Abstract
High-dose interleukin-2 (HDIL2) treatment of patients with metastatic melanoma and renal cell carcinoma is associated with durable responses, but therapy is accompanied by significant toxicity related to vascular leak syndrome (VLS). Currently, the cause of VLS is not well defined; however, based on the role of endothelial cell (EC) permeability in VLS and the commonly observed hypoalbuminemia in patients receiving HDIL2 therapy, we established an in vitro approach utilizing primary human pulmonary microvascular ECs to monitor the effect of HDIL2 therapy on albumin uptake. We found that HDIL2 treatment of ECs results in albumin colocalization with caveolin-1 leading to albumin uptake by ECs. This albumin uptake occurs through caveolae-mediated but not clathrin-mediated endocytosis and is abrogated with inhibition of the Src tyrosine kinase pathway. These findings provide insight into how IL-2 induces VLS and may help identify potential targets for prevention of toxicity without affecting the therapeutic activity of HDIL2.
Introduction
Interleukin-2 (IL-2) is a cytokine that plays a pivotal role in T- and NK cell homeostasis (Waldmann and others 2001; D'Cruz and Klein 2005). In the clinic, high-dose IL-2 (HDIL2) treatment has been associated with durable responses in a subset of patients with metastatic melanoma and renal cell carcinoma (Rosenberg and others 1994; Atkins 2006). Therapeutic responses are most often observed with high-dose (600,000–720,000 IU/kg), bolus, and intravenous infusions. However, therapy is often stopped for significant toxicity related to vascular leak syndrome (VLS) characterized by hypotension, tachycardia, third-space fluid sequestration, and end-organ failure (Rosenstein and others 1986; Edwards and others 1991). The cause of VLS is not well defined but may be associated with the hypoalbuminemia universally observed in patients receiving IL-2 (Deehan and others 1994; Pockaj and others 1994).
Here, we established an in vitro platform to evaluate the effect of IL-2 on microvascular endothelial cells (ECs) that allows direct visualization and quantitation of molecular changes. Previous studies have suggested that IL-2 mediates protein leak, including that of albumin, through disruption of EC integrity (Deehan and others 1994). Our data, however, support a role for IL-2-mediated direct albumin uptake by ECs. This process appears to occur through caveolin-mediated endocytosis and to be dependent on Src kinase signaling. A better understanding of how IL-2 induces VLS and regulates albumin homeostasis may identify potential targets for treating IL-2-mediated toxicity while preserving the immunologic and therapeutic function of IL-2 in patients with cancer.
Materials and Methods
EC culture
Primary human pulmonary microvascular ECs (Cambrex) were grown on dishes precoated with 4 μg/mL fibronectin. ECs were treated with IL-2 (10,000 IU/mL), and in the last 30 min of IL-2 incubation, 40 μg/mL FITC-labeled albumin (Sigma) was added. ECs were then washed with phosphate-buffered saline (PBS), fixed with 1% paraformaldehyde, and used in confocal microscopy evaluations.
Visualization of albumin uptake and caveolin-1 distribution
Fixed ECs were incubated with rhodamine phalloidin (Sigma) to visualize F-actin. In some experiments, cells were also incubated with a rabbit anti-caveolin-1 antibody (BD Biosciences) and subsequently with an Alexa 633-conjugated anti-rabbit IgG to visualize caveolin-1. Specificity of IL-2 effects was determined by pretreating cells for 30 min with 50 μg/mL anti-IL-2 antibody (clone 5334; R&D Systems) or an isotype control IgG. Slides were examined using a Leica TCS-SP confocal microscope. Serial images from the apical surface to the basolateral side of cells were acquired with a 0.5-μm step size. Two-dimensional projections were constructed using the Leica confocal software. Endocytosed albumin was semiquantitated using ImageJ64 software (NIH) (Schenkel and others 2013) by filtering out all colors other than green and removing background fluorescence.
Inhibition of endocytosis
To examine the role of endocytosis pathways, ECs were pretreated for 30 min with a vehicle (PBS), 5 μM chlorpromazine (an inhibitor of clathrin-mediated endocytosis), or 5 μM methyl-β-cyclodextrin (an inhibitor of caveolae-mediated endocytosis) (Wang and others 1993; Le and others 2000). To determine the role of Src, ECs were pretreated for 30 min with a vehicle or 20 μM PP2 (a Src inhibitor).
Statistical analysis
Data were analyzed using one-way ANOVA followed by post hoc comparisons (Bonferroni) using the GraphPad Prism v4.0 software. A P-value of <0.05 was considered significant.
Results and Discussion
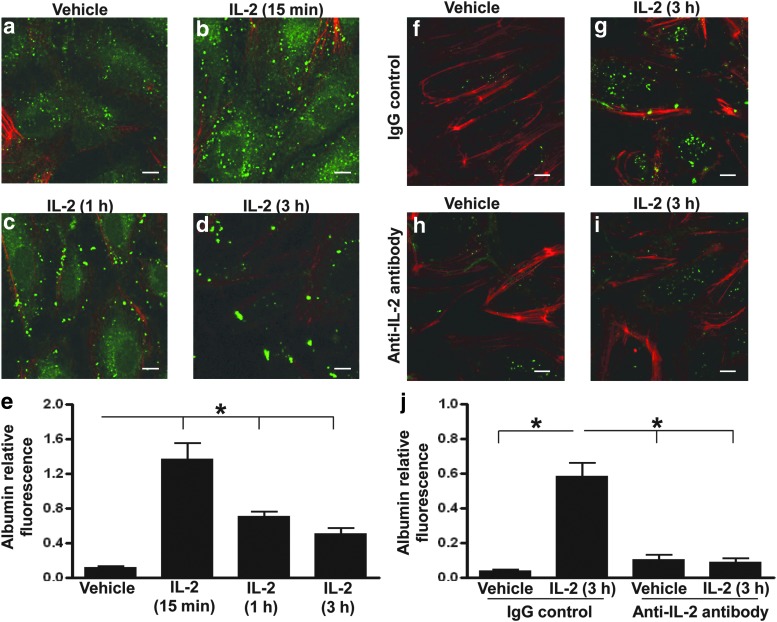
To determine whether HDIL2 treatment results in albumin uptake by ECs, we treated pulmonary microvascular ECs with 10,000 IU/mL of recombinant IL-2, a dose that corresponds to the mean serum IL-2 concentration found in clinical treatment (Sabatino and others 2009). IL-2 treatment induced a profound increase in albumin uptake by ECs (Fig. 1a–e). This uptake was apparent as early as 15 min after IL-2 exposure and persisted for at least 3 h. Direct imaging of ECs demonstrated that albumin was present as dispersed proteins at 15 min, which were uniform in size and measured 1–2 μm in length (Fig. 1b). After 3 h, larger aggregates that measured up to 3 μm were observed (Fig. 1d). IL-2-induced albumin uptake was completely blocked by the presence of an anti-IL-2 monoclonal antibody (Fig. 1f–j). HDIL2 did not cause a significant increase in the percentage of apoptotic ECs as determined by annexin V and propidium iodide staining (data not shown).
FIG. 1.
High-dose interleukin-2 (HDIL2) induces albumin uptake by human endothelial cells (ECs). (a–e) Confocal images of FITC-albumin (green) and F-actin (red) in ECs treated with a vehicle (phosphate-buffered saline) (a) or 10,000 IU/mL IL-2 for 15 min (b), 1 h (c), or 3 h (d). FITC-albumin was added to ECs in the last 30 min of IL-2 incubation for the 1- and 3-h time points. For the 15 min time point, FITC-albumin was added 15 min before the addition of IL-2 for a total incubation time of 30 min. Serial confocal images were collected at a 0.5-μm interval, and a 2D projection image representing all slices was constructed using confocal microscope software. Data in (a–d) represent more than 6 independent experiments with similar results. (e) Quantitation of the HDIL2-induced albumin uptake shown is based on the average FITC fluorescence of multiple fields from 1 experiment representing 6 independent experiments with similar results. (f–j) Confocal images from the experiment described in (a–e) of FITC-albumin (green) and F-actin (red) in ECs pretreated with 50 μg/mL isotype control (IgG) (f, g) or anti-IL-2 antibody (h, i) for 30 min before being treated with a vehicle (f, h) or HDIL2 (g, i) for 3 h. (j) Quantitation of the HDIL2-induced albumin uptake shown is based on the average FITC fluorescence of multiple fields from 1 experiment representing 6 independent experiments with similar results. All scale bars: 10 μm. *P<0.01.
These data suggest that albumin is rapidly taken up by ECs following exposure to high doses of recombinant IL-2. Previously, pulmonary microvascular ECs have been shown to express an intermediate affinity IL-2 receptor (Krieg and others 2010). Thus, IL-2 can directly influence physiological functions within ECs. This function may explain the common occurrence of hypoalbuminemia in patients on HDIL2 therapy. Although it is possible that hepatic dysfunction related to IL-2 may contribute to this phenomenon, our data provide a novel mechanism that may be equally or more important, since all patients experience hypoalbuminemia during IL-2 treatment, but hepatic toxicity is seen in <40% of patients (Palmer and others 1992; Atkins and others 1999). IL-2 can also induce apoptosis in some cells, but this was not observed in our system. This suggests that IL-2 may contribute to VLS through an uptake of albumin that adds to the fluid sequestration and the edema observed clinically in IL-2 treated patients.
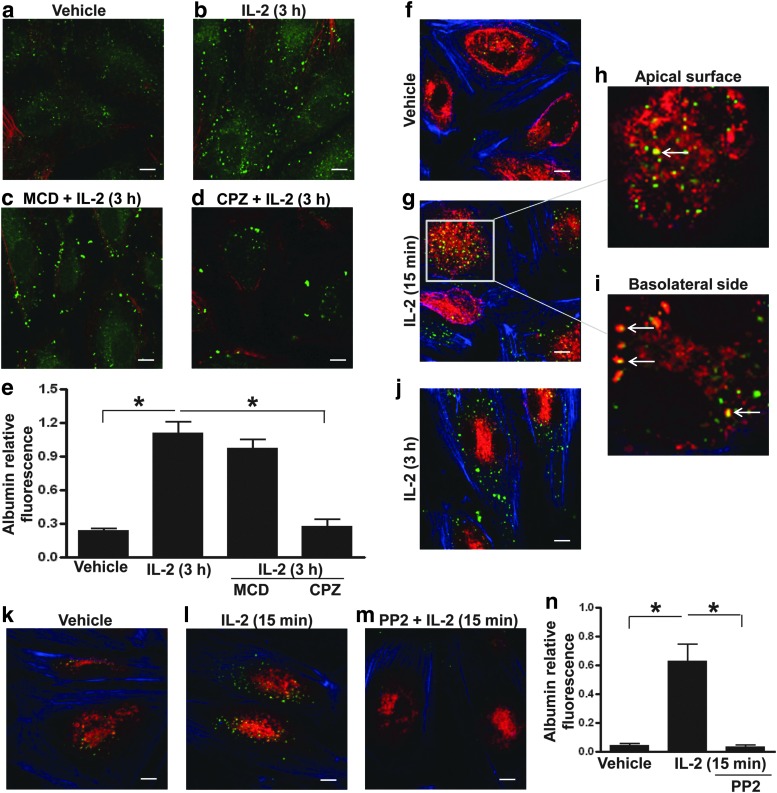
To define the endocytic pathways responsible for mediating albumin uptake induced by IL-2, ECs were exposed to IL-2 in the presence or absence of methyl-β-cyclodextrin, a cholesterol-depleting reagent that also inhibits caveolae-mediated endocytosis or chlorpromazine, an inhibitor of clathrin-mediated endocytosis. Methyl-β-cyclodextrin blocked IL-2-induced albumin uptake by ECs, whereas chlorpromazine had no effect (Fig. 2a–e). In addition, staining for caveolin-1 showed colocalization of albumin with caveolin-1 in response to IL-2 (Fig. 2f–j). IL-2-caveolin-1 complexes could be found near the apical surface of ECs (where the cells were exposed to albumin) and near the basolateral surfaces (Fig. 2h, i). The colocalization subsided within 3 h of IL-2 exposure (Fig. 2j). These data support caveolae-associated endocytosis as a mechanism of IL-2-induced albumin uptake.
FIG. 2.
HDIL2-induced albumin uptake is mediated through caveolae-mediated endocytosis and inhibited by a pharmacological inhibitor targeting Src tyrosine kinase. (a–e) Confocal images from the experiment described in Figure 1 of FITC-albumin (green) and F-actin (red) in ECs treated with a vehicle (a) or 10,000 IU/mL IL-2 for 3 h (b–d). Before stimulation, some ECs were also pretreated with 5 μM methyl-β-cyclodextrin, an inhibitor of caveolae-mediated endocytosis (c), or 5 μM chlorpromazine, an inhibitor of clathrin-mediated endocytosis (d). (e) Quantitation of the HDIL2-induced albumin uptake shown is based on the average FITC fluorescence of multiple fields from 1 experiment representing 3 independent experiments with similar results. (f–j) Confocal images from the experiment described in Figure 1 of FITC-albumin (green), F-actin (blue), and caveolin-1 (red) in ECs treated with a vehicle (f) or 10,000 IU/mL IL-2 for 15 min (g–i) or 3 h (j). The image in (h) shows colocalization of albumin and calveolin-1 (white arrows) of a slice near the apical surface and image in (i) shows similar colocalization near the basolateral side of the zoom area. (k–n) Confocal images from the experiment described in Figure 1 of FITC-albumin (green), F-actin (blue), and caveolin-1 (red) in ECs treated with a vehicle (k) or 10,000 IU/mL IL-2 for 15 min (l, m). ECs were pretreated with a vehicle (k, l) or 20 μM PP2, an Src tyrosine kinase inhibitor (m), before IL-2 stimulation. (n) The quantitation of HDIL2-induced albumin uptake shown is based on the average FITC fluorescence of multiple fields from 1 experiment representing 3 independent experiments with similar results. All scale bars: 10 μm. *P<0.01.
Since the activation of Src tyrosine kinase plays an important role in regulating caveolae-mediated albumin transport (Schubert and others 2001), the role of Src in regulating IL-2-induced albumin uptake by ECs was examined. Pretreatment with PP2, an Src inhibitor, prevented IL-2-induced albumin uptake by ECs (Fig. 2k–n). These data suggest that IL-2 induces early albumin uptake by ECs through mechanisms that depend on caveolae-mediated endocytosis, accumulation of IL-2-caveolin-1 complexes near the intracellular membrane, and activation of Src-mediated intracellular signaling.
These data support a pivotal role for Src kinase signaling in mediating albumin uptake and endocytosis in ECs exposed to high doses of IL-2. In T and NK cells, IL-2 is known to signal through the JAK-STAT pathway resulting in cell proliferation, cytokine production, and inhibition of apoptosis (Nelson and others 1994; Russell and others 1994; Moriggl and others 1999). This is the first report to suggest that IL-2 signals through the Src pathway in human pulmonary microvascular ECs. Future work will attempt to define the downstream factors involved in this signaling, which could lead to new therapeutic targets for blocking albumin uptake and thus possibly block the pathological consequences of IL-2-induced VLS.
The ability to differentiate specific signaling pathways or physiological processes associated with VLS from the lymphocyte signaling pathways responsible for IL-2-mediated therapeutic responses could have significant potential for improving the clinical management of patients receiving HDIL2 treatment. In this report, we have identified 2 such potential targets for therapeutic drug development. First, our finding that albumin uptake was dependent on caveolin-mediated endocytosis suggests that blocking caveolin-associated endocytosis could reverse albumin uptake. In contrast to clathrin-associated endocytosis, molecular internalization through caveolae depends on a complex signaling cascade (Dautry-Varsat 2000). Further in vitro studies using our pulmonary EC platform could help identify putative endocytosis inhibitors that could then be evaluated in animal models and considered for clinical translation. Here, we have also identified that the albumin uptake was dependent on Src tyrosine kinase signaling and showed that the process could be inhibited with an Src inhibitor. Thus, the use of an Src-specific inhibitor might be considered for in vivo blockade of albumin uptake, and it has potential for clinical development (Hanke and others 1996). The potential to pharmacologically block VLS might allow more patients to access HDIL2 therapy.
In summary, we have identified a new mechanism contributing to IL-2-mediated VLS in which IL-2 mediates albumin uptake in ECs through caveolae-mediated endocytosis in a manner dependent on Src kinase pathway signaling. These data support a direct effect of IL-2 on ECs. The identification of the signaling factors involved in this process might lead to new therapeutic targets for blocking IL-2-induced VLS. The potential to control serious side effects of HDIL2 therapy may improve patient's tolerability and allow more patients with cancer to benefit from IL-2 treatment.
Acknowledgments
The authors thank Dr. Qin Wang for original ideas and help in conceptual design of the pulmonary microvascular endothelial system.
Author Disclosure Statement
The authors declare that they have no competing interests.
References
- Atkins MB. 2006. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res 12(7 Pt 2):2353s–2358s [DOI] [PubMed] [Google Scholar]
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. 1999. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17(7):2105–2116 [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A. 2000. Clathrin-independent endocytosis. In: Marsh M. ed. Endocytosis. Frontiers in molecular biology, 1st ed., Oxford, United Kingdom: Oxford University Press. pp 26–57 [Google Scholar]
- D'Cruz LM, Klein L. 2005. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol 6(11):1152–1159 [DOI] [PubMed] [Google Scholar]
- Deehan DJ, Heys SD, Simpson W, Herriot R, Broom J, Eremin O. 1994. Correlation of serum cytokine and acute phase reactant levels with alterations in weight and serum albumin in patients receiving immunotherapy with recombinant IL-2. Clin Exp Immunol 95(3):366–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ, Schuschke DA, Abney DL, Miller FN. 1991. Interleukin-2 acutely induces protein leakage from the microcirculation. J Surg Res 50(6):609–615 [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brisette WH, Weringer EJ, Pollok BA, Connelly PA. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: study of Lck- and FynT-dependent T cell activation. J Biol Chem 271:695–701 [DOI] [PubMed] [Google Scholar]
- Krieg C, Letourneau S, Pantaleo G, Boyman O. 2010. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A 107(26):11906–11911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le PU, Benlimame N, Lagana A, Raz A, Nabi IR. 2000. Clathrin-mediated endocytosis and recycling of autocrine motility factor receptor to fibronectin fibrils is a limiting factor for NIH-3T3 cell motility. J Cell Sci 113(Pt 18):3227–3240 [DOI] [PubMed] [Google Scholar]
- Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D, Hoffmeyer A, van Deursen J, Sangster MY, Bunting KD, Grosveld GC, Ihle JN. 1999. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity 10(2):249–259 [DOI] [PubMed] [Google Scholar]
- Nelson BH, Lord JD, Greenberg PD. 1994. Cytoplasmic domains of the interleukin-2 receptor beta and gamma chains mediate the signal for T-cell proliferation. Nature 369(6478):333–336 [DOI] [PubMed] [Google Scholar]
- Palmer PA, Vinke J, Evers P, Pourreau C, Oskam R, Roest G, Vlems F, Becker L, Loriaux E, Franks CR. 1992. Continuous infusion of recombinant interleukin-2 with or without autologous lymphokine activated killer cells for the treatment of advanced renal cell carcinoma. Eur J Cancer 28A(6–7):1038–1044 [DOI] [PubMed] [Google Scholar]
- Pockaj BA, Yang JC, Lotze MT, Lange JR, Spencer WF, Steinberg SM, Topalian SL, Schwartzentruber DJ, White DE, Rosenberg SA. 1994. A prospective randomized trial evaluating colloid versus crystalloid resuscitation in the treatment of the vascular leak syndrome associated with interleukin-2 therapy. J Immunother Emphasis Tumor Immunol 15(1):22–28 [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. 1994. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271(12):907–913 [PubMed] [Google Scholar]
- Rosenstein M, Ettinghausen SE, Rosenberg SA. 1986. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol 137(5):1735–1742 [PubMed] [Google Scholar]
- Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, et al. . 1994. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science 266(5187):1042–1045 [DOI] [PubMed] [Google Scholar]
- Sabatino M, Kim-Schulze S, Panelli MC, Stroncek D, Wang E, Taback B, Kim DW, Deraffele G, Pos Z, Marincola FM, Kaufman HL. 2009. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol 27(16):2645–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. 2013. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol 14(5):509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP. 2001. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem 276(52):48619–48622 [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Dubois S, Tagaya Y. 2001. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity 14(2):105–110 [PubMed] [Google Scholar]
- Wang LH, Rothberg KG, Anderson RG. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol 123(5):1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]