Abstract
Objective
In Korea, early vertebroplasty (EVP) or delayed vertebroplasty (DVP, which is performed at least 2 weeks after diagnosis) were performed for the treatment of acute osteoporotic compression fracture (OCF) of the spine. The present study compared the outcomes of two surgical strategies for the treatment of single-level acute OCF in the thoracolumbar junction (T12-L2).
Methods
From 2004 to 2010, 23 patients were allocated to the EVP group (EVPG) and 27 patients to the DVP group (DVPG). Overall mean age was 68.3±7.9 and minimum follow-up period was 1.0 year. Retrospective study of clinical and radiological results was conducted.
Results
No significant differences in baseline characteristics were observed between the two groups. As expected, mean duration from onset to vertebroplasty and mean duration of hospital stay were significantly longer in the DVPG (17.1±2.1 and 17.5±4.2) than in the EVPG (3.8±3.3 and 10.8±5.1, p=0.001). Final clinical outcome including visual analogue scale (VAS), Oswestry Disability Index, and Odom's criteria did not differ between the two groups. However, immediate improvement of the VAS after vertebroplasty was greater in the EVPG (5.1±1.3) than in the DVPG (4.0±1.0, p=0.002). The proportion of cement leakage was lower in the EVPG (30.4%) than in the DVPG (59.3%, p=0.039). In addition, semiquantitative grade of cement interdigitation was significantly more favorable in the EVPG than in the DVPG (p=0.003). Final vertebral body collapse and segmental kyphosis did not differ significantly between the two groups.
Conclusion
Our findings suggest that EVP achieves a better immediate surgical effect with more favorable cost-effectiveness.
Keywords: Osteoporosis, Spine, Compression fracture, Vertebroplasty
INTRODUCTION
Percutaneous vertebroplasty is widely used for the treatment of painful osteoporotic compression fracture (OCF) of the spine. Numerous clinical and radiological features are considered relevant in selecting treatment strategy. Many previous studies, including guidance documents from the US Food and Drug Administration19), have recommended delayed vertebroplasty (DVP) only after a course of failed medical therapy, on the order of 2-6 weeks' duration2,3,13,17). However, classically, the indications of vertebroplasty for OCF include patients remain bedridden for more than 4 days due to severe pain, and the recently published VERTOS II study highlights the potential benefit of early vertebroplasty (EVP)10,16).
In South Korea, before 2007, EVP had become routine for treatment of OCF of the spine. However, since 2007, according to National Health Insurance guideline, only DVP has been allowed except for patients over 80 years-old or patients with severe co-morbidity.
In the current study, we compared the results of two surgical strategies by retrospective study in order to determine whether timing of surgery had an impact on outcome and cost-effectiveness.
MATERIALS AND METHODS
Patient selection
A retrospective chart review was performed on all patients diagnosed with OCF at our institution between January 2004 and December 2010. Based on the revision of laws in 2007, the study groups were classified according to two periods, from 2004 to 2006 and from 2009 to 2010.
In order to minimize the influence of multiple fractures or various fracture levels on outcomes, we identified patients who had been diagnosed with a single-level OCF of the thoracolumbar junction (T12-L2). In order to avoid potential confounding effect of fracture age, patients whose duration from symptom onset until diagnosis was more than three days were excluded. Also, patients over 80 years-old or patients with severe co-morbidity were excluded in order to avoid the bias of patient selection. Other exclusion criteria were compression fracture due to tumor or infection and previous lumbar spine surgery including vertebroplasty, kyphoplasty, and fusion.
Among patients who satisfied the above mentioned criteria, we identified all patients who had undergone vertebroplasty. Then, among patients who had undergone vertebroplasty, we enrolled the final cohort, which included patients who had available complete clinical and radiological evaluation up to one year after surgery.
Surgical indication and procedure
During 2004 and 2006, indications for EVP were severe back pain and patient's willingness to undergo vertebroplasty after sufficient explanation. On the other hand, during 2009 and 2010, indications for DVP were severe back pain resistant to a minimum of two weeks conservative treatment. During that period, although patients wanted EVP for rapid pain control, surgery had to be postponed for at least two weeks.
Three spine surgeons performed vertebroplasty using the same technique. All vertebroplasty procedures were performed in the prone position on a radiolucent bed using a C-arm. After local anesthesia and light sedation, a 10-G vertebroplasty needle was inserted into the vertebral body via a bilateral transpedicular approach. Then, polymethlymetacrylate (PMMA) was injected using a 1-cc syringe until satisfactory distribution of the cement, i.e., symmetrical filling of the central and anterior parts of the vertebral body, was achieved. Injected PMMA was the same product, and viscosity for each procedure was set up as evenly as possible, like toothpaste, approximately 5 minutes after mixing.
Outcome parameters
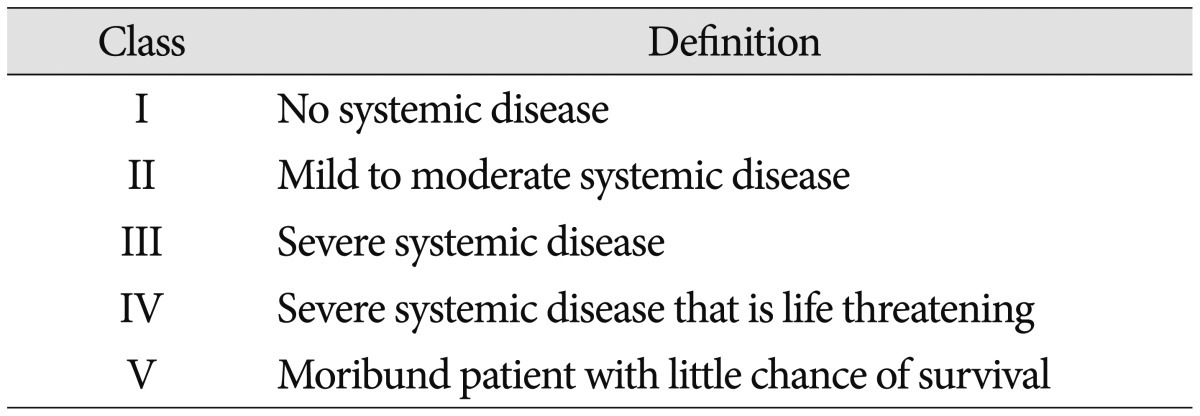
Duration from onset to diagnosis, duration from diagnosis to surgery, duration from onset to surgery, and duration of hospital stay were checked in all patients. In addition, etiology, surgery level, preoperative condition, preoperative body mass index (BMI), and bone mineral density (BMD) were checked in all patients. The American Society of Anesthesiologists (ASA) classification of physical status was used for assessment of preoperative medical conditions (Table 1)6).
Table 1.
American Society of Anesthesiologists classification
Clinical outcomes were assessed using a visual analogue scale (VAS) for low back pain, the Oswestry Disability Index (ODI), and Odom's criteria.
Preoperative lumbar magnetic resonance imaging (MRI) was acquired in all patients for diagnosis, and postoperative lumbar computed tomography (CT) was checked in all patients for evaluation of cement leakage and cement interdigitation. In addition, routine standing radiographs were taken preoperatively and during follow-up for all patients.
Fracture morphology type was denominated according to the classification from Genant et al.5), (type 1 : wedge, type 2 : biconcave, type 3 : crushed). Also, the grade of canal encroachment (grade 0 : none, grade 1 : <10%, grade 2 : 10-20%, grade 3 : >20% encroachment), disruption of cortical bone margin, and intravertebral cleft (i.e., intravertebral, abnormal, well-demarcated, linear or cystic lesion similar to air or fluid) were checked based on preoperative MRI findings.
The volume of injected PMMA bone cement was checked in all patients. Also, cement leakage, defined as the presence of any extravertebral cement, was assessed on the postoperative CT scan of the treated levels. Patterns of cement leakage were classified as four types; 1) epidural leakage via the vasivertebral vein, 2) cortical leakage through cortical defect, 3) disc space leakage through end plate defect, and 4) vascular leakage via the segmental vein.
The degree of interdigitation of bone cement on the postoperative CT scan was scored as a semiquantitative scale using the classification proposed by Nieuwenhuijse et al.15); grade 1 (complete interdigitation throughout the injected volume with clearly visible bone trabeculae), grade 2 (considerable interdigitation along the boundaries of a clearly recognizable cement deposit), grade 3 (a clump like filling pattern with sparsely gross interdigitation), and grade 4 (no interdigitation at all with sharp boundaries along the cement clump, comparable to cleft filling).
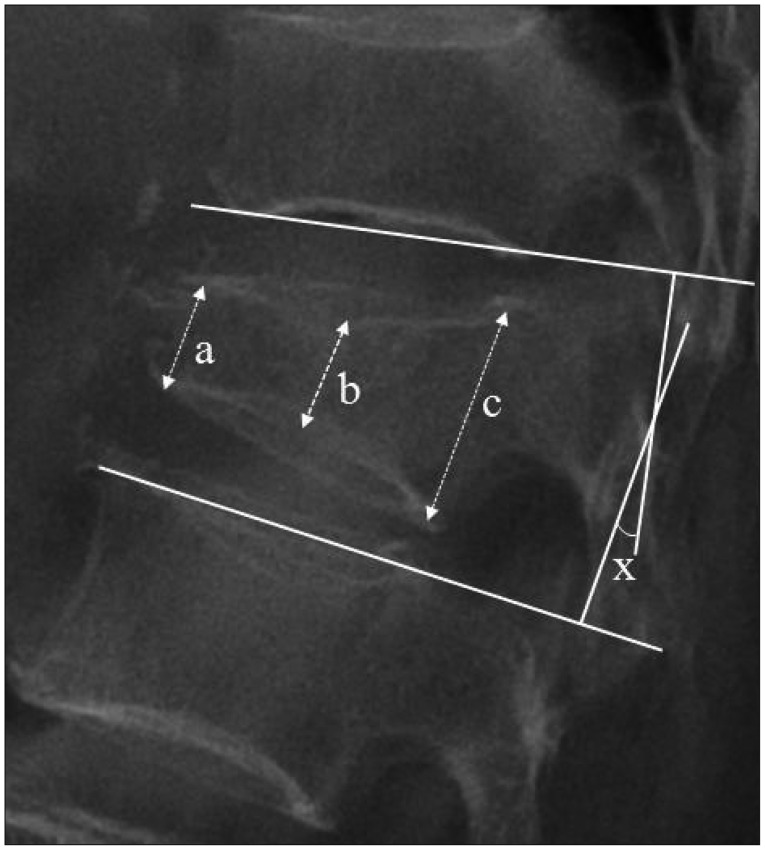
Compression ratio and segmental kyphotic angle were checked on the standing lateral radiographs in neutral position for evaluation of collapse progression (Fig. 1).
Fig. 1.
Occurrences of perioperative morbidities were checked. In addition, reoperation and development of new compression fracture in another level or the same level were documented.
Statistical analysis
SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used for analysis of all data. The Pearson's chi-square test, the independent two samples t-test, and the paired samples t-test were used depending on the characteristics of the variables being compared. The mean value was described as mean±1SD. Statistical significance was accepted for p values of <0.05.
RESULTS
Demographics
During 2004 and 2006, among 113 patients who satisfied the above mentioned inclusion criteria, 28 (24.8%) patients underwent EVP, whereas, during 2009 and 2010, among 142 patients who satisfied the inclusion criteria, 33 (23.2%) patients underwent DVP. Operation ratio was not different for the two periods (p=0.789, Pearson's chi-square test).
Among 28 patients who underwent EVP during 2004 and 2006, 23 patients were available for enrollment in the EVP group (EVPG). On the other hand, among 33 patients who underwent DVP during 2009 and 2010, 27 patients were available for enrollment in the DVP group (DVPG).
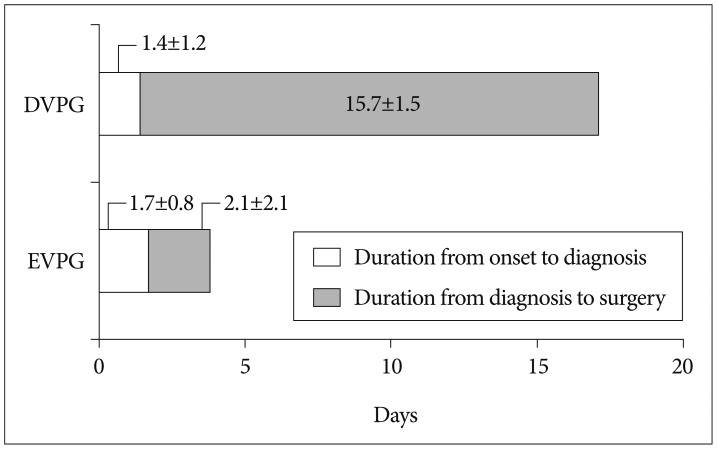
No statistical significance was observed in the mean duration from onset to diagnosis (p=0.684, the independent two samples t-test), whereas mean duration from diagnosis to surgery and duration from onset to surgery were significantly longer in the DVPG (p<0.001, the independent two samples t-test) (Fig. 2). Also, as expected, mean duration of hospital stay in the DVPG (17.5±4.2 days) was much longer than that in the EVPG (10.8±5.1 days) (p=0.001, the independent two samples t-test).
Fig. 2.
Mean duration from onset to diagnosis and from diagnosis to surgery in the two groups. DVPG : delayed vertebroplasty group, EVPG : early vertebroplasty group.
No significant difference in preoperative demographics was observed between the two groups. Also, the injected PMMA volume did not differ between the two groups (Table 2).
Table 2.
Summary of demographic data of both groups

EVPG : early vertebroplasty group, DVPG : delayed vertebroplasty group, BMI : body mass index, BMD : bone mineral density, PMMA : polymethlymetacrylate
Clinical outcomes
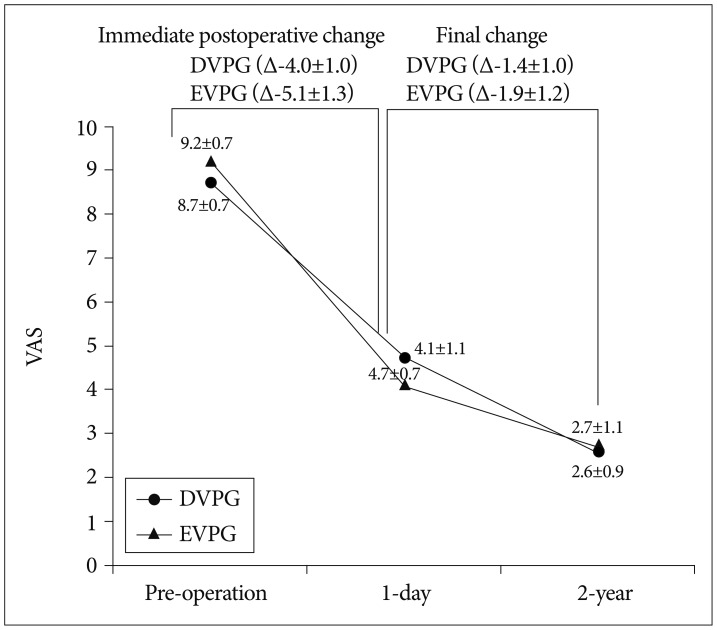
In both groups, VAS showed sequential decrease during follow-up (p<0.001, the paired samples t-test). In addition, final improvement of VAS did not differ between the two groups (p=0.703, the independent two samples t-test). However, immediate postoperative improvement of VAS was significantly greater in the EVPG (5.1±1.3) than in the DVPG (4.0±1.0, p=0.002, the independent two samples t-test) (Fig. 3).
Fig. 3.
Sequential changes of the mean visual analogue scale in the two groups. DVPG : delayed vertebroplasty group, EVPG : early vertebroplasty group.
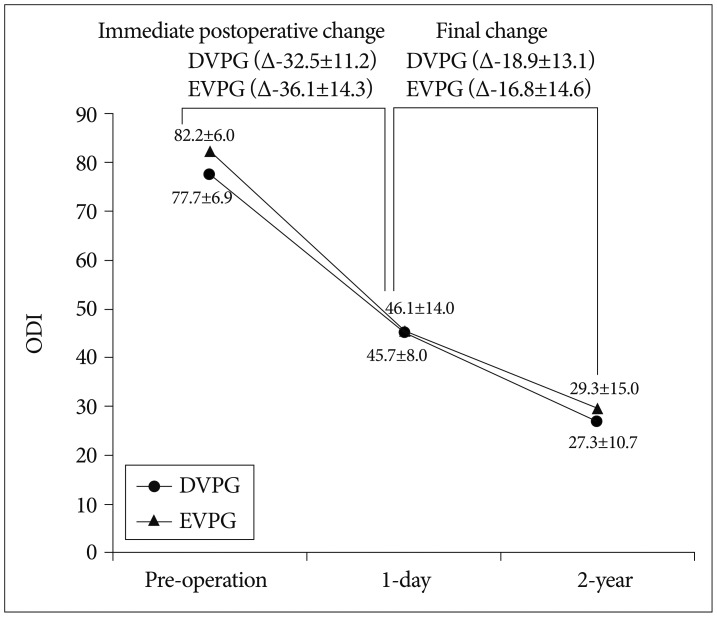
The functional aspects using ODI scores showed sequential improvement during the follow-up period in both groups (p<0.001, the paired samples t-test). Also, ODI scores did not differ significantly in the two groups at each follow-up (p=0.766 and 0.578, the independent two samples t test) (Fig. 4).
Fig. 4.
Sequential changes of the mean Oswestry Disability Indexs in the two groups. DVPG : delayed vertebroplasty group, EVPG : early vertebroplasty group.
According to Odom's criteria, there was no significant intergroup difference during follow-up (p=0.217 and 0.318, Pearson's chi-square test) (Table 3).
Table 3.
Summary of clinical outcome using Odom's criteria
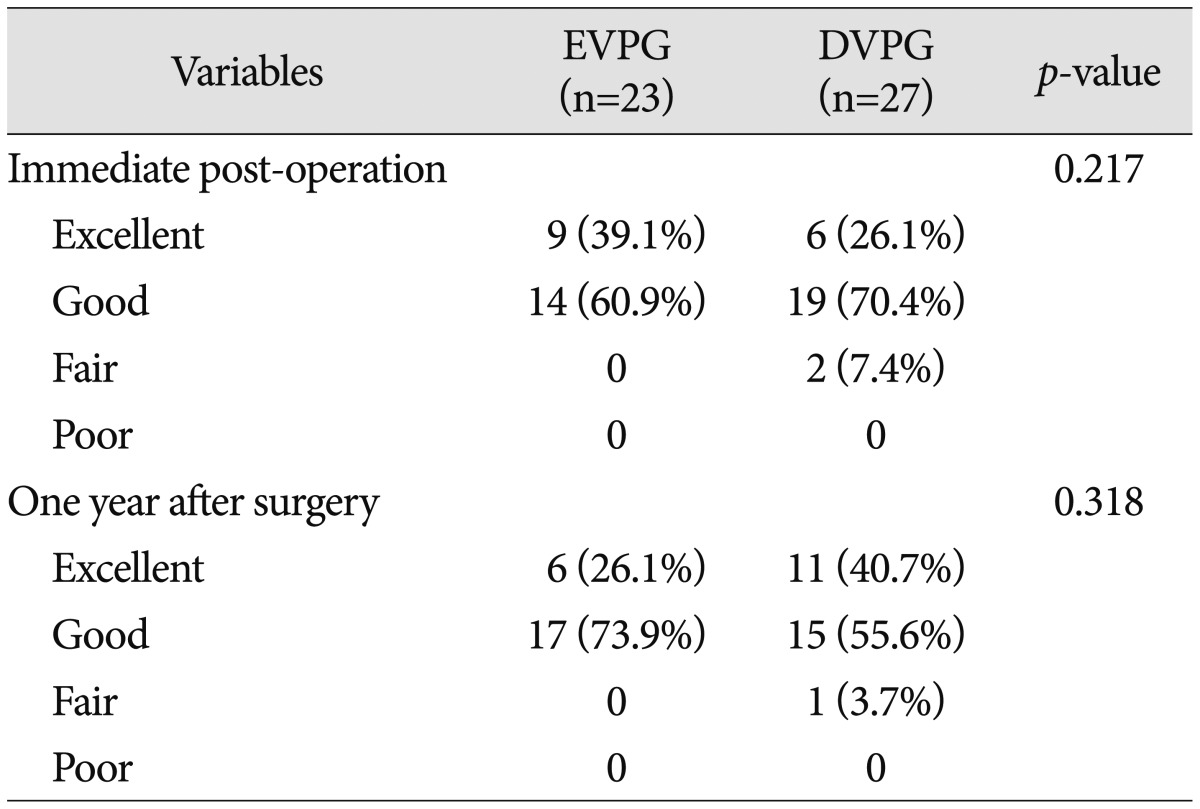
EVPG : early vertebroplasty group, DVPG : delayed vertebroplasty group
Fortunately, there was no postoperative mortality and no neurologic deterioration after surgery. Among a total of 50 patients, there was no major perioperative morbidity except for a single case of paralytic ileus in the EVPG.
Radiological outcomes
Based on preoperative MRI findings, preoperative characteristics were not significantly different between the two groups (Table 4).
Table 4.
Summary of preoperative MRI findings of both groups

EVPG : early vertebroplasty group, DVPG : delayed vertebroplasty group
Of particular interest, the proportion of vertebrae with detected cement leakage was significantly lower in the EVPG (30.4%) than in the DVPG (59.3%) (p=0.039, Pearson's chi-square test). In addition, the quantitative grade of cement interdigitation was significantly favorable in the EVPG (p=0.003, Pearson's chi-square test) (Table 5).
Table 5.
Summary of postoperative CT findings of both groups
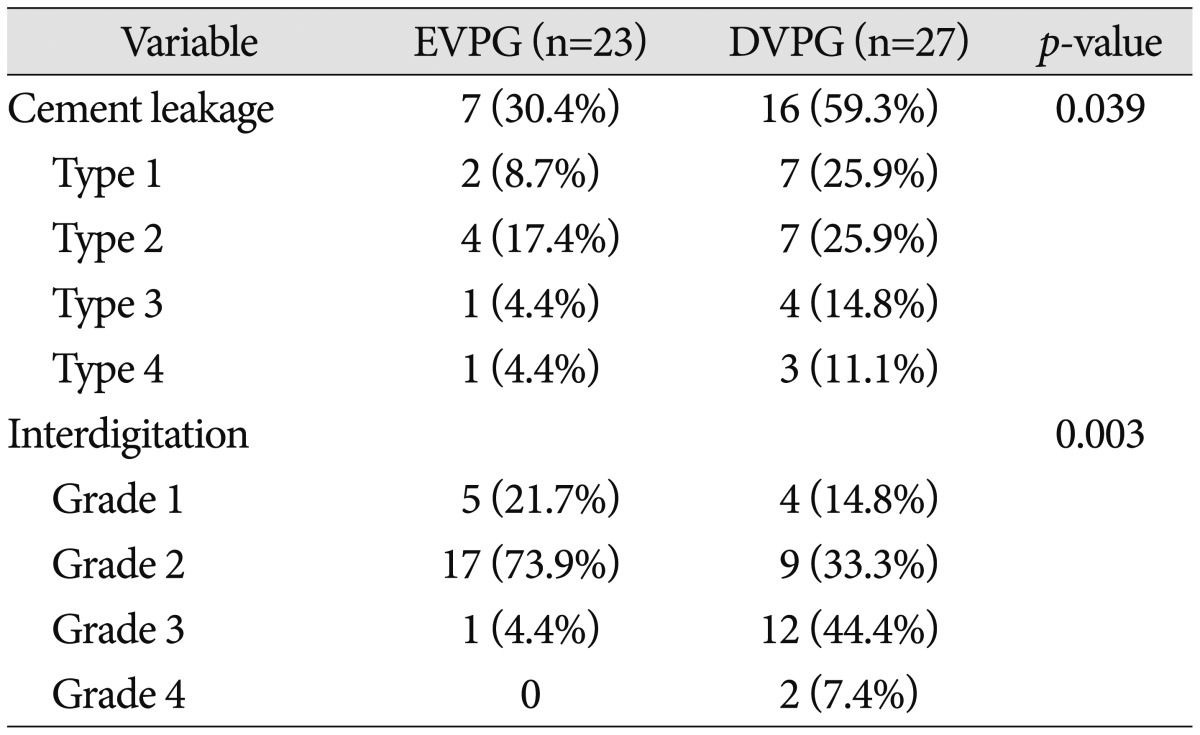
EVPG : early vertebroplasty group, DVPG : delayed vertebroplasty group
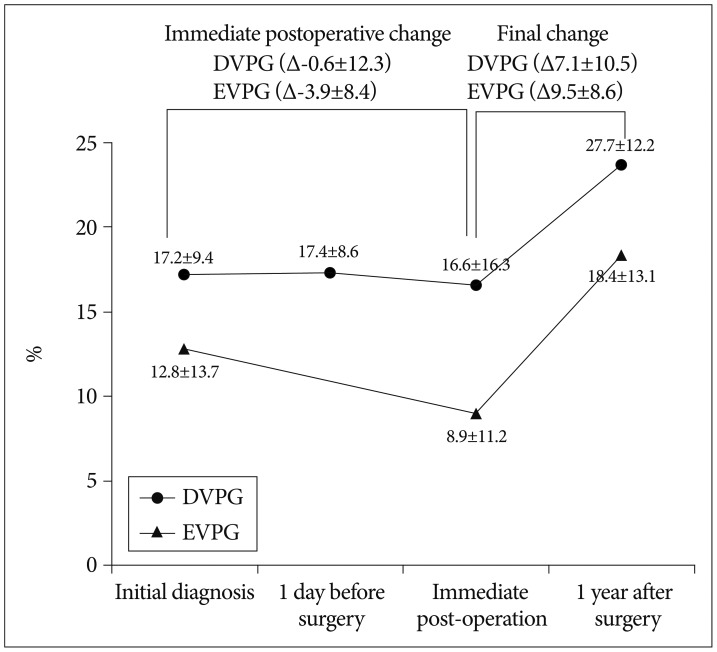
In the EVPG, the mean compression ratio decreased from 12.8±13.7% at initial diagnosis, to 8.9±11.2 at immediate post-operation, however, it increased to 18.4±13.1% at one year after surgery (p<0.001 and 0.016 sequentially, the paired samples t-test). In the DVPG, the mean compression ratio increased from 17.2±9.4% at initial diagnosis to 17.4±8.6% at one day before surgery, decreased to 16.6±16.3% at immediate post-operation, and increased to 23.7±12.2% at one year after surgery (p=0.584, 0.012, and 0.017 sequentially, the paired samples t-test). In the DVPG, the compression ratio between initial diagnosis and one day before surgery was not significantly different.
The compression ratio at each follow-up did not differ significantly between the two groups (p=0.188, 0.061, and 0.255 respectively, the independent two samples t-test). Also, the aggravation of compression ratio at final follow-up was not significantly different (p=0.562, the independent two samples t-test). However, there was a trend indicating that immediate postoperative compression ratio reduction was favorable in the EVPG, although statistically not significant (3.9±8.4% for EVPG and 0.6±12.3% for DVPG, p=0.131, the independent two samples t-test) (Fig. 5).
Fig. 5.
Sequential changes of the mean compression ratios (%) in the two groups. DVPG : delayed vertebroplasty group, EVPG : early vertebroplasty group.
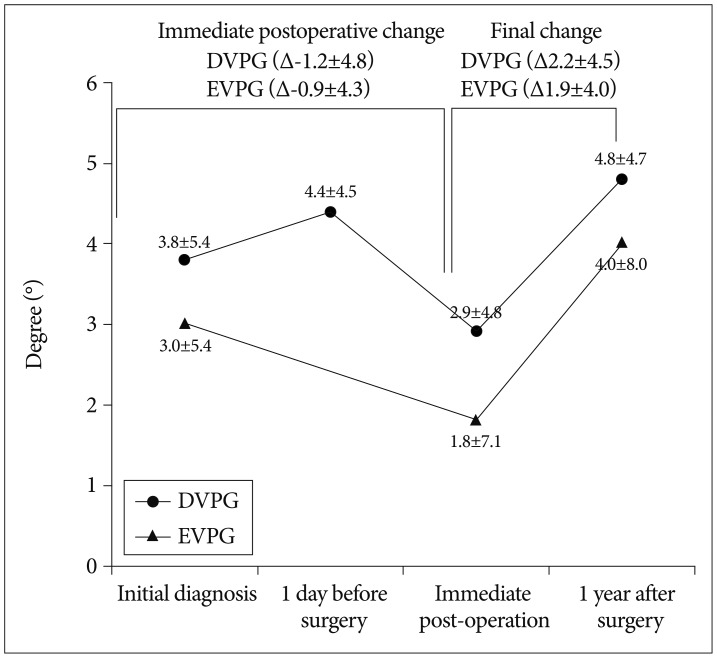
In the EVPG, the mean segmental angle decreased from 3.0±5.4° at initial diagnosis, to 1.8±7.1 at immediate post-operation, however, it increased to 4.0±8.0 at one year after surgery (p=0.008 and 0.286 sequentially). In the DVPG, the mean segmental angle increased from 3.8±6.0° at initial diagnosis to 4.4±4.5° at one day before surgery, decreased to 2.9±4.8° at immediate post-operation, and increased to 4.8±4.7° at one year after surgery (p=0.128, 0.006, and 0.262 sequentially, the paired samples t-test). In the DVPG, the increase of segmental angle between initial diagnosis and one day before surgery was not significantly different.
The segmental angle at each follow-up (i.e., at initial diagnosis, immediate post-operation, and one year after surgery) was not significantly different between the two groups (p=0.609, 0.158, and 0.899 respectively, the independent two samples t-test). Also, the aggravation of segmental kyphosis at one year after surgery was not significantly different between the two groups (p=0.877, the independent two samples t-test). However, there was a trend indicating that immediate postoperative segmental angle reduction was favorable in the EVPG, although statistically not significant (1.2±4.8° for EVPG and 0.9±4.3° for DVPG, p=0.275, the independent two samples t-test) (Fig. 6).
Fig. 6.
Sequential changes of the mean segmental angles (°) in the two groups. DVPG : delayed vertebroplasty group, EVPG : early vertebroplasty group.
In the EVPG, two new fractures adjacent to treated levels, occurred in two patients (8.7%), whereas, in the DVPG, two new fractures which all were not adjacent, occurred in two patients (7.4%). Proportion of patients with new compression fracture was comparable between the two groups. In addition, there was no case of reoperation to treated level.
DISCUSSION
Selection of EVP or DVP for patients with OCF is still controversial in several aspects. In general, once an OCF has been diagnosed, nonsurgical management with activity modification and symptomatic medication, with or without bracing, is universal for the majority of patients. This DVP strategy is rational, because unreasonable EVP may cause an increase of unnecessary surgery and medical expenses. However, interestingly, in the current study, the operation ratio was not significantly different between the two periods (24.8% during the period of EVP and 23.2% during the period of DVP). Namely, DVP strategy did not reduce the operation ratio compared with EVP strategy. Furthermore, in the current study, mean hospital stay was much longer in the DVPG than in the EVPG. This finding means that many patients in the DVPG had to spend more on medical expenses for preoperative hospitalization and conservative treatment. In addition, plenary DVP strategy may cause unnecessary delay of treatment for patients seeking rapid pain relief. In summary, if surgical indications of EVP are properly established (e.g., patients with intractable severe pain or patients who want rapid pain relief), EVP may be reasonable enough in the viewpoint of the operation ratio, waiting time, and cost-effectiveness.
Several single-center studies have reported outcomes after vertebroplasty according to fracture age; most of these studies failed to demonstrate differences in outcome based on pre-procedural fracture age1,7,18). On the other hand, some authors have reported that a shorter period between fracture and procedure provided relatively greater satisfaction with vertebroplasty among treated patients4). Our study adds to the previous literature by focusing on a relatively homogeneous population, that is, patients with single-level thoracolumbar fractures, and by providing data regarding not only pain relief but also functional outcome, and morbidity. In the current study, although final clinical outcomes, including VAS and ODI, were not different between the two groups, the degree of immediate pain relief after vertebroplasty was greater in the EVPG than in the DVPG. Our results suggest significant correlation between the age of the fracture and the immediate pain relief after vertebroplasty. In other words, the effect of vertebroplasty for pain control is better when the timing of surgery is sooner.
In the current study, cement leakage and interdigitation were significantly favorable in the EVPG compared with the DVPG. These results are assumed to be due to difference in the degree of disrupted trabecular microarchitecture at each phase. Because the state of the fracture of the trabecular bone is fresh, cement infiltration is better, and, as time goes by, somewhat already healed disrupted trabecular microarchitecture causes more uneven cement infiltration. This difficulty of cement infiltration to bone marrow and fixed cortical microdisruption as time elapsed may cause more common cement leakage. However, many reports have suggested that cement leakage appeared to be somewhat technique-dependent, and was rarely associated with clinical sequelae3,8,12). Also, correlation between the grade of cement interdigitation and clinical outcome has been controversial9,11). In the current study, such differences did not affect outcomes, including pain relief, surgical complication, and occurrence of adjacent fracture.
Although final vertebral body collapse did not differ significantly between the two groups, there was a trend indicating that immediate postoperative changes, that is, height gain of the vertebral body and reduction of segmental kyphotic angle were better in the EVPG than in the DVPG. We speculate that this result stems from the difference in the reversibility of fractured vertebra according to fracture age14).
This study had several limitations. First, the subsequent treatments with EVP and DVP were performed in study cohorts instead of randomized treatments. Therefore, effects of an operator learning curve could not be cancelled out. Second, three spinal surgeons were involved in this study, and minimal difference between surgical techniques could have influenced outcomes. Third, there was a lack of an objective way to measure viscosity of the injected cement. Finally, because we adapted strict inclusion criteria, the number of patients was too small. Conduct of a prospective randomized study with enrollment of more patients would provide greater validity and reliability to the results of this study.
CONCLUSION
If proper surgical indication is adapted, operation ratio of EVP strategy will not be higher than that of DVP strategy. In addition, in the viewpoint of waiting time and duration of hospital stay, EVP was more cost-effective than DVP.
Although final outcomes did not differ significantly between the two groups, immediate postoperative pain relief and radiological outcomes, including cement leakage and cement interdigitation, were more favorable in the EVPG than in the DVPG.
References
- 1.Alvarez L, Pérez-Higueras A, Granizo JJ, de Miguel I, Quiñones D, Rossi RE. Predictors of outcomes of percutaneous vertebroplasty for osteoporotic vertebral fractures. Spine (Phila Pa 1976) 2005;30:87–92. doi: 10.1097/00007632-200501010-00016. [DOI] [PubMed] [Google Scholar]
- 2.Anselmetti GC, Corrao G, Monica PD, Tartaglia V, Manca A, Eminefendic H, et al. Pain relief following percutaneous vertebroplasty : results of a series of 283 consecutive patients treated in a single institution. Cardiovasc Intervent Radiol. 2007;30:441–447. doi: 10.1007/s00270-006-0146-0. [DOI] [PubMed] [Google Scholar]
- 3.Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine (Phila Pa 1976) 2000;25:923–928. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Erkan S, Ozalp TR, Yercan HS, Okcu G. Does timing matter in performing kyphoplasty? Acute versus chronic compression fractures. Acta Orthop Belg. 2009;75:396–404. [PubMed] [Google Scholar]
- 5.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 6.Haynes SR, Lawler PG. An assessment of the consistency of ASA physical status classification allocation. Anaesthesia. 1995;50:195–199. doi: 10.1111/j.1365-2044.1995.tb04554.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann TJ, Jensen ME, Schweickert PA, Marx WF, Kallmes DF. Age of fracture and clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2001;22:1860–1863. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. 2006;6:479–487. doi: 10.1016/j.spinee.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Kim DJ, Kim TW, Park KH, Chi MP, Kim JO. The proper volume and distribution of cement augmentation on percutaneous vertebroplasty. J Korean Neurosurg Soc. 2010;48:125–128. doi: 10.3340/jkns.2010.48.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klazen CA, Lohle PN, de Vries J, Jansen FH, Tielbeek AV, Blonk MC, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II) : an open-label randomised trial. Lancet. 2010;376:1085–1092. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 11.Liebschner MA, Rosenberg WS, Keaveny TM. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine (Phila Pa 1976) 2001;26:1547–1554. doi: 10.1097/00007632-200107150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Lin EP, Ekholm S, Hiwatashi A, Westesson PL. Vertebroplasty : cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am J Neuroradiol. 2004;25:175–180. [PMC free article] [PubMed] [Google Scholar]
- 13.McGraw JK, Cardella J, Barr JD, Mathis JM, Sanchez O, Schwartzberg MS, et al. Society of Interventional Radiology quality improvement guidelines for percutaneous vertebroplasty. J Vasc Interv Radiol. 2003;14(9 Pt 2):S311–S315. doi: 10.1097/01.rvi.0000082822.75926.4c. [DOI] [PubMed] [Google Scholar]
- 14.Mellish RW, Garrahan NJ, Compston JE. Age-related changes in trabecular width and spacing in human iliac crest biopsies. Bone Miner. 1989;6:331–338. doi: 10.1016/0169-6009(89)90038-x. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwenhuijse MJ, Muijs SP, van Erkel AR, Dijkstra SP. A clinical comparative study on low versus medium viscosity polymethylmetacrylate bone cement in percutaneous vertebroplasty : viscosity associated with cement leakage. Spine (Phila Pa 1976) 2010;35:E1037–E1044. doi: 10.1097/BRS.0b013e3181ddd262. [DOI] [PubMed] [Google Scholar]
- 16.Norbert B, Mas A. Spinal disorders, Fundamentals of diagnosis and treatment. ed 1. New York: Springer; 2008. p. 938. [Google Scholar]
- 17.Peh WC, Gilula LA. Percutaneous vertebroplasty : indications, contraindications, and technique. Br J Radiol. 2003;76:69–75. doi: 10.1259/bjr/10254271. [DOI] [PubMed] [Google Scholar]
- 18.Rad AE, Kallmes DF. Correlation between preoperative pain duration and percutaneous vertebroplasty outcome. AJNR Am J Neuroradiol. 2011;32:1842–1845. doi: 10.3174/ajnr.A2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.USFDA. Guidance for Industry and FDA Staff - Clinical Trial Considerations : Vertebral Augmentation Devices to Treat Spinal Insufficiency Fractures. Silverspring: US FDA; 2004. [Google Scholar]