Abstract
Wamstad et al have provided a robust global analysis of histone markers and gene expression at 4 stages of murine embryonic stem (ES) cell differentiation into cardiac myocytes. This detailed data set will provide a rich opportunity for generating and testing hypotheses related to combinatorial transcriptional regulation of gene expression and epigenetic regulation of cell fate decisions in cardiac lineages.
Transcriptional regulation of cardiac development has been the focus of intense investigation for several decades. The recent elucidation of a histone code that characterizes epigenetic control of gene expression and the status of open and closed chromatin1 has revolutionized our understanding of the regulation of cell fate, cell differentiation, and environmentally mediated alterations in gene expression. At the same time, new techniques and technology, including affordable next-generation sequencing and genome-wide assays for chromatin occupancy by transcription factors, have offered new opportunities for observing the dynamic state of chromatin and gene expression during development.2,3 Bioinformatic programs are evolving that permit interpretation and organization of these large datasets into systems and networks. A recent report published in the Journal Cell by Wamstad et al4 is an example of the focused use of these new techniques to provide a global analysis of histone markers and transcriptional activity during cardiac myocyte specification and differentiation.
These investigators took advantage of recently optimized cell culture techniques for the expansion and differentiation of murine ES cells into contracting cardiac myocytes. They selected 4 stages of differentiation to study in detail: pluripotent ES cells, mesodermal cells characterized by expression of Mesp1 and Brachyury, cardiac progenitor cells expressing Nkx2.5, Tbx5, and Isl1, and functional cardiac myocytes expressing structural sarcomere proteins, such as cardiac troponin T (Figure). At each stage, they performed intensive global analysis of gene expression, including polyadenylated transcripts (eg, mRNA), microRNAs, and long noncoding RNAs that have recently been implicated in gene regulation.5 In addition, they performed chromatin immunoprecipitation with a series of antibodies that recognize modified histone residues, followed by sequence analysis of the precipitated DNA fragments (chromatin immunoprecipitation and sequencing [ChIP-seq]). For example, they analyzed histone 3 lysine 27 (H3K27) trimethylation mark characteristic of inactive chromatin and H3K4 trimethylation, which characterizes active promoters. H3K4 monomethylation and H3K27 acetylation, characteristic of active enhancers, were also analyzed at each time point. They also documented the binding of RNA polymerase II at active transcriptional start sites. This wealth of data provides a global snapshot of the state of chromatin and gene expression at 4 distinct stages of cardiac myocyte differentiation.
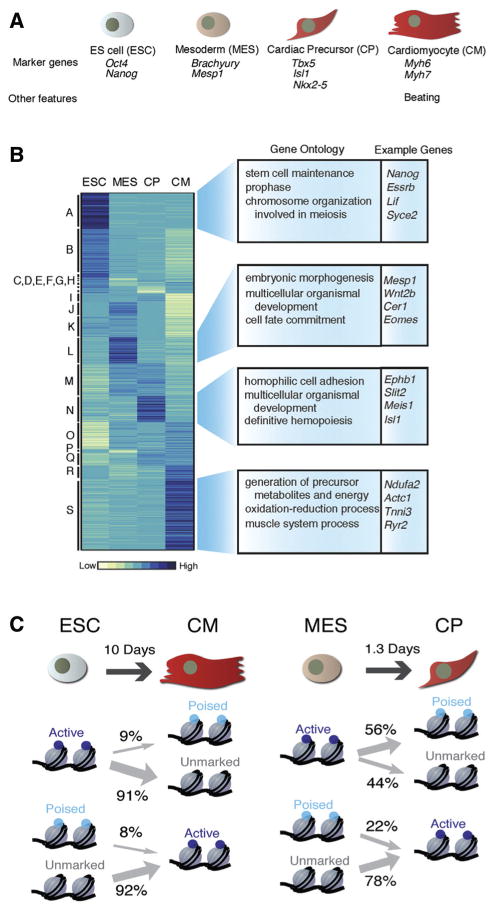
Figure.
A, The 4 stages of differentiation analyzed by Wamstad et al. B, Hierarchical clustering of coding and noncoding polyA+ gene expression in the 4 cell types. Enriched gene ontogeny (GO) terms and example genes are shown to the right. C, Enhancer state transitions between cell types. Note that many genes poised for expression at early time points (in ESC or MES) do not become active at later times (CP or CM). Figure panels reprinted from Wamstad et al4 with permission of the publisher. Copyright © 2013, Elsevier Inc.
The richness and complexity of this new dataset can be partially appreciated by analysis of some of the early conclusions drawn by Wamstad et al. For example, they interpret their combined results to predict the existence of >81 000 specific enhancer elements that are active during various stages of cardiac differentiation. Only a handful of these regulatory elements have been previously described and analyzed in detail, and further studies will be necessary to determine the functional accuracy of these predictions. However, many of these regulatory elements were previously implicated from related studies using murine or human heart cells, supporting the veracity of the present approach.6–8 The authors also predict a previously unappreciated significance for the H3K4 monomethyl mark that has previously been thought to indicate active enhancers. They found that this mark was also enriched at active cardiac promoters and, in the absence of H3K27 acetylation, seems to mark poised genes that are destined to be more fully activated at later stages of differentiation. Indeed, these authors found that a subset of genes marked at early time points by H3K4 monomethylation were destined to acquire H3K4 trimethylation and transcriptional activation at later time points of differentiation. However, other subgroups of genes marked by H3K4 monomethylation at their promoters subsequently lost this mark or failed to go on to exhibit H3K4 trimethylation or active gene expression. This could indicate the presence of classes of noncardiac genes that are transiently poised for expression in multipotential progenitor cells at later times in non-cardiac lineages, or this might represent a limitation of the ES cell-based assay system, and the time points chosen for examination, which may never reflect the gene expression programs of fully mature and robust adult cardiac myocytes.
Perhaps, not surprisingly, Wamstad et al found that cardiac-specific enhancers were enriched for binding motifs recognized by stage-specific cardiac transcription factors. Although this finding is not unexpected, it supports the conclusion that the algorithms used to identify enhancers are robust, and it offers an enormous and exciting dataset of predicted target genes that may be regulated by specific transcription factors. Some of these newly identified direct targets of cardiogenic transcription factors may play a role in the pathogenesis of common forms of congenital heart disease. In addition, analysis of the timing and number of enhancers that contain conserved combinations of binding motifs suggest combinatorial interactions that are likely to form regulatory networks during cardiac differentiation. For example, sets of genes activated at the cardiac progenitor stage were frequently found to contain a combination of Meis (a homeodomain-containing transcription factor), and GATA binding motifs, and many of these genes are known to have important functions in cardiac differentiation and during development of the conduction system. Indeed, luciferase reporter assays confirmed that 4 of 5 genes that were predicted to be coregulated by Meis and GATA could be synergistically activated by the combination of these 2 factors.
An important limitation of the study is that it was performed using ES cells differentiated in vitro into cardiac myocytes. Although this system closely mimics cardiac development in vivo, differences also exist. Patterning signals that inform chamber specification and subtypes of cardiac myocyte derivatives, including trabecular myocytes, compact zone myocytes, atrial and ventricular myocytes, and components of the specialized conduction system, are not fully recapitulated, and it is unclear whether all of these cell types are present in the culture system that was used. Indeed, it is unlikely that this system recapitulates all of the spatially and temporally regulated signals communicated between endocardial, myocardial, and epicardial cells in the embryonic heart. Moreover, despite recent advances,9 many of the cells in the ES culture system do not differentiate into cardiac muscle (perhaps 30%), and gene expression and ChIP seq signals from these cells contaminate the dataset provided by Wamstad et al. The assays were performed using pooled populations of cells at each time point, and the data represent averages of signals found in multiple cell types. It is therefore not possible to determine whether a specific cell exhibiting a certain transcriptional profile and set of histone markers is destined to be a cardiac myocyte, nor is it possible to determine whether any given cell exhibits the entire pattern of gene expression and chromatin configuration predicted by the pooled averages. Finally, the ability of cultured myocytes derived from ES cells to mature into fully differentiated adult-like muscle is limited, and the full program of adult myocyte gene expression is therefore unlikely to be duplicated in this dataset.
Nevertheless, these findings are likely to inspire further analysis of the coordinated regulation of chromatin structure and gene expression at all stages of cardiac differentiation both in vivo and in vitro. The initial interpretation of these findings, and the production of combinatorial networks of transcription factors that regulate a host of cardiac-specific enhancers, will need to be tested empirically, and the algorithms used to make these predictions will need to be modified and updated to incorporate experimental results. Indeed, an important challenge for the field will be to move beyond correlation to test the functional significance of the observed histone-modification patterns. Independent validation will help to eliminate noise introduced by the experimental system and by the rapidly evolving techniques themselves. It is likely that our understanding of the significance of specific histone modifications will continue to evolve, and a more complete analysis of global histone modifications will undoubtedly augment our understanding of the epigenetic regulation of gene expression during progressive lineage restriction and cardiac myocyte fate specification. Ultimately, this understanding will allow us to better control cardiac differentiation of various stem cell populations through the use of histone-modifying enzymes and small molecules that regulate their activity. Moreover, these data may also provide novel insights into the pathogenesis of cardiac adaptation to stress and cardiomyopathy.
Footnotes
The opinions expressed in this Commentary are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 2.Furey TS. ChIP-seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet. 2012;13:840–852. doi: 10.1038/nrg3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10:669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wamstad JA, Alexander JM, Truty RM, et al. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell. 2012;151:206–220. doi: 10.1016/j.cell.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muers M. RNA: Genome-wide views of long non-coding RNAs. Nat Rev Genet. 2011;12:742. doi: 10.1038/nrg3088. [DOI] [PubMed] [Google Scholar]
- 6.Blow MJ, McCulley DJ, Li Z, et al. ChIP-Seq identification of weakly conserved heart enhancers. Nat Genet. 2010;42:806–810. doi: 10.1038/ng.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He A, Kong SW, Ma Q, Pu WT. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc Natl Acad Sci USA. 2011;108:5632–5637. doi: 10.1073/pnas.1016959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May D, Blow MJ, Kaplan T, et al. Large-scale discovery of enhancers from human heart tissue. Nat Genet. 2012;44:89–93. doi: 10.1038/ng.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]