Abstract
The presence of chronic, unresolvable stresses leads to negative health outcomes, including development of clinical depression/depressive disorders, with outcome severity being correlated with depressive symptom severity. One of the major outcomes associated with chronic stress and depression is the development of cardiovascular disease (CVD) and an elevated CVD risk profile. However, in epidemiological research, sex disparities are evident, with premenopausal women suffering from depressive symptoms more acutely than men, but also demonstrating a relative protection from the onset of CVD. Given this, we investigated the differential effect of sex on conduit artery and resistance arteriolar function in male and female mice following 8 wk of an unpredictable chronic mild stress (UCMS) protocol. In males, plasma cortisol and depressive symptom severity (e.g., coat status, anhedonia, delayed grooming) were elevated by UCMS. Endothelium-dependent dilation to methacholine/acetylcholine was impaired in conduit arteries and skeletal muscle arterioles, suggesting a severe loss of nitric oxide bioavailability and increased production of thromboxane A2 vs. prostaglandin I2 associated with elevated reactive oxygen species (ROS) and an increased level of systemic inflammation. Endothelium-independent dilation was intact. In females, depressive symptoms and plasma cortisol increases were more severe than in males, although alterations to vascular reactivity were blunted, including the effects of elevated ROS and inflammation on dilator responses. These results suggest that compared with males, female rats are more susceptible to chronic stress in terms of the severity of depressive behaviors, but that the subsequent development of vasculopathy is blunted owing to an improved ability to tolerate elevated ROS and systemic inflammatory stress.
Keywords: vasodilation, endothelium-derived factors, peripheral vascular disease, nitric oxide, models of chronic stress, clinical depression
during the last decade, extensive evidence from epidemiologic and clinical studies has identified a complex relationship between depressive disorders and cardiovascular disease (CVD) outcomes (34, 41, 44, 45, 58). Depression is a powerful risk factor for development of CVD, and can be a predictor of cardiovascular pathologies, including myocardial infarction, coronary artery disease, and cardiomyopathies, regardless of prior history of overt CVD (41, 44).1
A consistent body of evidence indicates that chronic stress, a major contributor to depressive illnesses, may be a potent pathogenic factor linking depression and CVD, in part due to the stress-induced activation of the sympathetic-adrenal medullary and hypothalamic pituitary adrenal (HPA) axes (1, 2, 4, 7, 9, 47). Dysregulation of these two essential systems has been linked to depression and the development of several CVD risk factors, including sex-specific pathophysiological factors associated with autonomic nervous and immune dysfunction, and alterations to vascular reactivity and endothelial function (20, 22, 49, 56). Compared with age-matched male counterparts, premenopausal women are at lower risk of developing CVD (29, 30), but concurrently are twice as likely to suffer from depressive disorders (28, 35). This trend diminishes at menopause, and risk of CVD and neuropsychological disorders in women rapidly increases (32, 35). Current literature supports an association between estrogen and the progression of depressive symptoms and comorbid diseases (17, 35, 55), but also a relationship between estrogen and general cardiovascular protection and antioxidant defense (6, 27, 37).
We (12, 14, 26, 42) and others (3, 5, 27, 39) have previously reported that the impact of chronic stress and depression on aortic ring reactivity is mediated through altered endothelial dysfunction, characterized by an impaired dilator metabolite bioavailability. A comparable outcome, including poor endothelium-dependent reactivity (14, 25, 26, 36), nitric oxide (NO) bioavailability (42, 48), as well as a proinflammatory (24, 36, 39) and prothrombotic (43) environment, has also been observed in human patients with depression, and represents an independent predictor of risk for the development of CVD (18, 48). Because circulating levels of estrogen also exert a positive effect on processes associated with vascular function (6, 27, 35, 37), the effect of sex on CVD under conditions of chronic stress and depression is an area that warrants investigation.
The unpredictable chronic mild stress (UCMS) procedure is a widely used animal model of induced depressive symptoms and has been well established in behavioral studies as an extremely relevant rodent model of human depression on the basis of its ability to reproduce clinical symptoms of depression, including anhedonia and learned helplessness (25, 46, 59). Studies utilizing the UCMS protocol have reported increased behavioral vulnerability to UCMS in female rodents (23, 32, 45) associated with increased cortisol levels (15, 16), altered estrous cycle (15, 16, 23, 31), and decreased serotonergic and dopaminergic turnover ratios in brain regions related to stress and depression (16, 33). However, compared with male counterparts, UCMS female rodents show greater antioxidant defense and increased NO bioavailability, helping to maintain normal endothelial function and enhanced vascular anti-inflammatory and antioxidant capacities (27, 50).
The purpose of this study was to determine the differential susceptibility to depressive symptom development following UCMS between male and female mice, and to elucidate sex-specific mechanisms underlying vascular/endothelial dysfunction in both conduit vascular rings and in pressurized resistance arterioles. This study tested the hypothesis that 8 wk of UCMS will result in a more severe development of depressive symptoms in female mice (vs. males), but that vascular function will be preserved to a superior extent than in males. Of special note, this study utilized a protocol wherein the estrous cycle of female mice was not matched. Rather, female mice were randomized throughout their cycle to obtain a better assessment of a true sex difference rather than one that reflects cyclic variations in hormonal status.
MATERIALS AND METHODS
Animals.
Male and female BALB/cJ mice (Jackson) were fed standard chow and drinking water ad libitum and were housed in the animal care facility at the West Virginia University Health Sciences Center. All protocols received prior approval of the institutional animal care and use committee. At 9 wk of age, mice from each sex were divided into two groups, control and UCMS (below). After 8 wk duration under either condition, mice were anesthetized with injections of sodium pentobarbital (50 mg/kg ip) and a carotid artery was cannulated for determination of arterial pressure. Venous blood aliquots were collected for biochemical evaluation of plasma biomarkers of treatment outcomes and health status of the mice.
UCMS protocol.
All mice were doubly housed, with the control group in a separate quiet room in close proximity to the room used for UCMS treatments. Alternatively, in the UCMS group, mice were randomly exposed to the following stressors on multiple occasions throughout each 24-h period: 1) 10 oz. of water was added to each standard cage for the next 3 h (damp bedding); 2) all bedding was removed and ∼0.5 inch of water was added to empty cage for the next 3 h (water temperature was ∼30°C; room temperature was ∼24°C); 3) each cage was tilted to 45 degrees with or without bedding for 3 h; 4) each mouse was switched into a cage of a neighboring mouse for 3 h (social stress); 5) no bedding lasting for 3 h or, on two occasions each week, overnight; 6) succession of light/dark cycles, lasting 30 min throughout a 24-h period; and 7) exposure to predator smells (e.g., cat fur) and/or sounds (e.g., cat growling) for 8 h.
After 8 wk, all mice were subjected to a series of behavioral tests to evaluate the outcomes of the UCMS procedures.
Coat status.
This evaluation was carried out throughout the duration of the UCMS protocol. The total cumulative score was computed by giving an individual score of 0 (clean) or 1 (dirty) to eight body parts (head, neck, dorsal coat, ventral coat, tail, forelimb, hind-limb, and genital region).
Splash test.
This test was used to evaluate acute grooming behavior, defined as cleaning of the fur by licking or scratching. A 10% sucrose solution was sprayed on the dorsal coat of each mouse and grooming activity was recorded for 5 min. The viscosity of the sucrose solution will dirty the coat and induce grooming behavior, with depressive symptoms characterized by an increased latency (idle time between spray and initiation of grooming) and decreased frequency (number of times grooming a particular body part).
Tail suspension test.
Mice subjected to the short-term, inescapable stress of tail suspension, will develop an immobile posture, with longer periods of immobility in mice exhibiting a depressed behavior. Mice were completely suspended by the tail on a horizontal bar 35 cm from the base platform using adhesive tape such that contact with the laboratory benchtop was not possible. The latency between the first bout of immobility and the duration of total immobility was recorded for 6 min.
Measurements of vascular reactivity (skeletal muscle resistance arterioles).
From each anesthetized mouse, the intramuscular continuation of the gracilis artery was removed and cannulated, as described previously (8). These first-order arterioles were extended to their approximate in situ length and were equilibrated at 80% of mean arterial pressure to approximate in vivo intralumenal pressure. Following equilibration, the reactivity of isolated, pressurized arterioles was assessed in response to increasing concentrations of phenylephrine (10−10 M to 10−7 M) to assess adrenergic constrictor responses and increasing concentrations of acetylcholine (10−10 M to 10−6 M) and sodium nitroprusside (10−10 M to 10−6 M) to assess dilator reactivity. Vascular responses to stimuli were also determined following treatment with NG-nitro-l-arginine methyl ester (l-NAME; 10−4 M), indomethacin (INDO; 10−5 M), and 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL) (10−4 M) for 45–60 min, to assess the roles of NO, cyclooxygenase (COX), and reactive oxidant stress, respectively, in modulating reactivity.
Measurements of vascular reactivity (conduit arteries).
Following removal of the resistance arteriole in each mouse, the thoracic aorta was removed, rinsed in physiological salt solution, cleared of surrounding tissue, and cut in 2- to 3-mm ring segments. Each ring was mounted in a myobath chamber between a fixed point and a force transducer (World Precision Instruments), and set to 0.5 g tension for 45 min to equilibrate. The organ baths contained physiological salt solution at 37°C, and were aerated with 95% O2 and 5% CO2. Rings were preconditioned by treatment with 10−7 M phenylephrine for 5 min, at which time 10−5 M methacholine was added to the bath to assess endothelial integrity. Any ring that failed to demonstrate both a brisk constrictor response to phenylephrine and viable endothelial function was discarded. Subsequently, rings were treated with increasing concentrations of phenylephrine (10−10 M to 10−4 M) to assess constrictor reactivity. For assessment of dilator reactivity, rings were pretreated with 10−6 M phenylephrine and exposed to increasing concentrations of methacholine (10−10 M to 10−4 M) and sodium nitroprusside (10−10 M to 10−4 M). To assess the roles of NO, COX, and reactive oxidant stress in modulating vascular responses to the agonist treatments, concentration-response curves were also conducted following pretreatment of the rings for 45–60 min with l-NAME (10−4 M), INDO (10−5 M), and TEMPOL (10−4 M), respectively.
Measurement of vasoactive metabolite bioavailability.
From mice within each group, the remaining sections of the thoracic and abdominal aorta that were not used for measurements of reactivity were used to assess vascular NO, prostaglandin I2 (PGI2; from levels of 6-keto-PGF1α), and thromboxane A2 (TxA2; from levels of 11-dehydro-TxB2) bioavailability using amperometric sensors (World Precision Instruments) and a commercially available kits (Cayman), respectively. Briefly, aortae were isolated, cleaned, sectioned into ∼1 mm lengths and placed within a chamber filled with physiological salt solution equilibrated with 21% O2, 5% CO2, balance N2. Within this chamber, an NO sensor (ISO-NOPF 100) was inserted and a baseline current was obtained. Subsequently, methacholine (10−8, 10−6, and 10−4 M) was added to the chamber and the changes in current were determined. To verify that responses represented NO release, these procedures were repeated following addition of l-NAME (10−4 M) to the chamber. In response to challenge with 10−4 M methacholine, an aliquot of physiological salt solution was removed from the chamber and used for determination of PGI2 and TxA2 production.
Biochemical analyses.
In all samples, fasting blood glucose (8 h) was determined using a commercially available glucometer (Freestyle). Chronic oxidant stress was assessed through determination of plasma nitrotyrosine, and plasma cortisol was determined using standard kits (Cayman). Finally, using a multiplexed procedure, plasma insulin and biomarkers of inflammation were assessed using commercially available kits (Millipore).
Data and statistical analyses.
Mechanical responses following challenge with methacholine or phenylephrine were fit with the three-parameter logistic equation:
where y represents the isometric tension; min and max represent the lower (minimum) and upper (maximum) bounds, respectively, of the change in tone with agonist concentration; x is the logarithm of the agonist concentration; and logED50 represents the logarithm of the agonist concentration (x) where the response (y) is halfway between the bounds. The use of the three-parameter logistic equation is appropriate for the analysis of sigmoidal concentration-response relationships because it simultaneously provides estimates of the curve maximum (upper bound), minimum (lower bound), and the dose at which the dependent variable reaches 50% of maximum (ED50). In a dilator response in which the initial condition is set to 100% (the upper bound), the asymptotic minimum (the lower bound from the equation) is reflective of the degree of dilator reactivity for that vessel. If the vessel becomes more reactive, the lower bound will decrease toward reduced levels, whereas if the vessel becomes less reactive, the lower bound will increase toward higher values. This situation is reversed for constrictor responses, in which the vessel starts at the lower bound, and then goes through a range of increased tensions to reach an upper bound/asymptotic maximum. For the presentation of results, we have focused on the changes in the upper or lower bounds, because we did not determine a consistent or significant change to the ED50 values between relevant experiment groups.
All data are presented as means ± SE. Significant differences between groups were determined using ANOVA. In all cases, a Student-Newman-Keuls post hoc test was used when appropriate and P < 0.05 was taken to reflect statistical significance.
RESULTS
Table 1 presents data describing the characteristics of mouse groups in the present study. At the time of final use, plasma insulin and nitrotyrosine were significantly greater in UCMS mice than in controls. No consistent and significant differences were determined between body mass, mean arterial pressure, and blood glucose between conditions or sex. Plasma levels of cortisol were significantly increased with UCMS in both sexes compared with controls, and levels in UCMS females were significantly greater than those in UCMS males.
Table 1.
Baseline characteristics between mouse groups in the present study
| Males, n = 14 | Females, n = 9 | UCMS Male, n = 12 | UCMS Females, n = 12 | |
|---|---|---|---|---|
| Mass, g | 29 ± 2 | 28 ± 3 | 30 ± 3 | 29 ± 4 |
| Mean arterial pressure, mmHg | 87 ± 4 | 91 ± 4 | 94 ± 5 | 90 ± 5 |
| Insulinplasma, ng/ml | 1.1 ± 0.3 | 1.2 ± 0.4 | 4.1 ± 0.7* | 4.5 ± 0.5* |
| Glucoseblood, mg/dl | 82 ± 7 | 80 ± 8 | 94 ± 8 | 101 ± 10 |
| Cholesterolplasma, mg/dl | 71 ± 7 | 68 ± 8 | 78 ± 6 | 77 ± 10 |
| Triglyceridesplasma, mg/dl | 94 ± 6 | 101 ± 8 | 109 ± 8 | 116 ± 12 |
| Nitrotyrosineplasma, ng/ml | 12 ± 3 | 11 ± 4 | 29 ± 5* | 36 ± 6* |
| Cortisolplasma, pg/ml | 12 ± 3 | 14 ± 4 | 29 ± 5* | 44 ± 4* |
| TNF-αplasma, pg/ml | 2.3 ± 0.3 | 2.0 ± 0.2 | 4.1 ± 0.4* | 6.4 ± 0.4*† |
| MCP-1plasma, pg/ml | 2.8 ± 0.3 | 3.3 ± 0.5 | 10.2 ± 1.0* | 14.8 ± 1.3*† |
All mice were aged 17–18 wk.
P < 0.05 vs. control;
P < 0.05 vs. UCMS-males.
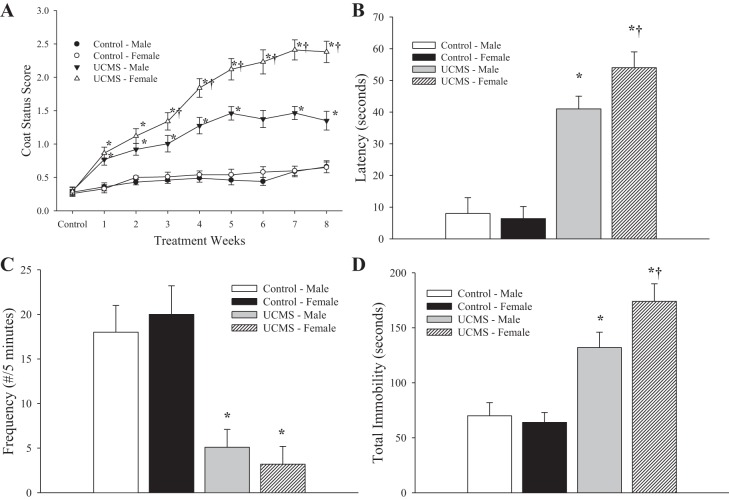
The effect of the UCMS protocol on depressive behaviors in mice is summarized in Fig. 1. Throughout 8 wk of UCMS, the coat status in mice undergoing the stress protocol was consistently poorer compared with that in control animals (Fig. 1A), although the severity of this degradation in coat status was significantly worse in UCMS females. In response to the sucrose spray, control mice demonstrated both a more rapid (Fig. 1B) and more frequent (Fig. 1C) grooming response compared with that exhibited by mice of either sex following the UCMS protocol. However, the latency of the grooming response was greater in UCMS females compared with that in UCMS males. Figure 1D presents the total period of immobility in response to tail suspension, where control mice demonstrated a significantly shorter period of immobility compared with that in UCMS mice of either sex. Consistent with the previous measurements, UCMS females demonstrated a longer period of immobility compared with UCMS males.
Fig. 1.
Depressive symptoms following 8 wk of the unpredictable chronic mild stress (UCMS) protocol in male and female BALB/cJ mice. Data are presented for coat status (A), latency (B), and frequency (C) of facial grooming following a 10% sucrose solution spray, and the total period of immobility during the tail suspension test (D) for control and UCMS mice. Control males n = 14; control females n = 9; UCMS males n = 12; UCMS females n = 12. *P < 0.05 vs. control in the sex; †P < 0.05 vs. UCMS male.
The constrictor responses of aortic rings from control and UCMS-mice in response to increasing concentrations of phenylephrine are summarized in Fig. 2. Compared with responses in aortic rings from control mice, rings from UCMS males exhibited a similar constrictor response to the adrenergic agonist (Fig. 2A). In contrast, the constrictor response of aortic rings from UCMS females was not different from that in controls. Following pretreatment of vessels from all groups with l-NAME, responses of aortic rings from the control and UCMS groups remained similar in response to increasing concentrations of phenylephrine (Fig. 2B). Pretreatment of aortic rings with INDO did not significantly alter responses to phenylephrine from that determined under untreated conditions in any group (data not shown).
Fig. 2.
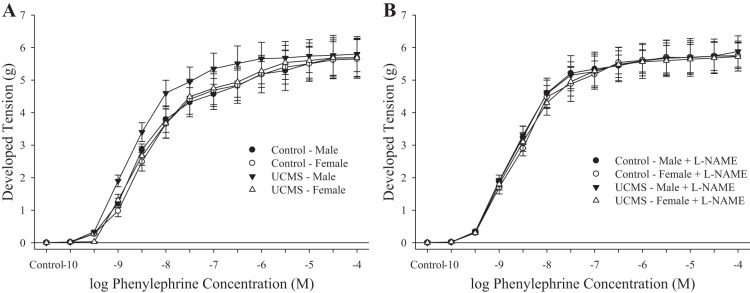
Constrictor responses of aortic rings to increasing concentrations of phenylephrine from mice under control conditions and after 8 wk of UCMS. Data are presented under untreated conditions (A) and in response to nitric oxide synthase inhibition with NG-nitro-l-arginine methyl ester (l-NAME) (B). Control males n = 12; control females n = 8; UCMS males n = 10; UCMS females n = 10.
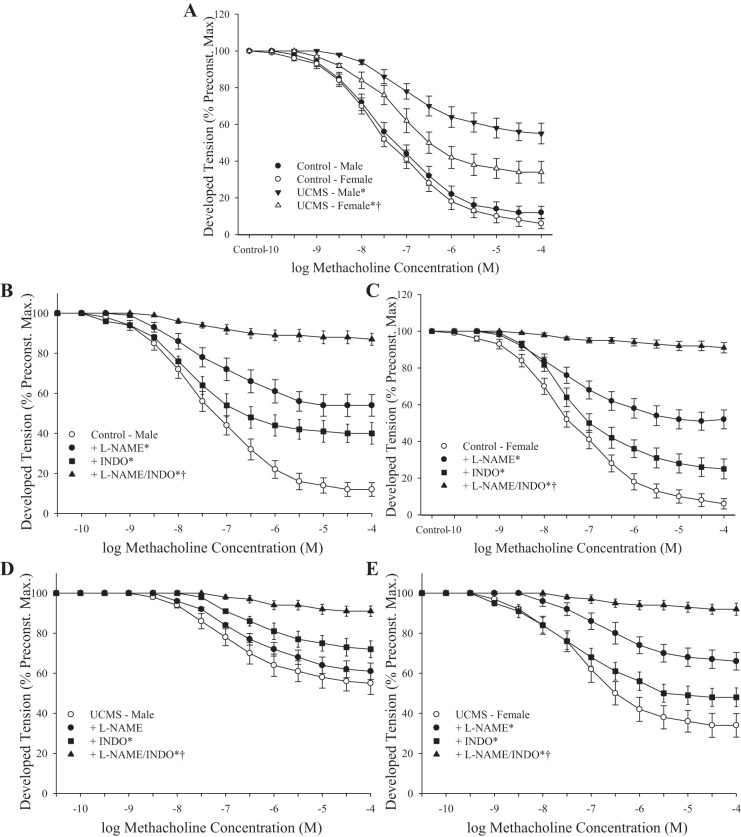
A summary of the methacholine-induced dilator responses of aortic rings from control and UCMS mice is presented in Fig. 3. Compared with responses from control mice, the dilation to increasing concentrations of methacholine was significantly reduced in both male and female UCMS mice, although the severity of this blunted response was greater in the aortic rings from UCMS males (Fig. 3A). In aortic rings from control animals, pretreatment with either l-NAME or INDO resulted in a significant inhibition of methacholine-induced dilator reactivity, whereas pretreatment with both nearly abolished all responses to the agonist (Fig. 3, B and C). In aortic rings from UCMS males, pretreatment with l-NAME did not further affect the reduced dilator responses to methacholine, although treatment with INDO reduced dilator responses from the untreated condition (Fig. 3D). In conduit artery segments from UCMS female rats, treatment with l-NAME reduced methacholine-induced dilation, whereas responses following treatment with INDO were less striking (Fig. 3E). In all cases, combined treatment with both l-NAME and INDO nearly abolished the reactivity of aortic rings to increasing concentrations of methacholine.
Fig. 3.
Dilator responses of aortic rings to increasing concentrations of methacholine from mice under control conditions and after 8 wk of UCMS. A: untreated conditions. B and C: dilator responses from male and female control mice, respectively, following pretreatment with l-NAME, indomethacin (INDO) or a combination of these. D and E: dilator responses from male and female UCMS mice, respectively, following pretreatment with l-NAME, INDO, or a combination. Control males n = 6–9; control females n = 5–7; UCMS males n = 8–10; UCMS females n = 7–10. *P < 0.05 vs. responses in untreated vascular rings from control mice of that sex; †P < 0.05 vs. responses in untreated vascular rings from UCMS male mice.
Fig. 4 summarizes the responses of aortic rings from control and UCMS mice to increasing concentrations of sodium nitroprusside. In all cases, application of the NO donor resulted in a significant dilator response that was not different between control and UCMS mice of either sex.
Fig. 4.
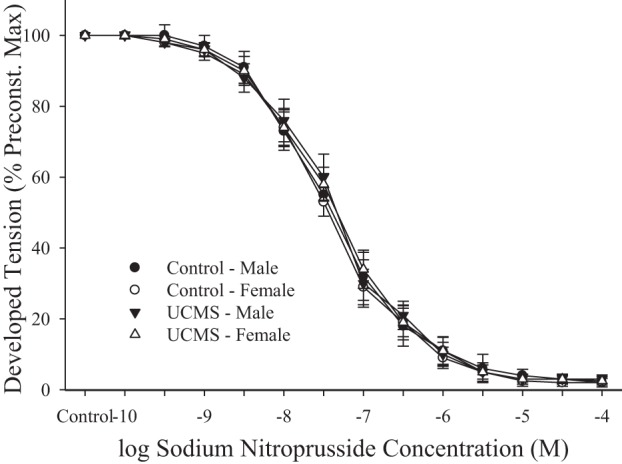
Dilator responses of aortic rings to increasing concentrations of sodium nitroprusside from mice under control conditions and after 8 wk of UCMS. Control males n = 10; control females n = 8; UCMS males n = 9; UCMS females n = 9.
The production of dilator metabolites in response to methacholine challenge is presented in Fig. 5. Vascular production of NO following application of methacholine was significantly reduced in conduit arteries of UCMS mice of either sex, although the severity of this reduction was greater in UCMS males (Fig. 5A). A similar pattern was determined for the vascular production of PGI2, with the methacholine-induced production of this metabolite, estimated from 6-keto-PGF1α, being reduced in UCMS mice of both sexes (male > female) compared with responses in arteries from control mice (Fig. 5B). Vascular production of TxA2 (Fig. 5C) was low and similar in male and female mice under control conditions. In response to the UCMS protocol, the vascular production of TxA2 was significantly elevated in both sexes, but was significantly increased in UCMS males compared with UCMS females.
Fig. 5.
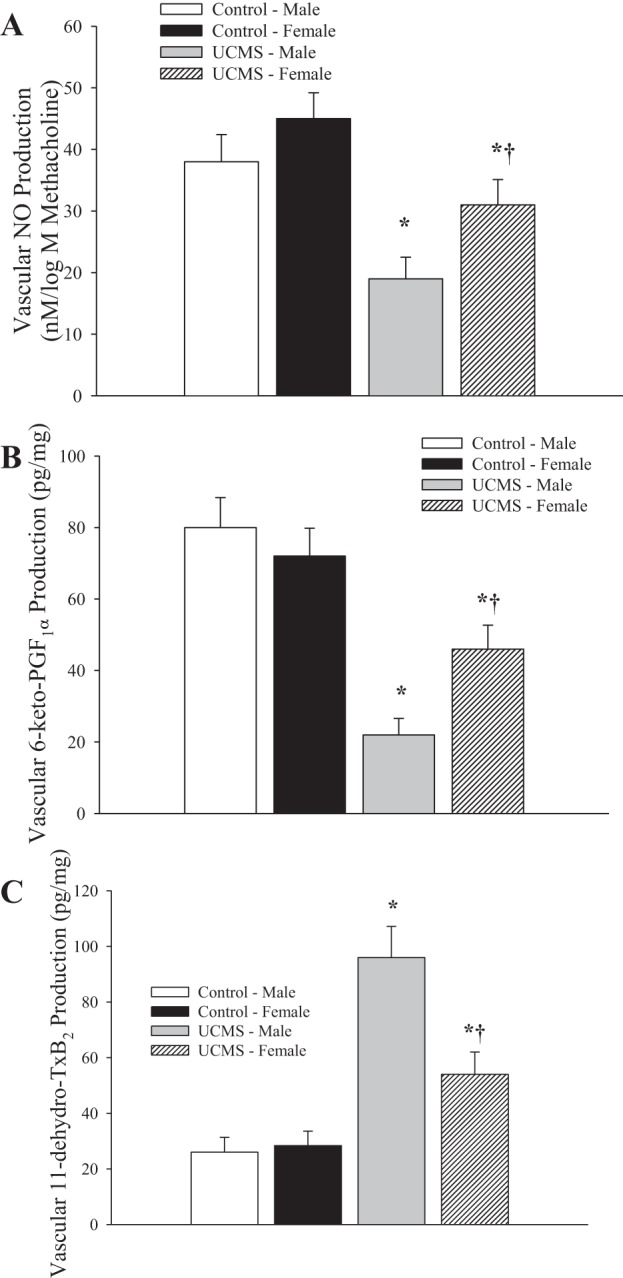
Vascular production of nitric oxide (A), prostaglandin I2 (PGI2) (B; estimated from 6-keto-PGF1α), and thromboxane A2 (TxA2) (C; estimated from 11-dehydro-TxB2) following methacholine challenge in mice under control conditions and following 8 wk of UCMS. Control males n = 9; control females n = 7; UCMS males n = 8; UCMS females n = 8. *P < 0.05 vs. responses in vessels from control mice; †P < 0.05 vs. responses in vessels from UCMS males.
The impact of pretreatment of aortic rings with the antioxidant TEMPOL on vascular production of NO following methacholine challenge is presented in Fig. 6. Although bioavailability of NO was attenuated in vessels from both male and female mice following UCMS treatment (males > females), pretreatment of vessels with TEMPOL significantly increased the bioavailability of NO in vessels from UCMS males. Although a trend for this was evident in UCMS females, this effect was not as striking, likely owing to the more modest reductions in NO bioavailability as a result of the UCMS protocol itself in these mice. Treatment of vessels with l-NAME abolished methacholine-induced NO bioavailability in all conditions.
Fig. 6.
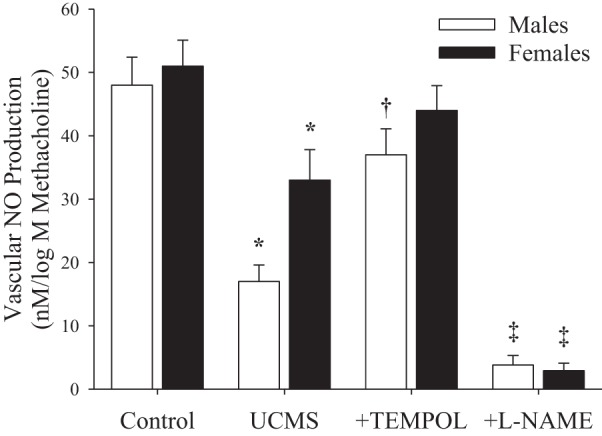
Vascular production of nitric oxide (NO) following methacholine challenge in male and female mice following 8 wk of UCMS. Data are presented for vessels under control (untreated) conditions, following treatment of vessels from UCMS animals with TEMPOL and following treatment of vessels from UCMS animals with l-NAME. Control males n = 9; control females n = 7; UCMS males n = 8; UCMS females n = 8. *P < 0.05 vs. responses in control vessels from mice of that sex; †P < 0.05 vs. responses in untreated vessels of UCMS mice of that sex; ‡P < 0.05 vs. responses in UCMS + TEMPOL-treated mice of that sex.
The effects of pretreatment of aortic rings from UCMS mice with TEMPOL on dilator responses are summarized in Fig. 7. In aortic rings from both UCMS male (Fig. 7A) and UCMS female (Fig. 7B) mice, pretreatment with TEMPOL significantly increased dilator responses to methacholine from the untreated responses; subsequent treatment of vessels with l-NAME or INDO significantly reduced dilation from this improved level of reactivity.
Fig. 7.
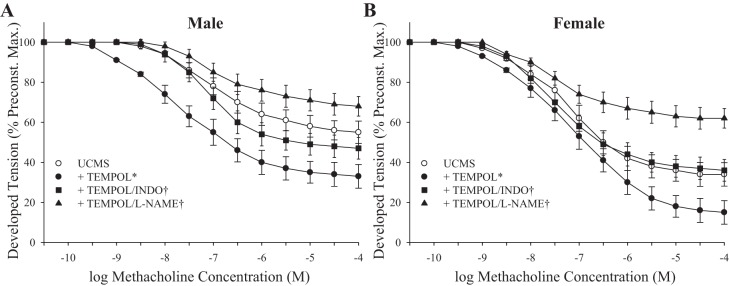
Dilator responses of aortic rings to increasing concentrations of methacholine from UCMS male (A) and UCMS female (B) mice. A: UCMS males under untreated conditions, following pretreatment with TEMPOL, following treatment with TEMPOL/INDO, and following treatment with TEMPOL/l-NAME. B: UCMS females under untreated conditions, following pretreatment with TEMPOL, following treatment with TEMPOL/INDO, and following treatment with TEMPOL/l-NAME. Control males n = 8–11; control females n = 7–9; UCMS males n = 8–9; UCMS females n = 7–9. *P < 0.05 vs. responses in untreated vessels; †P < 0.05 vs. responses in vessels that were pretreated with TEMPOL.
The constrictor responses of isolated skeletal muscle (gracilis) arterioles in response to increasing concentrations of phenylephrine are summarized in Fig. 8. In arterioles from male rats, imposition of the UCMS protocol resulted in a significant increase in the vasoconstrictor responses to phenylephrine compared with responses in vessels from control animals (Fig. 8A). Pretreatment of vessels with l-NAME abolished all differences in phenylephrine-induced constriction between sexes and treatment condition (Fig. 8B).
Fig. 8.
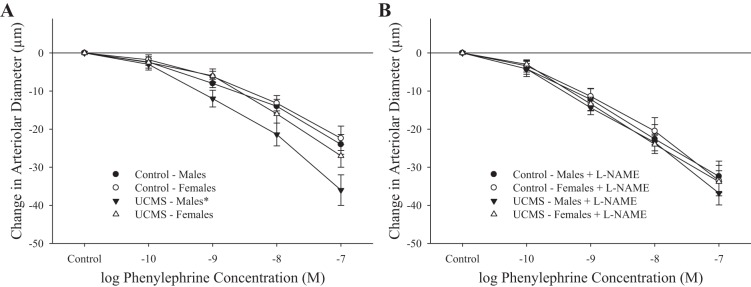
Constrictor responses of gracilis arterioles to increasing concentrations of phenylephrine from mice under control conditions and after 8 wk of UCMS. Data are presented under control conditions (A) and in response to nitric oxide synthase (NOS) inhibition with l-NAME (B). Control males n = 10; control females n = 8; UCMS males n = 10; UCMS females n = 10. *P < 0.05 vs. responses in arterioles from control mice.
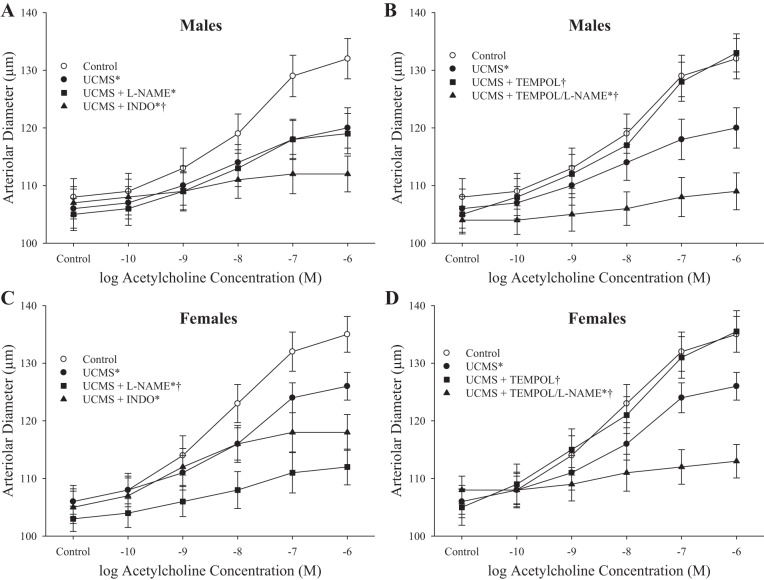
Figure 9 presents the dilator responses of gracilis muscle arterioles from control and UCMS-treated mice in response to increasing concentrations of acetylcholine. In males, UCMS treatment blunted dilator responses to acetylcholine from levels in controls; pretreatment of vessels from UCMS males with l-NAME had no effect on reactivity, whereas pretreatment with INDO further blunted this impaired response (Fig. 9A). However, treatment of arterioles from UCMS males with TEMPOL was very effective at restoring dilator reactivity to acetylcholine (Fig. 9B). The dilator response in UCMS females in response to acetylcholine was reduced compared with that from controls, and pretreatment with either l-NAME or INDO caused a further reduction in reactivity (Fig. 9C). Similar to that in UCMS males, treatment of vessels from UCMS females with TEMPOL restored dilator responses to increasing concentrations of acetylcholine (Fig. 9D).
Fig. 9.
Dilator responses of gracilis arterioles to increasing concentrations of acetylcholine from mice under control conditions and after 8 wk of UCMS. A: male mice under untreated conditions and following pretreatment with l-NAME or INDO. B: male mice after pretreatment with TEMPOL alone or with l-NAME. C: female mice under untreated conditions and following pretreatment with l-NAME or INDO. D: female mice after pretreatment with TEMPOL alone or with l-NAME. Control males n = 8–10; control females n = 7–8; UCMS males n = 9–10; UCMS females n = 8–10. *P < 0.05 vs. responses in vessels from control mice of that sex; †P < 0.05 vs. responses in vessels from UCMS mice of that sex.
The correlation between UCMS-induced changes in systemic inflammation and vascular dysfunction is summarized in Fig. 10 for tumor necrosis factor-α (TNF-α) (Fig. 10A) and monocyte chemoattractant protein-1 (MCP-1) (Fig. 10B). The degree of vascular dysfunction, estimated from the lower bound of the methacholine concentration-response relationship for aortic rings (determined from the logistic equation described above), demonstrated a positive relationship with the degree of systemic inflammation as a result of the UCMS protocol. However, when split into sexes, a more severe relationship was evident in males, in which similar levels of either TNF-α (Fig. 10A) or MCP-1 (Fig. 10B) was associated with a greater degree of vascular dysfunction (i.e., a higher lower bound) in males than in females. This was evident under both control and UCMS conditions. Interestingly, within either sex, imposition of the UCMS protocol did not appear to change the inherent relationship between markers of inflammation and vascular dysfunction. Rather, the relationship between the parameters remained similar, but was shifted to a higher severity of dysfunction.
Fig. 10.
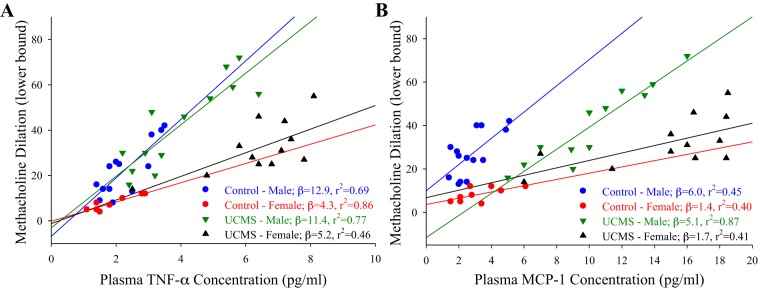
Correlations between vascular reactivity and individual markers of chronic inflammation in control male (blue), control female (red), UCMS male (green), and UCMS female (black) mice. Data are presented as the lower bound of the methacholine concentration-response curve for an individual aortic ring vs. plasma concentrations of tumor necrosis factor-α (TNF-α) (A) or monocyte chemoattractant protein-1 (MCP-1) (B). In this figure, the lower bound from methacholine concentration-response curve is reflective of the degree of dilator reactivity for that vessel. If the vessel is more reactive to methacholine, the lower bound is decreased, whereas if the vessel becomes less reactive, the lower bound is increased. Also presented are lines of best fit through the data for each group, with the resulting slope (β) and r2 values presented in the legend.
DISCUSSION
With an escalating body of clinical and epidemiological evidence demonstrating that sex differences influence the link between chronic stress, the onset of depressive symptoms and cardiovascular disease risk and outcomes, validated preclinical models offer a valuable translational tool for investigating the underlying mechanisms of disease and offer an efficacious method for studying novel interventional therapies and treatment options. This study utilized the UCMS protocol, a validated model of chronic stress-induced depressive symptoms in rodents, to interrogate sex-specific mechanisms of vascular dysfunction in UCMS mice.
The results of this study provide evidence that male and female mice experience graded outcomes related to the severity of depressive symptom development and an almost paradoxically gradation of the impairment to vascular reactivity after 8 wk of the UCMS protocol. Increased behavioral susceptibility to stress conditions was observed in female mice vs. male counterparts, as evidenced by worsened coat grooming score, increased immobility in the tail suspension test (behavioral despair), and elevated plasma levels of corticosterone. Estrogen levels are known to amplify the magnitude of the stress response at the HPA axis (4, 23, 57, 60, 61), offering a potential explanation for the increased corticosterone and behavioral symptom severity observed in UCMS female mice. UCMS-induced impairments to vascular reactivity and function were observed for both sexes, demonstrated by blunting of dilator responses to methacholine/acetylcholine in isolated aortic rings and in pressurized resistance arterioles, respectively. However, these physiological impairments were also sex-specific, in that female UCMS mice developed greater elevations to biomarkers of oxidative stress and inflammation, yet demonstrated a more modest impairment to vascular reactivity, despite more severe deterioration in the behavioral measures.
Building on our previous report that vasodilator reactivity is blunted in aortic rings of UCMS male mice (14, 26), our new findings suggest that the impaired dilator responses in UCMS mice of both sexes are due to endothelial dysfunction, because dilations to exogenous NO were similar across all groups, as were constrictor responses to phenylephrine, suggesting that an 8-wk UCMS protocol is not sufficient to significantly alter vascular wall mechanics or vascular smooth muscle function. Vasodilator responses were further blunted in aortic rings and gracilis arterioles of UCMS females following pretreatment with l-NAME; however, this treatment had minimal effects on vascular reactivity in UCMS males, likely owing to the preexisting severe attenuation of vascular NO bioavailability determined in UCMS males. Instead, the blunted dilator responses in vessels from UCMS males were further impaired as a result of COX inhibition with INDO, suggesting that a dependence on dilator influence of arachidonic acid metabolites (likely PGI2) helps to maintain vascular reactivity in the compromised state. However, vascular PGI2 production alone did not strongly correlate across all of the observed mechanical responses, suggesting a potential contributing role for other COX metabolites. Indeed, proinflammatory, oxidative stress conditions induced by chronic stress can alter the balance between constricting and dilating metabolites, specifically shifting arachidonic acid metabolism by COX in favor of increased production of vasoconstricting metabolites (e.g., thromboxane, TxA2) that can compete against the effect of stimulus-induced vasodilation by NO, PGI2, or other dilator metabolites (14, 26). Our results suggest that an elevation in stimulus-induced vascular TxA2 generation may contribute to the integrated vasoreactivity in this model. Given the integral role of inflammation and oxidant stress that has been demonstrated with chronic stress and depressive symptoms, and the impact of chronic elevations in vascular TxA2 production will have on negative cardiovascular outcomes in terms of perfusion control and the antithrombotic functions (among others) of the endothelium, the impact of chronic inhibition of these pathways on both the behavioral and vascular outcomes in UCMS-induced depressive symptoms clearly represents important areas for future interrogation.
Whereas the results of this study found only very limited evidence of an increased vascular reactivity to adrenergic constriction in UCMS males, they largely appear to be a reflection of the degree to which endothelial function was impaired as a result of the UCMS protocol. However, the vasoconstrictor response of all animal groups to increasing concentrations of phenylephrine converged at very similar levels following inhibition of NO synthase (NOS) activity via pretreatment with l-NAME, suggesting that the role endogenous NO bioavailability plays in moderating adrenergic constriction under normal conditions remains largely intact in females despite UCMS, but is diminished in males following UCMS protocols.
The relationship between systemic inflammation induced by UCMS and the severity of vascular dysfunction observed in males and females were investigated to determine sex differences in the effects of chronic inflammation on vascular reactivity. Circulating plasma levels of proinflammatory markers MCP-1 and TNF-α were associated with vascular dysfunction for all UCMS animals, but a greater degree of vascular dysfunction was observed in UCMS males than in UCMS females, despite the observation that the magnitude of the inflammatory markers was somewhat higher in female mice. When taken together with the higher plasma levels of ROS in female UCMS mice vs. males as well, these data suggest that males experience a substantially greater endothelial susceptibility to UCMS-induced dysfunction, raising the possibility of a protective mechanism for female sex hormones that effectively reduces impairment of endothelium-dependent vasodilation during exposure to chronic stress.
There is extensive evidence for the vasoprotective actions of estrogen against oxidative and inflammatory stressors. Estrogen increases NOS activity and promotes NO bioavailability from the vascular endothelium (15, 27, 60); it is also linked to increased responsiveness to β2-adrenergic-mediated vasodilation (19, 21, 38), partially through an NO-related mechanism (19, 51). In addition to supportive effects on NO, estrogen may also influence production of PGI2 via upregulation of the COX pathway, specifically COX-1 and prostacyclin synthase, in some vascular beds (40, 51, 53, 60). Although less is known about androgens, it has been reported that testosterone supplementation impairs vascular reactivity and antioxidant capacity (10) in females by mitigating vascular sensitivity to NO (11, 52). Females may therefore have a more robust and protected endothelium-dependent vasodilator capacity than males due to higher levels of circulating estrogen and significantly less testosterone, thus mitigating the inflammatory and oxidative effects of chronic stress on vascular reactivity (13, 54, 60).
That being stated, the purpose of the study was not to test the effects of cyclic variation in estrogen levels on vascular function; rather, it was to determine the potential effect of a true difference in sex. As such, the female mice used in this study were randomized within their estrous cycle. Given the robustness of the differences in outcomes (both behavioral and vascular) as a result of the UCMS protocol, the fact that these mice were not studied at the same points in their estrous cycle has significant implications. Most critically, these results suggest that the protective effect associated with the female sex may not be critically dependent on the specific time within the estrous cycle or on specific hormonal profiles at that time, but that it may be much more stable and afford a degree of protection that is relatively insensitive to the day-to-day fluctuations in hormonal profiles. This is especially compelling because it suggests that the potentially beneficial and protective mechanisms that are associated with the female sex remain in place throughout the estrous cycle and may not fluctuate along the same temporal pattern as the hormonal levels. The molecular basis for this temporal pattern, as well as the alternate hypothesis that the lower levels of androgen hormones is actually the basis for the protection in this model of chronic stress and depressive symptoms and poor cardiovascular outcomes, will require future targeted investigation.
Summary and conclusions.
In response to imposition of chronic unresolvable stresses onto male and female mice, the results of the present study indicate that behavioral impairments, elevations in plasma cortisol, and plasma markers of oxidant stress and inflammation demonstrated by female mice are significantly greater than those demonstrated by male mice. However, despite these elevated responses, the vasculopathy in both the conduit and resistance vasculature of female mice is less severe than that in males. Much of this blunted impairment appears to be a function of a superior maintenance of endothelial function, primarily via more normal levels of NO bioavailability and a more normal balance between the production of PGI2 and TxA2 to help maintain dilator reactivity. Because female rats were randomized with respect to their time in estrous cycle, these data appear to reflect a longer, more temporally stable, protective effect of being female rather than one that reflects the more labile temporal changes in hormonal levels. In addition, the correlational analyses between inflammatory biomarkers and vascular dysfunction suggest that fundamental differences exist between male and female sex with regard to these associations. However, imposition of the UCMS protocol does not appear to cause the differences in the sex-based protections to diminish. Rather, UCMS appears to right-shift these associations along the same sex-specific lines to a higher degree of vascular dysfunction. Future research into the specific nature of the temporal nature of these protective effects and how they can be exploited therapeutically to minimize the devastating effects of depressive symptoms on cardiovascular health outcomes represents a key area for future investigation.
GRANTS
Support for this study was provided by American Heart Association Grants IRG-14330015, PRE-16850005, and EIA-0740129N; and by National Institutes of Health Grants RR-2865AR and P20 RR-016477.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.C.S., S.D.B., J.T.B., A.C.d., S.J.F., and J.C.F. conception and design of research; S.C.S., S.D.B., J.T.B., and J.C.F. performed experiments; S.C.S., S.D.B., S.J.F., and J.C.F. analyzed data; S.C.S., S.D.B., J.T.B., A.C.d., S.J.F., and J.C.F. interpreted results of experiments; S.C.S., S.D.B., and J.C.F. drafted manuscript; S.C.S., S.D.B., A.C.d., and J.C.F. edited and revised manuscript; S.C.S., S.D.B., J.T.B., A.C.d., S.J.F., and J.C.F. approved final version of manuscript; J.C.F. prepared figures.
ACKNOWLEDGMENTS
We thank staff members in the Office of Laboratory Animal Research at West Virginia University for their expertise and assistance with procedures. We also acknowledge the support provided through Center for Cardiovascular and Respiratory Sciences at the West Virginia University Health Sciences Center.
Footnotes
This article is the topic of an Invited Editorial by DiVincenzo et al. (18a).
REFERENCES
- 1.Antonijevic IA. Depressive disorders – is it time to endorse different pathophysiologies? Psychoneuroendocrinology 31: 1–15, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci 29: 185–193, 2004 [PMC free article] [PubMed] [Google Scholar]
- 3.Bayramgurler D, Karson A, Yazir Y, Celikyurt IK, Kurnaz S, Utkan T. The effect of etanercept on aortic nitric oxide-dependent vasorelaxation in an unpredictable chronic, mild stress model of depression in rats. Eur J Pharmacol 710: 67–72, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav 75: 661–673, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 64: 43–51, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Borrás C, Gambini J, Gómez-Cabrera MC, Sastre J, Pallardó FV, Mann GE, Viña J. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell 4: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav 43: 48–59, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Butcher JT, Goodwill AG, Frisbee JC. The ex vivo isolated skeletal microvessel preparation for investigation of vascular reactivity. J Vis Exp. First published April 28, 2012; 10.3791/3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell T, Lin S, DeVries C, Lambert K. Coping strategies in male and female rats exposed to multiple stressors. Physiol Behav 78: 495–504, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Chainy GB, Samantaray S, Samanta L. Testosterone-induced changes in testicular antioxidant system. Andrologia 29: 343–349, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension 49: 1442–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Chrapko WE, Jurasz P, Radomski MW, Lara N, Archer SL, Le Melledo JM. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry 56: 129–134, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-κ. J Appl Physiol 105: 1333–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d'Audiffret AC, Frisbee SJ, Stapleton PA, Goodwill AG, Isingrini E, Frisbee JC. Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol 108: 1041–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience 135: 703–714, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, …Papadopoulou-Daifoti Z. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav 93: 595–605, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience 132: 57–64, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos N, Piperi C, Salonicioti A, Mitsonis C, Liappas I, Lea RW, Kalofoutis A. Elevation of plasma concentration of adhesion molecules in late-life depression. Int J Geriatr Psychiatry 21: 965–971, 2006 [DOI] [PubMed] [Google Scholar]
- 18a.DiVincenzo L, Reber M, Perera V, Chilian WM. Connecting the dots—Establishing causality between chronic stress, depression, and cardiovascular disease. J Appl Physiol; 10.1152/japplphysiol.00856.2014 [DOI] [PubMed] [Google Scholar]
- 19.Ferrer M, Meyer M, Osol G. Estrogen replacement increases beta-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res 33: 124–131, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Gallucci WT, Baum A, Laue L, Rabin DS, Chrousos GP, Gold PW, Kling MA. Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health Psychol 12: 420–425, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO, 3rd. Acute vascular effects of estrogen in postmenopausal women. Circulation 90: 786–791, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 30: 431–438, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 179: 769–780, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37: 137–162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isingrini E, Belzung C, Freslon JL, Machet MC, Camus V. Fluoxetine effect on aortic nitric oxide-dependent vasorelaxation in the unpredictable chronic mild stress model of depression in mice. Psychosom Med 74: 63–72, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Isingrini E, Surget A, Belzung C, Freslon JL, Frisbee J, O'Donnell J, Camus V, d'Audiffret A. Altered aortic vascular reactivity in the unpredictable chronic mild stress model of depression in mice: UCMS causes relaxation impairment to ACh. Physiol Behav 103: 540–546, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Kamper EF, Chatzigeorgiou A, Tsimpoukidi O, Kamper M, Dalla C, Pitychoutis PM, Papadopoulou-Daifoti Z. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol Behav 98: 215–222, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry 163: 109–114, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289: 3095–3105, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord 29: 85–96, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res 992: 227–238, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry 167: 1305–1320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert G, Johansson M, Agren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry 57: 787–793, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 66: 305–315, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: 948–954, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, Dal-Pizzol F, Gavioli EC, Quevedo J. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 54: 358–362, 2009 [DOI] [PubMed] [Google Scholar]
- 37.MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, Shaul PW. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res 81: 355–362, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR. Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol 547, Pt 1: 309–316, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munhoz CD, Garcia-Bueno B, Madrigal JL, Lepsch LB, Scavone C, Leza JC. Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res 41: 1037–1046, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol 284: R1055–R1062, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Patel V, Garrison P, de Jesus Mari J, Minas H, Prince M, Saxena S; Advisory Group of the Movement for Global Mental. The Lancet's series on global mental health: 1 year on. Lancet 372: 1354–1357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng YL, Liu YN, Liu L, Wang X, Jiang CL, Wang YX. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J Neuroinflammation 9: 75, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto VL, de Souza PF, Brunini TM, Oliveira MB, Moss MB, Siqueira MA, Ferraz MR, Mendes-Ribeiro AC. Low plasma levels of L-arginine, impaired intraplatelet nitric oxide and platelet hyperaggregability: implications for cardiovascular disease in depressive patients. J Affect Disord 140: 187–192, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Pizzi C, Manzoli L, Mancini S, Bedetti G, Fontana F, Costa GM. Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors. Atherosclerosis 212: 292–298, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Plante GE. Depression and cardiovascular disease: a reciprocal relationship. Metabolism 54, Suppl 1: 45–48, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci 11: 53–58, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160: 1554–1565, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol 88: 196–198, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Rivier C, Vale W. Effect of the long-term administration of corticotropin-releasing factor on the pituitary-adrenal and pituitary-gonadal axis in the male rat. J Clin Invest 75: 689–694, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circ Physiol 280: H1699–H1705, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17-estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol 293: H3713–H3719, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health–National Heart, Lung, and Blood Institute sponsored Women's Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 93: 1276–1284, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Singh H, Schwartzman ML. Renal vascular cytochrome P450-derived eicosanoids in androgen-induced hypertension. Pharmacol Rep 60: 29–37, 2008 [PubMed] [Google Scholar]
- 54.Takahashi M, Suzuki E, Takeda R, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, HirataY. Angiotensin II and tumor necrosis factor-α synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-κB, p38, and reactive oxygen species. Am J Physiol Heart Circ Physiol 294: H2879–H2888, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem 90: 155–163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tobet SA, Handa RJ, Goldstein JM. Sex-dependent pathophysiology as predictors of comorbidity of major depressive disorder and cardiovascular disease. Pflugers Arch 465: 585–594, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vamvakopoulos NV. Sexual dimorphism of stress response and immune/inflammatory reaction: the corticotropin releasing hormone perspective. Mediators Inflamm 4: 163–174, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382: 1575–1586, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134: 319–329, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens 24: 740–749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry 57: 1157–1162, 2000 [DOI] [PubMed] [Google Scholar]