Abstract
Adeno-associated viruses (AAVs) are established vectors for gene therapy of different human diseases. AAVs are assembled of 60 capsomers, which can be genetically modified, allowing high-density display of short peptide sequences at their surface. The aim of our study was to evaluate the immunogenicity and safety of an adeno-associated virus-like particle (AAVLP)-displayed B-cell peptide epitope taking ovalbumin (OVA) as a model antigen or allergen from egg, respectively. An OVA-derived B-cell epitope was expressed as fusion protein with the AAV-2 capsid protein of VP3 (AAVLP-OVA) and for control, with the nonrelated peptide TP18 (AAVLP-TP18). Cellular internalization studies revealed an impaired uptake of AAVLP-OVA by mouse BMDC, macrophages, and human HeLa cells. Nevertheless, BALB/c mice immunized subcutaneously with AAVLP-OVA formed similarly high titers of OVA-specific IgG1 compared to mice immunized with the native OVA. The extent of the immune response was independent whether aluminum hydroxide or water in oil emulsion was used as adjuvant. Furthermore, in mice immunized with native OVA, high OVA-specific IgE levels were observed, which permitted OVA-specific mast-cell degranulation in a β-hexosaminidase release assay, whereas immunizations with AAVLP-OVA rendered background IgE levels only. Accordingly, OVA-immunized mice, but not AAVLP-OVA immunized mice, displayed an anaphylactic reaction with a significant drop of body temperature upon intravenous OVA challenge. From this mouse model, we conclude that AAVLPs that display B-cell epitope peptides on their surface are suitable vaccine candidates, especially in the field of allergy.
Introduction
Adeno-associated viruses (AAV) are small (about 20 nm) nonenveloped icosahedric ssDNA viruses, which depend on helper viruses for replication (7). Until now, nine human serotypes have been characterized. About 80% of the population has detectable levels of anti-AAV antibodies, but there is no discernable pathology association with this virus. This fact and the ability of AAV to mediate transgene integration into a specific site in the human genome made it an important candidate for use in gene therapy. The resulting knowledge about capsid structure and tolerance to peptide insertions can be used for the design of genome-free AAV-like particles (AAVLPs) as a novel high-density system for peptide vaccines. Peptide insertion between amino acid positions 587 and 588 of the AAV2 capsid sequence is well established. Up to 34 amino acids can be inserted at this position, and the inserted peptides are repetitively displayed at the capsid surface 60 times, without impairment of capsid integrity (1). Integration of peptides at this position interrupts the heparin-binding domain of AAV2 and therefore reduces binding of the capsid to heparan sulfate proteoglycans (HSPG), if this is not compensated by positive charges in the insert (14). Natural human AAV isolates with approximately 90% amino acid identity to AAV2 do not have this heparin-binding site (2), indicating selection of heparin-binding AAV2 by in vitro cultivation of the virus.
The adaptive response to AAV2 is characterized by production of neutralizing antibodies (23), composed mostly of IgG1 and IgG2 subclasses, with little or no presence of IgG3 or IgG4 antibodies (11).
Compared to AAV2 of 20 nm, AAVLPs are of comparable size (25 nm) and, similar to the native virus, 60 subunits assemble to a viral capsid. Whereas AAV2 is composed of three types of capsid proteins (VP1, VP2, and VP3) that are arranged in an icosahedral capsid in a ratio of 1:1:8, AAVLPs are composed only of VP3.
In an alternative approach, we used here AAVLPs assembled in HEK293 cells for the surface display of peptide epitopes. Containing only VP3, these particles lack the N-terminal phospholipase A2 sequence necessary for endosome escape (10) and were shown to accumulate in the Golgi after endocytosis and passage through endosomes (9). On this quadrilateral “kite-shaped” molecule, as an exposed loop of VP3, we inserted a B-cell epitope from ovalbumin (OVA323–339 peptide) (15) or a control peptide (rabbit cholesterol ester transfer protein/CETP residues 215–229) between amino acids 587 and 588 of VP3, for investigating the immunogenicity and safety of AAVLPs in a BALB/c mouse model. The inserted OVA peptide encompasses B- and T-cell epitopes (restricted by the MHC class I-Ad molecule in mice) and was reported as being recognized by specific IgE antibodies (20).
In this study, we focused on the immunogenicity and safety of an AAVLP-displayed B-cell epitope, taking OVA as a model antigen or allergen respectively, and comparing a Th2 (aluminum hydroxide) versus a Th1 (Montanide® ISA 51) adjuvant.
Materials and Methods
Antigen and adjuvants
Ovalbumin (OVA) was obtained from Sigma-Aldrich (Vienna, Austria). The adjuvant aluminum hydroxide (Alu-Gel-S Suspension 1.3%, sterile) was purchased from Serva Electrophoresis (Heidelberg, Germany), while Montanide ISA 51 VG (sterile, endotoxin free) was obtained from Seppic (Cologne, Germany).
Cells
Human cervix carcinoma HeLa-H1 cells and murine RAW264.7 macrophages were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin G, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
For the generation of mouse bone marrow-derived dendritic cells (mBMDC), hindlimb bone marrow cells were flushed out, grinded through a cell strainer (BD Biosciences, Heidelberg, Germany), and sedimented by centrifugation. Red blood cells were lysed in 0.15 M ammonium chloride/Tris/HCl (pH 7.4) for 3 min, diluted with culture medium, centrifuged and resuspended in medium containing recombinant murine GM-CSF (PeproTech, Hamburg, Germany), and plated at a density of ∼1.5×106/cm2. On day 9, the loosely adherent dendritic cells were rinsed off and transferred to cover slips.
AAVLP plasmid generation
The generation of the AAVLP expression vector (pCIVP2mut) has been previously described (13). In order to introduce peptides into VP3, the vector was modified by introduction of Notl and BspEI sites at position 587 of VP3 (aa number relative to the VP1 protein of AAV2). The nucleotide sequence of an OVA epitope (aa 323–39) or CETP epitope (aa 215–229) flanked by an Ala/Gly linker was cloned into the Notl/BspEI site to generate the vectors for the expression of AAVLP-OVA or AAVLP-TP18.
Production and purification of AAVLPs
For production of AAVLP-OVA or AAVLP-TP18 (control peptide), HEK-293T cells were transfected with the corresponding expression vectors by calcium phosphate precipitation. Transfected cells were harvested after 3 days and lysed by three freeze–thaw cycles.
The medium containing lysed cells was clarified by filtration and diluted by adding HEPES/MgCl2 buffer. After adjusting the pH to 6.0, the diluted cleared lysate containing the AAVLPs was loaded on a chromatography column packed with Fractogel EMD SO3 (M) resin (Merck, Darmstadt, Germany) and bound particles were eluted by a step gradient using 0.5 M NaCl. After, the buffer was exchanged for Tris buffer, and particles were processed through a column packed with CaptoQ resin (GE Healthcare, Buckinghamshire, United Kingdom). The flow-through, containing the particles, was concentrated by filter centrifugation, and separated through a column packed with Superdex 200 (prep grade) resin (GE Healthcare). After column equilibration with HEPES/NaCl (pH 6.0), particles were processed through the column and eluted in the first fractions. Fractions were analyzed by SDS-PAGE and colloidal stained (Colloidal Blue staining Kit; Life Technologies, Carlsbad, CA).
AAVLP-TP18 were produced in a similar way to AAVLP-OVA, with only minor changes: after harvesting, the concentrated cell lysate was treated with Benzonase/MgCl2, and cleared by centrifugation and dialyzed against HEPES buffer, as before. The eluted particles were loaded on a column packed with SOURCE 15Q (GE Healthcare) and eluted as before.
Specificity enzyme-linked immunosorbent assay using OVA-specific rabbit antibody
AAVLP-OVA and AAVLP-TP18 were coated on 96-well-flat-bottom-plates (Nunc, Roskilde, Denmark) overnight at 4°C at a concentration of 0.5 μg/mL in Tris buffered saline (TBS), containing 0.1% milk powder. Wells were washed with TBS/0.05% Tween20 (TBST) and blocked with 1% milk powder/TBST for 1 h at room temperature and 1 h at 4°C. Polyclonal rabbit anti-ovalbumin antibody (PA-0323-100; Innovagen, Lund, Sweden) and control rabbit antibody (Life Technologies) were diluted from 1:1,000 to 1:1,000,000 in 0.1% milk powder/TBST (100 μL/well) and incubated for 1 h at room temperature and 1 h at 4°C. Anti-rabbit-IgG–HRP was diluted 1:2,000 and incubated for 1 h at room temperature and 1 h at 4°C. The reaction was developed with ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) solution (1 mg/mL ABTS and 0.1% H2O2, in citric acid buffer) and measured at OD405–490nm with a microplate reader (Spectra Max Plus 384, Sunnyvale, CA).
Immunization protocol of mice
Female BALB/c mice (8 weeks old; Charles River, Sulzfeld, Germany) were treated according to the rules of the European Community of animal care with the permission number of the Austrian Ministry of Science (BMWF-66.009/0003-II/3b/2011).
Each group (n=5) was immunized subcutaneously (s.c.) either with OVA (2 μg in 100 μL) or with equally calculated particles (100 μL of working concentrations of AAVLP-TP18: 106 μg/mL; 1.7 U+13 capsid/mL; AAVLP-OVA: 112 μg/mL, 1.8 U+13 capsid/mL) in combination with adjuvants, three times at 2 week intervals. Adjuvant concentrations were used 1:1 for Montanide (100 μL particles, 100 μL Montanide). For immunizations with aluminum, particles were adsorbed to 2% Al(OH3). To test the specific antibody production, pre-immune serum (PIS) was drawn from each mouse on day 0, and 1 day before each immunization for mouse immune sera (MIS). After completing the immunization protocol, on day 41, animals were sacrificed.
Titration of pooled mouse immune sera
Ninety-six-well flat-bottom plates were coated with 2 μg/mL OVA overnight at 4°C. Wells were washed with TBST and blocked with 1% milk powder/TBST for 1 h at room temperature and 1 h at 4°C. The pool of third MIS was composed of equal volumes of blood samples from each mouse and serially diluted from 1:1,000 to 1:1,000,000. After washing, goat anti-mouse-IgG HRP (Abcam, Cambridge, United Kingdom) 1:3,000 was incubated for 1 h at room temperature and 1 h at 4°C. After a final wash, detection was performed with TMB solution (BD Biosciences, Schwechat, Austria), according to the manufacturer's instructions, and read at an optical density of 450–630 nm in a microplate reader.
Detection of antigen- and carrier-specific antibodies by enzyme-linked immunosorbent assay
Ninety-six-well flat-bottom plates were coated either with 2 μg/mL OVA or with equally calculated wtAAVLP (w/o peptide insertion at position 587) overnight at 4°C. Wells were washed with TBST and blocked with 1% milk powder/TBST for 1 h at room temperature and 1 h at 4°C. Individual mouse serum samples were diluted 1:100 for IgG1, IgG2a, and IgG2b, and 1:10 for IgE in 0.1% milk powder/TBST. Isotype-specific primary detection antibodies rat-anti-mouse-IgG1, -IgG2a, -IgG2b, and -IgE were diluted 1:1,000 in 100 μL/well, the secondary detection antibody goat-anti-rat-IgG-HRP (Southern Biotech, Birmingham, AL) 1:3,000 in 0.1% milk powder/TBST and incubated for 1 h at room temperature and 1 h at 4°C. After repeated washing steps, detection was completed with ABTS solution as described above.
Internalization of wtAAVLP and AAVLP-OVA into different cell types
For internalization of AAVLPs or fluorescein isothiocyanate (FITC)-dextran (Sigma-Aldrich), HeLa-H1 carcinoma cells, RAW264.7 macrophages, and mBMDCs were plated on cover slips in 24-well plates and incubated overnight. After preincubation for 30 min at 37°C in serum-free medium, AAVLP-OVA, wtAAVLP (∼106 intact capsids/cell), or FITC-dextran (2.5 mg/mL) was added and incubated at 37°C for 2 h. After washing with PBS, cells were fixed or incubated with complete culture medium for further 18 h before fixation. Cells incubated with FITC-dextran were further processed for fluorescence microscopy without permeabilization.
For AAVLP detection, cells (including controls not treated with virus particles) were permeabilized and incubated overnight at 4°C with mouse monoclonal anti-AAV2 antibody A20 (Progen, Heidelberg, Germany) (22), followed by a secondary goat antibody labeled with Alexa Fluor 568 (Molecular Probes; Life Technologies). For labeling of CD11c, Alexa Fluor 647-conjugated hamster anti-mouse CD11c antibody (or isotype control) and unlabeled rat anti-mouse CD16/32 were used.
Nuclei were stained with Hoechst dye 33342 in PBS and viewed under a Zeiss Axioplan 2 fluorescence microscope using Axiovision software (Carl Zeiss AG, Jena, Germany). Confocal microscopy with a Zeiss Axiovert 200 microscope (equipped with a Perkin Elmer Ultraview ERS rapid confocal imager and the corresponding software) was used for detection of Alexa Fluor 647 fluorescence.
β-hexosaminidase-release assay
RBL-2H3 basophil cells (ATCC, Manassas, VA) were passively sensitized with serum pools from each mouse groups (diluted 1:10) by incubation for 2 h at 37°C and 5% CO2. After washing the cells, ovalbumin, Phl p 5 as irrelevant allergen (0.1 μg/mL, 0.5 μg/mL, or 1.0 μg/mL in 100 μL/well) or ionomycin as positive control (0.4 μg in 100 μL/well) were added and incubated for 30 min at 37°C and 5% CO2. The spontaneous release of β-hexosaminidase from RBL-2H3 cells was calculated using Tyrode's buffer alone, the maximal release using 10% Triton X-100. Fifty microliters of each supernatant mixed with 50 μL β-hexosaminidase assay solution was transferred into a flat-bottom plate. After incubation, the reaction was stopped by the addition of 100 μL of glycine buffer. Fluorescence was measured at 360/465 nm in a fluorometer (Cytofluor 2350; Millipore, Vienna, Austria), and results were calculated as relative percentage of total release, as achieved with Triton X-100.
In vivo anaphylaxis experiment
For the evaluation of systemic anaphylactic responses, all immunized mice were intravenously (i.v.) challenged with 50 μg OVA in 50 μL 0.9% NaCl.
Evaluation of body temperature by handheld thermometer
As a readout for anaphylactic reactions, rectal temperature was measured before and 20 min after the challenge. During the reaction time, physical activity was monitored in a blinded fashion.
Real-time temperature and physical activity measurements
The anaphylaxis cage allows two-dimensional heat images to be captured by a heat image camera, calculating center-of-heat points for a sequence of heat images and the distance between the center-of-heat points of sequential heat images. The distances between sequential center-of-heat points in each subenclosure can be used to determine a parameter related to the physical activity of the mouse. Four mice are placed into the cage and are recorded simultaneously. The time difference between two sequential images is 0.25 s, hence giving four images per second. The center-of-heat points are connected with a polyline calculating the distance travelled against the elapsed time.
Statistical analysis
Statistical comparison of antibody and cytokine levels between the differently immunized groups were analyzed by means of Mann–Whitney U-test, using PASW Statistics for Windows v18 (SPSS, Inc., Chicago, IL). Differences were considered statistically significant at *p<0.05, **p<0.01, ***p<0.001.
The statistical analysis of the IgE levels (Fig. 5A) was performed using one-way analysis of variance (ANOVA) with Tukey as post-hoc test. Data analysis and graphs were performed using GraphPad Prism6 software (GraphPad, San Diego, CA).
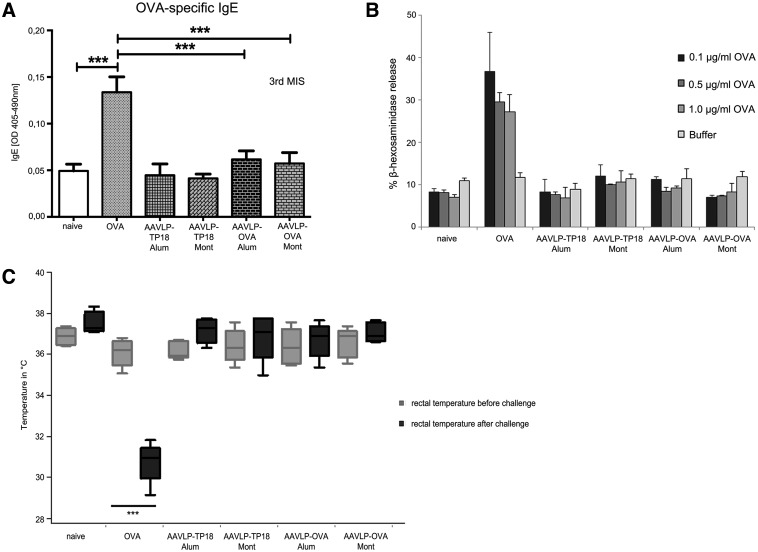
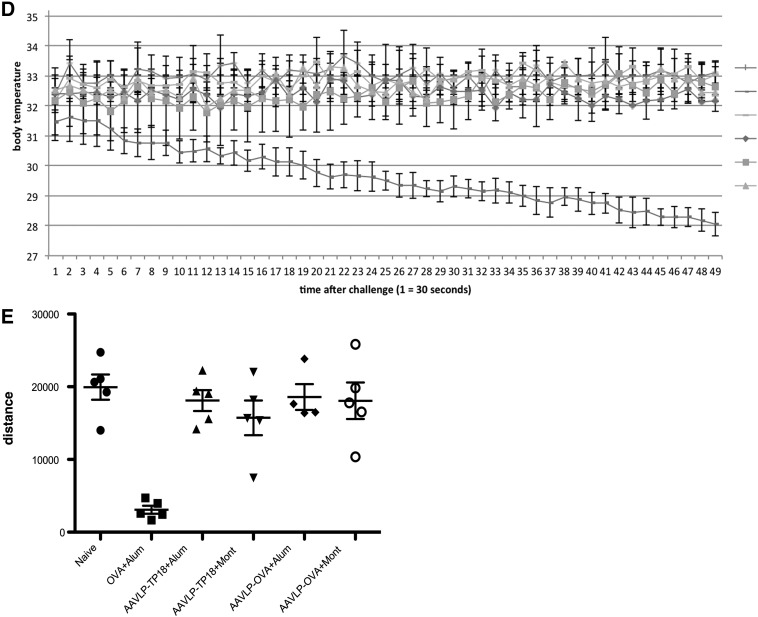
FIG. 5.
Testing IgE induction capacity of AAVLP in vitro and in vivo. (A) OVA was coated on ELISA plates, and single mouse sera of each group was diluted 1:10 for specific IgE detection and incubated. Black columns represent the mean values of x determinations at OD405–490nm values, y-axis. (B) Pooled sera were used for passive sensitization of RBL cells. After triggering with serially diluted OVA (x-axis), specific β–hexosaminidase release was recorded. (C) After completing the immunization with the different antigens (x-axis), all mouse groups were challenged i.v. with OVA. y-Axis indicates the body temperature evaluated by handheld rectal temperature measurements, before and 20 min after the OVA antigen challenge. The only group where significant temperature drop was detected in all the mice was immunized with OVA. In contrast, none of the AAVLP groups showed any signs of systemic anaphylaxis. (D) The anaphylactic reaction was recorded with real-time body temperature measurements using a heat-sensitive camera system and cage for simultaneous measurements of four mice. Each time point on the x-axis represents four seconds. The y-axis represents the body temperature. Screen pictures during the test are shown in the left panel. (E) Body activity was recorded as a further measure in the anaphylaxis cage using the data from the hottest point on the animals. Additionally, infrared laser beams in the cage allowed rising temperatures to be recorded. Distances moved are shown in an active versus anaphylactic mouse. The distance data are shown on the graph with mean±SD for different groups of mice. ***p<0.001.
Results
Construction and antigenicity of AAVLP-OVA
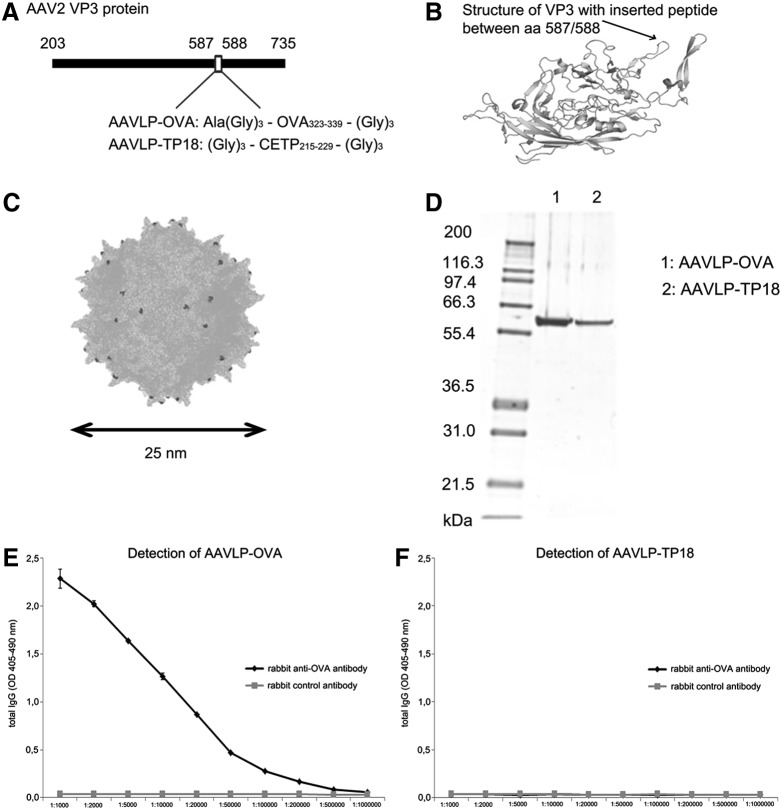
AAVLPs displaying 60 copies of a 16 amino acid OVA B-cell epitope at the capsid surface (Fig. 1A–C) were produced in HEK293 cells by transfection of plasmid DNA, followed by FPLC purification, and finally analyzed via SDS-PAGE for their purity. The recombinant VP3 proteins expressing either the OVA or TP18 epitopes migrate to 59 kDa on SDS-PAGE (Fig. 1D).
FIG. 1.
Design of the adeno-associated virus-like particle (AAVLP) display system and antigenicity testing of the AAVLPs. (A) OVA and control peptides were inserted between positions 587/588 of the AAV2 VP3 capsid protein. (B) Three-dimensional structure of VP3 capsid protein with the inserted peptide (as indicated by arrow). The image was created with The PyMOL Molecular Graphics System v1.3. (C) The AAVLP is composed of 60 copies of the VP3 capsid protein, also representing the inserted peptide (dark shapes) on the surface. The image was created with the PyMOL Molecular Graphic System v1.3. (D) AAVLPs were produced in HEK 293 cells by transfection of plasmid DNA, purified by FPLC, analyzed by SDS-PAGE for purity. Antigenicity was tested with enzyme-linked immunosorbent assay (ELISA). Therefore, AAVLP-OVA (E) and control AAVLP-TP18 (F) particles were coated on ELISA plates and incubated with polyclonal rabbit anti-OVA antibody or control rabbit antibody with irrelevant specificity, diluted as indicated at the x-axis. The y-axis shows the OD values. OD, optical density.
Next, we aimed to verify the surface accessibility and correct antigenic folding of the displayed peptides. AAVLP-OVA and, for negative control, AAVLP-TP18, presenting a peptide (rabbit CETP215–229) of the cholesterol-ester transfer protein 18 (TP-18) on the surface, were coated on ELISA plates and tested with a commercially available polyclonal rabbit anti-OVA antibody. As illustrated in Figure 1E and F, AAVLP-OVA were recognized in a highly specific manner, up to the dilution of 1:200,000, while the unspecific peptide remained negative.
Immunogenicity of AAVLPs by enzyme-linked immunosorbent assay
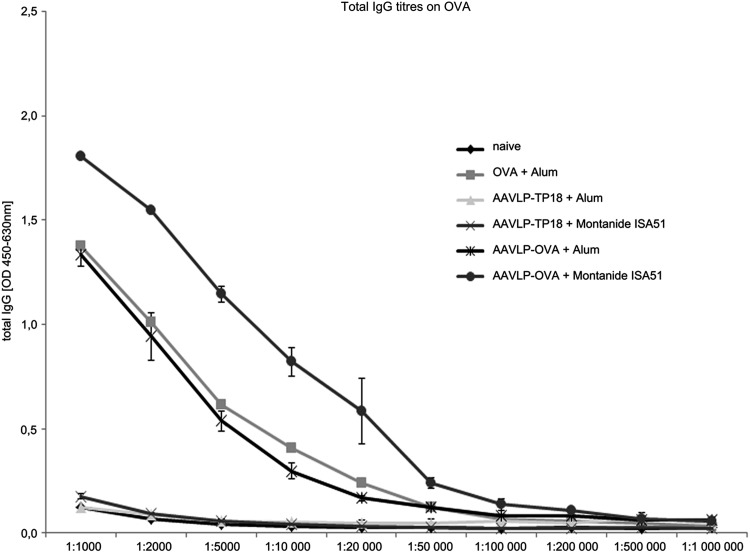
To examine the immunogenicity of AAVLPs, BALB/c mice were immunized with AAVLP-OVA or AAVLP-TP18, comparing aluminum hydroxide [Al(OH)3] versus Montanide ISA 51 VG adjuvants. The serial dilution of immune sera, illustrated in Figure 2, indicated that anti-OVA titers at 1:50,000 could be achieved in the two AAVLP-OVA groups. The highest specific antibody titer was detected in the group immunized with AAVLP-OVA when using Montanide, even higher than using the original allergen OVA.
FIG. 2.
Specificity and titer determination of mouse IgG induced by AAVLP vaccination. After immunization with OVA or different AAVLP/adjuvant combinations, third immune serum pools of each mouse group were tested, serially diluted 1:1,000–1:1,000,000 for total IgG titer determination by ELISA, using OVA for coating. Bound antibodies were detected by HRP-labeled subclass-specific antibodies.
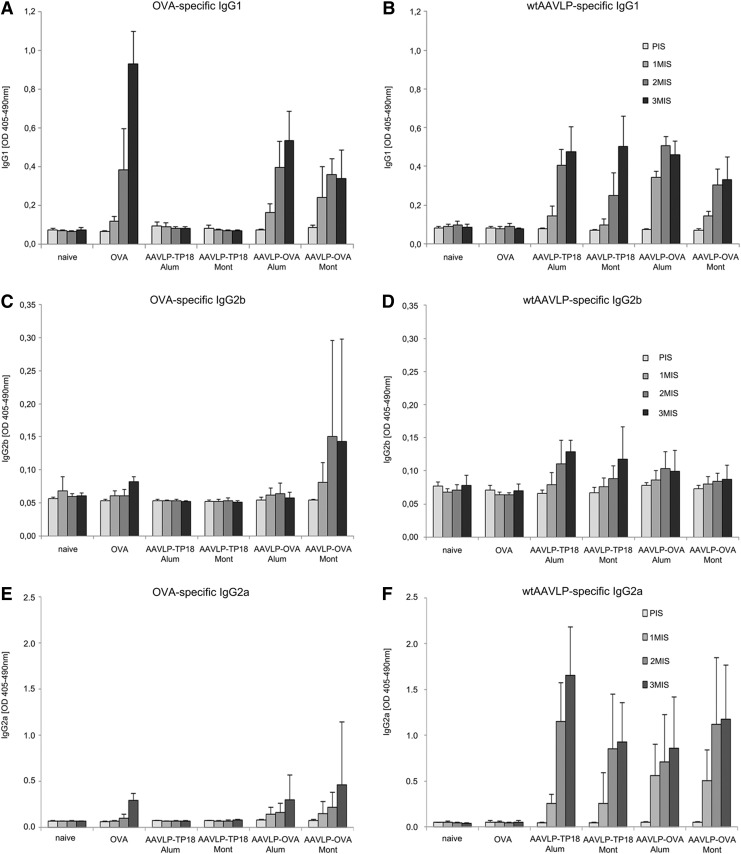
In accordance with the literature, our subsequent subclass analysis in Figure 3 indicated that the IgG response was mostly composed of IgG1, independent of the used adjuvant. Beside the OVA-specific IgG1 in the AAVLP-OVA groups, only anticarrier immune responses could be detected in all AAVLP immunized groups. The OVA-specific IgG levels achieved with Montanide were only marginally lower than using Alum, and IgG2a was only induced at very low levels. However, due to strong inflammatory granuloma formation at the injection site with Montanide, we concentrated on Alum for further studies.
FIG. 3.
Subclass determination in single sera of immunized mice. ELISA plates were coated either with OVA (A), (C), and (E) to determine antigen-specific response or with wtAAVLP (B), (D), and (F) for the evaluation of anticarrier response. Mouse sera (PIS-3MIS) were diluted 1:100 for subsequent antibody subclass detection (IgG1, IgG2b, and IgG2a). PIS, pre-immune sera; 1MIS, first immune sera; 2MIS, second immune sera; 3MIS, third immune sera. x-Axis indicates the antigen used for vaccination; y-axis the mean OD405–490nm values.
Internalization experiment of AAVLPs with different cell types
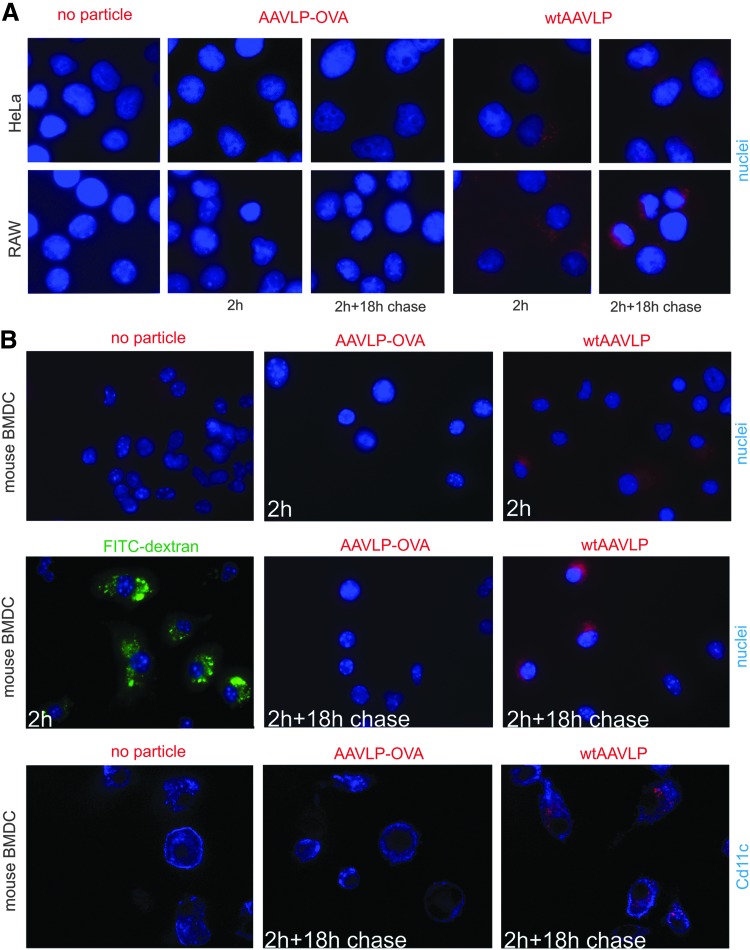
The uptake capacity of wtAAVLP and AAVLP-OVA into different cell types was compared by cellular internalization studies (Fig. 4) by immunofluorescence microscopy. After 2 h of incubation with the cells, wtAAVLP associated with the heparin sulfate proteoglycan (HSPG)-rich human HeLa epithelial cells, as well as with mouse RAW264.7 macrophages. In both cell lines, internalized wtAAVLPs were still detectable by A20 antibody after an 18 h chase in medium devoid of AAVLP, indicating that intact capsids were still present at this late time point (Fig. 4A), presumably in the Golgi. In contrast, AAVLP-OVA cell association after 2 h of incubation with the cells remained below the detection limit with both cell lines. Similar results were obtained with mouse bone marrow-derived dendritic cells (BMDC; Fig. 4B). While FITC-dextran and wtAAVLPs were internalized into the majority of cells in the course of 2 h, AAVLP-OVA internalization remained below the detection limit after 2 h of incubation with mouse BMDC and after an additional 18 h of confocal microscopy (upper and middle panels). The intracellular localization of wtAAVLPs in BMDC after co-incubation and chase was confirmed by confocal microscopy of anti-CD11c-labeled cells (lower panel). These data reaffirm that the insertion of the OVA-peptide into the capsid at amino acid position 587 inhibits its binding to HSPG and, as a consequence, its uptake into different cell types, including epithelial cells, macrophages, and dendritic cells.
FIG. 4.
Comparison of the uptake of AAVLP-OVA and wtAAVLP by different cell types by immunofluorescence microscopy. Cells grown on glass cover slips were incubated for 2 h with AAVLP-OVA, wtAAVLP, or FITC-dextran, followed by incubation for 18 h after removal of the AAVLPs. The cells were then fixed and processed for immunofluorescence microscopy. (A) AAVLP uptake into HeLa epithelial cells and RAW264.7 macrophages. The localization of the VLPs in close vicinity to the nucleus in both cell lines after 18 h of chase incubation is indicative of cellular uptake of the virus particles (compared to virus localization at 2 h after VLP addition). (B) Uptake of AAVLPs or FITC-dextran into mouse bone marrow–derived DC. In the last row confocal images of anti-CD11c-labeled cells are shown (one central layer), while the other images are non-confocal and the nuclei are labeled. The presented images are representative for the results of at least two independent experiments. The slight red staining seen in one cell in the anti-CD11c-labeled AAVLP-OVA-treated DC is most probably background staining, as a similar staining was also seen in some untreated DC. DC, dendritic cell.
Interestingly, despite this undetectable uptake into important antigen-presenting cell types (except B-lymphocytes), the in vivo immunization experiments above clearly showed that AAVLP-OVA were highly immunogenic and able to induce significant OVA-specific antibody levels.
Safety of AAVLPs: testing their anaphylactic and sensitizing capacity
It was our aim to investigate the ability of peptide-displaying AAVLPs for the formation of anaphylactogenic IgE antibody playing an important role in hypersensitivity reactions. Among all sera, only the i.p. OVA sensitized group, not any of the AAVLP-treated groups, showed increased levels of OVA-specific IgE (Fig. 5A). Accordingly, using these sera for passive sensitization of FcɛRI on rat basophil leukemia (RBL) cells showed that OVA can trigger the release of β-hexosaminidase only from the OVA-immunized group, not from the AAVLP-OVA peptide-treated group (Fig. 5B).
We considered that even at low levels of specific IgE, the injection of the specific antigen may trigger a systemic anaphylactic reaction. We addressed this question in an in vivo mouse model of anaphylaxis, where a systemic anaphylactic reaction typically leads to a drop in body temperature. The average rectal temperature of an active, healthy mouse is around 36.4°C. When we challenged all groups of immunized mice with the native OVA antigen i.v., a severe temperature drop (from 36.5°C to 30.5°C) was measured by real-time measurement of body temperature in four animals at the same time in an anaphylaxis cage. In this system, body temperature is recorded by a heat-imaging camera, recording the temperature at the hottest point of the monitored organism, which is usually the head in a healthy mouse. In Figure 5D, the resulting curves show that an immediate and continuous drop of temperature could be recorded in all animals immunized and challenged with OVA. The data were verified with a handheld rectal thermometer (Figure 5C). In contrast, mice in all other groups, including AAVLP-OVA-immunized groups, maintained their normal body temperature upon OVA challenge, indicating the absence of anaphylactogenic reactions, although moderate levels of specific IgG1 were detectable in these groups.
Systemic anaphylaxis in OVA/OVA-challenged mice was also associated with reduced physical activity. The anaphylaxis cage allows the parallel recording of the distances moved by collecting data points from the hottest point of the animal. Figure 5E shows the distances moved for each group (M±SD). Again, only the group sensitized and challenged with OVA showed significant impairment of the physical activity. Taken together, the AAVLP vaccine did not induce specific IgE-mediated allergy or anaphylaxis, even when the Th2 adjuvant Alum was used for immunization.
Discussion
Type I allergies belong to the most prevalent diseases worldwide. Around 10–30% of the population is affected by allergy in industrialized countries. Therefore, intensive research is going on for the optimization of allergen immunotherapies (3).
An allergy vaccine was recently constructed based on rhinovirus protein VP1 and a B-cell epitope derived from grass pollen allergen Phl p 1. The dual advantage of viral carriers for immunotherapy for type I allergy is the induction of protective antibodies both against the allergen but also against the viral infection (4,5).
Searching for improved display systems, we investigated AAVLPs as a novel carrier platform for peptides or mimotopes derived from allergenic molecules. We anticipated an improved peptide display and conformation in context with the AAVLP scaffold.
In our experiments, AAVLPs were assembled from 60 VP3 units per capsid. AAVLP-OVA323–339 and, for control purpose, AAVLP-TP18 (Rabbit CETP215–229) were generated, where selected peptides were inserted between amino acids 587 and 588 of VP3, and thus displayed 60 times on the surface (Fig. 1A–C). One of the advantages of this system is that with modifications, 3D structures of the peptide can be varied, and thus the conditions for specificity and immunogenicity can be optimized. The specificity of AAVLP-OVA and AAVLP-TP18 was controlled with a rabbit anti-OVA antibody (Fig. 1E and F), confirming the presentation of the OVA peptide on the particle surface.
On the other hand, we suspected that this insertion site could interfere with the binding to antigen-presenting cells, and inhibit the uptake of the particles in the absence of immunoglobulins or B-cells. The internalization studies indeed showed that a peptide insertion after position 587 severely inhibited the uptake of AAVLPs into important antigen-presenting cells such as BMDCs and macrophages as well as into HeLa epithelial cells, whereas wtAAVLPs were quickly internalized (Fig. 4). Nevertheless, immunizations with the constructs rendered a high and specific antibody response to OVA. We propose that preformed anti-OVA or anti-AAVLP antibodies, or the B-lymphocytes via membrane immunoglobulins mediate uptake and antigen presentation in this setting. Subsequently, T-bystander cells might provide the cytokine cocktail for an isotype switch to IgG1. A similar phenomenon could be observed in a previous study with a “mimotope” (i.e. B-cell epitope peptide mimetic) display system (19). Antibodies against the AAV capsid have been shown to enhance virus uptake into human monocytic cell lines (12). In this respect, the existing AAV-seropositivity in the population may be beneficial for the efficacy of the vaccine.
Generally, carrier-specific antibodies do not necessarily interfere with the induction of a protective immune response against the presented epitope, as could be shown for VLPs based on the hepatitis B virus core antigen (16). Moreover, it was already shown, that non-heparin-binding particles might reach the lymph nodes where dendritic cells reside in a high concentration with a substantial fraction of them being phenotypically and functionally immature and able to process new antigen (21).
In the immunization studies, we compared the effect of Th1 (Montanide ISA 51) versus Th2 adjuvant (Aluminum hydroxide, Alum). Alum is administered mostly in the context of infectious disease and allergy (8), while Montanide is used in vaccines against HIV and cancer, as well as infectious diseases (6,17,18). Our hypothesis was that the Th1 adjuvant would counterbalance an ongoing Th2 response. However, Montanide produced several local side effects, whereas the Th2 adjuvant Alum performed as desired in our model and prevented the formation of anaphylactogenic antibodies equally well. Specific antibodies to the empty wtAAVLP carrier were observed in all AAVLP-immunized groups, regardless of the adjuvant. Anticarrier-specific IgG1 and IgG2b increased during immunizations in all particle-immunized groups (Fig. 3), again independent of the chosen adjuvant.
With respect to safety, Figure 5A and B shows that no OVA-specific IgE was formed in any of the AAVLP-immunized mouse groups, and that accordingly the in vitro rat basophilic leukemia (RBL) cell assay remained negative. Figure 5C and D shows by two independent methods that AAVLP-OVA immunized groups resisted even an intravenous challenge with OVA, whereas OVA-immunized mice reacted anaphylactic with an immediate drop in body temperature associated with a significantly reduced walking distance (Fig. 5E). These results prove that the AAVLP-displayed vaccines induce the desired specific immune response, but do not nourish undesired hypersensitivity against the vaccine or the natural allergen.
Taken together, in the present study, we used ovalbumin as a model allergen and AAVLP-OVA as a paradigm of an allergy vaccine. Our results demonstrate that immunization with B-cell epitopes displayed by AAVLP induced high titers of allergen-specific IgG1, low levels of specific IgE, and had no anaphylactogenic potency in vitro and in vivo. Hence, we propose AAVLPs for the construction of B-cell epitope vaccines, being especially suited for allergy shots.
Acknowledgments
We acknowledge the support by MediGene, Martinsried, Germany, and by Biomed Int. R+D, Vienna, Austria. The study was also supported by the Austrian Science Fund (FWF) grant DK W 1205-B09 (CCHD: CS, JS), and in part by SFB-F4606-B19.
Author Disclosure Statement
The basic technology of AAVLP is covered by patent WO 2008/145401 A2 moreover the number WO 2013/037961 holds the patent for the use of AAVLPs for vaccination in cancer therapy. The method “anaphylaxis cage” is covered by patent application EP13159620.8 held by Biomed International R+D, Vienna, Austria. The study was financed by MediGene, Martinsried, Germany and Biomed International R+D, Vienna, Austria.
The authors have not received funding or sponsorship that impacts on this study. There are no conflicts of interest.
Erika Jensen-Jarolim and Ingo Flaschberger are inventors on the patent: Non-invasive Temperature and Physical Activity Measurement of Animals. Priority date March 11, 2013: Appl. Number EP13158620.8), 2013, and Erika Jensen-Jarolim is shareholder in Biomed Int. R+D GmbH; Vienna, Austria. MediGene, Martinsried, Germany, holds patents on the AAVLP technology for vaccines.
References
- 1.Boucas J, Lux K, Huber A, et al. Engineering adeno-associated virus serotype 2-based targeting vectors using a new insertion site-position 453-and single point mutations. J Gene Med 2009;11:1103–1113 [DOI] [PubMed] [Google Scholar]
- 2.Chen CL, Jensen RL, Schnepp BC, et al. Molecular characterization of adeno-associated viruses infecting children. J Virol 2005;79:14781–14792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douwes J, Brooks C, van Dalen C, et al. Importance of allergy in asthma: an epidemiologic perspective. Curr Allergy Asthma Rep 2011;11:434–444 [DOI] [PubMed] [Google Scholar]
- 4.Edlmayr J, Niespodziana K, Focke-Tejkl M, et al. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. Curr Top Microbiol Immunol 2011;352:121–140 [DOI] [PubMed] [Google Scholar]
- 5.Edlmayr J, Niespodziana K, Linhart B, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol 2009;182:6298–6306 [DOI] [PubMed] [Google Scholar]
- 6.Fox CB, Baldwin SL, Vedvick TS, et al. Effects on immunogenicity by formulations of emulsion-based adjuvants for malaria vaccines. Clin Vaccine Immunol 2012;19:1633–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henckaerts E, and Linden RM. Adeno-associated virus: a key to the human genome? Future Virol 2010;5:555–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogenesch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol 2012;3: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JS, Li C, DiPrimio N, et al. Mutagenesis of adeno-associated virus type 2 capsid protein VP1 uncovers new roles for basic amino acids in trafficking and cell-specific transduction. J Virol 2010;84:8888–8902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Zhang L, Johnson JS, et al. Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium. Mol Ther 2009;17:2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen D, Cantwell ER, O'Brien T, et al. Adeno-associated virus serotype 2 induces cell-mediated immune responses directed against multiple epitopes of the capsid protein VP1. J Gen Virol 2009;90:2622–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori S, Takeuchi T, and Kanda T. Antibody-dependent enhancement of adeno-associated virus infection of human monocytic cell lines. Virology 2008;375:141–147 [DOI] [PubMed] [Google Scholar]
- 13.Nieto K, Weghofer M, Sehr P, et al. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PloS One 2012;7:e39741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perabo L, Goldnau D, White K, et al. Heparan sulfate proteoglycan binding properties of adeno-associated virus retargeting mutants and consequences for their in vivo tropism. J Virol 2006;80:7265–7269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renz H, Bradley K, Larsen GL, et al. Comparison of the allergenicity of ovalbumin and ovalbumin peptide 323–339. Differential expansion of V beta-expressing T cell populations. J Immunol 1993;151:7206–7213 [PubMed] [Google Scholar]
- 16.Ruedl C, Schwarz K, Jegerlehner A, et al. Virus-like particles as carriers for T-cell epitopes: limited inhibition of T-cell priming by carrier-specific antibodies. J Virol 2005;79:717–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabbatini P, Tsuji T, Ferran L, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 2012;18:6497–6508 [DOI] [PubMed] [Google Scholar]
- 18.Sadat SM, Zabihollahi R, Aghasadeghi MR, et al. Application of SCR priming VLP boosting as a novel vaccination strategy against HIV-1. Curr HIV Res 2011;9:140–147 [DOI] [PubMed] [Google Scholar]
- 19.Scholl I, Wiedermann U, Forster-Waldl E, et al. Phage-displayed Bet mim 1, a mimotope of the major birch pollen allergen Bet v 1, induces B cell responses to the natural antigen using bystander T cell help. Clin Exper Allergy 2002;32:1583–1588 [DOI] [PubMed] [Google Scholar]
- 20.Sun LZ, Elsayed S, Aasen TB, et al. Comparison between ovalbumin and ovalbumin peptide 323–339 responses in allergic mice: humoral and cellular aspects. Scand J Immunol 2010;71:329–335 [DOI] [PubMed] [Google Scholar]
- 21.Wilson NS, El-Sukkari D, Belz GT, et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood 2003;102:2187–2194 [DOI] [PubMed] [Google Scholar]
- 22.Wobus CE, Hugle-Dorr B, Girod A, et al. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J Virol 2000;74:9281–9293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaiss AK, and Muruve DA. Immune responses to adeno-associated virus vectors. Curr Gene Ther 2005;5:323–331 [DOI] [PubMed] [Google Scholar]