Abstract
Argonautes are highly conserved proteins found in almost all eukaryotes and some bacteria and archaea. In humans, there are eight argonaute proteins evenly distributed across two clades, the Ago clade (AGO1-4) and the Piwi clade (PIWIL1-4). The function of Ago proteins is best characterized by their role in RNA interference (RNAi) and cytoplasmic post-transcriptional gene silencing (PTGS) – which involves the loading of siRNA or miRNA into argonaute to direct silencing of genes at the posttranscriptional or translational level. However, nuclear-localized, as opposed to cytoplasmic, argonaute-small RNA complexes may also orchestrate the mechanistically very different process of transcriptional gene silencing, which results in prevention of transcription from a gene locus by the formation of silent chromatin domains. More recently, the role of argonaute in other aspects of epigenetic regulation of chromatin, alternative splicing and DNA repair is emerging. This review focuses on the activity of nuclear-localized short RNA-argonaute complexes in a mammalian setting and discusses recent in vivo studies employing nuclear-directed sRNA for therapeutic interventions. These studies heed the potential development of RNA-based drugs which induce epigenetic changes in the cell.
Keywords: Ago, argonaute, microRNA, miRNA, nuclear, RNAa, siRNA, TGS, therapy
Introduction
The argonautes are a family of highly evolutionarily conserved proteins that bind small-RNAs (sRNA) and have essential roles in the RNA interference and microRNA pathways (reviewed in ref. 1). Argonaute proteins are broadly classified into two main evolutionary clades; the Ago clade binds small ~20–30 nt non-coding RNAs which include microRNAs (miRNAs) and small interfering RNAs (siRNAs), while the Piwi clade Ago binds P-element induced wimpy testis (PIWI)-interacting RNAs (piRNAs). Wago, a third clade, is particular to nematode worms.2 Argonautes are characterized by a Piwi-Argonaute-Zwille (PAZ) domain and a PIWI domain. This PAZ domain functions by binding specifically to the 3′ end of single-stranded RNA and is also found in the Dicer enzyme.3 The Piwi pathway is thought to be confined to regulating RNA expression in germ cells (reviewed in ref. 4). The Ago pathway is ubiquitously expressed and in mammals is composed of four enzymes, Ago1, Ago2, Ago3, and Ago4 which are all capable of loading small RNA. This review will focus on the Ago pathway.
Ago2 is well studied as it is the “catalytic engine” that drives mRNA cleavage at miRNA target sites5,6 and is required for embryonic development.5,7 Ago2 is best characterized in its role in post-transcriptional gene silencing (PTGS). PTGS is a cytoplasmic process which involves the loading of the guide strand of the ~20–24 nt sRNA species, siRNA or miRNA, into the Ago2-containing RNA induced silencing complex (RISC), which can then recognize a complementary mRNA target and bind to inhibit translation or destabilize through the Ago2 endonuclease activity (reviewed in ref. 8). While PTGS was first characterized as a genome/viral defence, there are many small endogenous RNAs, in particular, miRNAs and endo-siRNA in mammalian embryonic stem cells.9 siRNA are generated by Dicer RNase cutting of long dsRNA, such as that produced in virus replication, while miRNA are processed from nuclear hairpin dsRNA into short hairpin pre-miRNA by DGCR8 and Drosha, an RNAse III–type endonuclease, with subsequent transport to the cytoplasm and further cleavage by Dicer (reviewed in ref. 10) (Figure 1a). Except, in the case of miR-451, as its pre-miRNA is processed directly by Ago2 instead of Dicer.11,12 The loading of the sRNA guide strand into Ago requires the Dicer and TRBP containing RISC, with subsequent activation requiring the C3PO complex for efficient passenger strand removal.13,14 In humans, it is thought C3PO may be the sole loader and activator of Ago2.15
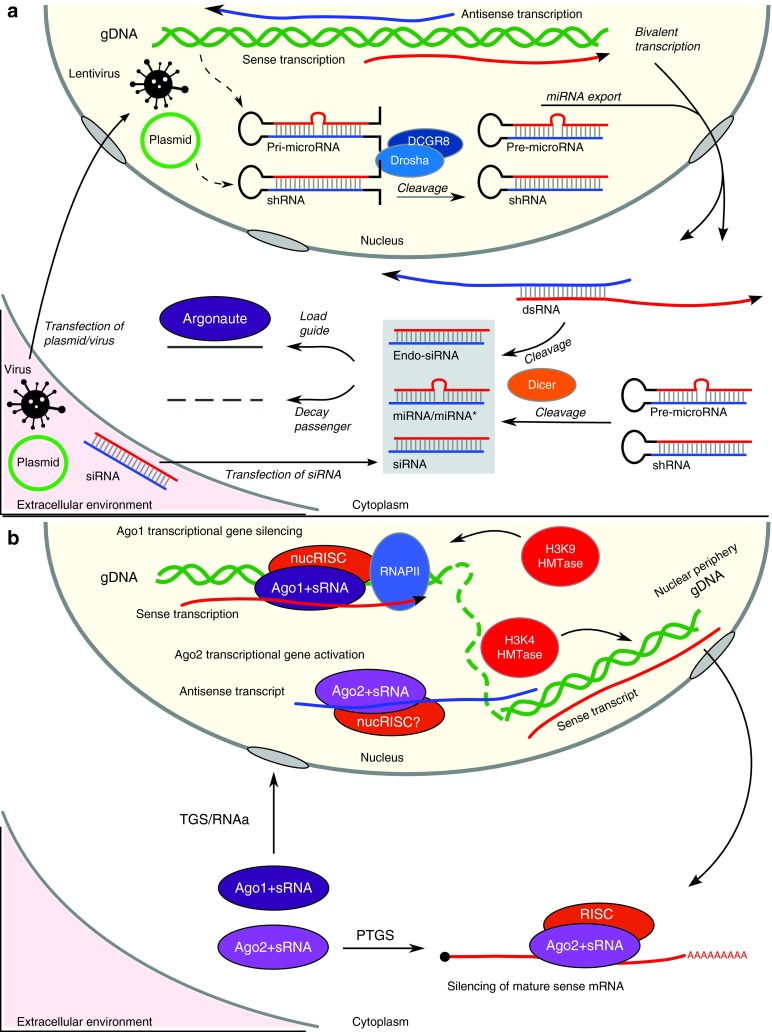
Figure 1.
The role of argonaute in transcriptional gene silencing (TGS) and activation (RNAa). (a) The dicer-generated small RNA (sRNA) species which are loaded into argonaute (gray box) can be derived from shRNA expressed from plasmids or viral vectors transfected into the cell, or from endogenous processes, such as miRNA biogenesis or dsRNA resulting from bivalent transcription. Alternatively, siRNA directly transfected into the cell may be loaded. The sRNA species have a single “guide” strand loaded into argonaute while the other “passenger” strand is degraded. (b) Loaded Ago1 (Ago1+sRNA) may enter the nucleus and together with a nuclear RISC (nucRISC) and in association with active RNAPII and sense transcripts, initiate chromatin remodelling and transcriptional gene silencing (TGS) by such enzymes as histone 3 lysine 9 methyltransferases (H3K9 HMTase). The loaded Ago2 (Ago2+sRNA) is catalytically active and may direct the silencing of mature mRNA in the cytoplasm via the post-transcriptional gene silencing (PTGS) pathway, or it may be migrate to the periphery of the nucleus and (perhaps in association with a nucRISC), degrade primarily antisense transcripts. This process is associated with remodeling to active chromatin by histone 3 lysine 4 histone methyltransferases (H3K4 HMTase) and subsequent transcriptional gene activation (RNAa). There is also evidence nuclear Ago2 is associated with TGS.
While Ago2 is well studied, much less is known about the function of the other argonautes. Ago1 is 80% identical to Ago2 but lacks a key catalytic residue and cannot cleave RNA efficiently. It is associated with the loading of specific sRNAs derived from the Epstein–Barr virus16 and Ago1 and/or Ago3 is required for optimal resistance to influenza-A in mice.17 Little is known about the function of Ago1 except that its overexpression slows neuroblastoma growth.18 Ago3, like Ago2, contains the catalytic residues essential for cleavage and is required to induce human embryonic stem cell proliferation arrest through binding of sRNA generated from transcribed Alu repeats and subsequent PTGS of critical stem cell mRNAs.19 Ago4 has been reported to localise to mouse spermatocyte nuclei during meiotic prophase and regulate meiotic entry.20
Sequencing or microarray analysis of endogenous sRNA bound to human Ago1, Ago2 and Ago3 shows that the great majority is miRNA, which has been loaded with no particular strand bias towards the pre-miRNA 3p (antisense) or 5p (sense) strand.21,22,23 However, those miRNA preferentially loaded into Ago1 or Ago2 had distinct antisense and sense bias, respectively.23 Other endogenous sRNA are also bound to Ago, as well as sRNA from intronic and exonic coding gene regions and promoter regions of coding genes.24 Many of these sRNA species were longer than the 21 nt canonical length, in particular promoter-derived RNAs were mostly of 21–24 nucleotides in length and mostly associated with Ago1 and Ago3. The differential loading between Ago1 and Ago2 might be due, in part, to editing of the 3′-end, with miRNA terminating in 3′ adenine and uracil preferentially loaded into Ago1 and Ago2, respectively.22 This differs from piRNA loaded into PIWI-clade Ago, as these are 2′-O-methylated at the 3′ termini and often include uracil at the 5′ end.25,26,27 Recent findings show that in Ago1-4 knockout mouse embryonic stem cells, expression of inducible Ago2 confers miRNA stability.28 Interestingly, this same work unveiled a new class of Ago/Dicer-dependent miRNAs which arise from the transcription start site (TSS-miRNA) of RNAPII protein-coding gene promoters.
Function in the Nucleus
Over the last decade or so, Ago has been recognized as having a role in the nucleus. RNA-induced silencing complexes (RISCs) containing Ago1 and Ago2 are present in both the cytosolic and nuclear fractions of human cells29,30,31,32 and Ago1 and Ago2 are known to associate with promoter DNA.33 Argonaute–RNA complexes can regulate nuclear events such as transcriptional silencing and activation as well as alternative splicing and DNA repair.34,35,36 Nuclear Ago1 directly interacts with RNA polymerase II (RNAPII)30,37,38 and binds to the promoters of actively transcribed genes.30,38 There are two contrary reports that describe human nuclear Ago2 as part of a multiprotein complex together with Dicer, TRBP and TRNC6A/GW182,39 or conversely in solitary form.40 However, both of these studies agree that Ago2 is loaded in the cytoplasm and imported into the nucleus (Figure 1b).
Using immunofluorescence microscopy, two groups have shown that tagged Ago1 and Ago2 have different nuclear distributions. Ago1 is scattered throughout the nuclear interior, whereas Ago2 co-localizes with siRNA primarily in the inner nuclear envelope.30,41 ChIP-seq data shows that Ago1 is associated with thousands of chromosomal loci throughout the genome, but in particular with the promoters of actively transcribed genes and distributed in highly punctate peaks mostly overlapping with histone 3 lysine 4 tri-methylation (H3K4me3).30 In the same study, there was no evidence to suggest Ago2 interacts with chromatin. Sequencing of sRNA bound to argonaute in whole-cell lysate from mouse T cells, shows that ~0.02% of reads map to promoter regions, with twofold more bound to Ago1 and Ago3, than to Ago2. Around 0.2% of Ago1 tags mapped to coding regions, which was six- and twofold more than Ago2 and Ago3, respectively. Over 80% of tags mapping in the antisense direction were bound to Ago1, while for sense tags, the split was closer across Ago proteins.22
Dicer also has a nuclear localization signal, in the dsRNA binding domain42 and there is clear evidence it is nuclear-imported. Nuclear Dicer has been variously reported as located in the nuclear periphery in association with NUP153, a component of the nuclear pore complex,43 or spread throughout the nucleoplasm with a punctate distribution.44 Nuclear Dicer is associated with RNAPII at actively transcribed genes and is required for Ago1-mediated TGS.44 Furthermore, nuclear Dicer is catalytically active and will cleave target RNA,29,39,44,45 with knockdown resulting in accumulation of endogenous double-stranded RNA (dsRNA), induction of the interferon-response pathway and cell death.44 Dicer is known to be important for the epigenetic regulation of the cell as its activity is required for the correct formation of heterochromatin structure in mammalian cells.46
Collectively, evidence suggests nuclear Ago1 binds mostly antisense sRNA and is distributed throughout the nucleus at promoters of active genes in association with RNAPII and Dicer, while nuclear Ago2 complexes are at the nuclear periphery and contain both sense and antisense sRNA.
Exogenous Small RNA Transcriptional Silencing and Activation
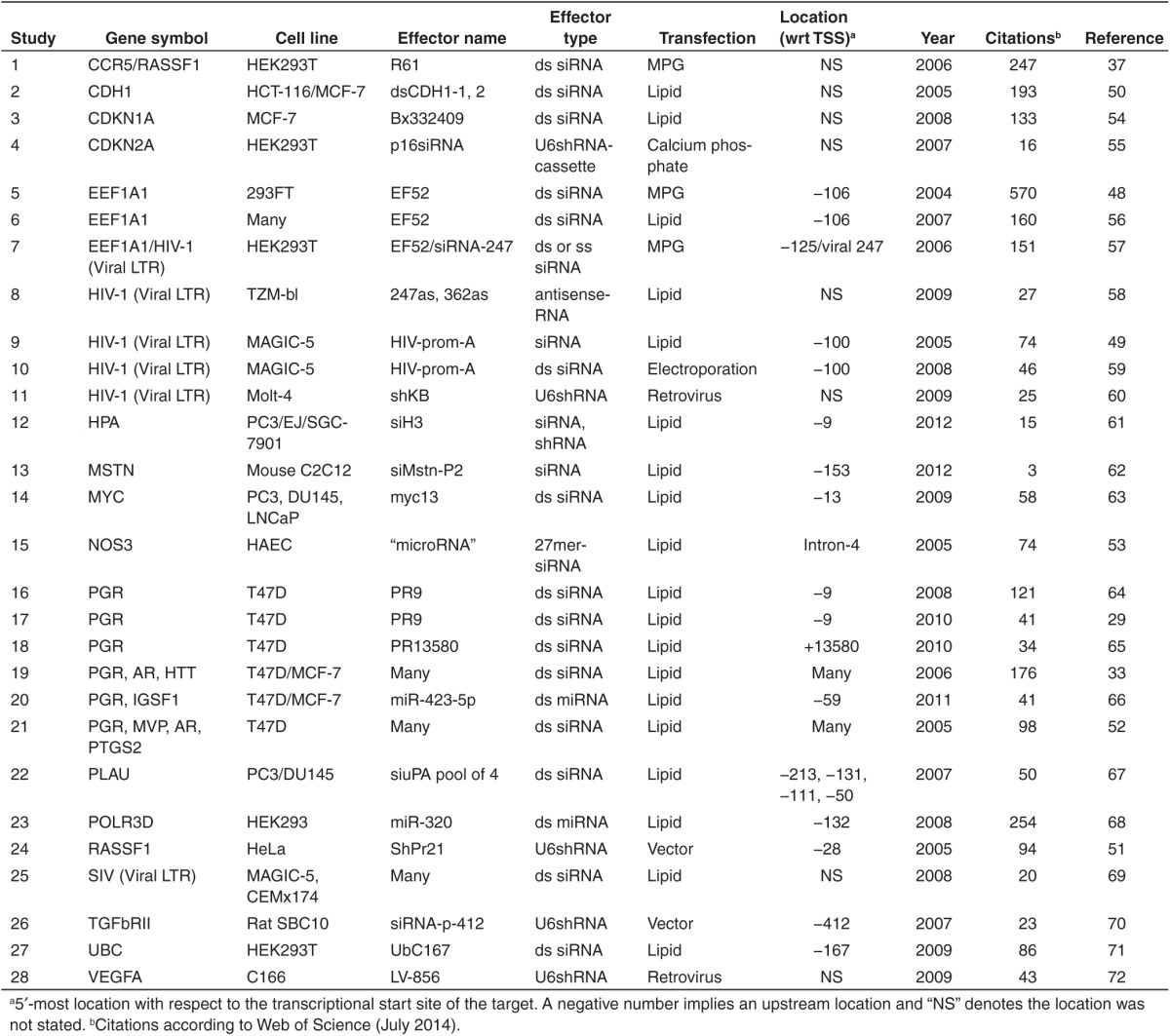
RNA-directed transcriptional gene silencing (TGS) is an important means of regulation, evident by the conservation of mechanism from yeast to plants to higher mammals (reviewed in ref. 47). The phenomenon of mammalian TGS was first reported by Morris et al. in 2004.48 They described transcriptional silencing of both endogenous and integrated proviral elongation factor 1-alpha 1 (EEF1A1) by delivery of a siRNA targeting the promoter region. This study was closely followed by those from several other groups also reporting TGS driven by promoter-directed siRNA targeting the HIV-1 viral LTR,49 CDH1,50 RASSF1,51 PGR, MVP, both AR and PTGS2 (ref. 52) and NOS3.53 The phenomenon has continued to be observed and clarified (Table 1).29,33,37,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72
Table 1. List of transcriptional gene silencing (TGS) studies.
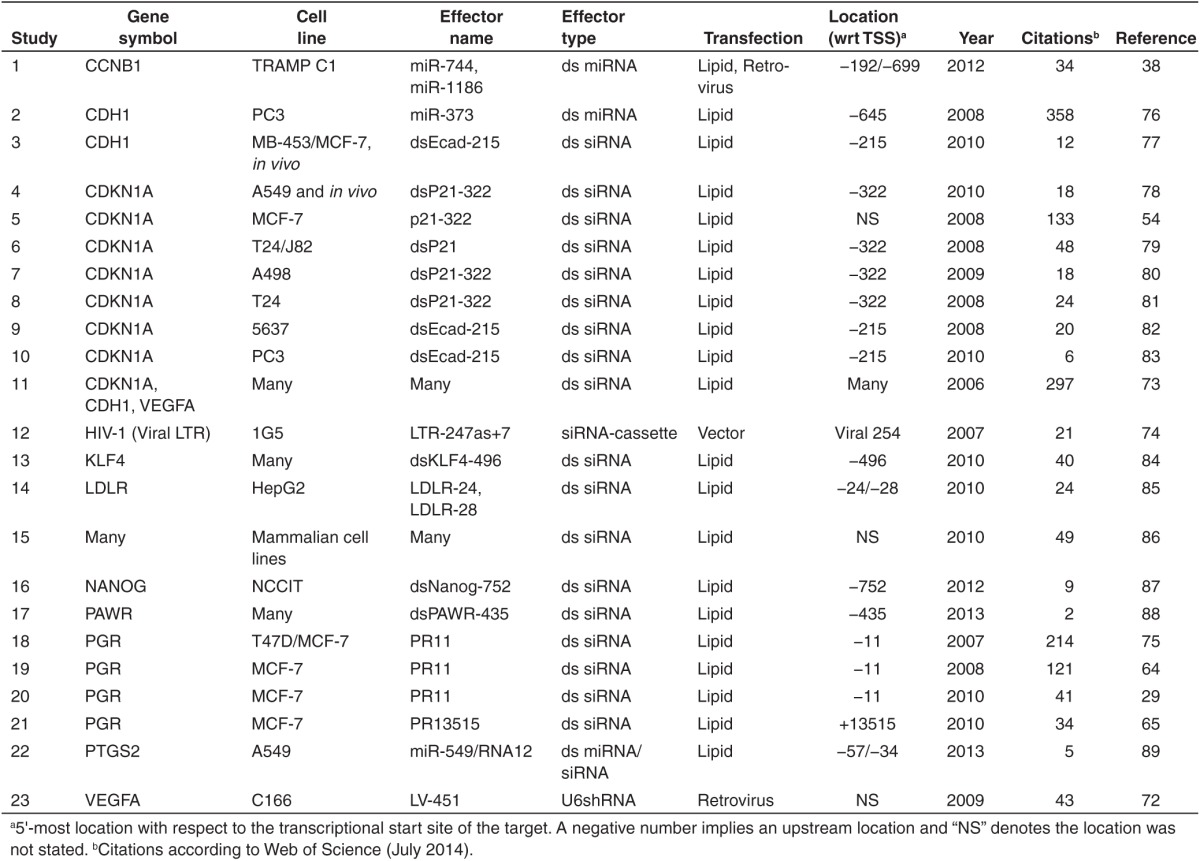
The counter-phenomenon of mammalian sRNA-induced transcriptional gene activation (RNAa) was reported in 2006 by Li et al.73 who observed that transfecting siRNA complementary to the promoter regions of CDKN1A, CDH1, and VEGFA resulted in increased transcription of the respective gene. Unlike the early TGS studies, their promoter-directed dsRNA designs avoided promoter CpG-rich regions. These observations were soon supported by work from two other groups that reported RNAa of the HIV-1 viral LTR74 and PGR,75 respectively. RNAa resulting from a single transfection could be observed for between 9 and 13 days.73,75 Paradoxically, Janowski et al. showed that 21-mer ds siRNA designed against the progesterone receptor (PGR) as little as a few nucleotides apart could profoundly affect the activation potential and in some cases, cause TGS instead of RNAa.75 Subsequently, there are many more reports of small-RNA directed RNAa, including by groups also demonstrating TGS (Table 2).29,51,54,64,65,72,76,77,78,79,80,81,82,83,84,85,86,87,88,89
Table 2. List of transcriptional gene activation (RNAa) studies.
Results showing suppression or activation of transcription by promoter-directed siRNA need to be interpreted with some caution. It is possible that alteration of observed transcriptional levels attributed to RNAa or TGS may in fact be the result of sequence-specific off-target effects. These off-target effects have been reported for siRNA designed against the VEGFA promoter90 and the HIV-1 LTR promoter.74
Requirement for Argonaute in Nuclear Processes
Knockdowns of argonaute protein have shown it is required for small-RNA induced TGS or RNAa (Tables 1 and 2). Some controversy exists about whether Ago1 or Ago2 is the main argonaute recruited to enact TGS. The first studies reported Ago1 as the key argonaute driving TGS, while some latter studies show evidence that only knockdown of Ago2 resulted in abrogation of TGS, with knockdown of Ago1, Ago3, or Ago4 not overly reducing the effect. In an early study, the Corey group reported that both Ago1 and Ago2 were required for TGS,33 however in later work they rationalized that off-target knockdowns may explain their earlier results.29 In the case of RNAa, fewer studies have examined the requirement for argonaute; however, there is no disagreement; all suggest that only Ago2 is required (Figure 2).
Figure 2.
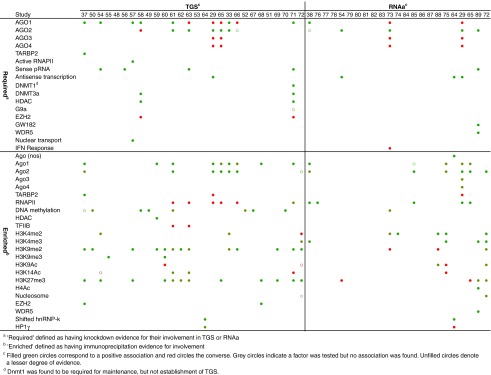
Required and enriched factors for transcriptional gene silencing and activation.
Association with Transcription, Epigenetic Regulation, and RISC Subunits
Early models speculated that siRNA might directly base-pair with DNA and recruit DNA methyltransferase (DNMT), but work by ourselves and others has shown that DNMTs cannot methylate DNA:RNA hybrid structures91 and that instead, Ago-loaded nuclear siRNA binds to nascently transcribed RNA.56,64 There are a number of reports that TGS requires active transcription by RNAPII and expression of sense-strand mRNA through the gene promoter.56,57,63,71 However, other data shows that Ago1 and Ago2 bind to antisense transcripts during TGS.29 Interestingly, transfection of only the siRNA anti-sense strand is sufficient to silence the EEF1A1 gene.56,57 Matsui et al.89 report Ago2-dependent transcriptional gene activation of PTGS2 by the endogenous miRNA, miR-589. As only one strand of miR-589 is complementary to the promoter RNA, they posit that the sense strand is the only potential partner for gene activation. In agreement, microarray data shows that in MCF7 cells, miRNA with biased Ago2 association are predominantly derived from sense strands of the corresponding pre-miRNA, while the majority of Ago1-associated miRNAs originate from the antisense strand.23
In accord with expectation, there is clear evidence that, after TGS, less RNAPII is found at promoters,29,33,53,61,62,63,64,65,66 while after RNAa, there is enrichment for RNAPII.29,38,65,76,85 Most studies that have examined chromatin regulation found promoters after TGS to be enriched for histones with silencing marks and those after RNAa with active marks (Figure 2). In particular, TGS is associated with enrichment for the repressive histone 3, lysine 9 di- and tri-methylation (H3K9me2, H3K9me3) and lysine 27 tri-methylation (H3K27me3) marks, with loss of the active histone 3 lysine 9 acetylation(H3K9Ac), lysine 14 acetylation (H3K14Ac) marks and in some cases loss of the active H3K4me3 mark. For RNAa, histone regulation is reversed, with loss of repressive H3K9me2 and H3K27me3 marks and gain of the activating H3K4me2 and H3K4me3 marks. Intriguingly, like TGS, RNAa is associated with the loss of H3K9ac and H3K14ac marks. These marks are associated with active and bivalent promoters in mouse cells.92 Some TGS studies report an increase in DNA methylation at the targeted promoter (Figure 2). The de novo methyltransferase Dnmt3a is required to establish DNA methylation at the targeted region of the promoter and Dnmt1 is required for maintenance of that methylation.58,71
Knockdown experiments suggest a TGS requirement for the chromatin condensation-associated histone deacetylase HDAC, perhaps the H3K9 methyltransferase G9a, but not the polycomb-group H3K27 methyltransferase, EZH2.58,71 However, in ChIP experiments from two TGS studies, EZH2 is enriched at target promoters.37,68 There is also a requirement in TGS for the RISC-loading complex subunit, TARBP2.37 For RNAa, it has been reported that activation of the PTGS2 locus required the WD repeat-containing protein 5 (WDR5) and the argonaute-interacting, GW182.89 Interestingly, the long intergenic ncRNA (lncRNA), HOTTIP, transcribed from the 5′-end of the HOXA locus, binds to WDR5 and in turn, mixed-lineage leukaemia (MLL) histone methyltransferase, which drives gene activation and H3K4 trimethylation (H3K4me3). This same histone mark is known to be enriched in RNAa (Table 2).72,75,89 This suggests transcriptional gene activation by short RNA and lncRNA may share a number of features in common.
Endogenous Small RNA Transcriptional Silencing and Activation
miRNA
The initial observation of exogenous small-RNAs directing TGS suggested endogenous sRNAs such as miRNA or endo-siRNA may elicit the same phenomenon. The first examples of endogenous TGS and RNAa in mammals were of genes with predicted miRNA binding sites in their promoters; in particular the silencing of POLR3D by miR-320 (ref. 68) and PGR and IGSF1 by miR-423-5p66 and the activation of CDKN1 transcription by miR-373, (ref. 76) PTGS2 transcription by miR-549 (ref. 89) and CCNB1 by miR-744 and miR-1186 (ref. 38) (Tables 1 and 2). Huang et al. found Ago1 was enriched at the CCNB1 promoter, just proximal to the predicted miR-744-binding site, but did not find any enrichment for Ago2.38 Particular miRNA 5′ sequence elements may direct import of the mature miRNA back into the nucleus93 with this re-importation dependent on the association of Importin8 with Ago2.31,94 There is evidence that Ago2 is involved in control of chromatin structure at a genomic miRNA site,95,96 with Ago2 knockdown correlated with upregulation of expression of the miRNA-155 host gene primary transcript (miR-155HG), the overlapping antisense long noncoding RNA transcript and an increase in acetylation of histone 4 in the promoter region.95
Benhamed et al. describe senescence-associated transcriptional gene silencing (SA-TGS); during cellular senescence miRNA such as let-7 direct Ago2 to promoters of the tumor suppressor gene/transcription factors repressor complex (RB1/E2F) target genes (such as CDC2 and CDCA8). These miRNA/Ago2 complexes block RNAPII engagement at target promoters and cooperate with E2F/RB1 complex to repress E2F-target promoters, resulting in increased H3K9me2 and H3K27me3 heterochromatin associated marks and a decrease in the active mark, H3K4me3.96 Ago2 accumulates in the nucleus of senescent cells, with Ago2 knockdown resulting in delayed senescence. This transcriptional repression of proliferation-promoting genes by SA-TGS may contribute to tumor suppression.
lncRNA
miRNA have an epigenetic TGS role either in cis as antisense targeting its own genomic location to silence adjacent gene transcription, or by associating with a methyltransferase such as EZH2, they may also function more broadly in TGS to target gene promoters in trans.68 lncRNA can also have both a cis and trans epigenetic role and can function in cis as antisense at the transcriptional level, regulating protein coding gene expression or in trans acting as scaffolds to mediate interactions that guide enzyme complexes to specific RNA or DNA target sites in order to exert their effect.
Despite lncRNA frequently being nuclear-localized, many have been found to interact with miRNA,97,98 suggestive of a role for nuclear Ago-miRNA complexes; however, direct evidence is lacking. Functionally, these miRNA-binding lncRNA, known as competing endogenous RNA (ceRNA), act like a “microRNA sponge” effectively reducing available miRNA.99 By antagonizing miRNAs, lncRNA are known to regulate several developmental processes.100,101,102 lncRNA interacting with miRNA can also result in histone modification and subsequent gene repression.103,104 For example, the lncRNA-miRNA complex of HULC and miR-372 recruits the histone modifying enzyme P300, a histone acetyltransferase and subsequently causes heterochromatin formation resulting in TGS.105
Sense-antisense transcription frequently results in formation of dsRNA.106 It has long been known in Schizosaccharomyces pombe that dsRNAs target complementary mRNAs for degradation via the Ago1 RNAi pathway (reviewed in ref. 107) and these interactions result in heterochromatin formation at specific DNA loci. Recently, antisense ncRNAs have been implicated in the silencing of tumor suppressor genes through epigenetic remodeling events in humans.54,57 miRNAs can recruit Ago2 to antisense lncRNA transcripts that overlap their target gene promoter.66,73,108 Younger et al. found miR-423-5p binds to RNA within the target progesterone receptor (PR) gene promoter, as well as ncRNA transcribed from the PR promoter, with TGS associated with recruitment of Ago2 to this ncRNA with subsequent decrease in promoter RNAPII occupancy and increase in the H3K9me2 mark.66 Other data shows nuclear Ago2-mediated regulation of the lncRNAs MALAT1 and the star-strand HOTAIR* by miR-9 and miR-141, respectively.99,109 It appears that both MALAT1 and HOTAIR and most likely other lncRNAs may be functioning as a ceRNA110 and competing for miRNA target sites, mediated by Ago2. Evidence for this appears in recent global analyses of Ago-bound transcripts.111,112,113
In the human genome, antisense transcription is widespread,106 which suggests a large potential for convergent transcription of overlapping transcripts to form endogenous dsRNA and to regulate the transcriptome. In S. pombe and mammals, convergent transcription induces TGS in trans.45 Antisense ncRNAs have been implicated in the silencing of tumor suppressor genes through epigenetic remodeling events in humans.54,57
Argonaute Association with Other Endogenous Processes
Alternative splicing
The introduction of exogenous duplex RNAs into the nucleus has been shown to redirect exon splicing of aberrant splice sites of the disease-associated SMN2 and dystrophin genes.35 The duplex RNAs recruited Ago2 to pre-mRNA transcripts and altered splicing without nuclear cleavage of the pre-mRNA. The first reported involvement of endogenous sRNA pathways in alternative-splicing was of the regulation of pre-mRNAs by MALAT.114 Subsequently, Ago1 and Ago2 have been identified in physical association with MALAT, chromatin modifiers and splicing factors.36 Using the CD44 gene as a model, the authors show that Dicer-dependent recruitment of Ago1 and Ago2 facilitated spliceosome recruitment and modulated RNAPII elongation rate thereby affecting alternative splicing. The recruitment of Ago1 and Ago2 to CD44 transcribed regions required the Dicer and histone modifying enzymes which resulted in increased H3K9 methylation on variant exons associated with heterochromatin and TGS.36
Double strand break repair
The double strand break (DSB) repair role of Ago in plants is well known, however, this function has been recently found conserved in humans, with a reduction of DSB repair ability observed after knockdown of Dicer or Ago2.115 These DSB-induced small RNAs (diRNA) are produced from sense and antisense strands of DSB proximal sequence. In mammals, like in plants and Drosophila, diRNAs may function as guide molecules directing chromatin modifications which cause heterochromatin formation or the recruitment of protein complexes to DSB sites to facilitate repair. diRNA binding and catalytic activity of Ago2 are required for recruitment of the repair protein Rad51 to DSBs.34
Model of Action
In the literature, there is conflicting information about the involvement of Ago1 and Ago2 in enacting transcriptional gene silencing and activation (see previous sections). More recent evidence suggests reconciliation into one central model is unnecessary. Instead, there is evidence for two discrete but overlapping pathways involved in TGS and RNAa (Figure 1b).
Generally, exogenous promoter-directed sRNA data shows that Ago1 is associated with TGS and transcription of sense promoter-associated RNA, while Ago2 is associated with both TGS and RNAa and antisense transcription (Figure 2). With regard to endogenous nuclear miRNA bound to Ago, the evidence suggests overlapping functionality of Ago1 and Ago2, but with some clear exceptions. Some miRNA species exhibit pre-miRNA antisense strand loading bias into Ago1 and the sense strand into Ago2.23 The interactions and location of argonautes within the nucleus reinforce the idea of discrete pathways of action. Ago1, but not Ago2, interacts directly with RNAPII and binds to the promoters of actively transcribed genes30,37 and only Ago1 has readily observable interactions with chromatin.30 Furthermore, Ago1 is dispersed throughout the nucleus, whereas Ago2 is primarily localized to the inner nuclear envelope.30,41 At the nuclear periphery are lamina associated domains (LADs), DNA which is known to be mostly maintained in a silent state with activation upon cellular differentiation (reviewed in ref. 116). The distribution of Ago2 in the vicinity of LADs is consistent with its involvement primarily in RNAa.
Ago2-induced RNAa has some seemingly paradoxical observations, in particular, the observation that the PGR promoter-directed siRNAs PR9 and PR11 (Tables 1 and 2), designed only two nucleotides apart, may induce TGS in high PGR-expressing T47D cells and RNAa in low PGR-expressing MCF-7 cells, respectively.29,33,64 RNAa at the PGR locus is known to depend upon antisense transcription and involves the PR11-loaded Ago2.29,64
Perhaps these results can be explained by consideration of the findings by Morris et al.54 In their 2008 study, they provide evidence that sense transcription from the p21 (CDKN1A) locus is held in balance by transcription from overlapping antisense transcripts; i.e., Ago2-mediated PTGS of antisense transcription results in activation of p21 sense transcription, while—conversely—PTGS of p21 sense mRNA results in activation of p21 antisense transcription and the recruitment of Ago1 at the p21 promoter. They also show the presence of p21 antisense transcript and Ago1 are required for regulation of promoter-associated sense RNA and suppression of the p21 promoter. Their findings imply that apparent RNAa is, in fact, the result of post-transcriptional repression of antisense RNA at bidirectionally transcribed loci. If instead PTGS is directed towards the sense RNA, this may result in increased promoter RNA transcription and reinforcement of silencing through recruitment of Ago1. This suggests that at bidirectional promoters, Ago1 and Ago2 may work in concert in the nucleus to reinforce cytoplasmic PTGS of gene loci. Indeed, genome-wide data shows that candidate antisense promoter-associated ncRNAs (pancRNAs) are associated with active chromatin marks, with the forced expression or knockdown of these pancRNAs causing DNA demethylation and methylation at the gene promoter, respectively.117,118
The association of Ago1, active RNAPII, Dicer and promoter sense transcription with TGS suggests a rapid monitoring system for aberrant transcription such as might be expected by transcription from transposons, repeated sequences and proviruses, or from chromosome abnormalities.29,48,117,118,119
Presently, it is unknown why there is a distinct strand loading bias between Ago1 and Ago2. These argonautes are structurally very similar and it remains to be seen whether the loading bias is an intrinsic property of argonaute or of co-factors in a loading complex.
Disease Therapy
The demonstrated specificity and potentially long-term efficacy of exogenous sRNA in vitro, raises the potential for a new class of RNA-based drugs. It may be possible to develop cancer therapies or to stably suppress HIV-1 replication in the CD-4+ T cells of HIV patients. Indeed, many TGS and RNAa studies target genes associated with cancer and the HIV-1 virus in cultured cells (Tables 1 and 2). To this end, the field has now matured to the point where in vivo experiments are being undertaken. The efficacy in vivo of sRNA-directed transcriptional silencing or activation to control tumor growth has been demonstrated using mouse xenograft models. In vivo, stable RNAa activation of CCNB1 by miRNA constructs resulted in tumors with reduced size compared to controls.38 Similarly, treating established tumors with lipid transfections of siRNA targeting CDKN1A78 or CDH1 (ref. 77) every three days showed reduced tumor growth, relative to controls. In addition, dsRNA chemically modified for lipidoid-encapsulated nanoparticle delivery is efficacious in promoting RNAa of CDKN1A in mouse xenografts.120,121
Effective lentiviral delivery of shRNA has also been demonstrated in vivo. The lentiviral transfer of VEGF promoter targeted shRNAs has been observed to increase blood flow in the hindlimbs of ischemic mice72 and recently, in this journal, Suzuki et al. showed that shRNA targeting the HIV-1 LTR was able to inhibit HIV-1 replication in lentiviral transduced human peripheral blood mononuclear cells circulating in humanized mice.122 These results show promise in the translation of exogenous sRNA therapy to the clinic. It is foreseeable that RNA-based drugs may eventually be used to reprogram epigenetic state at a targeted locus.
Also, as we better understand endogenous nuclear sRNA processes, the mechanisms behind some diseases may be uncovered. In a now classic case, Tufarelli et al. documented a rare α-thalassaemia resulting from a deletion truncating LUC7L and bringing it, juxtaposed, within proximity of the HBA2 hemoglobingene. Expression of the LUC7L antisense transcript resulted in epigenetic silencing of HBA2.123
Acknowledgments
The authors thank Peter Molloy (CSIRO) and Lloyd Graham (CSIRO) for their critical review of this manuscript. This work is supported by the CSIRO Preventative Health National Research Flagship. The authors declare no conflict of interest.
References
- Meister G. Argonaute proteins: functional insights and emerging roles. Nat Rev Genet. 2013;14:447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- Yigit E, Batista PJ, Bei Y, Pang KM, Chen CC, Tolia NH, et al. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell. 2006;127:747–757. doi: 10.1016/j.cell.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457:405–412. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- Bortvin A. PIWI-interacting RNAs (piRNAs) - a mouse testis perspective. Biochemistry Mosc. 2013;78:592–602. doi: 10.1134/S0006297913060059. [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. 2008;22:2773–2785. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465:584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes D, Xue H, Taylor DW, Patnode H, Mishima Y, Cheloufi S, et al. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science. 2010;328:1694–1698. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae IJ, Ma E, Zhou M, Robinson CV, Doudna JA. In vitro reconstitution of the human RISC-loading complex. Proc Natl Acad Sci USA. 2008;105:512–517. doi: 10.1073/pnas.0710869105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ye X, Jiang F, Liang C, Chen D, Peng J, et al. C3PO, an endoribonuclease that promotes RNAi by facilitating RISC activation. Science. 2009;325:750–753. doi: 10.1126/science.1176325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Huang N, Liu Y, Paroo Z, Huerta C, Li P, et al. Structure of C3PO and mechanism of human RISC activation. Nat Struct Mol Biol. 2011;18:650–657. doi: 10.1038/nsmb.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa N, Okuyama K, Ogata J, Kanai A, Helwak A, Takamatsu M, et al. Novel functional small RNAs are selectively loaded onto mammalian Ago1. Nucleic Acids Res. 2014;42:5289–5301. doi: 10.1093/nar/gku137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stry M, Oguin TH, 3rd, Cheloufi S, Vogel P, Watanabe M, Pillai MR, et al. Enhanced susceptibility of Ago1/3 double-null mice to influenza A virus infection. J Virol. 2012;86:4151–4157. doi: 10.1128/JVI.05303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi C, Giorgi C, Batassa EM, Braccini L, Maresca G, D'agnano I, et al. Ago1 and Ago2 differentially affect cell proliferation, motility and apoptosis when overexpressed in SH-SY5Y neuroblastoma cells. FEBS Lett. 2011;585:2965–2971. doi: 10.1016/j.febslet.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Hu Q, Tanasa B, Trabucchi M, Li W, Zhang J, Ohgi KA, et al. DICER- and AGO3-dependent generation of retinoic acid-induced DR2 Alu RNAs regulates human stem cell proliferation. Nat Struct Mol Biol. 2012;19:1168–1175. doi: 10.1038/nsmb.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewski AJ, Holmes RJ, Hilz S, Grimson A, Cohen PE. AGO4 regulates entry into meiosis and influences silencing of sex chromosomes in the male mouse germline. Dev Cell. 2012;23:251–264. doi: 10.1016/j.devcel.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang Z, O'Loughlin E, Lee T, Houel S, O'Carroll D, et al. Quantitative functions of Argonaute proteins in mammalian development. Genes Dev. 2012;26:693–704. doi: 10.1101/gad.182758.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikepahad S, Corry DB. Profiling of T helper cell-derived small RNAs reveals unique antisense transcripts and differential association of miRNAs with argonaute proteins 1 and 2. Nucleic Acids Res. 2013;41:1164–1177. doi: 10.1093/nar/gks1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchinovich A, Burwinkel B. Distinct AGO1 and AGO2 associated miRNA profiles in human cells and blood plasma. RNA Biol. 2012;9:1066–1075. doi: 10.4161/rna.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs AM, Ando Y, de Hoon MJ, Tomaru Y, Suzuki H, Hayashizaki Y, et al. Deep-sequencing of human Argonaute-associated small RNAs provides insight into miRNA sorting and reveals Argonaute association with RNA fragments of diverse origin. RNA Biol. 2011;8:158–177. doi: 10.4161/rna.8.1.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science. 2007;318:761–764. doi: 10.1126/science.1146484. [DOI] [PubMed] [Google Scholar]
- Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, et al. A slicer-mediated mechanism for repeat-associated siRNA 5' end formation in Drosophila. Science. 2007;315:1587–1590. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio JR, Kelly TJ, Sharp PA. Argonaute-bound small RNAs from promoter-proximal RNA polymerase II. Cell. 2014;156:920–934. doi: 10.1016/j.cell.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Yue X, Younger ST, Janowski BA, Corey DR. Involvement of argonaute proteins in gene silencing and activation by RNAs complementary to a non-coding transcript at the progesterone receptor promoter. Nucleic Acids Res. 2010;38:7736–7748. doi: 10.1093/nar/gkq648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang V, Zheng J, Qi Z, Wang J, Place RF, Yu J, et al. Ago1 Interacts with RNA polymerase II and binds to the promoters of actively transcribed genes in human cancer cells. PLoS Genet. 2013;9:e1003821. doi: 10.1371/journal.pgen.1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann L, Höck J, Ivacevic T, Ohrt T, Mütze J, Schwille P, et al. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell. 2009;136:496–507. doi: 10.1016/j.cell.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Robb GB, Brown KM, Khurana J, Rana TM. Specific and potent RNAi in the nucleus of human cells. Nat Struct Mol Biol. 2005;12:133–137. doi: 10.1038/nsmb886. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Huffman KE, Schwartz JC, Ram R, Nordsell R, Shames DS, et al. Involvement of AGO1 and AGO2 in mammalian transcriptional silencing. Nat Struct Mol Biol. 2006;13:787–792. doi: 10.1038/nsmb1140. [DOI] [PubMed] [Google Scholar]
- Gao M, Wei W, Li MM, Wu YS, Ba Z, Jin KX, et al. Ago2 facilitates Rad51 recruitment and DNA double-strand break repair by homologous recombination. Cell Res. 2014;24:532–541. doi: 10.1038/cr.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hu J, Corey DR. Expanding the action of duplex RNAs into the nucleus: redirecting alternative splicing. Nucleic Acids Res. 2012;40:1240–1250. doi: 10.1093/nar/gkr780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameyar-Zazoua M, Rachez C, Souidi M, Robin P, Fritsch L, Young R, et al. Argonaute proteins couple chromatin silencing to alternative splicing. Nat Struct Mol Biol. 2012;19:998–1004. doi: 10.1038/nsmb.2373. [DOI] [PubMed] [Google Scholar]
- Kim DH, Villeneuve LM, Morris KV, Rossi JJ. Argonaute-1 directs siRNA-mediated transcriptional gene silencing in human cells. Nat Struct Mol Biol. 2006;13:793–797. doi: 10.1038/nsmb1142. [DOI] [PubMed] [Google Scholar]
- Huang V, Place RF, Portnoy V, Wang J, Qi Z, Jia Z, et al. Upregulation of Cyclin B1 by miRNA and its implications in cancer. Nucleic Acids Res. 2012;40:1695–1707. doi: 10.1093/nar/gkr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KT, Li L, Chu Y, Janowski BA, Corey DR. RNAi factors are present and active in human cell nuclei. Cell Rep. 2014;6:211–221. doi: 10.1016/j.celrep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrt T, Mütze J, Staroske W, Weinmann L, Höck J, Crell K, et al. Fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy reveal the cytoplasmic origination of loaded nuclear RISC in vivo in human cells. Nucleic Acids Res. 2008;36:6439–6449. doi: 10.1093/nar/gkn693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenstiel CL, Lim HG, Cooper DA, Ishida T, Kelleher AD, Suzuki K. Direct evidence of nuclear Argonaute distribution during transcriptional silencing links the actin cytoskeleton to nuclear RNAi machinery in human cells. Nucleic Acids Res. 2012;40:1579–1595. doi: 10.1093/nar/gkr891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M, Badertscher L, Jaskiewicz L, Güttinger S, Jurado S, Hugenschmidt T, et al. The double-stranded RNA binding domain of human Dicer functions as a nuclear localization signal. RNA. 2013;19:1238–1252. doi: 10.1261/rna.039255.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando Y, Tomaru Y, Morinaga A, Burroughs AM, Kawaji H, Kubosaki A, et al. Nuclear pore complex protein mediated nuclear localization of dicer protein in human cells. PLoS ONE. 2011;6:e23385. doi: 10.1371/journal.pone.0023385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E, Schlackow M, Kamieniarz-Gdula K, Proudfoot NJ, Gullerova M. Human nuclear Dicer restricts the deleterious accumulation of endogenous double-stranded RNA. Nat Struct Mol Biol. 2014;21:552–559. doi: 10.1038/nsmb.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullerova M, Proudfoot NJ. Convergent transcription induces transcriptional gene silencing in fission yeast and mammalian cells. Nat Struct Mol Biol. 2012;19:1193–1201. doi: 10.1038/nsmb.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa T, Nogami M, Yoshikawa M, Ikeno M, Okazaki T, Takami Y, et al. Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat Cell Biol. 2004;6:784–791. doi: 10.1038/ncb1155. [DOI] [PubMed] [Google Scholar]
- Castel SE, Martienssen RA. RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nat Rev Genet. 2013;14:100–112. doi: 10.1038/nrg3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Shijuuku T, Fukamachi T, Zaunders J, Guillemin G, Cooper D, et al. Prolonged transcriptional silencing and CpG methylation induced by siRNAs targeted to the HIV-1 promoter region. J RNAi Gene Silencing. 2005;1:66–78. [PMC free article] [PubMed] [Google Scholar]
- Ting AH, Schuebel KE, Herman JG, Baylin SB. Short double-stranded RNA induces transcriptional gene silencing in human cancer cells in the absence of DNA methylation. Nat Genet. 2005;37:906–910. doi: 10.1038/ng1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanotto D, Tommasi S, Li M, Li H, Yanow S, Pfeifer GP, et al. Short hairpin RNA-directed cytosine (CpG) methylation of the RASSF1A gene promoter in HeLa cells. Mol Ther. 2005;12:179–183. doi: 10.1016/j.ymthe.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Huffman KE, Schwartz JC, Ram R, Hardy D, Shames DS, et al. Inhibiting gene expression at transcription start sites in chromosomal DNA with antigene RNAs. Nat Chem Biol. 2005;1:216–222. doi: 10.1038/nchembio725. [DOI] [PubMed] [Google Scholar]
- Zhang MX, Ou H, Shen YH, Wang J, Wang J, Coselli J, et al. Regulation of endothelial nitric oxide synthase by small RNA. Proc Natl Acad Sci USA. 2005;102:16967–16972. doi: 10.1073/pnas.0503853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV, Santoso S, Turner AM, Pastori C, Hawkins PG. Bidirectional transcription directs both transcriptional gene activation and suppression in human cells. PLoS Genet. 2008;4:e1000258. doi: 10.1371/journal.pgen.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Feng Y, Pan L, Wang Y, Xu X, Lu J, et al. The proximal GC-rich region of p16(INK4a) gene promoter plays a role in its transcriptional regulation. Mol Cell Biochem. 2007;301:259–266. doi: 10.1007/s11010-007-9427-4. [DOI] [PubMed] [Google Scholar]
- Han J, Kim D, Morris KV. Promoter-associated RNA is required for RNA-directed transcriptional gene silencing in human cells. Proc Natl Acad Sci USA. 2007;104:12422–12427. doi: 10.1073/pnas.0701635104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Villeneuve LM, Ehsani A, Amarzguioui M, Aagaard L, Chen ZX, et al. The antisense strand of small interfering RNAs directs histone methylation and transcriptional gene silencing in human cells. RNA. 2006;12:256–262. doi: 10.1261/rna.2235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AM, De La Cruz J, Morris KV. Mobilization-competent Lentiviral Vector-mediated Sustained Transcriptional Modulation of HIV-1 Expression. Mol Ther. 2009;17:360–368. doi: 10.1038/mt.2008.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Juelich T, Lim H, Ishida T, Watanebe T, Cooper DA, et al. Closed chromatin architecture is induced by an RNA duplex targeting the HIV-1 promoter region. J Biol Chem. 2008;283:23353–23363. doi: 10.1074/jbc.M709651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi M, Ishida T, Miyake A, Cooper DA, Kelleher AD, Suzuki K, et al. Retroviral delivery of promoter-targeted shRNA induces long-term silencing of HIV-1 transcription. Microbes Infect. 2009;11:500–508. doi: 10.1016/j.micinf.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Jiang GS, Zheng LD, Pu JR, Mei H, Zhao J, Huang K, et al. Small RNAs targeting transcription start site induce heparanase silencing through interference with transcription initiation in human cancer cells. PLoS ONE. 2012;7:e31379–. doi: 10.1371/journal.pone.0031379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TC, Andaloussi SE, Morris KV, McClorey G, Wood MJ. Small RNA-Mediated Epigenetic Myostatin Silencing. Mol Ther Nucleic Acids. 2012;1:e23. doi: 10.1038/mtna.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli S, Pastori C, Magistri M, Carbone GM, Catapano CV. Promoter-specific transcriptional interference and c-myc gene silencing by siRNAs in human cells. EMBO J. 2009;28:1708–1719. doi: 10.1038/emboj.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JC, Younger ST, Nguyen NB, Hardy DB, Monia BP, Corey DR, et al. Antisense transcripts are targets for activating small RNAs. Nat Struct Mol Biol. 2008;15:842–848. doi: 10.1038/nsmb.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Schwartz JC, Chu Y, Younger ST, Gagnon KT, Elbashir S, et al. Transcriptional regulation by small RNAs at sequences downstream from 3' gene termini. Nat Chem Biol. 2010;6:621–629. doi: 10.1038/nchembio.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger ST, Corey DR. Transcriptional gene silencing in mammalian cells by miRNA mimics that target gene promoters. Nucleic Acids Res. 2011;39:5682–5691. doi: 10.1093/nar/gkr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulukuri SM, Rao JS. Small interfering RNA directed reversal of urokinase plasminogen activator demethylation inhibits prostate tumor growth and metastasis. Cancer Res. 2007;67:6637–6646. doi: 10.1158/0008-5472.CAN-07-0751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim DH, Saetrom P, Snøve O, Jr, Rossi JJ. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci USA. 2008;105:16230–16235. doi: 10.1073/pnas.0808830105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HG, Suzuki K, Cooper DA, Kelleher AD. Promoter-targeted siRNAs induce gene silencing of simian immunodeficiency virus (SIV) infection in vitro. Mol Ther. 2008;16:565–570. doi: 10.1038/sj.mt.6300380. [DOI] [PubMed] [Google Scholar]
- Kim JW, Zhang YH, Zern MA, Rossi JJ, Wu J. Short hairpin RNA causes the methylation of transforming growth factor-beta receptor II promoter and silencing of the target gene in rat hepatic stellate cells. Biochem Biophys Res Commun. 2007;359:292–297. doi: 10.1016/j.bbrc.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins PG, Santoso S, Adams C, Anest V, Morris KV. Promoter targeted small RNAs induce long-term transcriptional gene silencing in human cells. Nucleic Acids Res. 2009;37:2984–2995. doi: 10.1093/nar/gkp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen MP, Lehtola T, Heinonen SE, Assefa GS, Korpisalo P, Girnary R, et al. Efficient regulation of VEGF expression by promoter-targeted lentiviral shRNAs based on epigenetic mechanism: a novel example of epigenetherapy. Circ Res. 2009;105:604–609. doi: 10.1161/CIRCRESAHA.109.200774. [DOI] [PubMed] [Google Scholar]
- Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, et al. Small dsRNAs induce transcriptional activation in human cells. Proc Natl Acad Sci USA. 2006;103:17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Barichievy S, Schaffer L, Han J, Morris KV. An RNA targeted to the HIV-1 LTR promoter modulates indiscriminate off-target gene activation. Nucleic Acids Res. 2007;35:7303–7312. doi: 10.1093/nar/gkm847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat Chem Biol. 2007;3:166–173. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci USA. 2008;105:1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junxia W, Ping G, Yuan H, Lijun Z, Jihong R, Fang L, et al. Double strand RNA-guided endogeneous E-cadherin up-regulation induces the apoptosis and inhibits proliferation of breast carcinoma cells in vitro and in vivo. Cancer Sci. 2010;101:1790–1796. doi: 10.1111/j.1349-7006.2010.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Zhao J, Long M, Han Y, Wang X, Lin F, et al. p21WAF1/CIP1 gene transcriptional activation exerts cell growth inhibition and enhances chemosensitivity to cisplatin in lung carcinoma cell. BMC Cancer. 2010;10:632. doi: 10.1186/1471-2407-10-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Place RF, Jia ZJ, Pookot D, Dahiya R, Li LC. Antitumor effect of dsRNA-induced p21(WAF1/CIP1) gene activation in human bladder cancer cells. Mol Cancer Ther. 2008;7:698–703. doi: 10.1158/1535-7163.MCT-07-2312. [DOI] [PubMed] [Google Scholar]
- Whitson JM, Noonan EJ, Pookot D, Place RF, Dahiya R. Double stranded-RNA-mediated activation of P21 gene induced apoptosis and cell cycle arrest in renal cell carcinoma. Int J Cancer. 2009;125:446–452. doi: 10.1002/ijc.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Zheng XY, Qin J, Wang YB, Bai Y, Mao QQ, et al. Up-regulation of p21WAF1/Cip1 by saRNA induces G1-phase arrest and apoptosis in T24 human bladder cancer cells. Cancer Lett. 2008;265:206–214. doi: 10.1016/j.canlet.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Mao Q, Li Y, Zheng X, Yang K, Shen H, Qin J, et al. Up-regulation of E-cadherin by small activating RNA inhibits cell invasion and migration in 5637 human bladder cancer cells. Biochem Biophys Res Commun. 2008;375:566–570. doi: 10.1016/j.bbrc.2008.08.059. [DOI] [PubMed] [Google Scholar]
- Mao Q, Zheng X, Yang K, Qin J, Bai Y, Jia X, et al. Suppression of migration and invasion of PC3 prostate cancer cell line via activating E-cadherin expression by small activating RNA. Cancer Invest. 2010;28:1013–1018. doi: 10.3109/07357900802620844. [DOI] [PubMed] [Google Scholar]
- Wang J, Place RF, Huang V, Wang X, Noonan EJ, Magyar CE, et al. Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Res. 2010;70:10182–10191. doi: 10.1158/0008-5472.CAN-10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Sakurai F, Elbashir S, Foster DJ, Manoharan M, Corey DR. Activation of LDL receptor expression by small RNAs complementary to a noncoding transcript that overlaps the LDLR promoter. Chem Biol. 2010;17:1344–1355. doi: 10.1016/j.chembiol.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang V, Qin Y, Wang J, Wang X, Place RF, Lin G, et al. RNAa is conserved in mammalian cells. PLoS ONE. 2010;5:e8848. doi: 10.1371/journal.pone.0008848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang J, Huang V, Place RF, Li LC. Induction of NANOG expression by targeting promoter sequence with small activating RNA antagonizes retinoic acid-induced differentiation. Biochem J. 2012;443:821–828. doi: 10.1042/BJ20111491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Shen J, Xie YQ, Lin YW, Qin J, Mao QQ, et al. Promoter-targeted double-stranded small RNAs activate PAWR gene expression in human cancer cells. Int J Biochem Cell Biol. 2013;45:1338–1346. doi: 10.1016/j.biocel.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Matsui M, Chu Y, Zhang H, Gagnon KT, Shaikh S, Kuchimanchi S, et al. Promoter RNA links transcriptional regulation of inflammatory pathway genes. Nucleic Acids Res. 2013;41:10086–10109. doi: 10.1093/nar/gkt777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses J, Goodchild A, Rivory LP. Intended transcriptional silencing with siRNA results in gene repression through sequence-specific off-targeting. RNA. 2010;16:430–441. doi: 10.1261/rna.1808510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JP, Rand KN, Molloy PL. Hypomethylation of repeated DNA sequences in cancer. Epigenomics. 2010;2:245–269. doi: 10.2217/epi.10.2. [DOI] [PubMed] [Google Scholar]
- Karmodiya K, Krebs AR, Oulad-Abdelghani M, Kimura H, Tora L. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics. 2012;13:424. doi: 10.1186/1471-2164-13-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HW, Wentzel EA, Mendell JT. A hexanucleotide element directs microRNA nuclear import. Science. 2007;315:97–100. doi: 10.1126/science.1136235. [DOI] [PubMed] [Google Scholar]
- Wei Y, Li L, Wang D, Zhang CY, Zen K. Importin 8 regulates the transport of mature microRNAs into the cell nucleus. J Biol Chem. 2014;289:10270–10275. doi: 10.1074/jbc.C113.541417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosue I, Quaranta R, Masciarelli S, Fontemaggi G, Batassa EM, Bertolami C, et al. Argonaute 2 sustains the gene expression program driving human monocytic differentiation of acute myeloid leukemia cells. Cell Death Dis. 2013;4:e926. doi: 10.1038/cddis.2013.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamed M, Herbig U, Ye T, Dejean A, Bischof O. Senescence is an endogenous trigger for microRNA-directed transcriptional gene silencing in human cells. Nat Cell Biol. 2012;14:266–275. doi: 10.1038/ncb2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali S, Bhartiya D, Lalwani MK, Sivasubbu S, Scaria V. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS ONE. 2013;8:e53823. doi: 10.1371/journal.pone.0053823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang M, Gao W, Xu J, Wang P, Shu Y. The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun. 2014;448:315–322. doi: 10.1016/j.bbrc.2013.12.126. [DOI] [PubMed] [Google Scholar]
- Leucci E, Patella F, Waage J, Holmstrøm K, Lindow M, Porse B, et al. microRNA-9 targets the long non-coding RNA MALAT1 for degradation in the nucleus. Sci Rep. 2013;3:2535. doi: 10.1038/srep02535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 2013;25:69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, et al. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res. 2014;114:1377–1388. doi: 10.1161/CIRCRESAHA.114.302476. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang F, Yuan JH, Yuan SX, Zhou WP, Huo XS, et al. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis. 2013;34:577–586. doi: 10.1093/carcin/bgs381. [DOI] [PubMed] [Google Scholar]
- Li CH, To KF, Tong JH, Xiao Z, Xia T, Lai PB, et al. Enhancer of zeste homolog 2 silences microRNA-218 in human pancreatic ductal adenocarcinoma cells by inducing formation of heterochromatin. Gastroenterology. 2013;144:1086–1097.e9. doi: 10.1053/j.gastro.2013.01.058. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu X, Wu H, Ni P, Gu Z, Qiao Y, et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38:5366–5383. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelin R, Dahary D, Sorek R, Levanon EY, Goldstein O, Shoshan A, et al. Widespread occurrence of antisense transcription in the human genome. Nat Biotechnol. 2003;21:379–386. doi: 10.1038/nbt808. [DOI] [PubMed] [Google Scholar]
- Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425:3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyomaru T, Fukuhara S, Saini S, Majid S, Deng G, Shahryari V, et al. Long non-coding RNA HOTAIR is targeted and regulated by miR-141 in human cancer cells. J Biol Chem. 2014;289:12550–12565. doi: 10.1074/jbc.M113.488593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Ghosal S, Sen R, Chakrabarti J. lnCeDB: database of human long noncoding RNA acting as competing endogenous RNA. PLoS ONE. 2014;9:e98965. doi: 10.1371/journal.pone.0098965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Mele A, Fak JJ, Ule J, Kayikci M, Chi SW, et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456:464–469. doi: 10.1038/nature07488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, et al. PAR-CliP–a method to identify transcriptome-wide the binding sites of RNA binding proteins. J Vis Exp. 2010; 41:2034. doi: 10.3791/2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Ba Z, Gao M, Wu Y, Ma Y, Amiard S, et al. A role for small RNAs in DNA double-strand break repair. Cell. 2012;149:101–112. doi: 10.1016/j.cell.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Shevelyov YY, Nurminsky DI. The nuclear lamina as a gene-silencing hub. Curr Issues Mol Biol. 2012;14:27–38. [PubMed] [Google Scholar]
- Tomikawa J, Shimokawa H, Uesaka M, Yamamoto N, Mori Y, Tsukamura H, et al. Single-stranded noncoding RNAs mediate local epigenetic alterations at gene promoters in rat cell lines. J Biol Chem. 2011;286:34788–34799. doi: 10.1074/jbc.M111.275750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka M, Nishimura O, Go Y, Nakashima K, Agata K, Imamura T. Bidirectional promoters are the major source of gene activation-associated non-coding RNAs in mammals. BMC Genomics. 2014;15:35. doi: 10.1186/1471-2164-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C, Bühler M. Chromatin-associated ncRNA activities. Chromosome Res. 2013;21:627–641. doi: 10.1007/s10577-013-9390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place RF, Wang J, Noonan EJ, Meyers R, Manoharan M, Charisse K, et al. Formulation of Small Activating RNA Into Lipidoid Nanoparticles Inhibits Xenograft Prostate Tumor Growth by Inducing p21 Expression. Mol Ther Nucleic Acids. 2012;1:e15. doi: 10.1038/mtna.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MR, Yang G, Place RF, Charisse K, Epstein-Barash H, Manoharan M, et al. Intravesical delivery of small activating RNA formulated into lipid nanoparticles inhibits orthotopic bladder tumor growth. Cancer Res. 2012;72:5069–5079. doi: 10.1158/0008-5472.CAN-12-1871. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Hattori S, Marks K, Ahlenstiel C, Maeda Y, Ishida T, et al. Promoter Targeting shRNA Suppresses HIV-1 Infection In vivo Through Transcriptional Gene Silencing. Mol Ther Nucleic Acids. 2013;2:e137. doi: 10.1038/mtna.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufarelli C, Stanley JA, Garrick D, Sharpe JA, Ayyub H, Wood WG, et al. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat Genet. 2003;34:157–165. doi: 10.1038/ng1157. [DOI] [PubMed] [Google Scholar]