Abstract
Pneumoperitoneum for laparoscopic surgery is known to stiffen the chest wall and respiratory system, but its effects on resting pleural pressure in humans are unknown. We hypothesized that pneumoperitoneum would raise abdominal pressure, push the diaphragm into the thorax, raise pleural pressure, and squeeze the lung, which would become stiffer at low volumes as in severe obesity. Nineteen predominantly obese laparoscopic patients without pulmonary disease were studied supine (level), under neuromuscular blockade, before and after insufflation of CO2 to a gas pressure of 20 cmH2O. Esophageal pressure (Pes) and airway pressure (Pao) were measured to estimate pleural pressure and transpulmonary pressure (Pl = Pao − Pes). Changes in relaxation volume (Vrel, at Pao = 0) were estimated from changes in expiratory reserve volume, the volume extracted between Vrel, and the volume at Pao = −25 cmH2O. Inflation pressure-volume (Pao-Vl) curves from Vrel were assessed for evidence of lung compression due to high Pl. Respiratory mechanics were measured during ventilation with a positive end-expiratory pressure of 0 and 7 cmH2O. Pneumoperitoneum stiffened the chest wall and the respiratory system (increased elastance), but did not stiffen the lung, and positive end-expiratory pressure reduced Ecw during pneumoperitoneum. Contrary to our expectations, pneumoperitoneum at Vrel did not significantly change Pes [8.7 (3.4) to 7.6 (3.2) cmH2O; means (SD)] or expiratory reserve volume [183 (142) to 155 (114) ml]. The inflation Pao-Vl curve above Vrel did not show evidence of increased lung compression with pneumoperitoneum. These results in predominantly obese subjects can be explained by the inspiratory effects of abdominal pressure on the rib cage.
Keywords: laparoscopy, respiratory mechanics, esophageal pressure, esophageal balloon, diaphragm
pneumoperitoneum is usually induced before laparoscopic surgery by insufflating CO2 or air into the abdomen, and it is known to increase respiratory system elastance (Ers) (i.e., decrease respiratory system compliance) by stiffening the chest wall. Pneumoperitoneum has also been reported to stiffen the lung in some studies (3, 6, 16, 22), but not in others (4, 12), and it decreases the end-expiratory lung volume (EELV)(4, 8, 22). Ventilation strategies recommended to mitigate the respiratory effects of pneumoperitoneum include raising positive end-expiratory pressure (PEEP) and applying recruitment maneuvers to reexpand collapsed air spaces (3, 8, 16, 29, 32).
The mechanisms whereby the chest wall and lung become stiffer have not been fully described. Although increasing intraperitoneal volume would logically raise abdominal pressure and expand the abdominal wall to a less compliant region of its pressure-volume characteristic, this alone cannot explain chest wall stiffening, because application of PEEP after pneumoperitoneum, which would only further expand the abdominal wall volume, causes a decrease in chest wall (Ecw) and lung elastance (El) (3, 4, 16). However, acute increases in abdominal volume also stretch the diaphragm and stiffen the chest wall, as demonstrated in studies in dogs and head-up pigs (13, 14, 22). Therefore, pneumoperitoneum could stiffen the abdominal wall and diaphragm while raising pleural pressure and compressing the lungs. The lungs, being exposed to a sustained high pleural pressure, might become stiffer secondary to airway closure, as in obesity (2, 23). Although pleural pressure during pneumoperitoneum has been estimated in animals using pleural or esophageal balloons (4, 12, 22), esophageal pressure (Pes) measurements have not been reported at resting lung volume (Vl) during pneumoperitoneum in humans. The present study was undertaken to test the hypothesis that raising intra-abdominal pressure would raise pleural (esophageal) pressure, compress the lungs, and reduce Vl at equilibrium, and thereby stiffen the lungs by mechanisms similar to those described in obese subjects (2).
METHODS
Subjects.
Twenty-nine subjects were recruited before elective laparoscopic surgery using a protocol approved by our research ethics committee. Operations included cholecystectomy (2), exploratory laparoscopy, fundoplication, gastric banding (3), gastric bypass (7), gastric sleeve, herniorrhaphy, and hysterectomy (3). No subject had a history of lung or respiratory disease or any contraindication for esophageal manometry. Patients were anesthetized, pharmacologically paralyzed, intubated, and mechanically ventilated while supine and level. Airflow was measured with a Fleisch pneumotachograph and integrated later to obtain volume change. Airway pressure (Pao) was measured at the endotracheal tube, and Pes was measured using an esophageal balloon catheter, filled with 0.5 ml air, and passed to position the tip of the balloon 40–45 cm from the nares or incisors. Transpulmonary pressure (Pl) was calculated as Pl = Pao − Pes. Pressure and flow signals were digitized, recorded, and analyzed using WinDaq hardware and software (Dataq Instruments, Akron, OH).
Protocol.
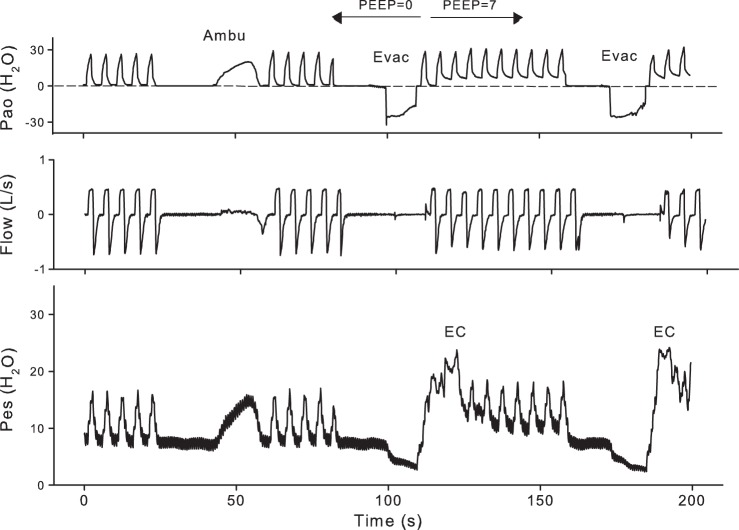
Measurements were made before and after insufflation of the abdomen using a clinical CO2 insufflator to maintain a peritoneal gas pressure of 15 mmHg (20 cmH2O). Respiratory mechanics were measured during volume-controlled mechanical ventilation with a PEEP of 0 and then 7 cmH2O (Fig. 1), with tidal volume (Vt) and frequency set by the anesthesiologist. To measure the minimum Pao [threshold pressure (Pao,thr)] required to initiate lung inflation from relaxation volume (Vrel), the ventilator was disconnected to let the subject exhale to atmospheric pressure for 15 s. An Ambu bag was connected in place of the ventilator, and Pao was gradually raised over several seconds by squeezing the Ambu bag while watching the Pao trace to produce a ramp increase in Pao (Ambu in Fig. 1). Then the subject was returned to mechanical ventilation.
Fig. 1.
Continuous recording from subject P7 illustrating the measurements before pneumoperitoneum. See text for explanation. Esophageal pressure (Pes) was not measured in periods of esophageal contraction (EC) like those after the negative pressure evacuations (Evac). (The small expiratory flow at Evac is not apparent at this scale.) Pao, airway pressure; PEEP, positive end-expiratory pressure; Ambu, Ambu bag.
To measure changes in Vrel caused by pneumoperitoneum, we measured the volume difference between Vrel and minimal gas volume (here assumed to be residual volume). The subject was allowed to exhale to atmospheric pressure for 15 s, after which Pao was reduced to −25 cmH2O for ∼5 s, and the expired volume was measured by integrating flow during the period of negative Pao (Evac in Fig. 1). The exhaled volume determined in this way was termed the expiratory reserve volume (ERV). Assuming that the minimal gas volume did not change with pneumoperitoneum, the measured change in ERV was equal to the change in Vrel. After a period of mechanical ventilation with 7-cmH2O PEEP, the ERV was determined again (Fig. 1). Then pneumoperitoneum was induced with insufflation of CO2, and the entire sequence of measurements was repeated.
Analysis.
During tidal ventilation before each respiratory maneuver, pressures, flow, and volume were measured in a representative breath at end expiration (ee) and during the 150 ms end-inspiratory pause (ei), and at the end of the exhalation to Vrel. Ers, Ecw, and El were calculated as follows: Ers = (Pao,ei − Pao,ee)/Vt; Ecw = (Pes,ei − Pes,ee)/Vt; El = (Pl,ei − Pl,ee)/Vt. The data from tidal breathing during the two periods on 0-cmH2O PEEP were averaged. Pes values measured during relaxation after exhalations to Vrel (Pes,rel) were averaged before and again after pneumoperitoneum. An increase in Pes,rel or decrease in ERV during pneumoperitoneum would indicate compression of the lungs at Vrel.
The possibility that pneumoperitoneum caused lung compression and airway closure near Vrel was assessed in two ways that do not require Pes measurements. In the first method, we determined the minimum Pao required to initiate inflation. Volume-time data during the slow increase in Pao were fit to two straight lines: a line of constant volume before inflation, and a line fit to the initial linear portion of the inflation volume-time curve. The minimum Pao required to initiate inflation, the Pao,thr, was estimated as the Pao measured at the intersection of these two lines (Fig. 2). Significant compression of the lungs by pneumoperitoneum would be expected to increase Pao,thr.
Fig. 2.
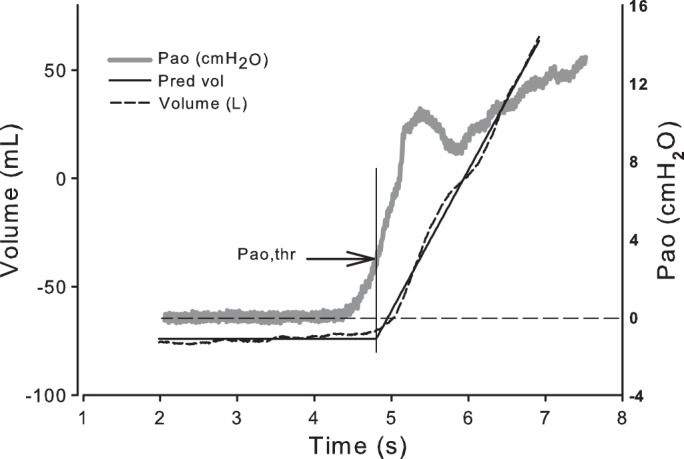
Tracings of volume and Pao during slow inflation before pneumoperitoneum in subject P5. Lines fit to the volume trace intersect at the time when Pao is 3.9 cmH2O (arrow), indicating the threshold Pao (Pao,thr) needed to start inflating the lungs. This pressure was determined before and during pneumoperitoneum in all subjects. Pred vol, predicted volume.
In the second method, the Pao-Vl curves from the slow inflations of each subject were examined for a lower “knee” or region of maximal curvature, indicating increasing compliance (decreasing elastance) with increasing Vl, suggesting progressive recruitment of lung (Fig. 3). We compared Pao-Vl curves before and during pneumoperitoneum to see if they were displaced to higher inflation pressures by pneumoperitoneum, suggesting lung compression.
Fig. 3.
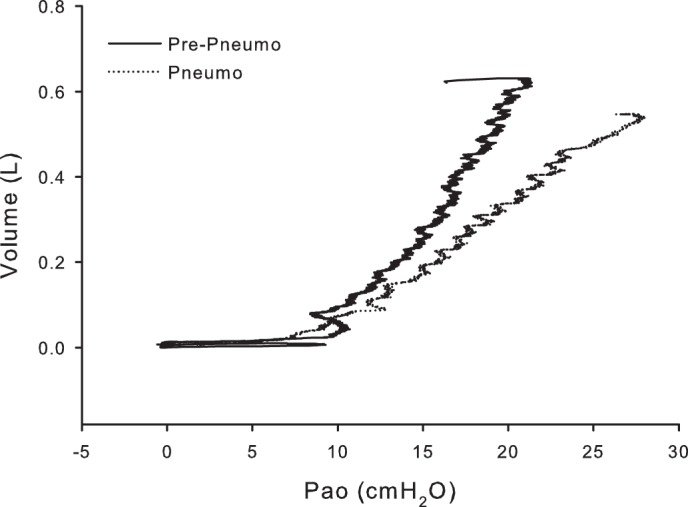
Pressure-volume curve during the slow inflations before and during pneumoperitoneum in subject P5. In this subject, there is no apparent shift in the Pao,thr required to begin inflating the lungs.
Statistics.
Results are presented as means (SD) and as percentages for categorical data. We utilized paired t-test for the pre-pneumoperitoneum vs. pneumoperitoneum and PEEP 0 vs. 7 cmH2O analyses. Significance was assumed for P < 0.05 (two-tailed).
RESULTS
Of the 29 patients recruited, 3 were excluded because of a change in experimental protocol, and 7 others were excluded due to technical problems or major deviations from the protocol. The 19 subjects analyzed averaged 43 yr in age (range 20–74 yr), and 4 of 19 were men. The body mass index (BMI) averaged 39.8 (9.8) [mean (SD)], reflecting the study population that included patients undergoing bariatric surgery; 14 were obese (BMI > 30), 3 were overweight (BMI = 25–30), and 2 were of normal weight (BMI = 18.5–25).
At Vrel, pneumoperitoneum did not significantly affect either Pes or ERV (Fig. 4, A and B). There were small, inconsistent changes in Pes,rel, which decreased on average from 8.7 (3.4) to 7.6 (3.2) cmH2O (P = 0.26), and ERV, which decreased from 183 (142) to 155 (114) ml (P = 0.089). Pneumoperitoneum did not significantly change Pao,thr required to initiate inflation, which changed from 1.1 (0.9) to 0.7 (1.4) cmH2O (P = 0.16). In addition, no subject whose inflation Pao-Vl curve from Vrel showed a pronounced upward curvature (knee), suggesting airway closure and recruitment, showed an increase in the transrespiratory pressure required to begin inflation after pneumoperitoneum (Fig. 3). These findings suggest that at Vrel, pneumoperitoneum does not increase pleural pressure or compress the lungs.
Fig. 4.
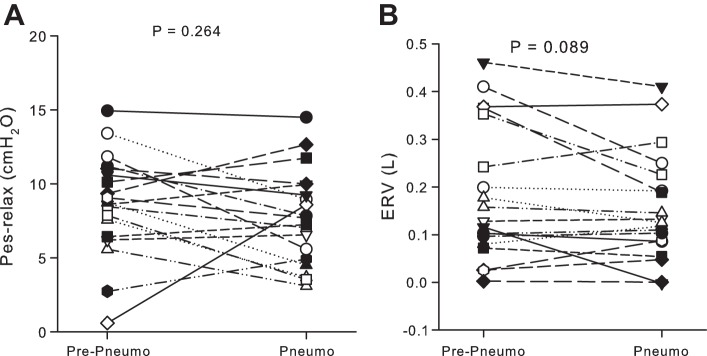
Effects of pneumoperitoneum at relaxation volume. A: Pes at relaxation volume. B: expiratory reserve volume (ERV), the volume exhaled during application of negative Pao. There was no significant effect of pneumoperitoneum. Each symbol represents a subject.
Pneumoperitoneum increased Ers and Ecw during tidal ventilation with 0- and 7-cmH2O PEEP (P < 0.0001). El was not significantly affected (Table 1).
Table 1.
Effects of pneumoperitoneum and PEEP on elastance
| PEEP = 0 cmH2O |
PEEP = 7 cmH2O |
|||||
|---|---|---|---|---|---|---|
| Pre | Pneumo | P Value | Pre | Pneumo | P Value | |
| Ers | 29.6 (11.2) | 42.9 (11.9) | <.0001 | 27.7 (14.7) | 38.7 (12.9)‡ | <0.0001 |
| El | 20.0 (12.2) | 21.6 (10.1) | 0.253 | 19.0 (15.2) | 19.6 (11.9) | 0.66 |
| Ecw | 10.5 (3.8) | 21.4 (4.6) | <.0001 | 9.0 (3.7)* | 18.2 (6.1)† | <0.0001 |
Values are means (SD) in cmH2O/l. Ers, respiratory elastance; El, lung elastance; Ecw, chest wall elastance; PEEP, positive end-expiratory pressure; Pneumo, pneumoperitoneum; Pre, pre-pneumoperitoneum. Elastance difference at 7-cmH2O PEEP from that at 0-cmH2O PEEP:
P < 0.02, †P < 0.01,
P < 0.0001.
Increasing PEEP from 0 to 7 cmH2O affected elastances differently before and during pneumoperitoneum (Table 1). Increasing PEEP reduced Ers with pneumoperitoneum (P < 0.0001), but not before. Increasing PEEP reduced Ecw both before (P = 0.011) and with pneumoperitoneum (P = 0.009). Increasing PEEP had no significant effect on El, either before or during pneumoperitoneum.
DISCUSSION
Principal findings of this study are that, contrary to our expectations, pneumoperitoneum induced at Vrel did not significantly change Pes, ERV, or Pao,thr required to initiate inflation from Vrel. Pneumoperitoneum did not significantly affect El, consistent with some studies in animals (4, 12) but not others in which El increased (3, 6, 16, 22). However, pneumoperitoneum did markedly increase Ecw and Ers, as expected from previous studies (3, 4, 6, 8, 12, 16, 22, 25, 27, 30). These findings suggest that pneumoperitoneum does not substantially increase pleural pressure or compress the lungs at Vrel. The addition of 7-cmH2O PEEP caused a substantial decrease in the Ers with pneumoperitoneum, but not before, and the El was not affected by PEEP. In what follows, we discuss previously described mechanisms to explain each of these findings.
Pneumoperitoneum increases the Ecw.
Insufflating gas into the abdomen necessarily stretches the abdominal wall and expands the chest wall as a whole (i.e., the rib cage and abdomen). This cannot by itself account for an increase in Ecw, because raising PEEP, which further increases the abdominal wall stretch and rib cage volume, causes a decrease in Ecw (3, 4, 16, 28). Furthermore, the relaxed rib cage itself becomes more compliant as its volume increases above FRC (26). A more likely explanation for stiffening of the chest wall during pneumoperitoneum is the diaphragm's interactions with the abdomen and rib cage.
The influence of the diaphragm on the elastance of the relaxed chest wall is apparent when the chest wall pressure-volume curve is obtained in the usual way, by changing Vl. As Vl in a passive subject is reduced below FRC, the chest wall becomes progressively stiffer. This stiffening is usually attributed to stretching and passive tension in the diaphragm, which stiffens the diaphragm-abdomen pathway for volume displacement (1). In addition, movement of the diaphragm into the thorax reduces the surface area of the lungs apposed to the rib cage, reducing the lungs' mechanical advantage in displacing the rib cage, thus stiffening the rib cage pathway for Vl displacement (20). In a similar way, pneumoperitoneum would increase intra-abdominal volume and stretch both the diaphragm and the abdominal wall and reduce the area of lung-apposed rib cage, thus stiffening both the diaphragm-abdomen and rib cage pathways for volume displacement.
Pneumoperitoneum does not cause significant change in Pes or ERV at Vrel.
Raising abdominal pressure might be expected to push the diaphragm into the rib cage, raise pleural pressure, and cause a decrease in Vl. This is the explanation for the major expiratory action of abdominal muscles. It is, therefore, surprising that, in our subjects, pneumoperitoneum caused only small, inconsistent changes in Pes at Vrel. Assuming that pressure at the top of the peritoneal cavity was atmospheric before pneumoperitoneum, abdominal insufflation would have raised intra-abdominal pressure by 20 cmH2O. Could a correspondingly large increase in pressure in the pleural space have been obscured by artifacts in the measurement of Pes? Several observations support the opposite conclusion: that pleural pressure changes were small and physiologically insignificant. First, we found only small and inconsistent changes in ERV with pneumoperitoneum (Fig. 4B), leading to the conclusion that Vrel is not changed with pneumoperitoneum. Second, in the slow inflation of the respiratory system after pneumoperitoneum, no subject exhibited a rightward shift of a knee in the pressure-volume curve that would have suggested pneumoperitoneum-induced lung compression (Fig. 3). Similarly, Pao,thr measured at the inflection of the volume-time trace during slow inflations showed that the minimal pressure required to initiate inflation did not increase with pneumoperitoneum, evidence against significant compression of the lungs by a high pleural pressure.
Why does a substantial rise in abdominal pressure not increase pleural pressure and decrease Vl substantially? We speculate that the explanation involves the inspiratory effect that increasing abdominal pressure has on the rib cage. Theoretical analyses and experimental evidence suggest when the diaphragm contracts during inspiration, it pulls on its insertions on the lower rib cage, applying a cephalad “insertional” force that is inspiratory to the rib cage (15, 33). Furthermore, diaphragm contraction raises abdominal pressure, which pushes outward on the area of diaphragm apposed to the rib cage (18), moving the lower ribs outward to expand the rib cage (5, 15).
When abdominal pressure increases without diaphragmatic contraction, the diaphragm's inspiratory action on the rib cage is normally insufficient to completely counteract the expiratory effect of diaphragmatic movement into the thorax. Thus a rise in abdominal pressure is usually expiratory. However, when the diaphragm is relatively stiff, increasing abdominal pressure can stretch and raise tension in the diaphragm, resulting in an inspiratory rib cage expansion. This apparently paradoxical effect was demonstrated in tetraplegic subjects, whose only significant inspiratory muscle is the diaphragm. When these subjects made maximal inspirations to TLC, they achieved a higher maximal Vl when their abdominal pressure was increased by an elastic abdominal binder (17). In this situation, the diaphragm, contracted during maximal inspiratory efforts, was relatively stiff and, therefore, not easily pushed into the thorax by abdominal compression. The increased abdominal pressure caused increased tension in the diaphragm, which became inspiratory to not only the rib cage, but the entire respiratory system (17).
The inspiratory effects on the rib cage of increased abdominal pressure could also result from abdominal pressure increases in the absence of diaphragm contraction, if the diaphragm is relatively stiff, for example at Vrel when the muscle is stretched enough to develop passive tension. As abdominal pressure increases, the stiffened diaphragm resists displacement into the rib cage, and increasing diaphragmatic tension causes an inspiratory insertional force, while increased abdominal pressure pushes outward to expand the rib cage. The resulting inspiratory effects on the rib cage would be greatest at low Vl values (15) and could counteract the expiratory effect of increased abdominal pressure in displacing the diaphragm. Indeed, infusing fluid into the abdomen of dogs stretched the diaphragm and displaced the diaphragmatic dome cephalad without reducing resting Vl, as measured plethysmographically (10). Thus increasing abdominal volume and pressure can result in negligible changes in pleural pressure and Pes, as observed in upright pigs (22). Consistent with these ideas, Gilroy et al. (9) found that, when gastric volume was acutely changed in seated subjects, increased abdominal volume caused unexpectedly small decreases in Vl and large increases in rib cage volume, suggesting an inspiratory effect of abdominal pressure on the rib cage.
It is worth distinguishing the effects of pneumoperitoneum during laparoscopic surgery from those of a gradual increase in intra-abdominal volume caused by less acute conditions, such as pregnancy, obesity, and ascites. Although conditions like obesity are associated with increased abdominal pressure (2, 28), they do not usually achieve levels seen with pneumoperitoneum (>20 cmH2O). In chronic conditions, there is likely sufficient time for length-adaptation of skeletal muscle in the abdominal wall, and possibly the diaphragm, to accommodate the additional intra-abdominal volume without causing a large rise in abdominal pressure. Length adaptation of diaphragmatic muscle would reduce diaphragmatic tension and transdiaphragmatic pressure, allowing the diaphragm to be displaced further into the thorax, thus increasing pleural pressure at rest.
Increasing PEEP reduces the Ers with pneumoperitoneum, but not without.
As noted above, when the pressure-volume characteristic of the relaxed chest wall is obtained in the usual way by changing pressure applied to the airway, chest wall stiffening at volumes below Vrel has been attributed to passive diaphragmatic tension. The passive tension stiffens the diaphragm-abdomen pathway for volume displacement and reduces the mechanical advantage of the lung in displacing the rib cage. In this circumstance, raising Vl by raising Pao decreases diaphragmatic tension, decreasing Ecw. As the lungs are inflated above Vrel, the passive chest wall pressure-volume characteristic is relatively linear, and, therefore, further increases in Pao (or PEEP) do not change Ecw. The same mechanisms would apply after pneumoperitoneum. At relaxation in the supine position, pneumoperitoneum produces substantial diaphragmatic tension that stiffens the chest wall. Raising Vl decreases diaphragmatic tension and reduces Ecw.
Unlike Ers, Ecw was reduced in our subjects by increasing PEEP, both with and without pneumoperitoneum. The decrease in Ecw was not correlated with BMI, so this result was not likely due to the preponderance of obese subjects in our study. Increases in PEEP did not affect the El, either with or without pneumoperitoneum. This suggests that the lungs were on the linear portion of their inflation pressure-volume curves above Vrel, and that pneumoperitoneum does not cause substantial compression of the lungs at Vrel.
Critique of method and comparison with previous results.
Because most of our subjects were obese and only two were of normal weight, the possibility must be considered that the principal findings of our study depend on obesity and would not have occurred in normal-weight subjects. The first principal finding is that pneumoperitoneum did not affect Pes at Vrel. An increase in Pes due to obesity should not prevent a further increase due to pneumoperitoneum, unless the obesity caused an increase in diaphragmatic tension and prevented the rise in abdominal pressure from being transmitted to the thorax. However, obesity does not appear to increase diaphragmatic tension, as the resting Pes and gastric pressures in obese subjects are increased to the same extent, with the result that the resting transdiaphragmatic pressure and presumably tension are not greater than in normal subjects (28). Also, there was no relation in our subjects between BMI and the change in Pes with pneumoperitoneum.
The second principal finding is that pneumoperitoneum did not change ERV at Vrel. This might be explained if obesity raised pleural pressure enough to compress the lungs, reduce ERV, and thus prevent any further decrease in ERV caused by pneumoperitoneum. This also seems unlikely, as there was no relation between BMI and either ERV at baseline or the change in ERV with pneumoperitoneum. Thus the available data suggest that our principal findings could apply to obese and normal subjects, although we cannot exclude the alternative.
Previous studies found that EELV, measured by nitrogen washout during mechanical ventilation, decreased with pneumoperitoneum in swine (4, 24) and patients (8). Futier et al. (8) found that pneumoperitoneum reduced EELV 530 ml in normal-weight patients and 136 ml in obese patients, whereas we found a nonsignificant decrease in ERV of only 29 ml. The previous studies using gas dilution techniques to measure the volume in the lungs during tidal ventilation above Vrel could have been sensitive to pneumoperitoneum-induced changes in the distribution of tidal ventilation above Vrel, whereas our measurements of ERV to determine changes in Vrel assumed a constant minimal gas volume of the lung at residual volume. To the extent that the pressure-volume characteristics of the lung and minimal gas volume can change gradually and progressively during ventilation with low PEEP (e.g., by gas absorption), gas dilution techniques could have indicated a decreased gas volume in the absence of a significant increase in pleural pressure. As a result, these different methods of assessing Vl need not necessarily agree.
Estimation of pleural pressure by Pes is subject to gravitational artifacts. In the supine position, Pes is apparently increased several centimeters of H2O by the weight of the heart and overlying mediastinal structures (7, 11, 19, 21, 31). Pneumoperitoneum, by elevating the ventral rib cage, might lift the heart off the esophagus, causing an artifactual decrease in Pes while the actual pleural pressure increased. Similarly, a change in the shape of the lung and chest wall brought about by pneumoperitoneum could cause interregional differences in tidal pleural pressure changes. Such effects could cause small differences between changes in Pes and pleural pressures. For these reasons, we measured Pao,thr at the onset of inflation and ERV to assess the impact of pleural pressure change during pneumoperitoneum, methods that do not depend on Pes measurement. On the other hand, our measurements based on Pes agree with other methods, suggesting negligible pleural pressure change with pneumoperitoneum, which supports the utility of Pes in estimating pleural pressure.
Conclusions.
We found that, although pneumoperitoneum markedly increases the Ecw and Ers, at Vrel pneumoperitoneum does not raise pleural pressure or compress the lungs in our predominantly obese subjects. This apparent incongruity can be explained by the inspiratory action of diaphragmatic tension on the rib cage, which counteracts the expiratory effect of increased abdominal pressure. With inflation above Vrel, the transrespiratory (airway) pressure and pleural pressure are higher with pneumoperitoneum due to increased Ecw.
Do the results of this study suggest a change in our approach to mechanical ventilation during pneumoperitoneum? Probably not. The approach often recommended is to apply moderate levels of PEEP and perhaps recruitment maneuvers (3, 29, 32), both of which have been shown to improve respiratory mechanics and gas exchange to some degree, and neither of which carries appreciable risk for patients with healthy respiratory systems. Added PEEP would increase Pes values, and Pes values are higher than normal in obese subjects (2, 28), but obesity of our subjects was not obviously responsible for our findings. High Pes values are also common in patients with acute lung injury, although this is likely due largely to intrathoracic and abdominal edema, whose effects may differ from those of acute abdominal volume expansion. The principal implication of our results is that the passive respiratory system has a remarkable ability to accommodate acute increases in abdominal volume and pressure without squeezing the lungs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants, HL-52586 and HL-108724 and the Beth Israel Anesthesia Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.H.L. and N.B. conception and design of research; S.H.L., N.B., A.N., and S.B.J. performed experiments; S.H.L., N.B., A.N., V.N., and C.R.O. analyzed data; S.H.L., N.B., A.N., V.N., C.R.O., and D.S.T. interpreted results of experiments; S.H.L. and A.N. prepared figures; S.H.L. and N.B. drafted manuscript; S.H.L., N.B., A.N., V.N., S.B.J., C.R.O., and D.S.T. edited and revised manuscript; S.H.L., N.B., A.N., V.N., S.B.J., C.R.O., and D.S.T. approved final version of manuscript.
REFERENCES
- 1.Agostoni E, Mead J. Statics of the respiratory system. In: Handbook of Physiology. Respiration. Washington, DC: Am. Physiol. Soc., 1964, sect. 3, vol. I, chapt. 13, p. 387–409 [Google Scholar]
- 2.Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol 108: 212–218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cinnella G, Grasso S, Spadaro S, Rauseo M, Mirabella L, Salatto P, De Capraris A, Nappi L, Greco P, Dambrosio M. Effects of recruitment maneuver and positive end-expiratory pressure on respiratory mechanics and transpulmonary pressure during laparoscopic surgery. Anesthesiology 118: 114–122, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Cortes-Puentes GA, Gard KE, Adams AB, Faltesek KA, Anderson CP, Dries DJ, Marini JJ. Value and limitations of transpulmonary pressure calculations during intra-abdominal hypertension. Crit Care Med 41: 1870–1877, 2013 [DOI] [PubMed] [Google Scholar]
- 5.De Troyer A, Boriek AM. Mechanics of the respiratory muscles. Compr Physiol 1: 1273–1300, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Fahy BG, Barnas GM, Flowers JL, Nagle SE, Njoku MJ. The effects of increased abdominal pressure on lung and chest wall mechanics during laparoscopic surgery. Anesth Analg 81: 744–750, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Ferris BG, Jr, Mead J, Frank NR. Effect of body position on esophageal pressure and measurement of pulmonary compliance. J Appl Physiol 14: 521–524, 1959 [Google Scholar]
- 8.Futier E, Constantin JM, Pelosi P, Chanques G, Kwiatkoskwi F, Jaber S, Bazin JE. Intraoperative recruitment maneuver reverses detrimental pneumoperitoneum-induced respiratory effects in healthy weight and obese patients undergoing laparoscopy. Anesthesiology 113: 1310–1319, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Gilroy RJ, Jr, Lavietes MH, Loring SH, Mangura BT, Mead J. Respiratory mechanical effects of abdominal distension. J Appl Physiol 58: 1997–2003, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Hubmayr RD, Sprung J, Nelson S. Determinants of transdiaphragmatic pressure in dogs. J Appl Physiol 69: 2050–2056, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Knowles JH, Hong SK, Rahn H. Possible errors using esophageal balloon in determination of pressure-volume characteristics of the lung and thoracic cage. J Appl Physiol 14: 525–530, 1959 [Google Scholar]
- 12.Kubiak BD, Gatto LA, Jimenez EJ, Silva-Parra H, Snyder KP, Vieau CJ, Barba J, Nasseri-Nik N, Falk JL, Nieman GF. Plateau and transpulmonary pressure with elevated intra-abdominal pressure or atelectasis. J Surg Res 159: e17–e24, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Leduc D, Cappello M, Gevenois PA, De Troyer A. Mechanics of the canine diaphragm in ascites: a CT study. J Appl Physiol 104: 423–428, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Leduc D, De Troyer A. Dysfunction of the canine respiratory muscle pump in ascites. J Appl Physiol 102: 650–657, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Loring SH, Mead J. Action of the diaphragm on the rib cage inferred from a force-balance analysis. J Appl Physiol 53: 756–760, 1982 [DOI] [PubMed] [Google Scholar]
- 16.Maracaja-Neto LF, Vercosa N, Roncally AC, Giannella A, Bozza FA, Lessa MA. Beneficial effects of high positive end-expiratory pressure in lung respiratory mechanics during laparoscopic surgery. Acta Anaesthesiol Scand 53: 210–217, 2009 [DOI] [PubMed] [Google Scholar]
- 17.McCool FD, Pichurko BM, Slutsky AS, Sarkarati M, Rossier A, Brown R. Changes in lung volume and rib cage configuration with abdominal binding in quadriplegia. J Appl Physiol 60: 1198–1202, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Mead J. Functional significance of the area of apposition of diaphragm to rib cage [proceedings]. Am Rev Respir Dis 119: 31–32, 1979 [DOI] [PubMed] [Google Scholar]
- 19.Mead J, Gaensler EA. Esophageal and pleural pressures in man, upright and supine. J Appl Physiol 14: 81–83, 1959 [DOI] [PubMed] [Google Scholar]
- 20.Mead J, Loring SH. Analysis of volume displacement and length changes of the diaphragm during breathing. J Appl Physiol 53: 750–755, 1982 [DOI] [PubMed] [Google Scholar]
- 21.Milic-Emili J, Mead J, Turner JM. Topography of esophageal pressure as a function of posture in man. J Appl Physiol 19: 212–216, 1964 [DOI] [PubMed] [Google Scholar]
- 22.Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK. Abdominal distension alters regional pleural pressures and chest wall mechanics in pigs in vivo. J Appl Physiol 70: 2611–2618, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest 109: 144–151, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Regli A, Hockings LE, Musk GC, Roberts B, Noffsinger B, Singh B, van Heerden PV. Commonly applied positive end-expiratory pressures do not prevent functional residual capacity decline in the setting of intra-abdominal hypertension: a pig model. Crit Care 14: R128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Runck H, Schumann S, Tacke S, Haberstroh J, Guttmann J. Effects of intra-abdominal pressure on respiratory system mechanics in mechanically ventilated rats. Respir Physiol Neurobiol 180: 204–210, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Smith JC, Loring SH. Passive mechanical properties of the chest wall. In: Handbook of Physiology. The Respiratory System. Mechanics of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. III, pt. 2, chapt. 25, p. 429–442 [Google Scholar]
- 27.Sprung J, Whalley DG, Falcone T, Warner DO, Hubmayr RD, Hammel J. The impact of morbid obesity, pneumoperitoneum, and posture on respiratory system mechanics and oxygenation during laparoscopy. Anesth Analg 94: 1345–1350, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Steier J, Lunt A, Hart N, Polkey MI, Moxham J. Observational study of the effect of obesity on lung volumes. Thorax 69: 752–759, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Valenza F, Chevallard G, Fossali T, Salice V, Pizzocri M, Gattinoni L. Management of mechanical ventilation during laparoscopic surgery. Best Pract Res Clin Anaesthesiol 24: 227–241, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Valenza F, Chevallard G, Porro GA, Gattinoni L. Static and dynamic components of esophageal and central venous pressure during intra-abdominal hypertension. Crit Care Med 35: 1575–1581, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Washko GR, O'Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol 100: 753–758, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Whalen FX, Gajic O, Thompson GB, Kendrick ML, Que FL, Williams BA, Joyner MJ, Hubmayr RD, Warner DO, Sprung J. The effects of the alveolar recruitment maneuver and positive end-expiratory pressure on arterial oxygenation during laparoscopic bariatric surgery. Anesth Analg 102: 298–305, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Wilson TA, De Troyer A. Effects of the insertional and appositional forces of the canine diaphragm on the lower ribs. J Physiol 591: 3539–3548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]