Abstract
Background
Sleep disturbance is a common feature of depression. However, recent work has found that individuals who are vulnerable to depression report poorer sleep quality compared to their low-risk counterparts, suggesting that sleep disturbance may precede depression. In addition, both sleep disturbance and depression are related to deficits in cognitive control processes. Thus we examined if poor sleep quality predicts subsequent increases in depressive symptoms and if levels of cognitive control mediated this relation.
Methods
Thirty-five undergraduate students participated in 2 experimental sessions separated by 3 weeks. Participants wore an actigraph watch between sessions, which provided an objective measure of sleep patterns. We assessed self-reported sleep quality and depressive symptoms at both sessions. Last, individuals completed an exogenous cuing task, which measured ability to disengage attention from neutral and negative stimuli during the second session.
Results
Using path analyses, we found that both greater self-reported sleep difficulty and more objective sleep stability measures significantly predicted greater difficulty disengaging attention (i.e., less cognitive control) from negative stimuli. Less cognitive control over negative stimuli in turn predicted increased depression symptoms at the second session. Exploratory associations among the circadian locomotor output cycles kaput gene, CLOCK, single nucleotide polymorphism (SNP), rs11932595, as well as sleep assessments and depressive symptoms also are presented.
Conclusions
These preliminary results suggest that sleep disruptions may contribute to increases in depressive symptoms via their impact on cognitive control. Further, variation in the CLOCK gene may be associated with sleep quality.
Keywords: sleep, circadian rhythm, actigraphy, cognitive control, depression, CLOCK gene
1. Introduction
Sleep disturbance is a common feature of depression and as such represents one of 9 symptoms of a depressive episode [1]. Some estimates suggest that up to 90% of individuals with major depression also are diagnosed with insomnia [2]. There also is empirical evidence that depression is associated with nonclinical sleep disturbances (e.g., reduced total sleep time, increased sleep-onset latency) [3]. Importantly, recent research shows that sleep disturbance may not only be a symptom of depression but may in fact precede the disorder. For example, Chen et al [4] found that daughters of depressed mothers reported poorer sleep quality than girls at low familial risk for depression. In addition, sleep restriction has been shown to increase depressive symptoms among otherwise healthy individuals [5]. In sum, sleep difficulties characterize depressive episodes and may serve as a phenotype of risk for the disorder. Therefore, our study sought to identify the underlying mechanisms by which sleep difficulties may contribute to depression symptomatology.
It is possible that sleep disturbance influences depressive symptoms via its impact on cognition. Indeed, sleep difficulties and depression have been independently linked to deficits in cognitive control. For example, sleep deficits are associated with poor performance on tasks measuring mental flexibility [6], working memory [7], attentional set shifting [8], and inhibition [9]. Depression also has been linked to cognitive control deficits, particularly during emotional information processing. Depressed individuals exhibit difficulties inhibiting the processing of negative material [10,11], disengaging attention from negative material [12] and removing negative material from working memory [13]. Given these results, our study examined if sleep difficulties are associated with reduced cognitive control over emotional stimuli and if these reductions are linked to increases in depressive symptoms.
Notably, much of the work on sleep and depression has relied on self-report measures. However, it is possible that negative reporting biases that often precede and characterize the disorder may account for observed group differences. Therefore, any study of sleep and depressive symptoms should consider including both self-report and objective measures of sleep disturbance. Actigraphy represents one method to objectively examine sleep in a naturalistic setting [14]. Actigraph devices use an accelerometer to measure movement across time and researchers can use movement data to infer periods of sleep and wakefulness. In contrast to other sleep methodologies (e.g., sleep deprivation, polysomnography [PSG]), actigraphy allows researchers to objectively measure naturalistic sleep outside the laboratory. In addition to measuring traditional sleep measures such as total sleep time, actigraphy also can provide measures of circadian rhythms [15]. Especially in the cognition literature, there is evidence that circadian rhythm measures account for outcomes above and beyond those accounted for by average sleep duration or changes in sleep duration [16–18]. Additionally, although some individuals have conceptualized depression as a disorder of disrupted circadian rhythms [19], research on the relation between sleep-wake cycles and depression is lacking. Therefore, our study utilized both subjective and objective measures of sleep difficulties and circadian rhythms to examine their relation to cognitive control and depressive symptoms.
Our study recruited a sample of undergraduate students to identify factors that may underlie the relation between sleep difficulties and depressive symptoms. In doing so, we first examined the relation between sleep and depressive symptoms. In line with previous findings, we hypothesized that poorer sleep quality and reduced sleep duration would predict increases in depressive symptoms. Second, because previous research has linked both sleep and depression to deficits in cognitive control, we hypothesized that cognitive control over negative stimuli would mediate the relation between sleep quality and change in depressive symptoms. As a final exploratory objective of the study, we explored if variation in a single nucleotide polymorphism (SNP) in the circadian locomotor output cycles kaput gene, CLOCK, relates to our primary variables of interest (i.e., sleep, cognitive control, depressive symptoms). The CLOCK SNP, rs11932595, was chosen because it has previously been linked to variation in daily sleep time [20].
2. Methods
2.1. Participants
Researchers posted flyers at The University of Texas at Austin advertising a study on sleep and cognition. Current undergraduate students at The University of Texas at Austin who were at least 18 years of age were eligible to participate in the study. Individuals who responded to the flyers via e-mail received a written description of the study. Individuals who remained interested in the study and who met inclusion criteria were scheduled for the first laboratory session.
Fifty-two individuals (22 women and 28 men) were originally enrolled into the study. One man and one woman failed to complete the second session, and consequentially they were not included in our sample. We excluded 13 individuals who were missing greater than 10% of their actigraph data, one individual who did not complete the emotional cuing task, and one individual with missing actigraph and emotional cuing task data. Therefore, we used a sample of 35 individuals (14 women and 21 men) who had complete data across all measures in our analyses. Participants were on average 19.83 years of age (standard deviation [SD], 1.25; range 18–23 y). Individuals received $80 for their participation in the study ($20 after completion of the first session and $60 after the second). The Institutional Review Board of the University of Texas at Austin approved all study procedures, and all participants provided both verbal and written informed consent.
2.2. Materials
2.2.1. Questionnaires
Participants completed 3 questionnaires. First, participants completed a demographics and health questionnaire. Second, they completed the Center for Epidemiologic Studies Depression Scale (CES-D) [21]. The CES-D is a 20-item self-report measure of depressive symptoms across the past week. The CES-D has an internal consistency of 0.85 in the general population and 0.90 in patient samples. It has moderate test-retest reliabilities, ranging from 0.45 to 0.70. Higher reliability is obtained when tested across shorter time periods. The CES-D has been found to moderately correlate with clinical interviewer ratings of depression [21]. Last, participants completed the Pittsburgh Sleep Quality Index (PSQI) [22]. The PSQI is a 19-item self-report measure of sleep quality and disturbances across the past month. Scores on individual items generate 7 component scores that together yield a global score of sleep quality. In our study, we used the global measure of sleep quality. The PSQI has an internal reliability coefficient of 0.83 and a test-retest reliability of 0.85 across approximately 1 month [22].
2.2.2. Actigraphy
We used Motionlogger Actigraphs (Ambulatory Monitoring, Inc., Ardsley, NY) to objectively measure real-world sleep patterns. Motionlogger Actigraphs are watch like actigraphs that are worn on the wrist. Participants were instructed to continuously wear an actigraph for the 3 weeks, which separated the first and second laboratory session. They were instructed to only remove the watch during situations when it could get wet or damaged. Motionlogger Actigraphs contain an internal accelerometer that generates a signal when motion is detected across 3 planes. Depending on the time resolution set, the actigraph surveys the number of summated voltages generated per unit time. For our study, we continuously examined 60-second epochs of movement data across the 3-week period. In addition, the Motionlogger Actigraph measures epidermal microvibrations to generate an index (LIFE) of whether or not someone is wearing the watch at any given time. We removed data in which the LIFE measure indicated the actigraph was off for periods greater than 30 minutes (missing data). As previously stated, individuals missing more than 10% of actigraph data were excluded from our sample.
2.2.2.1. Actigraph data generation
From the raw actigraph data, a number of measures were generated across the 3-week intersession period: 2 measures of sleep (average sleep duration and sleep standard deviation) and 2 circadian rhythm variables (described below). It is important to note that an actigraph does not directly measure sleep; it measures motor activity from which sleep-wake periods may be inferred. We used the Cole-Kripke PCD ZCM algorithm with an epoch length of 1 minute via Action 4 software (Ambulatory Monitoring, Inc., Ardsley, NY) to estimate sleep periods from activity data. This algorithm is widely used among automatic actigraphic sleep scoring [23]. Output from the algorithm defines each minute as either a period of sleep or wakefulness. From this, we calculated the mean and SD of 24-hour sleep periods across 3 weeks, resulting in an estimate of average daily sleep and its SD across the 3-week period.
To examine aspects of circadian rhythms, we applied the nonparametric circadian rhythm analysis provided as part of the Action 4 software [24]. Two measures were generated. The first was interdaily stability (IS), which reflects the degree to which individuals have a stable sleep-wake rhythm between 24-hour periods and the ratio between the variance of the average 24-hour pattern around the mean and the total variance [25,26]. This calculation ranges from 0 to 1, in which a higher value indicates a more consistent sleep-wake schedule. We also calculated intradaily variability (IV). The IV is the period of rest and sleep activity that is fragmented. It is calculated as a ratio of the mean squares of the difference between successive hours (first derivative) and the mean squares around the grand mean (overall variance) [26]. The value indicates how often and to what extent there are transitions between rest and activity. The calculation ranges from 0 to 2, with a higher value indicating more fragmented rhythms. These measures have previously been used to reveal differences in motor activity between schizophrenic and depressed individuals [27].
2.2.3. Emotional cuing task
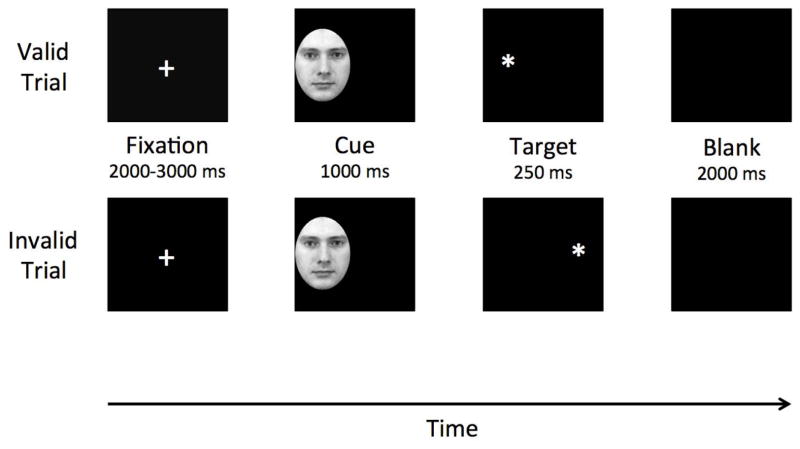
Originally developed by Posner [28], the emotional cuing task (ECT) has since been modified to incorporate emotional stimuli [12]. As such, the ECT measures individuals’ ability to disengage from irrelevant but emotionally salient stimuli. Each trial began with a fixation cross presented at the center of the computer screen. Fixation cross presentation varied from 2000 to 3000 ms. Next, either a neutral or sad face (cue) appeared on the left or right side of the visual field. These cues were presented for 1000 ms. After the cue disappeared, either a single or double asterisk (target) appeared in the same location as the cue (valid cue trial) or on the opposite side of visual field (invalid cue trial) for 250 ms. Participants were instructed to identify target type as quickly and accurately as possible, by pressing the “1” key if one asterisk appeared and the “2” key if two asterisks appeared. Participants had 2000 ms to respond. Fig. 1 provides an example of a valid and invalid cue trial. Cue stimuli were faces from the Karolinska Directed Emotional Faces set [29]. We converted images into black and white, cropped the images around the facial expression to remove any distracting and extraneous features (e.g., hair, ears), and fit into an oval with a height of 205 pixels and a width of 155 pixels (see Fig. 1). There were 48 sad face cues and 48 neutral face cues.
Fig. 1.
Trial sequence for valid and invalid trials on the Emotional Cuing Task. Faces and targets are not to scale for purposes of this Fig.
The main task was composed of 192 trials (128 valid cue trials and 64 invalid cue trials). Cues and targets were with equal frequency on the left and right side of the screen. Each cue was presented twice, and cue stimuli were randomized across trials. Participants first completed 10 practice trials of the task. Participants must have correctly identified the target on 8 of the 10 trials to advance to the main task. Individuals who failed to correctly identify the target on at least 8 trials had to repeat the set of 10 practice trials before they could advance to the main task.
To assess cognitive control, we computed a cue validity score for each individual for each cue type using the following formula: cue validity (CV)=mean RT (invalid trials)−mean RT (valid trials). Positive CV scores putatively reflected difficulty disengaging attention from invalid cues to identify the target on opposite side of visual field. Lower CV scores reflected relatively little difficulty with attention disengagement. We focused on difficulty with disengagement from sad cues, as this is the primary cognitive control deficit typically observed in depression [30].
2.2.4. Genotyping
Ethanol precipitation was used to extract DNA from collected saliva samples. Samples were genotyped using a Mass EXTENDSequenom assay based on the annealing of an oligonucleotide primer adjacent to the SNP of interest. The addition of DNA polymerase along with a mixture of terminator nucleotides allows extension of the primer through the polymorphic site and generates allele-specific extension products, each having a unique molecular mass. The resultant masses of the extension products are then analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and a genotype is assigned. The assay was performed in multiplex with 27 reactions in a single well. Primer sequences for rs11932595 are as follows: polymerase chain reaction (PCR) primer 1 (forward): ACGTTGGATGATCTCATCCATCTTGAGTGC; PCR primer 2 (reverse): ACGTTGGATGAGACTGGTGAACACTGTAGC; and extension primer: ttGTTTAGACCCCTGCC. The genotyping fail rate was 2.80% (n=1) for rs11932595 for all included participants. Genotypes were determined by investigators blinded to phenotypic data.
2.4. Procedure
Our study consisted of 2 experimental sessions, which were separated by 3 weeks. In addition to the measures discussed here, participants also completed a number of cognitive and affective measures both at baseline and after the 3-week period. A list of these additional measures can be made available by the authors on request. During the first experimental session (time 1), the study was described to participants who then provided informed consent, completed a set of questionnaires (demographics and health questionnaire, CES-D and PSQI), and were equipped with an actigraph watch. Between the 2 experimental sessions, participants continuously wore the actigraph and completed a daily sleep log, in which they reported the duration of each sleep period. Following this 3-week period, participants were scheduled for a second experimental session (time 2), when they completed the CES-D, the PSQI and the ECT. Participants also returned their actigraph watch and gave two 2-mL saliva samples for genotyping. The first laboratory session lasted approximately 30 minutes and the second session lasted approximately 90 minutes. Individuals were compensated, debriefed, and thanked for their participation.
3. Results
3.1. Descriptive statistics
Means, SDs, and correlations among the relevant variables are presented in Table 1. We first examined the relation between sleep and depressive symptoms. Depressive symptoms and self-reported sleep difficulties were moderately correlated. Total sleep was moderately associated with depression symptoms at time 2 but fell short of statistical significance (P=.08). Sleep stability (IS), fragmentation (IV), and variability (SD) were weakly associated with depressive symptoms at both time points. Cognitive control over negative stimuli was associated with depressive symptoms at time 2, self-reported sleep difficulties, and sleep stability. Mean depressive symptoms were relatively stable from time 1 to time 2, as there was only a point difference in CES-D between sessions.
Table 1.
Correlations, means, and standard deviations for depression severity, self-reported sleep difficulty, cognitive control of emotion stimuli, objective sleep measures, and the clock circadian locomotor output cycles kaput gene, CLOCK, single nucleotide polymorphisms.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. CESD-T1 | |||||||||
| 2. CESD-T2 | .78* | ||||||||
| 3. PSQI-G | .32 | .43* | |||||||
| 4. CV (ms) | .24 | .53* | .41* | ||||||
| 5. IS | −.00 | .20 | .14 | .46* | |||||
| 6. IV | .12 | −.04 | −.20 | −.30 | −.67* | ||||
| 7. Sleep duration | .19 | .29 | .04 | .08 | .30 | −.25 | |||
| 8. Sleep variability | .18 | −.02 | .05 | −.23 | −.51* | .44* | −.16 | ||
| 9. rs11932595 | .13 | .02 | .35* | .07 | .10 | −.17 | .21 | −.10 | |
|
| |||||||||
| Mean | 13.34 | 12.43 | 5.37 | 17.04 | 0.55 | 0.50 | 7.16 | 1.94 | 1.42 |
| SD | 8.75 | 7.71 | 1.78 | 34.27 | 0.13 | 0.08 | 0.79 | 0.51 | 0.61 |
Abbreviations: CES-D, Center for Epidemiological Studies–Depression Scale; PSQI-G, Pittsburgh Sleep Quality Inventory–General Functioning; CV, cue validity; IS, interdaily stability; IV, intradaily variability; SD, standard deviation.
Sleep duration is mean sleep duration; sleep variability is sleep standard deviation (SD).
indicates P<.05; n=35 for all correlations except those with rs11932595, in which n=34.
3.2. Path analyses
Path analysis was used to test hypotheses about relations between sleep, cognitive control, and depressive symptoms. Specifically, we examined if self-reported sleep difficulties and sleep stability contributed to altered cognitive control over negative stimuli, which in turn predicted changes in depressive symptoms. Several indices often are used to determine quality of path model fit. Among the most commonly used are the χ2 test, the comparative fit index (CFI), and root mean squared error of approximation (RMSEA). A model fit that includes CFI ≥.90 and RMSEA ≤.10 generally is acceptable [31]. These criteria were used in our study.
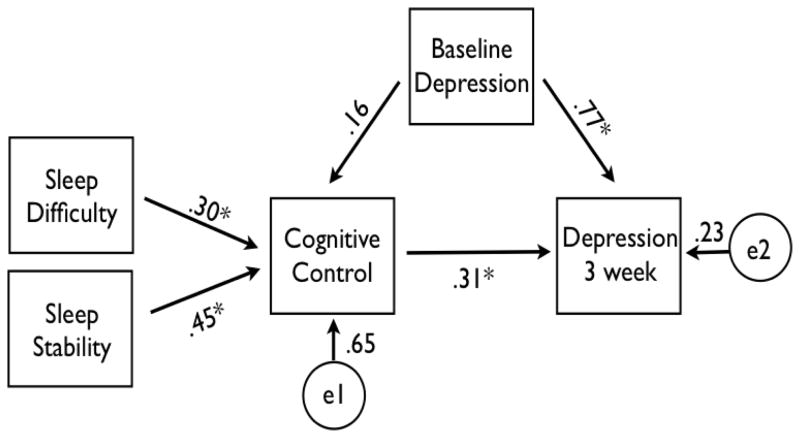
The initial path model posited direct effects between self-reported sleep difficulties and sleep stability to cognitive control over negative stimuli. A direct effect between cognitive control over negative stimuli and depressive symptoms at time 2 controlling for depression symptoms at time 1 also was estimated. This model is presented in Fig. 2 and provides good fit to the data χ2 ([df=2]=0.95, P=.62; RMSEA=.00, CFI=1.00). Greater sleep difficulty and more sleep stability both significantly predicted greater difficulty disengaging attention (i.e., less cognitive control) from negative stimuli. Less cognitive control over sad stimuli in turn predicted greater depressive symptoms at the 3-week time point, controlling for baseline depressive symptoms. Depressive symptoms at time 1 were not strongly correlated with cognitive control after 3 weeks, but they were strongly correlated with time 2 depressive symptoms.
Fig. 2.
Path analysis predicting change in depressive symptoms.
A separate nested model found that removing the path from cognitive control over negative stimuli to time 2 depressive symptoms significantly degraded model fit Δχ2 ([df=1]=12.06; P<.001). This finding suggests that the initial model should be retained, and more importantly cognitive control over emotion stimuli provides significant prediction of time 2 depressive symptoms beyond the contribution of baseline depressive symptoms.
Finally, we tested for indirect effects among associations in the initial model for which there was a possible intervening (or mediating) variable. For time 2 depressive symptoms, there was a marginally significant indirect effect for self-reported sleep difficulty (P=.07) and a significant indirect effect for sleep stability (P=.014). Cognitive control over negative stimuli was the intervening variable for these indirect effects. Taken together, these data suggest that sleep quality and circadian rhythm measures (self-reported and objectively measured, respectively) over a 3-week period can contribute to increases in depression symptoms via its impact on cognitive control (see Fig. 2).
3.3. Exploratory SNP analyses
Individuals were genotyped for polymorphisms in the CLOCK gene SNP, rs11932595. Genotype data were missing for 1 participant, so the following analyses reflect data from 34 participants. Given the association between the CLOCK gene and sleep, we were primarily interested in the association between the CLOCK gene variation and sleep variables; however, we also included other variables for exploratory analyses. For correlation analyses, rs11932595 was coded to reflect number of G alleles (i.e., 0, 1, 2). The rs11932595 was not associated with any objective measures of sleep, cognitive control, or depressive symptoms at either time point. However, number of G alleles in rs11932595 was positively associated with self-reported sleep difficulty. Correlations between rs11932595, self-reported sleep quality, cognitive control, and depressive symptoms are presented in Table 1.
4. Discussion
Our study explored the relation among sleep, cognitive control, and depressive symptoms in a 2-session study with a sample of undergraduate students. First, we found that self-reported sleep quality between the 2 laboratory sessions predicted depressive symptoms at time 2 after controlling for depressive symptoms at time 1. Specifically, poorer sleep quality was associated with greater increases in depressive symptoms. This finding supports those of Chen et al [4], suggesting that sleep disturbance is not solely a by-product of depression but instead may precede the disorder.
Although clinically reduced sleep (i.e., insomnia) also has been found to predict depression [32], we did not observe a relation between sleep duration and change in depressive symptoms. However, it is possible that insomnia may be a unique predictor of depressive symptoms, as opposed to nonclinical sleep reductions, and that the null finding in our study is a result of its nonclinical sample. Previous research has found adequate correspondence between insomnia and PSQI global scores greater than 8 [33], and indeed only one participant in the sample was above this cutoff point. In fact, our null finding is consistent with that of Chen et al [4], who found no differences in sleep duration between individuals at high and low risks for depression.
Second, we tested a comprehensive theoretically informed model which incorporated sleep, cognitive control, and depressive symptoms. Poor sleep quality was associated with greater difficulty disengaging attention from negative stimuli, which in turn predicted increases in depressive symptoms. An indirect relation between sleep-wake cycle stability (IS) and depressive symptoms also was observed. IS did not predict depressive symptoms at either time point. However, it did predict cognitive control over emotional information, which again predicted changes in depressive symptoms. Interestingly, stable sleepers exhibited poorer cognitive control than individuals with less consistent sleep patterns. We hypothesize that a third possibly trait like variable (e.g., behavioral inhibition) may be associated with both sleep stability and cognitive control, and therefore may account for this unexpected relation. However, this possibility is merely speculation and thus reflects an important area for future research. In sum, altered sleep quality may contribute to the development of depression by impairing cognitive control.
Finally, we explored the relation of the CLOCK gene to the variables of interest in our study. Although the CLOCK gene SNP, rs11932595, has previously been linked to sleep duration [20], it did not correlate with any objective measures of sleep in our study. Further, it was not related to cognitive control or depressive symptoms at either time point. However, rs11932595 was associated with sleep quality, such that a greater number of G alleles was associated with higher levels of self-reported sleep difficulties. In a recent commentary, Goel [34] noted that the fields of sleep and mood disorders share the overlap of phenotypes and genotypes and that future research should “exploit these commonalities.” Therefore, our study presents these findings as preliminary evidence to suggest that polymorphisms within the CLOCK gene may influence sleep quality. Future research should continue to explore the relation among these variables within a single model to develop a more comprehensive understanding of depression than 1-dimensional models that focus on one particular domain.
There are several limitations to our study. Perhaps the most important limitation is sample size. Indeed, effects in smaller samples are at greater risk for type I error than those initially found in larger samples [35]. In addition, we provided monetary compensation for participation in the study, and such financial incentive may have resulted in selection bias in the sample. Replication with a larger randomly selected sample is necessary before further development of the proposed model. Second, our study used correlational analyses and several of the associations tested in the path model were cross-sectional. As such, causality cannot be inferred. Future research using experimental manipulations (e.g., sleep deprivation, cognitive training paradigms) therefore is needed to better understand the causal role that sleep and cognition play in the development of depressive symptoms. Third, examination of sleep quality and depressive symptoms were limited to self-report assessments. Future work also should consider obtaining interviewer-based assessments, as self-report and interviewer ratings often do not entirely overlap and may provide unique information. A final limitation was the use of actigraphy over PSG to examine sleep characteristics. One weakness of actigraphy is that long periods of stillness may be inferred as sleep even though an individual may actually be awake, potentially resulting in an overestimation of sleep. However, previous findings show that the correspondence between actigraphy and PSG in differentiating sleep from wake is high [36], and there are in fact some noteworthy advantages to using actigraphy over PSG. For example, actigraphy allows an individual to measure sleep patterns outside of the laboratory for 24 hours a day across extended periods of time. Nevertheless, more research comparing the validity and reliability of actigraphy to PSG is needed.
Despite these limitations, we present a model in which perceived sleep quality and sleep-wake cycle stability predicts cognitive control over negative stimuli, which in turn predicts changes in depressive symptoms. To our knowledge, our study is the first to report that sleep disturbance may contribute to changes in depressive symptoms via its impact on cognitive control over emotional stimuli. In addition, a SNP within the CLOCK gene was associated with sleep quality. We believe our proposed model and exploratory SNP analyses are an excellent starting point for the development of interdisciplinary models of depression vulnerability that incorporate findings across levels of analysis.
Acknowledgments
The authors would like to thank the Chief of the Army—Grant to West Point Network Science Center, subcontracted to The University of Texas at Austin, for funding this study.
Footnotes
Conflict of interest
The authors report no conflict of interest.
References
- 1.Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–69. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 3.Karaz SS. Insomnia in psychiatric disorders. In: Attarian HP, Schuman C, editors. Clinical Handbook of Insomnia. New York, NY: Humana Press; 2010. pp. 229–41. [Google Scholar]
- 4.Chen MC, Burley HW, Gotlib IH. Reduced sleep quality in healthy girls at risk for depression. J Sleep Res. 2012;21:68–72. doi: 10.1111/j.1365-2869.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WD. The effects of sleep deprivation on symptoms of psychopathology in healthy adults [published online ahead of print March 26, 2007] Sleep Med. 2007;8:215–21. doi: 10.1016/j.sleep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehvioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep 2004;27:600] Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 7.Choo WC, Lee WW, Venkatraman V, Sheu FS, Chee MW. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone [published online ahead of print January 27, 2005] Neuroimage. 2005;25:579–87. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults [published online ahead of print February 9, 2009] J Gerontol B Psychol Sci Soc Sci. 2009;64B:180–7. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenuit P, Kerkhofs M. Effects of sleep restriction on cognition in women [published online ahead of print October 3, 2007] Biol Psychol. 2008;77:81–8. doi: 10.1016/j.biopsycho.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Joormann J. Attentional bias in dysphoria: the role of inhibitory processes. Cogn Emotion. 2004;18:125–47. [Google Scholar]
- 11.Goeleven E, De Raedt R, Baert S, Koster EH. Deficient inhibition of emotional information in depression. J Affect Dis. 2006;93:149–57. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Koster EHW, De Raedt R, Goeleven E, Franck E, Crombez G. Mood-congruent attentional bias in dysphoria: maintained attention to and impaired disengagement from negative information. Emotion. 2005;5:446–55. doi: 10.1037/1528-3542.5.4.446. [DOI] [PubMed] [Google Scholar]
- 13.Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. J Abnorm Psychol. 2008;117:182–92. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- 14.Waters F, Bucks RS. Neuropsychological effects of sleep loss: implication for neuropsychologists. J Int Neuropsychol Soc. 2011;17:571–86. doi: 10.1017/S1355617711000610. [DOI] [PubMed] [Google Scholar]
- 15.Calogiuri G, Weydahl A, Carandente F. Methodological sssues for studying the rest-activity cycle and sleep disturbances: a chronobiological approach using actigraphy data [published online ahead of print August 5, 2011] Biol Res Nurs. 2013;15:5–12. doi: 10.1177/1099800411416224. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. J Sleep Res. 1985;8(1):11–9. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet MH. Performance and sleepiness following moderate sleep disruption and slow wave sleep deprivation. Physiol Behav. 1986;37:915–8. [PubMed] [Google Scholar]
- 18.Lim AS, Yu L, Costa MD, Leurgans SE, Buchman AS, Bennett DA, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35:633–40. doi: 10.5665/sleep.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turek FW. From circadian rhythms to clock genes in depression. Int Clin Psychopharmacol. 2007;22(suppl 2):S1–8. doi: 10.1097/01.yic.0000277956.93777.6a. [DOI] [PubMed] [Google Scholar]
- 20.Allebrandt KV, Teder-Laving M, Akyol M, Pichler I, Müller-Myhsok B, Pramstaller P, et al. CLOCK gene variants associate with sleep duration in two independent populations [published online ahead of print February 10, 2010] Biol Psychiatry. 2010;67:1040–7. doi: 10.1016/j.biopsych.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 22.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 24.Van Someren EJ. Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J Sleep Res. 2007;16:269–75. doi: 10.1111/j.1365-2869.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 25.Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–60. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- 26.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:563–72. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 27.Berle J, Hauge E, Oedegaard K, Holsten F, Fasmer OB. Actigraphic registration of motor activity reveals a more structured behavioural pattern in schizophrenia than in major depression. BMC Res Notes. 2010;3:149. doi: 10.1186/1756-0500-3-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 29.Lundqvist D, Flykt A, Ohman A. The Karolinka Directed Emotional Faces-KDEF, CDROM. Department of Clinical Neuroscience, Psychology section. The Czech Republic: Karolinska Institutet; 1998. [Google Scholar]
- 30.Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:127–35. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 31.Kline RB. Principles and Practice of Structural Equation Modeling. New York, NY: Guilford Press; 1998. [Google Scholar]
- 32.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies [published online ahead of print February 5, 2011] J Affect Dis. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Fictenberg NL, Putnam SH, Mann NR, Zafonte RD, Millard AE. Insomnia screening in postacute traumatic brain injury: utility and validity of the Pittsburgh Sleep Quality Index. Am J Phys Med Rehabil. 2001;80:339–45. doi: 10.1097/00002060-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Goel N. Searching for genetic clues at the interface of sleep and mood. Sleep. 2012;35:739–40. doi: 10.5665/sleep.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maxwell SE. The persistence of underpowered studies in psychological research: causes, consequences, and remedies. Psychol Methods. 2004;9:147–63. doi: 10.1037/1082-989X.9.2.147. [DOI] [PubMed] [Google Scholar]
- 36.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]