Abstract
Laribacter hongkongensis is a gram-negative emerging bacterium associated with invasive bacteremic infections in patients with liver disease and fish-borne community-acquired gastroenteritis and traveler's diarrhea. Although the complete genome of L. hongkongensis has been sequenced, no animal model is available for further study of its pathogenicity mechanisms. In this study, we showed that adult zebrafish infected with L. hongkongensis by immersion following dermal abrasion or intraperitoneal injection suffered mortality in a dose-dependent manner, with lethal dose 50 (LD50) of 2.1×104 and 1.9×104 colony-forming units (CFU)/mL, respectively. All mortalities occurred in the first four days post-infection. Zebrafish that died showed characteristic clinicopathological features: swimming near water surface, marked lethargy and sidestroke; abdominal hemorrhage, ulcers and marked swelling with ascites; and hydropic degeneration and necrosis of hepatocytes around central vein and inflammatory cells infiltration. L. hongkongensis was recovered from the ascitic fluid and tissues of zebrafish that died. Of the 30 zebrafish infected with 2.1×104 CFU/mL (LD50) L. hongkongensis isolated from dead zebrafish using the immersion following dermal abrasion method, 18 (60%) died. All zebrafish that died also showed the characteristic clinical and pathological features. Histopathological studies also showed dilation of hepatic central vein and hydropic degeneration. L. hongkongensis was isolated from the zebrafish that died. The Koch's postulates for L. hongkongensis as an infectious agent have been fulfilled. This highly reproducible and effective zebrafish model is of crucial importance for future studies on virulence factors for L. hongkongensis infection.
Keywords: animal model, infection, Laribacter hongkongensis
INTRODUCTION
Laribacter hongkongensis is a facultative anaerobic gram-negative bacterium of the Neisseriaceae family.1 The bacterium was initially discovered from the blood and thoracic empyema pus cultures in a patient with alcoholic liver cirrhosis.2 Subsequently, it was also isolated from the blood culture of another patient with Wilson's disease and liver cirrhosis.3 Recently, the first fatal case associated with L. hongkongensis infection in a patient with colonic carcinoma with liver metastasis has been described.4 In addition to invasive blood stream infections, L. hongkongensis is also isolated from fecal samples of patients with community-acquired gastroenteritis and traveler's diarrhea and is found to be associated with these clinical conditions.5,6,7,8,9,10 Travel histories from patients suggested that L. hongkongensis is globally distributed.2,3,5,6,7,11 L. hongkongensis is most abundantly isolated from the intestinal samples of commonly consumed freshwater fish of the carp family, such as grass carps and big head carps.6,12,13,14,15,16 Moreover, it has also been found in drinking water reservoirs, Chinese tiger frogs and little egrets.14,15,17,18,19 Molecular typing experiments by pulsed-field gel electrophoresis and multilocus sequence analysis revealed that the L. hongkongensis strains isolated from patients and fish formed different clusters, which suggested that some clones may be more virulent or better adapted to the intestinal environment of human.12,20 Although the complete genome of L. hongkongensis has been sequenced21 and its acid resistance mechanisms revealed recently,22 currently there is no animal model available for further study of its pathogenicity and virulence mechanisms.
Zebrafish has been established as an animal model for the study of a variety of pathogenic bacteria, such as Mycobacterium tuberculosis, Mycobacterium marinum, Staphylococcus aureus, Streptococcus agalactiae and Salmonella Typhimurium.23,24,25,26,27 Since L. hongkongensis is abundantly found in freshwater fish of the carp family and zebrafish is also a carp, we hypothesized that zebrafish could be used as an animal model for L. hongkongensis infection. In this article, we report the results on the use of different routes of infection and the associated clinical features and histopathological changes in using an adult zebrafish model for L. hongkongensis infection.
MATERIALS AND METHODS
Ethical statement
All experimental protocols and animals used in this research were approved by the Ethical Committee of Southern Medical University (2013-062).
Bacterial strain and culture conditions
Wild-type L. hongkongensis HLHK9, a clinical strain isolated from a patient with severe gastroenteritis in Hong Kong, for which the complete genome has been sequenced,21 was used in all experiments. L. hongkongensis was grown in tryptic soy broth overnight at 37 °C with shaking. Overnight culture was diluted 1∶10 in tryptic soy broth and was further incubated at 37 °C with shaking (200 rpm) until logarithmic phase (OD600 nm of 1.0). Bacterial cells were washed with phosphate-buffered saline (PBS) three times before infection. Escherichia coli ATCC11775 was used as control and was cultured and prepared in the same way as L. hongkongensis.
Zebrafish care and maintenance
Healthy wild-type adult zebrafish were kindly provided by Professor Wen-Qing Zhang, Southern Medical University, Guangzhou, China. Their body lengths were 2.7±0.3 cm and weights were 0.3–0.4 g. The zebrafish were reared in water circulation and aeration system with the water temperature maintained at 28±1 °C with a flow rate of 150 L/min, as described in the Westerfield method.28 All experiments were commenced only when the mortality rate of zebrafish was <2% for at least one week. Feeding was stopped 24 h before all experiments.
Zebrafish infection by L. hongkongensis
Zebrafish were infected by L. hongkongensis using three different methods with E. coli and PBS as controls. All experiments were performed in triplicate. Lethal dose 50 (LD50) was calculated by the improved Karber's method in IBM SPSS Statistics version 19.
Exposure by immersion
Five groups of zebrafish (20 per group) were immersed in L. hongkongensis at 2.4×104 colony-forming units (CFU)/mL, 2.4×105 CFU/mL and 2.4×106 CFU/mL, E. coli at 2.4×106 CFU/mL, or PBS respectively for 5 h. The five groups were then kept in five separate 3-L aquaria and observed for 21 days.
Exposure by immersion following dermal abrasion
Nine groups of zebrafish (20 per group) were anesthetized in 160 µg/mL tricaine (MS-222). Each anesthetized zebrafish was lightly scraped along the lateral surface behind the pectoral fins with a sterile scalpel to remove several scales and scratch the underlying dermis with a technique described by Neely et al.29,30 After recovery from anesthesia, the nine groups of zebrafish were immersed in L. hongkongensis at 2.4×102 CFU/mL, 2.4×103 CFU/mL, 2.4×104 CFU/mL, 2.4×105 CFU/mL, 2.4×106 CFU/mL and 2.4×107 CFU/mL, E. coli at 2.4×106 CFU/mL and 2.4×104 CFU/mL, or PBS respectively for 10 min. The nine groups were then kept in nine separate 3-L aquaria and observed for 21 days.
Exposure by intraperitoneal injection
Nine groups of zebrafish (20 per group) were anesthetized in 160 µg/mL tricaine (MS-222). After anesthetization, the zebrafish of the nine groups were injected intraperitoneally with L. hongkongensis at 2.4×102 CFU/mL, 2.4×103 CFU/mL, 2.4×104 CFU/mL, 2.4×105 CFU/mL, 2.4×106 CFU/mL and 2.4×107 CFU/mL, E. coli at 2.4×106 CFU/mL and 2.4×104 CFU/mL, or PBS respectively. The nine groups were then kept in nine separate 3-L aquaria and observed for 21 days.
Histological studies
Two zebrafish from each group were sampled for histological studies at their deaths. The zebrafish were dissected into four parts: head, chest/abdomen, hip and tail and each part was fixed in 10 mL of neutral buffered 10% formalin. After 24 h, the fixative was replaced with 10 mL of fresh buffered 10% (v/v) formalin and the fixed samples were stored at 4 °C and embedded in paraffin. Paraffin-embedded sections were stained with hematoxylin–eosin and were examined using a Nikon Eclipse TE2000-U inverted microscope.
Recovery and identification of L. hongkongensis
Two zebrafish from each group were sampled for recovery of L. hongkongensis at their deaths. Ascitic fluid was aspirated and the wounds, livers and pancreas were separately homogenized in 10 mL of tryptic soy broth using a stomacher (Lab Blender 400; Tekmar, Clicinnati, OH, USA). The ascitic fluid and homogenized tissues were plated on cefoperazone MacConkey agar and incubated at 37 °C for 24 h.31 For zebrafish that survived, the livers and pancreas of two zebrafish from each group were also sampled for recovery of L. hongkongensis at day 21 using the method as described above.
Suspected bacterial isolates were identified phenotypically by standard conventional biochemical methods.2,6 Isolates suspected to be L. hongkongensis were subject to partial 16S rRNA gene amplification using L. hongkongensis-specific primers designed using the Primer 3 program.32 For bacterial DNA extraction, 80 µL of NaOH (0.05 M) was added to 20 µL of bacterial cells suspended in distilled water and the mixture was incubated at 60 °C for 45 min, followed by addition of 6 µL of Tris-HCl (pH 7.0), achieving a final pH of 8.0. The resultant mixture was diluted ×100 and 5 µL of the diluted extract was used for polymerase chain reaction (PCR).
For PCR amplification, DNase I-treated distilled water and PCR master mix (which contains deoxynucleoside triphosphates, PCR buffer, and Taq polymerase) were used in all PCR reactions by adding 1 U of DNase I (Invitrogen, Grand Island, New York, USA) to 40 µL of distilled water or PCR master mix, incubating the mixture at 25 °C for 15 min and subsequently at 95 °C for 10 min to inactivate the DNase I. The bacterial DNA extract and control were amplified with 0.5 µM primers (5′-TTG AGG GTG CCC GAA AGG GA-3′ and 5′-CTA CCC ACT TCT GGC GGA TT-3′) (Gibco BRL, Rockville, MD, USA). The PCR mixture (50 µL) contained bacterial DNA, PCR buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, 2 mM MgCl2 and 0.01% gelatin), 200 µM of each deoxynucleoside triphosphates and 1.0 U Taq polymerase (Boehringer, Mannheim, Germany). The mixtures were amplified in 30 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, and a final extension at 72 °C for 5 min in an automated thermal cycler (Perkin-Elmer Cetus, Gouda, The Netherlands). DNase I-treated distilled water was used as the negative control. 10 µL of each amplified product was electrophoresed in 1.0% (w/v) agarose gel, with a molecular size marker (DL1000 DNA Marker; TaKaRa Biotechnology, Dalian, China) in parallel. Electrophoresis in Tris-borate-EDTA buffer was performed at 100 V for 1.5 h. The gel was stained with ethidium bromide (0.5 µg/mL) for 15 min, rinsed and photographed under ultraviolet light illumination.
Infection of zebrafish using L. hongkongensis isolated from dead zebrafish
An isolate of L. hongkongensis recovered from the liver of a zebrafish infected by immersion following dermal abrasion was cultured using the method as described above. Two groups of zebrafish (30 per group) were infected with an LD50 of this L. hongkongensis isolate or PBS using the immersion following dermal abrasion method as described above. Histological studies and recovery and identification of L. hongkongensis were performed as described above.
RESULTS
Zebrafish infection by L. hongkongensis
Exposure by immersion
No mortality was observed in all three groups of zebrafish infected with the three different doses of L. hongkongensis and the control groups.
Exposure by immersion following dermal abrasion
Zebrafish infected with L. hongkongensis by immersion following dermal abrasion suffered mortality in a dose-dependent manner (Figure 1A). No mortality was observed for zebrafish infected with 2.4×102 CFU/mL L. hongkongensis whereas the mortality was 100% for zebrafish infected with 2.4×107 CFU/mL L. hongkongensis. All mortalities occurred in the first four days post-infection. Zebrafish infected with high concentrations of L. hongkongensis died more quickly than those infected with intermediate or low concentrations of L. hongkongensis. The LD50 was 2.1×104 (95% confidence interval: 1.9×104–2.4×104) CFU/mL (Figure 1B). No mortality was observed in the control groups.
Figure 1.
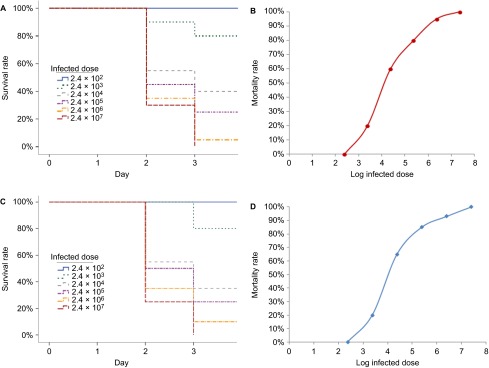
Survival of zebrafish infected with graded doses of L. hongkongensis by immersion following dermal abrasion and intraperitoneal injection. (A) Survival curve of zebrafish after L. hongkongensis infection by immersion following dermal abrasion. (B) Plot of zebrafish mortality after L. hongkongensis infection by immersion following dermal abrasion against logarithm of infected doses of L. hongkongensis. (C) Survival curve of zebrafish after L. hongkongensis infection by intraperitoneal injection. (D) Plot of zebrafish mortality after L. hongkongensis infection by intraperitoneal injection against logarithm of infected doses of L. hongkongensis. All results are expressed as mean of three experiments.
Exposure by intraperitoneal injection
Zebrafish infected with L. hongkongensis by intraperitoneal injection suffered mortality in a dose-dependent manner (Figure 1C). No mortality was observed for zebrafish infected with 2.4×102 CFU/mL L. hongkongensis, whereas the mortality was 100% for zebrafish infected with 2.4×107 CFU/mL L. hongkongensis. All mortalities occurred in the first four days post-infection. Zebrafish infected with high concentrations of L. hongkongensis died more quickly than those infected with intermediate or low concentrations of L. hongkongensis. The LD50 was 1.9×104 (95% confidence interval: 1.7×104–2.2×104) CFU/mL (Figure 1D). No mortality was observed in the control groups.
Clinical and pathological features associated with L. hongkongensis infection
Irrespective of the route of infection, all zebrafish that died swam near the surface of the water and showed marked lethargy and sidestroke before their deaths. In addition, they all developed characteristic abdominal hemorrhage, ulcers and marked swelling with ascites (Figure 2).
Figure 2.
Zebrafish after L. hongkongensis infection. (A and C) Lateral and ventral views of zebrafish two days after L. hongkongensis infection by immersion following dermal abrasion, showing abdominal hemorrhage and marked swelling with ascites. (B and D) Lateral and ventral views of zebrafish two days after E. coli infection by immersion following dermal abrasion.
Histological studies
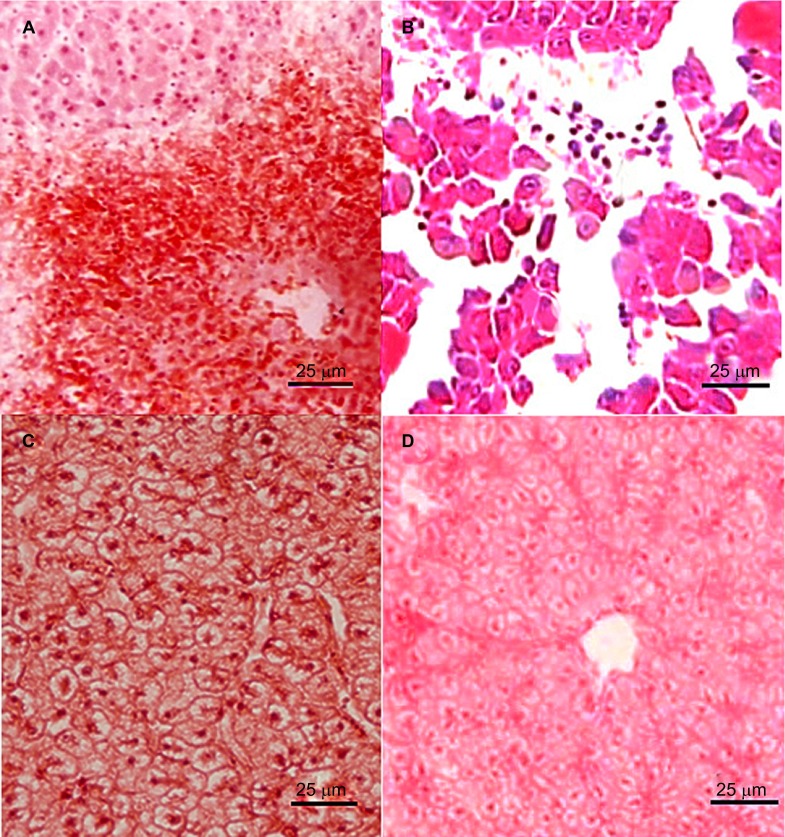
Histological sections of the livers of the dead zebrafish infected with 2.4×104–2.4×107 CFU/mL L. hongkongensis by immersion following dermal abrasion or intraperitoneal injection showed mild congestion and dilation of the hepatic central vein (Figure 3A), moderate hydropic degeneration and vague demarcation of hepatocytes. In addition, for the dead zebrafish infected with 2.4×106 and 2.4×107 CFU/mL L. hongkongensis by either method, structural disorder of hepatic lobules, atrophy and necrosis of hepatocytes, mild congestion and dilation in hepatic sinusoid with abundant inflammatory cells were also observed (Figure 3B and Supplementary Figure S1). For the dead zebrafish infected with 2.4×103 CFU/mL L. hongkongensis by either methods, mild hydropic degeneration of hepatocytes was observed (Figure 3C). No obvious changes were observed in the other organs. No obvious changes were observed in the liver sections of zebrafish infected with E. coli (Figure 3D).
Figure 3.
Histopathological changes in liver sections of zebrafish two days after L. hongkongensis infection by immersion following dermal abrasion. (A) Necrosis of hepatocytes and infiltration of inflammatory cells around the central vein. (B) Structural disorder of hepatic lobules with detachment of several groups of hepatocytes; necrosis and apoptosis of hepatocytes and dilatation of hepatic sinusoid with abundant inflammatory cells. (C) Hydropic degeneration with swollen and edematous hepatocytes. (D) No obvious changes were observed in liver sections of zebrafish two days after E. coli infection by immersion following dermal abrasion.
Recovery and identification of L. hongkongensis
For the zebrafish that died, abundant bacterial colonies in pure culture were recovered from the ascitic fluid and homogenized tissues on cefoperazone MacConkey agar. These bacteria were gram-negative, S-shaped, motile and catalase, cytochrome oxidase, urease and arginine dihydrolase positive. Partial 16S rRNA gene amplification using L. hongkongensis-specific primers resulted in specific bands at 424 bp for all colonies tested (Figure 4 and Supplementary Figure S2). No L. hongkongensis was recovered from the homogenized tissues of zebrafish that survived because the bacteria have been cleared by the host's immune system over time.
Figure 4.
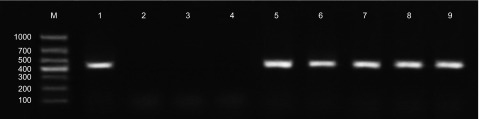
Identification of bacterial colonies from ascitic fluid and homogenized tissues of zebrafish infected with L. hongkongensis by immersion following dermal abrasion using L. hongkongensis-specific primers. Lane M, DL1000 DNA marker; lane 1, DNA extracted from L. hongkongensis HLHK9; lanes 2 and 3, ascitic fluid of two zebrafish infected with E. coli by immersion following dermal abrasion; lane 4, liver of zebrafish infected with E. coli by immersion following dermal abrasion; lanes 5–7, ascitic fluid of three zebrafish infected with L. hongkongensis by immersion following dermal abrasion; lanes 8 and 9, liver of zebrafish infected with L. hongkongensis by immersion following dermal abrasion.
Infection of zebrafish using L. hongkongensis isolated from dead zebrafish
Eighteen (60%) out of 30 zebrafish died over a period of 21 days after they were infected with 2.1×104 CFU/mL (LD50) L. hongkongensis using the immersion following dermal abrasion method. All zebrafish that died showed characteristic features of swimming near the surface of the water, marked lethargy and sidestroke before their deaths as well as abdominal hemorrhage, ulcers and marked swelling with ascites. Histopathological studies showed dilation of hepatic central vein and hydropic degeneration. L. hongkongensis was isolated from the zebrafish that died.
DISCUSSION
We have developed an animal model for L. hongkongensis infection using adult zebrafish, which have fulfilled Koch's postulates. In the last decade, zebrafish has been used as an animal model to study mammalian infections caused by a variety of gram-positive and gram-negative bacterial pathogens. In this study, we showed that L. hongkongensis was recovered in abundance and in pure culture from all sampled zebrafish that died, but the bacterium was not recovered in zebrafish that did not die. When an isolate of L. hongkongensis recovered from the zebrafish was used to infect another group of healthy zebrafish, it caused disease with the same characteristic clinical, pathological and histopathological features. L. hongkongensis was re-isolated from the zebrafish that died. It is of note that two different methods, immersion following dermal abrasion or intraperitoneal injection of L. hongkongensis, could be successfully used to infect the zebrafish. For the method of intraperitoneal injection, the dose of L. hongkongensis can be precisely controlled, whereas the immersion following dermal abrasion method can provide a more natural way of L. hongkongensis, infection but the exact amount of L. hongkongensis that enters the zebrafish is difficult to ascertain.
Underlying liver pathologies are risk factors for invasive L. hongkongensis infections in human, whereas the liver is the main target organ for L. hongkongensis infection in zebrafish. As for human invasive infections, L. hongkongensis was first discovered from the blood culture of a patient with alcoholic liver cirrhosis and ascites.2 Recently, L. hongkongensis was isolated from the blood culture of another patient with liver cirrhosis due to Wilson's disease.3 Since L. hongkongensis was acquired through the oral route as it is associated with community-acquired gastroenteritis and traveler's diarrhea, intestinal mucosal edema and local immunosuppression secondary to portal venous congestion vasculopathy due to liver cirrhosis in these patients predisposed the patients to L. hongkongensis invasion through the gastrointestinal mucosa. Interestingly, in the present animal model, L. hongkongensis also predominantly caused pathological changes in the livers of the zebrafish. In zebrafish infected either by immersion following dermal abrasion or intraperitoneal injection, the animals developed hydropic degeneration, necrosis and apoptosis of hepatocytes, hepatic central vein dilation and hepatic sinusoid dilation with infiltration of abundant inflammatory cells. These were associated with abdominal hemorrhage, ulcers and marked swelling with ascites as well as swimming near surface of water, marked lethargy and sidestroke. In zebrafish, abdominal hemorrhage, ulcers and ascites have been reported to be associated with severe infections due to other gram-negative bacteria, such as Aeromonas hydrophila and Pseudomonas aeruginosa;33,34 whereas swimming near the surface of water, marked lethargy and sidestroke are common symptoms that developed in moribund zebrafish.35,36 Of note is that only zebrafish infected with L. hongkongensis by immersion following dermal abrasion and intraperitoneal injection, but not immersion, developed disease and died. This indicates that an intact integument is a very important defense mechanism for L. hongkongensis infection in adult zebrafish,29 and L. hongkongensis cannot infect adult zebrafish through the gills or intact skin.
This zebrafish model is of crucial importance for future studies on virulence factors for L. hongkongensis infection. The complete genome of L. hongkongensis was sequenced and published in 2009.21 In the L. hongkongensis genome, genes and novel mechanisms for adaption to different temperatures and habitats were observed.21,37 Moreover, in addition to genes encoding putative proteins responsible for acid and bile resistance and mucosal adhesion, a number of genes encoding potential virulence factors were observed.21,38 These include genes encoding putative superoxide dismutase and catalases for evasion of host defense by decomposing superoxide and hydrogen peroxide respectively, enzymes for lipopolysaccharide biosynthesis and hence, eliciting the endotoxin response pathway, cell surface acting (RTX toxin and hemolysins) and intracellular cytotoxins (patatin-like proteins) and enzymes for tissue invasion (outer membrane phospholipase A). Although studies on the biochemical roles of these proteins are possible,39 the examination of their biological role as virulence factors in a live animal has been greatly hampered by the lack of an animal model. In the present study, adult zebrafish infected by either immersion following dermal abrasion or intraperitoneal injection was shown to be a highly reproducible animal model for L. hongkongensis infection, with easily observable clinical, pathological and histopathological features as well as mortality in a dose-dependent manner. Since all clinicopathological features and mortalities developed in the first four days post-infection, this is a very convenient and effective animal model for studying L. hongkongensis pathogenesis. Potential virulence factors can therefore be easily studied by deleting the genes encoding for the potential virulence factors in wild-type L. hongkongensis and observing for a decrease in mortality when the isogenic mutant is used for infecting the zebrafish by either immersion following dermal abrasion or intraperitoneal injection.
Acknowledgments
We thank Professor Wen-Qing Zhang (Southern Medical University, Guangzhou, China) for provision of healthy adult zebrafish. This work is partly supported by the Guangdong Province College Students' Innovative Experiment Project (212111030).
Footnotes
Note: Supplementary Information for this article can be found on Emerging Microbes and Infections' website (http://www.nature.com/emi/).
Supplementary Information
References
- Woo PC, Lau SK, Teng JL, Yuen KY. Current status and future directions for Laribacter hongkongensis, a novel bacterium associated with gastroenteritis and traveller's diarrhoea. Curr Opin Infect Dis. 2005;18:413–419. doi: 10.1097/01.qco.0000180162.76648.c9. [DOI] [PubMed] [Google Scholar]
- Yuen KY, Woo PC, Teng JL, Leung KW, Wong MK, Lau SK. Laribacter hongkongensis gen. nov., sp. nov., a novel gram-negative bacterium isolated from a cirrhotic patient with bacteremia and empyema. J Clin Microbiol. 2001;39:4227–4232. doi: 10.1128/JCM.39.12.4227-4232.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Wi YM, Choi JY, Peck KR, Song JH, Ko KS. Bacteremia caused by Laribacter hongkongensis misidentified as Acinetobacter lwoffii: report of the first case in Korea. J Korean Med Sci. 2011;26:679–681. doi: 10.3346/jkms.2011.26.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse CW, Curreem SO, Cheung I, et al. Novel MLST sequence type clustered with other sequence types of human isolates discovered in first fatal case of Laribacter hongkongensis bacteremia. Emerg Microbes Infect. 2014;3:e41. doi: 10.1038/emi.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo PC, Kuhnert P, Burnens AP, et al. Laribacter hongkongensis: a potential cause of infectious diarrhea. Diagn Microbiol Infect Dis. 2003;47:551–556. doi: 10.1016/s0732-8893(03)00161-5. [DOI] [PubMed] [Google Scholar]
- Woo PC, Lau SK, Teng JL.et al. L Hongkongensis Study Group Association of Laribacter hongkongensis in community-acquired gastroenteritis with travel and eating fish: a multicentre case–control study Lancet 20043631941–1947. [DOI] [PubMed] [Google Scholar]
- Ni XP, Ren SH, Sun JR, et al. Laribacter hongkongensis isolated from a patient with community-acquired gastroenteritis in Hangzhou City. J Clin Microbiol. 2007;45:255–256. doi: 10.1128/JCM.01400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos LA, DuPont HL. Advances in defining etiology and new therapeutic approaches in acute diarrhea. J Infect. 2007;55:385–393. doi: 10.1016/j.jinf.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Schlenker C, Surawicz CM. Emerging infections of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2009;23:89–99. doi: 10.1016/j.bpg.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Raja MK, Lulu SS, Ghosh AR. A novel pathogen for gastroenteritis: Laribacter hongkongensis. Indian J Med Microbiol. 2013;31:204. doi: 10.4103/0255-0857.115240. [DOI] [PubMed] [Google Scholar]
- Verboeket SO, van den Berk GE, Arends JE, van Dam AP, Peringa J, Jansen RR. Hookworm with hypereosinophilia: atypical presentation of a typical disease. J Travel Med. 2013;20:265–267. doi: 10.1111/jtm.12042. [DOI] [PubMed] [Google Scholar]
- Teng JL, Woo PC, Ma SS, et al. Ecoepidemiology of Laribacter hongkongensis, a novel bacterium associated with gastroenteritis. J Clin Microbiol. 2005;43:919–922. doi: 10.1128/JCM.43.2.919-922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Woo PC, Fan RY, Lee RC, Teng JL, Yuen KY. Seasonal and tissue distribution of Laribacter hongkongensis, a novel bacterium associated with gastroenteritis, in retail freshwater fish in Hong Kong. Int J Food Microbiol. 2007;113:62–66. doi: 10.1016/j.ijfoodmicro.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Feng JL, Hu J, Lin JY, et al. The prevalence, antimicrobial resistance and PFGE profiles of Laribacter hongkongensis in retail freshwater fish and edible frogs of southern China. Food Microbiol. 2012;32:118–123. doi: 10.1016/j.fm.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Chen DQ, Yang L, Luo YT, Mao MJ, Lin YP, Wu AW. Prevalence and characterization of quinolone resistance in Laribacter hongkongensis from grass carp and Chinese tiger frog. J Med Microbiol. 2013;62:1559–1564. doi: 10.1099/jmm.0.059451-0. [DOI] [PubMed] [Google Scholar]
- Feng JL, Yan H, Chowdhury N, et al. Identification and characterization of integron-associated antibiotic resistant Laribacter hongkongensis isolated from aquatic products in China. Int J Food Microbiol. 2011;144:337–341. doi: 10.1016/j.ijfoodmicro.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lau SK, Woo PC, Fan RY, et al. Isolation of Laribacter hongkongensis, a novel bacterium associated with gastroenteritis, from drinking water reservoirs in Hong Kong. J Appl Microbiol. 2007;103:507–515. doi: 10.1111/j.1365-2672.2006.03263.x. [DOI] [PubMed] [Google Scholar]
- Lau SK, Lee LC, Fan RY, et al. Isolation of Laribacter hongkongensis, a novel bacterium associated with gastroenteritis, from Chinese tiger frog. Int J Food Microbiol. 2009;129:78–82. doi: 10.1016/j.ijfoodmicro.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Ni X, Sun J, Kong Q, et al. Isolation of Laribacter hongkongensis from Little Egrets (Egretta garzetta) in Hangzhou, China. Lett Appl Microbiol. 2011;52:465–467. doi: 10.1111/j.1472-765X.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- Woo PC, Teng JL, Tsang AK, et al. Development of a multi-locus sequence typing scheme for Laribacter hongkongensis, a novel bacterium associated with freshwater fish-borne gastroenteritis and traveler's diarrhea. BMC Microbiol. 2009;9:21. doi: 10.1186/1471-2180-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo PC, Lau SK, Tse H, et al. The complete genome and proteome of Laribacter hongkongensis reveal potential mechanisms for adaptations to different temperatures and habitats. PLoS Genet. 2009;5:e1000416. doi: 10.1371/journal.pgen.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Teng JL, Watt RM, Kan B, Lau SK, Woo PC. Arginine deiminase pathway is far more important than urease for acid resistance and intracellular survival in Laribacter hongkongensis: a possible result of arc gene cassette duplication. BMC Microbiol. 2014;14:42. doi: 10.1186/1471-2180-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RD, Ramakrishnan L. Insights into tuberculosis from the zebrafish model. Trends Mol Med. 2012;18:689–690. doi: 10.1016/j.molmed.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Parikka M, Hammarén MM, Harjula SK, et al. Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog. 2012;8:e1002944. doi: 10.1371/journal.ppat.1002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajsnar TK, Cunliffe VT, Foster SJ, Renshaw SA. A novel vertebrate model of Staphylococcus aureus infection reveals phagocyte-dependent resistance of zebrafish to non-host specialized pathogens. Cell Microbiol. 2008;10:2312–2325. doi: 10.1111/j.1462-5822.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- Patterson H, Saralahti A, Parikka M, et al. Adult zebrafish model of bacterial meningitis in Streptococcus agalactiae infection. Dev Comp Immunol. 2012;38:447–455. doi: 10.1016/j.dci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- van der Sar AM, Musters RJ, van Eeden FJ, Appelmelk BJ, Vandenbroucke-Grauls CM, Bitter W. Zebrafish embryos as a model host for the real time analysis of Salmonella typhimurium infections. Cell Microbiol. 2003;5:601–611. doi: 10.1046/j.1462-5822.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- Westerfield M.The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio*)3rd ed.Eugene, OR: University of Oregon Press; 1995 [Google Scholar]
- Neely MN, Pfeifer JD, Caparon M. Streptococcus–zebrafish model of bacterial pathogenesis. Infect Immun. 2002;70:3904–3914. doi: 10.1128/IAI.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressley ME, Phelan PE 3rd, Witten PE, Mellon MT, Kim CH Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev Comp Immunol. 2005;29:501–513. doi: 10.1016/j.dci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lau SK, Woo PC, Hui WT, et al. Use of cefoperazone MacConkey agar for selective isolation of Laribacter hongkongensis. J Clin Microbiol. 2003;41:4839–4841. doi: 10.1128/JCM.41.10.4839-4841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S, Skaletsky HJ.Primer3 on the WWW for general users and for biologist programmersIn: Krawetz S, Misener S (ed.)Bioinformatics methods and protocols: methods in molecular biology Totowa, NJ: Humana Press; 2000365–386. [DOI] [PubMed] [Google Scholar]
- Rodríguez I, Novoa B, Figueras A. Immune response of zebrafish (Danio rerio) against a newly isolated bacterial pathogen Aeromonas hydrophila. Fish Shellfish Immunol. 2008;25:239–249. doi: 10.1016/j.fsi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Clatworthy AE, Lee JS, Leibman M, Kostun Z, Davidson AJ, Hung DT. Pseudomonas aeruginosa infection of zebrafish involves both host and pathogen determinants. Infect Immun. 2009;77:1293–1303. doi: 10.1128/IAI.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research—advantages and current limitations. Toxicol Pathol. 2003;31:62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghmans S, Jette C, Langenau D, et al. Making waves in cancer research: new models in the zebrafish. Biotechniques. 2005;39:227–237. doi: 10.2144/05392RV02. [DOI] [PubMed] [Google Scholar]
- Lau SK, Fan RY, Ho TC, et al. Environmental adaptability and stress tolerance of Laribacter hongkongensis: a genome-wide analysis. Cell Biosci. 2011;1:22. doi: 10.1186/2045-3701-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Wong GK, Tsang AK, et al. Virulence determinants, drug resistance and mobile genetic elements of Laribacter hongkongensis: a genome-wide analysis. Cell Biosci. 2011;1:17. doi: 10.1186/2045-3701-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chen WY, Wang H, et al. Structural and functional insight into the mechanism of an alkaline exonuclease from Laribacter hongkongensis. Nucleic Acids Res. 2011;39:9803–9819. doi: 10.1093/nar/gkr660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.