Abstract
Calcium (Ca2+) is an important multifaceted second messenger that regulates a wide range of cellular events. A Ca2+-signaling toolkit has been shown to exist in the nucleus and to be capable of generating and modulating nucleoplasmic Ca2+ transients. Within the nucleus, Ca2+ controls cellular events that are different from those modulated by cytosolic Ca2+. This review focuses on nuclear Ca2+ signals and their role in regulating physiological and pathological processes.
The role of calcium (Ca2+) signaling in regulating a variety of cellular processes has been widely described, showing the multifunctional nature of this ion. Even though the mechanisms that allow Ca2+ to modulate a wide range of events have not been completely elucidated, it is appreciated that the amplitude, the spatiotemporal profile (e.g., single transient, wave, oscillation), as well as the intracellular compartments where Ca2+ signals occur are all involved in orchestrating Ca2+ increases that correspond to specific cellular functions mediated by Ca2+. In this review, we will briefly discuss the machinery that regulates intracellular Ca2+ signaling and then highlight mechanisms of Ca2+ signaling in the nucleus as well as cellular events that are modulated by nucleoplasmic Ca2+.
Intracellular Ca2+-Signaling Toolkit
Although the variety of biological processes regulated by Ca2+ can be partially explained by the spatiotemporal organization of Ca2+ increases within the cell, a crucial question in this signaling cascade is how cells can generate, regulate, and coordinate such Ca2+-evoked responses. The remarkable plasticity of Ca2+ signaling emerges not only from the origin of the intracellular Ca2+ signals (i.e., endoplasmic reticulum, mitochondria, or nucleus) but also from the molecular toolkit expressed by different cell types, as well as from the interplay between the cellular compartments. Below, we will briefly review some of the components of the Ca2+ toolkit that have been described to date. Although we will not discuss the components at length and we will not present a complete list of the Ca2+ signaling molecules, the discussion will provide a sense of the variety of components of the Ca2+ signaling network that can be used by different cell types to generate a specific cellular event.
Intracellular Ca2+ signals can be initiated by the influx of Ca2+ from the extracellular environment or by the mobilization of Ca2+ from intracellular stores. The latter depends mainly on the binding of hormones or growth factors to specific receptor recruiting and generating a variety of second messengers that, in turn, will control Ca2+ mobilization from different intracellular stores (reviewed in Refs. 10, 87). One of the major signaling cascades triggered by the activation of transmembrane receptors involves phospholipase C (PLC), which generates inositol-1,4,5-trisphosphate (InsP3). InsP3 diffuses throughout the cell to bind to its specific receptor (InsP3R), which is a ligand-gated Ca2+ release channel (reviewed in Refs. 19, 30). Three isoforms of InsP3R have been described, and although these receptors are mainly located in the endoplasmic reticulum, they can also be detected at the plasma membrane (25), portions of the Golgi complex (83), acidic stores (35), and within the nucleus (29, 45, 59). Moreover, the expression pattern of InsP3R isoforms varies among different cell types (108). Although InsP3 is required to activate InsP3R, the opening of the channel can be modulated by Ca2+ itself, ATP, and proteins such as Chromogranin-B (reviewed in Refs. 19, 24, 30). It is also known that the affinity of InsP3R for InsP3 is highest for InsP3R-II, intermediate in InsP3R-I, and lowest for InsP3R-III (52, 74).
The intracellular increase in Ca2+ concentrations itself may activate a second class of receptors: the ryanodine receptors (RyR). Similar to InsP3R, RyR forms channels that are permeable to Ca2+. So far, three isoforms of RyR have been identified (reviewed in Refs. 57, 112). RyR-I is abundantly expressed in skeletal muscle but also is found in cardiac muscle, cerebellum, and other tissues (33, 40, 75). RyR-II is the major isoform found in cardiac muscle (71, 79) but is also expressed in other tissues (33, 55, 72, 96), whereas RyR-III is expressed within hippocampal neuron cerebellum as well as in smooth muscle cells of many organs (33, 40, 46). Ca2+ is the major trigger of RyR opening, but some RyR channel subtypes are also activated by cyclic ADP-ribose (cADPR) (70, 100). Moreover, FK506 binding protein, magnesium, calmodulin, and a number of small molecules such as ATP are able to modulate the activity of RyRs (Ref. 68; reviewed in Refs. 11, 112).
In addition to the well known mechanisms above, a growing body of evidence shows that members of a third and a fourth class of intracellular Ca2+ channels can be involved in controlling Ca2+ release from organelles within the cell. One family of these receptors, named two pore channels (TPCs), acts as Ca2+ channels and is responsible for the mobilization of Ca2+ from acidic organelles in response to stimulation by nicotinic acid adenine dinucleotide phosphate (NAADP) (15, 34). There are three isoforms of TPCs in mammals, with TPC1 and TPC3 expressed in endosomes and TPC2 present primarily in lysosomes (15, 34, 92). It has been shown that TPC-induced Ca2+ release may trigger further Ca2+ release through the activation of juxtaposed InsP3R or RyR, suggesting a broader role of TPCs in the regulation of cellular activities (Refs. 16, 34; reviewed in Ref. 102).
Another channel shown to be involved in intracellular Ca2+ signaling is the TRP2 that belongs to the transient receptor potential (TRP) superfamily of ion channels (Refs. 53, 54; reviewed in Refs. 5, 104). Besides its location at the plasma membrane and the primary cilium, TRP2 is also found in high concentrations in the endoplasmic reticulum membrane functioning as an intracellular Ca2+-release channel that augments InsP3-induced intracellular Ca2+ release (54).
Within a cell, Ca2+ signaling may be seen as a shifting balance that controls cell physiology. When this signaling pathway is activated but tightly controlled, Ca2+ regulates a broad spectrum of biological processes, including secretion, motility, gene transcription, proliferation, differentiation, contraction, cell death, and others (10). Therefore, it is important that the cells express a toolkit not only to allow Ca2+ release from intracellular stores but also to reestablish the intracellular free Ca2+ to baseline levels. To accomplish this, cytosolic Ca2+ can be 1) extruded into the extracellular environment by the action of the plasma membrane Ca2+-ATPase (PMCA) or the sodium Ca2+ exchanger (NCX), or 2) pumped back into the lumen of the Ca2+ stores, such as endoplasmic reticulum and mitochondria, by the action of proteins like the sarco-/endoplasmic Ca2+-ATPase (SERCA) and mitochondrial Ca2+ uptake 1 (MCU1) (Ref. 82; reviewed in Refs. 12, 84).
Although understanding the components of the intracellular Ca2+ toolkit constitutes a powerful strategy to familiarize the reader with the diversity and peculiarities of the molecular repertoire involved in intracellular Ca2+ signaling, the reader should be reminded that these components act in a coordinated fashion to regulate specific cellular functions triggered by increases in Ca2+. Also, despite the broad expression of the components of the Ca2+ toolkit, Ca2+ signaling may occur in distinct microdomains of the cell (9). Evidence showing components of the Ca2+ toolkit in the nucleus identifies this cellular compartment as a pivotal microdomain for independent and localized Ca2+ signaling. In the following sections, we will describe the mechanisms underlying nuclear Ca2+ signaling as well as its relevance for physiological and pathological processes.
Nucleoplasmic Ca2+ Signaling
There are several reports in the literature suggesting that nuclear Ca2+ signals can result from passive diffusion of Ca2+ from the cytosol to the nucleoplasm (14, 48, 61, 98). This view evolved from observations made by numerous groups showing not only the existence of nuclear-cytosolic Ca2+ gradients (94, 107) but also the presence of several components of the Ca2+ toolkit within the nucleus capable of autonomously controlling nuclear Ca2+ homeostasis (reviewed in Refs. 37, 87, 91).
For instance, InsP3-sensitive pools (77), as well as a high-affinity, InsP3 binding site, were demonstrated to exist in isolated liver nuclei, with InsP3 being able to release 45Ca2+ directly from isolated nuclei (63). Also using isolated rat liver nuclei, it was shown that ATP increases the free Ca2+ concentration in the nucleoplasm (76). However, it was not initially clear which intranuclear structures contained Ca2+ nor into which intranuclear compartments Ca2+ was released by InsP3. Indeed, the first report demonstrating that InsP3 is able to cause the release of Ca2+ from the nuclear envelope into the nucleoplasm came a few years later. It was then shown that the nuclear envelope actively takes up Ca2+ via a Ca2+-activated ATPase and that InsP3 releases Ca2+ from this envelope store into the nucleoplasm (39). A subsequent study, in which InsP3 was injected in the nucleus of Xenopus laevis oocytes, also demonstrated that InsP3 causes an increase in nuclear Ca2+, even when cytosolic InsP3Rs were blocked (48). Moreover, photorelease of caged InsP3 in the nucleus of starfish oocytes was shown to trigger nucleoplasmic Ca2+ increase (94). Like InsP3, cADPR also could raise Ca2+ in isolated hepatocyte nuclei (1, 39) and induce Ca2+ oscillations in the nucleus of starfish oocytes (94). These findings illustrate some of the initial evidence that supported the hypothesis that the nucleus could autonomously trigger nucleoplasmic Ca2+ transients. This hypothesis was strengthened by additional data showing that the inner and/or the outer membranes of the nuclear envelope express several components of the Ca2+ toolkit. In particular, it was shown that the nuclear envelope accumulates Ca2+ via a Ca2+-ATPase pump (SERCA) (56, 76) located in its outer leaflets (38, 49) and a Na+/Ca2+-exchanger (109, 110) located in its inner membrane (109), and releases Ca2+ via channels that are sensitive to InsP3 (39, 76), cADPR (1, 39), and NAADP (36).
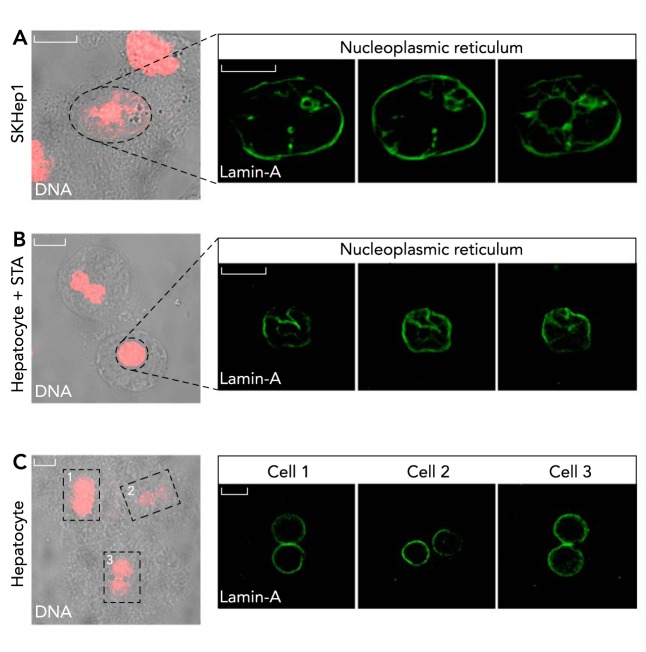
There are reports that the InsP3R (17, 49, 101) and the RyR (93) can be present on both leaflets of the nuclear envelope, whereas ADP-ribosyl (CD38), an enzyme required for generation of cADPR, is found to be only associated with the inner membrane of the nuclear envelope (1). Although it is widely accepted that the nucleus also holds the apparatus required to produce nuclear InsP3, including PIP2 as well as PLC (Ref. 65; reviewed in Ref. 27), and that PLC can be found associated with the nuclear membrane (28, 64), it is still not completely clear whether PIP2 is localized in the nucleus. It has been proposed that nuclear PIP2 could be confined within a lipid bilayer forming intranuclear membrane invaginations (51). Alternatively, there are reports that PIP2 is located in the nucleoplasm rather than at the nuclear envelope (13, 78, 106). Nevertheless, there are several reports showing that nuclear PIP2 hydrolysis involves the translocation of activated tyrosine kinase receptors from the plasma membrane to the nucleus (2, 20, 44, 89). For instance, IGF-1 (66), integrins (20), hepatocyte growth factor (44), and insulin (89) represent some agonists known to preferentially cause nuclear PIP2 breakdown, producing InsP3 and consequently Ca2+ signals in the nucleus. Moreover, it has been demonstrated that, upon growth factor stimulation, activation of nuclear PLC can occur through an additional pathway that involves relocation of MAP kinase to the nucleus (93). Even though the identification of several components of the Ca2+ toolkit within the nucleus represented solid evidence that could explain how Ca2+ is released from the nuclear envelope directly into the nucleoplasm, recognition and characterization of a structure named the nucleoplasmic reticulum (NR) (29) increased appreciation for the role of nuclear Ca2+ signaling, as it provided direct evidence of a compartment that stores Ca2+ deep in the nucleus and releases it within the nucleoplasm in a process that could occur completely independent of the cytosolic Ca2+ increases. The NR was shown to be continuous with the endoplasmic reticulum/nuclear envelope (29), as observed by lamin-A staining (FIGURE 1). Additional morphological characterization showed that the NR can be divided into two types (reviewed in Ref. 62). The type I NR is characterized by invagination of the inner nuclear membrane, whereas NR type II is formed by invagination of both the outer and inner nuclear membrane; and, although structurally different, both NR subtypes can coexist within a single nucleus (31, 32, 62). Indeed, similar intranuclear extensions of the endoplasmic reticulum had been described previously and were thought to be widespread among mammalian cell types (32) and to be dynamic structures that become altered during cell proliferation or disease states (105). The NR has also been identified in plant cells, and it has been suggested that it regulates nuclear Ca2+ signaling in these cell types as well (22, 81). By showing that the NR gives rise to localized Ca2+ gradients in the nucleoplasm, a potential mechanism was revealed by which Ca2+-dependent events can be regulated differentially in the nucleus, just as they are in the cytosol.
Figure 1.
The nucleus contains the nucleoplasmic reticulum structure
The nuclear envelope was labeled with an antibody against lamin-A (green) in SkHep1 (A), an adenocarcinoma liver cell line, and freshly isolated primary hepatocytes treated (B) or not treated (C) with staurosporine (STA) for 4 h, respectively. DNA was stained with propidium iodide. Note the presence of the nucleoplasmic reticulum (NR) in a cell line as well as in a primary culture. In the two top panels, the images represent three focal planes, depicting the three dimensionality of the NR. In the cell lineage used here, the NR seems to be constitutively present (A). In the primary culture of hepatocytes, the NR, not present under control condition, can be induced by drugs, such as STA (compare B with C), corroborating the findings that the NR is a dynamic and inducible structure. Bar in A = 5 μm; bars in B and C = 10 μm.
The NR was shown to express functional InsP3R (29) and RyR (67), giving rise to local Ca2+ signals in the nuclear interior, triggered by either InsP3 (29) or Ca2+, respectively (67). To date, it is not known whether the TPCs or TRPC2 are also expressed along the NR. It is also not known whether and how the various intracellular Ca2+ channels in the NR interact to coordinate nuclear Ca2+ signals and cellular functions. Although more studies are needed to clarify these points, there are suggestions that the NR expresses InsP3-kinase isoform B (IP3KB) (73), which can inactivate InsP3 (26, 111) and SERCA (21), indicating that nuclear Ca2+ signals can be locally initiated and terminated within the nucleus.
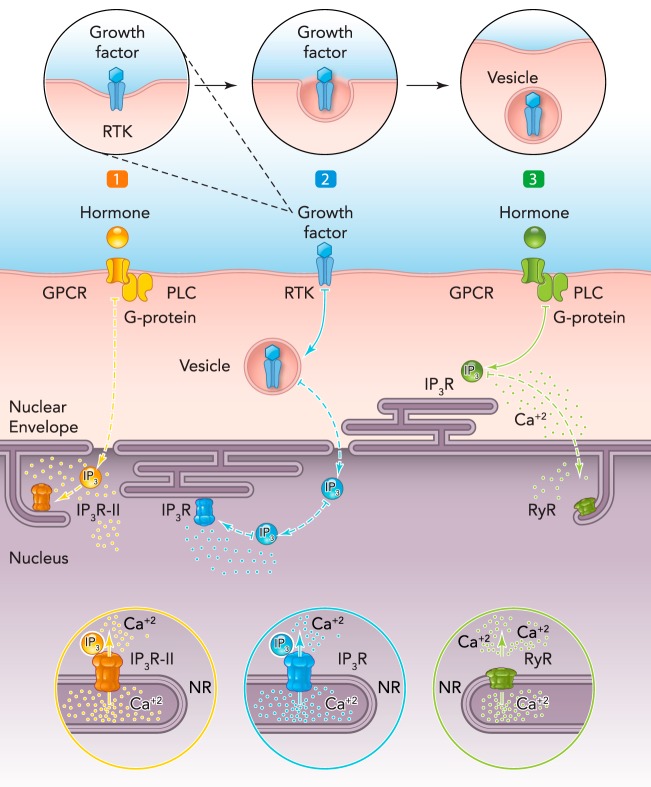
Although the data described above demonstrate the potential independent behavior of the nucleus relative to the cytosol, they cannot rule out that, in response to some stimuli, the nuclear Ca2+ signaling machinery needs to be activated by intermediate effectors located in the cytosol. For instance, it was shown in a liver cell line that extracellular ATP preferentially activates nuclear Ca2+ release via InsP3 that diffuses from the cytosol to the nucleus to activate nuclear InsP3R (59). Indeed, at present, different mechanisms for nuclear Ca2+ increases have been considered. One mechanism relies on the expression pattern of InsP3R isoforms in the cell (FIGURE 2A). It involves cytosolic to nucleoplasmic InsP3 diffusion to preferentially bind to the type II InsP3R concentrated in the nucleus. The main question regarding this model is how InsP3, as it diffuses across the cytosol, does not trigger Ca2+ release from the InsP3R located in the endoplasmic reticulum. The answer may rely on the differing affinity of the various InsP3R isoforms to InsP3 as described above: InsP3R-II > InsP3R-I > InsP3R-III (52, 74). Therefore, if type II InsP3R expression is concentrated in the nucleus, Ca2+ signals are more likely to start there (59). A second mechanism consists of nuclear InsP3 formation (FIGURE 2B). In support of this, it has been demonstrated that growth factors and their respective receptors translocate from the plasma membrane to the nucleus to stimulates the hydrolysis of nuclear PIP2 with the formation of InsP3 within the nucleus and consequently nuclear Ca2+ signals (2, 23, 44, 86, 89). Another possible mechanism is the stimulation of nuclear RyR (FIGURE 2C). This might occur when Ca2+ released from nuclear stores stimulates further Ca2+ release from RyR present along the NR. Although more experimental evidence is needed to support the importance of this pathway, the presence of RyR in the NR and its functionality was already described in C2C12 cells and in cardiomyocytes (45, 67). Finally, but no less important, is the model that predicts the juxtaposition of the components of the Ca2+ toolkit with the nuclear envelope. Recently, it was shown using cardiomyocytes that the invaginations of the plasma membrane, known as T-tubules, concentrate the machinery to generate InsP3 in direct apposition to the components of the nuclear Ca2+ toolkit. Thus, after stimulation from an extracellular ligand, InsP3 is generated in the vicinity of the nucleus diffusing into it and triggering independent nuclear Ca2+ signals (50).
Figure 2.
Working hypotheses to trigger nuclear Ca2+
Different pathways to generate nuclear Ca2+ signals have been proposed. In 1 (left), the binding of an agonist to its transmembrane G-protein-coupled receptor (GPCR) produces InsP3 (IP3) in the cytosol. IP3 diffuses to the nucleus and binds to the high-affinity, type II InsP3R (IP3R-II), located along the nucleoplasmic reticulum (NR), to release Ca2+ in the nucleoplasm. In 2 (middle), the binding of a growth factor to a receptor tyrosine kinase (TKR) may result in the translocation of the receptor to the nucleus to generate nuclear IP3. IP3 binds to the IP3R to release Ca2+ in the nucleoplasm. The topology of internalized TRK is not known, and the cartoon shows TKR as being inside the vesicle merely for simplicity. In 3 (right), another putative pathway is initiated following the binding of a hormone to GPCR at the plasma membrane. The IP3 generated within the cytosol can release Ca2+ from the endo-/sarcoplasmic reticulum, and Ca2+ may reach the nucleus to trigger Ca2+-induced Ca2+ release by activating the ryanodine receptor (RyR) in the NR.
These studies have clearly shown that the nucleus can be completely autonomous in terms of Ca2+ regulation. The presence of the Ca2+ signaling machinery in the nuclear interior has several implications, in physiological and pathological states, some of which will be described below.
Cellular Functions Mediated by Nuclear Ca2+
Ca2+ signaling regulates a variety of cellular functions, and, more recently, it has been shown that Ca2+ increases in the nucleoplasm regulate events that are distinct from the ones mediated by cytosolic Ca2+ (2, 6, 29, 43, 87, 89, 91). For instance, nuclear Ca2+ promotes translocation of protein kinase C (PKC) to the nuclear envelope, whereas cytosolic Ca2+ causes PKC translocation to the plasma membrane (29). Also, nuclear but not cytosolic Ca2+ generated by insulin receptor activation regulates hepatocyte proliferation after partial hepatectomy (2). This finding was in agreement with previous data that showed that buffering nuclear Ca2+ regulates the cell cycle by arresting cells in an early phase of mitosis (90). In fact, it was found that nuclear Ca2+ signals regulate the rate of cell proliferation both in vitro and in xenographic tumors (3, 89). One possible mechanism by which nuclear Ca2+ may control growth is by activating the promoter region of genes involved in cell proliferation (4). In addition to its direct effect on tumor growth, impaired nuclear Ca2+ signaling can sensitize adenocarcinoma cells to radiotherapy, in part by downregulating metalloproteinase and growth factor receptor expression and activation induced by X-ray irradiation (3).
Nuclear Ca2+ is also involved in cardiomyocyte hypertrophy. For example, buffering Ca2+ in the nucleus causes nuclear enlargement in neonatal cardiomyocytes, an early sign of cardiac hypertrophy (45). Nuclear Ca2+ activates the calcineurin/NFAT signaling cascade, culminating in increased ANP expression (45), a well known cardiomyocyte hypertrophic marker (88). However, cardiac hypertrophy is an adaptive response that can be induced in pathological and physiological conditions. Indeed, nuclear Ca2+ is indispensable for induction of hypertrophy in both health and disease. Regardless of the precise cellular compartment in which InsP3 is produced, it must reach the nucleus interior to trigger nuclear Ca2+-mediating hypertrophy (6).
Also dependent on the spatial properties of Ca2+ signals are transcriptional responses, since they are strongly influenced by the intracellular localization of Ca2+ transients. For example, neuronal gene expression is differentially controlled by nuclear and cytoplasmic Ca2+ signals: signaling pathways activated by cytoplasmic Ca2+ target the serum-response element (SRE), whereas increases in nuclear Ca2+ are critical for cyclic-AMP-response element (CRE)-dependent calcium-activated transcription (8, 47). Also, the transcriptional factor cyclic-AMP response element-binding protein (CREB) can function as a nuclear Ca2+-responsive transcription factor (97) that is involved in mediating Ca2+-dependent transcription of a number of genes, including c-fos (41). Nuclear Ca2+ buffering inhibits CREB-mediated gene transcription (47). In addition, nuclear Ca2+ controls, through nuclear calcium-/calmodulin (CaM)-dependent protein kinases, especially CaM Kinase IV, the activity of the coactivator CREB-binding protein (CBP) (18). CBP interacts with CREB and with many transcription factors (42), a mechanism whereby nuclear Ca2+ can modulate the expression of different genes. Nuclear Ca2+ is similarly required for epidermal growth factor (EGF)-mediated transcriptional activation of Elk-1 (85). An additional target directly regulated by nuclear Ca2+ signaling is the downstream regulatory element antagonist modulator (DREAM) (69). All DREAM family members bind specifically to DNA and regulate transcription negatively. An increase in the concentration of nuclear Ca2+ causes DREAM to dissociate from its DNA binding sites and thereby allows the transcription of its target genes (58). On the other hand, nuclear Ca2+ also can negatively regulate the activity of transcription factors. For example, chelation of nuclear Ca2+ raises the activity of the transcription enhancer factor (TEAD) (103).
It is widely known that Ca2+ transients are crucial for synaptic activity and neuro-adaptations (reviewed in Ref. 7) including survival (114), memory consolidation (60), and pain (99). For example, histone deacetylases (HDACs) have a pivotal role linking external stimuli with gene expression. Inhibition of nuclear Ca2+ signaling interferes with the subcellular distribution of members of class IIa HDACs in hippocampal neurons, representing a novel transcriptional pathway regulated by nuclear Ca2+ (95). Also, synaptic activity is required for neuronal survival. Part of this neuroprotection is afforded by the resulting increase in Ca2+ in the cell nucleus due to activation of CREB (80) and inhibition of DREAM family members (58). Together, these changes increase the transcriptional activity of neurons, as already discussed in this review, and result in the triggering of a neuroprotective program referred to as Activity Inhibitor of Death (AID) (114). The core of this program consists of the robust induction of nuclear Ca2+-related genes involved in survival and differentiation processes, including ATF3, GADD45β, GADD45γ, IFI202b, NPAS4, NR4A1, SERPINb2, Inhibin β (114), and BTG2 (113). Another important role of nuclear Ca2+ signaling in the nervous system is its relationship to pain. Inducing Ca2+ signals in the nucleoplasm increases the expression of genes that modulate neuronal excitation and morphology. In this case, neurons became more susceptible to painful stimuli, contributing to the amplification of nociceptive sensitivity (99).
Together, these data show that a large amount of information regarding nuclear Ca2+ signaling and cell function has been acquired in recent years. These findings not only contribute to our understanding of basic aspects of several physiological processes but also permit nuclear Ca2+ to be considered as a potential therapeutic target to treat diseases.
Future Perspectives for Nuclear Ca2+ Signaling
The characterization of the NR as a nuclear Ca2+ compartment, together with evidence demonstrating the presence of components of the components of the Ca2+ toolkit in the nuclear interior, add one more level of complexity to the already multifaceted field of Ca2+ signaling. A remaining intriguing question would be to understand whether and how the different intracellular Ca2+ channels present in the nucleus interact to decode Ca2+ signals to produce a specific cellular response. Moreover, the existence of micro-domains within the nucleus might also be important to tightly regulate nuclear Ca2+ signals and should be considered and investigated.
Acknowledgments
We thank Dr. Michael H. Nathanson (Yale University) for carefully reading the manuscript.
Footnotes
The authors acknowledge the supporting agencies CAPES, FAPEMIG, FAPEMIG-Pronex, CNPq, and HHMI. We also acknowledge the technical assistance of Gilson Nogueira.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: A.G.O. prepared figures; A.G.O., E.S.G., L.M.A., G.B.M., and M.F.L. drafted manuscript; A.G.O., G.B.M., and M.F.L. edited and revised manuscript; A.G.O., E.S.G., L.M.A., G.B.M., and M.F.L. approved final version of manuscript.
References
- 1.Adebanjo OA, Anandatheerthavarada HK, Koval AP, Moonga BS, Biswas G, Sun L, Sodam BR, Bevis PJ, Huang CL, Epstein S, Lai FA, Avadhani NG, Zaidi M. A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nat Cell Biol 1: 409–414, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Amaya MJ, Oliveira AG, Guimaraes ES, Casteluber MC, Carvalho SM, Andrade LM, Pinto MC, Mennone A, Oliveira CA, Resende RR, Menezes GB, Nathanson MH, Leite MF. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology 59: 274–283, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade LM, Geraldo JM, Gonçalves OX, Leite MTT, Catarina AM, Guimarães MM, Paes Leme AF, Yokoo S, Machado CR, Rajão MA, Carvalho SM, Gomes DA, Aguiar CJ, Souza-Fagundes EM, Zani CL, Resende RR, Martins-Filho OA, Leite MF. Nucleoplasmic calcium buffering sensitizes human squamous cell carcinoma to anticancer therapy. J Cancer Sci Ther 4: 131–139, 2012 [Google Scholar]
- 4.Andrade V, Guerra M, Jardim C, Melo F, Silva W, Ortega JM, Robert M, Nathanson MH, Leite F. Nucleoplasmic calcium regulates cell proliferation through legumain. J Hepatol 55: 626–635, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anyatonwu GI, Ehrlich BE. Calcium signaling and polycystin-2. Biochem Biophys Res Commun 322: 1364–1373, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Arantes LA, Aguiar CJ, Amaya MJ, Figueiro NC, Andrade LM, Rocha-Resende C, Resende RR, Franchini KG, Guatimosim S, Leite MF. Nuclear inositol 1,4,5-trisphosphate is a necessary and conserved signal for the induction of both pathological and physiological cardiomyocyte hypertrophy. J Mol Cell Cardiol 53: 475–486, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Bading H. Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci 14: 593–608, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Bading H, Hardingham GE, Johnson CM, Chawla S. Gene regulation by nuclear and cytoplasmic calcium signals. Biochem Biophys Res Commun 236: 541–543, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium 40: 405–412, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol 37: 417–429, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Boronenkov IV, Loijens JC, Umeda M, Anderson RA. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol Biol Cell 9: 3547–3560, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brini M, Murgia M, Pasti L, Picard D, Pozzan T, Rizzuto R. Nuclear Ca2+ concentration measured with specifically targeted recombinant aequorin. EMBO J 12: 4813–4819, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459: 596–600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature 398: 74–76, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Cardenas C, Escobar M, Garcia A, Osorio-Reich M, Hartel S, Foskett JK, Franzini-Armstrong C. Visualization of inositol 1,4,5-trisphosphate receptors on the nuclear envelope outer membrane by freeze-drying and rotary shadowing for electron microscopy. J Struct Biol 171: 372–381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science 281: 1505–1509, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE 2006: re15, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science 268: 233–239, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Collado-Hilly M, Shirvani H, Jaillard D, Mauger JP. Differential redistribution of Ca2+-handling proteins during polarisation of MDCK cells: effects on Ca2+ signalling. Cell Calcium 48: 215–224, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Collins DA, Carter CN, Rink JC, Scott AC, Wyatt CN, Allen NS. Plant nuclei can contain extensive grooves and invaginations. Plant Cell 12: 2425–2440, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Angelis Campos AC, Rodrigues MA, de Andrade C, de Goes AM, Nathanson MH, Gomes DA. Epidermal growth factor receptors destined for the nucleus are internalized via a clathrin-dependent pathway. Biochem Biophys Res Commun 412: 341–346, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decrock E, De Bock M, Wang N, Gadicherla AK, Bol M, Delvaeye T, Vandenabeele P, Vinken M, Bultynck G, Krysko DV, Leybaert L. IP3, a small molecule with a powerful message. Biochim Biophys Acta 1833: 1772–1786, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Dellis O, Dedos SG, Tovey SC, Taufiq Ur R, Dubel SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science 313: 229–233, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Dewaste V, Moreau C, De Smedt F, Bex F, De Smedt H, Wuytack F, Missiaen L, Erneux C. The three isoenzymes of human inositol-1,4,5-trisphosphate 3-kinase show specific intracellular localization but comparable Ca2+ responses on transfection in COS-7 cells. Biochem J 374: 41–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Divecha N, Banfic H, Irvine RF. Inositides and the nucleus and inositides in the nucleus. Cell 74: 405–407, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Divecha N, Banfic H, Irvine RF. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J 10: 3207–3214, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol 5: 440–446, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fricker M, Hollinshead M, White N, Vaux D. The convoluted nucleus. Trends Cell Biol 7: 181, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol 136: 531–544, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci 14: 4794–4805, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galione A, Morgan AJ, Arredouani A, Davis LC, Rietdorf K, Ruas M, Parrington J. NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem Soc Trans 38: 1424–1431, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Gerasimenko JV, Lur G, Sherwood MW, Ebisui E, Tepikin AV, Mikoshiba K, Gerasimenko OV, Petersen OH. Pancreatic protease activation by alcohol metabolite depends on Ca2+ release via acid store IP3 receptors. Proc Natl Acad Sci USA 106: 10758–10763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerasimenko JV, Maruyama Y, Yano K, Dolman NJ, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol 163: 271–282, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerasimenko O, Gerasimenko J. New aspects of nuclear calcium signalling. J Cell Sci 117: 3087–3094, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Gerasimenko OV, Gerasimenko JV, Petersen OH, Tepikin AV. Short pulses of acetylcholine stimulation induce cytosolic Ca2+ signals that are excluded from the nuclear region in pancreatic acinar cells. Pflügers Arch 432: 1055–1061, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell 80: 439–444, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol 128: 893–904, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginty DD, Bonni A, Greenberg ME. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell 77: 713–725, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Goldman PS, Tran VK, Goodman RH. The multifunctional role of the co-activator CBP in transcriptional regulation. Recent Prog Horm Res 52: 103–119; discussion 119–120, 1997 [PubMed] [Google Scholar]
- 43.Gomes DA, Leite MF, Bennett AM, Nathanson MH. Calcium signaling in the nucleus. Can J Physiol Pharmacol 84: 325–332, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, Bennett AM, Nathanson MH. c-Met must translocate to the nucleus to initiate calcium signals. J Biol Chem 283: 4344–4351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guatimosim S, Amaya MJ, Guerra MT, Aguiar CJ, Goes AM, Gomez-Viquez NL, Rodrigues MA, Gomes DA, Martins-Cruz J, Lederer WJ, Leite MF. Nuclear Ca2+ regulates cardiomyocyte function. Cell Calcium 44: 230–242, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Hakamata Y, Nakai J, Takeshima H, Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett 312: 229–235, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 385: 260–265, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Hennager DJ, Welsh MJ, DeLisle S. Changes in either cytosolic or nucleoplasmic inositol 1,4,5-trisphosphate levels can control nuclear Ca2+ concentration. J Biol Chem 270: 4959–4962, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Humbert JP, Matter N, Artault JC, Koppler P, Malviya AN. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. Discrete distribution of inositol phosphate receptors to inner and outer nuclear membranes. J Biol Chem 271: 478–485, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Ibarra C, Vicencio JM, Estrada M, Lin Y, Rocco P, Rebellato P, Munoz JP, Garcia-Prieto J, Quest AF, Chiong M, Davidson SM, Bulatovic I, Grinnemo KH, Larsson O, Szabadkai G, Uhlen P, Jaimovich E, Lavandero S. Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ Res 112: 236–245, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Irvine RF. Nuclear inositide signalling: expansion, structures and clarification. Biochim Biophys Acta 1761: 505–508, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwai M, Michikawa T, Bosanac I, Ikura M, Mikoshiba K. Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J Biol Chem 282: 12755–12764, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Kottgen M, Walz G. Subcellular localization and trafficking of polycystins. Pflügers Arch 451: 286–293, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Lai FA, Dent M, Wickenden C, Xu L, Kumari G, Misra M, Lee HB, Sar M, Meissner G. Expression of a cardiac Ca2+-release channel isoform in mammalian brain. Biochem J 288: 553–564, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanini L, Bachs O, Carafoli E. The calcium pump of the liver nuclear membrane is identical to that of endoplasmic reticulum. J Biol Chem 267: 11548–11552, 1992 [PubMed] [Google Scholar]
- 57.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2: a003996, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ledo F, Kremer L, Mellstrom B, Naranjo JR. Ca2+-dependent block of CREB-CBP transcription by repressor DREAM. EMBO J 21: 4583–4592, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leite MF, Thrower EC, Echevarria W, Koulen P, Hirata K, Bennett AM, Ehrlich BE, Nathanson MH. Nuclear and cytosolic calcium are regulated independently. Proc Natl Acad Sci USA 100: 2975–2980, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Limback-Stokin K, Korzus E, Nagaoka-Yasuda R, Mayford M. Nuclear calcium/calmodulin regulates memory consolidation. J Neurosci 24: 10858–10867, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lipp P, Thomas D, Berridge MJ, Bootman MD. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J 16: 7166–7173, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malhas A, Goulbourne C, Vaux DJ. The nucleoplasmic reticulum: form and function. Trends Cell Biol 21: 362–373, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Malviya AN, Rogue P, Vincendon G. Stereospecific inositol 1,4,5-[32P]trisphosphate binding to isolated rat liver nuclei: evidence for inositol trisphosphate receptor-mediated calcium release from the nucleus. Proc Natl Acad Sci USA 87: 9270–9274, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manzoli L, Martelli AM, Billi AM, Faenza I, Fiume R, Cocco L. Nuclear phospholipase C: involvement in signal transduction. Prog Lipid Res 44: 185–206, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Maraldi NM, Cocco L, Capitani S, Mazzotti G, Barnabei O, Manzoli FA. Lipid-dependent nuclear signalling: morphological and functional features. Adv Enzyme Regul 34: 129–143, 1994 [DOI] [PubMed] [Google Scholar]
- 66.Maraldi NM, Zini N, Ognibene A, Martelli AM, Barbieri M, Mazzotti G, Manzoli FA. Immunocytochemical detection of the intranuclear variations of phosphatidylinositol 4,5-bisphosphate amount associated with changes of activity and amount of phospholipase C beta 1 in cells exposed to mitogenic or differentiating agonists. Biol Cell 83: 201–210, 1995 [DOI] [PubMed] [Google Scholar]
- 67.Marius P, Guerra MT, Nathanson MH, Ehrlich BE, Leite MF. Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium 39: 65–73, 2006 [DOI] [PubMed] [Google Scholar]
- 68.McPherson PS, Campbell KP. Characterization of the major brain form of the ryanodine receptor/Ca2+ release channel. J Biol Chem 268: 19785–19790, 1993 [PubMed] [Google Scholar]
- 69.Mellstrom B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev 88: 421–449, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Meszaros LG, Bak JZ, Chu A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature 364: 76–79, 1993 [DOI] [PubMed] [Google Scholar]
- 71.Nakai J, Imagawa T, Hakamat Y, Shigekawa M, Takeshima H, Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett 271: 169–177, 1990 [DOI] [PubMed] [Google Scholar]
- 72.Nakanishi S, Kuwajima G, Mikoshiba K. Immunohistochemical localization of ryanodine receptors in mouse central nervous system. Neurosci Res 15: 130–142, 1992 [DOI] [PubMed] [Google Scholar]
- 73.Nalaskowski MM, Fliegert R, Ernst O, Brehm MA, Fanick W, Windhorst S, Lin H, Giehler S, Hein J, Lin YN, Mayr GW. Human inositol 1,4,5-trisphosphate 3-kinase isoform B (IP3KB) is a nucleocytoplasmic shuttling protein specifically enriched at cortical actin filaments and at invaginations of the nuclear envelope. J Biol Chem 286: 4500–4510, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newton CL, Mignery GA, Sudhof TC. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J Biol Chem 269: 28613–28619, 1994 [PubMed] [Google Scholar]
- 75.Neylon CB, Richards SM, Larsen MA, Agrotis A, Bobik A. Multiple types of ryanodine receptor/Ca2+ release channels are expressed in vascular smooth muscle. Biochem Biophys Res Commun 215: 814–821, 1995 [DOI] [PubMed] [Google Scholar]
- 76.Nicotera P, McConkey DJ, Jones DP, Orrenius S. ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci USA 86: 453–457, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nicotera P, Orrenius S, Nilsson T, Berggren PO. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc Natl Acad Sci USA 87: 6858–6862, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Osborne SL, Thomas CL, Gschmeissner S, Schiavo G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J Cell Sci 114: 2501–2511, 2001 [DOI] [PubMed] [Google Scholar]
- 79.Otsu K, Willard HF, Khanna VK, Zorzato F, Green NM, MacLennan DH. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem 265: 13472–13483, 1990 [PubMed] [Google Scholar]
- 80.Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci 25: 4279–4287, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pauly N, Knight MR, Thuleau P, van der Luit AH, Moreau M, Trewavas AJ, Ranjeva R, Mazars C. Control of free calcium in plant cell nuclei. Nature 405: 754–755, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467: 291–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pinton P, Pozzan T, Rizzuto R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J 17: 5298–5308, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev 74: 595–636, 1994 [DOI] [PubMed] [Google Scholar]
- 85.Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, Hoek JB, Karpen SJ, Nathanson MH, Bennett AM. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem 277: 27517–27527, 2002 [DOI] [PubMed] [Google Scholar]
- 86.Reilly JF, Maher PA. Importin beta-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J Cell Biol 152: 1307–1312, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Resende RR, Andrade LM, Oliveira AG, Guimaraes ES, Guatimosim S, Leite MF. Nucleoplasmic calcium signaling and cell proliferation: calcium signaling in the nucleus. Cell Commun Signal 11: 14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roderick HL, Higazi DR, Smyrnias I, Fearnley C, Harzheim D, Bootman MD. Calcium in the heart: when it's good, it's very very good, but when it's bad, it's horrid. Biochem Soc Trans 35: 957–961, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Rodrigues MA, Gomes DA, Andrade VA, Leite MF, Nathanson MH. Insulin induces calcium signals in the nucleus of rat hepatocytes. Hepatology 48: 1621–1631, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, Cheng YC, Bennett AM, Nathanson MH. Nucleoplasmic calcium is required for cell proliferation. J Biol Chem 282: 17061–17068, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodrigues MA, Gomes DA, Nathanson MH, Leite MF. Nuclear calcium signaling: a cell within a cell. Braz J Med Biol Res 42: 17–20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, Cheng X, Churchill GC, Zhu MX, Platt FM, Wessel GM, Parrington J, Galione A. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr Biol 20: 703–709, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santella L, Carafoli E. Calcium signaling in the cell nucleus. FASEB J 11: 1091–1109, 1997 [PubMed] [Google Scholar]
- 94.Santella L, Kyozuka K. Effects of 1-methyladenine on nuclear Ca2+ transients and meiosis resumption in starfish oocytes are mimicked by the nuclear injection of inositol 1,4,5-trisphosphate and cADP-ribose. Cell Calcium 22: 11–20, 1997 [DOI] [PubMed] [Google Scholar]
- 95.Schlumm F, Mauceri D, Freitag HE, Bading H. Nuclear calcium signaling regulates nuclear export of a subset of class IIa histone deacetylases following synaptic activity. J Biol Chem 288: 8074–8084, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci 13: 3051–3063, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sheng M, Thompson MA, Greenberg ME. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252: 1427–1430, 1991 [DOI] [PubMed] [Google Scholar]
- 98.Shirakawa H, Miyazaki S. Spatiotemporal analysis of calcium dynamics in the nucleus of hamster oocytes. J Physiol 494: 29–40, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simonetti M, Hagenston AM, Vardeh D, Freitag HE, Mauceri D, Lu J, Satagopam VP, Schneider R, Costigan M, Bading H, Kuner R. Nuclear calcium signaling in spinal neurons drives a genomic program required for persistent inflammatory pain. Neuron 77: 43–57, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sonnleitner A, Conti A, Bertocchini F, Schindler H, Sorrentino V. Functional properties of the ryanodine receptor type 3 (RyR3) Ca2+ release channel. EMBO J 17: 2790–2798, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stehno-Bittel L, Luckhoff A, Clapham DE. Calcium release from the nucleus by InsP3 receptor channels. Neuron 14: 163–167, 1995 [DOI] [PubMed] [Google Scholar]
- 102.Taylor CW, Dale P. Intracellular Ca2+ channels: a growing community. Mol Cell Endocrinol 353: 21–28, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Thompson M, Andrade VA, Andrade SJ, Pusl T, Ortega JM, Goes AM, Leite MF. Inhibition of the TEF/TEAD transcription factor activity by nuclear calcium and distinct kinase pathways. Biochem Biophys Res Commun 301: 267–274, 2003 [DOI] [PubMed] [Google Scholar]
- 104.Tsiokas L, Kim S, Ong EC. Cell biology of polycystin-2. Cell Signal 19: 444–453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wamhoff BR, Dixon JL, Sturek M. Atorvastatin treatment prevents alterations in coronary smooth muscle nuclear Ca2+ signaling in diabetic dyslipidemia. J Vasc Res 39: 208–220, 2002 [DOI] [PubMed] [Google Scholar]
- 106.Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J 363: 657–666, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waybill MM, Yelamarty RV, Zhang Y, Scaduto RC, LaNoue KF, Hsu CJ, Smith BC, Tillotson DL, Yu FTS, Cheung JY. Nuclear calcium gradients in cultured rat hepatocytes. Am J Physiol Endocrinol Metab 261: E49–E57, 1991 [DOI] [PubMed] [Google Scholar]
- 108.Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem 270: 11678–11683, 1995 [DOI] [PubMed] [Google Scholar]
- 109.Wu G, Xie X, Lu ZH, Ledeen RW. Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc Natl Acad Sci USA 106: 10829–10834, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xie X, Wu G, Lu ZH, Ledeen RW. Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J Neurochem 81: 1185–1195, 2002 [DOI] [PubMed] [Google Scholar]
- 111.Yu JC, Lloyd-Burton SM, Irvine RF, Schell MJ. Regulation of the localization and activity of inositol 1,4,5-trisphosphate 3-kinase B in intact cells by proteolysis. Biochem J 392: 435–441, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 76: 367–385, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Zhang SJ, Steijaert MN, Lau D, Schutz G, Delucinge-Vivier C, Descombes P, Bading H. Decoding NMDA receptor signaling: identification of genomic programs specifying neuronal survival and death. Neuron 53: 549–562, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Zhang SJ, Zou M, Lu L, Lau D, Ditzel DA, Delucinge-Vivier C, Aso Y, Descombes P, Bading H. Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLos Genet 5: e1000604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]