Abstract
Data suggest women are more sensitive to the lipolytic action of epinephrine compared with men while maintaining similar glucoregulatory effects (Horton et al. J Appl Physiol 107: 200–210, 2009). This study aimed to determine the specific adrenergic receptor(s) that may mediate these sex differences. Lean women (n = 14) and men (n = 16) were studied on 4 nonconsecutive days during the following treatment infusions: saline (S: control), epinephrine [E: mixed β-adrenergic (lipolytic) and α2-adrenergic (antilipolytic) stimulation], epinephrine + phentolamine (E + P: mixed β-adrenergic stimulation only), and terbutaline (T: selective β2-adrenergic stimulation). Tracer infusions of glycerol, palmitate, and glucose were administered to determine systemic lipolysis, free fatty acid (FFA) release, and glucose turnover, respectively. Following basal measurements, substrate and hormone concentrations were measured in all subjects over 90 min of treatment and tracer infusion. Women had greater increases in glycerol and FFA concentrations with all three hormone infusions compared with men (P < 0.01). Glycerol and palmitate rate of appearance (Ra) and rate of disappearance (Rd) per kilogram body weight were greater with E infusion in women compared with men (P < 0.05), whereas no sex differences were observed with other treatments. Glucose concentration and kinetics were not different between sexes with any infusion. In conclusion, these data support the hypothesis that the greater rate of lipolysis in women with infusion of E was likely due to lesser α2 antilipolytic activation. These findings may help explain why women have greater lipolysis and fat oxidation during exercise, a time when epinephrine concentration is elevated.
Keywords: epinephrine, adrenergic receptors, lipolysis, sex differences, glucose kinetics
men and women differ in their fat metabolism during exercise (2, 10, 19, 27, 31), and this may be partially due to sex differences in the adrenergic stimulation of lipolysis (5, 19). Delineating the fundamental reasons for these sex differences will help our understanding of what constitutes “normal” vs. “abnormal” lipid metabolism in men relative to women. This is important as disorders in lipid metabolism form the cornerstone of metabolic diseases such as obesity, diabetes, and cardiovascular disease (20). If the normal physiological regulation of substrate and especially lipid metabolism differs between lean, healthy men and women, then this has implications for what represents aberrant metabolism in men vs. women. Such information may help identify what intervention and preventive strategies are most effective in disease prevention and whether the magnitude of effectiveness differs between the sexes.
It has been shown that during exercise of the same relative intensity, women derive proportionally more of their total energy expended from fat oxidation, whereas men derive proportionally more energy from carbohydrate oxidation (2, 5, 19, 27, 37). This is observed despite the fact that during exercise, men have higher catecholamine concentrations than women (19). When similar levels of catecholamines are infused, mimicking the levels observed during moderate-intensity exercise, women exhibit a greater increase in systemic levels of glycerol and free fatty acids (FFA) compared with men and significantly higher rates of glycerol turnover (17). These data imply that catecholamines are more effective at stimulating lipolysis in women compared with men during either exercise or controlled catecholamine infusions. The question arises as to how this sex difference in catecholamine effectiveness is mediated, and what the implications of this sex difference are.
Catecholamines stimulate lipolysis via the activation of β-adrenergic receptors in target tissues, mainly adipose tissue and muscle (23, 39). The catecholamines, epinephrine (E) and norepinephrine (NE), bind to both the lipolytic β-adrenergic receptors and the antilipolytic α2-adrenergic receptors (24). The balance between activation of the α2- and β-receptors determines the overall level of lipolysis within a tissue (24). The systemic (whole body) response to catecholamines represents the summation of lipolytic activity from predominantly peripheral tissues (subcutaneous adipose tissue beds and skeletal muscle) with little contribution from visceral adipose tissue (20, 28). It also represents the combined effects of adrenergic stimulation or blockade on tissue lipolysis and blood flow. The whole body lipolytic response to α2- and/or β-adrenergic stimulation has not been systematically addressed in women compared with men. Differences in the lipolytic effectiveness of catecholamines may be related to differences in the balance of the response between different adrenergic receptors under conditions of elevated catecholamines. If the “normal” lipolytic response differs between lean, healthy men and women, then this may provide insight for sex-specific treatment and prevention strategies aimed at normalizing and/or optimizing lipid metabolism, as the manipulation of lipolysis has therapeutic potential in metabolic disorders that are associated with obesity. This study is the first to directly determine the adrenergic regulation of systemic lipolysis in men vs. women, while avoiding confounding factors such as variations in activity level and diet between subjects, as well as controlling for hormonal status in women.
With respect to systemic glycerol and FFA concentrations, systemic glycerol and FFA turnover rates, and relative lipid oxidation, it was hypothesized that 1) women would have a significantly greater increase in these parameters in response to β-adrenergic stimulation due mainly to a lower α2-adrenergic response compared with men, and 2) men would have a significantly greater increase in these parameters in response to E infusion with α2-adrenergic blockade compared with women.
MATERIALS AND METHODS
Subjects
Normal weight, healthy women and men (20–45 yr) were recruited from the University of Colorado and surrounding community. Women were required to be eumenorrheic and not using any form of hormonal contraceptive. Medical exclusions included past or present history of cardiovascular disease, high blood pressure, diabetes, and any hormonal imbalance or metabolic abnormality. Highly active individuals (≥30 min of mild to moderate intensity exercise/day) were also excluded. The study protocol was approved by the Colorado Multiple Institutional Review Board. All subjects read and signed an informed consent form prior to admission into the study.
Preliminary assessments.
A health and physical examination was performed along with the measurement of resting metabolic rate (RMR) and determination of body composition as previously described (17). Resting metabolic rate was used to determine energy intake of subjects during the period of prestudy control diet.
Prestudy diet and exercise control.
Subjects were fed a controlled diet for 2 days prior to each study day as previously described (17). All food was prepared by the metabolic kitchen of the Clinical and Translational Research Center (CTRC) at the University of Colorado, School of Medicine, with a diet composition of 25% fat, 15% protein, and 60% carbohydrate and an initial energy intake calculated at 1.6–1.75 × RMR based on subjects self-reported habitual activity level. Subjects were allowed to follow their usual activity routine for the first day of the diet and on the second day they refrained from any planned exercise. In women and men the average energy intake was 50 and 48 kcal/kg FFM, respectively.
Study Days
The evening before each test day, study participants were admitted to the inpatient unit of the CTRC and consumed their evening meal between 1900 and 2000. Subjects slept on the unit and remained fasted until the end of the study the following day. Women were studied in the follicular phase of the menstrual cycle (measured progesterone ≤2.5 ng/ml). Treatment order was randomly assigned for each subject.
Infusion and sampling procedures.
Between 0645 and 0730 of the study day an infusion intravenous catheter was placed in an antecubital vein for delivery of stable isotopes and the test infusion. On the contralateral side a sampling catheter was placed retrograde fashion into a dorsal hand, or wrist vein, for obtaining arterialized blood samples using the heated hand technique (26). Initial blood samples were drawn for determination of background enrichment of isotopes followed by a primed (2 μmol/kg), constant (0.09 μmol·kg−1·min−1) infusion of [1,1,2,2,3,-2H5]glycerol and a primed (17.6 μmol/kg), constant (0.2 μmol·kg−1·min−1) infusion of [6,6-2H2]glucose (Cambridge Isotopes, Andover, MA). One hour following the start of the glycerol and glucose tracer infusions an infusion of [1-13C]palmitate (Cambridge Isotopes) bound to human albumin (0.08 μmol·kg−1·min−1 continuous infusion of palmitate) began. All infusates were prepared by the Research Pharmacist at University Hospital, University of Colorado Anschutz Medical Campus, and were tested for sterility and pyrogenicity prior to use. The palmitate infusate was combined with 250 ml of 5% human albumin the morning of each study, as previously delineated (18). Blood samples were taken over the final 30 min of a 120-min baseline phase (t = 90, 100, 110, and 120 min) for measurement of basal substrate kinetics and concentrations. The test infusion then commenced at t = 130 min and continued for 90 min up to 220 min. Test infusions were diluted and delivered in 0.9% saline to give a total volume of 50 ml. Solutions containing epinephrine included 1 mg/ml ascorbic acid, to prevent oxidation. Infusion rate of fluid was based on the hormone concentration(s) and body weight of subject. Epinephrine alone (E) was infused at a rate of 8 ng·kg−1·min−1, epinephrine + phentolamine (E + P) at 8 ng·kg−1·min−1 and 7.0 μg·kg−1·min−1, respectively, terbutaline (T) at 14 ng·kg−1·min−1, and saline (S) at the same rate as the E infusion. The infusion rate of E was the same as that previously used (17) and elevates circulating epinephrine levels to those observed during moderate exercise and results in significantly greater lipolysis in women compared with men (17). The infusion rate of T was selected to give a similar degree of metabolic stimulation as observed with the dose of E infused, but not to result in significant changes in heart rate (HR), blood pressure (BP), and insulin (14, 34). The infusion rate of phentolamine was selected based on previous studies that have shown this dose blocks the α-adrenergic receptors (6, 16, 32) without significantly changing insulin or glucose concentrations or glucose kinetics. At the onset of the treatments the infusion rate of the glycerol and palmitate was increased to 1.3 × basal in an attempt to avoid large fluctuations in tracer enrichment due to increased substrate turnover. Blood samples were drawn at t = 140, 150, 160, 170, 180, 190, 200, 210, and 220 min during treatments for sample as described below.
Determination of circulating hormone and substrate levels.
Measurements of glycerol, palmitate, total FFA, and glucose were made on all blood samples. Catecholamines (epinephrine and norepinephrine) and insulin were measured on samples drawn at 100 and 120 min of baseline and at 150, 170, 190, and 210 min of the test infusion. Glucagon and cortisol levels were measured at baseline (t= 100) and twice during the infusions (t = 170 and 210). Testosterone, estradiol and progesterone were measured on baseline samples (t0). Details of sample collection and processing for tracer enrichment and plasma substrate concentrations have been previously described as have methods for catecholamine, glucagon, progesterone, and estradiol collection and analysis (17). Within subjects, samples from each study day were run in the same batch.
Blood pressure and heart rate measurement.
Heart rate and blood pressure were monitored by an automatic blood pressure cuff. Measurements were made immediately after blood draws using the sampling arm.
Respiratory gas exchange.
Indirect calorimetry (Sensormedics 2900, Sensormedics, Yorba Linda, CA) (17) was used to measure respiratory gas exchange at baseline (60–90 min) and during adrenergic agonist/antagonist infusions (135–155, 165–185, and 195–215 min). Gas exchange data were used, along with urinary nitrogen excretion, to estimate metabolic rate and nonprotein respiratory exchange ratio as previously described (17). Urine was collected over the entire study period for each trial, and urinary nitrogen excretion was averaged over the entire time period.
Determination of glycerol and glucose isotope enrichment and concentration.
For the pentacetate derivative, samples (100 μl for glucose or 50 μl for glycerol) were first spiked with 25 μl of 500 μg/ml internal standard (IS) (12.5 μg) [U-13C]glucose or 20 μl of 1 μg/ml IS (10.5 μmol/l) [1,2,3,-13C3]glycerol, then deproteinized with 1 ml iced methanol, and spun at 10,000 g for 5 min. The supernatant was dried completely under N2 at 65°C. Samples were then derivatized using 100 μl of acetic anhydride-pyridine solution (2:1), capped, and heated for 30 min at 100°C. Samples were dried under N2 completely, then reconstituted with 100 μl of ethyl acetate, vortexed, and transferred to GC-MS vials with inserts for analysis. Glucose standards from 10–200 mg/dl were prepared and spiked with 25 μl of 500 μg/ml IS (12.5 μg) [U-13C]glucose. Glucose concentration was determined by comparing the known ratio of glucose:[U-13C]glucose to the measured area ratio of 331:337. Enrichment was of glucose was determined by the 331:333 ratio. Glycerol standards were prepared from 54 to 217 μmol/l and spiked with 20 μl of 10.5 μmol/l of the internal standard [1,2,3,-13C3]glycerol. Glycerol concentration was determined by comparing the known ratio of glycerol:[1,2,3,-13C3]glycerol to the measured area ratio of 159:162. Enrichment of glycerol was determined by the 164:159 ratio.
Glucose and glycerol enrichments were measured via gas chromatography-mass spectrometry (GC-MS; GC Model 7890 and 5975C, Agilent). Injector temperature of the GC-MS was set at 250°C and initial oven temperature was set at 110°C. The column used was an Agilent DB-5MS 0.25 mm × 30 m with a 0.25-μm film thickness. Oven temperature was increased 35°C/min (glucose) or 45°C/min (glycerol) until a final temperature of 290°C was achieved. Helium was used as a carrier gas with a 65:1 ml/min split injection ratio; transfer line temperature was set at 280–290°C, source temperature at 280°C, and quadruple temperature at 150°C, with methane chemical ionization (41).
Determination of palmitate enrichment and concentration.
These measurements were made using gas chromatography-mass spectrometry (GC-MS; GC Model 6890 and 5975C, Agilent, Palo Alto, CA) using the method of Patterson and Wolfe (29). Plasma samples were spiked with 100 μl heptadecanoate (∼200 μM) to determine palmitate concentration. The methyl ester derivative of palmitate and internal standard were generated as follows: plasma (250 μl) was extracted with 3 ml hexane and the hexane layer removed by evaporation with nitrogen gas at 60°C. Samples were derivatized to the methyl esters using 250 μl of iodomethane-dichloromethane solution (1:10 vol:vol) at room temperature. After vortexing for 10 min, methyl esters were extracted with 3 ml hexane and the hexane layer transferred into a new tube. Hexane was evaporated to dryness and then another 100 μl hexane added. The derivatized sample was transferred to GC-MS vials for analysis. Injector temperature of the GC-MS was set at 280°C and initial oven temperature was set at 100°C. The column used was a Phenomenex ZB-1MS 0.25 mm × 30 m with a 1.00-μm film thickness. Oven temperature was increased 30°C/min until a final temperature of 325°C was achieved. Helium was used as the carrier gas; transfer line temperature was set at 280°C, source temperature at 250°C, and quadruple temperature was set at 150°C. Electron ionization was used to monitor selective ions with mass-to-charge ratios of 270 (M + 0 from natural palmitate), 271 (M + 1 from [13C]palmitate), and 284 (heptadecanoate internal standard).
Natural palmitate standards were prepared from 10 to 1,000 μmol/l and spiked with 227 μmol/l of the internal standard heptadecanoate to generate a standard curve for determining palmitate concentration. An enrichment calibration curve was constructed by comparing known ratios of palmitate:[13C]palmitate to the measured area ratio of 270:271. A linear equation obtained from the calibration curve was used to calculate the palmitate enrichment (30). Due to a freezer malfunction that was not detected for a number of days, defrosting compromised the integrity of a number of plasma samples making them unusable for tracer analysis (7 women and 9 men). Hence, tracer analysis was only possible on samples from 7 women and 7 men. For glucose and glycerol turnover calculations, concentrations measured by GC-MS were used. Substrate concentrations measured by GC-MS and enzymatic analysis closely paralleled each other; hence, for consistency's sake, statistical analysis of concentration data used values from enzymatic analysis.
Calculations.
The non-steady-state equation was used to calculate substrate turnover
where Ra is rate of appearance of tracee (μmol/min), F is infusion rate of tracer (μmol/min), pV is effective volume of tracee distribution (230 ml/kg body wt for glycerol, 40 ml/kg body wt for palmitate, and 100 ml/kg body wt for glucose) (33, 36), t1 is time 1 of sampling, t2 is time 2 of sampling, C1 is [tracee] at t1, C2 is [tracee] at t2, E1 is tracer enrichment (tracer:tracee ratio) at t1, E2 is enrichment at t2, and Rd is rate of disappearance.
Data analysis
One-way ANOVA was used to compare subject characteristics. For substrate concentrations (full data set) and kinetics (reduced data set), data were analyzed using a repeated-measures ANOVA. This was used to evaluate differences in the pattern of the time course of response between the sexes on each study day. In this model, time and infusion (S, E, E + P, and T) were included as the repeated within-subject factors, and between-subject factors included sex (male or women). The model evaluated 2-way interactions (time × sex, and time × infusion), as well as any 3-way interaction (time × sex × infusion). Post hoc analyses were performed using Bonferroni's test. In the subjects who had samples run for both tracer and substrates levels, data were unavailable from 2 men on the S test day due to one subject not completing the study and for the other there was a problem with the tracer infusion. One woman did not complete the T study day (tracer data available on other days) as well as one male (substrate levels but no tracer data available from other study days).
For glycerol concentration and kinetics, there was a clear change in the pattern of response from rest to the first 30 min of the hormone infusion (t = 140–160) and then the last 60 min of infusion. Physiologically, this may be explained by the onset of tachyphylaxia of the adrenergic β-receptors about 30 min into the catecholamine infusion. Therefore, data over three time periods was averaged (t = 140–160 min; t = 170–190 min; t = 200–220 min) and further compared between the sexes and study days. There were dynamic changes during these time periods; therefore, the three averages were analyzed separately for the FFA and glucose as well. For catecholamines, insulin, and glucagon, values for the entire 90 min infusion were averaged and compared with average rest values.
In the reduced number of subjects on whom the tracer data were available, hormone and substrate concentrations closely reflected those of the larger group in general and are not presented separately. In this smaller group, data for glycerol and palmitate kinetics were expressed as absolute rates as well as relative to body weight, the more traditional method of data presentation. As adipose tissue is the major site of lipolysis, it could be considered that expressing data relative to fat mass, or statistically covarying for fat mass, may be the best approach for comparing glycerol Ra. This was not necessary, however, as the men and women for whom tracer turnover data were available had identical fat masses, and the difference in body weight was almost entirely due to differences in FFM. For glucose kinetics, data were expressed in terms of body weight and FFM as organs (liver and kidney) are the source of glucose production in the body and lean tissue mass the main sight of glucose disposal.
Results are presented as mean ± standard error of the mean (SE) except in Table 1, where SD is given. Statistical significance was set at P < 0.05. A borderline significance of P < 0.09 is reported for tracer data, where applicable, due to the reduced sample size that restricted the statistical power of the analysis, but suggests consideration of the data as potentially relevant.
Table 1.
Subject characteristics
| Women: n = 14 (n = 7) | Men: n = 16 (n = 7) | |
|---|---|---|
| Age, yr | 32 ± 7 (32 ± 9) | 31 ± 7 (32 ± 8) |
| Body weight, kg | 60.0 ± 7.0a (58.3 ± 6.8) | 77.3 ± 9.3 (77.3 ± 10.9) |
| BMI, kg/m2 | 21.2 ± 2.0b (21.0 ± 2.6) | 23.3 ± 2.2 (23.7 ± 2.4) |
| Body fat, % | 25.3 ± 4.4a (24.5 ± 5.4) | 17.3 ± 4.7 (18.1 ± 4.1) |
| Fat mass, kg | 15.0 ± 3.6 (14.4 ± 4.3) | 13.5 ± 4.5 (14.2 ± 4.2) |
| Fat-free mass, kg | 43.9 ± 4.8a (43.9 ± 4.8) | 63.8 ± 6.9 (63.1 ± 4.2) |
| VAT, cm2* | 29.2 ± 17.1b (45.8 ± 20.4) | 54.2 ± 27.5 (61.4 ± 27.1) |
| Estradiol, pg/ml | 60.2 + 28.4a (58.3 + 32.2) | 29.1 + 12.3 (34.8 ± 7.8) |
| Testosterone, ng/dl | 41.8 ± 15.5a (43.2 ± 13.0) | 544.7 ± 117.4 (546.0 ± 65.5) |
Values are means ± SD. Data for subgroup on whom substrate kinetic measurements were made are given in parentheses. BMI, body mass index; VAT, visceral adipose tissue;
n = 12 (n = 7) women, n = 14 (n = 7) men. Sex difference:
P < 0.0001, bP < 0.01.
RESULTS
A total of 14 women and 16 men took part in the study (Table 1). Subjects were young, lean, and healthy, but not highly trained. As expected, women had a higher percent body fat and lower fat-free mass and body weight than men, but they did not differ in the absolute amount of body fat.
Hormone Concentrations
Table 2 shows the circulating epinephrine and norepinephrine concentrations during each study day. Men and women had similar epinephrine and norepinephrine levels, although there was a significant main effect of sex, with men having slightly higher levels of epinephrine than women (P < 0.05). After 20 min of infusion with the E or E + P treatments, the concentration of epinephrine was between 0 and 30 pg/ml of the final hormone sample (t = 150 min vs. t = 210 min). These differences are within the analytical error range for HPLC analysis and demonstrate a rapidly achieved steady state for the infused hormone levels. By design, circulating epinephrine levels were significantly increased above baseline for the E and E + P infusions (P < 0.0001 both). Although there was no infusion of norepinephrine, circulating norepinephrine significantly increased from baseline with both the E + P and T treatments (P < 0.0001) but not S or E. Infusion of E + P resulted in a significantly greater norepinephrine level relative to all other treatments (P < 0.0001). Across the four treatments, insulin concentration was not significantly different between men and women at baseline (men: 4.1 ± 0.6; women: 5.7 ± 0.6 uU/ml, P = 0.07). Insulin concentration significantly increased with the infusion of E + P and T (time × treatment interaction, P < 0.0001) but did not change with either S or E treatments. For both sexes, average insulin levels with the E + P and T treatments were significantly greater than with S (P < 0.0001 both sexes) or E (P < 0.01 and P < 0.001, for women and men, respectively). Relative to baseline, insulin levels increased on average by 3.9 ± 0.8 and 6.6 ± 0.9 uU/ml in women with the E + P and T treatments, respectively and by 5.7 ± 0.9 and 6.3 ± 0.9 μU/ml in men, respectively. Importantly, this change in insulin concentration was not different between the sexes.
Table 2.
Circulating catecholamine levels at rest and during test infusions for each study day
| Saline |
Epi |
Epi + Phent |
Terb |
|||||
|---|---|---|---|---|---|---|---|---|
| Infusion: | [E], pg/ml | [NE], pg/ml | [E], pg/ml | [NE], pg/ml | [E], pg/ml | [NE], pg/ml | [E], pg/ml | [NE], pg/ml |
| Before infusion | ||||||||
| Women | 23 ± 2 | 124 ± 14 | 25 ± 3 | 136 ± 24 | 27 ± 3 | 139 ± 19 | 26 ± 3 | 134 ± 16 |
| Men* | 31 ± 2 | 139 ± 12 | 32 ± 4 | 165 ± 16 | 35 ± 4 | 151 ± 16 | 26 ± 2 | 151 ± 14 |
| During infusion | ||||||||
| Women | 24 ± 2 | 113 ± 14 | 188 ± 22a | 151 ± 26 | 190 ± 16a | 434 ± 51b | 21 ± 1 | 184 ± 18c |
| Men | 29 ± 2 | 141 ± 13 | 221 ± 10a | 172 ± 52 | 223 ± 8a | 550 ± 28b,d | 25 ± 2 | 215 ± 15c |
Values are means ± SE.
[E], circulating epinephrine concentration; [NE], circulating norepinephrine concentration; Saline, saline infusion only (control); Epi, epinephrine infusion only; Epi + Phent, epinephrine + phentolamine infusion; Terb, terbutaline infusion. Significant time × infusion interaction for [E] and [NE] (P < 0.0001).
Main effect of sex for [E] (P = 0.001). Post hoc analysis [E]:
P < 0.0001 compared with same-day preinfusion and compared with Saline. Post hoc analysis [NE]:
P < 0.0001 compared with same-day preinfusion and compared Saline, Epi, or Terb,
P < 0.0001 compared with same-day preinfusion and compared with Saline.
P < 0.05 for men vs. women.
Glucagon concentration was not different between sexes for any treatment or time point (P > 0.05). Glucagon concentration from baseline to the end of the infusions did not change with the S, E, or E + P treatments (P > 0.05), but it did significantly decrease from resting values with the infusion of T in both men (53.4 ± 3.4 to 47.5 ± 3.6 pg/ml, P < 0.01) and women (49.5 ± 3.2 to 43.6 ± 3.6 pg/ml, P < 0.05). For cortisol concentration, there were no time × sex or time × treatment interactions (P > 0.05); however, there was a main effect of time with a fall in cortisol from pre- to posttreatment (P < 0.0001). Baseline cortisol ranged from 8.5 ± 0.9 to 10.0 ± 0.9 μg/dl in men and 8.1 ± 0.7 to 9.8 ± 1.5 μg/dl in women, and posttreatment cortisol concentrations decreased by an average of 2.6 and 2.0 μg/dl in men and women, respectively.
Heart Rate and Blood Pressure
Pretreatment HR rate over the four treatment days averaged 59 ± 2 in men and 62 ± 3 beats/min in women. Values for the individual treatments are reported in Table 3. There were no significant changes in HR in response to the S treatment, but in men there was a significant increase in HR with the E (P = 0.01), E + P (P < 0.0001), and T (P = 0.002) treatments, and women with the E + P and T (P < 0.0001 for both). Blood pressure significantly decreased with saline (control) treatment in both men and women (P < 0.01) with a similar decrease observed for all active treatments (Table 3) except for T treatment in men where the fall was not significant.
Table 3.
Baseline heart rate and blood pressure
| Saline |
Epi |
Epi + Phent |
Terb |
|||||
|---|---|---|---|---|---|---|---|---|
| Infusion: | Basal | Infusion | Basal | Infusion | Basal | Infusion | Basal | Infusion |
| Heart rate beats/min | ||||||||
| Women | 63 ± 1 | 59 ± 1 | 61 ± 1 | 65 ± 1 | 63 ± 1 | 77 ± 1b | 61 ± 1 | 74 ± 1b |
| Men | 59 ± 1 | 56 ± 1 | 60 ± 1 | 63 ± 1a | 61 ± 1 | 81 ± 1b | 58 ± 1 | 71 ± 1c |
| Systolic blood pressure, mmHg | ||||||||
| Women | 112 ± 1 | 101 ± 0.5a | 111 ± 1 | 100 ± 0.5a | 111 ± 1 | 100 ± 0.4a | 114 ± 1 | 102 ± 1a |
| Men | 117 ± 1 | 108 ± 0.5a | 118 ± 1 | 114 ± 0.5a | 122 ± 1 | 113 ± 0.5a | 115 ± 1 | 113 ± 0.5 |
| Diastolic blood pressure, mmHg | ||||||||
| Women | 69 ± 1 | 60 ± 0.5 | 68 ± 1 | 57 ± 0.5 | 69 ± 1 | 54 ± 0.5 | 64 ± 1 | 58 ± 1 |
| Men | 66 ± 1 | 62 ± 0.5 | 71 ± 0.5 | 64 ± 1 | 73 ± 1 | 60 ± 0.5 | 67 ± 1 | 62 ± 0.5 |
Values are means ± SE. Baseline heart rate and blood pressure and average values during study infusions. Infusion data represent the average of 9 values measured every 10 min during the 90-min treatment. Saline, saline infusion only; Epi, epinephrine infusion only; Epi + Phent, epinephrine + phentolamine infusion; Terb, terbutaline infusion.
P < 0.01 compared with basal;
P < 0.0001 compared with basal;
P < 0.005 compared with basal.
Substrate Concentration and Kinetics
Glycerol.
Figure 1 shows the glycerol concentration at baseline and during each 90-min study infusion. There was a significant time × sex (P < 0.05) interaction for glycerol concentration, and a significant main effect of sex (P < 0.001), with women having greater glycerol concentrations across the three infusion conditions compared with men (average concentrations during treatments, S: 87 ± 7 vs. 73 ± 6 μmol/l, P > 0.05; E: 120 ± 7 vs. 89 ± 5, P < 0.01; E + P: 145 ± 11 vs. 104 ± 6, P < 0.01; T: 132 ± 9 vs. ± 5, P < 0.01, for women and men, respectively). Glycerol enrichment during each study day, measured on the subset of subjects, is shown in Fig. 2. Despite the increase in isotope infusion rate, glycerol MPE% fell following the start of each active treatment and then remained relatively stable for the remainder of the infusions. There was a significant effect of sex, with men having greater enrichment than women (P = 0.01); however, there was no infusion × sex interaction (P > 0.05), indicating that the change with treatments was similar for both sexes. Figure 3 shows the glycerol Ra (absolute rates) throughout each experimental day. There was a significant time × treatment interaction (P < 0.0001) due to an initial increase in glycerol Ra after the start of each active treatment followed by a decrease, with no change in the S infusion. There was not a significant treatment × sex interaction (P = 0.25) for absolute glycerol Ra, but a trend for women to have higher glycerol Ra compared with men with E (Table 4). By contrast, with E + P treatment, men had significantly higher glycerol absolute Ra than women at t = 170–190 min and t = 200–220 min (P < 0.05 for both). No other sex differences were observed for glycerol absolute Ra across the different treatments. When data were expressed per kilogram of body weight (Ra/kg body wt), women had higher glycerol Ra/kg body wt throughout the E treatment (P < 0.05). No other significant sex differences were observed for glycerol Ra/kg body wt (Table 4). Similar results were observed for glycerol Rd (absolute and per body wt). As noted previously, fat mass was the same for men and women; therefore, absolute Ra/Rd reflects data relative to fat mass.
Fig. 1.
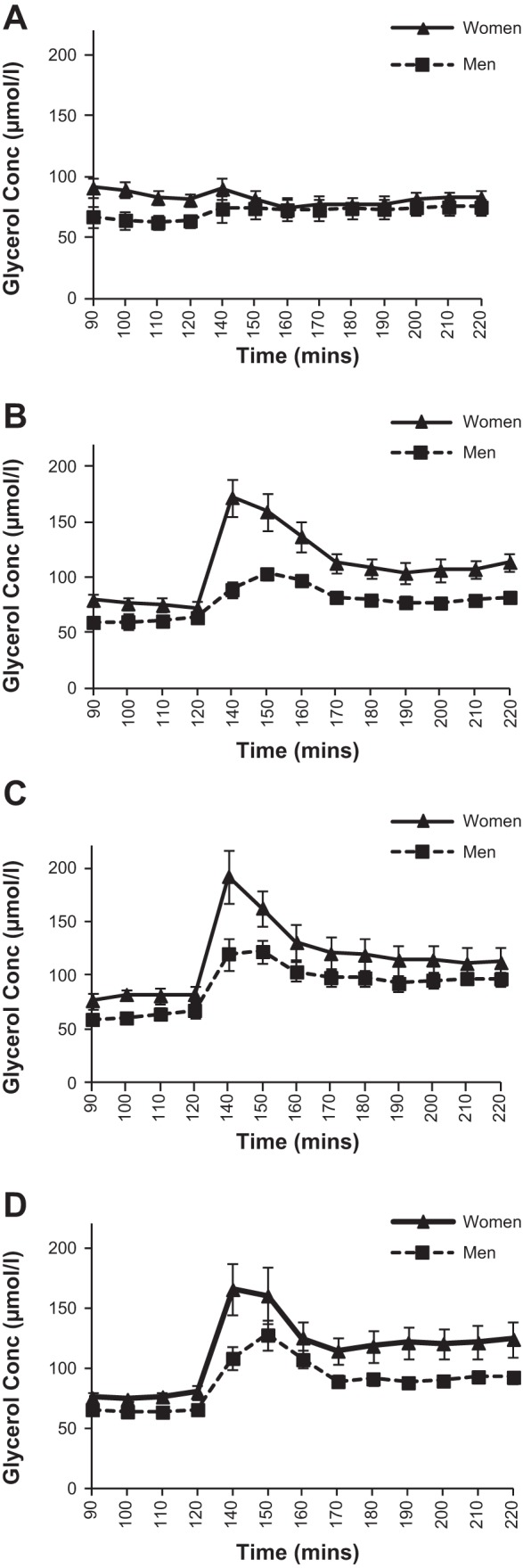
Glycerol concentration at baseline and during each 90-min study infusion (n = 14 women, 16 men). A: saline infusion. B: epinephrine infusion. C: epinephrine and phentolamine infusion. D: terbutaline infusion.
Fig. 2.
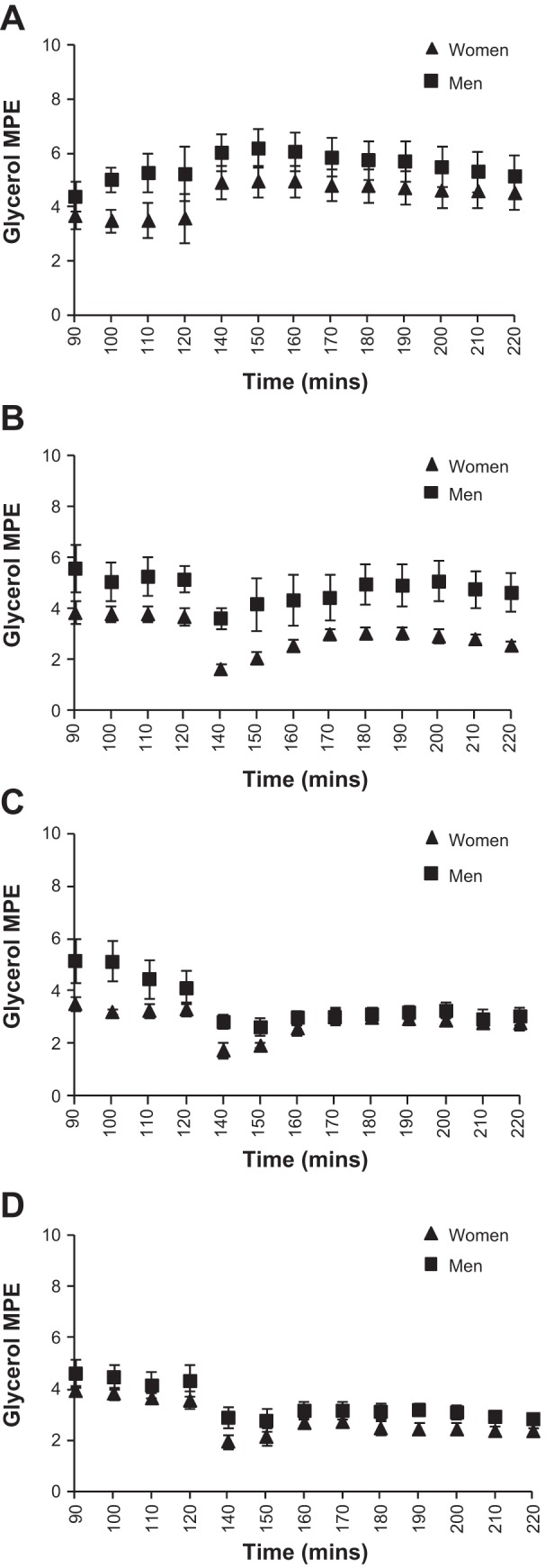
Glycerol enrichment at baseline and during each 90-min study infusion (n = 7 women, 7 men). MPE, mole percent excess. A: saline infusion. B: epinephrine infusion. C: epinephrine and phentolamine infusion. D: terbutaline infusion.
Fig. 3.
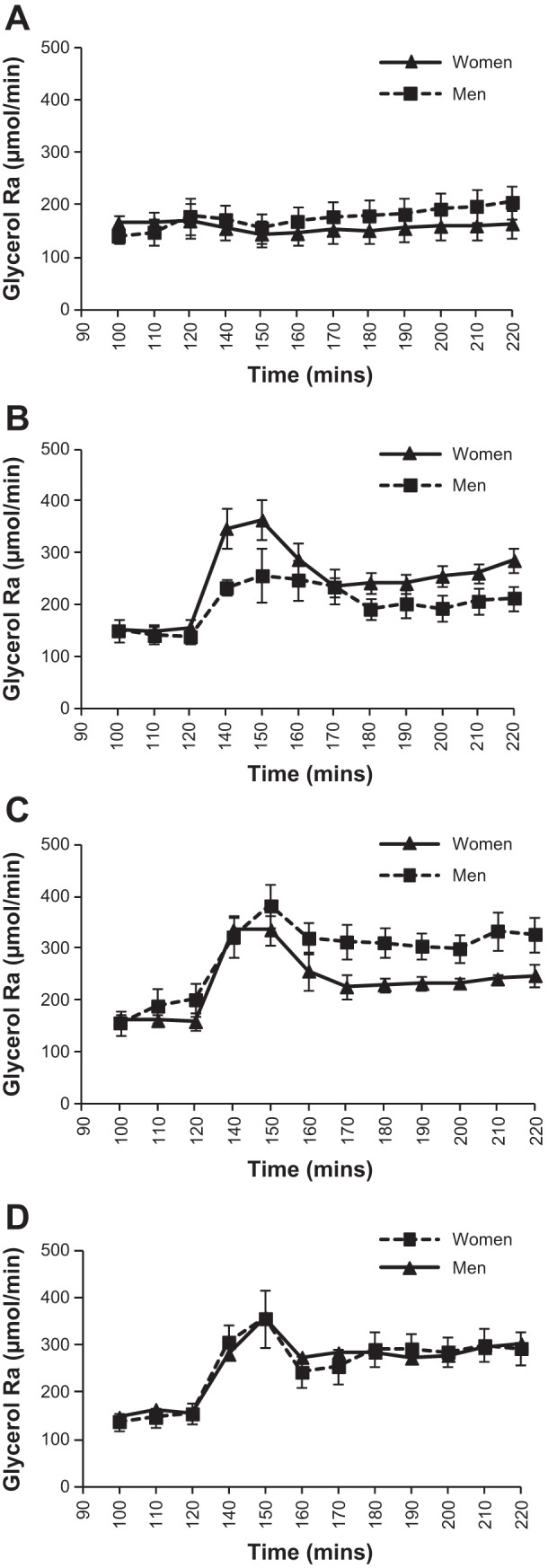
Glycerol rate of appearance (absolute rates) in μmol/min at baseline and during each 90-min study infusion (n = 7 women, 7 men). A: saline infusion. B: epinephrine infusion. C: epinephrine and phentolamine infusion. D: Terbutaline infusion.
Table 4.
Glycerol kinetics
| Average Rest |
Average 140–160 min |
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline | Epi | Epi + Phent | Terb | Saline | Epi | Epi + Phent | Terb | |
| Ra, μmol/min | ||||||||
| Men | 156 ± 29 | 144 ± 17 | 182 ± 29 | 156 ± 24 | 166 ± 42 | 246 ± 33b | 341 ± 33 | 205 ± 50 |
| Women | 167 ± 16 | 152 ± 13 | 161 ± 10 | 147 ± 13 | 148 ± 8 | 333 ± 32 | 309 ± 23 | 302 ± 27 |
| Ra, μmol·kg body wt−1·min−1 | ||||||||
| Men | 2.04 ± 0.36 | 1.83 ± 0.18a | 2.30 ± 0.33 | 2.05 ± 0.37 | 2.08 ± 0.42 | 3.18 ± 0.47c | 4.38 ± 0.43 | 3.96 ± 0.71 |
| Women | 2.89 ± 0.27 | 2.63 ± 0.26 | 2.78 ± 0.22 | 2.57 ± 0.25 | 2.55 ± 0.15 | 5.75 ± 0.57 | 5.33 ± 0.41 | 5.28 ± 0.49 |
| Average 170–190 min |
Average 200–220 min |
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline | Epi | Epi + Phent | Terb | Saline | Epi | Epi + Phent | Terb | |
| Ra, μmol/min | ||||||||
| Men | 180 ± 45 | 210 ± 22 | 309 ± 28a | 280 ± 43 | 199 ± 45 | 205 ± 23b | 320 ± 28a | 292 ± 42 |
| Women | 153 ± 8 | 240 ± 18 | 230 ± 15 | 279 ± 18 | 161 ± 11 | 268 ± 19 | 241 ± 11 | 293 ± 13 |
| Ra, μmol·kg body wt−1·min−1 | ||||||||
| Men | 2.27 ± 0.50 | 2.71 ± 0.34a | 3.97 ± 0.39 | 3.63 ± 0.58 | 2.54 ± 0.54 | 2.63 ± 0.31c | 4.10 ± 0.38 | 3.78 ± 0.57 |
| Women | 2.64 ± 0.18 | 4.17 ± 0.39 | 4.00 ± 0.37 | 4.91 ± 0.41 | 2.79 ± 0.24 | 4.63 ± 0.39 | 4.19 ± 0.33 | 5.13 ± 0.32 |
Values are means ± SE. Ra, rate of appearance. Significant difference between sex:
P < 0.05;
P < 0.10;
P < 0.01.
Total FFA and palmitate.
Figure 4 shows the total FFA concentrations over the baseline and each 90-min study treatment. Women had significantly greater FFA concentrations during all treatment infusions compared with men (average concentrations during treatments: S: 723 ± 61 vs. 533 ± 44 μmol/l, P < 0.05; E: 1,053 ± 65 vs. 782 ± 43, P < 0.001; E + P: 1,223 ± 51 vs. 982 ± 42, P < 0.01; T: 1,101 ± 66 vs. 893 ± 46, P < 0.05). In the data subset from subjects on whom tracer measurements were made, total FFA concentration reflected that of the total FFA in the entire group. Women also tended to have higher palmitate concentrations compared with men for all treatments (Fig. 5), although this only met statistical significance for the E infusion. In this subgroup, palmitate enrichment was not different between men and women at baseline: 4.7 ± 0.3 MPE% and 5.1 ± 0.3, respectively. Mirroring changes in glycerol enrichment, palmitate enrichment also dropped initially with the start of each active treatment but remained stable thereafter in both men (3.5 ± 0.2 MPE%) and women (3.8 ± 0.3 MPE%). Table 5 shows the palmitate Ra for each experimental day and time period. For absolute Ra, there was a significant time × treatment interaction (P < 0.01) for all subjects' average for minutes 140–220. This was due to all active treatments being significantly higher than control (S) treatment (E: P = 0.04; E + P: P = 0.01; T: P = 0.02). When body weight was taken into account, women had higher palmitate Ra/kg body wt with E infusion (Table 5). Notably, no other significant sex differences were observed for palmitate Ra/kg body wt among the other treatments, including treatment of E + P. Palmitate absolute Rd and Rd/kg body wt results were similar to those for Ra.
Fig. 4.
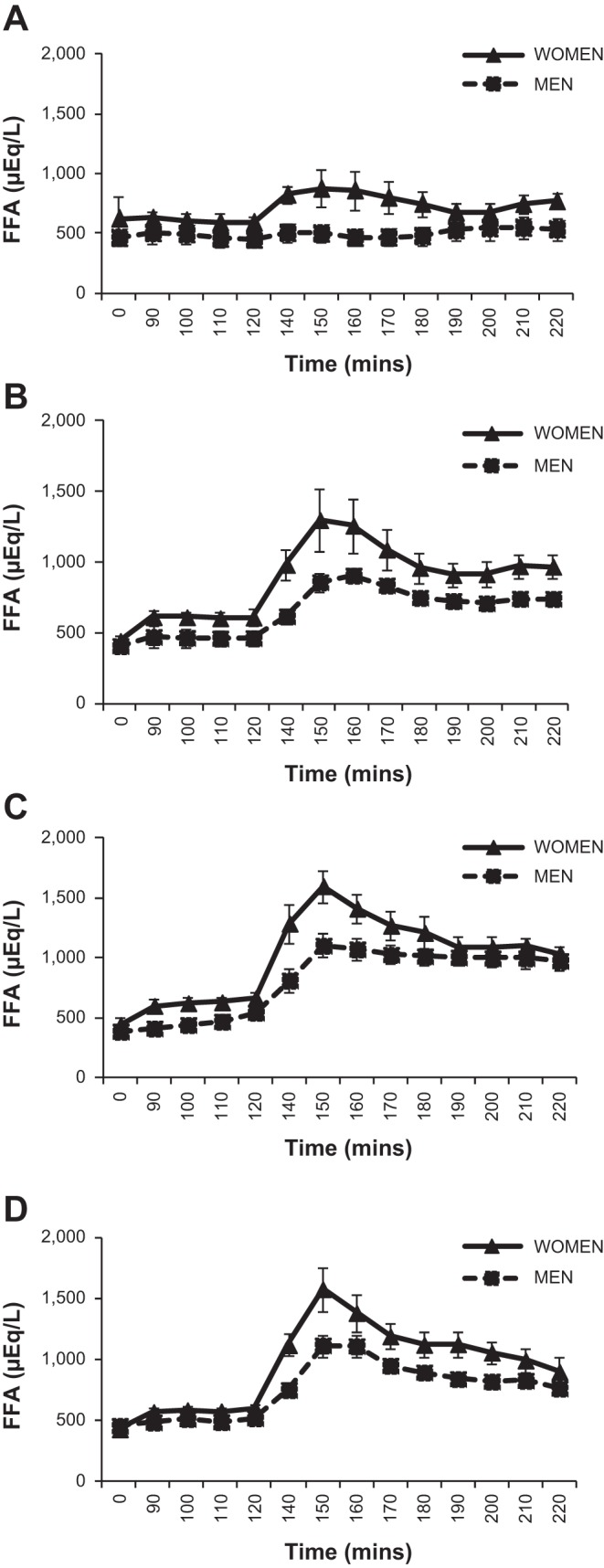
Free fatty acid (FFA) concentration at baseline and during each 90-min study infusion (n = 7 women, 7 men). A: saline infusion. B: epinephrine infusion. C: epinephrine and phentolamine infusion. D: terbutaline infusion.
Fig. 5.
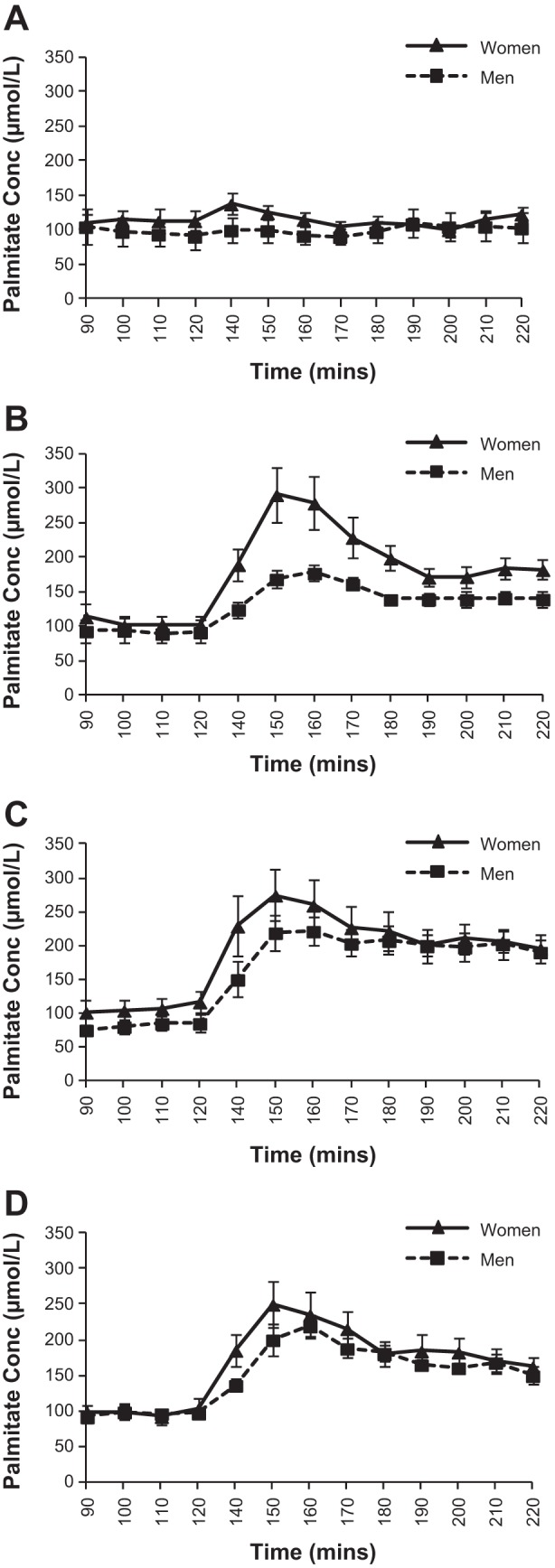
Palmitate concentration at baseline and during each 90 min study infusion (n = 7 women, n = 7 men). A: saline infusion. B: epinephrine infusion. C: epinephrine and phentolamine infusion. D: terbutaline infusion.
Table 5.
Palmitate kinetics
| Average Rest |
Average 140–160 min |
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline | Epi | Epi + Phent | Terb | Saline | Epi | Epi + Phent | Terb | |
| Ra, μmol/min | ||||||||
| Women | 128 ± 19 | 94 ± 12 | 102 ± 9 | 94 ± 10 | 118 ± 7 | 188 ± 19 | 191 ± 20 | 185 ± 20 |
| Men | 121 ± 24 | 116 ± 19 | 125 ± 21 | 129 ± 12 | 120 ± 27 | 169 ± 17 | 217 ± 23 | 218 ± 18 |
| Ra, μmol·kg body wt−1·min−1 | ||||||||
| Women | 1.83 ± 0.17 | 1.60 ± 0.16 | 1.73 ± 0.10 | 1.63 ± 0.14 | 2.01 ± 0.10 | 3.21 ± 0.27 | 3.22 ± 0.63 | 3.19 ± 0.28 |
| Men | 1.56 ± 0.25 | 1.47 ± 0.23 | 1.59 ± 0.24 | 1.71 ± 0.24 | 1.49 ± 0.25 | 2.17 ± 0.22a | 2.77 ± 0.30 | 2.89 ± 0.37 |
| Average 170–190 min |
Average 200–220 min |
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline | Epi | Epi + Phent | Terb | Saline | Epi | Epi + Phent | Terb | |
| Ra, μmol/min | ||||||||
| Women | 102 ± 17 | 151 ± 13 | 160 ± 16 | 178 ± 28 | 106 ± 6 | 144 ± 9 | 160 ± 11 | 174 ± 18 |
| Men | 121 ± 28 | 162 ± 12 | 227 ± 20a | 213 ± 18 | 128 ± 31 | 154 ± 12 | 227 ± 20a | 203 ± 16 |
| Ra, μmol·kg body wt−1·min−1 | ||||||||
| Women | 1.76 ± 0.11 | 2.57 ± 0.17 | 2.73 ± 0.24 | 3.06 ± 0.40 | 1.81 ± 0.11 | 2.47 ± 0.12 | 2.73 ± 0.24 | 3.00 ± 0.24 |
| Men | 1.51 ± 0.27 | 2.06 ± 0.14a | 2.89 ± 0.21 | 2.82 ± 0.35 | 1.60 ± 0.31 | 1.96 ± 0.13a | 2.88 ± 0.21 | 2.67 ± 0.31 |
Values are means ± SE. Ra, rate of appearance; Rd, rate of disappearance. Significant difference between sex:
P < 0.05.
Glucose.
Glucose concentrations at baseline vs. the 90 min of each treatment were relatively stable, with S: 4.8 ± 0.1 vs. 4.8 ± 0.1 mmol/l; E: 5.1 ± 0.01 vs. 5.4 ± 0.03; E + P: 5.0 ± 0.01 vs. 4.9 ± 0.01; and T: 4.9 ± 0.02 vs. 5.3 ± 0.1, respectively, for men, and the corresponding data for women were S: 4.6 ± 0.01 vs. 4.6 ± 0.01; E: 4.7 ± 0.01 vs. 5.2 ± 0.04; E + P: 4.8 ± 0.001 vs. 4.6 ± 0.04; and T: 4.7 ± 0.01 vs. 5.2 ± 0.1, respectively. There was no sex × infusion interaction; however, there was a main effect of infusion, with post hoc tests revealing a significantly higher glucose concentration with E and T infusion compared with saline (P < 0.001 for both). There was also a main effect of sex, with males having higher glucose than females (5.1 ± 0.08 vs. 4.8 ± 0.09 mmol/l, P < 0.05). Tracer data in the subset of subjects showed that glucose enrichment at baseline and during the 90 min of each treatment was stable in men and women. Pretreatment values were 1.4 ± 0.2 MPE% in men and 1.5 ± 0.3 in women, and with treatment 1.5 ± 0.2 MPE% and 1.6 ± 0.1, respectively. For glucose absolute Ra, there was no sex × infusion or infusion × time interaction; however, there was a main effect of time (P < 0.0001), with glucose Ra decreasing throughout all of the infusions (S: 950 ± 59 vs. 887 ± 66 μmol/min; E: 1,036 ± 81 vs. 960 ± 75; E + P: 999 ± 96 vs. 874 ± 87; T: 966 ± 72 vs. 946 ± 80, respectively, for men, and the corresponding data for women were S: 751 ± 54 vs. 699 ± 60; E: 855 ± 74 vs. 813 ± 68; E + P: 679 ± 87 vs. 628 ± 79; T: 860 ± 66 vs. 825 ± 73, respectively). There was also a significant main effect of sex, with men having greater absolute Ra than women (952 ± 45 μmol/min vs. 764 ± 41, P < 0.05). Post hoc Bonferroni showed no significant differences between treatments for glucose absolute Ra (P > 0.05). When data were expressed as glucose Ra/kg FFM, there were no significant differences between sex or among the infusions (P > 0.1). Glucose Rd/kg FFM followed a similar pattern.
Energy expenditure and nonprotein respiratory exchange ratio.
In both men and women the metabolic rate (kcal/min) increased from baseline with each active treatment (Table 6). There was a significant main effect of sex, with men having greater metabolic rate than females (P < 0.001). For women, metabolic rate significantly increased from resting values vs. the average for the final 50 min of treatment in the E (+0.08 ± 0.02 kcal/min; P < 0.001), E + P (+0.10 ± 0.02; P < 0.0001) and T (+0.13 ± 0.03; P = 0.003) infusions, with no change in the S infusion (−0.02 ± 0.02; P > 0.05). For men, metabolic rate also increased with the active treatment infusions, with significant increases in E (+0.08 ± 0.01; P < 0.0001), E + P (+0.15 ± 0.02 P < 0.0001), and T (+0.23 ± 0.01 P < 0.0001) and no change with S (+0.03 ± 0.02 P > 0.05). For each treatment and time point, men had significantly greater metabolic rate than women (P < 0.0001); however, when FFM was taken into account, there were no significant differences between sex in the change in metabolic rate for any treatment (P > 0.05). Protein oxidation rates during infusions, estimated from urinary nitrogen excretion, in men vs. women were similar to what we found in our previous study (9): during S: 0.057 ± 0.02 vs. 0.049 ± 0.018 g/min (P = 0.002); E: 0.059 ± 0.006 vs. 0.052 ± 0.003; (P = 0.12); E + P: 0.051 ± 0.006 vs. 0.076 ± 0.03 (P = 0.9); and during T: 0.056 ± 0.026 vs. 0.064 ± 0.013 (P = 0.001). The nonprotein respiratory exchange ratio (NPRER) significantly decreased during E and E + P treatments (P < 0.0001), but did not significantly change with T or S (P > 0.05) in men and women (Table 6). Treatment × sex and time × sex interactions were not significant (P > 0.05), and there were no sex-based differences at any time point in NPRER among the different treatments (P > 0.05). Due to NPRER values falling below 0.70 in a number of subjects, particularly with the E and E + P treatments, substrate oxidation was not calculated. An NPRER under 0.70 implies nonoxidative contribution to gas exchange, which, under these experimental conditions, is difficult to explain. Rather, the result could be related to slight errors in both the indirect calorimetry measures of gas exchange and the estimation of protein oxidation from urinary nitrogen excretion, resulting in an artifact that lowered NPRER < 0.70. In such instances, it is probably more appropriate to assume CHO oxidation equaled zero rather than a negative value. Given the need for multiple assumptions, substrate oxidation was not calculated from the NPRER data.
Table 6.
Energy expenditure (EE) and nonprotein respiratory exchange ratio (NPRER)
| Infusion: | Saline |
Epi |
Epi + Phent |
Terb |
||||
|---|---|---|---|---|---|---|---|---|
| EE, kcal/min | NPRER | EE, kcal/min | NPRER | EE, kcal/min | NPRER | EE, kcal/min | NPRER | |
| Before infusion | ||||||||
| Women | 0.99 ± 0.05 | 0.75 ± 0.02 | 0.98 ± 0.04 | 0.78 ± 0.02 | 1.0 ± 0.05 | 0.77 ± 0.02 | 1.0 ± 0.04 | 0.77 ± 0.01 |
| Men | 1.2 ± 0.06a | 0.77 ± 0.03 | 1.3 ± 0.05a | 0.77 ± 0.03 | 1.3 ± 0.06a | 0.77 ± 0.03 | 1.2 ± 0.06a | 0.76 ± 0.03 |
| During infusion | ||||||||
| Women | 0.96 ± 0.04 | 0.74 ± 0.01 | 1.1 ± 0.04** | 0.70 ± 0.02** | 1.1 ± 0.05** | 0.69 ± 0.02** | 1.2 ± 0.06* | 0.76 ± 0.02 |
| Men | 1.3 ± 0.07a | 0.75 ± 0.02 | 1.4 ± 0.06**a | 0.72 ± 0.03** | 1.4 ± 0.06**a | 0.72 ± 0.03** | 1.4 ± 0.07**a | 0.76 ± 0.02 |
Values are means ± SE. Energy expenditure (EE) and nonprotein respiratory exchange ratio (NPRER) for women and men before and during the final 50 min of each treatment infusion.
P < 0.01 vs. before infusion, within sex;
P < 0.001 vs. before infusion, within sex;
P < 0.001 vs. women during same infusion.
DISCUSSION
We have previously shown that epinephrine is more effective at increasing systemic lipolysis in women compared with men while maintaining similar glucoregulatory effects (17). The present study was conducted to begin to delineate the contribution of different adrenergic receptors to these previously demonstrated sex-based differences in epinephrine-stimulated lipolysis. We measured sex-based responses to moderate elevations in 1) epinephrine, which stimulates α2- and mixed β-adrenergic receptors, antilipolytic and lipolytic, respectively; 2) epinephrine + phentolamine (α-adrenergic antagonist), which results in mixed β-stimulation only; and 3) terbutaline, which selectively stimulates the β2-adrenergic receptors. In agreement with previous observations (17), we found that women have a greater rate of lipolysis than men when epinephrine was infused alone. Furthermore, data suggest that this sex-based difference was at least partly due to greater α2 antilipolytic activation in men, given that, when antilipolytic α-receptor activation was blocked (E + P treatment) or when β2-adrenergic receptors were predominantly stimulated (T treatment), men and women did not significantly differ in the stimulation of lipolysis. This study is the first to show a potential mechanism by which men and women differ in their lipolytic response to elevated epinephrine.
Adrenergic receptor distribution varies in the different adipose tissue locations throughout the body (22, 24, 25, 26); therefore, sex-based differences in whole body lipolysis may be related to sex-based differences in body fat distribution. This seems to be more plausible than differences in total body fat as in the present study, similar to previous observations (17), absolute fat mass was not different between the sexes, despite men having a lower percent body fat than women. Therefore, greater systemic lipolysis in the women could not be explained simply by the fact that they have more absolute adipose tissue mass. Characteristic body fat distribution differences between men and women include greater subcutaneous adipose tissue (AT), particularly gluteal-femoral AT in women vs men, but lower visceral AT. Gluteal-femoral adipose tissue is characterized by a greater α2 adrenergic response (antilipolytic) (25) and a much lower β-adrenergic response (22, 24) compared with subcutaneous abdominal adipose tissue. There is less expression of the β-adrenergic receptors in gluteal vs abdominal subcutaneous adipose tissue (26) and in particular, fewer β2 adrenergic receptors (24). Visceral adipose tissue is the most lipolytic adipose tissue bed (25, 27, 28), having more β-adrenergic receptors, compared with subcutaneous adipose tissue (28, 29), and little α2 adrenergic activity (30). Men did have higher VAT than females in the present investigation, but this most lipolytic of the fat depots has been shown to have little impact on whole body lipolysis in lean individuals (20, 28) and even if it did, it would be expected to give the opposite results to those observed, i.e., greater systemic lipolysis in men vs. women. It is likely, therefore, that the sex-based differences in epinephrine's effect on lipolysis reflect differences in the stimulation of receptors in subcutaneous adipose tissue. We did not measure subcutaneous AT distribution, but given the small differences in the VAT between men and women in the study, it might be assumed that subcutaneous AT also was similar in absolute mass. If the typical sex-based differences in subcutaneous AT are assumed, women would be predicted to have a propensity for higher α2-receptors due to greater gluteal-femoral AT and thus a greater increase in systemic lipolysis with α2-receptor blockade (E + P treatment in the present study). This was not what we observed, however, and it was men who had the greater increase in systemic lipolysis with α2-receptor blockade. An explanation for the sex-based differences in systemic lipolysis with adrenergic stimulation is, therefore, difficult to reconcile with current cell-based studies of adrenergic receptors. It is possible that differences in lipolysis with epinephrine infusion may be due to men having a higher density of antilipolytic α2-receptors in subcutaneous adipose tissue, or the α2 receptors are more sensitive to epinephrine action compared with these receptors in women. Future work is needed to directly compare sex-based differences in the regulation of lipolysis looking at the responses from the cellular and tissue level integrated into a whole body response.
Current data suggest that in this group of nonobese, untrained men and women, β-adrenergic receptors were not playing a major role in the observed sex-based differences in systemic lipolysis. Along with the mixed β-receptor agonist epinephrine, we used the selective β2-receptor agonist terbutaline (11), a weak agonist at α receptors (25), to partly address this. Men and women had similar levels of lipolysis with T infusion, which suggests that differences in the stimulation of β2-receptors do not explain the sex differences seen in lipolysis. A previous study that examined the effect of T infusion also found that there was no difference in lipolysis between men and women (25). In the present study, we did not confirm that β2-receptors alone were stimulated by terbutaline but this drug has been previously used to investigate the effects of β2-stimulation of lipolysis in humans (21). Even though it is possible that there was some slight activation of β1- or β3-receptors with terbutaline, the fact that men and women had no difference in lipolysis with this treatment still supports our contention that the difference in systemic lipolysis was unlikely to be due to greater β2-adrenergic receptor activation in women, rather greater α2, antilipolytic activation in men.
All active treatments in the present study significantly increased metabolic rate, which would be expected given the cellular activation following stimulation of β-adrenergic receptors. After differences in body composition were taken into account, however, there were no sex differences in the degree to which metabolic rate was stimulated. Interestingly, T treatment (β2-receptor stimulation) resulted in the greatest increase in metabolic rate in both sexes, significantly so for men. Nonprotein respiratory exchange ratio, however, did not change with T treatment whereas treatment with E or E + P led to a significant reduction in NPRER in men and women. Unlike in our previous study (17), no sex difference was observed in the decrease in NPRER with E treatment. It is unclear what might explain this difference in study results but it may be related to a marginal statistical power, as the P value for the difference in change in NPRER approached significance at P = 0.165. As NPRER fell below 0.70 for the E and E + P treatments in a number of subjects, it would have been inaccurate to use the NPRER to quantify fat and carbohydrate oxidation. It appears that nonselective stimulation of β-adrenergic receptors results in these phenomena (17). Given that we did not observe this with T treatment, this suggests that β2-stimulation alone leads to less of an increase in fat oxidation. This suggests an additive effect from the stimulation of the β1-and β3-receptors with E and E + P, as it has been shown that selective stimulation of β1- and β3-adrenergic receptors can increase lipolysis and decrease RER (8, 35, 40). The stimulation of β2 adrenergic receptors alone may not have been sufficient to induce as large of a decrease in NPRER.
Men had higher testosterone concentrations than women, and testosterone has been shown to reduce catecholamine-stimulated lipolysis in human preadipocytes from abdominal subcutaneous adipose tissue (7). Catecholamine-induced lipolysis in polycystic ovary syndrome (PCOS; a condition where women have elevated testosterone) is also decreased in subcutaneous fat cells (1). Despite the data that support the idea that higher testosterone in men may explain the lower systemic lipolysis with epinephrine infusion, there are also data showing that estrogen may decrease lipolysis (9, 38), although these studies did not measure whole body catecholamine-induced lipolysis. Testosterone has been shown to increase the number of antilipolytic α2-adrenergic receptors in white adipocytes (3, 4), which supports the idea that people with higher testosterone have lower systemic lipolysis due to greater antilipolytic activation with epinephrine.
Although there was no infusion of norepinephrine on any study day, circulating NE significantly increased from baseline with both the E + P and the T treatments. Only with infusion of E + P, however, did NE increase significantly compared with the other treatments. Phentolamine is generally considered to be a pure α-adrenergic blocking agent, although it is also thought to have some direct vasodilatory effects (42). Although we did not test the adequacy of the α2-blockade in the present study, we based the dose of phentolamine used on the dose of phentolamine shown to induce α2-blockade in other investigations (22, 32). Phentolamine infusion alone has been shown to increase norepinephrine concentration in humans (15, 22, 32); therefore the elevation in this catecholamine that we observed was not unexpected. The magnitude of the norepinephrine increase from baseline was significantly greater in men compared with women for the E + P treatment; however, we showed in a previous study that infusion of norepinephrine has significantly less impact on whole body lipolysis compared with epinephrine alone or norepinephrine plus epinephrine infused together (17). In addition, norepinephrine in the presence of epinephrine has not been shown to induce a sex difference in lipolysis (17). The greater lipolysis (absolute glycerol Ra) with the E + P infusion in men was no longer apparent when body weight was accounted for, and the elevated norepinephrine likely has little effect on our contention that when α-receptors are blocked, men and women have a similar sensitivity to epinephrine-induced lipolysis.
We based our conclusions for sex-based differences in the adrenergic regulation of epinephrine-stimulated lipolysis upon the measurement of circulating glycerol and glycerol Ra. Unfortunately, our sample size for the tracer determinations of substrate turnover was at most half that available for substrate concentration determination, due to the loss of plasma samples described. This greatly reduced the statistical power when performing statistical analysis on the tracer data. In our previous study, using a larger sample size, the significantly greater epinephrine stimulated lipolysis in women compared with men was observed for glycerol Ra in absolute terms and relative to body weight and was also closely mirrored by glycerol concentration changes (17). Similar results were obtained in the present study and the marginal lack of statistical difference for absolute glycerol Ra (reflective also of glycerol Ra relative to fat mass) was likely due to the small sample size. The same would most likely be true for palmitate kinetics had we measured this in our previous study (17) as suggested by similar patterns of FFA concentrations between the two studies. We believe, therefore, that current data strongly support the conclusion of higher systemic lipolysis in women with E treatment compared with men, and that this sex difference was abolished with the simultaneous infusion of phentolamine to block antilipolytic α2-adrenergic receptors.
Men and women differ in the effect of a recent exercise bout on resting metabolic rate, lipolysis, and fat oxidation as indicated previously by our group and by other groups (12, 19). This sex difference appears to track with adrenergic signaling. There are also sex differences in lipolysis and fat oxidation after exercise, when catecholamine levels return to baseline (12, 13). This phenomenon has not been well-studied from a mechanistic standpoint but may be related to changes in the sensitivity of adrenergic receptors after exercise in men vs. women. Alternatively, postexercise differences in lipid utilization may not be driven by catecholamine signaling but may be mediated by factors such as changes in growth hormone levels following exercise or release of natriuretic peptides.
In conclusion, in lean healthy subjects, we have shown that infusion of epinephrine in women induces a greater lipolytic response than in men. The increased lipolytic response in women was likely due to men having greater antilipolytic receptor activity in response to epinephrine. It does not appear that there are differences in lipolysis when only lipolytic β-receptors are stimulated. These findings may help explain why women have greater lipolysis and fat oxidation during exercise: the exercise-induced rise in epinephrine likely stimulates α2 antilipolytic receptors to a greater extent in men, which results in a lesser stimulation of lipolysis and fat oxidation during an exercise bout compared with women.
GRANTS
This study was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-071155-04 (T. J. Horton and D. H. Bessesen) and K24-DK-02935 (D. H. Bessesen). S. L. Schmidt received support from Grant T32-DK-007446. This study was also supported in part by the Colorado Nutrition Obesity Research Center (Grant P30-DK-048520) and NIH/NCATS Colorado CTSI Grant UL1-TR000154. Isotope analysis by B. F. Miller and F. F. Peeler III was supported by Grant K01-AG-031829-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.L.S. and T.J.H. analyzed data; S.L.S. and T.J.H. interpreted results of experiments; S.L.S. and T.J.H. prepared figures; S.L.S. and T.J.H. drafted manuscript; S.L.S., D.H.B., and T.J.H. edited and revised manuscript; S.L.S. and T.J.H. approved final version of manuscript; D.H.B. and T.J.H. conception and design of research; D.H.B., S.A.S., F.F.P., B.F.M., and T.J.H. performed experiments.
ACKNOWLEDGMENTS
E. Donovan contributed to the isotope analysis.
The contents of this manuscript are the authors' sole responsibility and do not necessarily represent official National Institutes of Health (NIH) views.
REFERENCES
- 1.Arner P. Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie 87: 39–43, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 4: 499–502, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bouloumie A, Valet P, Dauzats M, Lafontan M, Saulnier-Blache JS. In vivo upregulation of adipocyte alpha 2-adrenoceptors by androgens is consequence of direct action on fat cells. Am J Physiol Cell Physiol 267: C926–C931, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Bouloumie A, Valet P, Daviaud D, Prats H, Lafontan M, Saulnier-Blache JS. Adipocyte alpha 2A-adrenoceptor is the only alpha 2-adrenoceptor regulated by testosterone. Eur J Pharmacol 269: 95–103, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Braun B, Horton T. Endocrine regulation of exercise substrate utilization in women compared to men. Exerc Sport Sci Rev 29: 149–154, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Burns TW, Langley PE, Robison GA. Adrenergic receptors and cyclic AMP in the regulation of human adipose tissue lipolysis. Ann NY Acad Sci 185: 115–128, 1971 [DOI] [PubMed] [Google Scholar]
- 7.Dicker A, Ryden M, Naslund E, Muehlen IE, Wiren M, Lafontan M, Arner P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 47: 420–428, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Enocksson S, Shimizu M, Lonnqvist F, Nordenstrom J, Arner P. Demonstration of an in vivo functional beta 3-adrenoceptor in man. J Clin Invest 95: 2239–2245, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gormsen LC, Host C, Hjerrild BE, Pedersen SB, Nielsen S, Christiansen JS, Gravholt CH. Estradiol acutely inhibits whole body lipid oxidation and attenuates lipolysis in subcutaneous adipose tissue: a randomized, placebo-controlled study in postmenopausal women. Eur J Endocrinol 167: 543–551 [DOI] [PubMed] [Google Scholar]
- 10.Hellstrom L, Blaak E, Hagstrom-Toft E. Gender differences in adrenergic regulation of lipid mobilization during exercise. Int J Sports Med 17: 439–447, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Hellstrom L, Wahrenberg H, Reynisdottir S, Arner P. Catecholamine-induced adipocyte lipolysis in human hyperthyroidism. J Clin Endocrinol Metab 82: 159–166, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Henderson GC, Alderman BL. Determinants of resting lipid oxidation in response to a prior bout of endurance exercise. J Appl Physiol (1985) 116: 95–103, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Mau TL, Luke-Zeitoun M, Brooks GA. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol 584: 963–981, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeks J, van Baak MA, Hesselink MK, Hul GB, Vidal H, Saris WH, Schrauwen P. Effect of beta1- and beta2-adrenergic stimulation on energy expenditure, substrate oxidation, and UCP3 expression in humans. Am J Physiol Endocrinol Metab 285: E775–E782, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hoffman RP, Sinkey CA, Dopp JM, Phillips BG. Lack of effect of alpha- and beta-adrenergic inhibition on forearm glucose uptake despite differences in forearm blood flow in healthy humans. Metabolism 51: 1506–1513, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Hoffman RP, Sinkey CA, Dopp JM, Phillips BG. Systemic and local adrenergic regulation of muscle glucose utilization during hypoglycemia in healthy subjects. Diabetes 51: 734–742, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Horton TJ, Dow S, Armstrong M, Donahoo WT. Greater systemic lipolysis in women compared with men during moderate-dose infusion of epinephrine and/or norepinephrine. J Appl Physiol 107: 200–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton TJ, Miller EK, Bourret K. No effect of menstrual cycle phase on glycerol or palmitate kinetics during 90 min of moderate exercise. J Appl Physiol 100: 917–925, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Horton TJ, Pagliassotti MJ, Hobbs K, Hill JO. Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol 85: 1823–1832, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Jensen MD. Adipose tissue metabolism—an aspect we should not neglect? Horm Metab Res 39: 722–725, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kendall MJ, Clark NW, Haffner CA, Kong J, Hughes BA. Investigation of the effects of beta-2 stimulation on free fatty acids in man. J Clin Pharm Ther 16: 31–40, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Kiowski W, Buhler FR, van Brummelen P, Amann FW. Plasma noradrenaline concentration and alpha-adrenoceptor-mediated vasoconstriction in normotensive and hypertensive man. Clin Sci (Lond) 60: 483–489, 1981 [DOI] [PubMed] [Google Scholar]
- 23.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48: 275–297, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Large V, Arner P. Regulation of lipolysis in humans. Pathophysiological modulation in obesity, diabetes, and hyperlipidaemia. Diabetes Metab 24: 409–418, 1998 [PubMed] [Google Scholar]
- 25.Lima JJ, Mauras N, Kissoon N, Wang J, Wiltrout SA, Sylvester JE. Influence of sex and beta2 adrenergic receptor haplotype on resting and terbutaline-stimulated whole body lipolysis. Metabolism 54: 492–499, 2005 [DOI] [PubMed] [Google Scholar]
- 26.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol 41: 565–573, 1976 [DOI] [PubMed] [Google Scholar]
- 27.Mittendorfer B, Horowitz JF, Klein S. Effect of gender on lipid kinetics during endurance exercise of moderate intensity in untrained subjects. Am J Physiol Endocrinol Metab 283: E58–E65, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 113: 1582–1588, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson BW, Wolfe RR. Concentration dependence of methyl palmitate isotope ratios by electron impact ionization gas chromatography/mass spectrometry. Biol Mass Spectrom 22: 481–486, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 40: 2118–2124, 1999 [PubMed] [Google Scholar]
- 31.Perreault L, Lavely JM, Kittelson JM, Horton TJ. Gender differences in lipoprotein lipase activity after acute exercise. Obes Res 12: 241–249, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Rizza RA, Cryer PE, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest 64: 62–71, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab 265: E380–E391, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Schiffelers SL, Saris WH, Boomsma F, van Baak MA. Beta1- and beta2-adrenoceptor-mediated thermogenesis and lipid utilization in obese and lean men. J Clin Endocrinol Metab 86: 2191–2199, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Schiffelers SL, van Harmelen VJ, de Grauw HA, Saris WH, van Baak MA. Dobutamine as selective β1-adrenoceptor agonist in in vivo studies on human thermogenesis and lipid utilization. J Appl Physiol 87: 977–981, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187: 15–24, 1956 [DOI] [PubMed] [Google Scholar]
- 37.Tarnopolsky MA. Gender differences in substrate metabolism during endurance exercise. Can J Appl Physiol 25: 312–327, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Van Pelt RE, Gozansky WS, Hickner RC, Schwartz RS, Kohrt WM. Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity (Silver Spring) 14: 2163–2172, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wahrenberg H, Lonnqvist F, Hellmer J, Arner P. Importance of beta-adrenoceptor function in fat cells for lipid mobilization. Eur J Clin Invest 22: 412–419, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Weyer C, Tataranni PA, Snitker S, Danforth E, Jr., Ravussin E. Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective beta3-adrenoceptor agonist in humans. Diabetes 47: 1555–1561, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Wolfe RR. Tracers in metabolic research: radioisotope and stable isotope/mass spectrometry methods. Lab Res Methods Biol Med 9: 1–287, 1984 [PubMed] [Google Scholar]
- 42.Zahir M, Gould L. Phentolamine and beta-adrenergic receptors. J Clin Pharmacol New Drugs 11: 197–203, 1971 [PubMed] [Google Scholar]


