Summary
The cause of elevated level of amyloid β-peptide (Aβ42) in common late-onset sporadic (AD) has not been established. Here we show that the membrane lipid peroxidation product 4-hydroxynonenal (HNE) is associated with amyloid and neurodegenerative pathologies in AD, and that it enhances γ-secretase activity and Aβ42 production in neurons. The γ-secretase substrate receptor, nicastrin was found to be modified by HNE in cultured neurons and in brain specimens from AD patients, in which HNE-nicastrin levels were found to be correlated with increased γ-secretase activity and Aβ plaque burden. Furthermore, HNE modification of nicastrin enhanced its binding to the γ-secretase substrate, amyloid precursor protein (APP) C99. In addition, the stimulation of γ-secretase activity and Aβ42 production by HNE were blocked by an HNE-scavenging histidine analog in a 3xTgAD mouse model of AD. These findings suggest a specific molecular mechanism by which oxidative stress increases Aβ42 production in AD, and identify HNE as a novel therapeutic target upstream of the γ-secretase cleavage of APP.
Keywords: Alzheimer’s disease, oxidative stress, amyloid, lipid peroxidation, γ-secretase, nicastrin
Introduction
Alzheimer’s disease (AD), the most common form of dementia, is characterized pathologically by the presence of large numbers of neuritic plaques and neurofibrillary tangles (Markesbery 1997a). The major protein component of these plaques is amyloid β-peptide (Aβ), a 40–42 amino acid protein derived from amyloid precursor protein (APP) through sequential proteolytic cleavages by β-secretase (BACE) and γ-secretase (Hardy & Selkoe 2002). Molecular genetic analyses have demonstrated that mutations in presenilin-1 (PS1) cause many cases of early-onset familial AD, and subsequent studies have established that PS1 is an essential enzymatic component of γ-secretase. Furthermore, mutations in PS1 have shown to increase the production of Aβ42 and the ratio of Aβ42/Aβ40, which is believed to cause synaptic dysfunction and neuronal death in AD (Mattson 2004). Three additional transmembrane proteins have been identified as components of the γ-secretase complex – nicastrin, Pen-2 and Aph-1; it has been reported that the ectodomain of nicastrin functions as a γ-secretase substrate receptor (Shah et al. 2005). However, although mutations in APP and presenilins can result in increased Aβ42 production and AD, the cause of the high accumulation of Aβ42 in the most common late-onset sporadic cases of AD has not been determined.
Aβ pathology has been associated with increased cellular oxidative stress as demonstrated by elevated levels of oxidatively modified proteins and lipids at sites of Aβ deposits in AD patients, transgenic mouse models of AD, and cultured neurons exposed to synthetic Aβ (McLellan et al. 2003; Murray et al. 2007; Sultana et al. 2009). Furthermore, it has been reported that lipid peroxidation precedes Aβ deposition in a mouse model of AD (Pratico et al. 2001), which suggests that oxidative stress plays a role in Aβ production and accumulation. In fact, the membrane lipid peroxidation product 4-hydoxynonenal (HNE) has been shown accumulate in the brain during normal aging and to be associated with AD pathology (Montine et al. 1997; Sayre et al. 1997; Cutler et al. 2004; Williams et al. 2006). The mechanism whereby lipid peroxidation damages neurons involves the aldehyde HNE, which is liberated from peroxidized membrane fatty acids. In addition, HNE can covalently modify proteins and may thereby alter their structures and functions. Furthermore, it has been suggested that HNE accumulates in membranes at concentrations of 10 µM to 5 mM in response to oxidative insults (Uchida 2003). HNE is known to modify the functions of several membrane-associated proteins in neurons including ion-motive ATPases, the neuronal glucose transporter GLUT3, the astrocyte glutamate transporter GLT-1, GTP-binding proteins, and tau (Uchida 2003). Moreover, the major Aβ degrading protease, neprilysin is modified by HNE in the AD brain and in neuronal cells (Wang et al. 2009).
Brain samples of AD patients homozygous for the apoE e4 allele exhibit greater HNE adduct immunoreactivity than those of AD patients with other apoE genotypes, which suggests that the capacities of apoE isoforms to detoxify HNE differ (Montine et al. 1997; Pedersen et al. 2000). However, it has not been determined whether or how HNE affects the amyloidogenic process in AD.
Results
The membrane lipid peroxidation product HNE increases γ-secretase activity and Aβ production
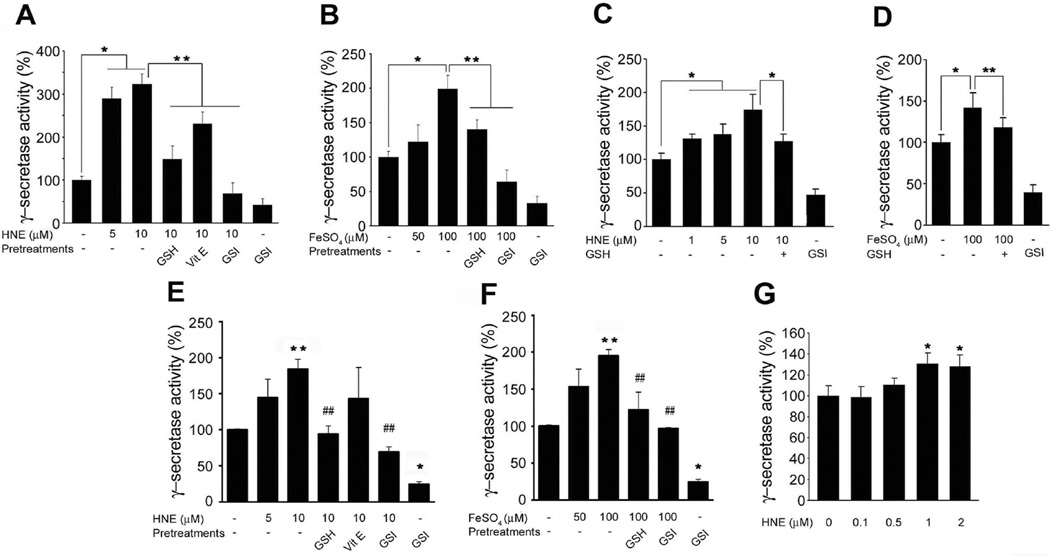
To determine if membrane-associated oxidative stress can affect γ-secretase activity, we treated primary cultured rat cerebral cortical neurons with HNE for 3 h and then measured γ-secretase activity. Neurons exposed to HNE at concentrations (of 1–10 µM), which have been previously reported to occur in AD and in experimental models of AD (Mark et al. 1997; McGrath et al. 2001), exhibited significantly greater γ-secretase activity than vehicle-treated control neurons (Fig. 1A–C). Iron (Fe2+), which induces membrane lipid peroxidation and HNE production, was also found to significantly increase γ-secretase activity. Furthermore, treatment of neurons with glutathione-ethyl ester (GSH), a cell permeant form of reduced glutathione that scavenges HNE (Kruman et al. 1997; Mark et al. 1997), largely prevented HNE- and Fe2+-induced increases in γ-secretase activity (Fig. 1A–C, D). Vitamin E, which inhibits membrane lipid peroxidation but does not directly interact with HNE, was found to be less effective than GSH at inhibiting the effect of HNE on γ-secretase activity. It was further observed that the γ-secretase inhibitor L-685,458 (GSI) significantly suppressed HNE and Fe2+-induced γ-secretase activities (Fig. 1A, D) suggesting a possible direct effect of HNE on γ-secretase protein.
Figure 1.
The lipid peroxidation product HNE enhances γ-secretase activity. Cultured rat cortical neurons (A and B), hippocampal neurons (C and D), and SH-SY5Y cells (E and F) were pre-incubated with 500 µM glutathione-ethyl ester (GSH) or 2 µM γ-secretase inhibitor (GSI) for 1 h, or with vitamin E (50 ng/ml) for 24 h before being treated with HNE. Cells were collected after 3 h of incubation with HNE or FeSO4. Lysates of primary cultured cortical (A and B) and hippocampal (C and D) neurons were tested for γ-secretase activity. Values are the mean ± S.D. of at least 3 independent experiments. *p < 0.01, **p < 0.05. (E and F) SH-SY5Y cells were transfected with the constructs of C99-GVP with UAS-luciferase reporter gene. Cells were collected after 3h of incubation with HNE or FeSO4. Values are the mean ± S.D. of at least 3 independent experiments. *p < 0.01, **p < 0.05 vs. controls. ##p < 0.05 vs. HNE or FeSO4 treated samples. (G) Total extracts of 2×108 SH-SY5Y cells were immunoprecipitated with anti-PS1 antibody. Immunoprecipitants were incubated with HNE at 37 °C for 30 min and analyzed for γ-secretase activity in vitro. Values are the mean ± S.D. of at least 3 independent experiments. *p < 0.01 vs. non-treated control.
In addition, HNE-induced γ-secretase activity was confirmed using a luciferase or green fluorescent protein (GFP) γ-secretase reporter assay. Exposure of SH-SY5Y cells to HNE, Fe2+ or Aβ42 was found to increase luciferase activity (Fig. 1E, F) and GFP fluorescence intensity (Supplementary Fig. S1), and pretreatment with GSH or GSI blocked these γ-secretase-dependent increases in GFP fluorescence. Because Aβ can induce membrane lipid peroxidation and HNE production in neurons (Mark et al. 1997), the ability of GSH to block Aβ42-induced γ-secretase activity suggests that Aβ may amplify the amyloidogenic processing of APP via HNE-mediated positive feedback activation of γ-secretase. No significant changes in cell viability were detected under these experimental conditions (Supplementary Fig. S1).
To explore the mechanistic basis for the enhancement of γ-secretase activity by HNE, γ-secretase complex was immunoprecipitated with anti-PS1-CTF antibody from SH-SY5Y cell lysates, and the isolated complex obtained was then incubated with HNE that at concentrations that have been reported in various biological tissues (0.1 to 5 µM). It was found that incubation of γ-secretase complex with 1 or 2 µM HNE for 30 min, significantly increased the activity of γ-secretase by 30% as compared with vehicle-treated controls, which demonstrated that HNE has a direct effect on γ-secretase complex (Fig. 1G).
The membrane lipid peroxidation product HNE increases Aβ42/Aβ40 ratio and AICD production
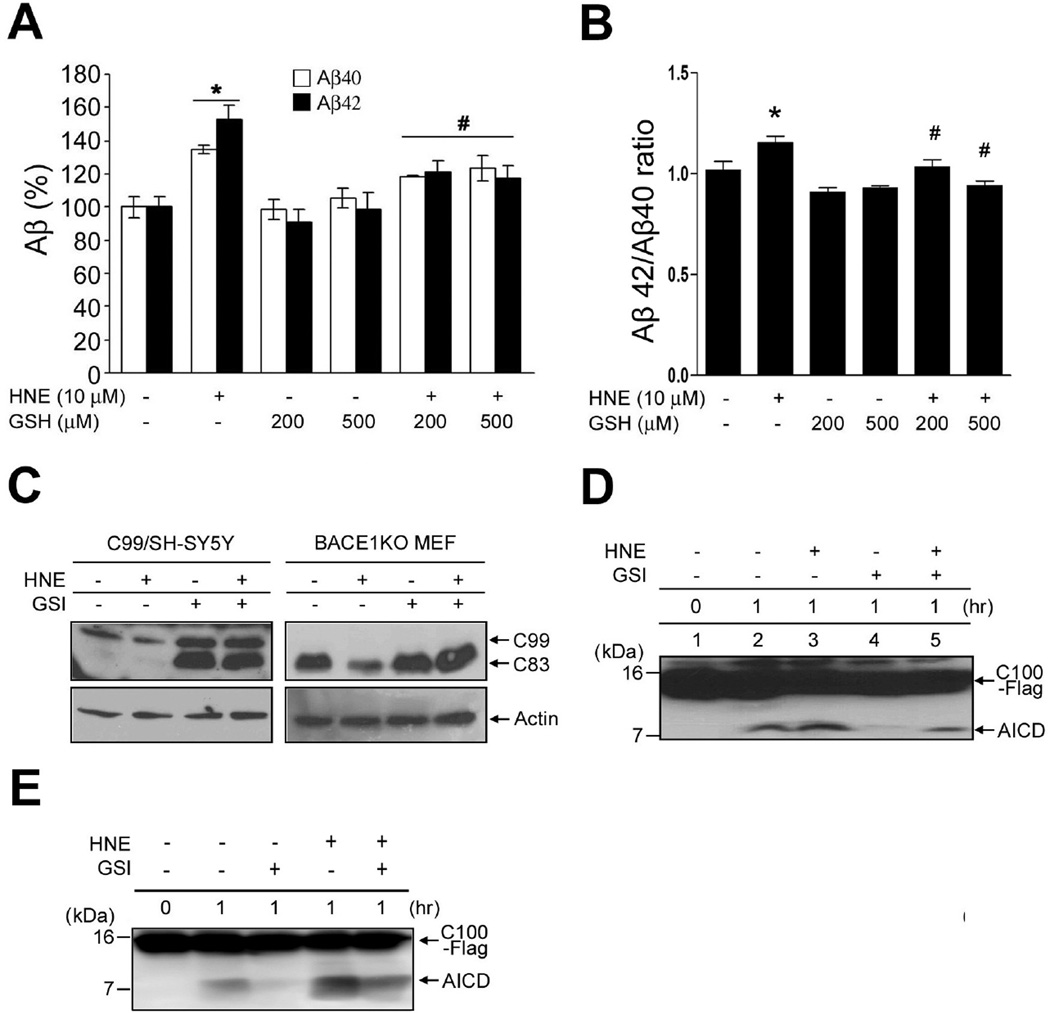
We next examined whether the stimulation of γ-secretase activity by HNE increases the ratio of Aβ42/Aβ40. It was found that HNE increased the production of both Aβ40, Aβ42 and ratio of Aβ42/Aβ40 in SH-SY5Y cells stably overexpressing the Swedish APP mutant, and that this was inhibited by GSH (Fig. 2A, B). It is also possible that oxidative stress-mediated Aβ production was caused by elevated β-secretase activity, rather than by increased γ-secretase activity (Jo et al. ; Tamagno et al. 2002). Therefore, the levels of C99 and C83 in BACE1-deficient cells were used to quantify γ-secretase activity. As expected, C99 levels were diminished by HNE treatment in cells overexpressing C99 (Fig. 2C, left panel), and in BACE1 knock-out mouse embryonic fibroblasts (BACE1KO MEF), HNE diminished C83 levels, which is the only APP-derived γ-secretase substrate in the β-secretase-deficient cells (Fig. 2C, right panel). Furthermore, treatment with a γ-secretase inhibitor blocked the ability of HNE to reduce C99 and C83 levels (Fig. 2C).
Figure 2.
The membrane lipid peroxidation product HNE increases Aβ42/Aβ40 ratio and AICD production. (A and B) HNE treating increased the amounts of secreted Aβ40, Aβ42 and Aβ42/Aβ40. After treating SH-SY5Y cells stably expressing mutant (Swedish) APP with HNE for 24 h, media were harvested and analyzed by sandwich ELISA for secreted Aβ40 (dark bars) and Aβ42 (light bars). In control cultures, the concentrations of Aβ40 and Aβ42 were 2067 ± 134 pg/ml protein and 848 ± 56 pg/ml, respectively (mean ± SD; n=3). *p < 0.01 vs. controls, #p < 0.01 vs. HNE-treated samples. (C) C99/SH-SY5Y cells (left panel) and BACE1KO MEF (right panel) were treated for 3 h with HNE (10 µM) in the presence or absence of the γ-secretase inhibitor DAPT (GSI; 1 µM). C99 and C83 levels were then analyzed using anti-APP-CTF antibody. (D and E) C100-Flag was incubated with the CHAPSO (D) or dodecyl-maltoside (E) solubilized lysate of SH-SY5Y cells at 37 °C; and the reaction was terminated at the indicated times by placing reaction tubes on ice. Reaction mixtures were separated in a 16% Tricine gel and subjected to immunoblotting for AICD using APP-CTF antibody.
The CHAPSO-solubilized γ-secretase assay system was employed to confirm that HNE increases γ-secretase activity (Li et al. 2000; Fraering et al. 2004). CHAPSO-solubilized γ-secretase was prepared from HNE (10 µM)- or vehicle-treated SH-SY5Y cells, and then incubated with the APP-based recombinant substrate C100-Flag. AICD production was found to be significantly increased by the solubilized γ-secretase from HNE-treated cells (Fig. 2D, lane 3) as compared with control cells (Fig. 2D, lane 2). In addition, AICD production was found to be completely blocked in GSI-treated membranes (Fig. 2D, lane 4), and GSI treatment was found to significantly reduce the production of AICD in the membranes of HNE-treated cells (Fig. 2D, lane 5). Next, the dodecyl-maltoside-solubilized γ-secretase assay system was employed to confirm that HNE increase γ-secretase activity (Fig. 2E).
HNE modifies nicastrin, one of the four γ-secretase proteins
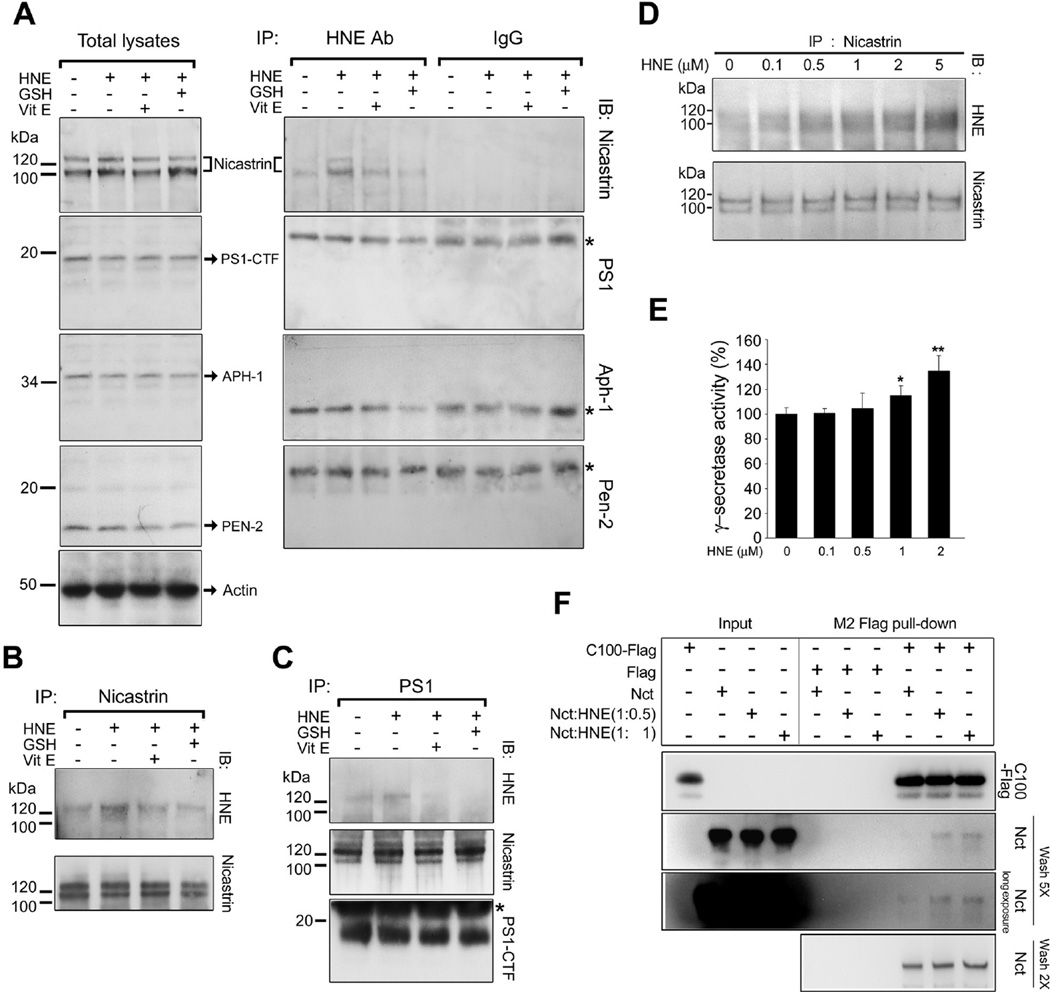
HNE can covalently modify cysteine, lysine, and the histidine residues of proteins by Michael addition (Uchida 2003). Futhermore, GSH (a tripeptide with a cysteine residue) scavenges HNE, and thereby prevents binding between HNE and cellular proteins. To determine whether γ-secretase components are susceptible to HNE modification, SH-SY5Y cells were exposed to HNE, and HNE-modified proteins were immunoprecipitated using a monoclonal antibody against HNE-modified proteins, and then immunoblotted using antibodies against nicastrin, PS1, Aph-1, or Pen-2. It was found that nicastrin was modified by HNE, but that PS1, Aph-1, and Pen-2 were not (Fig. 3A). Furthermore, the modification of nicastrin by HNE was markedly lower in cells pretreated with GSH. Nicastrin was then immunoprecipitated from HNE-treated cells and nicastrin-associated proteins were immunoblotted using an HNE antibody (Fig. 3B). It was found that HNE immunoreactivity was present in the expected band for nicastrin (~120 kDa) in HNE-treated cells but not in vehicle-treated cells. In addition, GSH or vitamin E pretreatment reduced the amount of HNE-modified nicastrin (Fig. 3B). PS1 was then immunoprecipitated from HNE-treated cells and it was found that HNE-modified nicastrin was present as a complex, which showed that nicastrin remains associated with γ-secretase complex when modified by HNE (Fig. 3C). Furthermore levels of PS1, nicastrin, Aph-1 and Pen-2 were found to be unchanged in cells exposed to HNE for 3 h (Fig. 3A), which suggested that HNE does not increase γ-secretase activity by increasing the levels of γ-secretase proteins. To confirm HNE modification of nicastrin in vitro, the γ-secretase complex was immunoprecipitated with nicastrin antibody from SH-SY5Y cell lysates, and the immunoprecipitate obtained was incubated for 30 min with increasing concentrations of HNE. Immunoblot analysis of the samples demonstrated that HNE modified nicastrin in a concentration-dependent manner (Fig. 3D). These HNE-treated samples were then examined for γ-secretase activity (Fig. 3E). Incubation with 1 or 2 µM HNE for 30 min was found to increase the activity of γ-secretase significantly as compared with vehicle-treated controls (Fig. 3E).
Figure 3.
Nicastrin is modified by HNE. (A) Left) SH-SY5Y cells were pre-incubated with GSH (500 µM) for 1 h before being treated with HNE (10 µM) or vitamin E (50 ng/ml), both for 24 h, before HNE treatment (3 h). The cell lysates (25 µg/lane) obtained were then immunoblotted using antibodies for the indicated-γ-secretase subunits. Right) Cell lysates (800 µg) in SDS-IP buffer were immunoprecipitated with an anti-HNE antibody and then subjected to immunoblot analysis using antibodies against nicastrin (Nct), PS1, Aph-1, or Pen-2 (* indicates the band corresponding to the light chain of anti-HNE antibody). (B) Cell lysates in SDS-IP buffer were immunoprecipitated with anti-nicastrin antibody and HNE-nicastrin conjugates were then detected using an antibody against HNE protein adducts. (C) HNE-modified nicastrin remained in the γ-secretase complex. Cell lysates in CHAPSO-IP buffer were co-immunoprecipitated with anti-PS1 antibody and then immunoblotted using antibodies against HNE-protein adducts or nicastrin. (D) Nicastrin was modified by HNE in vitro. Total extracts of 2×108 SH-SY5Y cells were immunoprecipitated with anti-nicastrin antibody. The immunopurified nicastrin obtained was incubated with increasing concentrations of HNE for 30 min at 37 °C. After incubation, samples were separated by SDS-PAGE, blotted, and lipid-protein conjugates and immunopurified nicastrin were detected using antibodies against HNE protein adducts or nicastrin. (E) Immunoprecipitants in (D) were incubated with HNE for 30 min at 37 °C and analyzed for γ-secretase activity. Values are the mean and ± SD of at least 3 independent experiments. *p < 0.01, **p < 0.05 vs. the controls. (F) The binding affinity of HNE-modified nicastrin for C100-Flag. To estimate the relative strength of binding between C100-Flag and non-modified and HNE-modified nicastrins, purified nicastrin ectodomain (Nct(ECD)) was preincubated with HNE at molar ratios of 1:0, 1:0.5 or 1:1 and then incubated with purified C100-Flag. After 5 washes, HNE-modified Nct(ECD) co-precipitated with C100-Flag. The addition of Flag peptide to this mixture prevented the precipitation.
It has been revealed that a nicastrin ectodomain binds γ-secretase substrates directly, which suggests that nicastrin is a receptor for γ-secretase substrates (Shah et al. 2005). Therefore, we decided to compare the γ-secretase substrate binding activities of non-modified nicastrin and HNE-modified nicastrin using highly purified proteins (Fig. 3F). Purified nicastrin ectodomain (Nct(ECD)) (Shah et al. 2005) was preincubated with HNE at 1:0, 1:0.5 or 1:1 (mol:mol) molar ratios and then incubated with purified C100-Flag, and a mixture of Nct(ECD) and C100-Flag was then subjected to immunoprecipitation with anti-Flag M2-Agarose. HNE-modified Nct(ECD) was found to show higher binding affinity with C100-Flag than unmodified Nct(ECD) after washing five times (Fig. 3F, second and third panels), whereas both HNE-modified and unmodified Nct(ECD) proteins were found to remain bound to C100-Flag after washing the precipitants twice (Fig. 3F, fourth panel). In the presence of Flag peptides, Nct(ECD) did not bind to C100-Flag (Fig. 3F).
The levels of γ-secretase activity and HNE-modified nicastrin were enhanced in sporadic AD brains
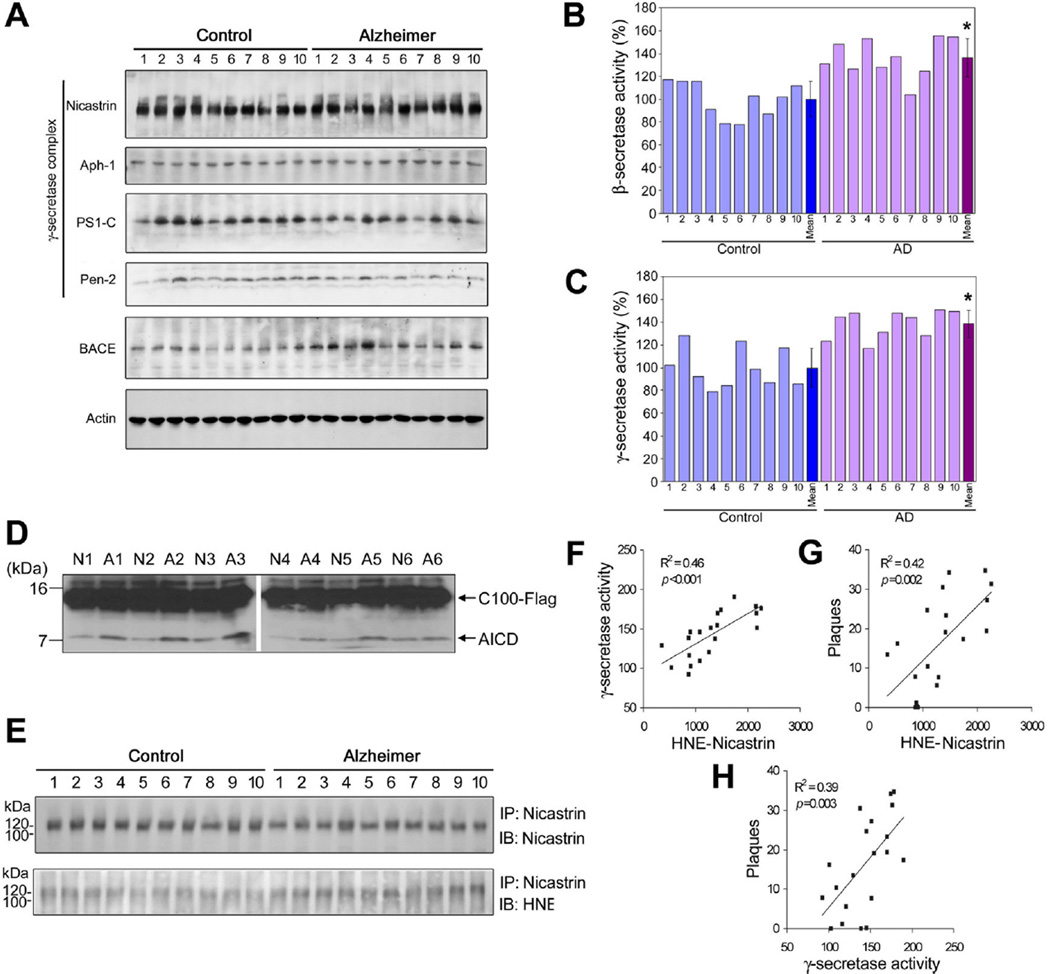
To determine whether HNE plays a role in amyloidogenesis in AD, levels of γ-secretase proteins, γ-secretase activity, and HNE modification of γ-secretase components were measured in rapidly autopsied (PMI=2.98 h ± 0.89) specimens from the inferior parietal lobule of AD patients and age-matched control subjects. The age, sex, times postmortem interval, and amyloid plaque counts are shown in Supplementary Table S1. Levels of nicastrin, PS1, Aph-1, and Pen-2 were no different in two specimen types (Fig. 4A), but BACE1 levels were greater in AD subjects (Fig. 4A), and these increases corresponded with increases in β-secretase activity (Fig. 4B), as has been previously reported (Li et al. 2004). In addition, a positive correlation was found between BACE1 protein levels and β-secretase enzymatic activities, and between plaque numbers and β-secretase activities (Supplementary Fig. S2). Furthermore, despite no change in γ-secretase protein levels, γ-secretase activity was significantly greater in samples from AD subjects (Fig. 3C). We then reexamined γ-secretase activities in human brain tissue samples using the CHAPSO-solubilized assay system. CHAPSO-solubilized γ-secretase was prepared from brain tissue samples of AD patients and neurologically normal control subjects, and then incubated with purified C100-Flag peptide. Considerably more AICD was found in the AD samples than non-demented samples (Fig. 4D). This latter observation is consistent with the result of the γ-secretase assay performed using a fluorogenic peptide substrate (Fig. 4C). In samples from cerebellum, a brain region with little or no amyloid pathology, no significant differences were found between the β-secretase and γ-secretase activities of AD patients and controls (Supplementary Fig. S3). To determine if levels of HNE-nicastrin adducts were increased in AD, nicastrin protein was immunoprecipitated from AD and control brain samples and immunoblotted using a HNE antibody (Fig. 4E). It was found that levels of HNE-modified nicastrin were greater in samples from AD subjects. Linear regression analysis revealed a significant positive correlation between levels of HNE-nicastrin adducts and γ-secretase activity in our samples (Fig. 4F). In addition, significant correlations were found between HNE-nicastrin levels and Aβ plaque numbers (Fig. 4G), and between γ-secretase activities and Aβ plaque numbers (Fig. 4H).
Figure 4.
HNE-modified nicastrin is associated with increased γ-secretase and BACE1 enzymatic activities in sporadic AD brain tissue samples. (A) Immunoblots showing relative levels of each protein in γ-secretase complex and BACE in samples from the inferior parietal lobule of AD and control subjects. Loaded protein levels were normalized versus β-actin. Experiments were performed at least three times. (B and C) BACE1 (B) and γ-secretase (C) enzymatic activities were evaluated using AD-enzymatic crude extracts incubated with fluorescent-labeled peptides bearing the β-site or γ-site of APP. BACE1 and γ-secretase enzymatic activities in AD brain extracts were normalized versus mean values in controls. BACE1 and γ-secretase activities were significantly greater in AD subjects (p < 0.0001). (D) C100-Flag was incubated with CHAPSO-solubilized membrane lysates of inferior parietal lobule specimens from the brains of AD patients (A1-6) and neurologically normal subjects (N1-6) at 37 °C; reactions were terminated after 1 h by placing reaction tubes on ice. Reaction mixtures were separated in 16% Tricine gel and immunoblotted for AICD. (E) Nicastrin was modified by HNE in the AD brain. Proteins in brain tissue samples from AD and control subjects were immunoprecipitated using antibodies against nicastrin, and then immunoblotted. (F) Linear regression analysis of γ-secretase activity versus HNE-nicastrin levels in inferior parietal lobes from clinically diagnosed and neuropathologically confirmed AD patients, and non-demented control subjects. (G) Linear regression analysis of HNE-nicastrin levels versus neuritic Aβ plaque numbers in AD and control brains. (H) Linear regression analysis of γ-secretase activities versus numbers of neuritic Aβ plaques.
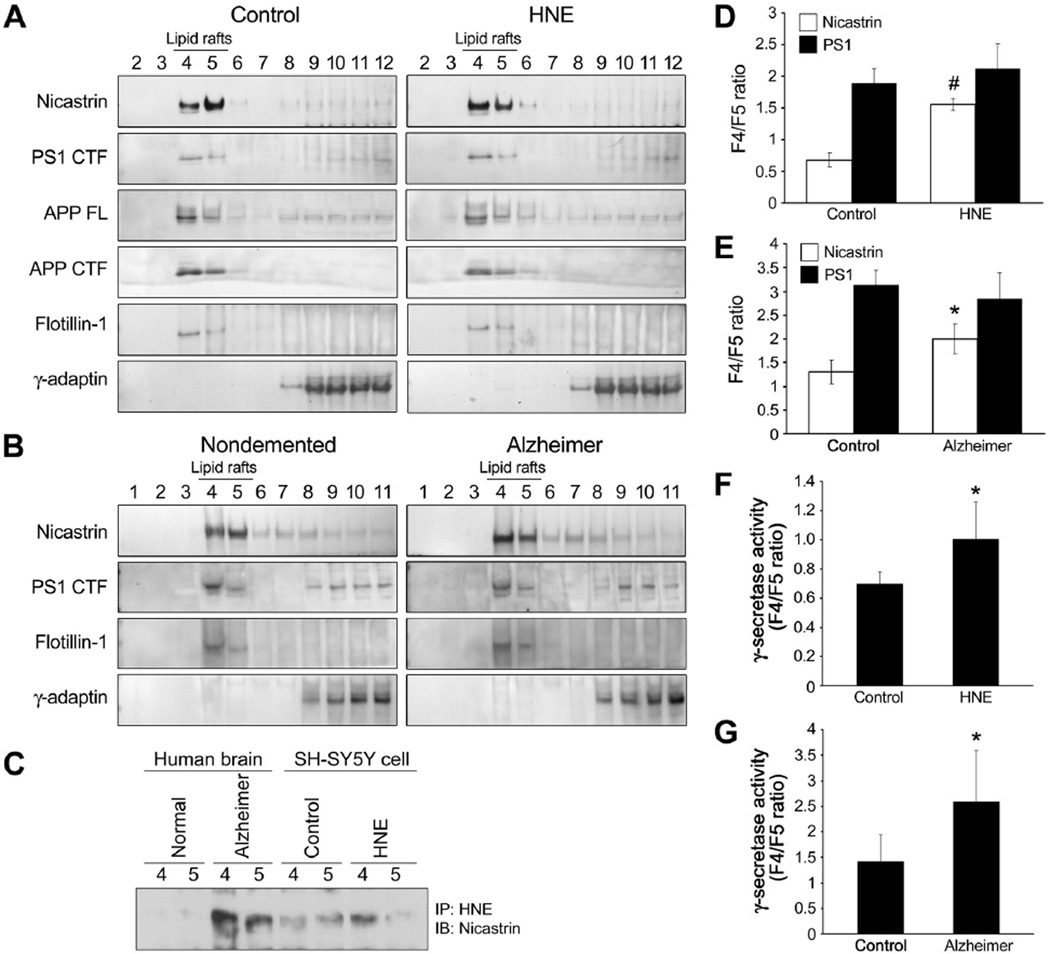
Nicastrin is redistributed to an APP-CTF enriched lipid raft fraction in response to HNE
APP processing is believed to occur in lipid rafts, regions of membrane rich in cholesterol and sphingomyelin, which contain all components of γ-secretase complex (Vetrivel et al. 2004; Grimm et al. 2005). To gain mechanistic insight of γ-secretase activity regulation by lipid peroxidation, we examined the distributions of APP, nicastrin, PS1, and γ-secretase activity in the lipid rafts of HNE-treated SH-SY5Y cells, and inferior parietal lobule specimens of AD patients and controls (Fig. 5). Two lipid raft fractions (4 and 5) contained the raft marker protein flotillin-1. In untreated cells the amounts of APP and PS1 were found to be greater in lipid raft fraction 4 than in lipid raft fraction 5, and this was unchanged in cells treated with HNE (Fig. 5A). In contrast, HNE was found to induce significant increase in the amount of nicastrin in fraction 4 and a corresponding decrease in the amount of nicastrin in fraction 5 (Fig. 5A, D). A similar enrichment of nicastrin in fraction 4 was observed in samples from AD patients but not in controls (Fig. 5B, E). The redistribution of HNE-modified nicastrin to fraction 4 in samples from AD brains and HNE-treated cells was then examined (Fig. 5C). Lipid raft fractions 4 and 5 were immunoprecipitated using antibody against HNE-modified proteins, and precipitated proteins were immunoblotted for nicastrin (Fig, 5C). It was found that nicastrin and HNE-modified nicastrin were elevated in lipid raft fraction 4 of HNE-treated cells and in AD brains (Fig. 5A–C, E), and that these increases were associated with commensurate increases in γ-secretase activity in fraction 4 (Fig. 5F, G). These results suggest that modification of nicastrin by HNE alters its location within the membrane in a manner that increases its associations with APP-CTF and PS1, and that these changes increase Aβ production. These results concur with our previous observation of increased binding affinity between HNE-modified Nct(ECD) and C100-Flag (Fig. 3F).
Figure 5.
Nicastrin is redistributed to an APP-enriched lipid raft fraction in AD brains and in response to direct exposure of neural cells to HNE. (A and B) Control and HNE-treated (10 µM for 3 h) SH-SY5Y cells (A), and AD and control inferior parietal lobes (B) were lysed in sodium carbonate buffer and subjected to flotation sucrose gradient centrifugation to isolate lipid rafts. Equal volumes of fractions were immunoblotted with antibodies against PS1 CTF, nicastrin, APP, APP CTF, flotillin-1 (a lipid raft marker), and γ-adaptin (a marker of clathrin-coated, non-raft membranes). (C) Lipid raft fractions (fractions 4 and 5) were immunoprecipitated using an antibody against HNE-modified proteins, and precipitated proteins were immunoblotted using anti-nicastrin antibody. (D and E) The signal intensities of PS1 CTF and nicastrin were quantified and fraction 4 versus fraction 5 (F4/F5) ratios were calculated for these proteins. The values shown are mean and S.D. (n=5; #p<0.01, *p<0.05 vs. controls). (F and G) γ-secretase activities in fractions 4 and 5, are reported as F4/F5 ratios. Values are the mean and S.D (n=5; *p<0.05 compared to control).
Administration of HNE-Scavenging Agent Reduces γ-Secretase Activity and Aβ42 Production in a Murine Model of AD
We reported that the histidine analogue AG/01 is highly effective at scavenging HNE, and that it significantly protects against focal ischemia-induced brain damage (Tang et al. 2007). Accordingly, we sought to determine whether AG/01 is effective at preventing HNE-mediated increases in γ-secretase activity, Aβ42 production, Aβ42/Aβ40 ratio and Nct modification. Indeed HNE-induced γ-secretase activity was found to be suppressed by AG/01 in primary cultured rat cortical neurons (Supplementary Fig. S4), and AG/01 was also found to inhibit HNE-induced Aβ42 production in SH-SY5Y cells stably overexpressing the Swedish APP mutant (Supplementary Fig. S4). We then employed a 3xTg-AD mouse model of AD (Oddo et al. 2003; Halagappa et al. 2007) to confirm the abilities of AG/01 in vivo. Mice were administered AG/01 intraperitoneally every other day, at 20 mg/kg for a month. It was found that the levels of brain γ-secretase activity, Aβ42 level, HNE-modified Nct, and Aβ42/Aβ40 ratio were significantly lower in 3xTgAD mice administered AG/01 than in vehicle-treated controls (Fig. 6).
Figure 6.
The histidine analogue histidyl hydrazide (AG/01) reduces the ratio of Aβ42/Aβ40, γ-secretase activity and HNE-modified Nct in the brains of AD mice. Seven month-old male 3xTg-AD mice were treated with AG/01 (20 mg/kg) or vehicle (PBS) intraperitoneally every other day for one month. (A–C) Aβ40 and Aβ42 levels, and the ratio of Aβ42/Aβ40 in the hippocampal and neocortical tissues of 3xTg-AD mice treated with PBS or AG/01. Values are the mean and S.D. (6 mice/group). **p < 0.01 vs. vehicle-treated mice. (D) Hippocampal and neocortical tissues were homogenized and γ-secretase activity was measured using fluorogenic substrates. The values shown are the means and S.D. (6 mice/group). *p < 0.05, **p < 0.01 vs. vehicle-treated mice. (E and F) Hippocampal and neocortical lysates were immunoprecipitated with anti-nicastrin antibody, and HNE-nicastrin conjugates were detected using an antibody against HNE-protein adducts. Signal intensities of HNE-Nct and total amounts of immunoprecipitated nicastrin were quantified and HNE-Nct/total Nct ratios were calculated. Values (panel F) are the mean and S.D. *p < 0.05 vs. vehicle-treated mice.
Discussion
The present study provides first evidence that amyloidogenic γ-secretase activity is increased in sporadic AD, and suggests a mechanism responsible for increased γ-secretase activity and Aβ42 production. We show that the membrane lipid peroxidation product HNE is associated with amyloid pathology and neuronal degeneration in AD and that it enhances γ-secretase activity and Aβ42 production in neurons. Nicastrin, one of the four γ-secretase proteins, found to be covalently modified by HNE in the cultured neurons and brain tissues of AD patients, in which HNE-nicastrin levels were found to be correlated with increased γ-secretase activities and Aβ levels. Furthermore, HNE modification of nicastrin increases its binding to the C99, a γ-secretase substrate. In addition, the results of our examination of lipid rafts suggests that modification of nicastrin by HNE alters its location within the membrane in a manner that increases its association with substrates of γ-secretase and PS1, and that these association result in increased Aβ production.
We also found that γ-secretase activity and Aβ production were significantly reduced by an HNE-scavenging histidine analog (AG/01) in the 3xTgAD mouse model of AD. We recognize that it is possible that GSH can attenuate the effects of HNE on γ-secretase activity and Aβ42 production by a mechanism in addition to directly binding HNE. Indeed, that is why we determined whether a different agent AG/01 that binds HNE also inhibits HNE-induced γ-secretase activity and Aβ42 production. Aside from binding HNE, the properties of AG/01 are dissimilar to those of GSH, and we therefore conclude that the abilities of both GSH and AG/01 to inhibit HNE-induced γ-secretase activity and Aβ42 production are indeed due ‘scavenging’ of HNE.
Interestingly, recent FRET based studies show that pathogenic changes in PS1/γ-secretase conformation occur in sporadic AD brain, and HNE can alter PS1/γ-secretase conformation in vitro towards the pathogenic state favoring production of Aβ42 (L. Wahlster and O. Berezovska, Harvard Medical School, USA, personal communication). These findings establish correlations between the HNE modification of nicastrin, γ-secretase activity, and amyloid deposition in AD, and when taken together with the finding that HNE modifies nicastrin and increases γ-secretase activity and Aβ production in cultured neurons, suggest a key role for lipid peroxidation and HNE in the amyloidogenic processing of APP in AD.
Increasing evidence suggests that oxidative stress in neurons precedes and accompanies the accumulation of Aβ in AD (Bonda et al.). Levels of HNE-lysine and HNE-histidine adducts increase progressively in the brain during normal aging (Cutler et al. 2004), and in association with Aβ deposition and neuronal degeneration in AD (Montine et al. 1997; Sayre et al. 1997). Furthermore, increased levels of oxidative stress, including membrane lipid peroxidation, have been detected prior to and during Aβ accumulation in transgenic mouse models of AD (Pratico et al. 2001; Yao et al. 2004) and in human subjects with AD and mild cognitive impairment (Nunomura et al. 2001; Pratico et al. 2002; Markesbery et al. 2005). In addition, manipulations known to reduce oxidative stress in brain, such as, caloric restriction and antioxidants, reduce Aβ production and accumulation in AD mice (Wang et al. 2005; Halagappa et al. 2007; Nishida et al. 2009). Our findings suggest a mechanism whereby oxidative stress increases Aβ production. In particular, HNE was found to increase γ-secretase activity and modify nicastrin in cultured neurons, and this modification was found to be correlated with increased γ-secretase activities and Aβ levels in AD brain tissue samples.
Oxidative stress is known to be associated with increased BACE1 expression and β-secretase activity (Jo et al. ; Tamagno et al. 2002), and recently, we found that γ-secretase regulates oxidative stress (HNE)-induced BACE1 expression in AD (Jo et al. ; Tamagno et al. 2008). In addition, we found that ischemic and hypoxic conditions (Arumugam et al. 2006) and oxidative stress (HNE) increase γ-secretase activity, which further supports the idea that BACE1 induction by oxidative stress is mediated by γ-secretase. The increased Aβ production observed under conditions of oxidative stress may therefore result from a combination of elevated activities of β- and γ-secretase, and the generation of Aβ42 as the result of the HNE-induced γ-secretase cleavage of APP may be of particular importance to the pathophysiology of AD because of the neurotoxic actions of Aβ42 (Markesbery 1997a; Mattson 2004).
Aβ42 can induce membrane lipid peroxidation in neurons, and this can result in synaptic dysfunction and neuronal death by HNE-mediated mechanisms (Markesbery 1997a; Markesbery 1997b). The findings of the present study therefore suggest a scenario for the pathogenesis of AD in which membrane lipid peroxidation increases Aβ42 production, which, in turn, induces further lipid peroxidation resulting in extensive localized Aβ deposition and associated synaptic dysfunction. An understanding of the molecular processes involved in the production and accumulation of Aβ would accelerate the developments of treatments that prevent neuronal dysfunction and death. In fact, inhibitors of γ-secretase or β-secretase have been developed that can inhibit Aβ production, but γ-secretase cleaves several different protein substrates, including Notch, which suggests that γ-secretase inhibitors are likely to have serious side effects (Pollack & Lewis 2005). Importantly, we found that treatment of cultured neurons and 3xTgAD mice with the HNE scavenger AG/01 reduced γ-secretase activity and Aβ42 production. These observations provide direct evidence of a role for HNE in the amyloidogenic processing of APP in vivo. Our findings suggest that HNE can be targeted for therapeutic intervention upstream of Aβ42 production.
Experimental Procedures
Cell culture and experimental treatments
Human SH-SY5Y, Swedish APP mutant expressing SH-SY5Y (swAPP/SH-SY5Y; kindly provided by W. Araki), and APP-C99 expressing SH-SY5Y (C99/SH-SY5Y; kindly provided by T. Hartmann) neuroblastoma cells were cultured in Dulbecco’s minimum Eagle’s medium (DMEM) (GIBCO) supplemented with 10% fetal bovine serum (FBS) (GIBCO) and 1% penicillin/streptomycin (GIBCO). Cells were maintained at 37 °C in a humidified incubator under 95% air and 5% CO2. Primary dissociated cell cultures of hippocampal and cortical neurons were prepared from 18-day-old rat embryos as previously described (Mattson et al. 1995). 4-Hydroxy-2,3-nonenal (HNE) (Cayman Chemical) was prepared as a 1000× stock in ethanol; glutathione ethyl ester (Sigma) as a 50× stock in water; FeSO4 as a 100× stock in water; and vitamine E (α-tocopherol) as a 500× stock in ethanol. In all experiments, an equivalent volume of vehicle was added to control cultures.
Fluorometric detection of of β- and γ-secretases activities
The activities of β- and γ-secretases in primary cultured neurons were determined using commercially available secretase kits (R&D Systems). The fluorometric detection of activities of β- and γ-secretases in human brain tissues was performed as previously described (Farmery et al. 2003).
Luciferase or GFP-based γ-secretase reporter assay
UAS-reponsive reporter gene construct MH100 and C99-GVP plasmid have been described previously (Karlstrom et al. 2002). The cleavage of C99-GVP by γ-secretase releases the transcription factor that activates luciferase expression, providing a quantitative measurement of γ-secretase activity. Transfections for GFP-based γ-secretase reporter assays were carried out in 6-well culture plates as previously described (Jo et al. 2005).
Membrane preparations and immunoprecipitation
Co-immunoprecipitation assays were performed as described previously (Farmery et al. 2003), except Figures 2A and 2B. Detailed methods are included in the Supporting Information.
Preparation of membrane fractions and γ-secretase assays
CHAPSO-solubilized γ-secretase preparations (Li et al. 2000; Fraering et al. 2004) and dodecyl-maltoside-solubilized γ-secretase preparations (Wakabayashi et al. 2009) were prepared as previously described. Detailed methods are included in the Supporting Information.
HNE modification and in vitro binding assay
Recombinant APP derived γ-secretase substrate C100-Flag was prepared as described previously (Li et al. 2000), except that it was purified by M2 Flag chromatography (Sigma). Briefly, purified Nct(ECD) was incubated with HNE at Nct(ECD):HNE molar ratios of 1:0, 1:0.5, and 1:1 for 2 h at 37°C. HNE-treated Nct(ECD) were incubated at room temperature for an additional 1 h with NaBH4 (final concentration 5 mM), and then desalted using a Microcon filter. HNE treated Nct(ECD) was incubated with either C99-Flag or Flag peptide (Sigma) in binding buffer (1% Triton X-100, 1mM EDTA (pH7.5), 10mM Tris-HCl (pH7.5), 150 mM NaCl) at 4°C for 6 h. M2 Flag Sepharose was then added for 2 h. Samples were then centrifuged for three minutes and the pellets washed 5 times with binding buffer. The pellets were incubated for 10 minutes at 90°C in a buffer containing 10% DTT and 20% of LDS sample buffer (Invitrogen), centrifuged for three minutes, and supernatants were loaded onto Bis-Tris 4–15% SDS-polyacrylamide gels.
Antibodies
HNE-modified proteins were immunoprecipitated using mouse monoclonal antibodies (clone 1G4 and 1H4) that specifically recognize proteins covalently modified by HNE (Waeg et al. 1996). Anti-APP polyclonal antibody (AB5300), and anti-PS1 CTF (MAB5232) and BACE1 (MAB5308) monoclonal antibodies were purchased from Chemicon; rabbit anti-nicastrin (N1660) and anti-APP C-terminal polyclonal antibodies were purchased from Sigma; monoclonal anti-nicastrin (612290) and rabbit anti-HNE-Michael adducts antibodies (393207) were purchased from Calbiochem; anti-BACE1 polyclonal antibody was purchased from ProSci; anti-Aph-1 antibody H2-D2 and anti-Pen-2 antibodies were kindly provided by G. Yu and G. Thinakaran, respectively. Antibodies against γ-adaptin and flotillin-1 were from BD Transduction Laboratories.
Aβ40 and Aβ42 quantitative assays
Quantitative ELISA analyses of secreted Aβ40 and Aβ42 were performed as previously described with slight modification (Jo et al. 2001).
Animals and treatment with the histidine analogue histidyl hydrazide (AG/01)
3xTgAD mice (swAPP, PS1-M146V, tau-P301L) that had been backcrossed to C57BL/6 mice for 8 generations were maintained in our animal facility under pathogen-free conditions on a 12 hr light/12 hr dark cycle with continuous access to food and water. Seven month-old male mice were treated with AG/01 (20 mg/kg, intraperitoneally), or vehicle (PBS) every other day for a month. Animals were then euthanized and brains were removed for processing. All procedures were approved by the NIA Animal Care and Use Committee.
Supplementary Material
Acknowledgements
We thank G. Yu for his valuable comments during the preparation of the manuscript. This research was supported by the Intramural Research Program of the National Institute on Aging, by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0000593), and Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A092042).
Footnotes
Author contributions
A.R.G., J.S.P, T.V.A., D.G.J, and M.P.M designed the project. A.R.G., J.S.P., T.V.A., Y.K.K., S.L.C., S.H.B., S.Y., Y.K.Y., Y.C., S.K., B.K., S.C.T., D.H.H., A.C., and M.B. performed research. S.H.K., J.L., K.K., C.E.D., Y.K.J., and W.R.M. contributed new reagents/analytic tools. A.R.G., J.S.P., T.V.A., M.P.M., and D.G.J. analyzed data. M.P.M and D.G.J. wrote the manuscript.
References
- Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmery MR, Tjernberg LO, Pursglove SE, Bergman A, Winblad B, Naslund J. Partial purification and characterization of gamma-secretase from post-mortem human brain. J Biol Chem. 2003;278:24277–24284. doi: 10.1074/jbc.M211992200. [DOI] [PubMed] [Google Scholar]
- Fraering PC, Ye W, Strub JM, Dolios G, LaVoie MJ, Ostaszewski BL, van Dorsselaer A, Wang R, Selkoe DJ, Wolfe MS. Purification and characterization of the human gamma-secretase complex. Biochemistry. 2004;43:9774–9789. doi: 10.1021/bi0494976. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Jo DG, Arumugam TV, Woo HN, Park JS, Tang SC, Mughal M, Hyun DH, Park JH, Choi YH, Gwon AR, Camandola S, Cheng A, Cai H, Song W, Markesbery WR, Mattson MP. Evidence that gamma-secretase mediates oxidative stress-induced beta-secretase expression in Alzheimer's disease. Neurobiol Aging. 2010;31:917–925. doi: 10.1016/j.neurobiolaging.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo DG, Jang J, Kim BJ, Lundkvist J, Jung YK. Overexpression of calsenilin enhances gamma-secretase activity. Neurosci Lett. 2005;378:59–64. doi: 10.1016/j.neulet.2004.12.078. [DOI] [PubMed] [Google Scholar]
- Jo DG, Kim MJ, Choi YH, Kim IK, Song YH, Woo HN, Chung CW, Jung YK. Pro-apoptotic function of calsenilin/DREAM/KChIP3. FASEB J. 2001;15:589–591. doi: 10.1096/fj.00-0541fje. [DOI] [PubMed] [Google Scholar]
- Karlstrom H, Bergman A, Lendahl U, Naslund J, Lundkvist J. A sensitive and quantitative assay for measuring cleavage of presenilin substrates. J Biol Chem. 2002;277:6763–6766. doi: 10.1074/jbc.C100649200. [DOI] [PubMed] [Google Scholar]
- Kruman I, Bruce-Keller AJ, Bredesen D, Waeg G, Mattson MP. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci. 1997;17:5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, Wong P, Price D, Shen Y. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer's disease patients. Proc Natl Acad Sci U S A. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Lai MT, Xu M, Huang Q, DiMuzio-Mower J, Sardana MK, Shi XP, Yin KC, Shafer JA, Gardell SJ. Presenilin 1 is linked with gamma-secretase activity in the detergent solubilized state. Proc Natl Acad Sci U S A. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Neuropathological criteria for the diagnosis of Alzheimer's disease. Neurobiol Aging. 1997a;18:S13–S19. doi: 10.1016/s0197-4580(97)00064-x. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997b;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann Neurol. 2005;58:730–735. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Barger SW, Begley JG, Mark RJ. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- McGrath LT, McGleenon BM, Brennan S, McColl D, Mc IS, Passmore AP. Increased oxidative stress in Alzheimer's disease as assessed with 4-hydroxynonenal but not malondialdehyde. QJM. 2001;94:485–490. doi: 10.1093/qjmed/94.9.485. [DOI] [PubMed] [Google Scholar]
- McLellan ME, Kajdasz ST, Hyman BT, Bacskai BJ. In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J Neurosci. 2003;23:2212–2217. doi: 10.1523/JNEUROSCI.23-06-02212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine KS, Olson SJ, Amarnath V, Whetsell WO, Jr, Graham DG, Montine TJ. Immunohistochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer's disease is associated with inheritance of APOE4. Am J Pathol. 1997;150:437–443. [PMC free article] [PubMed] [Google Scholar]
- Murray IV, Liu L, Komatsu H, Uryu K, Xiao G, Lawson JA, Axelsen PH. Membrane-mediated amyloidogenesis and the promotion of oxidative lipid damage by amyloid beta proteins. J Biol Chem. 2007;282:9335–9345. doi: 10.1074/jbc.M608589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y, Ito S, Ohtsuki S, Yamamoto N, Takahashi T, Iwata N, Jishage K, Yamada H, Sasaguri H, Yokota S, Piao W, Tomimitsu H, Saido TC, Yanagisawa K, Terasaki T, Mizusawa H, Yokota T. Depletion of vitamin E increases amyloid beta accumulation by decreasing its clearances from brain and blood in a mouse model of Alzheimer disease. J Biol Chem. 2009;284:33400–33408. doi: 10.1074/jbc.M109.054056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Chan SL, Mattson MP. A mechanism for the neuroprotective effect of apolipoprotein E: isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 2000;74:1426–1433. doi: 10.1046/j.1471-4159.2000.0741426.x. [DOI] [PubMed] [Google Scholar]
- Pollack SJ, Lewis H. Secretase inhibitors for Alzheimer's disease: challenges of a promiscuous protease. Curr Opin Investig Drugs. 2005;6:35–47. [PubMed] [Google Scholar]
- Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Butterfield DA. Oxidatively modified proteins in Alzheimer's disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis. Acta Neuropathol. 2009;118:131–150. doi: 10.1007/s00401-009-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo DG, Mattson MP, Tabaton M. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104:683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Cutler RG, Jo DG, Magnus T, Chan SL, Mughal MR, Telljohann RS, Nassar M, Ouyang X, Calderan A, Ruzza P, Guiotto A, Mattson MP. Neuroprotective actions of a histidine analogue in models of ischemic stroke. J Neurochem. 2007;101:729–736. doi: 10.1111/j.1471-4159.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeg G, Dimsity G, Esterbauer H. Monoclonal antibodies for detection of 4-hydroxynonenal modified proteins. Free Radic Res. 1996;25:149–159. doi: 10.3109/10715769609149920. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Craessaerts K, Bammens L, Bentahir M, Borgions F, Herdewijn P, Staes A, Timmerman E, Vandekerckhove J, Rubinstein E, Boucheix C, Gevaert K, De Strooper B. Analysis of the gamma-secretase interactome and validation of its association with tetraspanin-enriched microdomains. Nat Cell Biol. 2009;11:1340–1346. doi: 10.1038/ncb1978. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wang R, Wang S, Malter JS, Wang DS. Effects of HNE-modification induced by Abeta on neprilysin expression and activity in SH-SY5Y cells. J Neurochem. 2009;108:1072–1082. doi: 10.1111/j.1471-4159.2008.05855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer's disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yao J, Petanceska SS, Montine TJ, Holtzman DM, Schmidt SD, Parker CA, Callahan MJ, Lipinski WJ, Bisgaier CL, Turner BA, Nixon RA, Martins RN, Ouimet C, Smith JD, Davies P, Laska E, Ehrlich ME, Walker LC, Mathews PM, Gandy S. Aging, gender and APOE isotype modulate metabolism of Alzheimer's Abeta peptides and F-isoprostanes in the absence of detectable amyloid deposits. J Neurochem. 2004;90:1011–1018. doi: 10.1111/j.1471-4159.2004.02532.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.