Support
The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1). Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Keywords: HIV, ARV, neurodevelopment, children, cognition, academic achievement
Vertical transmission of HIV has been substantially reduced since antiretroviral medications (ARVs) were introduced, leading to increasing numbers of HIV-uninfected children who have been exposed to multiple ARVs.1-3 As efforts to increase ARV coverage to all pregnant women with HIV reach their full potential in low- to mid-resource countries, roughly 1.5 million women and their infants may be exposed to ARVs each year.4
ARVs such as nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) are known to cross the placental barrier.5,6 Animal studies suggest that NRTIs can cause mitochondrial toxicities.7-10 In addition, structural and functional changes in the central nervous system (CNS) have been found in animal and human studies, suggesting that the CNS may be an important target of zidovudine (Retrovir; GlaxoSmithKline, Brentford, Middlesex, UK) or other ARV agents.11-14 Thus, the long-term effect of ARV exposure on neurodevelopmental functioning has become a particular area of concern.
Research on the neurodevelopmental functioning of children exposed to ARVs in utero and in the neonatal period has focused on infants and very young children, and the results have been variable. Most studies have not shown a risk for lowered cognitive performance or behavior problems.15-19 While data from 12-month-old infants in the Surveillance Monitoring for Antiretroviral Treatment Toxicities (SMARTT) study indicated that ARV regimens used in pregnancy and the neonatal period are safe overall, some individual medications were associated with negative effects on infant development.20 Additionally, the French Perinatal Cohort study reported a higher than expected rate of mitochondrial dysfunction and developmental delays in infants and children exposed to HIV and ARVs.21 Subsequently, Brogly et al.22 observed an association between third trimester exposure to zidovudine/lamivudine (Combivir;GlaxoSmithKline, Brentford, Middlesex, UK) and possible mitochondrial dysfunction, including developmental delays. To date, the impact of in utero and neonatal ARV exposure on the neurodevelopmental functioning of HIV-exposed, uninfected school-age children and adolescents has not been studied.23
The results of studies comparing HIV-infected and HIV-exposed, uninfected children (the majority of whom were also ARV-exposed) with normative samples have been variable. In a group of children age 9-16 years, Brackis-Cott et al.24 found that about one-third of HIV-infected and HIV-exposed, uninfected children fell below the tenth percentile on measures of word reading and receptive vocabulary. Smith et al.25 in their study of cognitive and adaptive functioning in HIV-infected and HIV-exposed, uninfected children age 7-16 years found that overall mean scores fell within the low normal range in both groups. Although providing important information regarding the impact of HIV on developmental and cognitive functioning, previous studies of school-age children and adolescents have not addressed the specific contribution of ARVs prescribed during pregnancy and the neonatal period to cognitive outcomes. It is important to evaluate individual ARVs because certain medications or combinations of medications may be more toxic than others to the developing fetus, but their adverse effects on cognitive, academic, or behavioral outcomes might not manifest until later in life.
According to Ciaranello et al.,26 many prior studies failed to control adequately for important maternal/caregiver characteristics relevant to child development such as obstetric complications, maternal viral load and CD4, pre- and postnatal substance use, psychiatric health, and socio-economic status.21,22,26 In addition, environmental factors associated with poverty have been shown to have adverse effects on children's development.27-30 Examining the factors contributing to developmental, cognitive, or behavioral disturbances that may be seen in children exposed to HIV and ARVs is crucial to their prevention and treatment. Therefore, it is important to follow children into later childhood and adolescence to examine the potential latent effect of their early exposure to ARVs. The current study of HIV-exposed, uninfected children examines how cognitive outcomes, as well as academic functioning, relate to prenatal and neonatal ARV exposure, while considering the effects of important pre- and postnatal influences on development.
Materials and Methods
Participants
Study participants were enrolled in SMARTT, a multisite cohort of HIV-exposed, uninfected children. SMARTT is designed to evaluate the safety of in utero and neonatal exposure to ARVs and is conducted by the Pediatric HIV/AIDS Cohort Study (PHACS) network at 22 sites in the United States, including Puerto Rico. SMARTT includes infants followed from birth (Dynamic cohort) and infants and children enrolled past infancy or in earlier HIV-related studies (Static cohort). For this study, all children enrolled in the SMARTT Static cohort (HIV-exposed) were eligible if they had completed a valid, age-appropriate measure of cognition and/or academic achievement in English (at ages 5, 7, 9, 11, and/or 13 years) and had information regarding in utero and neonatal ARV exposure.
The institutional review board at each participating site and at Harvard School of Public Health approved the study. Children provided assent and parents or legal guardians provided written informed consent for research participation.
Procedures
Cognitive and academic assessments were administered at protocol-specific time points (± 3 months of the children's birthdays) and according to standardized procedures by licensed psychologists or psychometricians under supervision of a licensed psychologist. The cognitive and academic tests used in this study were only available in English and were therefore administered only to children who could complete the tests in English.
ARV exposure
Combination ARV regimens (cARV) were defined as any maternal regimen containing at least three ARVs from at least two drug classes. ARV regimens were categorized as follows: (1) cARV with protease inhibitor (PI; with or without NNRTI), (2) cARV with NNRTI (no PI), (3) non-cARV (mono or dual therapy or three or more NRTIs), and (4) no ARV. Neonatal prophylaxis was defined as use of ARV medications during the first eight weeks of life, dichotomized into zidovudine monotherapy versus zidovudine with another ARV.
Cognitive outcomes
Two standardized measures of intelligence were used. The Wechsler Preschool and Primary Scale of Intelligence--III31 (WPPSI-III) was administered to 5-year-old children and the Wechsler Abbreviated Scale of Intelligence32 (WASI) to 7-, 9-, 11-, and 13-year-old children. The batteries provide comparable Verbal and Performance Intelligence Quotients (VIQ and PIQ) with population norm mean = 100 (standard deviation [SD] = 15). The VIQ and PIQ were the primary measures of cognitive functioning.
Academic outcomes
The Wechsler Individual Achievement Test, Second Edition, Abbreviated33 (WIAT-II-A) is a standardized measure of academic achievement (mean = 100 [SD = 15]), providing assessment of acquired skills in word reading, spelling, and written arithmetic. It was administered to children at ages 7, 9, 11, and 13 years of age.
Because of the longitudinal nature of SMARTT, some children had completed both a WPPSI-III and a WASI at the appropriate ages. This analysis included only the first cognitive and academic assessment for each study participant.
Covariates and potential confounders
Prenatal and postnatal factors that could serve as potential confounders of the relationship between ARV exposure and cognitive and academic outcomes were evaluated. Covariates and potential confounders were identified a priori and included the following: severity of maternal HIV disease (as measured by first and last CD4% and viral load prior to delivery), maternal substance use during pregnancy and at the time of the child's assessment, sexually transmitted infection (STI) during pregnancy, child's year of birth, sex, and ethnicity, gestational age and birth weight, household income, and maternal/caregiver variables of education, cognitive and psychiatric status, and functional limitations. Demographic and maternal health data were obtained through medical record review and maternal/caregiver interviews at study entry. Maternal/caregiver cognition was measured at study entry with the WASI, providing a Full Scale IQ (FSIQ) score. Maternal/caregiver mental health status was assessed with the Client Diagnostic Questionnaire34 (CDQ), a screening instrument developed and validated for populations affected by HIV. The CDQ was administered as a face-to-face interview to assess the presence of symptoms of psychiatric illness, including depression, anxiety, alcohol and illicit substance abuse, post-traumatic stress disorder, and psychosis. Virtually all of the maternal/caregiver CDQ data (97-99%) were collected within 90 days of the child's cognitive and academic testing.
Statistical Methods
To evaluate potential selection bias, statistical comparisons of background characteristics for children included versus not included in the analyses (due to missing or invalid assessments) were conducted using two-sample t-tests or chi-square tests, as appropriate. Univariate analyses were performed to evaluate the associations of each of the cognitive and academic outcomes with the selected covariates and with prenatal and neonatal ARV exposures. For each outcome, a multivariable general linear model was constructed including all covariates with p < 0.20 in univariate analyses. The model was further reduced to a core model containing only the covariates with p < 0.10. Adjusted associations of each outcome with ARV exposures were estimated with general linear models, controlling for the core model covariates.
Use of cARV and other ARV regimens during pregnancy was analyzed as follows. Children with no in utero ARV exposure were excluded (3-7% within each sample). For the children exposed to ARVs in utero, individual ARVs were evaluated by comparing children exposed to a specific ARV versus children not exposed to that ARV. A separate analysis was performed to compare the outcomes of children exposed to PIs either boosted or not boosted with ritonavir to the outcomes of children with no exposure to PIs.
Sensitivity analyses were conducted to adjust for prematurity35 and low birth weight. Since these birth characteristics could be on the causal pathway between in utero ARV exposure and later cognitive or academic functioning, they were not considered in the primary analyses. Due to the potential for inherent differences among research sites, a sensitivity analysis was conducted to adjust for site by considering site as a random effect in a mixed effect model.
Analyses were based on the data collected as of April 1, 2012. SAS Version 9.2 (SAS Institute Inc., Cary, NC) was used to conduct all statistical analyses. Two-sided p-values < 0.05 were considered statistically significant. Because SMARTT is a safety study, no correction for multiple comparisons was made to minimize the Type II error rate (the probability of failing to detect true associations), thus the findings warrant confirmation in future studies.
Results
Participant Characteristics
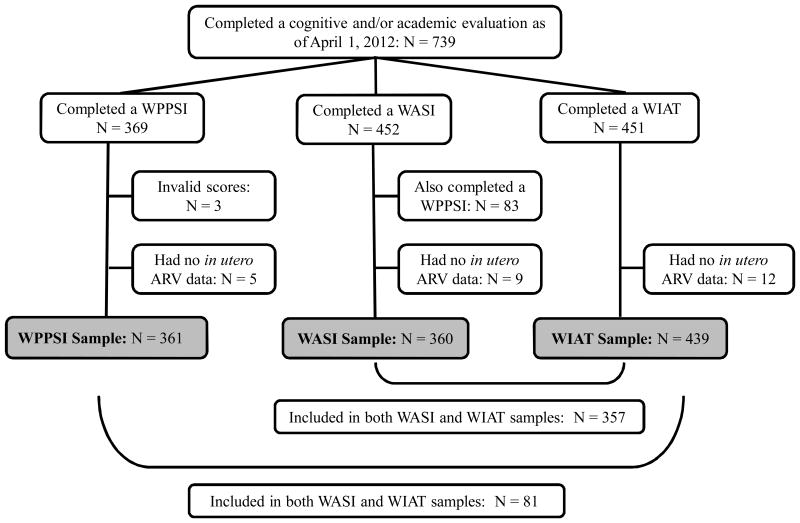
A total of 739 children with HIV-exposure in utero had completed at least one Wechsler test and were considered eligible for this analysis (Figure 1). The numbers of assessments were: WPPSI-III (n = 369), WASI (n = 452), and WIAT-II-A (n = 451). Some children (n = 216) were considered ineligible for the analyses because they had a study visit at which one or more Wechsler tests were expected to be administered but were not. The reasons for this were primarily that the test could not be completed in English, lack of time, or scheduling problems. Of the eligible assessments, three WPPSI-IIIs were confirmed invalid and excluded from the analysis. In addition, 83 children who completed both a WPPSI-III and a WASI test were excluded from the sample for WASI analysis, since this analysis used only the first cognitive assessment for each child. Of the children with a valid cognitive or academic assessment, 26 additional children without available prenatal ARV data were excluded, as follows: WPPSI-III, n = 5 (1.4%); WASI, n = 9 (2.4%); and WIAT-II-A, n = 12 (2.7%). The final sample size for analysis was WPPSI-III, n = 361; WASI, n = 360; and WIAT-II-A, n = 439. There were some significant differences in demographic characteristics between children who were included in the analyses versus those who were not included. For all three outcomes, the differences were associated primarily with the child's race, ethnicity, and primary language (p ≤ 0.001).
Figure 1. Derivation of study samples.
Several of the demographic characteristics of the sample included in this study (Table 1) are of note. The proportion of pre-term births (< 37 weeks) ranged from 17-21% and low birth weight (< 2500 grams), 18-20%. Additionally, 59-69% of the children lived in households with ≤ $20,000 annual income. Approximately one third of the caregivers had less than a high school education, and 9-10% had a FSIQ of < 70.
Table 1. Demographic Characteristics Among Children Who Completed a Cognitive or Academic Assessment.
| Characteristic | WPPSI-III (n = 361) | WASI (n = 360) | WIAT-II-A (n = 439) |
|---|---|---|---|
| Male sex | 176 (49%) | 188 (52%) | 221 (50%) |
| Black race | 262 (75%) | 270 (77%) | 331 (77%) |
| Hispanic ethnicity | 91 (25%) | 69 (19%) | 87 (20%) |
| Birth year | |||
| 1995 - 1999 | 0 (0%) | 157 (44%) | 156 (35%) |
| 2000 - 2001 | 0 (0%) | 144 (40%) | 143 (33%) |
| 2002 - 2003 | 108 (30%) | 59 (16%) | 140 (32%) |
| 2004 - 2005 | 152 (42%) | 0 (0%) | 0 (0%) |
| 2006 - 2007 | 101 (28%) | 0 (0%) | 0 (0%) |
| Primary language of child is English | 319 (88%) | 330 (92%) | 408 (93%) |
| Number of people in home (excluding child) | |||
| 1-2 | 104 (29%) | 103 (29%) | 131 (30%) |
| 3-4 | 177 (50%) | 188 (52%) | 229 (52%) |
| 5 or more | 76 (21%) | 68 (19%) | 77 (18%) |
| Preterm (gestational age < 37 weeks) | 73 (21%) | 59 (17%) | 76 (18%) |
| Birth weight < 2500 g | 72 (20%) | 66 (18%) | 82 (19%) |
| Caregiver identity: biological parent | 342 (96%) | 327 (91%) | 398 (91%) |
| Primary language of caregiver is English | 265 (74%) | 289 (81%) | 354 (81%) |
| Maternal age at delivery ≥ 35 | 53 (15%) | 47 (14%) | 57 (14%) |
| Annual household income | |||
| ≤ $20,000 | 234 (69%) | 205 (59%) | 259 (61%) |
| $20,000 – 40,000 | 81 (24%) | 100 (29%) | 114 (27%) |
| > $40,000 | 25 (7%) | 45 (13%) | 54 (13%) |
| Caregiver education less than high school | 113 (32%) | 121 (34%) | 149 (34%) |
| Single parent | 176 (49%) | 195 (54%) | 235 (54%) |
| Number of caregiver functional limitations | |||
| 0 | 202 (59%) | 210 (61%) | 252 (60%) |
| 1-2 | 79 (23%) | 90 (26%) | 115 (27%) |
| 3 or more | 59 (17%) | 43 (13%) | 54 (13%) |
| Caregiver: any psychiatric diagnosis | 115 (34%) | 95 (29%) | 122 (30%) |
| Caregiver: any postnatal substance abuse disorder | 34 (10%) | 19 (6%) | 34 (9%) |
| Maternal alcohol use during pregnancy | 20 (6%) | 17 (6%) | 23 (6%) |
| Maternal tobacco use during pregnancy | 74 (23%) | 49 (16%) | 70 (19%) |
| Maternal illicit drug use during pregnancy | 31 (10%) | 23 (8%) | 29 (8%) |
| Caregiver FSIQ, Mean (SD) | 86.3 (13.1) | 86.1 (13.6) | 86.1 (13.7) |
| Caregiver FSIQ < 85 | 135 (46%) | 145 (49%) | 175 (48%) |
| Caregiver FSIQ < 70 | 28 (9%) | 27 (9%) | 36 (10%) |
| Any obstetric complication | 206 (59%) | 193 (56%) | 236 (56%) |
| Sexually transmitted infection (STI) during pregnancy | 129 (38%) | 116 (34%) | 145 (35%) |
| First CD4% during pregnancy ≤ 25% | 145 (45%) | 120 (41%) | 161 (44%) |
| Last CD4% prior to delivery ≤ 25% | 93 (29%) | 93 (32%) | 114 (32%) |
| First viral load during pregnancy > 400 | 199 (60%) | 195 (72%) | 241 (70%) |
| Last viral load prior to delivery > 400 | 62 (19%) | 86 (33%) | 104 (32%) |
Participants with missing data were excluded from the relevant calculations, as follows. WPPSI-III: race (12), ethnicity (1), number of people in home (4), prematurity (10), birth weight (2), caregiver identity (4), caregiver primary language (4), maternal age at delivery (4), household income (21), caregiver education (4), single parent (4), number of caregiver functional limitations (21), caregiver psychiatric disorder (22), caregiver postnatal substance abuse disorders (30), alcohol/tobacco/illicit substance use during pregnancy (37), caregiver FSIQ (66), obstetrical complications (11), STI during pregnancy (21), first /last CD4% during pregnancy (38), first/last RNA during pregnancy (29). WASI: race (9), number of people in home (1), prematurity (7), birth weight (1), caregiver identity (1), caregiver primary language (1), maternal age at delivery (11), household income (10), caregiver education (1), single parent (1), number of caregiver functional limitations (17), caregiver psychiatric disorder (31), caregiver postnatal substance abuse disorders (38), alcohol/tobacco/illicit substance use during pregnancy (62), caregiver FSIQ (64), obstetrical complications (15), STD during pregnancy (23), first /last CD4% during pregnancy (67), first/last RNA during pregnancy (90). WIAT-II-A: race (10), number of people in home (2), prematurity (13), birth weight (2), caregiver identity (2), caregiver primary language (1), maternal age at delivery (14), household income (12), caregiver education (1), single parent (1), number of caregiver functional limitations (18), caregiver psychiatric disorder (31), caregiver postnatal substance abuse disorders (39), alcohol/tobacco/illicit substance use during pregnancy (71), caregiver FSIQ (73), obstetrical complications (17), STD during pregnancy (29), first/last CD4% during pregnancy (75), first/last RNA during pregnancy (97).
ARV Exposures
Among the WPPSI-III sample, 97% of children were exposed in utero to some type of ARV; 81% were exposed to cARV and 66% to a PI-containing cARV regimen. In the WASI sample, 93% were exposed in utero to some type of ARV; 60% to cARV and 45% to a PI-containing cARV regimen. In the WIAT-II-A sample, 94% were exposed in utero to some type of ARV; 63% to cARV and 47% to a PI-containing cARV regimen. Regarding neonatal prophylaxis, 91% of the WPPSI-III sample, 86% of the WASI sample, and 87% of the WIAT-II-A sample were exposed to zidovudine alone. In each sample, only 1% received no neonatal ARV prophylaxis.
Cognitive and Academic Outcomes
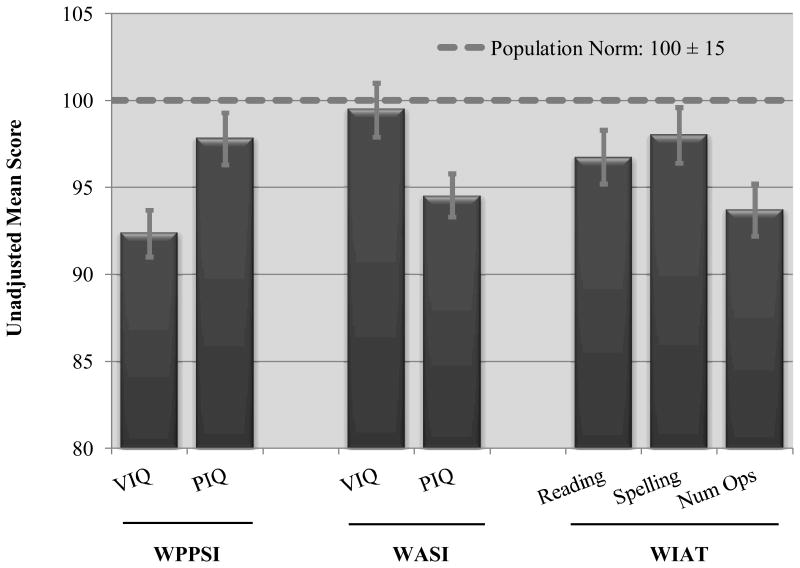
Unadjusted WPPSI-III, WASI, and WIAT-II-A scores are presented in Figure 2. The mean scores were significantly below population norms (p = 0.01 to p < 0.001) with the exception of the WASI VIQ (p = 0.48), yet these mean scores (ranging from 93 to 99) still fell within the expected range for the children's ages.
Figure 2. Unadjusted mean cognitive and academic scores with 95% confidence limits.
Associations of Cognitive and Academic Outcomes with ARV Exposure
By prenatal ARV regimen
Table 2 presents the adjusted mean cognitive and academic scores for each prenatal ARV regimen. The means were adjusted for the core confounders shown in the table note. No significant differences in adjusted mean scores were observed between the groups exposed prenatally to cARV versus non-cARV regimens. There were no significant differences in mean scores for any cognitive or academic outcome when comparing NNRTI-based cARV regimens and non-cARV regimens with PI-based cARV regimens. No significant differences were found when comparing outcomes for children not exposed to any PI with those exposed to boosted or non-boosted PIs. In addition, no significant associations were observed between the cumulative duration (in months) of prenatal cARV exposure and cognitive and academic outcomes.
Table 2. Adjusted Mean Cognitive and Academic Scores by Prenatal and Neonatal ARV Regimen Exposure in HIV-exposed Children with In Utero ARV Exposure.
| Cognitive/Academic Domain | Prenatal ARV Regimen | Neonatal ARV Regimen | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| cARV with PI | cARV with NNRTI (no PI) | Non-cARV | ZDV + Other ARV | ZDV Alone | ||||||
|
| ||||||||||
| Adjusted Mean (SE) | p | Adjusted Mean (SE) | p | Adjusted Mean (SE) | p | Adjusted Mean (SE) | p | Adjusted Mean (SE) | p | |
| WPPSI-III (n = 350) | n = 236 (68%) | n = 56 (16%) | n = 57 (16%) | n = 26 (7%) | n = 324 (93%) | |||||
| Verbal IQ | 91.5 (1.6) | Ref. | 89.8 (2.2) | 0.37 | 88.9 (2.2) | 0.17 | 89.6 (2.8) | 0.61 | 90.9 (1.5) | Ref. |
| Performance IQ | 97.4 (1.9) | Ref. | 98.3 (2.6) | 0.67 | 94.8 (2.7) | 0.26 | 97.9 (3.4) | 0.81 | 97.2 (1.8) | Ref. |
|
| ||||||||||
| WASI (n = 337) | n = 157 (49%) | n = 50 (15%) | n = 117 (36%) | n = 45 (13%) | n = 291 (87%) | |||||
| Verbal IQ | 94.7 (2.4) | Ref. | 95.5 (2.9) | 0.73 | 94.1 (2.3) | 0.76 | 100.0 (2.8) | 0.004 | 93.6 (2.1) | Ref. |
| Performance IQ | 97.2 (1.6) | Ref. | 94.2 (2.2) | 0.12 | 96.7 (1.7) | 0.71 | 99.7 (2.2) | 0.08 | 96.6 (1.5) | Ref. |
|
| ||||||||||
| WIAT-II-A (n = 415) | n = 197 (49%) | n = 72 (18%) | n = 133 (33%) | n = 50 (12%) | n = 364 (88%) | |||||
| Word Reading | 90.0 (2.3) | Ref. | 88.8 (2.8) | 0.61 | 89.8 (2.2) | 0.93 | 97.2 (3.0) | <0.001 | 88.9 (1.9) | Ref. |
| Spelling | 91.7 (2.3) | Ref. | 89.6 (2.9) | 0.37 | 91.0 (2.3) | 0.72 | 96.7 (3.0) | 0.01 | 90.3 (2.0) | Ref. |
| Numerical Operations | 91.3 (1.7) | Ref. | 90.1 (2.3) | 0.57 | 92.4 (1.6) | 0.58 | 95.9 (2.4) | 0.09 | 91.9 (1.3) | Ref. |
Note. Non-cARV regimens include monotherapy or dual therapy or three or more NRTIs. There were no significant differences in the unadjusted mean scores for participants taking three or four NRTIs versus other regimens. General linear regression models were adjusted for all child and maternal covariates with p < 0.10, as follows: WPPSI-III Verbal IQ (household income, caregiver FSIQ, alcohol use during pregnancy, and tobacco use during pregnancy); WPPSI-III Performance IQ (race, birth year, number of people in the household, maternal age at delivery, caregiver FSIQ, and alcohol use during pregnancy); WASI Verbal IQ (age at testing, child's primary language, number of people in the household, household income, caregiver FSIQ, illicit drug use during pregnancy, and maternal first CD4% during pregnancy); WASI Performance IQ (caregiver's primary language, caregiver FSIQ, caregiver postnatal substance abuse, and number of caregiver functional imitations); WIAT-II-A Word Reading (sex, age at testing, birth year, number of people in the household, caregiver FSIQ, alcohol use during pregnancy, and maternal last CD4% during pregnancy); WIAT-II-A Spelling (sex, age at testing, birth year, number of people in the household, caregiver FSIQ, alcohol use during pregnancy, and maternal last CD4% during pregnancy); WIAT-II-A Numerical Operations (age at testing, caregiver's primary language, and caregiver FSIQ). Participants with missing information about ARV regimens were excluded from the calculations of exposure prevalence, as follows: WPPSI-III (1), WASI (13), and WIAT-II-A (13). Participants with no exposure to neonatal ARV regimens were excluded from the analyses, as follows: WASI (1) And WI AT-II-A (1).
By prenatal exposure toindividual ARVs
No individual ARV was associated with significantly lower mean cognitive or academic outcomes. However, prenatal exposure to regimens containing tenofovir (Viread; Gilead Sciences, Inc., Foster City, CA) was associated with higher mean WPPSI-III PIQ scores compared to regimens without tenofovir (100.8 versus 96.1, p = 0.03) (Table 3).
Table 3. Adjusted Mean Differences between Children Exposed In Utero versus Unexposed to Individual ARV Medications.
| Exposure during Pregnancy | WPPSI-III (N = 350) | WASI (N = 337) | WIAT-II-A (N = 415) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| VIQ | PIQ | VIQ | PIQ | Word Reading | Spelling | Numerical Operations | |
|
| |||||||
| Zidovudine | -3.5 | -1.8 | 1.9 | -3.7 | 2.9 | 3.0 | 2.7 |
| Lamivudine | -1.6 | 0.5 | 3.0 | -0.7 | 2.9 | 1.1 | 1.7 |
| Abacavir | 0.3 | -0.5 | -4.6 | -1.3 | 0.1 | 0.8 | -0.6 |
| Tenofovir | 3.2 | 4.7* | --- | --- | --- | --- | --- |
| Stavudine | 3.7 | 2.6 | -3.2 | -0.6 | -4.1 | -3.4 | -4.1 |
| Didanosine | 1.4 | 1.1 | -4.3 | 0.3 | -2.5 | 0.6 | -4.4 |
| Emtricitabine | 1.9 | 3.4 | --- | --- | --- | --- | --- |
| Nevirapine | -1.4 | 0.6 | 0.5 | -2.3 | -0.9 | -2.3 | -1.2 |
| Nelfinavir | -0.7 | -0.5 | 1.3 | 1.1 | 0.1 | 0.7 | -0.8 |
| Indinavir | --- | --- | -1.4 | -0.7 | 4.9 | 4.8 | 2.7 |
| Atazanavir | 4.0 | 5.8 | --- | --- | --- | --- | --- |
| Lopinavir/RTV | 1.3 | -0.6 | --- | --- | --- | --- | --- |
| Ritonavir | 2.3 | 0.3 | -4.9 | -2.8 | 0.6 | -2.4 | -1.7 |
|
| |||||||
| Neonatal Prophylaxis | |||||||
|
| |||||||
| ZDV with another ARV (vs ZDV alone) | -1.3 | 0.7 | 6.4* | 3.1 | 8.3* | 6.4* | 3.9 |
p < 0.05
Note. “---” indicates the ARV was not evaluated because too few children (< 5%) were exposed to it. General linear regression models were adjusted for all child and maternal covariates with p < 0.10, as follows: WPPSI-III Verbal IQ (household income, caregiver FSIQ, alcohol use during pregnancy, and tobacco use during pregnancy); WPPSI-III Performance IQ (race, birth year, number of people in the household, maternal age at delivery, caregiver FSIQ, and alcohol use during pregnancy); WASI Verbal IQ (age at testing, child's primary language, number of people in the household, household income, caregiver FSIQ, illicit drug use during pregnancy, and maternal first CD4% during pregnancy); WASI Performance IQ (caregiver's primary language, caregiver FSIQ, caregiver postnatal substance abuse, and number of caregiver functional imitations); WIAT-II-A Word Reading (sex, age at testing, birth year, number of people in the household, caregiver FSIQ, alcohol use during pregnancy, and maternal last CD4% during pregnancy); WIAT-II-A Spelling (sex, age at testing, birth year, number of people in the household, caregiver FSIQ, alcohol use during pregnancy, and maternal last CD4% during pregnancy); WIAT-II-A Numerical Operations (age at testing, caregiver's primary language, and caregiver FSIQ). Participants with missing information about ARV regimens were excluded from the calculations of exposure prevalence, as follows: WPPSI-III (1), WASI (13), and WIAT-II-A (13).
By neonatal exposure
The majority of children received zidovudine alone; only 46 (13%) and 52 (12%) of the WASI and WIAT-II-A samples, respectively, were exposed to combination neonatal prophylaxis. Children who received zidovudine in combination with another ARV during the first eight weeks of life had significantly higher WASI VIQ and WIAT-II-A Word Reading and Spelling scores (by 6.4 to 8.3 points) as compared to children who received zidovudine alone.
Sensitivity Analyses
Sensitivity analyses including prematurity and low birth weight in the adjusted models resulted in similar adjusted effects of ARV regimen, cARV exposure, individual ARVs, and neonatal prophylaxis on cognitive and academic outcomes. A significant association of in utero exposure to zidovudine or to zidovudine/lamivudine (Combivir; GlaxoSmithKline, Brentford, Middlesex, UK) with lower WPPSI-III VIQ was observed; these findings were not observed in the primary analysis.
The results of sensitivity analyses including research site in the adjusted models were similar to the results of the primary analyses. The significant association of tenofovir with higher WPPSI-III PIQ was attenuated. Exposure to didanosine (Videx; Bristol-Myers Squibb, New York, NY) was significantly associated with lower WASI VIQ; this result was not observed in the primary analysis.
Discussion
The results of the present study indicated that mean scores for the cognitive and academic outcomes in this sample of HIV-exposed children, age 5-13 years fell slightly below the average range. There were no significant associations between any ARV regimen or drug class and any cognitive or academic outcome. In addition, no individual ARV drug was associated with significantly lower cognitive or academic outcomes. Overall, these results support the safety of ARV use during pregnancy and the neonatal period and increase our knowledge regarding the long-term effects of in utero and neonatal ARV exposure on the cognitive and academic functioning of HIV-exposed, uninfected school-age children.
The present study found no differences in verbal intelligence when comparing school-aged children with and without atazanavir (Reyataz; Bristol-Myers Squibb, New York, NY) exposure, contrary to earlier analyses of SMARTT data by Sirois et al.20 and Rice et al.36 that found small but significant negative associations between atazanavir exposure and language development in infants at one and two years of age. Atazanavir is a relatively new ARV approved for use during pregnancy; the cohorts of infants and young children in the earlier studies were more likely to have been exposed to atazanavir than the cohort of school-age children in the present study. The finding of no difference in our study may therefore be due to the small number of children with atazanavir exposure. This question should be further examined in future studies.
Children in this study with in utero tenofovir exposure attained higher mean WPPSI-III PIQ scores and those exposed to combination neonatal prophylaxis obtained higher WASI VIQ and WIAT-II-A Word Reading and Spelling scores. While the finding regarding PIQ suggests a possible relative protective effect of tenofovir exposure, it should be interpreted with caution, given the multiple comparisons included in the analysis. In addition, although we controlled for maternal health factors during pregnancy and socioeconomic factors that may have predicted maternal use of specific ARV regimens or individual ARV drugs, as well as receipt of combination prophylaxis in infants, these findings could be at least partially attributable to residual confounding from unmeasured or imperfectly measured factors.
In the general population, many variables have been found to affect cognitive and academic functioning, specifically, maternal cognitive status, prematurity, small for gestational age and household income.37-39 This study of children with in utero HIV exposure corroborated the findings of previous research. Our study participants were more likely to live in low income households, with mothers or caregivers whose mean cognitive scores fell below average. Prenatal alcohol exposure was significantly associated with several of the outcomes. Although we could reasonably have expected that HIV and ARV exposure, in addition to these factors, might have been associated with even lower scores, this was not seen in the present study and is an encouraging finding. Thus, while prenatal HIV and ARV exposure may be important factors to consider, this study highlights the significant influence of other biological and environmental factors on development of children exposed to HIV.
The strengths of this study included the large sample of children with perinatal HIV and ARV exposure and valid cognitive and academic data. The study included detailed information concerning maternal immunologic status, ARV history during pregnancy, and duration of ARV use. Detailed information about the children's birth history and exposure to ARVs in the neonatal period and other relevant confounders was also included.
The generalizability of our results may be limited by demographic differences between the children included versus not included in the analyses. The requirement for fluency in English limited the number of Spanish-speaking children who completed the assessments for this study. Although there was a significant difference between the children included and not included in the WPPSI-III sample with respect to their exposures to cARV in utero, the proportion of children with prenatal exposure to cARV was high in both groups; thus, the difference was unlikely to affect study conclusions. Additionally, we did not have information on maternal adherence to ARV regimens and the analyses did not include all possible combinations of ARVs, thus limiting our ability to detect potential effects of any specific combination.
In conclusion, these results support the safety of ARV use during pregnancy and the neonatal period with respect to later cognitive and academic outcomes in school-aged children. As access to ARVs to prevent mother-to-child transmission increases across the globe, it is anticipated that an increasing number of children will experience in utero ARV exposure. Thus, it is critical that more information be obtained on the potential short- and long-term toxicities of such exposures on the child. New drugs and combinations of drugs continue to become available and will be used during pregnancy for treatment of women with HIV. Therefore, continued follow-up studies of HIV- and ARV-exposed youth are critical to monitor for potential neurobehavioral consequences of such exposure and to help determine if there are optimal approaches to choice of ARV drug regimens during pregnancy.
Acknowledgments
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
Abbreviations
- ARV
antiretroviral medication
- cARV
combination antiretroviral regimen
- CDQ
Client Diagnostic Questionnaire
- CNS
central nervous system
- FSIQ
Full Scale Intelligence Quotient
- HIV
human immunodeficiency virus
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- SGA
small for gestational age
- SMARTT
Surveillance Monitoring for ART Toxicities
- STI
sexually transmitted infection
- Viral load
HIV RNA plasma level (copies/mL)
- WASI
Wechsler Abbreviated Scale of Intelligence
- WIAT-II-A
Wechsler Individual Achievement Test, Second Edition, Abbreviated
- WPPSI-III
Wechsler Preschool and Primary Scale of Intelligence, Third Edition
Footnotes
Conflicts of interest: The authors have no conflicts of interest or funding to disclose.
Presentation of data: Data from this analysis were presented at the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA, March 2013.
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2012, in alphabetical order: Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Anna Cintron; Children's Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Doyle Patton, Deyana Leon; Ann & Robert H. Lurie Children's Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Helen Rozelman; St. Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Hermann Mendez, Ava Dennie, Susan Bewley; Tulane University Health Sciences Center: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Newana Beatty, Dan Marullo; University of California, San Diego: Stephen Spector, Jean Manning, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Florida/Jacksonville: Mobeen Rathore, Kristi Stowers, Ann Usitalo; University of Illinois, Chicago: Kenneth Rich, Lourdes Richardson, Delmyra Turpin, Renee Smith; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Claudia Florez, Elizabeth Willen; University of Southern California: Toinette Frederick, Mariam Davtyan, Maribel Mejia; University of Puerto Rico Medical Center: Zoe Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
References
- 1.Griner R, Williams PL, Read JS, et al. Pediatric HIV/AIDS Cohort Study In utero and postnatal exposure to antiretrovirals among HIV-exposed but uninfected children in the United States. AIDS Patient Care STDs. 2011;25(7):385–394. doi: 10.1089/apc.2011.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidari S, Mofenson L, Cotton MF, Marlink R, Cahn P, Katabira E. Antiretroviral drugs forpreventing mother-to-child transmission of HIV: A review of potential effects on HIV-exposed but uninfected children. J Acquir Immune Defic Syndr. 2011;57:290–296. doi: 10.1097/QAI.0b013e318221c56a. [DOI] [PubMed] [Google Scholar]
- 3.Whitmore SK, Zhang X, Taylor A, Blair JM. Estimated number of infants born to HIV-infected women in the United States and five dependent areas, 2006. J Acquir Immune Defic Syndr. 2011;57:218–222. doi: 10.1097/QAI.0b013e3182167dec. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Mother-to-child transmission of HIV data and statistics. [Accessed January 28, 2014]; Available at: http://www.who.int/hiv/topics/mtct/data/en/index1.html.
- 5.Ivanovic J, Nicastri E, Anceschi MM, et al. Transplacental transfer of antiretroviral drugs and newborn birth weight in HIV-infected pregnant women. Current HIV Research. 2009;7:620–625. doi: 10.2174/157016209789973628. [DOI] [PubMed] [Google Scholar]
- 6.Thorne C, Newell ML. Safety of agents used to prevent mother-to-child transmission of HIV: Is there any cause for concern? Drug Saf. 2007;30:203–213. doi: 10.2165/00002018-200730030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Divi RL, Leonard SL, Walker BL, et al. Erythrocebus patas monkey offspring exposed perinatally to NRTIs sustain skeletal muscle mitochondrial compromise at birth and at 1 year of age. Toxicol Sci. 2007;99:203–213. doi: 10.1093/toxsci/kfm143. [DOI] [PubMed] [Google Scholar]
- 8.Divi RL, Einem TL, Leonard-Fletcher SL, et al. Progressive mitochondrial compromise in brains and livers of primates exposed in utero to nucleoside reverse transcriptase inhibitors (NRTIs) Toxicol Sci. 2010;118(1):191–201. doi: 10.1093/toxsci/kfq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerschenson M, Nguyen V, Ewings EL, et al. Mitochondrial toxicity in fetal Erythrocebus patas monkeys exposed transplacentally to zidovudine plus lamivudine. AIDS Res Hum Retroviruses. 2004;20:91–100. doi: 10.1089/088922204322749530. [DOI] [PubMed] [Google Scholar]
- 10.Poirier MC, Olivero OA, Walker DM, Walker VE. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drug. Toxicol Appl Pharmacol. 2004;199:151–161. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Calamandrei G, Valanzano A, Puopolo MY, Aloe L. Developmental exposure to the antiretroviral drug zidovudine increases brain levels of brain-derived neurotrophic factor in mice. Neurosci Lett. 2002;333:111–114. doi: 10.1016/s0304-3940(02)01023-6. [DOI] [PubMed] [Google Scholar]
- 12.Fundaro C, Genovese O, Rendeli C, Tamburrini E, Salvaggio E. Myelomeningocele in a child with intrauterine exposure to efavirenz. AIDS. 2002;25:299–300. doi: 10.1097/00002030-200201250-00025. [DOI] [PubMed] [Google Scholar]
- 13.Rajlakshmi C, Bhattacharyya A, Trigunayat A, Pandey BL. Neurobehavioral profile of F1 and F2 generation mice following one stage zidovudine exposure through pregnancy and lactation. Annals of Neurosciences. 2008;15:69–74. [Google Scholar]
- 14.Venerosi A, Valanzano A, Puopolo M, Calamandrei G. Neurobehavioral effects of prenatal exposure to AZT: a preliminary investigation with the D1 receptor agonist SKF 38393 in mice. Neurotoxicol Teratol. 2005;27:169–173. doi: 10.1016/j.ntt.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Alimenti A, Forbes JC, Oberlander TF, et al. A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics. 2006;118:e1139–e1145. doi: 10.1542/peds.2006-0525. [DOI] [PubMed] [Google Scholar]
- 16.Culnane M, Fowler M, Lee SS, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. JAMA. 1999;281:151–157. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- 17.Kammerer B, Kacanek D, Mayondi G, et al. Pediatric neurodevelopmental functioning following in utero exposure to triple-NRTI-vs PI-based ART in a randomized trial, Botswana. XIX International AIDS Conference; Washington, D.C: Jul 22-27, 2012. abstract WEPE208. [Google Scholar]
- 18.Lindsey JC, Malee KM, Brouwers P, Hughes MD, PACTG 219C Study Team Neurodevelopmental functioning in HIV-infected infants and children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119:e681–693. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- 19.Williams PL, Marino M, Malee K, Brogly S, Hughes MD, Mofenson LM. PACTG 219C Team. Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics. 2010 Feb;125(2):e250–260. doi: 10.1542/peds.2009-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirois PA, Huo Y, Williams PL, et al. Pediatric HIV/AIDS Cohort Study. Safety of perinatal exposure to antiretroviral medications: Developmental outcomes in infants. Pediatr Infect Dis J. 2013;32:648–655. doi: 10.1097/INF.0b013e318284129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 22.Brogly SB, Ylitalo N, Mofenson LM, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 23.LeDoare K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130:e1326. doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- 24.Brackis-Cott E, Kang E, Dolezal C, Abrams E, Mellins CA. The impact of perinatal HIV infection on older school-aged children's and adolescents' receptive language and word recognition skills. AIDS Patient Care and STD's. 2009;23 doi: 10.1089/apc.2008.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith R, Chernoff M, Williams P, et al. Pediatric HIV/AIDS Cohort Study. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J. 2012;31:592–598. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciaranello AL, Seage GR, III, Freedberg KA, Weinstein MC, Lockman S, Walensky RP. Antiretroviral drugs for preventing mother-to-child transmission of HIV in sub-Saharan Africa: Balancing efficacy and infant toxicity. AIDS. 2008;22:2359–2369. doi: 10.1097/QAD.0b013e3283189bd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellinger D. Very low lead exposures and children's neurodevelopment. Current Opinion in Pediatrics. 2008;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- 29.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 30.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Future Child. 1997;7(2):55–71. [PubMed] [Google Scholar]
- 31.Wechsler Preschool and Primary Scales of Intelligence — Third Edition. San Antonio: The Psychological Corporation; 2002. [Google Scholar]
- 32.Wechsler Abbreviated Scale of Intelligence. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- 33.Wechsler Individual Achievement Test—Second Edition—Abbreviated. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- 34.Aidala A, Havens J, Mellins CA, et al. Development and validation of the Client Diagnostic Questionnaire (CDQ): A mental health screening tool for use in HIV/AIDS service settings. Psychol Health Med. 2004;9:362–379. [Google Scholar]
- 35.Powis KM, Kitch D, Ogwu A, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis. 2011;204:506–514. doi: 10.1093/infdis/jir307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice ML, Zeldow B, Siberry GK, et al. Pediatric HIV/AIDS Cohort Study Evaluation of risk for late language emergence after in utero antiretroviral drug exposure in HIV-exposed uninfected infants. Pediatr Infect Dis J. 2013 Oct;32(10):e406–13. doi: 10.1097/INF.0b013e31829b80ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohaugen G, Ostgard H, Martinussen M. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J Pediatr. 2013;163:447–453. doi: 10.1016/j.jpeds.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 38.McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 39.Breslau N, Chilcoat HD, Susser ES, et al. Stability and change in childrens intelligence quotient scores: a comparison of two socioeconomically disparate communities. Amer J of Epidem. 2011;154(8):711–717. doi: 10.1093/aje/154.8.711. [DOI] [PubMed] [Google Scholar]