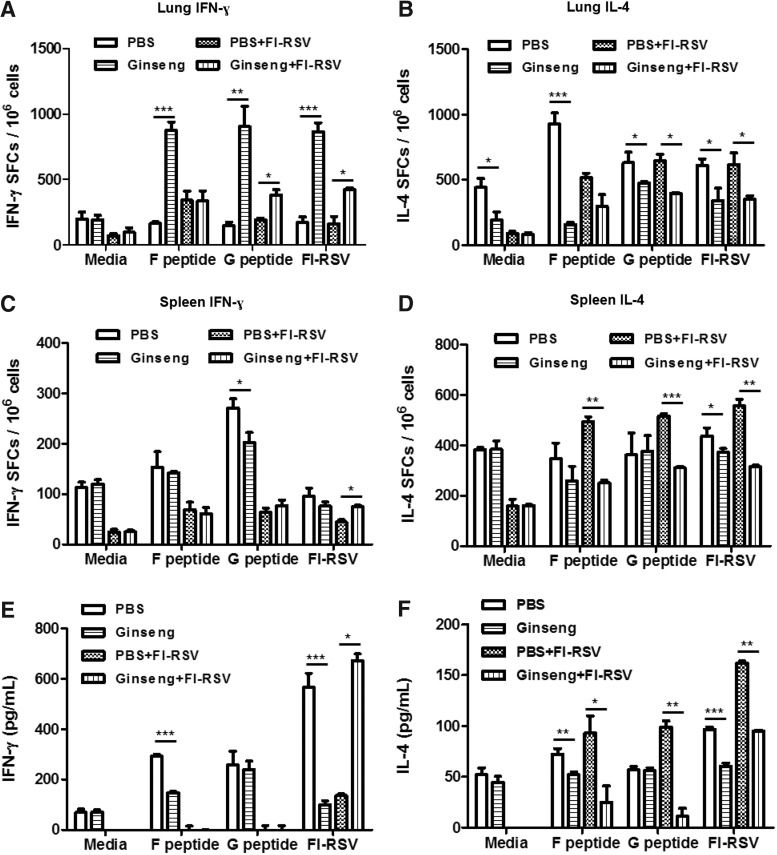
To further explore this interesting finding that ginseng treatment exhibits differential effects on IL-4 and IFN-γ secreting cell response, C57BL/6 mice were analyzed after FI-RSV immunization during ginseng treatment and then RSV infection (Fig. 6). In line with a BALB/c mouse model (Fig. 5), we observed a similar trend of ginseng effects on reducing the IL-4 secreting cells and increasing IFN-γ secreting cells in C57BL/6 mice (Fig. 6). It is important to note that higher levels of IFN-γ secreting lung cells were prominently observed in ginseng mock control mice compared with PBS mock control mice (Fig. 6A). In contrast, IL-4 levels were lower in ginseng mock control mice compared with PBS mock control mice (Fig. 6B). FI-RSV mice with ginseng treatment resulted in higher levels of IFN-γ producing lung cells on RSV G peptide or FI-RSV stimulation (Fig. 6A) and IFN-γ producing spleen cells on FI-RSV stimulation (Fig. 6C, E) when compared with FI-RSV mice without ginseng treatment. In contrast, ginseng treatment significantly reduced IL-4 cytokine producing spleen cells (Fig. 6D) and IL-4 production by spleen cells (Fig. 6F). In addition, there was a pattern of suppressing IL-4 producing lung cells in the ginseng-treated C57BL/6 mouse groups (Fig. 6B). Ginseng effects on IFN-γ producing cells were more prominent in lungs locally where RSV replication is mainly occurring but not in spleens systemically (Fig. 6A, C, E). It would be a desirable outcome for ginseng to induce lower levels of cytokines systemically in spleen cells from ginseng mock control mice (Fig. 6C–F). These results indicate that ginseng treatment endows a trend of suppressing IL-4 production while enhancing IFN-γ production, a Th1 response.
FIG. 6.
Ginseng modulates a pattern of cytokine-producing splenocytes and lung cells from C57BL/6 mice. Cytokine (IFN-γ and IL-4)-producing lung cells (A, B) and splenocytes (C, D) isolated at day 5 postinfection (n=5 C57BL/6 mice per group) were determined by ELISpot after stimulation with FI-RSV (2 μg/mL), RSV F peptide (4 μg/mL), or RSV G peptide (4 μg/mL). The spots for cytokine-producing cells from the spleen or lung were counted and expressed based on 1×106 cells. (E, F) In vitro cytokine production by spleen cell culture. Splenocytes were incubated in the absence or presence of FI-RSV, RSV F peptide, or RSV G peptide for in vitro stimulation and incubated for 72 h. Culture supernatants were harvested, and cytokines were determined by ELISA. The group labeling and treatments are the same as in the Fig. 3. Values are the mean±SEM. *P<0.05; **P<0.01; ***P<0.001.
Ginseng treatment modulates bronchoalveolar cell populations
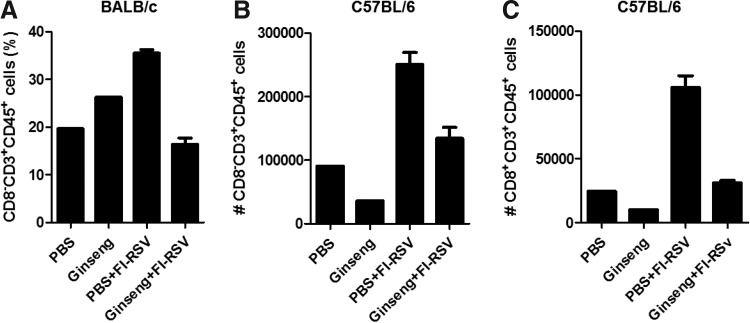
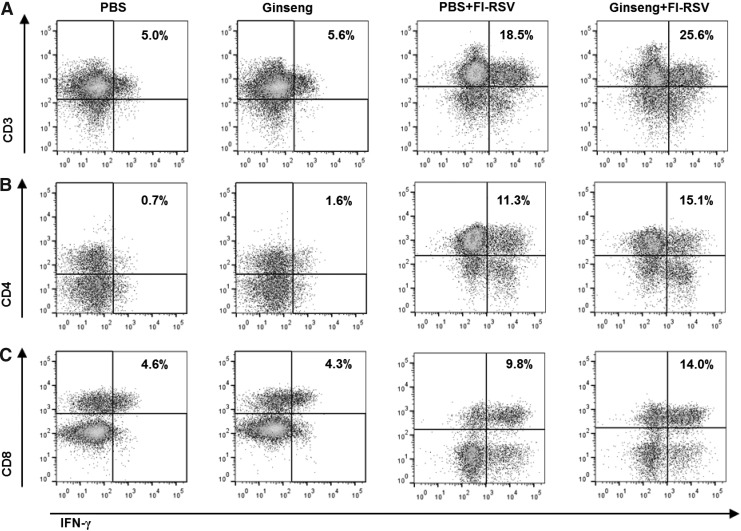
Previous studies reported that a higher number of CD4 T cells in BAL fluids was associated with an enhanced pulmonary inflammation response after RSV challenge in FI-RSV-immunized BALB/c mice (Waris and others 1996, 1997). Therefore, we determined the effects of ginseng on T-cell populations in BAL cells from mice after FI-RSV vaccination and then RSV challenge (Fig. 7). Oral administration of mice with ginseng significantly decreased the numbers of CD4 T cells (CD45+CD3+CD8−) infiltrating BAL fluids after FI-RSV vaccination and RSV infection in both BALB/c mice (Fig. 7A) and C57BL/6 mice (Fig. 7B). Ginseng mock control mice also showed a pattern of reducing CD4 T cell population in BALB/c mice (Fig. 7A) and C57BL/6 mice (Fig. 7B), although the data were presented using pooled samples. In addition, ginseng treatment resulted in decreased CD8 T-cell populations in C57BL/6 mice in FI-RSV immunization comparing groups (PBS+FI-RSV vs. Ginseng+FI-RSV) or in mock controls (Fig. 7C). We also found a moderate increase in IFN-γ producing CD3+ T (Fig. 8A), CD4+ T (Fig. 8B), and CD8+ T (Fig. 8C) cells in BAL fluids from ginseng-treated mice with FI-RSV immunization and RSV infection compared with their counterparts without ginseng treatment as determined by an intracellular cytokine staining assay (Fig. 8). In line with local lung IFN-γ producing cells (Figs. 5 and 6), ginseng mock control mice showed a moderate increase in lung IFN-γ producing CD4 T cells (Fig. 8). These results suggest ginseng-mediated inhibition of CD4+ T-cell infiltration into lung airways (BAL fluids) as well as enhancing Th1 type INF-γ producing cells. Thus, ginseng mediated anti-inflammatory immune modulation seems to contribute to conferring better clinical outcomes and protection in FI-RSV-immunized mice after RSV challenge.
FIG. 7.
Ginseng modulates infiltrates of T cells into BAL in FI-RSV-immunized mice. As a flow cytometry gating strategy, CD45+ and CD3+ cells were gated first. Then, CD8+ or CD8− cells were gated and analyzed. (A) CD4 T cells (CD45+CD3+CD8−) in BAL fluids from BALB/c mice. (B) CD4 T cells (CD45+CD3+CD8−) in BAL fluids from C57BL/6 mice. (C) CD8 T cells (CD45+CD3+CD8+) in BAL fluids from C57BL/6 mice. BALB/c mice (A) or C57BL/6 mice (B, C) (n=5 mice per group) were infected with RSV (2×106 PFU) after oral administration of ginseng or PBS in mice immunized with or without FI-RSV. BAL cells from PBS and Ginseng were pooled (n=5), stained, and analyzed by flow cytometry. The group labeling and treatments are the same as in Fig. 3. Average values of data are shown out of 2 independent experiments, and the error bars indicate SEM.
FIG. 8.
Influence of ginseng on bronchoalveolar cells producing IFN-γ in FI-RSV-immunized mice. (A) Intracellular IFN-γ-positive CD3+ T cells. (B) Intracellular IFN-γ-positive CD3+CD4+ T cells. (C) Intracellular IFN-γ-positive CD3+CD8+ T cells. BALB/c mice were orally administered with PBS or ginseng, immunized with FI-RSV or no immunization mock controls (PBS, Ginseng), and challenged as described in Materials and Methods. BAL cells were harvested, pooled (n=5 per BALB/c mice group), followed by surface staining with CD3, CD4, CD8 antibodies, and intracellular staining with IFN-γ antibodies, and analyzed by flow cytometry. As a gating strategy for intracellular IFN-γ-positive T cells, CD3+CD45+ cells were gated first, and then CD3+ and IFN-γ+ (A) or CD4+ and IFN-γ+ (B), or CD8+ and IFN-γ+ cells (C) were gated. The numbers in dot plots indicate the cell percentages of double-positive population. The group labeling and treatments are the same as in Fig. 2.
Discussion
In this article, we investigated the effects of ginseng on protection and pulmonary lung disease by FI-RSV or mock (PBS, ginseng) immunization after RSV infection using different strains of mouse models. Oral administration of BALB/c and C57BL/6 mice with ginseng resulted in beneficial effects on improving clinical illness by mitigating body weight losses and/or modulating host immune responses after FI-RSV or mock (PBS, ginseng) immunization and respiratory infection with live RSV. Ginseng treatment modulated host immune responses in the direction of inducing higher levels of IgG2a antibodies after FI-RSV immunization. Ginseng treatment (mock control) improves clinical outcomes of illness due to RSV infection most likely by alleviating weight losses and modulating host cytokine production toward Th1 type immune responses regardless of mouse strains. After live RSV challenge of FI-RSV-immunized mice, ginseng-treated groups showed less severe weight loss, diminishing effects on pulmonary inflammatory response, and reduced CD4 T-cell infiltration as well as lower levels of IL-4 cytokine and increased levels of IFN-γ producing cells. All these combined effects by ginseng treatment might have contributed to inducing Th1-type immune responses and conferring better protection against pulmonary inflammatory disease caused by FI-RSV or mock immunization after live RSV infection. We observed a similar pattern of protective effects of oral treatment with ginseng for 80 or 130 days. The optimum duration of ginseng treatment remains to be determined.
Failure of FI-RSV vaccine to protect has been attributed to its induction of Th2-biased immune response, a major contributing factor for causing vaccine-enhanced RSV disease (Waris and others 1997). Induction of a higher level of RSV-specific IgG1 isotype antibodies is an indicator for inducing a Th2-type host immune response (Coffman 1988). In the present study, enhanced pulmonary disease was reproduced by FI-RSV immunization and infectious RSV challenge similar to that as previously reported (Murawski and others 2010).
Cell-culture contaminants in vaccine (FI-RSV) and challenge virus (RSV) preparations are known to be potential factors contributing to RSV pulmonary lesions in animal models (Shaw and others 2013). Thus, it is possible that the severe lung RSV disease in the FI-RSV immunized groups and mock control mice after RSV infection might be partially due to the non-RSV antigens present in the FI-RSV and RSV preparations. In another study, FI-RSV, as a nonreplicating vaccine, failed to protect because of its inefficient innate immune stimulation, leading to strong IgG1 antibody response (Delgado 2009). Thus, FI-RSV immunization undergoing RSV challenge infection would be an in vivo mouse model to investigate protective effects of an agent against inflammatory disease.
In this study, we found that ginseng oral administration could modulate host immune responses to FI-RSV toward increasing IgG2a isotype antibodies. The mechanism that ginseng treatment increased the levels of IgG2a isotype antibody response remains unclear. Cytokine environment is an important factor affecting the isotype switching of B cells, as IL-4 is known to induce more IgG1 isotype antibodies and IFN-γ enhances the output of IgG2a isotype antibodies (Coffman 1988; Graham 1995). Therefore, it is speculated that modulating cytokine production of lowering IL-4 and increasing IFN-γ by ginseng treatment could be a major contributing factor in inducing host immune responses toward Th1-type immunity.
We do not know the underlying mechanisms of how oral administration of red ginseng extracts play a role in priming the host immune system in a Th1 environment. The most likely candidate active components of ginseng extracts are ginsenosides (saponins) and acidic polysaccharides that were reported to act as adjuvants shaping the immune system to induce Th1-type immune responses (Song and others 2010b; den Brok and others 2012; Su and others 2012). Alternatively, oral administration of ginseng extracts would result in more active metabolites contributing to improved outcomes of inflammatory disease. Fermented red ginseng extracts were demonstrated to exhibit more potent anti-inflammatory actions (Jung and others 2012a). Ginsenosides and acidic polysaccharides are also known to have anti-inflammatory effects on macrophages and dendritic cells (Kim and others 2009; Paul S 2012). Modulation of macrophage and dendritic cells would shape the types of T-cell immune responses, leading to a Th1-type cytokine environment.
It is not very well known what causes weight loss in mock (PBS) control and FI-RSV-immunized mice after infectious RSV infection. PBS mock control mice showed more severe weight loss than FI-RSV-immunized mice although lung inflammation in mock control mice was not as severe as FI-RSV immunized mice. Strong signaling through STAT4 such as tumor necrosis factor-α or a high level of IL-4 has been shown to be associated with weight loss disease (Tang and Graham 1994; Castilow and others 2008a). Ginseng mock control mice showed less severe weight loss compared with PBS control mice. This might be due to modulating host cytokine immune responses through ginseng treatment, as too high levels of IFN-γ or IL-4 would contribute to weight loss and RSV disease. FI-RSV-immunized BALB/c and C57BL/6 mice showed significant weight loss as well as severe pulmonary disease after exposure to RSV challenge infection. Ginseng treatment resulted in diminished weight loss and moderately reduced lung inflammation but substantial levels of weight loss and lung histopathology were observed in FI-RSV-immunized BALB/c and C57BL/6 mice after RSV challenge. We found that FI-RSV immunization resulted in good control of lung viral loads after RSV challenge, and ginseng treatment only moderately improved the lung viral clearance (data not shown). The result that FI-RSV immunization significantly lowered lung viral loads is consistent with previous studies, demonstrating that FI-RSV-immunized mice or cotton rats effectively controlled lung viral loads (Prince and others 2001a; Johnson and others 2004; Kamphuis and others 2012).
PBS mock control MHCII KO mice showed significantly more weight loss compared with other wild-type mice or ginseng mock control MHCII KO mice. Thus, in the absence of immunization, the intact CD4 T cells would contribute to protection against RSV infection. It is possible that ginseng treatment contributes to protection against RSV in a CD4 T-cell independent manner by modulating innate immune cells such as macrophage or dendritic cells. FI-RSV-immunized MHCII KO mice did not show body weight loss after RSV challenge regardless of ginseng treatment. A lower degree of lung histopathology was observed in the MHCII knockout mouse group compared with those in the other wild-type BLAB/c and C57BL/6 mouse groups after FI-RSV immunization and RSV challenge. MHCII KO mice lack CD4 T cells that are likely to contribute to FI-RSV vaccine-induced RSV disease. Therefore, CD4 T cells in FI-RSV-immunized mice seem to play an important role in inducing severe pulmonary inflammatory response, which is consistent with an antibody-mediated CD4 T-cell depletion study (Graham and others 1991; Connors and others 1992). Nevertheless, substantial lung inflammation was observed in the FI-RSV-immunized MHCII knockout mice after RSV challenge. Importantly, ginseng treatment of MHCII knockout mice was found to prominently inhibit pulmonary inflammation response, preventing the low degree of lung inflammation. Thus, this study supports the evidence that ginseng exerts anti-inflammatory effects on respiratory viral infection at a moderate level.
Previous studies over decades have been directed toward characterizing enhanced disease that occurs after FI-RSV immunization and RSV infection. This enhanced disease caused by FI-RSV immunization is characterized by an unbalanced Th2-biased cytokine response, an increased number of CD4 T cells, prominent interstitial cellular infiltrates, enhanced mucous secretion, and pronounced eosinophilia. In contrast, a few immunological studies have been conducted to correct RSV disease induced by FI-RSV immunization and RSV challenge. FI-RSV formulation with monophosphorly lipid A adjuvant was shown to induce a balanced Th1/Th2 cytokine profile and reduced pulmonary disease on RSV challenge (Prince and others 2001b). The possible action mechanism of ginseng may be different from a traditional adjuvant effect, because there was no significant difference in total antibody levels between ginseng-treated and nontreated FI-RSV immunized groups.
In this study, we have described full measure characteristics of enhanced pulmonary inflammatory response using different mouse strains. Ginseng oral treatment was found to modulate host responses to FI-RSV immunization and RSV challenge, resulting in multiple immunological and clinical different parameters. In addition to increasing IgG2a antibodies and IFN-γ producing cells as well as lowering IL-4 producing cells, ginseng also decreased cell numbers of CD4+ T (CD3+CD8−) cells, increasing the ratio of CD8+/CD4+ T cells. The cellularity of granulocytes and eosinophils was also reduced in BAL cell populations from the ginseng-treated groups. A recent study demonstrated that oral administration of ginseng reduced IL-6 production but increased IFN-γ production in mice infected with 2009 pandemic H1N1 virus (Yoo and others 2012). Ginseng was shown to stimulate the production of IFN-γ in an asthma mouse model (Jung and others 2012b). It was suggested that ginseng could stimulate dendritic cells, resulting in a high level of IFN-γ production (Kim and others 2009). Overall, it is considered that a combined effect of ginseng via its propensity to induce Th1-type immune responses and anti-inflammatory functions is contributing to improved protection of less weight loss and diminishing pulmonary inflammatory response. The molecular mechanisms by which ginseng extract works on modulating host responses in response to RSV infection or FI-RSV immunization and RSV challenge are not yet clear. Further studies are needed for a better understanding of multiple effects by ginseng oral treatment.
Acknowledgments
This work was supported in part by NIH/NIAID grants AI105170 (S.M.K.) and AI093772 (S.M.K.), and the Korean Ginseng Corp. (S.M.K.). The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the funding agents.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, Song JY. 2006. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol 36(1):37–45 [DOI] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan CS. 1999. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 58(11):1685–1693 [DOI] [PubMed] [Google Scholar]
- Boelen A, Andeweg A, Kwakkel J, Lokhorst W, Bestebroer T, Dormans J, Kimman T. 2000. Both immunisation with a formalin-inactivated respiratory syncytial virus (RSV) vaccine and a mock antigen vaccine induce severe lung pathology and a Th2 cytokine profile in RSV-challenged mice. Vaccine 19(7–8):982–991 [DOI] [PubMed] [Google Scholar]
- Castilow EM, Legge KL, Varga SM. 2008a. Cutting edge: Eosinophils do not contribute to respiratory syncytial virus vaccine-enhanced disease. J Immunol 181(10):6692–6696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilow EM, Meyerholz DK, Varga SM. 2008b. IL-13 is required for eosinophil entry into the lung during respiratory syncytial virus vaccine-enhanced disease. J Immunol 180(4):2376–2384 [DOI] [PubMed] [Google Scholar]
- Chan P, Fu P. 2007. Toxicity of panax ginseng–an herbal medicine and dietary supplement. J Food Drug Anal 15(4):416–427 [Google Scholar]
- Coffman RL, Seymour BW, Lebman DA, Hiraki DD, Christiansen JA, Shrader B, Cherwinski HM, Savelkoul HF, Finkelman FD, Bond MW, et al. 1988. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev 102:5–28 [DOI] [PubMed] [Google Scholar]
- Collins PL, Graham BS. 2008. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 82(5):2040–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC, 3rd, Murphy BR. 1994. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol 68(8):5321–5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M, Kulkarni AB, Firestone CY, Holmes KL, Morse HC, 3rd, Sotnikov AV, Murphy BR. 1992. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol 66(12):7444–7451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 15(1):34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Brok MH, Nierkens S, Wagenaars JA, Ruers TJ, Schrier CC, Rijke EO, Adema GJ. 2012. Saponin-based adjuvants create a highly effective anti-tumor vaccine when combined with in situ tumor destruction. Vaccine 30(4):737–744 [DOI] [PubMed] [Google Scholar]
- Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 89(4):435–448 [DOI] [PubMed] [Google Scholar]
- Girard MP, Cherian T, Pervikov Y, Kieny MP. 2005. A review of vaccine research and development: human acute respiratory infections. Vaccine 23(50):5708–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS. 1995. Pathogenesis of respiratory syncytial virus vaccine-augmented pathology. Am J Respir Crit Care Med 152(4 Pt 2):S63–S66 [DOI] [PubMed] [Google Scholar]
- Graham BS. 2011. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev 239(1):149–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS, Bunton LA, Wright PF, Karzon DT. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest 88(3):1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253(5026):1417–1420 [DOI] [PubMed] [Google Scholar]
- Hong CE, Lyu SY. 2011. Anti-inflammatory and anti-oxidative effects of Korean red ginseng extract in human keratinocytes. Immune Netw 11(1):42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen B, Faller S, Krempl CD, Ehl S. 2011. Major histocompatibility complex-dependent cytotoxic T lymphocyte repertoire and functional avidity contribute to strain-specific disease susceptibility after murine respiratory syncytial virus infection. J Virol 85(19):10135–10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TR, Teng MN, Collins PL, Graham BS. 2004. Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. J Virol 78(11):6024–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Choi H, Lim HW, Shin D, Kim H, Kwon B, Lee JE, Park EH, Lim CJ. 2012a. Enhancement of anti-inflammatory and antinociceptive actions of red ginseng extract by fermentation. J Pharm Pharmacol 64(5):756–762 [DOI] [PubMed] [Google Scholar]
- Jung ID, Kim HY, Park JW, Lee CM, Noh KT, Kang HK, Heo DR, Lee SJ, Son KH, Park HJ, Shin SJ, Park JH, Ryu SW, Park YM. 2012b. RG-II from Panax ginseng C.A. Meyer suppresses asthmatic reaction. BMB reports 45(2):79–84 [DOI] [PubMed] [Google Scholar]
- Kamphuis T, Meijerhof T, Stegmann T, Lederhofer J, Wilschut J, de Haan A. 2012. Immunogenicity and protective capacity of a virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice. PLoS One 7(5):e36812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89(4):405–421 [DOI] [PubMed] [Google Scholar]
- Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, Polack FP, Randolph VB, Deatly A, Hackell J, Gruber W, Murphy BR, Collins PL. 2005. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis 191(7):1093–1104 [DOI] [PubMed] [Google Scholar]
- Kim CM, Yi SJ, Cho IJ, Ku SK. 2013a. Red-koji fermented red ginseng ameliorates high fat diet-induced metabolic disorders in mice. Nutrients 5(11):4316–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89(4):422–434 [DOI] [PubMed] [Google Scholar]
- Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, Kang SM. 2013b. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther 21(2):485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Byon YY, Ko EJ, Song JY, Yun YS, Shin T, Joo HG. 2009. Immunomodulatory activity of ginsan, a polysaccharide of panax ginseng, on dendritic cells. Korean J Physiol Pharmacol 13(3):169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JM, Yao Q, Chen C. 2009. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7(3):293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok H, Lee S, Utley TJ, Shepherd BE, Polosukhin VV, Collier ML, Davis NL, Johnston RE, Crowe JE., Jr.2007. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J Virol 81(24):13710–13722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG. 2010. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol 84(2):1110–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw PJ, Tregoning JS. 2005. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev 18(3):541–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EH, Yum J, Ku KB, Kim HM, Kang YM, Kim JC, Kim JA, Kang YK, Seo SH. 2014. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J Ginseng Res 38(1):40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Shin HS, Kang SC. 2012. Inhibition of inflammations and macrophage activation by ginsenoside-Re isolated from Korean ginseng (Panax ginseng C.A. Meyer). Food Chem Toxicol 50(5):1354–1361 [DOI] [PubMed] [Google Scholar]
- Prince GA, Curtis SJ, Yim KC, Porter DD. 2001a. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol 82(Pt 12):2881–2888 [DOI] [PubMed] [Google Scholar]
- Prince GA, Denamur F, Deschamps M, Garcon N, Prieels JP, Slaoui M, Thiriart C, Porter DD. 2001b. Monophosphoryl lipid A adjuvant reverses a principal histologic parameter of formalin-inactivated respiratory syncytial virus vaccine-induced disease. Vaccine 19(15–16):2048–2054 [DOI] [PubMed] [Google Scholar]
- Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, Moore ML, Compans RW. 2011. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis 204(7):987–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. 2010. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol 84(15):7760–7769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw CA, Galarneau JR, Bowenkamp KE, Swanson KA, Palmer GA, Palladino G, Markovits JE, Valiante NM, Dormitzer PR, Otten GR. 2013. The role of non-viral antigens in the cotton rat model of respiratory syncytial virus vaccine-enhanced disease. Vaccine 31(2):306–312 [DOI] [PubMed] [Google Scholar]
- Shibata S. 2001. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci 16Suppl:S28–S37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. 2010a. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 405(1):165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Chen J, Sakwiwatkul K, Li R, Hu S. 2010b. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int Immunopharmacol 10(3):351–356 [DOI] [PubMed] [Google Scholar]
- Stark JM, McDowell SA, Koenigsknecht V, Prows DR, Leikauf JE, Le Vine AM, Leikauf GD. 2002. Genetic susceptibility to respiratory syncytial virus infection in inbred mice. J Med Virol 67(1):92–100 [DOI] [PubMed] [Google Scholar]
- Su F, Yuan L, Zhang L, Hu S. 2012. Ginsenosides Rg1 and Re act as adjuvant via TLR4 signaling pathway. Vaccine 30(27):4106–4112 [DOI] [PubMed] [Google Scholar]
- Takei M, Tachikawa E, Umeyama A. 2008. Dendritic cells promoted by ginseng saponins drive a potent Th1 polarization. Biomark Insights 3:269–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YW, Graham BS. 1994. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Invest 94(5):1953–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp RA, Hou S, Etchart N, Prinz A, Moore D, Winter J, Anderson LJ. 2001. CD4(+) T cell frequencies and Th1/Th2 cytokine patterns expressed in the acute and memory response to respiratory syncytial virus I-E(d)-restricted peptides. Cell Immunol 207(1):59–71 [DOI] [PubMed] [Google Scholar]
- Waris ME, Tsou C, Erdman DD, Day DB, Anderson LJ. 1997. Priming with live respiratory syncytial virus (RSV) prevents the enhanced pulmonary inflammatory response seen after RSV challenge in BALB/c mice immunized with formalin-inactivated RSV. J Virol 71(9):6935–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. 1996. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol 70(5):2852–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo DG, Kim MC, Park MK, Song JM, Quan FS, Park KM, Cho YK, Kang SM. 2012. Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J Med Food 15(10):855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CS, Wang CZ, Wicks SM, Qi LW. 2010. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Ginseng Res 34(3):160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]