Abstract
Purpose of review
Prediction models in critical illness are generally limited to short-term mortality and uncommonly include patient-centered outcomes. Current outcome prediction tools are also insensitive to individual context or evolution in healthcare practice, potentially limiting their value over time. Improved prognostication of patient-centered outcomes in critical illness could enhance decision making quality in the ICU.
Recent Findings
Patient-reported outcomes (PROs) have emerged as precise methodological measures of patient-centered variables and have been successfully employed using diverse platforms and technologies, enhancing the value of research in critical illness survivorship and in direct patient care. The learning health system is an emerging ideal characterized by integration of multiple data sources into a smart and interconnected health information technology infrastructure with the goal of rapidly optimizing patient care. We propose a vision of a smart, interconnected learning health system with integrated electronic PROs (ePRO) to optimize patient-centered care including critical care outcome prediction.
Summary
A learning health system infrastructure integrating ePROs may aid in the management of critical illness associated conditions and yield tools to improve prognostication of patient-centered outcomes in critical illness.
Keywords: critical illness, prediction models, decision support, shared decision making, patient reported outcomes
Introduction and purpose: how can we address problems with patient-centered prediction models?
Imagine being able to predict the future in detail for a critically ill patient who is the focus of a treatment decision in the intensive care unit (ICU). What would the patient, their family, and their doctor want to know? Survival would naturally be one of the first questions, yet many would also wonder what to expect from different choices in terms of their future quality of life, ability to interact meaningfully with family, functional independence, and symptom burdens. Others may wish to know how likely it is that they can reach certain life milestones, such as the birth of a grandchild. The list of prognostic concerns would be unique depending on each unique patient, situation, or decision. While families and clinicians engage in these discussions daily, extrapolating data from other studies or personal experiences, there are few validated prediction models that can add specificity to patient-centered outcome prognostication in ICU settings.
In this article, we will discuss the informational needs of ICU decision making participants, limitations of current patient-centered prediction models, and describe possible future approaches that could address these limitations. We will focus on the development of validated and practical patient generated or patient-reported outcome measures (PROs) and integration of these measures within electronic health care records (EHR) systems, an innovation that could expedite the development of individualized prediction models that are sensitive to the outcomes about which patients care most.
Important problems with patient-centered outcomes prediction in the ICU exist
Decision making is a universally important element of medicine. The key clinical tasks include basic information gathering, the process of deciding, taking action, and then evaluating the outcome of the decision. Yet, the first step—understanding what the problem is and what might happen next—is perhaps the most challenging.1 There is currently a stark contrast between the ideal and the actual state of critical care outcome prediction—the scientific approach to quantifying an answer to the question, “What should I expect?”
First, the repertoire of outcomes and the time horizon of our tools are limited. Traditional outcome measures in critical care research are often short-term and procedure/process-driven—28-day mortality, ventilator-free days, and ICU length of stay. Though these models can improve physicians’ accuracy in short-term survival estimates2, intensivists are fairly good at estimating short-term outcomes without use of these tools, likely because of their internal database of experience.3 Intensivists’ accuracy diminishes significantly, however, when predicting outcomes at 1 year or longer.4 Few intensivists personally witness ICU patients’ long-term outcomes, which may foster a subconscious disconnect between expectations and actual outcomes experienced, potentially limiting their ability to appropriately counsel patients and families in an ICU setting. To help address this, some have created long-term prediction tools targeting 1-year mortality.5 However, these models still fail to provide a multi-dimensional prognostic picture about what to expect for functional ability, symptoms, and other patient-centered outcomes in the months to years following a course of ICU care.6
Also, many prediction models are poorly sensitive to context and individual circumstance. Perhaps the most common reason that physicians fail to incorporate model-based prognostic estimates into practice is a sense of uncertainty about their validity as applied to a patient who may not have been well represented in the models’ original derivation. These models are expensive to develop, though, and can rarely accommodate large inception cohorts with numerous statistically robust subgroups. Furthermore, decisional participants often have a sense that there are important unmeasured factors that may place someone in the 30% of survivors (vs. the 70% of non-survivors) for example.7
Finally, current models are static and not optimized to changing healthcare environments, epidemiology, or standards of care because they are derived in one point of time and may not be easily updated. Only a minority of the models incorporate continuous data analysis (such as through electronic health records) to provide feedback and enhance accuracy. As a result, we know little about how prediction models hold up in the face of subsequent developments in health care technologies or changes in practice patterns. Many, in fact, are simply abandoned as time goes on because of a lack of funding for upkeep. It is prohibitively expensive to continually address the shortcomings of current outcome prediction with the current paradigm or infrastructure.
Patient reported outcomes measures could play a key role in ICU care and prediction models
Patients certainly care about mortality. In fact, they want to be as healthy as possible, maximizing productivity and minimizing personal costs and symptom burden. Yet as part of this goal, they also care about the personal impact of the potentially profound functional, cognitive, neuropsychiatric, and even financial effects of critical illness.8–12 These commonly form the core of the discussion during difficult decisions in ICU settings. But there is clear evidence of discordance in expectations for survival and functional status between physicians and families (as well as between individuals’ expectations and reality).4 While this discordance may reflect mistrust, inadequate communication, poor understanding of medical or prognostic information, or many other factors, we believe this may also reflect the fact that current prediction models are not based on patient-centered outcomes—that is, outcomes that patients value.13
In contrast to traditional, observed outcomes, a patient generated, or patient reported, outcome (PRO) is a patient’s subjective assessment of personal experience and how they feel.14 Classically, PROs reflect quality of life, symptoms, satisfaction with care, and other experiential concerns; the list is growing. PROs are inherently patient-centered. PRO data can be valuable both as static (cross-sectional) and dynamic (longitudinal, multiple measures) reflections of personal outcomes when measuring effectiveness.14 PROs are increasingly accepted as precise methodological tools that enhance the value of both clinical trials and clinical care.14 They can be assessed using a variety of technologies including remotely over the telephone and with electronic interfaces; mixing modes of data collection (e.g., paper plus mobile technology solutions) increases the ability to align data collection with a patient’s personal needs, and evolving analytic strategies support this approach.15
Information about PRO-based predictors of patient-centered outcomes in critical care is emerging. While understanding these predictors might improve the immediate holistic management of the critically ill person, there is no current prognostic tool that can predict the trajectories of patient-centered long-term functional outcomes. And, the current health information technology infrastructure and research paradigm impedes the development of such models. Is there a way forward?
Four key current barriers to integrating patient generated outcomes measures into our practice
PROs could play an important role in allowing outcome prediction to become more patient-centered. However, to evolve from their current primary role as a research tool to a respected aspect of critical care delivery, PROs must overcome several key challenges. First, there are not universally accepted PRO measures applicable for critical care. Lack of a common outcomes language, difficulty of prioritizing outcomes for individuals, and cognitive and communication difficulties among patients have slowed progress on this front. Second, not all current PROs are validated in an electronic format for convenient use and data transmission or aggregation; full-scale psychometric testing of every instrument that is transitioned from paper to electronic may not be necessary, but better understanding the performance of digitally-collected survey instruments among the variety of complex patient scenarios encountered in the ICU is fundamental.16 Third, in most health systems, there is flood of data from multiple poorly connected sources that cannot be easily integrated and viewed by the clinician, researcher, and patient/family users; better integration of PRO data with diverse datasets is fundamental to developing longitudinal prediction models that incorporate PROs. Finally, most EHRs do not have PRO capabilities; even when patient portals are in place, the systems generally do not make it simple and user-friendly to gather and present PRO data, as that was not their primary purpose. The sum result is an unsupportive, disconnected system of health care data that may frustrate its users.
Smart and connected health systems: a novel way to address the current barriers to integrating patient generated outcomes measures within electronic health records systems
A smart and connected healthcare system that incorporates web- and mobile-based PROs into EHRs could expedite patient-centered care and, subsequently, decision-making for ICU patients and survivors. The goal is to make PRO data easily collected, accessible, and actionable – driving care in the ICU to be most in line with desired outcomes of patients and families. Simply put, our prosed supportive digital health environment is comprised of a series of data inputs (computer, smartphone, clinical activities), storage of continuously aggregating data, and data translation into output that can support a variety of clinical tasks.
The system envisioned for tracking PROs following critical illness is one example of what is sometimes termed a learning healthcare system, or rapid learning healthcare.17 Such infrastructure has been endorsed by a variety of industry and thought leaders in medicine,18 including the Institute of Medicine.19 Implementing a learning health system has benefits far beyond critical illness outcome prediction, but in our current example, the learning health system model is intended to be a supportive and shared digital infrastructure than can help bridge the gaps in our increasingly complex and fragmented healthcare system.
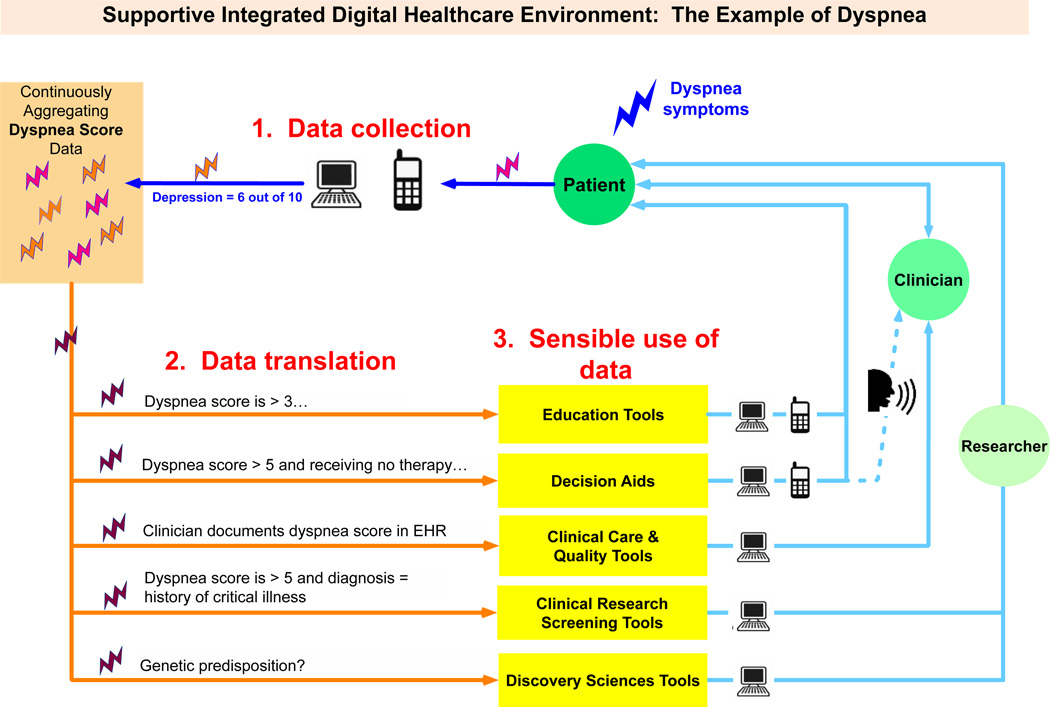
Figure 1 shows this supportive integrated digital healthcare environment of integrated data inputs and outputs addressing dyspnea symptoms, a topic relevance to ICU survivors. In this figure as an example, dyspnea is measured using a simple single item scale at pre-defined intervals (e.g. once a week while in an inpatient setting, each time the patient comes to the clinic, and at least monthly using a personal smartphone). The results for each timepoint are summarized as a single clinically-interpretable 0-10 scale that can be monitored over time (0 = no dyspnea, 10 = severe dyspnea). The 0-10 scale is chosen because it parallels the 0-10 numerical pain scale with which so many clinicians are familiar. Plotted over time, the pattern of the patient’s experience with dyspnea is reflected over the entire trajectory of illness and helps to individualize the holistic management of the condition. Note that many other symptoms could be assessed similarly, though multi-item instruments may require transformation to easily interpretable scales.
Figure 1.
In Step 1, data entry and capture, patients could record dyspnea symptoms with a phone or computer via web-based portals as frequently as they wished. Inpatient and outpatient clinicians could do the same via documentation during clinical interactions in EHRs. Data are integrated from myriad sources into a seamless stream; because a common questionnaire format is being used, the ePRO data integration process is much more efficient. The depression measures are archived within a continuously aggregating pool of data, along with other possible measures such as pain, breathlessness, or satisfaction, to name a few; the overall stream of PRO data can be integrated with other data sources (e.g. EHR, administrative, research databases) to generate a more complete picture of the patient’s depression and symptom experience in context.
But how can one use all of these data sensibly? In Step 2, data rules are applied to translate this dyspnea score information into a format that is usable by patients, clinicians, and researchers. The performance of different clinical tasks (Step 3) requires different uses of the same data. For example, all persons with a dyspnea scale score >3 for longer than a week or two could be directed toward electronic resources for education (e.g., ‘what to expect after a critical illness’). Those with a dyspnea severity scores >5 for over two weeks might be offered electronic decision support after the clinical encounter to decide on a preference for therapy if warranted. And all with a clinically important persistent burden of symptoms could be flagged for researchers to study their course further in the context of surviving critical illness. Importantly, each clinical interaction and intervention that is performed, along with the symptom impact, continues to contribute to the database—as well as the clinical tools that draw from these data.
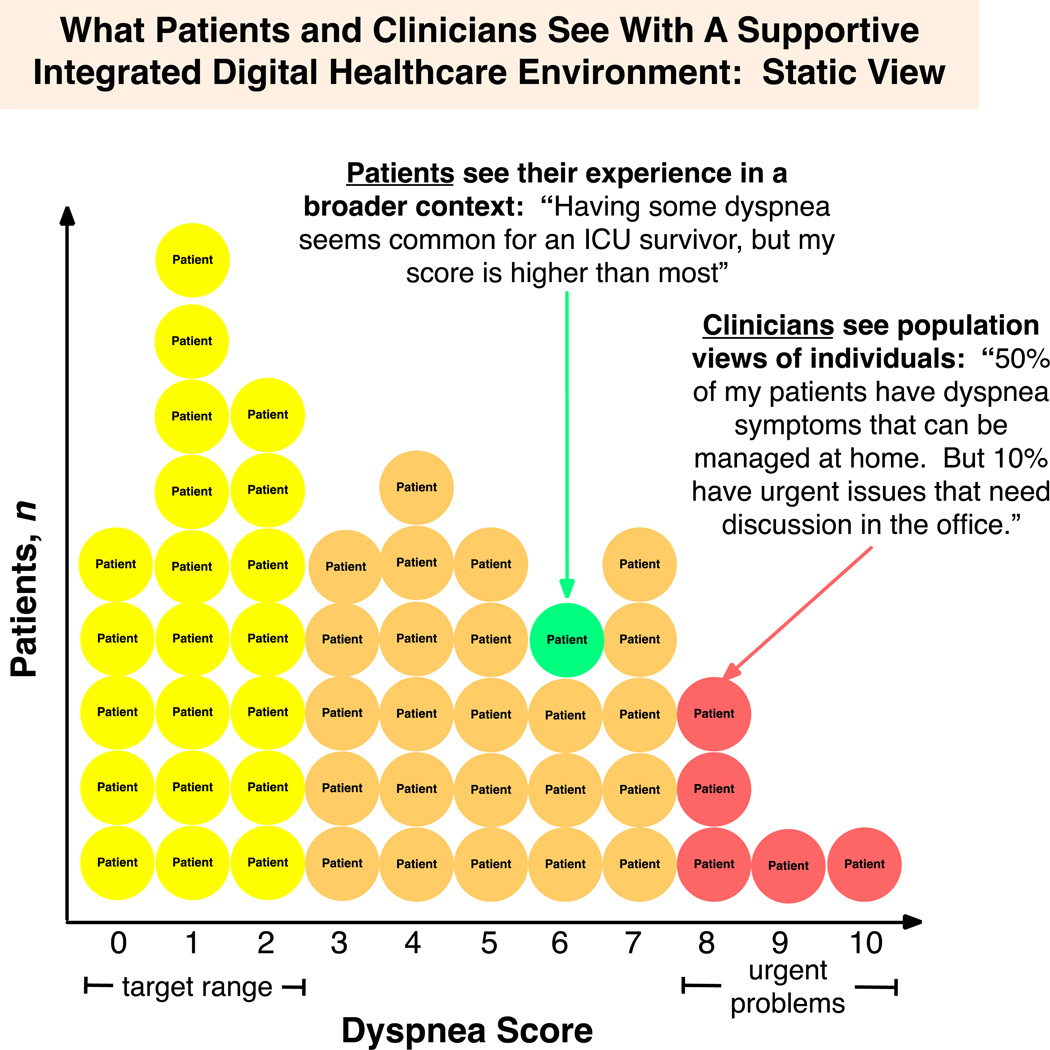
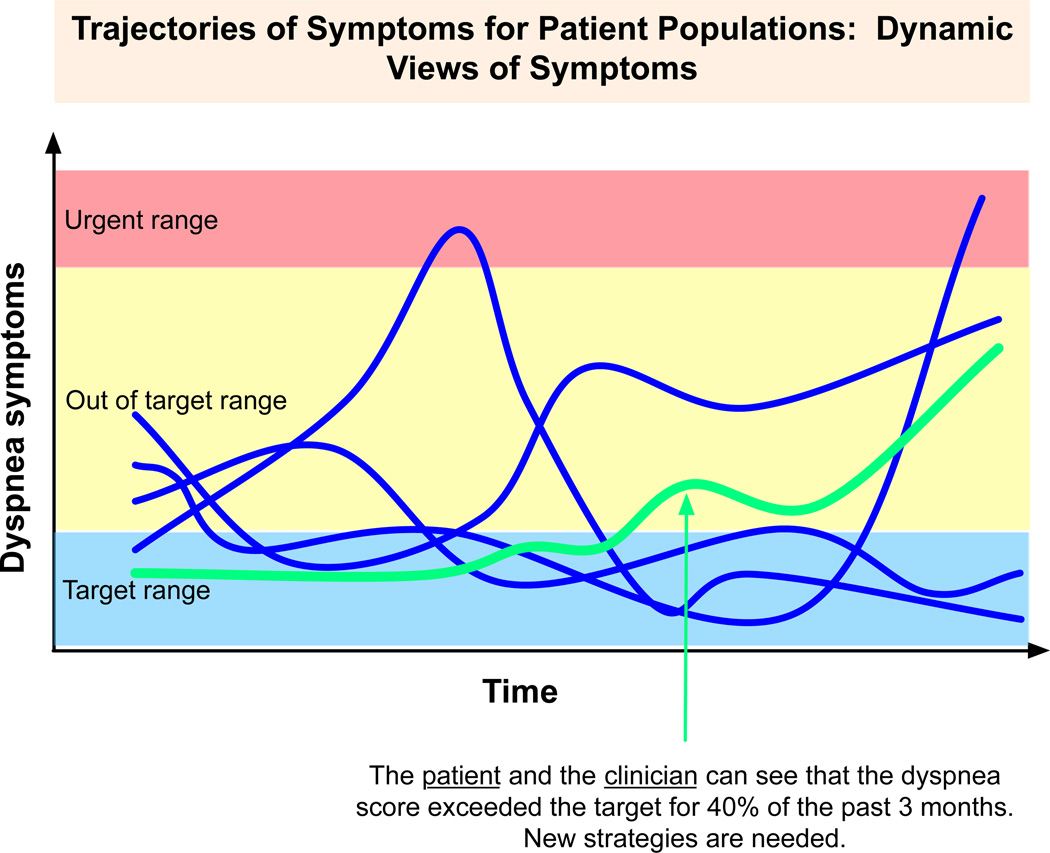
From the perspective of a clinician, such a system would present many advantages for the efficient management of both individuals and populations in both static (cross-sectional) and dynamic (longitudinal) ways. Figure 2 displays a static view in which the clinician and patient can both see how, given an anchor to some time reference, the patient compares to others. This view could also assist physicians in identifying patients at high risk for poor outcomes who need prioritized care in a face-to-face (as opposed to user-directed care) setting. Figure 3 shows how a physician could view dynamic PRO data to appreciate changes of symptoms over time and the duration of time within a target range, better visualizing the symptom burden. Then, both the slope and/or specific ‘alert’ parameters could be flagged as demanding a different therapeutic approach. Nurses, social workers, or case managers could view these collections of symptoms in a dashboard to efficiently direct resources in a timely manner to those in greatest need. This supportive digital data environment of smart, connected healthcare using PROs and patient-centered outcomes trajectory data tools could also inform the development of the multi-dimensional, patient-centered predictive models that we noted earlier are currently so few in number.
Figure 2.
Figure 3.
From a patient/family perspective, these novel, adaptive, patient-centered prediction models could then help to better answer their most common question: “What should a person like me expect to happen with different treatment choices?” The system-derived multi-dimensional prognostic information could help to avert potentially burdensome treatments, align desired outcomes with the optimal management plan, assist in anticipatory preparation for caregiving needs, or identify patients/families who need specific psychological, social, and emotional supports. Importantly, this PRO-based data could be aggregated and analyzed for predictors of patient-centered outcomes, to inform comparative effectiveness of various therapeutic approaches, and to alert patient and clinician users about the financial stresses associated with different treatments.20 The applications of this prognostic power could expand from the individual patient to the health of populations more efficiently and effectively than a series of costly prospective studies.
Challenges and next steps: developing a learning health care system with PROs
There are several important barriers to a learning health system with integrated PROs. Additional PROs need to be developed and/or validated in critical care populations for ICU-relevant symptom complexes and disease states, including critical care survivorship. These PRO tools should be inexpensive, accessible, acceptable to patients, and useful for the care of individuals who interface with the ICU if they are going to gain widespread adoption. We must also address regulatory concerns and technical challenges related to privacy of health information and data sharing between health care institutions, payers, systems, and EHRs. And the siloes that have been erected within the health information technology infrastructure need to be torn down21. The good news is that progress is being made on all of these fronts, even if currently nascent; acceleration of progress, convergence of goals, and the growing expectation of patient-centricity within all healthcare actions will help to lead us there. These preliminary steps, along with buy-in of users, will push us towards a vision where integrated prognostic tools that can support individualized patient-centric decision-making in the ICU with an eye towards longitudinal outcomes. We must set the vision now so that we know for what we are aiming.
Conclusion
The current state of outcome prediction in critical illness is limited by a focus on short-term outcomes judged from the perspective of physicians or health systems, not from a patient-centered view. Many patients will survive their episode of critical illness but suffer severe functional limitations and poor quality of life, and we have little to help us predict which patients these might be or identify these patients for supportive services to help ameliorate their suffering. To address these deficiencies, there is a great potential value proposition for developing a supportive digital healthcare environment that integrates PROs with EHR data. Not only could this empower patients and bring informational equity to clinical interactions, the system’s capabilities could enable to efficient, expedient development of patient-centered outcomes models that could be continuously improved with the acquisition of data. With sufficient aggregated data, this system could be used to develop powerful outcome prediction tools adapted to the unique characteristics of the user and better personalize the delivery of healthcare.
Key Points.
Current outcome prediction in critical care does not address many relevant patient-centered outcomes.
Patient reported outcomes could be incorporated in electronic health records to improve the care of individual patients.
A learning health system that employed electronic patient-reported outcomes could develop more precise prediction of patient-centered outcomes and improve care.
Acknowledgement
We sincerely thank Matthew Harker for his thoughtful suggestions to the concepts.
Grant support: CC was supported by NIH grant R01 HL109823 and PCORI grant PFA 195.
Footnotes
Study design and concept: CC, AA
Writing and review: NW, CC, AA
Final approval: CC, NW, AA
Accountable for all aspects of work: NW
References
- 1. Longo DR, Woolf SH. Rethinking the information priorities of patients. JAMA. 2014;311(18):1857–1858. doi: 10.1001/jama.2014.3038. * Describes the development of several decision-aids and the opportunities offered by developments in mobile technology, but also the heterogeneity in patient's informational desires and needs.
- 2.Knaus WA, Harrell FE, Jr, Lynn J, et al. The SUPPORT prognostic model. Objective estimates of survival for seriously ill hospitalized adults. Study to understand prognoses and preferences for outcomes and risks of treatments. Ann Intern Med. 1995 Feb 1;122(3):191–203. doi: 10.7326/0003-4819-122-3-199502010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Rocker G, Cook D, Sjokvist P, et al. Clinician predictions of intensive care unit mortality. Crit Care Med. 2004 May;32(5):1149–1154. doi: 10.1097/01.ccm.0000126402.51524.52. [DOI] [PubMed] [Google Scholar]
- 4.Cox CE, Martinu T, Sathy SJ, et al. Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med. 2009 Nov;37(11):2888–2894. doi: 10.1097/CCM.0b013e3181ab86ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson SS, Kahn JM, Hough CL, et al. A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit Care Med. 2012 Apr;40(4):1171–1176. doi: 10.1097/CCM.0b013e3182387d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unroe M, Kahn JM, Carson SS, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010 Aug 3;153(3):167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd EA, Lo B, Evans LR, et al. "It's not just what the doctor tells me:" factors that influence surrogate decision-makers' perceptions of prognosis. Crit Care Med. 2010 May;38(5):1270–1275. doi: 10.1097/CCM.0b013e3181d8a217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011 Apr 7;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 9.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012 Jun 15;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covinsky KE, Goldman L, Cook EF, et al. The impact of serious illness on patients' families. JAMA. 1994 Dec 21;272(23):1839–1844. doi: 10.1001/jama.272.23.1839. [DOI] [PubMed] [Google Scholar]
- 11. Needham DM, Wozniak AW, Hough CL, et al. Risk Factors for Physical Impairment after Acute Lung Injury in a National, Multi-Center Study. Am J Respir Crit Care Med. 2014 May 15;189(10):1214–1224. doi: 10.1164/rccm.201401-0158OC. **Measured a patient-reported outcome measure (SF-36 Physical Function score) and 6-minute-walk distance in 6 and 12-month follow-up following course of ALI/ARDS, and found that corticosteroid dose and exposure and ICU duration correlated to poorer physical performance and quality of life.
- 12. Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. The Lancet Respiratory Medicine. 2014;2(5):369–379. doi: 10.1016/S2213-2600(14)70051-7. *Assesed multiple patient-reported outcomes at intervals up to a year following critical illness, finding that depression symptoms dominate and correlate with somatic complaints, suggesting targeting the somatic impairments of ICU survivors could improve their psychological and cognitive function.
- 13.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012 Mar 1;366(9):780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 14. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167. doi: 10.1136/bmj.f167. **Describes rationale of PROs, types of PROs, implementation of PROs in England, and opportunities in their widespread use.
- 15.Abernethy AP, Ahmad A, Zafar SY, Wheeler JL, Reese JB, Lyerly HK. Electronic patient-reported data capture as a foundation of rapid learning cancer care. Med Care. 2010 Jun;48(6 Suppl):S32–S38. doi: 10.1097/MLR.0b013e3181db53a4. [DOI] [PubMed] [Google Scholar]
- 16.Coons SJ, Gwaltney CJ, Hays RD, et al. Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2009 Jun;12(4):419–429. doi: 10.1111/j.1524-4733.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- 17.Abernethy AP, Kamal AH, Wheeler JL, Cox C. Management of dyspnea within a rapid learning healthcare model. Current opinion in supportive and palliative care. 2011 Jun;5(2):101–110. doi: 10.1097/SPC.0b013e32834582b3. [DOI] [PubMed] [Google Scholar]
- 18. Grumbach K, Lucey CR, Johnston SC. Transforming from centers of learning to learning health systems: the challenge for academic health centers. JAMA. 2014 Mar 19;311(11):1109–1110. doi: 10.1001/jama.2014.705. **Describes opportunities for academic health centers to become centers of learning healthcare
- 19.Grossmann C, Powers B, McGinnis JM Institute of Medicine (U.S.). Roundtable on Value & Science-Driven Health Care. Digital infrastructure for the learning health system : the foundation for continuous improvement in health and health care : workshop series summary. Washington, D.C.: National Academies Press; 2011. [PubMed] [Google Scholar]
- 20. Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. Oncologist. 2013;18(4):381–390. doi: 10.1634/theoncologist.2012-0279. *Employed patient reports of financial strain to estimate the financial impact of cancer treatments, highlighting an important patient-centered outcome of care.
- 21. Schneeweiss S. Learning from Big Health Care Data. New England Journal of Medicine. 2014;370(23):2161–2163. doi: 10.1056/NEJMp1401111. **Emphasizes the benefits of big healthcare data and point of care decision support in the direct care of patients, including the value and importance of rapidly updating predictive models. Also addresses the impediments to integrating health-record data into a usable common format.