Abstract
Introduction
Ethanol has been used for years in neonatal and infant liquid medications, yet the pharmacokinetics, pharmacodynamics, and safety of ethanol in this vulnerable population have not been well characterized. The purpose of this review is to raise awareness of ethanol use as an excipient in neonatal and infant medications and to provide insight, based on the available evidence, into clearance rates of ethanol in babies. We also discuss ethanol pharmacokinetics in adults, theoretical pharmacokinetic changes in neonates and infants as it may apply to ethanol disposition, and case reports involving ethanol exposure in neonates and infants.
Materials and methods
This study was a narrative review in which relevant papers were selected using databases and scientific search engines such as PubMed with the key words ethanol, infant, and newborninfant.
Results
It remains unclear what ethanol exposure is safe for neonates and infants. The Food and Drug Administration and American Academy of Pediatrics have both taken action, by either setting limits of ethanol content in over-the-counter medications or by recommending restricted exposure to ethanol-containing pediatric formulations.
Conclusions
Until the short- and long-term health effects of chronic ethanol administration can be further characterized, ethanol-containing medications should be used with caution.
Key words: Alcohol, Ethanol, Infant, Neonate, Pediatric
Introduction
Ethanol, also known as alcohol or ethyl alcohol, has been used medicinally and recreationally for centuries, and its pharmacokinetic properties have been studied widely for medical, legal, and forensic purposes. Ethanol serves as a solvent and a microbial preservative in oral liquid medications and is the second most commonly used solvent in liquid formulations following water.1,2 Often used to dissolve water-insoluble ingredients, it can be found in medicinal solutions, elixirs, and spirits. Table I shows some examples of commonly prescribed oral ethanol-containing medications in the United States, and their respective ethanol contents. Ethanol can also be found in parenteral formulations and functions as a co-solvent.
Table I.
Ethanol content of select oral pediatric medications.
| Drug | Ethanol content (%) |
|---|---|
| Acetaminophen with codeine elixir | 7 |
| Chlorothiazide oral suspension | 0.50 |
| Cyproheptadine hydrochloride syrup | 5 |
| Dexamethasone oral solution | 30 |
| Diazoxide oral suspension | 7.25 |
| Digoxin oral solution | 10 |
| Ferrous sulfate oral drops | 0.20 |
| Griseofulvin oral suspension | 0.20 |
| Hydroxyzine hydrochloride syrup | 0.50 |
| Lasix oral solution | 11.50 |
| Maalox oral suspension | <0.5 |
| Metoclopramide oral solution | <0.1 |
| Nystatin oral suspension | ≤1 |
| Phenobarbital elixir | 15 |
| Prednisolone oral solution | 2 |
| Propranolol hydrochloride | 0.60 |
| Ranitidine oral solution | 7.70 |
| Sulfamethoxazole and trimethoprim oral suspension | 0.26 |
| Zantac | 7.50 |
Despite widespread use of ethanol in medications for neonates and infants, the pharmacokinetics and safety of this pharmacologically active agent are not well described. There is limited information on the physiologic effects of ethanol in pediatric populations except in acute poisonings when signs of ethanol exposure can include hypoglycemia, acidosis, tachycardia, hypothermia, hyporesponsiveness, and disorders of consciousness.3,4 The Food and Drug Administration has set a maximum limit of 0.5% ethanol in oral over-the-counter products intended for children younger than age 6 years (21 CFR P328 2013). The American Academy of Pediatrics Committee on Drugs has recommended that the amount of ethanol contained in any preparation should not be able to produce a blood concentration >25 mg/dL after a single dose,5 whereas the European Medicines Agency recently proposed that blood ethanol levels should not exceed 1 mg/dL after a single dose (or a dose of 6 mg/kg) in children younger than age 6 years.6 These limits are intended to serve as safety standards, although they are not based on specific scientific evidence.
Ethanol intake secondary to medication administration can vary widely in pediatric patients. A pilot study of pediatric intensive care unit patients aged 26 weeks to 15 years in the United Kingdom found that 26 of 28 patients were prescribed an ethanol-containing medication with a daily intake of 0.006 to 2.18 mL uncorrected for weight. Authors estimated that these intakes were equivalent to about 0.07 to 15.2 adult UK alcohol units per week7 or 0.04 to 8.68 US alcohol units per week (ie, >0.5–121 g/wk). The United Kingdom considers 8 g to be 1 unit, or standard adult drink;8 the United States considers 14 g to be 1 unit or standard adult drink. The most commonly prescribed drugs containing ethanol included nystatin, ranitidine, furosemide, and morphine. Of 28 patients, 2 who had an intake of >15 UK units/wk (8.47 US units/wk) were both receiving oral morphine for weaning due to opioid abstinence. Since the study was completed, ethanol has been largely removed from oral morphine solutions for pediatric patients.
A second observational study in a UK neonatal intensive care unit (NICU) identified 38 preterm babies over a 1-year period with complete records that survived to discharge at a single hospital. Authors documented excipient intake rates from eight commonly used NICU medications. Two of these medications (iron and furosemide) contained ethanol as an excipient. Babies were exposed to 0.2 to 1.8 mL ethanol per week uncorrected for weight. Correlating to about 1 to 7 adult UK alcohol units/wk9 or 0.57 to 4 US units/wk (ie, >8–56 g/wk), these intake rates are substantial when you consider that the US weekly recommended limits of alcohol in adults are 14 units/wk for men and 7 units/wk for women.10
Ethanol pharmacokinetics in adults
Absorption
Ethanol is a small molecule with both hydrophilic and lipophilic characteristics. It is rapidly absorbed in the stomach (20%) and intestines (80%) by simple diffusion. Ethanol absorption is largely dependent on gastric emptying rates because it is absorbed to a large degree in the duodenum.11 In adults, delayed gastric emptying brought on by the ingestion of food reduces the absorption of alcohol independent of the relative macronutrient content of the meal.12 This phenomenon is the basis for the commonly employed adage of “not drinking on an empty stomach.” Differences in gastric emptying rates have an influence on the shape of the concentration time profile. Absorption rate is driven primarily by gastric motility, as evidenced by decreases in both Cmax and AUC at 30 minutes with cigarette smoking13 and a reduced Cmax and delayed tmax with aspirin administration.14 Similarly, gastric bypass surgery15 and use of promotility drugs16,17 increase peak blood ethanol levels. Absorption differences can also be linked to the quantity, concentration, and speed at which ethanol is ingested.18 For example, a drink with 10% to 30% ethanol is absorbed more rapidly than a more dilute drink; but drinks >30% ethanol are absorbed more slowly due to gastric irritation.19 Ethanol is metabolized presystemically in the gastric mucosa at low ethanol doses (0.3g/kg or 0.15g/kg);20,21 but the magnitude and importance of gastric first pass metabolism is debated.22
Distribution
Following absorption, ethanol readily distributes into tissues and body fluids and does not exhibit any protein binding. Its volume of distribution is relative to water content, which is partially responsible for age- and sex-related differences in pharmacokinetic parameters.23 In adults, total body water content depends on age, weight, and gender, and is approximately 50% to 60% of total body weight in men and 45% to 55% of body weight in women.24 Ethanol crosses the blood–brain and placental barriers, due in part to its amphiphilic nature.25 It has been described as following a 2-compartment model, with its distribution depending on elements that govern peripheral circulation, including vasoconstriction, hormone changes, muscular activity, temperature, and circulatory impairment.23
Metabolism
Less than 10% of ethanol is excreted in the breath, sweat, and urine. The remaining fraction of ingested ethanol is metabolized to acetaldehyde by 1 of 3 systems: alcohol dehydrogenase (ADH), microsomal ethanol-oxidizing system (MEOS), or catalase. Acetaldehyde is subsequently oxidized to acetate by aldehyde dehydrogenase (ALDH). Acetate leaves the liver and is converted to acetyl-coenzyme A to ultimately produce carbon dioxide and water. A small fraction of ethanol is cleared by nonoxidative pathways and involves ethanol conjugation to endogenous substrates such as fatty acids, phospholipids, sulfate, or glucuronic acid to form fatty acid ethyl esters, phosphaditylethanol, ethyl sulfate, and ethyl glucuronide.26,27 These metabolites have a longer half-life than ethanol and have been used as biomarkers of ethanol consumption and chronic ethanol use, although none are optimal for this purpose.28
A 70-kg adult can metabolize approximately 170 to 240 g of ethanol a day.26 Ethanol clearance is easily saturated and is best described by Michaelis-Menton (saturation) kinetics. At high concentrations, ethanol is eliminated by zero-order kinetics at a rate of approximately 20 mg/dL/h depending on previous exposure and genetic differences.29 Nontolerant individuals can eliminate ethanol at a rate of about 10 to 25 mg/dL/h,23 whereas habitual drinkers can have elimination rates of ≥30 mg/dL/h.30 Ethanol elimination switches from zero-order to first-order kinetics when blood ethanol concentrations begin to drop below 1 to 3 mM (4.6–13.8 mg/dL).31,32 Covariates that influence ethanol clearance include body weight, age, dissolved oxygen concentration,33 and genetic polymorphisms (Table II).34
Table II.
Sources of variability in adult ethanol pharmacokinetics.
| Parameter | Mechanism | Corresponding covariates |
|---|---|---|
| Absorption | Gastric motility | Fed state, concomitant medications, tobacco |
| Distribution | Negligible protein binding, correlation to total body water | Age, sex, body weight |
| Metabolism | Enzymatic polymorphism of ADH and ALDH | Ethnicity, sex |
| Induction of metabolism by CYP2E1 | Frequency of ethanol consumption |
ADH = alcohol dehydrogenase; ALDH = aldehyde dehydrogenase; CYP = cytochrome P450.
ADH accounts for the bulk of ethanol’s oxidative metabolism. It has a wide spectrum of activity, oxidizing many primary and secondary alcohols.26 ADH is primarily located in the liver, but can be found to a lesser extent in the gastrointestinal tract, kidneys, nasal mucosa, testes, and uterus.26 Humans have 5 main classes of ADH enzymes (Class I–V), each with differing affinities for ethanol. Class I ADH is primarily found in the liver and contributes the most to ethanol metabolism.26 Class I isozymes form hetero- or homodimers with α, β, and γ subunits, and are coded by ADH1, ADH2, and ADH3 genes, respectively. All Class I ADH isoforms, except the αα and relatively uncommon β3β3 isoforms, have Michaelis constant (Km) values ≤1 mM and therefore become saturated quickly. The Km of αα and β3β3 isoforms are about 4.2 and 24 mM, respectively.26 Class II ADH is also located in the liver and helps in metabolizing higher ethanol concentrations due to its higher Km (~34 mM) for ethanol.31 Class III ADH is distributed throughout the body, has a high Km for ethanol (>2 mM), and is important in metabolizing other alcohols.26 Class IV ADH is localized in the digestive tract and stomach, and class V ADH has only been recognized at the mRNA level. Overall in vivo metabolism by ADH is dependent on both enzyme kinetics and on the relative concentration of each isoform. ADH has numerous polymorphic forms, which are described extensively.35,36
MEOS is a cytochrome P450-dependent system involving cytochromes P4502E1 (CYP2E1), P4501A2 (CYP1A2), and P4503A4 (CYP3A4).37 MEOS accounts for <10% of ethanol oxidation by the liver.31 Within the MEOS system, CYP2E1 plays the major role in metabolism.38 CYP2E1 has broad substrate specificity and plays an important role in detoxification and defense mechanisms against xenobiotics.39 It has a relatively high Km for ethanol (10 mM) and is particularly important at high ethanol concentrations.37 Ethanol increases CYP2E1 levels upon chronic administration, and microsomal levels are up to 4-fold higher in alcoholics compared with nondrinkers.40 CYP2E1 induction is primarily due to posttranscriptional protein stabilization against degradation.41 In chronic ethanol administration, CYP2E1 increases ethanol clearance and contributes to metabolic tolerance.42 CYP2E1 metabolism generates reactive oxygen species from molecular oxygen, and has been linked to ethanol-induced hepatotoxicity.38,41
Catalase is an antioxidant enzyme that degrades hydrogen peroxide into water and oxygen. It can oxidize a wide variety of compounds, including ethanol, with the use of hydrogen peroxide. Although catalase is widely distributed in the body, the pathway is limited by the low amounts of hydrogen peroxide in the body. The catalase pathway accounts for about 2% of ethanol metabolism.26 Catalase accounts for a small percentage of the overall metabolism of ethanol, but it has been proposed to play an important role in the local production of acetaldehyde in the brain.43,44
ALDH enzymes irreversibly oxidize acetaldehyde in a nicotinamide adenine dinucleotide phosphate-dependent manner. To date, 19 human ALDH isozymes have been identified, with cytosolic ALDH1A1 and mitochondrial ALDH2 being the most important for acetaldehyde metabolism.45 They have Km values of 50 to 180 uM and <1 uM, respectively.45 Under normal physiologic conditions acetaldehyde concentrations are low, and ALDH2 is the primary isoform responsible for oxidizing acetaldehyde to acetate.31 ALDH1A1 and ADH2 are distributed throughout the body, including the liver, kidney, and brain.45
Elimination
The majority of ethanol is metabolized, with <10% excreted unchanged by exhalation, urine, and sweat. Further, after a single dose of ethanol, approximately 0.7% to 1.5% of ethanol is excreted unchanged in the urine.46
Neonate Pharmacokinetics: Focus on Ethanol absorption, distribution, metabolism, and excretion Pathways
Neonates are in a period of rapid growth and maturation, leading to altered pharmacokinetics. These changes may translate to increased toxicity or reduced efficacy of drugs. Numerous reviews are available on the subject of neonate and infant pharmacokinetics.47–50 Figure 1 identifies physiologic factors in neonates having the potential to affect the absorption, distribution, metabolism, and excretion of ethanol when compared with adults.
Figure 1.
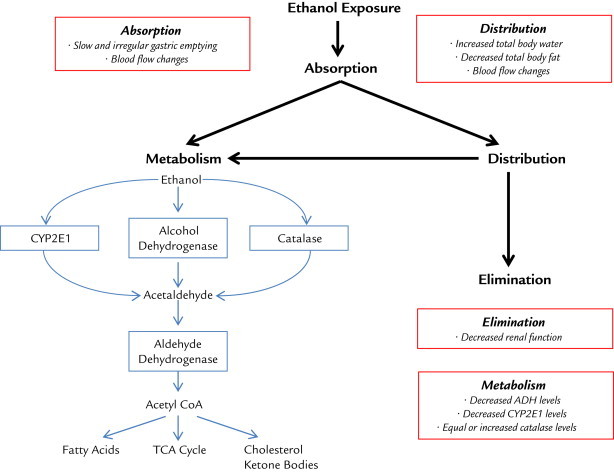
Ethanol disposition. Physiologic factors in neonates having the potential to affect the absorption, distribution, metabolism, and excretion of ethanol when compared with adults. Red boxes show infant-specific differences compared with adults.
Absorption
Generally, neonates have prolonged and variable gastric emptying times compared with adults,51,52 and predicting oral absorption can be difficult during this epoch. Additionally, changes in splanchnic blood flow during this period can alter the concentration gradient across the intestinal mucosa, leading to altered absorption rates.53 Ethanol absorption is largely dependent on gastric emptying rates and the rate and extent of absorption may therefore be reduced in neonates. Absorption is further complicated by extrauterine factors, including the rate of ethanol consumption and food or drug effects. In addition to normal maturation and development, diseases are likely to have an influence on oral absorption and must be considered on a case-to-case basis.
Distribution
Neonates have a very different body composition than adults. The total body water content decreases from about 92% of total body weight in premature newborns to 75% in full-term newborns and 60% by age 1 year.54,55 It does not approach adult values until age 12 to 13 years.55 In full-term neonates, total body fat increases from approximately 12% to 16% of total body weight to about 20% to 25% of body weight at age 1 year.55 These dynamic body composition changes have the potential to affect drug levels. For example, hydrophilic drugs generally have an increased volume of distribution in neonates on a per kilogram body weight basis, so higher doses may be required to achieve similar peak concentrations. Ethanol may therefore have a larger apparent volume of distribution and lower plasma concentrations in neonates when compared to adults. Other factors that influence drug distribution include regional blood flow, organ perfusion, permeability of membranes, and cardiac output.56 Cardiac output is redistributed in the first 5 days of life to metabolically active regions, such as the brain.53 Altered blood flow rates and increased perfusion to the brain may influence ethanol distribution in neonates.
Metabolism
In general, neonatal clearance mechanisms and metabolizing enzymes are immature. ADH activity is reported to be present in neonates and infants from age 9 days to 2 months, but it accounts for <20% of adult enzymatic activity (milliunits per gram liver wet weight of crude fetal and adult livers).57 One report estimated ADH content to be about 10-fold lower in perinatal infants than in adults (0.11 ± 0.09 vs 1.00 ± 0.37 g/kg liver wet weight respectively; n = 7 perinatal infants), with the αα isoenzyme predominating.58 ADH activity does not reach adult levels until about age 5 years.57 The work of Smith et al59 shows the progressive expression of ADH during development. Neonatal CYP enzymes have significant differences in their mRNA, protein, and enzyme activity when compared with adults.60 CYP2E1 levels increase within a few hours of birth and gradually reach 30% to 40% of adult levels by age 1 year.61,62 Catalase, on the other hand, may have a greater influence on neonatal ethanol metabolism. Autopsy examination reveals equal (0.32 ± 0.03 vs 0.37 ± 0.20 g/kg wet liver weight; P = not significant; n = 4 perinatal infants) or greater catalase content (1.55 ± 0.48 vs 0.37 ± 0.02 g/kg liver wet weight; P < 0.05; n = 3 perinatal infants) in perinatal infants compared with adults.58
Neonates may be more susceptible to drug–excipient or excipient–excipient pharmacokinetic interactions when compared with adults because of their reduced metabolic activity. For example, the Food and Drug Administration issued a warning in 2011 to avoid Kaletra (lopinavir/ritonavir) oral solution in premature babies until 14 days after their due date, or in full-term babies younger than age 14 days due to multiple postmarketing cases of life-threatening events.63 Kaletra oral solution contains 42.4% (v/v) ethanol and 15.3% (w/v) propylene glycol, both of which are metabolized by ADH in the liver. Thus, a theoretical pharmacokinetic interaction between ethanol and propylene glycol may explain Kaletra’s toxicity in newborns whom have reduced ADH activity.
Elimination
Renal clearance is determined by the combination of glomerular filtration (GFR), tubular secretion, and reabsorption. After birth, GFR matures more quickly than tubular function,64 although GFR does not approach adult levels until age 1 to 2 years.65 Tubular secretion and reabsorption rates are about 20% to 30% of adult values at birth.66 Drugs primarily eliminated by the kidney may have an increased half-life during the neonatal period when compared with adults. Renal elimination of ethanol in neonates may be decreased, but renal elimination accounts for <5% of ethanol elimination.
Case reports of ethanol toxicity in pediatric patients
A MeSH search on PubMed of ethanol AND infant OR newborn infant returned >500 hits, many with prenatal components, intoxications, and accidental exposures, or animal data. Numerous case reports are available describing ethanol toxicity in pediatrics. Ethanol can be found in alcoholic beverages, medications, and household products such as hand sanitizers, mouthwash, and cosmetics. One of the most common adverse effects of ethanol intoxication in pediatric patients is hypoglycemia, due to reduced glycogen stores.67 The lethal dose of ethanol is reported to be 3 g/kg in children.68
We describe several case reports, including clearance data for ethanol in neonates and infants (Table III).
Table III.
Ethanol clearance estimates in pediatric patients from case reports.
| Study design | Sample size | Postnatal age (mo) | Estimated clearance (mg/dL/h) | Source |
|---|---|---|---|---|
| Postnatal exposure | ||||
| Case report | 1 | 18 | 28.7 | 69 |
| Retrospective chart review | 8 | 18-156 | 28.4 | 70 |
| Case report | 1 | 0.95 | 10-25 | 71 |
| Case report | 1 | 1.15 | 17.1-21.2 | 72 |
| Case report | 1 | 7 | 49.7 | 74 |
| Perinatal exposure | ||||
| Case report | 1 | <1 d | 11.3 | 75 |
| Case report | 1 | <1 d | 17.3 | 75 |
| Review | 47 | <1 d | 8.3 | 76 |
A study from Charity Hospital in Louisiana described four pediatric patients having ethanol levels >300 mg/dL.69 One patient, an 18-month old boy had serial blood ethanol levels drawn. The boy had an initial ethanol level of 575 mg/dL and based on 3 subsequent blood draws, authors concluded that the ethanol level decreased at a rate of 28.7 mg/dL/h assuming zero-order elimination.69
In a retrospective chart review, authors identified 8 children (aged 18 months to 13 years) with ethanol ingestion and at least 2 documented blood ethanol levels from Calgary-area hospitals.70 Authors concluded that the mean rate of decline of ethanol in these children was 28.4 mg/dL/h, nearly twice the rate of adults.70 One of these patients, an 18-month old girl had an initial ethanol level 189 mg/dL and a calculated clearance of 41 mg/dL/h assuming zero-order elimination. Four of the 8 patients had a second ethanol level of 0, indicating that ethanol may be eliminated more rapidly than was reported.
A study from the Children’s Hospital of Philadelphia described a 29-day old baby with an initial ethanol level 301 mg/dL.71 The child had a mild clinical course with subtle neurologic symptoms and made a full recovery. From serial blood alcohol measurements, authors estimated ethanol was cleared at a rate of 10 to 25 mg/dL/h.
A case report described a 5-week old African American boy admitted to an emergency department with an initial serum ethanol level of 270 mg/dL.72 The infant recovered successfully and was “back to normal” within 24 hours. Based on three subsequent blood ethanol measurements (1 of which was undetectable for ethanol) they estimated an ethanol clearance of 17.1 to 21.2 mg/dL/h assuming zero-order elimination.
McCormick et al73 described 2 cases of ethanol intoxication in infants younger than age 2 months in a California pediatric emergency department.73 The infants presented with acute life-threatening events and had initial ethanol levels 278 mg/dL and 405 mg/dL, respectively. Subsequent ethanol levels were drawn following admission and authors concluded that ethanol follows Michaelis-Menten kinetics, changing from zero-order to first-order elimination at about 225 mg/dL. As described earlier in this review, elimination switches from zero-order to first-order at around 1 mM (4.6 mg/dL) in adults.
Finally, a study from the Children’s Hospital of Pittsburg described a 7-month old infant with an initial ethanol level of 183 mg/dL .74 The infant did not develop any complications, and was found to have an ethanol elimination rate of 49.7 mg/dL/h based on 3 blood draws (1 of which was undetectable for ethanol).
In addition to alcohol toxicity studies in pediatrics, studies on perinatal alcohol exposure are also available. The pharmacokinetics described in these studies may be complicated by the development of tolerance. A case study by Kvigne et al75 described 2 neonates with chronic alcohol exposure in utero. Mothers of the neonates had documented alcohol exposure before, during, and after their pregnancies. The first neonate had an ethanol level of 38.4 mg/dL at 129 minutes after birth and a calculated ethanol elimination rate of 11.3 mg/dL/h. The second neonate had an ethanol level 246.5 mg/dL at 67 minutes after birth and was found to have an elimination rate of 17.3 mg/dL/h. The first infant did not show any physical signs of fetal alcohol syndrome; the second infant was diagnosed with fetal alcohol syndrome.
In a recent review that identified 47 newborns with perinatal alcohol exposure,76 newborns had a mean blood ethanol concentration of 79.6 mg/dL (range = 5–212 mg/dL) at birth and a mean elimination rate of 8.3 mg/dL/h (range = 2.5–20 mg/dL/h). A comparison of neonate and maternal elimination of alcohol found that the neonates eliminated it at a rate that was one-third to twice that of their mothers.
We note that all of these reports should be interpreted with caution. Most calculated ethanol elimination assuming zero-order elimination and may only have used 2 time points. Furthermore, with the exception of the acute poisonings, they provided little information on the effects of ethanol in pediatric patients, especially for low chronic doses of ethanol. From limited case reports, it is obvious that there is a great deal of interindividual variability in ethanol clearance in neonates and infants.
Measurement of ethanol in blood
In adults, numerous analytic techniques are available for determining blood ethanol levels. Methods can be classified as chemical, enzymatic or chromatographic77 with enzymatic and chromatographic methods being the most popular. Enzymatic assays are often employed in the clinic. When enzymatic assays are used to quantify ethanol levels, ADH and oxidized nicotinamide adenine dinucleotide (NAD+) are added to blood samples to produce acetaldehyde and reduced nicotinamide adenine dinucleotide (NADH) from ethanol metabolism. The reduced coenzyme (NADH) can be monitored photometrically, flurimetrically, or voltammetrically and is proportional to the ethanol concentration in the sample.77 Gas chromatography (GC) is often used in forensic labs and is the gold standard for determining blood ethanol levels. GC is specific for ethanol and can concurrently measure other volatile alcohols such as methanol and isopropranol.78 Measuring blood ethanol levels in neonate presents a technical challenge because neonates have lower blood volumes than adults. Often, microassays and microanalytical methods must be used to allow for small volumes and sparse sampling of blood. Recently, Cordell et al79 developed a sensitive and reproducible headspace GC-mass spectrometry method for volatile organic compounds using microvolume samples (≤100 uL).79 The GC-mass spectrometry method was linear from 0.02 to 10 mg/dL, with limits of detection between 0.01 to 0.02 mg/dL and lower limits of quantification between 0.05 to 0.1 mg/L.
Ethanol is produced endogenously at low levels from reduction of the metabolic byproduct acetaldehyde via a reversible ADH reaction and through carbohydrate fermentation by gut flora. Candida albicans, which plays an important role in intestinal fermentation, has been shown to produce ethanol at a maximum rate of 1 mg/h/g intestinal content.80 Endogenous ethanol levels have substantial interindividual differences and levels vary depending on the methods used to measure ethanol. In healthy individuals, endogenous ethanol levels have been reported to range from below detection limits to 1.6 µg/mL (0.16 mg/dL).81 Metabolic conditions such as diabetes,82 short bowel syndrome,83 and nonalcoholic steatohepatitis can elevate endogenous ethanol levels.84 In a small study of 10 healthy volunteers, ethanol levels ranged from 0 to 0.377 µg/mL (0–0.0377 mg/dL) using a sensitive gas chromatography method.85
Discussion
The pathology and pathophysiology of adverse neurodevelopment due to antenatal ethanol exposure has been well described.86,87 Ethanol exposure during gestation has been shown to cause widespread damage throughout the brain, interfering with cell proliferation, migration, growth and differentiation, and cell death depending on the time and levels of alcohol exposure.88 However, the influence of ethanol on nervous system development in the postnatal period is less clear. There remains a lack of evidence of the adverse events of ethanol in the developing brain of infants. Challenges to establishing a dose that is considered to be harmful include variability in doses administered, likely changes in ethanol disposition based on developmental factors, difficulty in choosing a short term outcome to measure, and lack of long-term follow-up to assess outcomes. The applicability of animal models has been limited by the common use of prenatal or massive doses of ethanol.
Due to the lack of evidence on how ethanol affects developing brains, clear recommendations are impossible at this time, but it seems reasonable for clinicians to minimize the use of ethanol-containing solutions in neonates whenever possible. Furthermore, clinicians should work to reduce ethanol in existing formulations. It is acknowledged that ethanol has been used for years, and that there are certainly circumstances where the benefit of ethanol in a medication outweighs potential adverse effects. When ethanol exposure cannot be avoided, we must be cognizant of potential pharmacokinetic and pharmacodynamic drug interactions, especially in NICU patients taking multiple medications. Similar to adults, NICU patients are at risk of enhanced sedative and hypnotic effects on the central nervous system with agents such as barbiturates and benzodiazepines. Additionally, it may not be feasible to watch for subtle neurologic deficits, but it is possible to watch for more obvious adverse events, such as hypoglycemia, with ethanol use.
The pharmacodynamics of ethanol and the nature of an individual’s response may differ between neonates and adults. Although little is known in this regard as it applies to neonates, we do know that in adults, ethanol affects a large number of membrane proteins involved in signaling pathways. Specifically, ethanol enhances the action of γ-aminobutyric acid at γ-aminobutyric acidA receptors and inhibits the ability of glutamate to open N-methyl-D-aspartate cation channels.89 In addition, it has effects on calcium channels, dopamine and serotonin receptors, the hypothalamic-pituitary-adrenal axis, and the central adrenergic system.90
Acetaldehyde accumulation in the periphery contributes to some of the adverse effects in adults associated with drinking, including nausea, vomiting, vasodilation, and hypotension.91 For most people, acetaldehyde does not accumulate in the periphery due to the high efficiency of ALDH; although genetic polymorphisms in ALDH can lead to increased sensitivity to ethanol.92 Due to its nonpolar characteristics, acetaldehyde is extensively reabsorbed from the renal tubules and the route of elimination is primarily by metabolism. Acetaldehyde is a highly reactive molecule that can create adducts with proteins, lipids, and DNA.93 These adducts play a role in alcoholic liver disease,93 and there is evidence of adducts in other tissues such as the brain, gut, muscle, heart, and red blood cells.94 Current evidence suggests that acetaldehyde, formed locally in the brain from ethanol metabolism, crosses the blood–brain barrier when the ALDH enzymatic barrier is saturated.44 In rodents, catalase has been proposed to account for 60% of ethanol oxidation in the brain, whereas CYP2E1 may account for 20% of ethanol oxidation in the brain.43 Animal studies have shown that acetaldehyde adducts are expressed in neonatal brains exposed to ethanol in utero.95 As described above, neonates may have increased catalase levels compared with adults, but the localization and potential effects on ethanol oxidation in the brain are largely unknown.
The potential dysphoric effects of acetate produced after ethanol ingestion have been recently been explored.96 Acetate modulates the adenosine receptor. Caffeine, an adenosine receptor antagonist, is used frequently in neonatology and has well-described beneficial effects in ethanol-induced headache. The exact implications of such are unclear.
Questions remain about many of the other ways that ethanol may affect infants. In particular, little is known about the psychoactive effects of chronic ethanol administration in neonates. In neonates, some tissues may be more or less sensitive to ethanol, regardless of drug concentrations, based on their developmental stage, concomitant diseases, and treatment modalities. And for many drugs, including ethanol, the effect of ontogeny on the pharmacodynamics of the drug is not well established.56 Additionally, there are limited pharmacogenetic studies in neonates and infants.
We aimed to raise awareness of ethanol use as an excipient in pediatric medications. Not all excipients are safe, as has been seen with the use of benzyl alcohol and propylene glycol toxicity.97,98 Safety studies of excipients can be more challenging than those of the active pharmaceutical ingredient because exposure is generally less controlled and excipients may originate from numerous dose forms taken over various time periods. Although challenging, safety and pharmacokinetic studies of excipients in neonates are feasible, as has been demonstrated with propylene glycol.99,100
To help establish safe limits for excipients, the European and United States Paediatric Formulation Initiatives have developed a Safety and Toxicity of Excipients for Pediatrics (STEP) database.101 The database aims to centralize any available excipient safety and toxicity data, and to organize these data into a searchable, publicly available system. Similarly, the European Study of Neonatal Excipient Exposure project aims to assess neonatal exposure to potentially toxic excipients.102 As a part of the project, blood samples will be collected from neonates receiving select excipients (ie, propylene glycol, ethanol, propylhydroxybenzoate, sodium benzoate, polysorbate 80, and sorbitol) to determine how excipients are handled in neonates. Both the STEP database and European Study of Neonatal Excipient Exposure project have included ethanol as a prioritized excipient, highlighting the importance of additional research on ethanol use in neonates and infants.
Conclusions
The STEP database along with available pharmacokinetic data may provide sufficient evidence for a more rational use of ethanol in this venerable population. Until the effects of ethanol have been more clearly defined, formulations using ethanol should be minimized and the risk–benefit assessment should be taken into account.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
Dr. Marek was supported by National Institutes of Health Postdoctoral training grant no. T32GM008562. Elizabeth Marek and Walter Kraft contributed equally to this manuscript. Walter K. Kraft was supported by NIH/NIDA RO1 DA029076-01A1. We would like to acknowledge Jennifer Wilson of Thomas Jefferson University. She helped in reviewing the manuscript.
References
- 1.Ghosh T.K., Jasti B.R. CRC Press; Boca Raton: 2005. Theory and practice of contemporary pharmaceutics. [Google Scholar]
- 2.Remington: The science and practice of pharmacy. 21st ed. Philadelphia: Lippincott Williams & Wilkins; c2006. Remington, Joseph P. (Joseph Price),1847-1918. and Beringer Paul, eds.
- 3.Rayar P., Ratnapalan S. Pediatric ingestions of house hold products containing ethanol: A review. Clin Pediatr (Phila) 2013;52(3):203–209. doi: 10.1177/0009922812470970. [DOI] [PubMed] [Google Scholar]
- 4.Bitunjac K., Saraga M. Alcohol intoxication in pediatric age: Ten-year retrospective study. Croat Med J. 2009;50(2):151–156. doi: 10.3325/cmj.2009.50.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Committee on Drugs Ethanol in liquid preparations intended for children. Pediatrics. 1984;73(3):405–407. [PubMed] [Google Scholar]
- 6.Committee for Human Medicinal Products (CHMP). Questions and answers on ethanol in the context of the revision of the guideline on ‘Excipients in the label and package leaflet of medicinal products for human use’ (CPMP/463/00). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/02/WC500162033.pdf. Accessed 08/22, 2014.
- 7.Isaac R., Khan I., Langley C. Ethanol intake of paediatric intensive care patients. Archives of Disease in Childhood. 2013;98(6) 11-11. [Google Scholar]
- 8.International Center for Alcohol Policies . International drinking guidelines. International Center for Alcohol Policies; Washington, D.C.: 2003. [Google Scholar]
- 9.Whittaker A., Currie A.E., Turner M.A., Field D.J., Mulla H., Pandya H.C. Toxic additives in medication for preterm infants. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F236–F240. doi: 10.1136/adc.2008.146035. [DOI] [PubMed] [Google Scholar]
- 10.United States, Department of Health and Human Services, Department of Agriculture, Dietary Guidelines Advisory Committee . U.S. Dept. of Health and Human Services, U.S. Dept. of Agriculture; [Washington, D.C.]: 2010. Dietary Guidelines for Americans, 2010. [Google Scholar]
- 11.Swift R. Direct measurement of alcohol and its metabolites. Addiction. 2003;98(Suppl 2):73–80. doi: 10.1046/j.1359-6357.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones A.W., Jonsson K.A., Kechagias S. Effect of high-fat, high-protein, and high-carbohydrate meals on the pharmacokinetics of a small dose of ethanol. Br J Clin Pharmacol. 1997;44(6):521–526. doi: 10.1046/j.1365-2125.1997.t01-1-00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson R.D., Horowitz M., Maddox A.F., Wishart J.M., Shearman D.J. Cigarette smoking and rate of gastric emptying: Effect on alcohol absorption. BMJ. 1991;302(6767):20–23. doi: 10.1136/bmj.302.6767.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kechagias S., Jonsson K.A., Norlander B., Carlsson B., Jones A.W. Low-dose aspirin decreases blood alcohol concentrations by delaying gastric emptying. Eur J Clin Pharmacol. 1997;53(3-4):241–246. doi: 10.1007/s002280050369. [DOI] [PubMed] [Google Scholar]
- 15.Klockhoff H., Naslund I., Jones A.W. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54(6):587–591. doi: 10.1046/j.1365-2125.2002.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelbroek M.A., Horowitz M., Wishart J.M., Akkermans L.M. Effects of erythromycin on gastric emptying, alcohol absorption and small intestinal transit in normal subjects. J Nucl Med. 1993;34(4):582–588. [PubMed] [Google Scholar]
- 17.Amir I., Anwar N., Baraona E., Lieber C.S. Ranitidine increases the bioavailability of imbibed alcohol by accelerating gastric emptying. Life Sci. 1996;58(6):511–518. doi: 10.1016/0024-3205(95)02316-x. [DOI] [PubMed] [Google Scholar]
- 18.Roine R.P., Gentry R.T., Lim R.T., Jr, Helkkonen E., Salaspuro M., Lieber C.S. Comparison of blood alcohol concentrations after beer and whiskey. Alcohol Clin Exp Res. 1993;17(3):709–711. doi: 10.1111/j.1530-0277.1993.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 19.Levine B. American Association for Clinical Chemistry; [Washington, D.C.]: 1999. Principles of forensic toxicology. [Google Scholar]
- 20.Frezza M., di Padova C., Pozzato G., Terpin M., Baraona E., Lieber C.S. High blood alcohol levels in women. the role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322(2):95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 21.Caballeria J., Frezza M., Hernandez-Munoz R. Gastric origin of the first-pass metabolism of ethanol in humans: Effect of gastrectomy. Gastroenterology. 1989;97(5):1205–1209. doi: 10.1016/0016-5085(89)91691-0. [DOI] [PubMed] [Google Scholar]
- 22.Levitt D.G. PKQuest: Measurement of intestinal absorption and first pass metabolism - application to human ethanol pharmacokinetics. BMC Clin Pharmacol. 2002;2:4. doi: 10.1186/1472-6904-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norberg A., Jones A.W., Hahn R.G., Gabrielsson J.L. Role of variability in explaining ethanol pharmacokinetics: Research and forensic applications. Clin Pharmacokinet. 2003;42(1):1–31. doi: 10.2165/00003088-200342010-00001. [DOI] [PubMed] [Google Scholar]
- 24.Kyle U.G., Genton L., Slosman D.O., Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition. 2001;17(7-8):534–541. doi: 10.1016/s0899-9007(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 25.Hacker, Miles P, Messer, William S, Bachmann, Kenneth A. Pharmacology principles and practice. Updated 2009.
- 26.Cederbaum A.I. Alcohol metabolism. Clin Liver Dis. 2012;16(4):667–685. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maenhout T.M., De Buyzere M.L., Delanghe J.R. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322–329. doi: 10.1016/j.cca.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Ingall G.B. Alcohol biomarkers. Clin Lab Med. 2012;32(3):391–406. doi: 10.1016/j.cll.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Gershman H., Steeper J. Rate of clearance of ethanol from the blood of intoxicated patients in the emergency department. J Emerg Med. 1991;9(5):307–311. doi: 10.1016/0736-4679(91)90371-l. [DOI] [PubMed] [Google Scholar]
- 30.Jones A.W. Ultra-rapid rate of ethanol elimination from blood in drunken drivers with extremely high blood-alcohol concentrations. Int J Legal Med. 2008;122(2):129–134. doi: 10.1007/s00414-007-0181-7. [DOI] [PubMed] [Google Scholar]
- 31.Lands W.E. A review of alcohol clearance in humans. Alcohol. 1998;15(2):147–160. doi: 10.1016/s0741-8329(97)00110-9. [DOI] [PubMed] [Google Scholar]
- 32.Levitt M.D., Levitt D.G. Use of a two-compartment model to assess the pharmacokinetics of human ethanol metabolism. Alcohol Clin Exp Res. 1998;22(8):1680–1688. [PubMed] [Google Scholar]
- 33.Lee B.Y., Yoon H.K., Baek I.H., Kwon K.I. Population pharmacokinetics of multiple alcohol intake in humans. Alcohol. 2013;47(2):159–165. doi: 10.1016/j.alcohol.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Seng K.Y., Limenta L.M., Heng D., Lee E.J. Population pharmacokinetics and pharmacogenetics of alcohol in Chinese and Indians in Singapore. J Clin Pharm Ther. 2013;38(2):141–149. doi: 10.1111/jcpt.12003. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y.C., Peng G.S., Wang M.F., Tsao T.P., Yin S.J. Polymorphism of ethanol-metabolism genes and alcoholism: Correlation of allelic variations with the pharmacokinetic and pharmacodynamic consequences. Chem Biol Interact. 2009;178(1-3):2–7. doi: 10.1016/j.cbi.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Crabb D.W., Matsumoto M., Chang D., You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc. 2004;63(1):49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- 37.Lieber C.S. Microsomal ethanol-oxidizing system (MEOS): The first 30 years (1968-1998)--a review. Alcohol Clin Exp Res. 1999;23(6):991–1007. [PubMed] [Google Scholar]
- 38.Matsumoto H., Fukui Y. Pharmacokinetics of ethanol: A review of the methodology. Addict Biol. 2002;7(1):5–14. doi: 10.1080/135562101200100553. [DOI] [PubMed] [Google Scholar]
- 39.Lieber C.S. The discovery of the microsomal ethanol oxidizing system and its physiologic and pathologic role. Drug Metab Rev. 2004;36(3-4):511–529. doi: 10.1081/dmr-200033441. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi M., Lasker J.M., Shimizu M., Rosman A.S., Lieber C.S. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology. 1989;10(4):437–446. doi: 10.1002/hep.1840100407. [DOI] [PubMed] [Google Scholar]
- 41.Kessova I., Cederbaum A.I. CYP2E1: Biochemistry, toxicology, regulation and function in ethanol-induced liver injury. Curr Mol Med. 2003;3(6):509–518. doi: 10.2174/1566524033479609. [DOI] [PubMed] [Google Scholar]
- 42.Lieber C.S. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257(1):59–84. doi: 10.1016/s0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- 43.Zimatkin S.M., Pronko S.P., Vasiliou V., Gonzalez F.J., Deitrich R.A. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res. 2006;30(9):1500–1505. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- 44.Correa M., Salamone J.D. Segovia KN, et al. Piecing together the puzzle of acetaldehyde as a neuroactive agent. Neurosci Biobehav Rev. 2012;36(1):404–430. doi: 10.1016/j.neubiorev.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Marchitti S.A., Brocker C., Stagos D., Vasiliou V. Non-P450 aldehyde oxidizing enzymes: The aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4(6):697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones A.W. Excretion of alcohol in urine and diuresis in healthy men in relation to their age, the dose administered and the time after drinking. Forensic Sci Int. 1990;45(3):217–224. doi: 10.1016/0379-0738(90)90177-z. [DOI] [PubMed] [Google Scholar]
- 47.Van den Anker J.N. Developmental pharmacology. Dev Disabil Res Rev. 2010;16(3):233–238. doi: 10.1002/ddrr.122. [DOI] [PubMed] [Google Scholar]
- 48.Besunder J.B., Reed M.D., Blumer J.L. Principles of drug biodisposition in the neonate. A critical evaluation of the pharmacokinetic-pharmacodynamic interface. Clin Pharmacokinet. 1988;14(4):189–216. doi: 10.2165/00003088-198814040-00001. [DOI] [PubMed] [Google Scholar]
- 49.Besunder J.B., Reed M.D., Blumer J.L. Principles of drug biodisposition in the neonate. A critical evaluation of the pharmacokinetic-pharmacodynamic interface (part II) Clin Pharmacokinet. 1988;14(5):261–286. doi: 10.2165/00003088-198814050-00001. [DOI] [PubMed] [Google Scholar]
- 50.Funk R.S., Brown J.T., Abdel-Rahman S.M. Pediatric pharmacokinetics: Human development and drug disposition. Pediatr Clin North Am. 2012;59(5):1001–1016. doi: 10.1016/j.pcl.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Signer E., Fridrich R. Gastric emptying in newborns and young infants. Acta Pædiatrica. 1975;64(3):525–530. doi: 10.1111/j.1651-2227.1975.tb03874.x. [DOI] [PubMed] [Google Scholar]
- 52.Kaye J.L. Review of paediatric gastrointestinal physiology data relevant to oral drug delivery. Int J Clin Pharm. 2011;33(1):20–24. doi: 10.1007/s11096-010-9455-0. [DOI] [PubMed] [Google Scholar]
- 53.Martinussen M., Brubakk A.M., Linker D.T., Vik T., Yao A.C. Mesenteric blood flow velocity and its relation to circulatory adaptation during the first week of life in healthy term infants. Pediatr Res. 1994;36(3):334–339. doi: 10.1203/00006450-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Friis-Hansen B. Body water compartments in children: Changes during growth and related changes in body composition. Pediatrics. 1961;28(2):169–181. [PubMed] [Google Scholar]
- 55.Murphy, John E., American Society of Health-System Pharmacists . American Society of Health-System Pharmacists; Bethesda, MD: 2008. Clinical pharmacokinetics. [Google Scholar]
- 56.Tayman C., Rayyan M., Allegaert K. Neonatal pharmacology: Extensive interindividual variability despite limited size. J Pediatr Pharmacol Ther. 2011;16(3):170–184. doi: 10.5863/1551-6776-16.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pikkarainen P.H., Raiha N.C. Development of alcohol dehydrogenase activity in the human liver. Pediatr Res. 1967;1(3):165–168. doi: 10.1203/00006450-196705000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Tran M.N., Wu A.H., Hill D.W. Alcohol dehydrogenase and catalase content in perinatal infant and adult livers: Potential influence on neonatal alcohol metabolism. Toxicol Lett. 2007;169(3):245–252. doi: 10.1016/j.toxlet.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Smith M., Hopkinson D.A., Harris H. Developmental changes and polymorphism in human alcohol dehydrogenase. Ann Hum Genet. 1971;34(3):251–271. doi: 10.1111/j.1469-1809.1971.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 60.Alcorn J., McNamara P.J. Ontogeny of hepatic and renal systemic clearance pathways in infants: Part I. Clin Pharmacokinet. 2002;41(12):959–998. doi: 10.2165/00003088-200241120-00003. [DOI] [PubMed] [Google Scholar]
- 61.Vieira I., Sonnier M., Cresteil T. Developmental expression of CYP2E1 in the human liver. hypermethylation control of gene expression during the neonatal period. Eur J Biochem. 1996;238(2):476–483. doi: 10.1111/j.1432-1033.1996.0476z.x. [DOI] [PubMed] [Google Scholar]
- 62.Hines R.N., McCarver D.G. The ontogeny of human drug-metabolizing enzymes: Phase I oxidative enzymes. J Pharmacol Exp Ther. 2002;300(2):355–360. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- 63.Food and Drug Administration. Drug safety communication: serious health problems seen in premature babies given Kaletra (lopinavir/ritonavir) oral solution. http://www.fda.gov/Drugs/DrugSafety/ucm246002.htm. Accessed 08/14, 2014.
- 64.Milsap R.L., Jusko W.J. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(Suppl 11):107–110. doi: 10.1289/ehp.94102s11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhodin M.M., Anderson B.J., Peters A.M. Human renal function maturation: A quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 66.Reiter P.D. Neonatal pharmacology and pharmacokinetics. NeoReviews. 2002;3(11):e229–e236. [Google Scholar]
- 67.Rayar P., Ratnapalan S. Pediatric ingestions of house hold products containing ethanol: A review. Clin Pediatr (Phila) 2013;52(3):203–209. doi: 10.1177/0009922812470970. [DOI] [PubMed] [Google Scholar]
- 68.Vogel C., Caraccio T., Mofenson H., Hart S. Alcohol intoxication in young children. J Toxicol Clin Toxicol. 1995;33(1):25–33. doi: 10.3109/15563659509020212. [DOI] [PubMed] [Google Scholar]
- 69.Ragan F.A., Jr, Samuels M.S., Hite S.A. Ethanol ingestion in children. A five-year review. JAMA. 1979;242(25):2787–2788. [PubMed] [Google Scholar]
- 70.Leung A.K. Ethyl alcohol ingestion in children. A 15-year review. Clin Pediatr (Phila) 1986;25(12):617–619. doi: 10.1177/000992288602501207. [DOI] [PubMed] [Google Scholar]
- 71.Fong H.F., Muller A.A. An unexpected clinical course in a 29-day-old infant with ethanol exposure. Pediatr Emerg Care. 2014;30(2):111–113. doi: 10.1097/PEC.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 72.Ford J.B., Wayment M.T., Albertson T.E., Owen K.P., Radke J.B., Sutter M.E. Elimination kinetics of ethanol in a 5-week-old infant and a literature review of infant ethanol pharmacokinetics. Case Rep Med. 2013;2013:250716. doi: 10.1155/2013/250716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCormick T., Levine M., Knox O. Claudius I. Ethanol ingestion in two infants under 2 months old: A previously unreported cause of ALTE. Pediatrics. 2013;131(2):e604–e607. doi: 10.1542/peds.2012-1652. [DOI] [PubMed] [Google Scholar]
- 74.Chikwava K., Lower D.R., Frangiskakis S.H., Sepulveda J.L., Virji M.A., Rao K.N. Acute ethanol intoxication in a 7-month-old infant. Pediatr Dev Pathol. 2004;7(4):400–402. doi: 10.1007/s10024-004-1017-9. [DOI] [PubMed] [Google Scholar]
- 75.Kvigne V.L., Randall B., Simanton E.G., Brenneman G., Welty T.K. Blood alcohol levels for American Indian mothers and newborns. Pediatrics. 2012;130(4):e1015–e1018. doi: 10.1542/peds.2011-1400. [DOI] [PubMed] [Google Scholar]
- 76.Burd L., Blair J., Dropps K. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J Perinatol. 2012;32(9):652–659. doi: 10.1038/jp.2012.57. [DOI] [PubMed] [Google Scholar]
- 77.Ruz J., Fernandez A., Luque de Castro M.D., Valcarcel M. Determination of ethanol in human fluids--I. determination of ethanol in blood. J Pharm Biomed Anal. 1986;4(5):545–558. doi: 10.1016/0731-7085(86)80001-2. [DOI] [PubMed] [Google Scholar]
- 78.Tagliaro F., Lubli G., Ghielmi S., Franchi D., Marigo M. Chromatographic methods for blood alcohol determination. J Chromatogr. 1992;580(1-2):161–190. doi: 10.1016/0378-4347(92)80534-w. [DOI] [PubMed] [Google Scholar]
- 79.Cordell R.L., Pandya H., Hubbard M., Turner M.A., Monks P.S. GC-MS analysis of ethanol and other volatile compounds in micro-volume blood samples--quantifying neonatal exposure. Anal Bioanal Chem. 2013;405(12):4139–4147. doi: 10.1007/s00216-013-6809-1. [DOI] [PubMed] [Google Scholar]
- 80.Geertinger P., Bodenhoff J., Helweg-Larsen K., Lund A. Endogenous alcohol production by intestinal fermentation in sudden infant death. Z Rechtsmed. 1982;89(3):167–172. doi: 10.1007/BF01873798. [DOI] [PubMed] [Google Scholar]
- 81.Jones A.W., Mardh G., Anggard E. Determination of endogenous ethanol in blood and breath by gas chromatography-mass spectrometry. Pharmacol Biochem Behav. 1983;18(Suppl 1):267–272. doi: 10.1016/0091-3057(83)90184-3. [DOI] [PubMed] [Google Scholar]
- 82.Simic M., Ajdukovic N., Veselinovic I., Mitrovic M., Djurendic-Brenesel M. Endogenous ethanol production in patients with diabetes mellitus as a medicolegal problem. Forensic Sci Int. 2012;216(1-3):97–100. doi: 10.1016/j.forsciint.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Jansson-Nettelbladt E., Meurling S., Petrini B., Sjolin J. Endogenous ethanol fermentation in a child with short bowel syndrome. Acta Paediatr. 2006;95(4):502–504. doi: 10.1080/08035250500501625. [DOI] [PubMed] [Google Scholar]
- 84.Zhu L., Baker S.S., Gill C. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe-Suzuki K., Seno H., Ishii A., Kumazawa T., Suzuki O. Ultra-sensitive method for determination of ethanol in whole blood by headspace capillary gas chromatography with cryogenic oven trapping. J Chromatogr B Biomed Sci Appl. 1999;727(1-2):89–94. doi: 10.1016/s0378-4347(99)00063-8. [DOI] [PubMed] [Google Scholar]
- 86.Riley E.P., Infante M.A., Warren K.R. Fetal alcohol spectrum disorders: An overview. Neuropsychol Rev. 2011;21(2):73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guerri C., Bazinet A., Riley E.P. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44(2):108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de la Monte S.M., Kril J.J. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127(1):71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katzung B.G., Masters S.B., Trevor A.J. McGraw-Hill Medical; McGraw-Hill distributor; New York; London: 2012. Basic & clinical pharmacology. [Google Scholar]
- 90.Diamond I., Messing R.O. Neurologic effects of alcoholism. West J Med. 1994;161(3):279–287. [PMC free article] [PubMed] [Google Scholar]
- 91.Swift R. Direct measurement of alcohol and its metabolites. Addiction. 2003;98(Suppl 2):73–80. doi: 10.1046/j.1359-6357.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- 92.Edenberg H.J. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- 93.Setshedi M., Wands J.R., Monte S.M. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3(3):178–185. doi: 10.4161/oxim.3.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Symposium on Acetaldehyde Related Pathology: Bridging the Trans-Disciplinary Divide, Chadwick, Derek., Goode, Jamie. Novartis Foundation. Acetaldehyde-related pathology: Bridging the trans-disciplinary divide. 2007.
- 95.Hamby-Mason R., Chen J.J., Schenker S., Perez A., Henderson G.I. Catalase mediates acetaldehyde formation from ethanol in fetal and neonatal rat brain. Alcohol Clin Exp Res. 1997;21(6):1063–1072. [PubMed] [Google Scholar]
- 96.Maxwell C.R., Spangenberg R.J., Hoek J.B., Silberstein S.D., Oshinsky M.L. Acetate causes alcohol hangover headache in rats. PLoS One. 2010;5(12):e15963. doi: 10.1371/journal.pone.0015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gershanik J., Boecler B., Ensley H., McCloskey S., George W. The gasping syndrome and benzyl alcohol poisoning. N Engl J Med. 1982;307(22):1384–1388. doi: 10.1056/NEJM198211253072206. [DOI] [PubMed] [Google Scholar]
- 98.MacDonald M.G., Getson P.R., Glasgow A.M., Miller M.K., Boeckx R.L., Johnson E.L. Propylene glycol: Increased incidence of seizures in low birth weight infants. Pediatrics. 1987;79(4):622–625. [PubMed] [Google Scholar]
- 99.Allegaert K., Vanhaesebrouck S., Kulo A. Prospective assessment of short-term propylene glycol tolerance in neonates. Arch Dis Child. 2010;95(12):1054–1058. doi: 10.1136/adc.2010.190330. [DOI] [PubMed] [Google Scholar]
- 100.De Cock R.F., Knibbe C.A., Kulo A. Developmental pharmacokinetics of propylene glycol in preterm and term neonates. Br J Clin Pharmacol. 2013;75(1):162–171. doi: 10.1111/j.1365-2125.2012.04312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salunke S., Brandys B., Giacoia G., Tuleu C. The STEP (safety and toxicity of excipients for paediatrics) database: Part 2 - the pilot version. Int J Pharm. 2013;457(1):310–322. doi: 10.1016/j.ijpharm.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 102.Turner M.A., Duncan J., Shah U. European study of neonatal exposure to excipients: An update. Int J Pharm. 2013;457(1):357–358. doi: 10.1016/j.ijpharm.2013.08.078. [DOI] [PubMed] [Google Scholar]


